Developing a CeS2/ZnS Quantum Dot Composite Nanomaterial as a High-Performance Cathode Material for Supercapacitor
Abstract
1. Introduction
2. Experimental Section
2.1. Materials Synthesis
2.2. Synthesis of CeS2
2.3. Synthesis of ZnS QD
2.4. Synthesis of CeS2/ZnS QD Nanocomposite
2.5. Electrode Preparation
3. Characterization
3.1. Morphological and Structural Characterization
3.2. Electrochemical Characterizations
4. Results and Discussion
4.1. Structural and Morphological Analysis
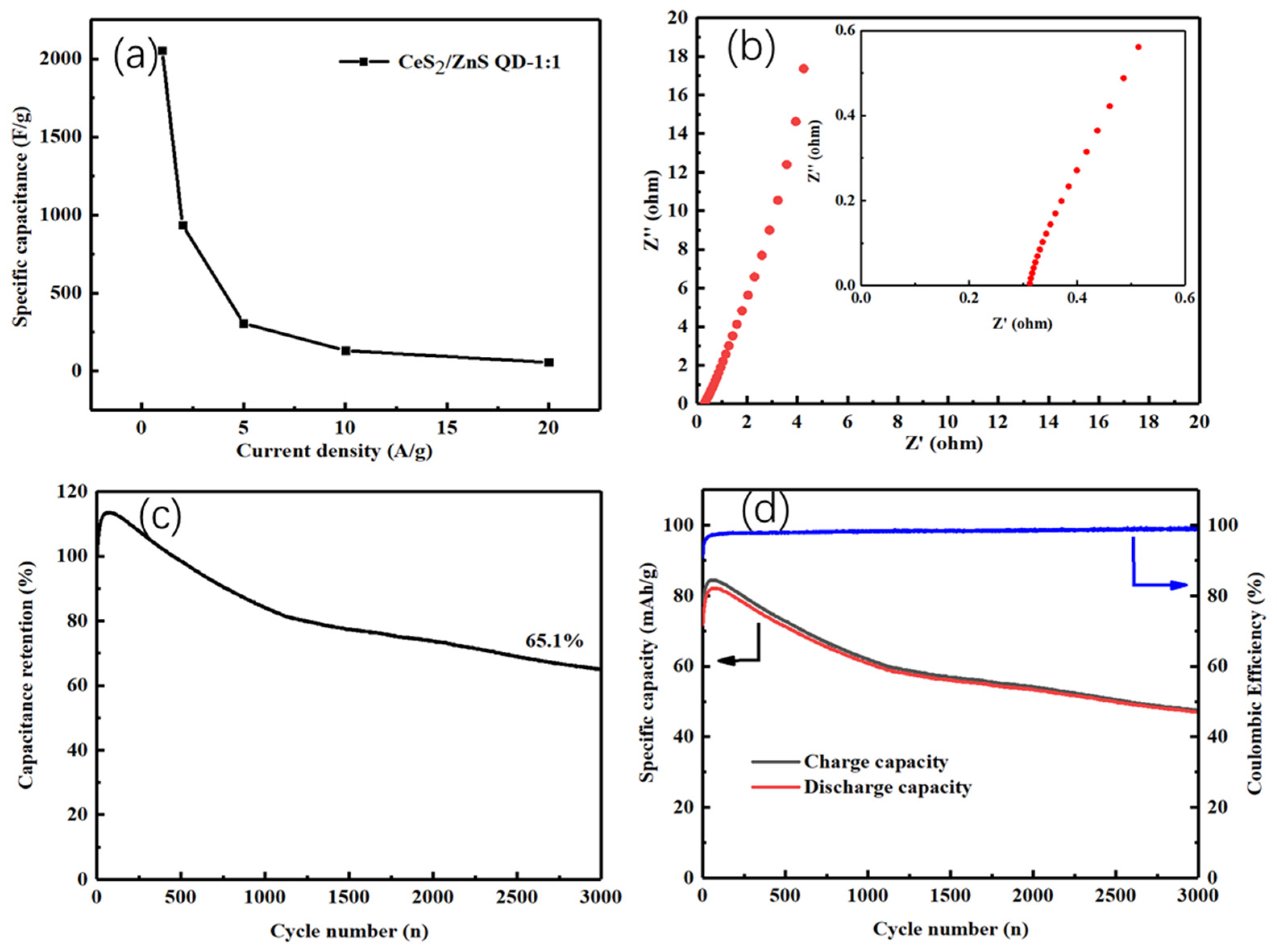
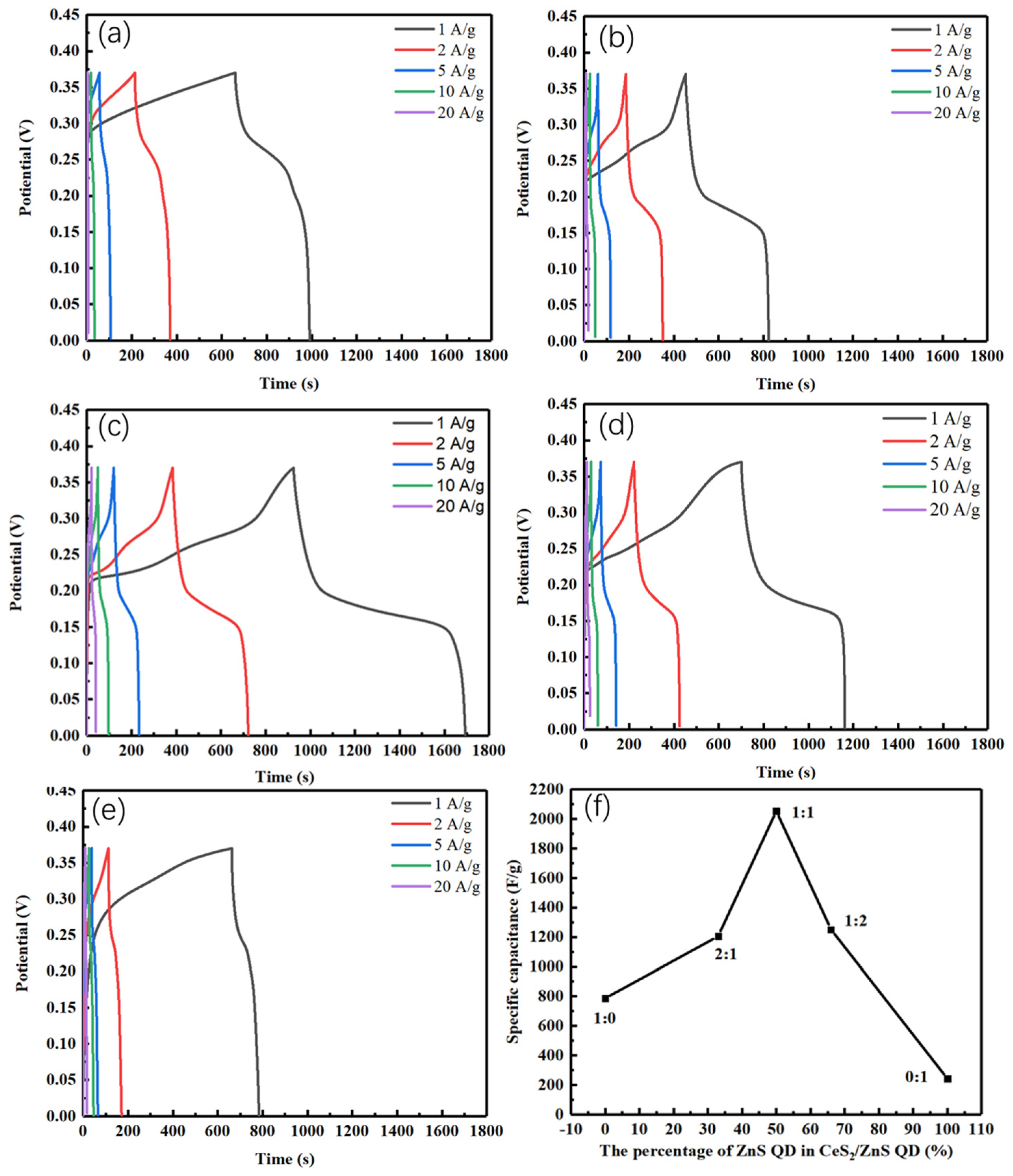
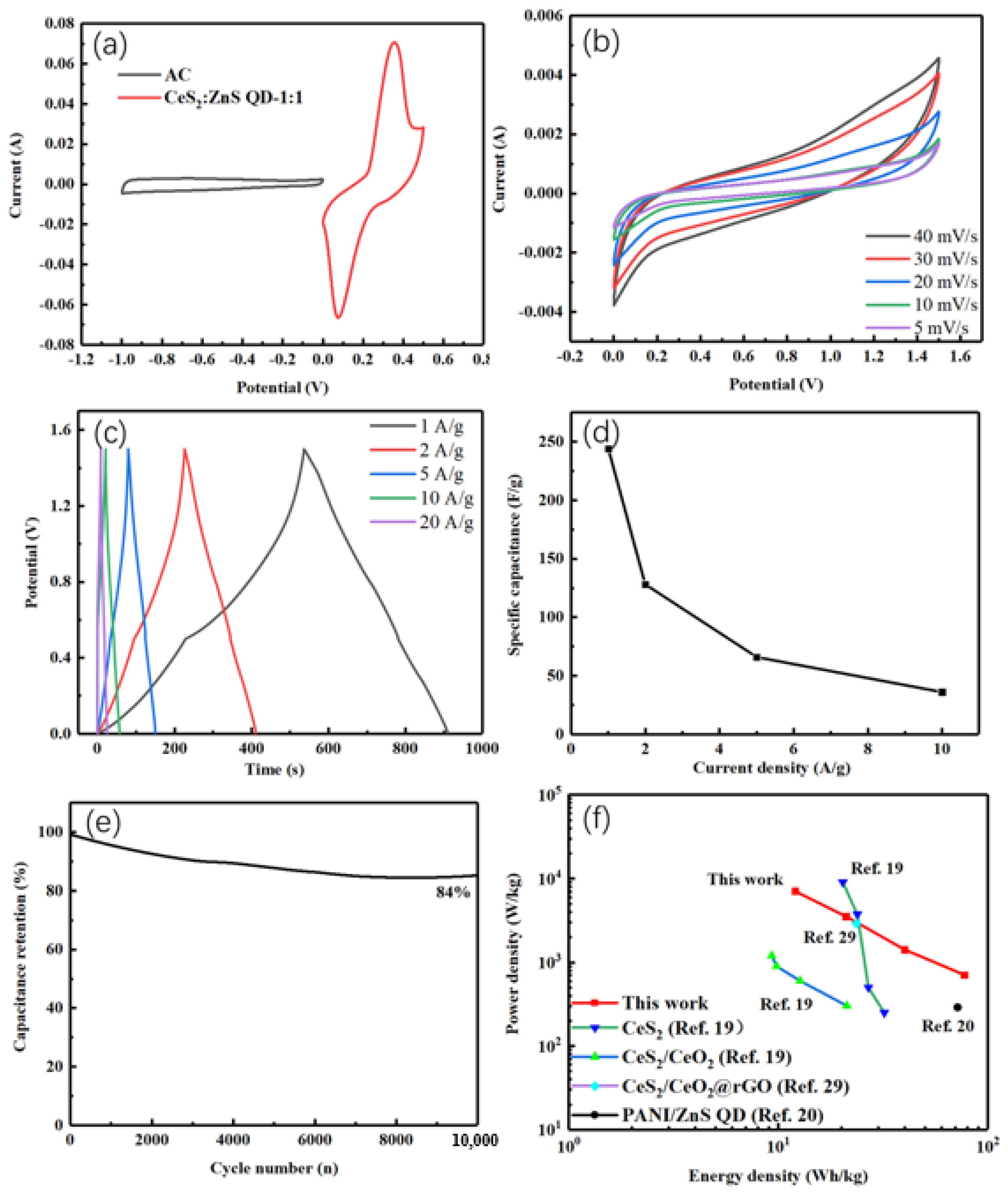
4.2. Electrochemical Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dissanayake, K.; Kularatna-Abeywardana, D. A review of supercapacitors: Materials, technology, challenges, and renewable energy applications. J. Energy Storage 2024, 96, 112563. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, H.; Kumar, G.; Sharma, S.; Aneja, R.; Sharma, A.K.; Kumar, R.; Kumar, P. Progress and challenges in electrochemical energy storage devices: Fabrication, electrode material, and economic aspects. Chem. Eng. J. 2023, 468, 143706. [Google Scholar] [CrossRef]
- Biradar, M.R.; Morajakar, P.P.; Bhosale, S.V.; Bhosale, S.V. A review on energy storage devices based on rylene imide dyes: Synthesis, applications and challenges. Fuel 2021, 310, 122487. [Google Scholar] [CrossRef]
