A Concise Overview of the Use of Low-Dimensional Molybdenum Disulfide as an Electrode Material for Li-Ion Batteries and Beyond
Abstract
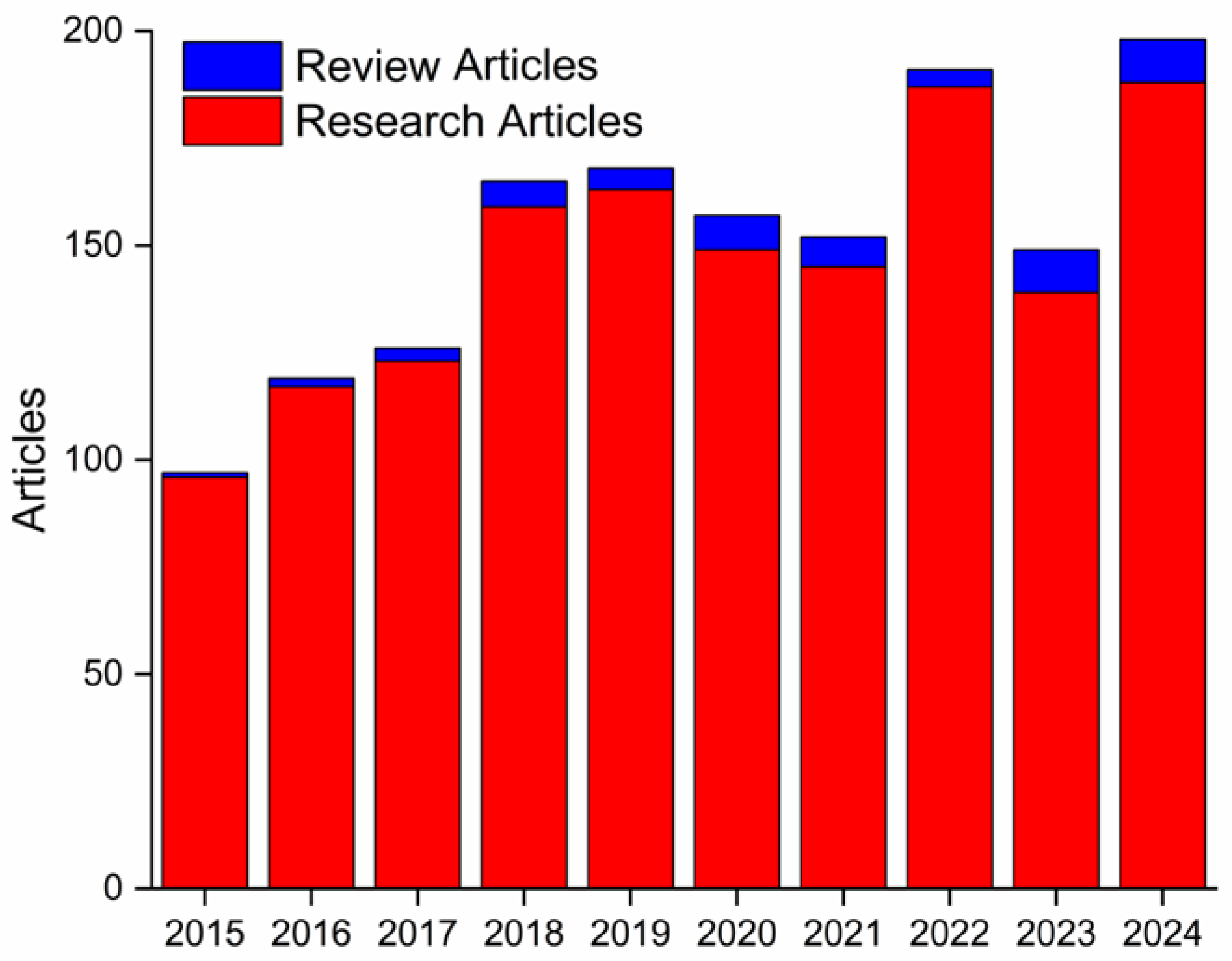
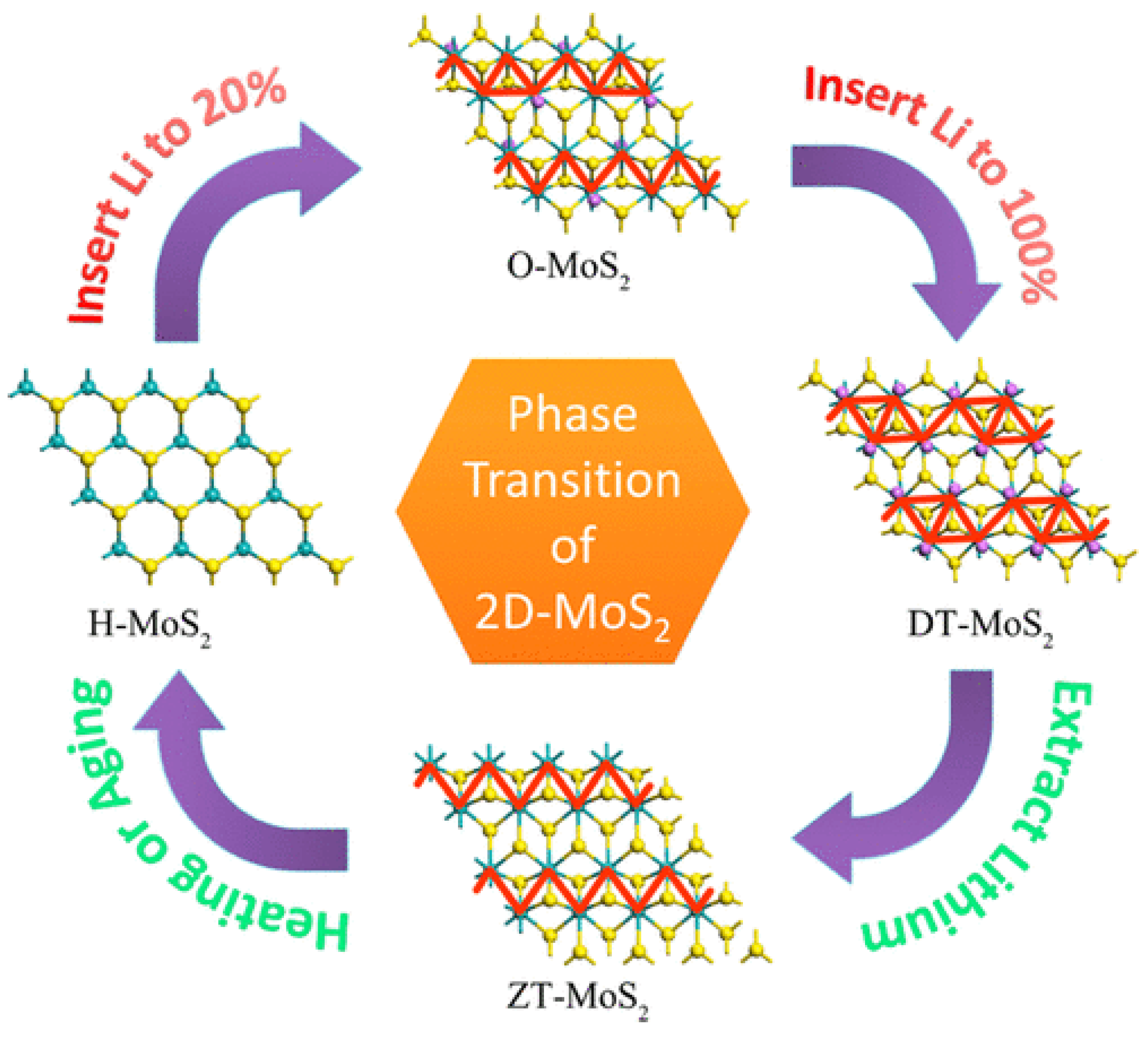
1. Introduction
2. An Overview of Key MoS2 Properties
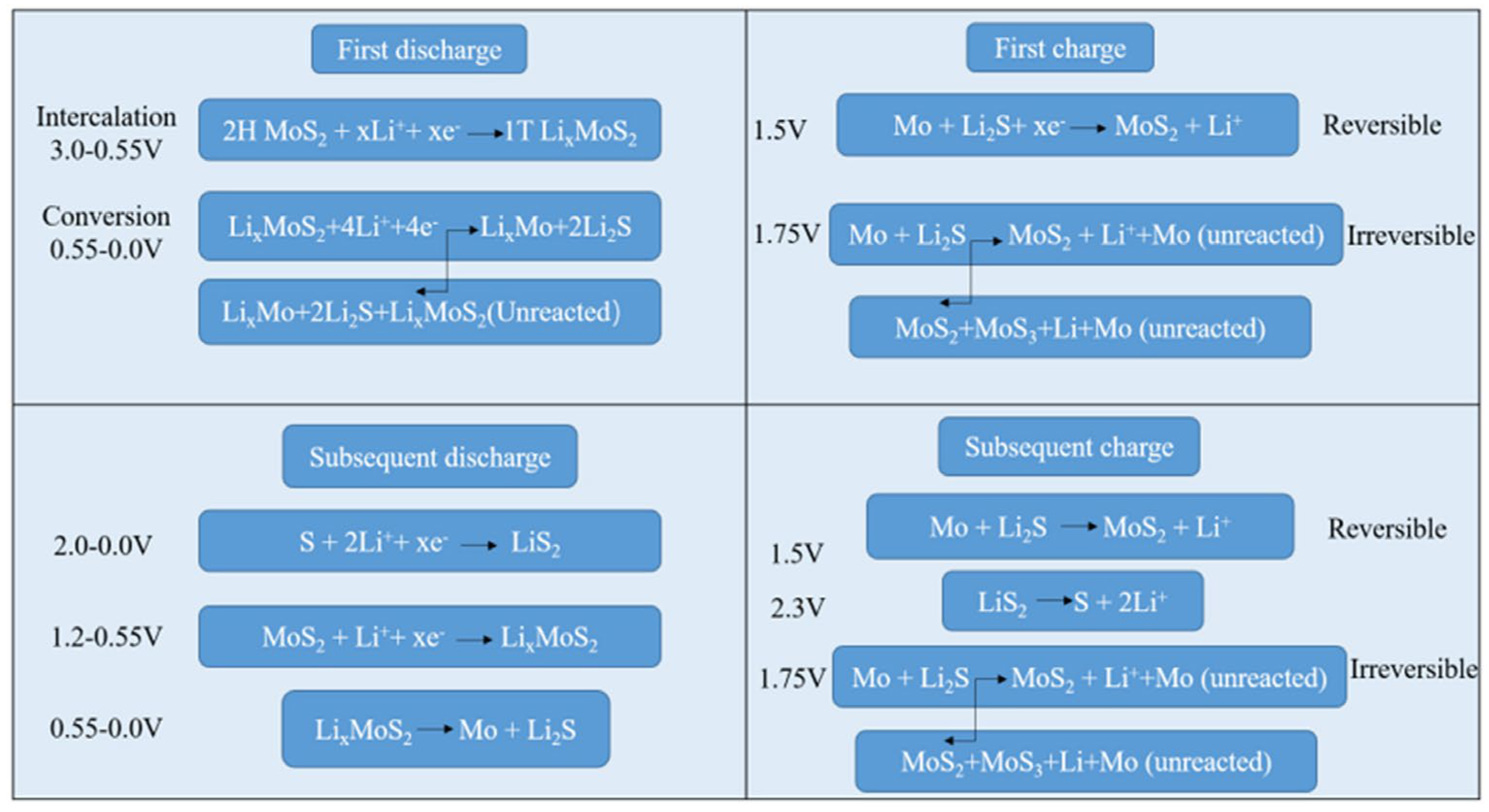
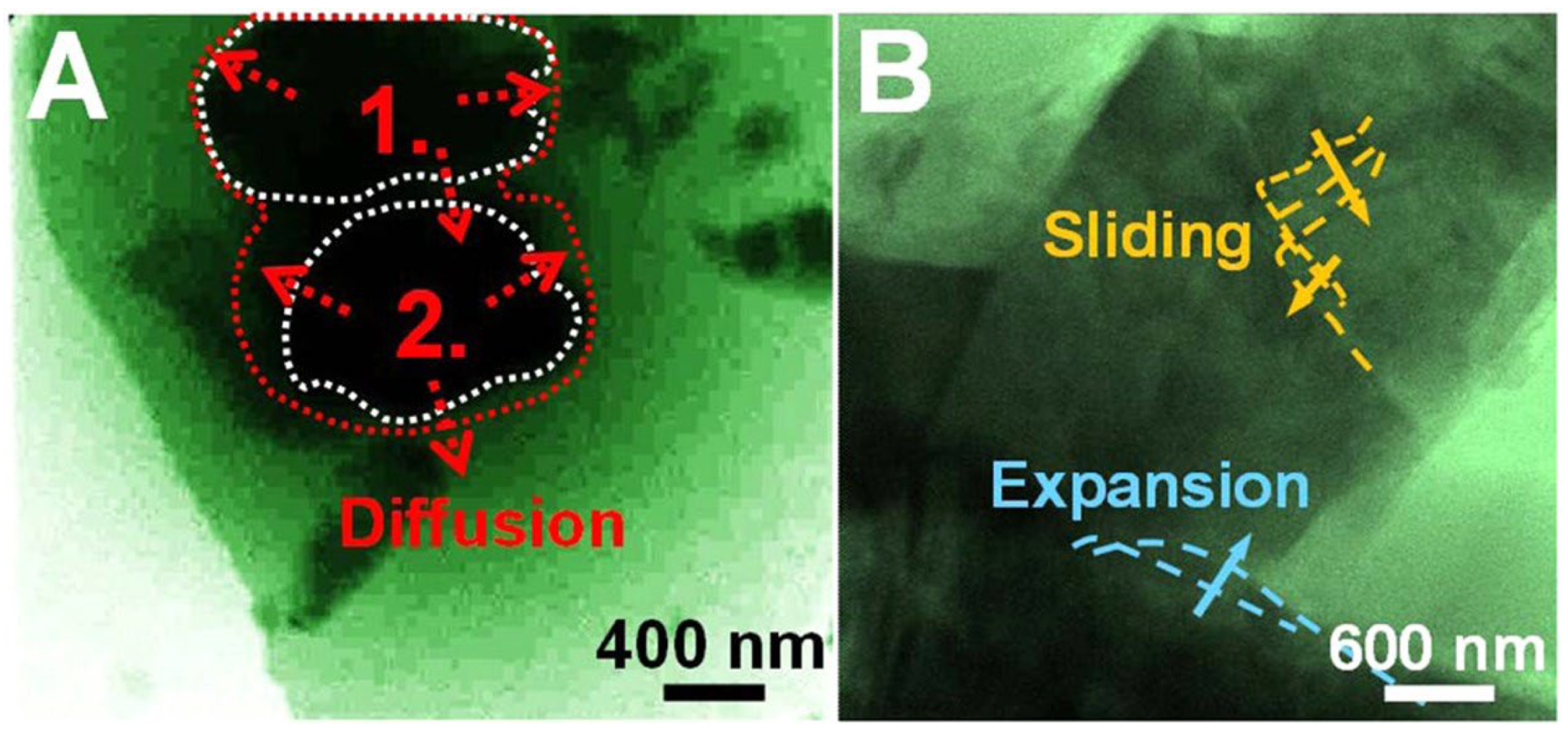
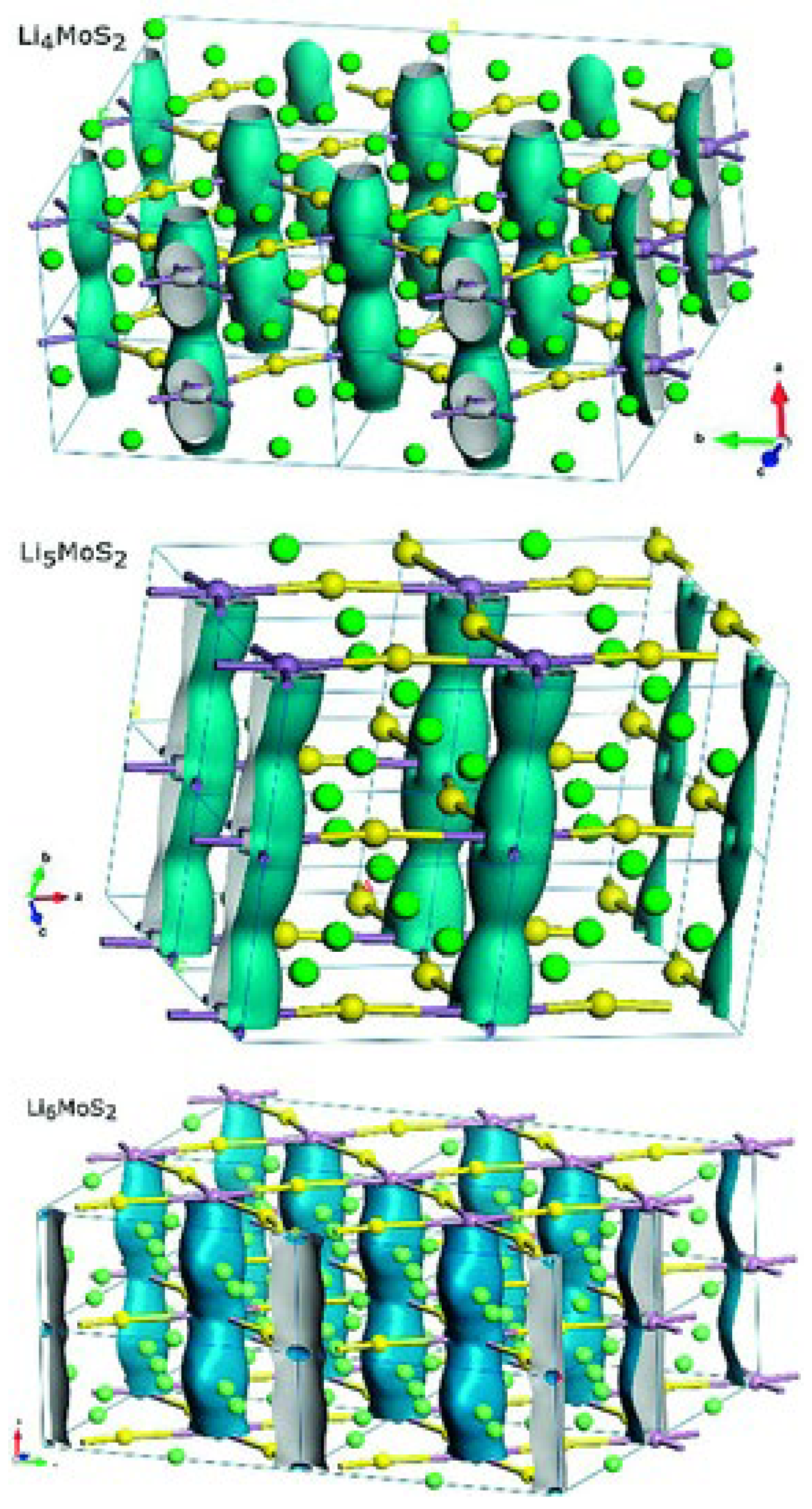
3. On the Use of MoS2 as a Li-Ion Battery Anode
4. A Snapshot of Recent and Future Trends in MoS2-Based 2D Materials for Li-Ion Batteries and Beyond
- ▪
- Phase engineering: Controlling the ratio of 1T to 2H phases can optimize the balance between conductivity and stability. Methods such as lithium intercalation, chemical doping, or plasma treatment are being used to manipulate the phase composition.
