Green Batteries: A Sustainable Approach Towards Next-Generation Batteries
Abstract
1. Introduction
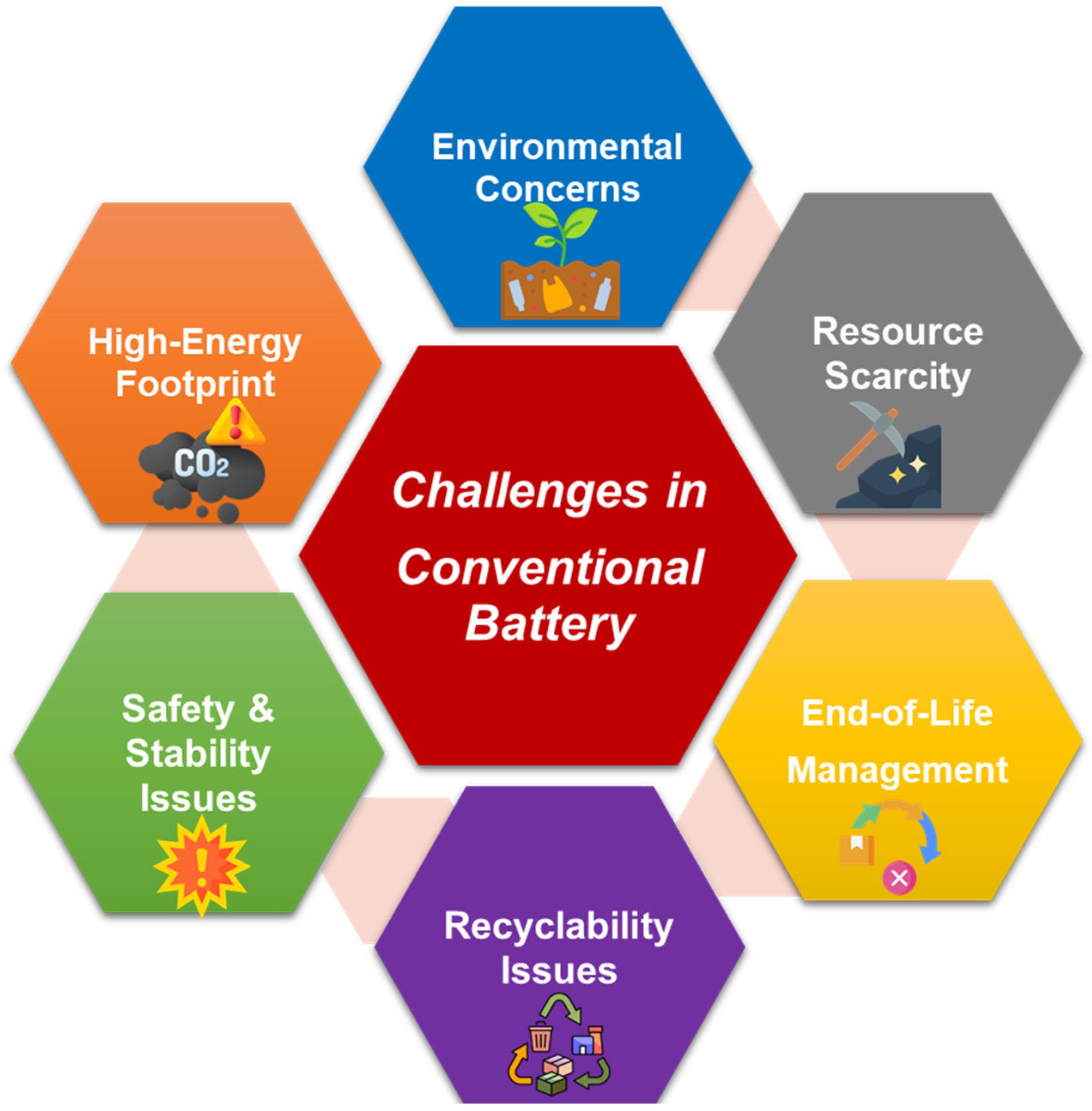
1.1. Why Green Batteries
1.2. Green Batteries: Concept and Principles
- (i).