- Biswas, S.; Chowdhury, A. Organic Supercapacitors as the Next Generation Energy Storage Device: Emergence, Opportunity, and Challenges. ChemPhysChem 2023, 24, e202200567. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Zhao, D.; Cheng, M.; Zhou, J.; Owusu, K.A.; Mai, L.; Yu, Y. A new view of supercapacitors: Integrated supercapacitors. Adv. Energy Mater. 2019, 9, 1901081. [Google Scholar] [CrossRef]
- Banerjee, S.; Mordina, B.; Sinha, P.; Kar, K.K. Recent advancement of supercapacitors: A current era of supercapacitor devices through the development of electrical double layer, pseudo and their hybrid supercapacitor electrodes. J. Energy Storage 2024, 108, 115075. [Google Scholar] [CrossRef]
- Shin, S.; Gittins, J.W.; Balhatchet, C.J.; Walsh, A.; Forse, A.C. Metal–Organic Framework Supercapacitors: Challenges and Opportunities. Adv. Funct. Mater. 2023, 34, 2308497. [Google Scholar] [CrossRef]
- Kumar, Y.A.; Yadav, A.A.; Al-Asbahi, B.A.; Kang, S.-W. Sulfur nanoparticle-decorated nickel cobalt sulfide hetero-nanostructures with enhanced energy storage for high-performance supercapacitors. Molecules 2022, 27, 7458. [Google Scholar] [CrossRef]
- Jiang, M.; Abdullah, M.; Chen, X.; E, Y.; Tan, L.; Yan, W.; Liu, Y.; Jiang, W. Two-step synthesis of ZnS-NiS2 composite with rough nanosphere morphology for high-performance asymmetric supercapacitors. Batteries 2023, 10, 16. [Google Scholar] [CrossRef]
- Ikhioya, I.L.; Nkele, A.C. A Novel Synthesis of Hydrothermally Prepared Europium Sulfide and Cerium Sulfide Nanomaterials Doped with Yttrium. Arab. J. Sci. Eng. 2023, 49, 1217–1225. [Google Scholar] [CrossRef]