- ▪
- Defect engineering and doping: Introducing defects or heteroatoms (e.g., N, S, or Se) into the MoS2 lattice can create additional active sites and modulate the electronic properties to improve performance.
- ▪
- Scalable and green synthesis: Large-scale production techniques (such as CVD, hydrothermal methods, and electrochemical exfoliation) are being optimized to meet industrial demands while minimizing environmental impacts and production costs.
- ▪
- Artificial intelligence (AI) and machine-learning-guided optimization: Data-driven approaches are being increasingly employed to predict material properties, screen potential hybrids, and optimize battery performance with minimal trial-and-error experimentation [171].
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ajayan, P.; Kim, P.; Banerjee, K. Two-dimensional van der Waals materials. Phys. Today 2016, 69, 38–44. [Google Scholar] [CrossRef]
- Marian, M.; Berman, D.; Rota, A.; Jackson, R.L.; Rosenkranz, A. Layered 2D nanomaterials to tailor friction and wear in machine elements—A review. Adv. Mater. Interfaces 2022, 9, 2101622. [Google Scholar] [CrossRef]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Carbon nanomaterials for electronics, optoelectronics, photovoltaics, and sensing. Chem. Soc. Rev. 2013, 42, 2824–2860. [Google Scholar] [CrossRef] [PubMed]
- Mannix, A.J.; Kiraly, B.; Hersam, M.C.; Guisinger, N.P. Synthesis and chemistry of elemental 2D materials. Nat. Rev. Chem. 2017, 1, 0014. [Google Scholar] [CrossRef]
- Choi, S.H.; Yun, S.J.; Won, Y.S.; Oh, C.S.; Kim, S.M.; Kim, K.K.; Lee, Y.H. Large-scale synthesis of graphene and other 2D materials towards industrialization. Nat. Commun. 2022, 13, 1484. [Google Scholar] [CrossRef] [PubMed]
- Kozhakhmetov, A.; Torsi, R.; Chen, C.Y.; Robinson, J.A. Scalable low-temperature synthesis of two-dimensional materials beyond graphene. J. Phys. Mater. 2020, 4, 012001. [Google Scholar] [CrossRef]
- Alli, U.; Hettiarachchi, S.J.; Kellici, S. Chemical functionalisation of 2D materials by batch and continuous hydrothermal flow synthesis. Chem. A Eur. J. 2020, 26, 6447–6460. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, S.; Lettieri, S.; Ferraro, G.; Bartoli, M.; Etzi, M.; Pirri, C.F.; Bocchini, S. A Concise Overview of Ultrasound-Assisted Techniques for the Production of 2D Materials. Processes 2024, 12, 759. [Google Scholar] [CrossRef]
- Zago, S.; Bartoli, M.; Muhyuddin, M.; Vanacore, G.M.; Jagdale, P.; Tagliaferro, A.; Santoro, C.; Specchia, S. Engineered biochar derived from pyrolyzed waste tea as a carbon support for Fe-N-C electrocatalysts for the oxygen reduction reaction. Electrochim. Acta 2022, 412, 140128. [Google Scholar] [CrossRef]
- Yu, J.; Hu, X.; Li, H.; Zhou, X.; Zhai, T. Large-scale synthesis of 2D metal dichalcogenides. J. Mater. Chem. C 2018, 6, 4627–4640. [Google Scholar] [CrossRef]
- Dean, C.R.; Young, A.F.; Meric, I.; Lee, C.; Wang, L.; Sorgenfrei, S.; Watanabe, K.; Taniguchi, T.; Kim, P.; Shepard, K.L.; et al. Boron nitride substrates for high-quality graphene electronics. Nat. Nanotechnol. 2010, 5, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Butler, S.Z.; Hollen, S.M.; Cao, L.; Cui, Y.; Gupta, J.A.; Gutiérrez, H.R.; Heinz, T.F.; Hong, S.S.; Huang, J.; Ismach, A.F.; et al. Progress, challenges, and opportunities in two-dimensional materials beyond graphene. ACS Nano 2013, 7, 2898–2926. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.A.; Yoffe, A. The transition metal dichalcogenides discussion and interpretation of the observed optical, electrical and structural properties. Adv. Phys. 1969, 18, 193–335. [Google Scholar] [CrossRef]
- Bandurin, D.A.; Tyurnina, A.V.; Yu, G.L.; Mishchenko, A.; Zólyomi, V.; Morozov, S.V.; Kumar, R.K.; Gorbachev, R.V.; Kudrynskyi, Z.R.; Pezzini, S.; et al. High electron mobility, quantum Hall effect and anomalous optical response in atomically thin InSe. Nat. Nanotechnol. 2017, 12, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.K.; Yang, S.J.; Zhai, T.Y. 2D Bi2Se3 materials for optoelectronics. Iscience 2021, 24, 103291. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th anniversary article: MXenes: A new family of two-dimensional materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Jezequel, G.; Pollini, I. Optical properties of layered transition-metal halides. J. Phys. Condens. Matter 1990, 2, 5439. [Google Scholar] [CrossRef]
- McGuire, M.A.; Dixit, H.; Cooper, V.R.; Sales, B.C. Coupling of crystal structure and magnetism in the layered, ferromagnetic insulator CrI3. Chem. Mater. 2015, 27, 612–620. [Google Scholar] [CrossRef]
- Ma, R.; Sasaki, T. Two-dimensional oxide and hydroxide nanosheets: Controllable high-quality exfoliation, molecular assembly, and exploration of functionality. Accounts Chem. Res. 2015, 48, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.H.; Stoumpos, C.C.; Farha, O.K.; Hupp, J.T.; Kanatzidis, M.G. 2D homologous perovskites as light-absorbing materials for solar cell applications. J. Am. Chem. Soc. 2015, 137, 7843–7850. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Cinquanta, E.; Chiappe, D.; Grazianetti, C.; Fanciulli, M.; Dubey, M.; Molle, A.; Akinwande, D. Silicene field-effect transistors operating at room temperature. Nat. Nanotechnol. 2015, 10, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Wang, H.; Huang, S.; Xia, F.; Dresselhaus, M.S. The renaissance of black phosphorus. Proc. Natl. Acad. Sci. USA 2015, 112, 4523–4530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, R.; Guo, Y.; Li, Y.; Li, K. A review on MoS2 structure, preparation, energy storage applications and challenges. J. Alloys Compd. 2024, 998, 174916. [Google Scholar] [CrossRef]
- Yadav, A.; Sharma, A.K.; Yadav, J.; Bhasker, S.; Mishra, G.; Bhasker, H.P.; Patel, S.P.; Dhawan, P.K.; Chaudhary, D.K. Morphological impact on energy storage properties of 2D-MoS2 and its nanocomposites: A comprehensive review. Z. Naturforsch. A 2025, 80, 345–362. [Google Scholar] [CrossRef]
- Sangwan, V.K.; Hersam, M.C. Electronic transport in two-dimensional materials. Annu. Rev. Phys. Chem. 2018, 69, 299–325. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, H.; Lebedev, D.; Hersam, M.C. Polymorphism in post-dichalcogenide two-dimensional materials. Chem. Rev. 2021, 121, 2713–2775. [Google Scholar] [CrossRef] [PubMed]
- Molaei, M.J.; Younas, M.; Rezakazemi, M. A comprehensive review on recent advances in two-dimensional (2D) hexagonal boron nitride. ACS Appl. Electron. Mater. 2021, 3, 5165–5187. [Google Scholar] [CrossRef]
- Fan, F.R.; Wang, R.; Zhang, H.; Wu, W. Emerging beyond-graphene elemental 2D materials for energy and catalysis applications. Chem. Soc. Rev. 2021, 50, 10983–11031. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Yu, K.; Zhou, Z.; Li, J.; Gao, L. Preparation of two-dimensional superconductors: A comprehensive review. J. Mater. Chem. C 2025, 13, 6963–6979. [Google Scholar] [CrossRef]
- Fiori, G.; Bonaccorso, F.; Iannaccone, G.; Palacios, T.; Neumaier, D.; Seabaugh, A.; Banerjee, S.K.; Colombo, L. Electronics based on two-dimensional materials. Nat. Nanotechnol. 2014, 9, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Tedstone, A.A.; Lewis, D.J.; Hao, R.; Mao, S.-M.; Bellon, P.; Averback, R.S.; Warrens, C.P.; West, K.R.; Howard, P.; Gaemers, S.; et al. Mechanical Properties of Molybdenum Disulfide and the Effect of Doping: An in Situ TEM Study. ACS Appl. Mater. Interfaces 2015, 7, 20829–20834. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, H. Two-dimensional MoS2: Properties, preparation, and applications. J. Materiomics 2015, 1, 33–44. [Google Scholar] [CrossRef]
- Ghorbani-Asl, M.; Zibouche, N.; Wahiduzzaman, M.; Oliveira, A.F.; Kuc, A.; Heine, T. Electromechanics in MoS2 and WS2: Nanotubes vs. monolayers. Sci. Rep. 2013, 3, 2961. [Google Scholar] [CrossRef] [PubMed]