- Eco-friendly material selection: Green batteries prioritize the use of abundant, renewable, and non-toxic materials. This includes replacing scarce or hazardous elements like cobalt and nickel with more sustainable alternatives such as iron, manganese, sodium, zinc, or organic redox-active materials. Natural polymers (e.g., cellulose, chitosan, alginate) are increasingly used in electrolytes and separators due to their biodegradability and functional properties.
- (ii).
- Sustainable synthesis processes: Green synthesis techniques emphasize low-energy, solvent-free, or water-based fabrication methods. Bioinspired or bio-assisted approaches, such as using plant extracts or microorganisms for electrode material synthesis, not only reduce the use of harmful chemicals but also introduce functional groups that enhance electrochemical performance. These methods contribute to a smaller carbon footprint and reduced environmental toxicity.
- (iii).
- Design for end-of-life and recyclability: A fundamental tenet of green battery development is circularity—designing batteries that can be easily disassembled and recycled at the end of their service life. This involves selecting materials and chemistries that allow for efficient recovery and reuse without generating hazardous waste, aligning with principles of the circular economy.
- (iv).
- Safety and environmental compatibility: Green batteries incorporate inherently safe materials that are thermally and chemically stable. Non-flammable solid-state or aqueous electrolytes reduce risks of leakage, combustion, and thermal runaway. This improves operational safety and ensures compliance with environmental and transportation regulations.
- (v).
- Efficient energy and resource: Minimizing the use of critical raw materials and energy during manufacturing enhances sustainability. Green batteries also strive for high energy density and long cycle life to reduce overall resource consumption over time.
2. Green Electrode Fabrication Techniques
| Green Synthesis/Fabrication Method | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Mechanochemical synthesis | Solvent-free, scalable, low environmental impact | Limited control over nanostructure, possible lower conductivity, scalability challenges | [34,35] |