- Ahmed, F.B.M.; Khalafallah, D.; Zhi, M.; Hong, Z. Porous nanoframes of sulfurized NiAl layered double hydroxides and ternary bismuth cerium sulfide for supercapacitor electrodes. Adv. Compos. Hybrid Mater. 2022, 5, 2500–2514. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, T.; Chen, R.; Fang, H.; Yang, Y.; Cao, Y.; Zhang, L. Molybdenum nitride and oxide quantum dot @ nitrogen-doped graphene nanocomposite material for rechargeable lithium Ion batteries. Batteries 2023, 9, 32. [Google Scholar] [CrossRef]
- Wu, P.; Xu, Y.; Zhan, J.; Li, Y.; Xue, H.; Pang, H. The research development of quantum dots in electrochemical energy storage. Small 2018, 14, 1801479. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Xu, B. Layered double hydroxide with interlayer quantum dots and laminate defects for high-performance supercapacitor. Adv. Funct. Mater. 2023, 33, 2300149. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Xu, C.; Liu, Y.; Xu, J.; Miao, Z.; Yu, H.; Yan, L.; Zhang, L.; Shu, J. Dual synergistic effects assisting Cu-SeS2 electrochemistry for energy storage. Proc. Natl. Acad. Sci. USA 2023, 120, e2220792120. [Google Scholar] [CrossRef]
- Bibi, N.; Xia, Y.; Ahmed, S.; Zhu, Y.; Zhang, S.; Iqbal, A. Highly stable mesoporous CeO2/CeS2 nanocomposite as electrode material with improved supercapacitor electrochemical performance. Ceram. Int. 2018, 44, 22262–22270. [Google Scholar] [CrossRef]