- Bertolazzi, S.; Brivio, J.; Kis, A. Stretching and Breaking of Ultrathin MoS2. ACS Nano 2011, 5, 9703–9709. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Gomez, A.; Poot, M.; Steele, G.A.; van der Zant, H.S.J.; Agraït, N.; Rubio-Bollinger, G. Elastic Properties of Freely Suspended MoS2 Nanosheets. Adv. Mater. 2012, 24, 772–775. [Google Scholar] [CrossRef] [PubMed]
- Halim, S.N.M.; Zuikafly, S.N.F.; Taib, M.F.M.; Ahmad, F. First Principles Study on Electronic and Optical Properties of Graphene/MoS2 for Optoelectronic Application. In Proceedings of the 2020 IEEE International Conference on Semiconductor Electronics (ICSE), Kuala Lumpur, Malaysia, 28–29 July 2020; pp. 29–32. [Google Scholar]
- Nalwa, H.S. A review of molybdenum disulfide (MoS2) based photodetectors: From ultra-broadband, self-powered to flexible devices. RSC Adv. 2020, 10, 30529–30602. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, J.-Z.; Wei, X.-X.; Guo, D.; Wu, B.; Yu, L.-W.; Wang, X.-R.; Shi, Y. Tuning Photoluminescence Performance of Monolayer MoS2 via H2O2 Aqueous Solution*. Chin. Phys. Lett. 2015, 32, 117801. [Google Scholar] [CrossRef]
- Johari, P.; Shenoy, V.B. Tuning the Electronic Properties of Semiconducting Transition Metal Dichalcogenides by Applying Mechanical Strains. ACS Nano 2012, 6, 5449–5456. [Google Scholar] [CrossRef] [PubMed]
- Kadantsev, E.S.; Hawrylak, P. Electronic structure of a single MoS2 monolayer. Solid State Commun. 2012, 152, 909–913. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Li, Y. Impact of Doping Concentration on Electronic Properties of Transition Metal-Doped Monolayer Molybdenum Disulfide. IEEE Trans. Electron Devices 2018, 65, 733–738. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Z.; Qiao, H.; Qi, X. Characteristics and performance of layered two-dimensional materials under doping engineering. Phys. Chem. Chem. Phys. 2024, 26, 17423–17442. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Li, M.; Li, E.; Ma, D.; Zhao, B. First-principles study of antimony-doped monolayer molybdenum disulfide: Electronic structure and optical properties. Phys. E Low-Dimens. Syst. Nanostruct. 2018, 104, 91–97. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Huang, S.; Zhang, Y.; Chen, Z. Integrated design of sandwich-like C@MoS2@C nanospheres as active anode material for lithium-ion batteries. J. Mater. Sci. 2022, 57, 14948–14958. [Google Scholar] [CrossRef]
- Zhang, H.; Song, J.; Li, J.; Feng, J.; Ma, Y.; Ma, L.; Liu, H.; Qin, Y.; Zhao, X.; Wang, F. Interlayer-expanded MoS2 nanoflowers vertically aligned on MXene@dual-phased TiO2 as high-performance anode for sodium-ion batteries. ACS Appl. Mater. Interfaces 2022, 14, 16300–16309. [Google Scholar] [CrossRef] [PubMed]
- Guguchia, Z.; Kerelsky, A.; Edelberg, D.; Banerjee, S.; von Rohr, F.; Scullion, D.; Augustin, M.; Scully, M.; Rhodes, D.A.; Shermadini, Z.; et al. Magnetism in semiconducting molybdenum dichalcogenides. Sci. Adv. 2018, 4, eaat3672. [Google Scholar] [CrossRef] [PubMed]
- Tongay, S.; Varnoosfaderani, S.S.; Appleton, B.R.; Wu, J.; Hebard, A.F. Magnetic properties of MoS2: Existence of ferromagnetism. Appl. Phys. Lett. 2012, 101, 123105. [Google Scholar] [CrossRef]
- Liang, S.; Yang, H.; Renucci, P.; Tao, B.; Laczkowski, P.; Mc-Murtry, S.; Wang, G.; Marie, X.; George, J.-M.; Petit-Watelot, S.; et al. Electrical spin injection and detection in molybdenum disulfide multilayer channel. Nat. Commun. 2017, 8, 14947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, H.; Qu, J.; Li, J. Two-dimensional layered MoS2: Rational design, properties and electrochemical applications. Energy Environ. Sci. 2016, 9, 1190–1209. [Google Scholar] [CrossRef]
- Wilson, J.A.; Di Salvo, F.; Mahajan, S. Charge-density waves and superlattices in the metallic layered transition metal dichalcogenides. Adv. Phys. 1975, 24, 117–201. [Google Scholar] [CrossRef]
- Xu, M.; Liang, T.; Shi, M.; Chen, H. Graphene-like two-dimensional materials. Chem. Rev. 2013, 113, 3766–3798. [Google Scholar] [CrossRef] [PubMed]
- Piatti, E.; Prando, G.; Meinero, M.; Tresca, C.; Putti, M.; Roddaro, S.; Lamura, G.; Shiroka, T.; Carretta, P.; Profeta, G.; et al. Superconductivity induced by gate-driven hydrogen intercalation in the charge-density-wave compound 1T-TiSe2. Commun. Phys. 2023, 6, 202. [Google Scholar] [CrossRef]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef] [PubMed]
- Piatti, E.; Galasso, S.; Tortello, M.; Nair, J.; Gerbaldi, C.; Bruna, M.; Borini, S.; Daghero, D.; Gonnelli, R. Carrier mobility and scattering lifetime in electric double-layer gated few-layer graphene. Appl. Surf. Sci. 2017, 395, 37–41. [Google Scholar] [CrossRef]
- Romanin, D.; Brumme, T.; Daghero, D.; Gonnelli, R.S.; Piatti, E. Strong band-filling-dependence of the scattering lifetime in gated MoS2 nanolayers induced by the opening of intervalley scattering channels. J. Appl. Phys. 2020, 128, 063907. [Google Scholar] [CrossRef]
- Narita, S.-I.; Terada, S.-I.; Mori, S.; Muro, K.; Akahama, Y.; Endo, S. Far-infrared cyclotron resonance absorptions in black phosphorus single crystals. J. Phys. Soc. Jpn. 1983, 52, 3544–3553. [Google Scholar] [CrossRef]
- Allain, A.; Kang, J.; Banerjee, K.; Kis, A. Electrical contacts to two-dimensional semiconductors. Nat. Mater. 2015, 14, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Meric, I.; Huang, P.Y.; Gao, Q.; Gao, Y.; Tran, H.; Taniguchi, T.; Watanabe, K.; Campos, L.M.; Muller, D.A.; et al. One-dimensional electrical contact to a two-dimensional material. Science 2013, 342, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Piatti, E.; Arbab, A.; Galanti, F.; Carey, T.; Anzi, L.; Spurling, D.; Roy, A.; Zhussupbekova, A.; Patel, K.A.; Kim, J.M.; et al. Charge transport mechanisms in inkjet-printed thin-film transistors based on two-dimensional materials. Nat. Electron. 2021, 4, 893–905. [Google Scholar] [CrossRef]
- Zheng, W.; Sun, B.; Li, D.; Gali, S.M.; Zhang, H.; Fu, S.; Di Virgilio, L.; Li, Z.; Yang, S.; Zhou, S.; et al. Band transport by large Fröhlich polarons in MXenes. Nat. Phys. 2022, 18, 544–550. [Google Scholar] [CrossRef]
- Haering, R.R.; Stiles, J.A.; Brandt, K. Lithium Molybdenum Disulphide Battery Cathode. U.S. Patent US4224390A, 23 September 1980. [Google Scholar]
- Samy, O.; El Moutaouakil, A. A review on MoS2 energy applications: Recent developments and challenges. Energies 2021, 14, 4586. [Google Scholar] [CrossRef]
- Cordeiro, N.J.A.; Gaspar, C.; de Oliveira, M.J.; Nunes, D.; Barquinha, P.; Pereira, L.; Fortunato, E.; Martins, R.; Laureto, E.; Lourenço, S.A. Fast and low-cost synthesis of MoS2 nanostructures on paper substrates for near-infrared photodetectors. Appl. Sci. 2021, 11, 1234. [Google Scholar] [CrossRef]
- Tao, R.; Gu, Y.; Du, Z.; Lyu, X.; Li, J. Advanced electrode processing for lithium-ion battery manufacturing. Nat. Rev. Clean Technol. 2025, 1, 116–131. [Google Scholar] [CrossRef]
- Fang, X.; Hua, C.; Guo, X.; Hu, Y.; Wang, Z.; Gao, X.; Wu, F.; Wang, J.; Chen, L. Lithium storage in commercial MoS2 in different potential ranges. Electrochim. Acta 2012, 81, 155–160. [Google Scholar] [CrossRef]