| Hydrothermal carbonization | Renewable precursors, high surface area, eco-friendly | May require high energy input, batch-to-batch variability, limited scalability | [36,37,38] |
| Biopolymer-assisted synthesis | Plant extracts, biopolymer binders, high stability | Variability in biopolymer sources, potential for lower mechanical/electrochemical performance | [39] |
| Molten salt/in situ electrochemical MXene | No hazardous acids, direct device integration | Lower mechanical strength, binder limitations, material compatibility | [40,41] |
| Water/ethanol-based electrode processing | Non-toxic, cost-effective, comparable performance | Lower mechanical strength, binder limitations, material compatibility | [9,10,11] |
| Electrophoretic deposition (EPD) | Green solvents, improved adhesion | Process complexity, scalability issues | [9] |
| Aqueous polymer binders | Fluoro-free, recyclable, closed-loop manufacturing | Adhesion/chemical stability concerns | [10,11] |
| Atomic layer deposition (ALD) | Stable coatings, improved organic electrode performance | Slow deposition rates, scalability, equipment cost | [16] |
| Green Electrolyte Preparation | Reduces hazardous waste and environmental impact | May have lower conductivity or performance than traditional methods | [42] |
| (Natural solvents, green synthesis) | Uses renewable, biodegradable materials | High viscosity, cost, and scalability issues for some solvents | [42] |
| Sustainable Separator Synthesis | Utilizes biodegradable, renewable material | Limited data on long-term stability and performance | [43] |
| Biomass-based, green processing) | Reduces reliance on petrochemicals and toxic solvents | Potentially higher production costs and process complexity | [43] |
| Waste Source | Battery Type | Electrochemical Performance | Ref. |
|---|---|---|---|
| Biomass (charcoal) | Na-ion, Li-ion | High capacity, long cycle life | [33] |
| Glass microfiber | Li-ion | Good cycling, high areal capacity | [46] |
| Silicon cutting waste | Li-ion | High reversible capacity, stable cycling | [47] |
| Cotton cloth | Vanadium redox flow | Higher efficiency than commercial electrodes | [48] |
| Tires | Li-ion | Potential for higher capacity than graphite | [49] |
| Pomelo peel | Zn-ion (flexible) | Outstanding electrochemical/mechanical properties | [50] |