- Salah, N.; Shehab, M.; El Nady, J.; Ebrahim, S.; El-Maghraby, E.; Sakr, A.-H. Polyaniline/ZnS quantum dots nanocomposite as supercapacitor electrode. Electrochim. Acta 2023, 449, 142174. [Google Scholar] [CrossRef]
- Sahoo, S.; Satpati, A.K.; Sahoo, P.K.; Naik, P.D. Incorporation of Carbon Quantum Dots for Improvement of Supercapacitor Performance of Nickel Sulfide. ACS Omega 2018, 3, 17936–17946. [Google Scholar] [CrossRef]
- Elnady, J.; Shaala, T.; Tork, H.M.; Soliman, M.; Ebrahim, S.; Elshaer, A.M. Acquisition of optimum co-sources of sulfur MAA capped-ZnS quantum dots conditions for photoluminescence chlorine sensor. J. Mater. Sci. Mater. Electron. 2021, 32, 16831–16844. [Google Scholar] [CrossRef]
- Lu, C.; Tu, C.; Yang, Y.; Ma, Y.; Zhu, M. Construction of Fe3O4@Fe2P heterostructures as electrode materials for supercapacitors. Batteries 2023, 9, 326. [Google Scholar] [CrossRef]
- Morariu, M.I.; Nicolaescu, M.; Hulka, I.; Duţeanu, N.; Orha, C.; Lăzău, C.; Bandas, C. Fabrication of Cu2O/CuO Nanowires by one-step thermal oxidation of flexible copper mesh for supercapacitor applications. Batteries 2024, 10, 246. [Google Scholar] [CrossRef]
- Shohag, M.R.H.; Khan, M.M.R.; Saleh, M.A.; Supta, A.S.; Chowdhury, M.S.H.; Marwani, H.M.; Rahman, M.M.; Okoli, O. Easy fabrication of L-glutamic acid/ZnS composites for efficient photo-catalytic and supercapacitor performance. RSC Adv. 2023, 13, 24343–24352. [Google Scholar] [CrossRef] [PubMed]
- Powder Diffraction File for Cerium Sulfide (CeS2); International Centre for Diffraction Data: Newtown Square, PA, USA, 2023.