- Bissett, M.A.; Kinloch, I.A.; Dryfe, R.A. Characterization of MoS2–graphene composites for high-performance coin cell supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 17388–17398. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, Z.J.; Moloney, J.; Yu, C.P.; Chhowalla, M. Quasi-solid-state electrolyte induced by metallic MoS2 for lithium–sulfur batteries. ACS Nano 2024, 18, 16041–16050. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Guo, W.; Song, J.; Fu, Y. Dynamic 1T-2H Mixed-Phase MoS2 Enables High-Performance Li-Organosulfide Battery. Small 2022, 18, 2105071. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Chen, M.; Li, Y.; Zhang, W.; Zhao, D.; Zhu, Q. Dendrite-free potassium metal anode induced by in-situ phase transitions of MoS2. Mater. Today Phys. 2023, 35, 101141. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, W.; Yu, R.; Qiao, F.; Wang, J.; Wang, J.; An, Q. Molecular Engineering to Construct MoS2 with Expanded Interlayer Spacing and Enriched 1T Phase for “Rocking-Chair” Aqueous Calcium-Ion Pouch Cells. ACS Nano 2024, 18, 35286–35295. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Ma, J.; Li, H.; Zeng, R.; Guo, Z.; Liu, H. Synthesis of molybdenum disulfide (MoS2) for lithium ion battery applications. Mater. Res. Bull. 2009, 44, 1811–1815. [Google Scholar] [CrossRef]
- Stephenson, T.; Li, Z.; Olsen, B.; Mitlin, D. Lithium ion battery applications of molybdenum disulfide (MoS2) nanocomposites. Energy Environ. Sci. 2014, 7, 209–231. [Google Scholar] [CrossRef]
- Miki, Y.; Nakazato, D.; Ikuta, H.; Uchida, T.; Wakihara, M. Amorphous MoS2 as the cathode of lithium secondary batteries. J. Power Sources 1995, 54, 508–510. [Google Scholar] [CrossRef]
- Jiao, Y.; Hafez, A.M.; Cao, D.; Mukhopadhyay, A.; Ma, Y.; Zhu, H. Metallic MoS2 for high performance energy storage and energy conversion. Small 2018, 14, 1800640. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Mu, Y.; Guo, J.; Wei, L.; Zeng, L.; Zhao, T. Monolayer MoS2 Fabricated by In Situ Construction of Interlayer Electrostatic Repulsion Enables Ultrafast Ion Transport in Lithium-Ion Batteries. Nano-Micro Lett. 2023, 15, 80. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, B.; Xue, Y.; Li, J.; Zhang, Y.; Gao, F. Fabrication of defect-rich MoS2 ultrathin nanosheets for application in lithium-ion batteries and supercapacitors. J. Mater. Chem. A 2015, 3, 19445–19454. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Zhao, Y.; Shen, T.; Yan, X.; Yu, C.; Wang, H.; Zeng, H. Novel plasma-engineered MoS2 nanosheets for superior lithium-ion batteries. J. Alloys Compd. 2019, 787, 996–1003. [Google Scholar] [CrossRef]
- Liu, Q.; Xia, W.; Wu, Z.; Huo, J.; Liu, D.; Wang, Q.; Wang, S. The origin of the enhanced performance of nitrogen-doped MoS2 in lithium ion batteries. Nanotechnology 2016, 27, 175402. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhao, K.; He, Y.; Zheng, J.; Xu, W.; Zhang, C.; Wang, X.; Guo, M.; Mai, L.; Wang, C.; et al. Interconnected Vertically Stacked 2D-MoS2 for Ultrastable Cycling of Rechargeable Li-Ion Battery. ACS Appl. Mater. Interfaces 2019, 11, 20762–20769. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bhoyate, S.; Kim, Y.-H.; Kim, Y.-M.; Lee, Y.H.; Conlin, P.; Cho, K.; Choi, W. Unusually high ion conductivity in large-scale patternable two-dimensional MoS2 film. ACS Nano 2021, 15, 12267–12275. [Google Scholar] [CrossRef] [PubMed]
- Venkateshwaran, S.; Partheeban, T.; Sasidharan, M.; Senthil Kumar, S.M. Mesoporous Silica Template-Assisted Synthesis of 1T-MoS2 as the Anode for Li-Ion Battery Applications. Energy Fuels 2021, 35, 2683–2691. [Google Scholar] [CrossRef]
- Wang, P.-P.; Sun, H.; Ji, Y.; Li, W.; Wang, X. Three-Dimensional Assembly of Single-Layered MoS2. Adv. Mater. 2014, 26, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, Z.; Xiao, W.; Huang, H.; Zhang, L.; Cheng, Y.; Zhang, J. Low Crystalline MoS2 Nanotubes from MoS2 Nanomasks for Lithium Ion Battery Applications. ACS Appl. Nano Mater. 2020, 3, 7580–7586. [Google Scholar] [CrossRef]
- Faizan, M.; Hussain, S.; Islam, M.; Kim, J.-Y.; Han, D.; Bae, J.-H.; Vikraman, D.; Ali, B.; Abbas, S.; Kim, H.-S.; et al. MoO3@MoS2 Core-Shell Structured Hybrid Anode Materials for Lithium-Ion Batteries. Nanomaterials 2022, 12, 2008. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Fu, J.; Shen, A.; Zhang, L.; Kong, S.; Feng, Y.; Gong, W.; Tian, K.; Li, Q. Interfacial coupling of MoS2/MoO3 hierarchical heterostructures as superior anodes for high-performance lithium-ion battery. J. Energy Storage 2023, 72, 108595. [Google Scholar] [CrossRef]
- Lei, D.; Shang, W.; Zhang, X.; Li, Y.; Qiao, S.; Zhong, Y.; Deng, X.; Shi, X.; Zhang, Q.; Hao, C. Facile synthesis of heterostructured MoS2–MoO3 nanosheets with active electrocatalytic sites for high-performance lithium–sulfur batteries. ACS Nano 2021, 15, 20478–20488. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zha, Z.; Liu, X.; Tian, H.; Wu, Z.; Li, W.; Sun, L.-B.; Liu, B.; Chen, Z. Core–sheath structured MoO3@MoS2 composite for high-performance lithium-ion battery anodes. Energy Fuels 2020, 34, 11498–11507. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, W.; Chen, M.; Wang, R.; Fang, Z. The synthesis of ZnS@MoS2 hollow polyhedrons for enhanced lithium storage performance. CrystEngComm 2018, 20, 7266–7274. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, C.; Xiao, F.; Wang, J.; Su, X. Synthesis of nano-TiO2-decorated MoS2 nanosheets for lithium-ion batteries. New J. Chem. 2015, 39, 683–688. [Google Scholar] [CrossRef]
- Xu, W.; Wang, T.; Yu, Y.; Wang, S. Synthesis of core-shell TiO2@MoS2 composites for lithium-ion battery anodes. J. Alloys Compd. 2016, 689, 460–467. [Google Scholar] [CrossRef]
- Chen, Z.; Yin, D.; Zhang, M. Sandwich-like MoS2@SnO2@C with High Capacity and Stability for Sodium/Potassium Ion Batteries. Small 2018, 14, e1703818. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Pan, Q.; Chen, J.; Zan, Y.; Huang, Y.; Yang, G.; Yan, Z.; Li, Q. Facile synthesis of Sn/MoS2/C composite as an anode material for lithium-ion batteries with outstanding performance. New J. Chem. 2016, 40, 1263–1268. [Google Scholar] [CrossRef]
- Ette, P.M.; Chithambararaj, A.; Prakash, A.S.; Ramesha, K. MoS2 Nanoflower-Derived Interconnected CoMoO4 Nanoarchitectures as a Stable and High Rate Performing Anode for Lithium-Ion Battery Applications. ACS Appl. Mater. Interfaces 2020, 12, 11511–11521. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, H.; Zhu, M.; Yuan, A.; Shen, X. Metal-organic framework-derived Co3O4 covered by MoS2 nanosheets for high-performance lithium-ion batteries. J. Alloys Compd. 2018, 744, 220–227. [Google Scholar] [CrossRef]
- Samad, A.; Shin, Y.-H. MoS2@VS2 Nanocomposite as a Superior Hybrid Anode Material. ACS Appl. Mater. Interfaces 2017, 9, 29942–29949. [Google Scholar] [CrossRef] [PubMed]
- Bindumadhavan, K.; Srivastava, S.K.; Mahanty, S. MoS2–MWCNT hybrids as a superior anode in lithium-ion batteries. Chem. Commun. 2013, 49, 1823–1825. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Z.; Lu, L.; Lotya, M.; Coleman, J.N.; Chou, S.-L.; Liu, H.-K.; Minett, A.I.; Chen, J. Development of MoS2–CNT composite thin film from layered MoS2 for lithium batteries. Adv. Energy Mater. 2013, 3, 798–805. [Google Scholar] [CrossRef]
- Yoo, H.; Tiwari, A.P.; Lee, J.; Kim, D.; Park, J.H.; Lee, H. Cylindrical nanostructured MoS2 directly grown on CNT composites for lithium-ion batteries. Nanoscale 2015, 7, 3404–3409. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Guan, B.Y.; Yu, L.; Lou, X.W. Rational Design of Three-Layered TiO2@Carbon@MoS2 Hierarchical Nanotubes for Enhanced Lithium Storage. Adv. Mater. 2017, 29, 1702724. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Shi, Y.; Shi, W.; Rui, X.; Yan, Q.; Kong, J.; Zhang, H. Preparation of MoS2-Coated Three-Dimensional Graphene Networks for High-Performance Anode Material in Lithium-Ion Batteries. Small 2013, 9, 3433–3438. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, Y.; Gao, Y.; Tronganh, N.; Chen, F.; Lu, M.; Jiang, Y.; Jiao, Z.; Zhao, B. Facile synthesis of ultrathin, undersized MoS2/graphene for lithium-ion battery anodes. RSC Adv. 2016, 6, 99833–99841. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, Z.; Gao, Y.; Chen, L.; Lu, M.; Jiao, Z.; Jiang, Y.; Ding, Y.; Cheng, L. Hydrothermal synthesis of layer-controlled MoS2/graphene composite aerogels for lithium-ion battery anode materials. Appl. Surf. Sci. 2016, 390, 209–215. [Google Scholar] [CrossRef]