| Electrolyte Type | Battery Type | Electrochemical Performance | Ref. |
|---|---|---|---|
| Water-in-salt (LiTFSI) | Li-ion, Na-ion, Zn | High voltage, safety, energy density | [51,52] |
| Semisolid (lake water) | Flexible supercapacitors | Low cost, flexibility, cycling stability | [53] |
| Water-in-polymer | Solid-state batteries | Recyclability, wide voltage window | [53] |
| Acetate-based WiSEs | Zn-ion | Corrosion suppression, long cycle life | [54] |
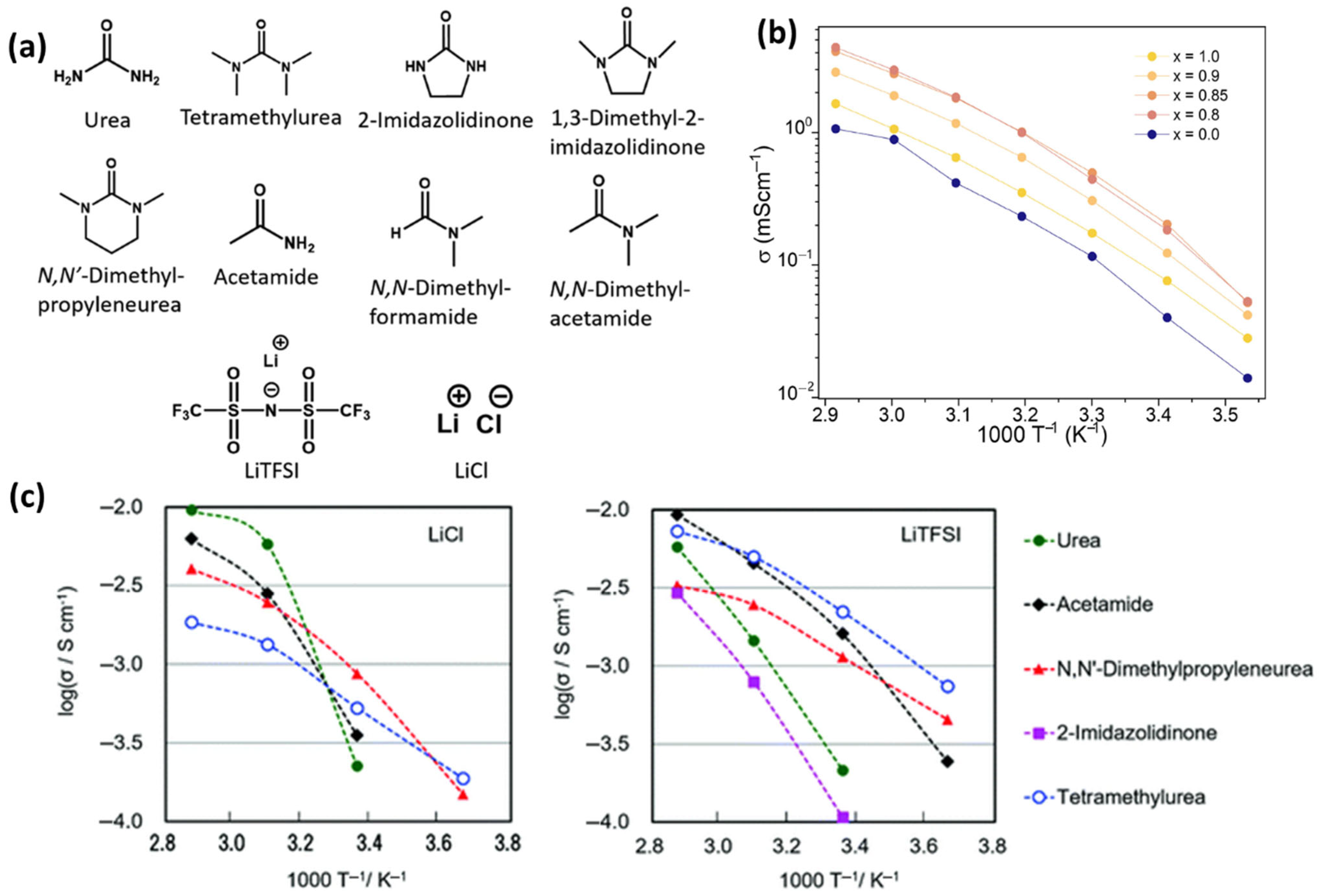
3. Green, Sustainable, and Efficient Electrolytes
3.1. Ionic Liquids and Deep Eutectic Solvents as Green Electrolytes
3.2. Water-Based and Water-in-Polymer Electrolytes
3.3. Biopolymer-Based Solid Electrolytes
3.3.1. Cellulose-Based Electrolytes
3.3.2. Chitosan-Based Electrolytes
3.3.3. Other Biopolymer-Based Electrolytes
3.4. Additive-Free and Renewable Electrolytes
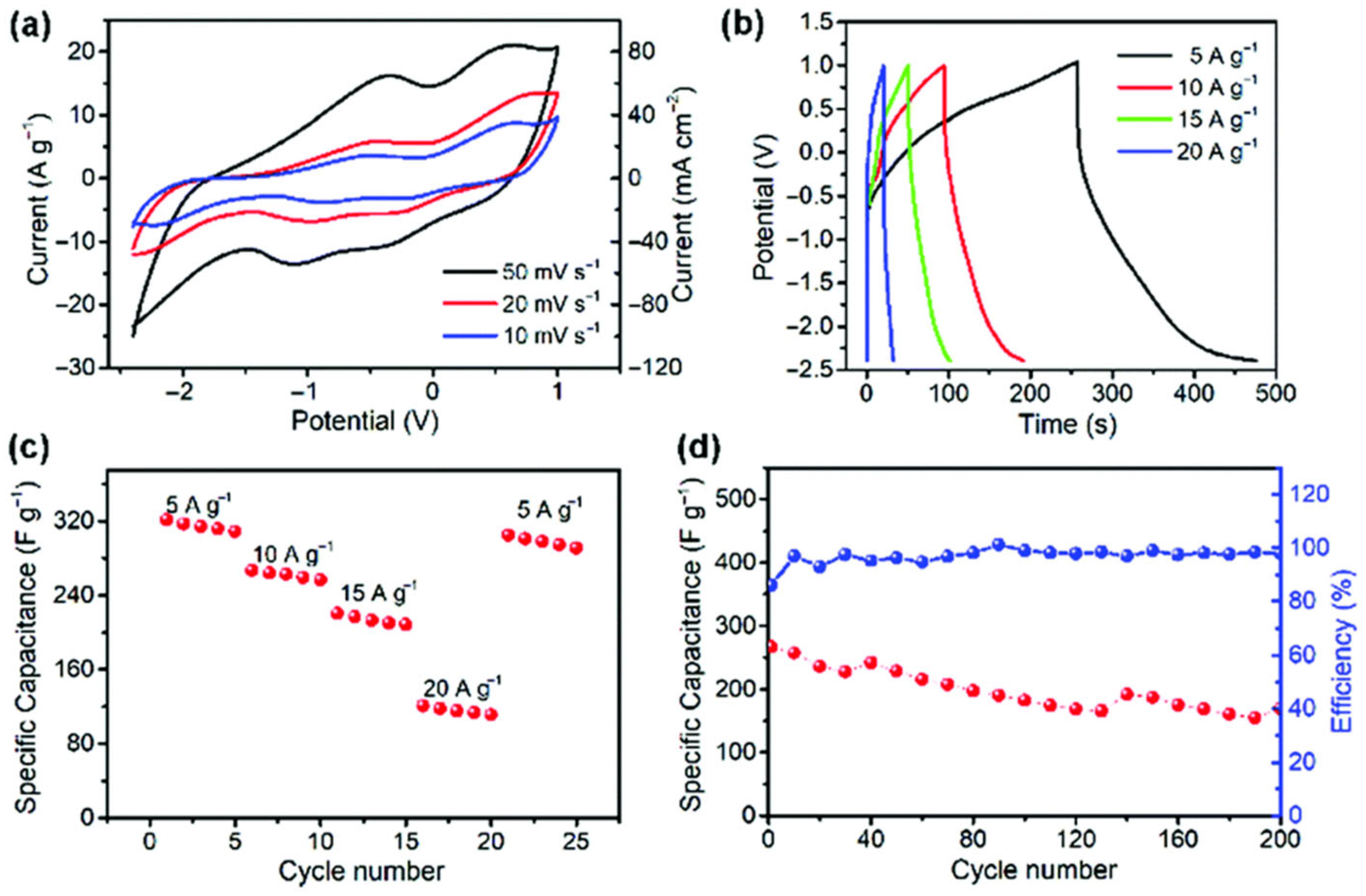
4. Green Separators for Sustainable Batteries
4.1. Cellulose and Chitosan-Derived Separators
4.2. Advanced Nanostructured Membranes
5. Overview of Advancements in Latest Sustainable Key Battery Technologies
5.1. Green and Sustainable Na-Ion Batteries
5.2. Green and Sustainable Zn-Ion/Air Batteries
5.3. Green and Sustainable Aluminum-Ion Batteries
5.4. Green and Conventional Battery Comparative Electrochemical Performance
6. Environment Assessment
6.1. Biodegradability Test
6.2. Recyclability
- Deep eutectic solvents, which have achieved over 90% leaching efficiency for lithium and cobalt.
- Ultrasonic-assisted leaching, reaching over 98% recovery of both metals.
- An enhanced oxalate process using hydrogen peroxide, which improves cost-effectiveness and reduces energy consumption.
- Electrolysis-based recovery, which can extract over 50% of LiCoO2 in a single step without toxic reagents or high temperatures.
- Vacuum metallurgy, allowing in situ lithium recycling with over 80% efficiency.
7. Application Field Strategies for Green Battery Technologies
8. Market Potential and Global Regulations
9. Challenges and Future Perspectives
- Molecular tailoring through chemical modification (e.g., grafting ionic moieties or blending with conductive fillers) will be essential to enhance ionic conductivity and mechanical stability.