- Powder Diffraction File for Zinc Sulfide (ZnS); International Centre for Diffraction Data: Newtown Square, PA, USA, 2023.
- Yang, X.; Xu, J.; Chen, X.; Lei, Y.; Wang, L.; Cheng, S.; Li, Y.; Lu, Y.; Zhu, Y.; Chen, N. Preparation and Characterization of Porous Carbon from Mixed Leaves for High-Performance Supercapacitors. Chin. J. Chem. 2021, 39, 353–359. [Google Scholar] [CrossRef]
- E, Y.; Shen, X.; Chen, X.; Jiang, M.; Yan, W.; Liu, Y.; Jiang, W.; Abdullah, M. Preparation of biomass composite activated carbon based supercapacitor materials and their application in energy storage devices. Chem. Eng. Sci. 2023, 282, 119193. [Google Scholar] [CrossRef]
- Moyseowicz, A. Scalable one-pot synthesis of bismuth sulfide nanorods as an electrode active material for energy storage applications. J. Solid State Electrochem. 2019, 23, 1191–1199. [Google Scholar] [CrossRef]
- Tong, Y.; Dai, M.; Xing, L.; Liu, H.; Sun, W.; Wu, X. Asymmetric Hybrid Capacitor Based on NiCo2O4 Nanosheets Electrode. Acta Phys. Chim. Sin. 2020, 36, 1903046. [Google Scholar] [CrossRef]
- Yan, W.; Chen, X.; Wang, Z.; Zhao, Z.; Liu, Y.; Muhammad, A. BI2O3/Bi2S3 rod/sheet nanocomposites as battery-type materials for supercapacitors. Chem. Eng. J. 2024, 504, 158864. [Google Scholar] [CrossRef]