- Wang, S.; Wang, R.; Zhao, Q.; Ren, L.; Wen, J.; Chang, J.; Fang, X.; Hu, N.; Xu, C. Freeze-drying induced self-assembly approach for scalable constructing MoS2/graphene hybrid aerogels for lithium-ion batteries. J. Colloid Interface Sci. 2019, 544, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Zhao, H.; Zhang, Z.; Li, Z.; Xia, Q.; Zhang, Y.; Zhao, L.; Du, X.; Du, Z.; Lv, P.; et al. MoS2 Nanosheets Vertically Grown on Graphene Sheets for Lithium-Ion Battery Anodes. ACS Nano 2016, 10, 8526–8535. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Cai, Z.; Qu, L.; Zhang, P.; Yuan, Z.; Asare, O.K.; Xu, W.; Lin, C.; Mai, L. Three-Dimensional Crumpled Reduced Graphene Oxide/MoS2 Nanoflowers: A Stable Anode for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 12625–12630. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, K.; Fu, H.; Guo, B.; Lei, X.; Zhu, Z. MoS2/Graphene Hybrid Nanoflowers with Enhanced Electrochemical Performances as Anode for Lithium-Ion Batteries. J. Phys. Chem. C 2015, 119, 7959–7968. [Google Scholar] [CrossRef]
- Wang, T.; Li, M.; Qi, L.; Jie, P.; Yang, W.; Li, Y. Multilevel Heterostructure of MoS2/GDYO for Lithium-Ion Batteries. Adv. Funct. Mater. 2023, 33, 2308470. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, X.; Kong, J.; Ang, J.M.; Lee, P.S.; Liu, Z.; Lu, X. Self-Assembly-Induced Alternately Stacked Single-Layer MoS2 and N-doped Graphene: A Novel van der Waals Heterostructure for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 2372–2379. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Du, K.; Wang, Y.; Sun, P.; Zhao, H.; Tang, P.; Fan, Q.; Tian, H.; Li, Q.; Xu, Q. N plasma treatment on graphene oxide-MoS2 composites for improved performance in lithium ion batteries. Mater. Chem. Phys. 2020, 240, 122169. [Google Scholar] [CrossRef]
- Zhou, J.; Qin, J.; Zhang, X.; Shi, C.; Liu, E.; Li, J.; Zhao, N.; He, C. 2D space-confined synthesis of few-layer MoS2 anchored on carbon nanosheet for lithium-ion battery anode. ACS Nano 2015, 9, 3837–3848. [Google Scholar] [CrossRef] [PubMed]
- Dinh, D.A.; Nguyen, T.L.; Cuong, T.V.; Hui, K.S.; Bui, T.H.; Wu, S.; Hui, K.N. Defect-Free MoS2-Flakes/Amorphous-Carbon Hybrid as an Advanced Anode for Lithium-Ion Batteries. Energy Fuels 2021, 35, 3459–3468. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, J.; Lu, H. Uniform Yolk–Shell MoS2@Carbon Microsphere Anodes for High-Performance Lithium-Ion Batteries. Chem. A Eur. J. 2017, 23, 9937–9945. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, Z.; Chen, Y.; Zou, M.; Yousaf, M.; Yang, Y.; Yang, L.; Cao, A.; Han, R.P.S. Controlled Synthesis of Core–Shell Carbon@MoS2 Nanotube Sponges as High-Performance Battery Electrodes. Adv. Mater. 2016, 28, 10175–10181. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Chen, F.; Wu, L.; Kuang, T.; Liu, X.; Yang, J.; Fan, P.; Fei, Z.; Zhao, Z.; Zhong, M. Heteroatoms-doped 3D carbon nanosphere cages embedded with MoS2 for lithium-ion battery. Electrochim. Acta 2020, 332, 135490. [Google Scholar] [CrossRef]
- Kong, J.; Zhao, C.; Wei, Y.; Lu, X. MoS2 Nanosheets Hosted in Polydopamine-Derived Mesoporous Carbon Nanofibers as Lithium-Ion Battery Anodes: Enhanced MoS2 Capacity Utilization and Underlying Mechanism. ACS Appl. Mater. Interfaces 2015, 7, 24279–24287. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Ren, Y.; Yang, H.; Xu, Q. Fabrication of 3D Hierarchical MoS2/Polyaniline and MoS2/C Architectures for Lithium-Ion Battery Applications. ACS Appl. Mater. Interfaces 2014, 6, 14644–14652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, W.; Zhang, S.; Ji, X.; Li, L. Synthesis of expanded graphite-based materials for application in lithium-based batteries. J. Energy Storage 2023, 60, 106678. [Google Scholar] [CrossRef]
- Guan, P.; Zhou, L.; Yu, Z.; Sun, Y.; Liu, Y.; Wu, F.; Jiang, Y.; Chu, D. Recent progress of surface coating on cathode materials for high-performance lithium-ion batteries. J. Energy Chem. 2020, 43, 220–235. [Google Scholar] [CrossRef]
- Lyu, Y.; Wu, X.; Wang, K.; Feng, Z.; Cheng, T.; Liu, Y.; Wang, M.; Chen, R.; Xu, L.; Zhou, J. An overview on the advances of LiCoO2 cathodes for lithium-ion batteries. Adv. Energy Mater. 2021, 11, 2000982. [Google Scholar] [CrossRef]
- Cui, C.; Li, X.; Hu, Z.; Xu, J.; Liu, H.; Ma, J. Growth of MoS2@C nanobowls as a lithium-ion battery anode material. RSC Adv. 2015, 5, 92506–92514. [Google Scholar] [CrossRef]
- Jiang, F.; Li, S.; Ge, P.; Tang, H.; Khoso, S.A.; Zhang, C.; Yang, Y.; Hou, H.; Hu, Y.; Sun, W.; et al. Size-tunable natural mineral-molybdenite for lithium-ion batteries toward: Enhanced storage capacity and quicken ions transferring. Front. Chem. 2018, 6, 389. [Google Scholar] [CrossRef] [PubMed]
- Francis, M.K.; Rajesh, K.; Bhargav, P.B.; Ahmed, N. Binder-free phosphorus-doped MoS2 flexible anode deposited on carbon cloth for high-capacity Li-ion battery applications. J. Mater. Sci. 2023, 58, 4054–4069. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Yang, Z.; Yan, Y.; Sun, K. Synthesis of MoS2 and MoO2 for their applications in H2 generation and lithium ion batteries: A review. Sci. Technol. Adv. Mater. 2013, 14, 043501. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, N.; Wang, S.; Li, Y.; Liang, C.; Yu, K. 3D nanoflower-like MoS2 grown on wheat straw cellulose carbon for lithium-ion battery anode material. J. Alloys Compd. 2023, 933, 167689. [Google Scholar] [CrossRef]
- Zhu, W.; Kamali, A.R. Green preparation of nanostructured β-MoO3/hexagonal-shaped MoS2/graphene with enhanced lithium-ion storage performance. J. Alloys Compd. 2023, 932, 167724. [Google Scholar] [CrossRef]
- Salah, M.; Hall, C.; Francis, C.; Rollo-Walker, G.; Fabretto, M. Binary silicon-based thin-film anodes for lithium-ion batteries: A review. J. Power Sources 2022, 520, 230871. [Google Scholar] [CrossRef]
- Li, L.; Zhang, D.; Deng, J.; Gou, Y.; Fang, J.; Cui, H.; Zhao, Y.; Cao, M. Carbon-based materials for fast charging lithium-ion batteries. Carbon 2021, 183, 721–734. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, Q. Dendrite-free lithium metal anodes: Stable solid electrolyte interphases for high-efficiency batteries. J. Mater. Chem. A 2015, 3, 7207–7209. [Google Scholar] [CrossRef]
- Nzereogu, P.; Omah, A.; Ezema, F.; Iwuoha, E.; Nwanya, A. Anode materials for lithium-ion batteries: A review. Appl. Surf. Sci. Adv. 2022, 9, 100233. [Google Scholar] [CrossRef]
- Hai, N.Q.; Kwon, S.H.; Kim, H.; Kim, I.T.; Lee, S.G.; Hur, J. High-performance MoS2-based nanocomposite anode prepared by high-energy mechanical milling: The effect of carbonaceous matrix on MoS2. Electrochim. Acta 2018, 260, 129–138. [Google Scholar] [CrossRef]
- Zu, G.; Yang, Y.; Li, H.; Wang, J.; Fu, Y.; Wang, X.; Zhou, W.; Wang, J. The compactness of 2H-MoS2 thin films determines their performance on lithium storage ability. Mater. Charact. 2023, 196, 112570. [Google Scholar] [CrossRef]
- Guo, B.; Feng, Y.; Chen, X.; Li, B.; Yu, K. Preparation of yolk-shell MoS2 nanospheres covered with carbon shell for excellent lithium-ion battery anodes. Appl. Surf. Sci. 2018, 434, 1021–1029. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Wei, C.; Huang, X.; Zhang, X.; Wen, G. MoS2-based anode materials for lithium-ion batteries: Developments and perspectives. Particuology 2024, 87, 240–270. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, X.; Bustillo, K.; Niu, K.; Gammer, C.; Xu, J.; Zheng, H. In situ study of lithiation and delithiation of MoS2 nanosheets using electrochemical liquid cell transmission electron microscopy. Nano Lett. 2015, 15, 5214–5220. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-J.; Zhu, Y.-L.; Zhang, T.; Tian, J.; Gao, F.; Zhao, Y.; Bu, X.-Y.; Quan, T. Competition between discharge reaction and side reaction for anode’s lithium during internal short circuit in lithium-ion batteries. J. Clean. Prod. 2024, 470, 143280. [Google Scholar] [CrossRef]