- Composite approaches, where biopolymers are integrated with nanomaterials (e.g., graphene, MXenes, or metal–organic frameworks), will offer enhanced thermal resilience, porosity control, and ion selectivity.
- Improved crosslinking strategies can yield biopolymer membranes with better electrolyte retention and long-term electrochemical stability.
- Standardization of biodegradability protocols under both aerobic and anaerobic conditions is necessary to support environmental risk assessments and ensure these materials decompose into benign by-products.
- Environmental risk perspective:
- Assessing end-of-life decomposition products and their interactions with soil and water ecosystems is critical.
- Potential allergenicity or toxicity of degradation intermediates must be systematically evaluated.
- Life cycle analysis (LCA) should become integral to biopolymer material development to validate their sustainability claims.
- Develop self-healing or multifunctional electrolyte systems that inherently stabilize SEI without additives.
- Investigate solvent–electrode interface chemistry to engineer stable interphases.
- Explore biologically derived solvents from lignin, glycerol, or sugars that can offer high polarity and low volatility.
- Scalable fabrication methods like roll-to-roll electrospinning or 3D printing will be pivotal.
- Future membranes may incorporate smart stimuli-responsive features (pH, temperature, or ion-triggered gating) for real-time control of ion flow and dendrite suppression.
- Biodegradable nanomaterials (e.g., nanocellulose, chitin nanofibers) will increasingly replace synthetic polymers.
- Environmental risk and biodegradability focus:
- Nanotoxicology must be rigorously studied, especially for nanomaterials released during degradation.
- Emphasis should be placed on membranes that degrade into non-toxic fragments and offer soil or marine compostability.
- Development of bio-derived and biodegradable ILs/DESs (e.g., based on amino acids, choline chloride, sugars) is critical.
- Lowering viscosity while preserving ionic conductivity is a primary research target.
- Future ILs/DESs may function as dual-purpose materials (electrolytes + binders or flame retardants).
- Investigate biocompatibility and long-term environmental fate of ILs/DESs.