- Runfa, L.; Chen, X.; Hongliang, C.; Wei, Y.; Yuanfang, Z.; Siyu, C.; Wenrui, J.; Qi, Z.; Yi, E.; Meng, J.; et al. Facile synthesis of Ni3Se4/Ni0.6Zn0.4O/ZnO nanoparticle as high-performance electrode materials for electrochemical energy storage device. Nanotechnology 2023, 34, 185401. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhang, H.; Duan, X.; Ma, J.; Sun, H.; Tian, W.; Wang, S. Carbonaceous materials in structural dimensions for advanced oxidation processes. Chem. Soc. Rev. 2025, 54, 2436–2482. [Google Scholar] [CrossRef]
- Rabanian, A.; Neghabi, M.; Zadsar, M.; Jafari, M. Theoretical studies of energy states of CdSe/ZnS/CdSe and ZnS/CdSe/ZnS quantum dots with an impurity. Mater. Sci. Eng. B 2021, 274, 115489. [Google Scholar] [CrossRef]
- Ðorđević, L.; Arcudi, F.; Cacioppo, M.; Prato, M. A multifunctional chemical toolbox to engineer carbon dots for biomedical and energy applications. Nat. Nanotechnol. 2022, 17, 112–130. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Y.; Jiang, Q.; Guo, S.; Huang, J.; Xu, L.; Wang, Y.; Li, G. Facile synthesis of cobalt cluster-CoNx composites: Synergistic effect boosts electrochemical oxygen reduction. J. Mater. Chem. A 2022, 10, 16920–16927. [Google Scholar] [CrossRef]