- Caputo, R.; Tekin, A.; Nesper, R. Topochemical Path in High Lithiation of MoS2. Z. Für Anorg. Und Allg. Chem. 2019, 645, 309–316. [Google Scholar] [CrossRef]
- Cheng, Y.; Nie, A.; Zhang, Q.; Gan, L.-Y.; Shahbazian-Yassar, R.; Schwingenschlogl, U. Origin of the phase transition in lithiated molybdenum disulfide. ACS Nano 2014, 8, 11447–11453. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Li, F.; Hu, C.; Liang, P.; Cao, D.; Chen, X. The capacity fading mechanism and improvement of cycling stability in MoS2-based anode materials for lithium-ion batteries. Nanoscale 2016, 8, 2918–2926. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Shu, H.; Shen, Z.; Hu, H.; Wang, J.; Chen, X. Electrochemical Lithiation Mechanism of Two-Dimensional Transition-Metal Dichalcogenide Anode Materials: Intercalation versus Conversion Reactions. J. Phys. Chem. C 2019, 123, 2139–2146. [Google Scholar] [CrossRef]
- Zhu, Z.; Xi, S.; Miao, L.; Tang, Y.; Zeng, Y.; Xia, H.; Lv, Z.; Zhang, W.; Ge, X.; Zhang, H.; et al. Unraveling the Formation of Amorphous MoS2 Nanograins during the Electrochemical Delithiation Process. Adv. Funct. Mater. 2019, 29, 1904843. [Google Scholar] [CrossRef]
- Py, M.A.; Haering, R.R. Structural destabilization induced by lithium intercalation in MoS2 and related compounds. Can. J. Phys. 1983, 61, 76–84. [Google Scholar] [CrossRef]
- Cook, J.B.; Kim, H.-S.; Yan, Y.; Ko, J.S.; Robbennolt, S.; Dunn, B.; Tolbert, S.H. Mesoporous MoS2 as a Transition Metal Dichalcogenide Exhibiting Pseudocapacitive Li and Na-Ion Charge Storage. Adv. Energy Mater. 2016, 6, 1501937. [Google Scholar] [CrossRef]
- Kan, M.; Wang, J.Y.; Li, X.W.; Zhang, S.H.; Li, Y.W.; Kawazoe, Y.; Sun, Q.; Jena, P. Structures and phase transition of a MoS2 monolayer. J. Phys. Chem. C 2014, 118, 1515–1522. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Q.; Zhu, J.; Duan, X.; Xu, Z.; Liu, Y.; Yang, H.; Lu, B. Nature of extra capacity in MoS2 electrodes: Molybdenum atoms accommodate with lithium. Energy Storage Mater. 2019, 16, 37–45. [Google Scholar] [CrossRef]
- Zhu, Z.; Tang, Y.; Lv, Z.; Wei, J.; Zhang, Y.; Wang, R.; Zhang, W.; Xia, H.; Ge, M.; Chen, X. Fluoroethylene Carbonate enabling a robust LiF-rich solid electrolyte interphase to enhance the stability of the MoS2 Anode for Lithium-ion storage. Angew. Chem. 2018, 130, 3718–3722. [Google Scholar] [CrossRef]
- Yu, L.; Su, Q.; Li, B.; Huang, L.; Du, G.; Ding, S.; Zhao, W.; Zhang, M.; Xu, B. Pre-lithiated Edge-enriched MoS2 nanoplates embedded into carbon nanofibers as protective layers to stabilize Li metal anodes. Chem. Eng. J. 2022, 429, 132479. [Google Scholar] [CrossRef]
- Jiao, Y.; Mukhopadhyay, A.; Ma, Y.; Yang, L.; Hafez, A.M.; Zhu, H. Ion transport nanotube assembled with vertically aligned metallic MoS2 for high rate lithium-ion batteries. Adv. Energy Mater. 2018, 8, 1702779. [Google Scholar] [CrossRef]
- Nasir, M.Z.M.; Sofer, Z.; Ambrosi, A.; Pumera, M. A limited anodic and cathodic potential window of MoS2: Limitations in electrochemical applications. Nanoscale 2015, 7, 3126–3129. [Google Scholar] [CrossRef] [PubMed]
- Burse, S.R.; Tyagaraj, H.B.; Safarkhani, M.; Marje, S.J.; Gagankumar, S.K.; Al Ghaferi, A.; Alhajri, E.; Chodankar, N.R.; Huh, Y.S.; Han, Y.-K. Unleashing potential: Engineering advancements in two-dimensional MoS2 for improved energy applications. Adv. Compos. Hybrid Mater. 2025, 8, 216. [Google Scholar] [CrossRef]
- Mammeri, F.; Le Bourhis, E.; Rozes, L.; Sanchez, C. Mechanical properties of hybrid organic–inorganic materials. J. Mater. Chem. 2005, 15, 3787–3811. [Google Scholar] [CrossRef]
- Rashidi, M.; Ghasemi, F. Thermally oxidized MoS2-based hybrids as superior electrodes for supercapacitor and photoelectrochemical applications. Electrochim. Acta 2022, 435, 141379. [Google Scholar] [CrossRef]
- Wang, R.; Wang, S.; Jin, D.; Zhang, Y.; Cai, Y.; Ma, J.; Zhang, L. Engineering layer structure of MoS2-graphene composites with robust and fast lithium storage for high-performance Li-ion capacitors. Energy Storage Mater. 2017, 9, 195–205. [Google Scholar] [CrossRef]
- Liu, C.; Bai, Y.; Zhao, Y.; Yao, H.; Pang, H. MoS2/graphene composites: Fabrication and electrochemical energy storage. Energy Storage Mater. 2020, 33, 470–502. [Google Scholar] [CrossRef]
- Jiang, L.; Lin, B.; Li, X.; Song, X.; Xia, H.; Li, L.; Zeng, H. Monolayer MoS2–Graphene Hybrid Aerogels with Controllable Porosity for Lithium-Ion Batteries with High Reversible Capacity. ACS Appl. Mater. Interfaces 2016, 8, 2680–2687. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Jiang, H.; Hu, Y.; Liu, Y.; Zhang, L.; Liu, H.; Li, C. 3D Ordered Macroporous MoS2@C Nanostructure for Flexible Li-Ion Batteries. Adv. Mater. 2017, 29, 1603020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lou, X.W. Hierarchical MoS2 Shells Supported on Carbon Spheres for Highly Reversible Lithium Storage. Chem. A Eur. J. 2014, 20, 5219–5223. [Google Scholar] [CrossRef] [PubMed]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.-J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Falco, M.; Muñoz-García, A.B.; Bonomo, M.; Brutti, S.; Pavone, M.; Gerbaldi, C. Solid-State Post Li Metal Ion Batteries: A Sustainable Forthcoming Reality? Adv. Energy Mater. 2021, 11, 2100785. [Google Scholar] [CrossRef]
- Meng, R.; Wu, J.; Zhu, M.; Chang, M.; Zhang, N.; Cao, P.; Tian, F.; Yao, X. Two-Dimensional MoS2 NS@Li7P3S11 Composite Cathode for All-Solid-State Lithium Batteries. ACS Appl. Energy Mater. 2024, 7, 4603–4608. [Google Scholar] [CrossRef]
- Marriam, I.; Tebyetekerwa, M.; Chen, H.; Chathuranga, H.; Motta, N.; Alarco, J.A.; He, Z.-J.; Zheng, J.-C.; Du, A.; Yan, C. Few-layer MoS2 nanosheets with and without silicon nanoparticles as anodes for lithium-ion batteries. J. Mater. Chem. A 2023, 11, 2670–2678. [Google Scholar] [CrossRef]
- Xu, R.-C.; Xia, X.-H.; Wang, X.-L.; Xia, Y.; Tu, J.-P. Tailored Li2S–P2S5 glass-ceramic electrolyte by MoS2 doping, possessing high ionic conductivity for all-solid-state lithium-sulfur batteries. J. Mater. Chem. A 2017, 5, 2829–2834. [Google Scholar] [CrossRef]
- Chen, Y.; Song, B.; Tang, X.; Lu, L.; Xue, J. Ultrasmall Fe3O4 Nanoparticle/MoS2 Nanosheet Composites with Superior Performances for Lithium Ion Batteries. Small 2014, 10, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liu, E.; Cao, T.; He, F.; Shi, C.; He, C.; Ma, L.; Li, Q.; Li, J.; Zhao, N. Controllable graphene incorporation and defect engineering in MoS2-TiO2 based composites: Towards high-performance lithium-ion batteries anode materials. Nano Energy 2017, 33, 247–256. [Google Scholar] [CrossRef]
- Zhuang, W.; Li, L.; Zhu, J.; An, R.; Lu, L.; Lu, X.; Wu, X.; Ying, H. Facile Synthesis of Mesoporous MoS2-TiO2 Nanofibers for Ultrastable Lithium Ion Battery Anodes. ChemElectroChem 2015, 2, 374–381. [Google Scholar] [CrossRef]
- Villevieille, C.; Wang, X.-J.; Krumeich, F.; Nesper, R.; Novák, P. MoS2 coating on MoO3 nanobelts: A novel approach for a high specific charge electrode for rechargeable Li-ion batteries. J. Power Sources 2015, 279, 636–644. [Google Scholar] [CrossRef]
- Younis, M.W.; Akhter, T.; Yousaf, M.; Ali, M.; Naeem, H. Controlled dynamic variation of interfacial electronic and optical properties of lithium intercalated ZrO2/MoS2 vdW heterostructure. J. Mol. Graph. Model. 2024, 127, 108694. [Google Scholar] [CrossRef] [PubMed]