- Khan, U.A.; Iqbal, N.; Noor, T.; Ahmad, R.; Ahmad, A.; Gao, J.; Amjad, Z.; Wahab, A. Cerium based metal organic framework derived composite with reduced graphene oxide as efficient supercapacitor electrode. J. Energy Storage 2021, 41, 102999. [Google Scholar] [CrossRef]
- Kumaresan, L.; Shanmugavelayutham, G.; Saravanan, P. Single-phase ferromagnetic iron nitride (ε-Fe3N) nanoparticles synthesized by thermal plasma method for oxygen evolution and supercapacitor applications. Appl. Phys. A 2022, 128, 1073. [Google Scholar] [CrossRef]
- Liu, S.; Chen, J.; Zhang, C.; Jin, L.; Yang, Q. Experimental study on lithium-ion cell characteristics at different discharge rates. J. Energy Storage 2021, 45, 103418. [Google Scholar] [CrossRef]
- Zheng, W. IR compensation for electrocatalysis studies: Considerations and recommendations. ACS Energy Lett. 2023, 8, 1952–1958. [Google Scholar] [CrossRef]
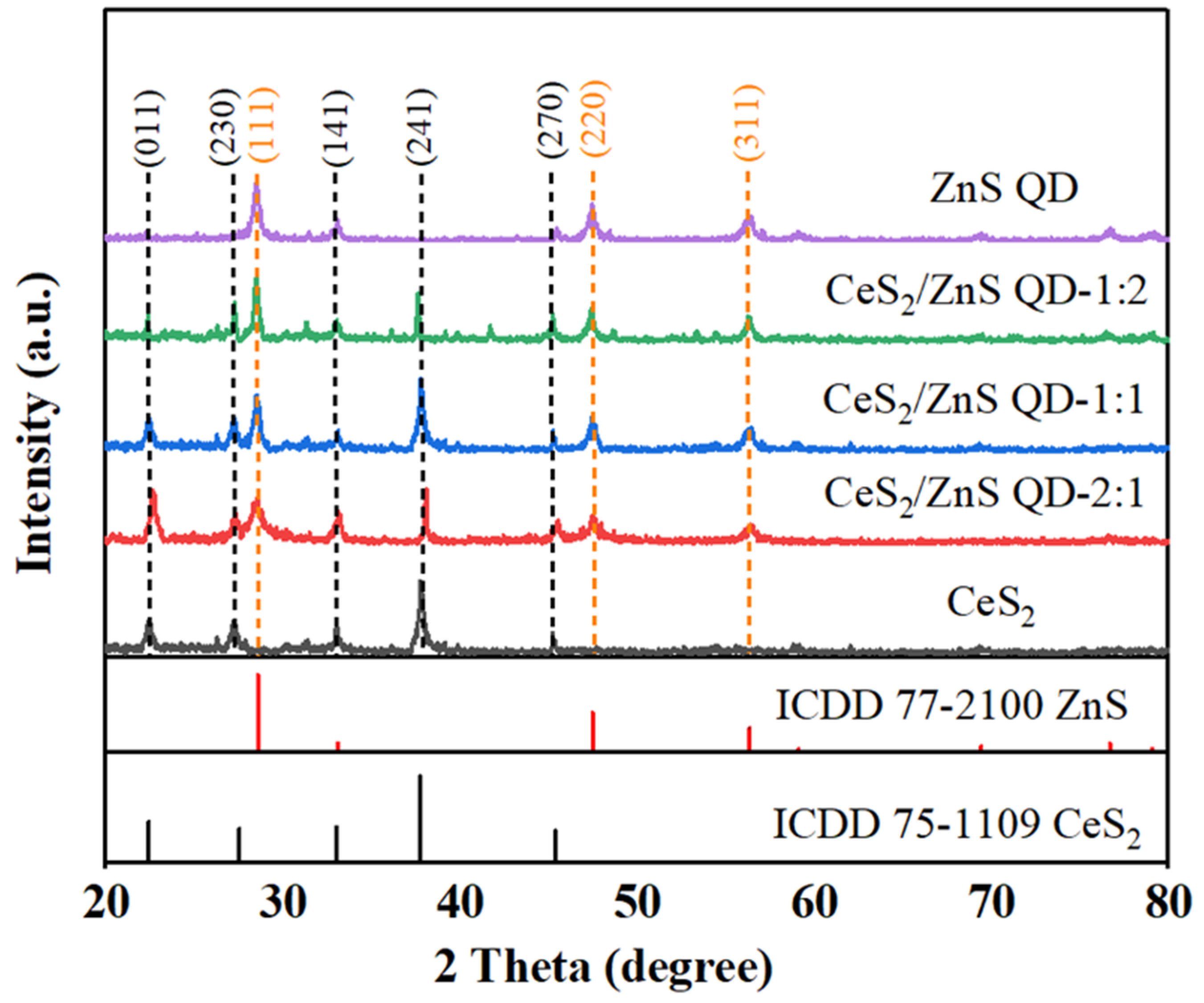

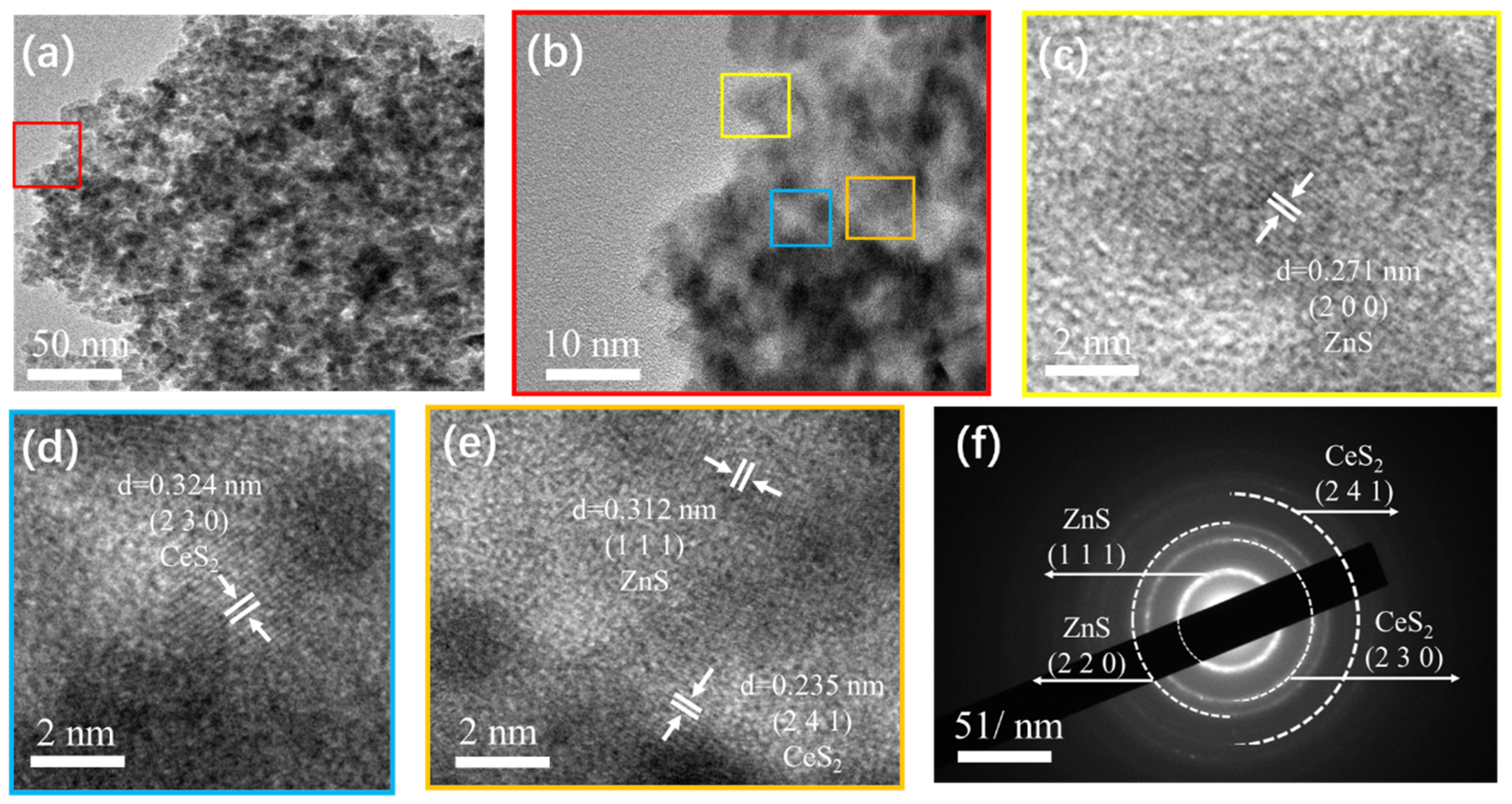
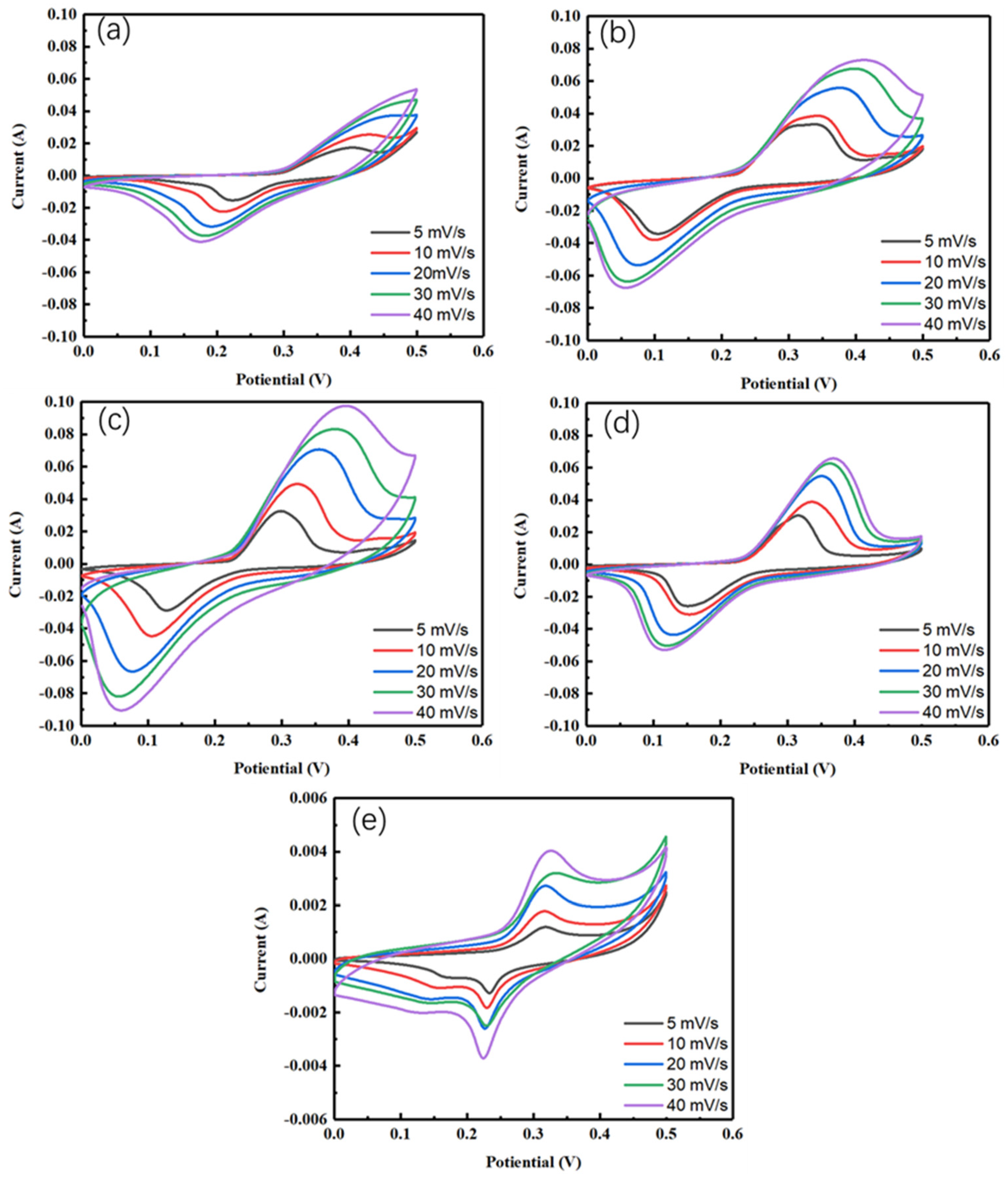
| Current Density (A/g) | CeS2 | CeS2/ZnS QD-2:1 | CeS2/ZnS QD-1:1 | CeS2/ZnS QD-1:2 | ZnS QD |
|---|---|---|---|---|---|
| 1 | 780 | 1205 | 2074 | 1250 | 242 |
| 2 | 346 | 595 | 935 | 690 | 128 |