- Shahmohammadi, A.; Dalvand, S.; Molaei, A.; Mousavi-Khoshdel, S.M.; Yazdanfar, N.; Hasanzadeh, M. Transition metal phosphide/ molybdenum disulfide heterostructures towards advanced electrochemical energy storage: Recent progress and challenges. RSC Adv. 2025, 15, 13397–13430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, J.; Han, L.; Li, H.; Wang, J.; Lu, T.; Pan, L. In-situ fabrication of few-layered MoS2 wrapped on TiO2-decorated MXene as anode material for durable lithium-ion storage. J. Colloid Interface Sci. 2021, 604, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, A.; Amirhosseini, M.H. Machine Learning in Lithium-Ion Battery: Applications, Challenges, and Future Trends. SN Comput. Sci. 2024, 5, 717. [Google Scholar] [CrossRef]
- Lu, M.; Ji, H.; Chen, Y.; Gao, F.; Liu, B.; Long, P.; Deng, C.; Wang, Y.; Tao, J. Machine learning assisted layer-controlled synthesis of MoS2. J. Mater. Chem. C 2024, 12, 8893–8900. [Google Scholar] [CrossRef]
- Chen, X.; Liu, X.; Shen, X.; Zhang, Q. Applying machine learning to rechargeable batteries: From the microscale to the macroscale. Angew. Chem. 2021, 133, 24558–24570. [Google Scholar] [CrossRef]
- Kilic, A.; Oral, B.; Eroglu, D.; Yildirim, R. Machine learning for beyond Li-ion batteries: Powering the research. J. Energy Storage 2023, 73, 109057. [Google Scholar] [CrossRef]
- Lai, C.-H.; Lu, M.-Y.; Chen, L.-J. Metal sulfide nanostructures: Synthesis, properties and applications in energy conversion and storage. J. Mater. Chem. 2012, 22, 19–30. [Google Scholar] [CrossRef]
- Chothe, U.; Ugale, C.; Kulkarni, M.; Kale, B. Solid-State Synthesis of Layered MoS2 Nanosheets with Graphene for Sodium-Ion Batteries. Crystals 2021, 11, 660. [Google Scholar] [CrossRef]
- Fang, Y.; Xiao, L.; Chen, Z.; Ai, X.; Cao, Y.; Yang, H. Recent Advances in Sodium-Ion Battery Materials. Electrochem. Energy Rev. 2018, 1, 294–323. [Google Scholar] [CrossRef]
- Szkoda, M.; Ilnicka, A.; Trzciński, K.; Zarach, Z.; Roda, D.; Nowak, A.P. Synthesis and characterization of MoS2-carbon based materials for enhanced energy storage applications. Sci. Rep. 2024, 14, 26128. [Google Scholar] [CrossRef] [PubMed]
- Rahmatinejad, J.; Ye, Z. Advanced MoS2 nanocomposites for post-lithium-ion batteries. Chem. Eng. J. 2024, 500, 156872. [Google Scholar] [CrossRef]
- Chinnappan, N.; Punniyakoti, S. Emerging advances of 2D molybdenum disulfide (MoS2) and their composites towards high-performance supercapacitors: A comprehensive review. J. Energy Storage 2024, 102, 114040. [Google Scholar] [CrossRef]
- Joseph, N.; Shafi, P.M.; Bose, A.C. Recent Advances in 2D-MoS2 and its Composite Nanostructures for Supercapacitor Electrode Application. Energy Fuels 2020, 34, 6558–6597. [Google Scholar] [CrossRef]
- Liu, F.; Wang, T.; Liu, X.; Fan, L.-Z. Challenges and Recent Progress on Key Materials for Rechargeable Magnesium Batteries. Adv. Energy Mater. 2021, 11, 2000787. [Google Scholar] [CrossRef]
- Kotobuki, M.; Yan, B.; Lu, L. Recent progress on cathode materials for rechargeable magnesium batteries. Energy Storage Mater. 2023, 54, 227–253. [Google Scholar] [CrossRef]
- Liang, H.; Cao, Z.; Ming, F.; Zhang, W.; Anjum, D.H.; Cui, Y.; Cavallo, L.; Alshareef, H.N. Aqueous Zinc-Ion Storage in MoS2 by Tuning the Intercalation Energy. Nano Lett. 2019, 19, 3199–3206. [Google Scholar] [CrossRef] [PubMed]


| Material | Specific Capacity (mAh g−1) | Coulombic Efficiency | Reference |
|---|---|---|---|
| Amorphous bulky MoS2 | 100 | 54% after 100 cycles | [75] |
| Nanoflake MoS2 | 1175 | 96% after 40 cycles | [76] |
| Cobalt-templated few-layered MoS2 | 1661 | 92% after 300 cycles | [77] |
| Lysine-templated few-layered MoS2 | 1160 | 51% after 70 cycles | [78] |
| Plasma-treated MoS2 | 1038 | 50% after 70 cycles | [79] |
| Nitrogen-doped MoS2 | 1130 | 86% after 100 cycles | [80] |
| Stacked 2D MoS2 | 569 | 90% after 200 cycles | [81] |
| Vertically aligned MoS2 nanotubes | 1100 | 83% after 200 cycles | [82] |
| Mesoporous silica-templated 1T-MoS2 | 1100 | 80% after 50 cycles | [83] |
| 3D self-assembled few-layered MoS2 | 1137 | 54% after 50 cycles | [84] |
| MoS2 nanotubes | 1253 | 16% after 50 cycles | [85] |
| MoO3@MoS2 | 564 | 99% after 50 cycles | [86] |
| MoO3@MoS2 | 864 | 39% after 1000 cycles | [87] |
| MoO3@MoS2 | 1531 | 78% after 100 cycles | [88] |
| Core–sheath MoO3@MoS2 | 1531 | 98% after 150 cycles | [89] |
| ZnS@MoS2 | 1346 | 73% after 300 cycles | [90] |
| TiO2@MoS2 | 827 | 73% after 100 cycles | [91] |
| TiO2@MoS2 | 871 | 54% after 80 cycles | [92] |
| SnO2@MoS2 | 530 | 75% after 150 cycles | [93] |
| SnO2@MoS2 | 707 | 71% after 100 cycles | [94] |
| CoMoO4@MoS2 | 1100 | 55% after 100 cycles | [95] |
| Co3O4@MoS2 | 1200 | 67% after 100 cycles | [96] |
| VS2@MoS2 * | 585 * | n.a. * | [97] |
| MoS2@MWCNTs | 1214 | 85% after 60 cycles | [98] |
| MoS2@SWCNTs thin film | 1066 | 93% after 60 cycles | [99] |
| MoS2@MWCNTs layered composites | 670 | 75% after 80 cycles | [100] |
| TiO2@MoS2@MWCNTs | 680 | 85% after 200 cycles | [101] |
| MoS2 supported on graphene | 877 | 62% after 60 cycles | [102] |
| MoS2 supported on a few layers of graphene | 1229 | 77% after 60 cycles | [103] |
| MoS2 supported on graphene aerogel | 1140 | 91% after 60 cycles | [104] |
| MoS2 supported on graphene cryo-aerogel | 863 | ~99% after 60 cycles | [105] |
| Vertically aligned MoS2 supported on GO | 1077 | 87% after 400 cycles | [106] |
| Nanoflowers of MoS2 supported on rGO | 1250 | 54% after 250 cycles | [107] |
| Nanoflowers of MoS2 supported on rGO | 1150 | 77% after 60 cycles | [108] |
| MoS2 supported on graphdiyne oxide | 653 | ~99% after 600 cycles | [109] |
| MoS2 supported on nitrogen-doped graphene | 1040 | 97% after 100 cycles | [110] |
| MoS2 supported on nitrogen-doped graphene | 727 | 97% after 100 cycles | [111] |
| 2D MoS2 supported on carbon nanosheets | 709 | 95% after 520 cycles | [112] |
| MoS2 supported on amorphous carbon | 521 | ~99% after 100 cycles | [113] |
| MoS2 incorporated into carbon spheres | 1813 | ~99% after 100 cycles | [114] |
| MoS2 incorporated into carbon sponges | 784 | 86% after 100 cycles | [115] |
| MoS2 incorporated into carbon nanocages | 1372 | 91% after 100 cycles | [116] |
| MoS2 supported on poly(dopamine) | 1210 | ~99% after 100 cycles | [117] |
| MoS2 supported on poly(aniline) | 888 | 97% after 100 cycles | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartoli, M.; Cinali, M.B.; Coşkun, Ö.D.; Porporato, S.; Pugliese, D.; Piatti, E.; Geobaldo, F.; Elia, G.A.; Gerbaldi, C.; Meligrana, G.; et al. A Concise Overview of the Use of Low-Dimensional Molybdenum Disulfide as an Electrode Material for Li-Ion Batteries and Beyond. Batteries 2025, 11, 269. https://doi.org/10.3390/batteries11070269
Bartoli M, Cinali MB, Coşkun ÖD, Porporato S, Pugliese D, Piatti E, Geobaldo F, Elia GA, Gerbaldi C, Meligrana G, et al. A Concise Overview of the Use of Low-Dimensional Molybdenum Disulfide as an Electrode Material for Li-Ion Batteries and Beyond. Batteries. 2025; 11(7):269. https://doi.org/10.3390/batteries11070269
Chicago/Turabian StyleBartoli, Mattia, Meltem Babayiğit Cinali, Özlem Duyar Coşkun, Silvia Porporato, Diego Pugliese, Erik Piatti, Francesco Geobaldo, Giuseppe A. Elia, Claudio Gerbaldi, Giuseppina Meligrana, and et al. 2025. "A Concise Overview of the Use of Low-Dimensional Molybdenum Disulfide as an Electrode Material for Li-Ion Batteries and Beyond" Batteries 11, no. 7: 269. https://doi.org/10.3390/batteries11070269
APA StyleBartoli, M., Cinali, M. B., Coşkun, Ö. D., Porporato, S., Pugliese, D., Piatti, E., Geobaldo, F., Elia, G. A., Gerbaldi, C., Meligrana, G., & Piovano, A. (2025). A Concise Overview of the Use of Low-Dimensional Molybdenum Disulfide as an Electrode Material for Li-Ion Batteries and Beyond. Batteries, 11(7), 269. https://doi.org/10.3390/batteries11070269