| 5 | 110 | 226 | 306 | 240 | 48 |
| 10 | 42 | 92 | 131 | 105 | 22 |
| 20 | 14 | 40 | 55 | 48 | 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.-D.; Wu, L.-C.; Adil, M.; Sheng, L.-F.; Zhao, Z.-Y.; Xu, K.; Chen, X. Developing a CeS2/ZnS Quantum Dot Composite Nanomaterial as a High-Performance Cathode Material for Supercapacitor. Batteries 2025, 11, 289. https://doi.org/10.3390/batteries11080289
Xu S-D, Wu L-C, Adil M, Sheng L-F, Zhao Z-Y, Xu K, Chen X. Developing a CeS2/ZnS Quantum Dot Composite Nanomaterial as a High-Performance Cathode Material for Supercapacitor. Batteries. 2025; 11(8):289. https://doi.org/10.3390/batteries11080289
Chicago/Turabian StyleXu, Shan-Diao, Li-Cheng Wu, Muhammad Adil, Lin-Feng Sheng, Zi-Yue Zhao, Kui Xu, and Xin Chen. 2025. "Developing a CeS2/ZnS Quantum Dot Composite Nanomaterial as a High-Performance Cathode Material for Supercapacitor" Batteries 11, no. 8: 289. https://doi.org/10.3390/batteries11080289
APA StyleXu, S.-D., Wu, L.-C., Adil, M., Sheng, L.-F., Zhao, Z.-Y., Xu, K., & Chen, X. (2025). Developing a CeS2/ZnS Quantum Dot Composite Nanomaterial as a High-Performance Cathode Material for Supercapacitor. Batteries, 11(8), 289. https://doi.org/10.3390/batteries11080289