- Standardized testing under environmental exposure conditions (light, moisture, microbial degradation) must be established.
- Development of ISO-compliant protocols to test real-world biodegradation of battery materials.
- Integration of microbial enrichment studies to understand and optimize decomposition kinetics.
- Implement predictive toxicity modeling and metabolomics to evaluate degradation pathways.
- Full life cycle assessment (LCA) to determine energy consumption, emissions, and recycling potential.
- Push for eco-labeling and certifications for green battery materials.
- Encourage international green materials registries and guidelines to harmonize safety and performance standards.
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Melin, H.E.; Rajaeifar, M.A.; Ku, A.Y.; Kendall, A.; Harper, G.; Heidrich, O. Global Implications of the EU Battery Regulation. Science 2021, 373, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Arya, N. India’s E-Vehicle Policy 2024: Paving the Way for a Greener Future in Sustainable Transportation. Available online: https://www.impriindia.com/insights/indias-e-vehicle-policy-2024/ (accessed on 24 May 2025).
- Wang, H.; Wang, Y.; Wu, Q.; Zhu, G. Recent Developments in Electrode Materials for Dual-Ion Batteries: Potential Alternatives to Conventional Batteries. Mater. Today 2021, 52, 269–298. [Google Scholar] [CrossRef]
- Xu, P.; Tan, D.; Chen, Z. Emerging Trends in Sustainable Battery Chemistries. Trends Chem. 2021, 3, 620–630. [Google Scholar] [CrossRef]
- Annu Park, S.S.; Alam, M.N.; Yewale, M.; Shin, D.K. Unraveling the Electrochemical Insights of Cobalt Oxide/Conducting Polymer Hybrid Materials for Supercapacitor, Battery, and Supercapattery Applications. Polymers 2024, 16, 2907. [Google Scholar] [CrossRef]
- Paul, D.; Pechancová, V.; Saha, N.; Pavelková, D.; Saha, N.; Motiei, M.; Jamatia, T.; Chaudhuri, M.; Ivanichenko, A.; Venher, M.; et al. Life Cycle Assessment of Lithium-Based Batteries: Review of Sustainability Dimensions. Renew. Sustain. Energy Rev. 2024, 206, 114860. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, T.; Zhang, M. Advances in Intelligent Regeneration of Cathode Materials for Sustainable Lithium-Ion Batteries. Adv. Energy Mater. 2022, 12, 2201526. [Google Scholar] [CrossRef]
- Shivani; Thakur, R.C.; Thakur, A.; Sharma, A.; Sharma, R. Unravelling the Prospects of Electrolytes Containing Ionic Liquids and Deep Eutectic Solvents for Next Generation Lithium Batteries. J. Energy Chem. 2025, 105, 482–500. [Google Scholar] [CrossRef]
- Jin, X.; Zhu, Z.; Miao, Q.; Fang, C.; Huang, D.; Giovine, R.; Chen, L.; Dun, C.; Urban, J.; Fu, Y.; et al. Green Electrode Processing Enabled by Fluoro-Free Multifunctional Binders for Lithium-Ion Batteries. Adv. Sci. 2025, 12, 2416995. [Google Scholar] [CrossRef]
- Xia, Z.; Li, Z.-H.; Xu, J.; Sasidharan, S.; Sanchez, J.; Palermo, V.; Asp, L. Green Synthesis of Positive Electrodes for High Performance Structural Batteries—A Study on Graphene Additives. Compos. Sci. Technol. 2024, 251, 110568. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Yang, T.; Ge, D.; Wood, D.; Li, Z. Water-Based Electrode Manufacturing and Direct Recycling of Lithium-Ion Battery Electrodes—A Green and Sustainable Manufacturing System. iScience 2020, 23, 101081. [Google Scholar] [CrossRef]
- Ri, V.; Kim, H.; Lee, H.; Ku, J.; Lee, P.G.; Kim, C.; Shin, H. Directly Electrostatic-Spray Deposited Cross-Linked Nanocomposites for the High-Performance Lithium-Ion Battery Anode. J. Alloys Compd. 2024, 980, 173613. [Google Scholar] [CrossRef]
- Hiroya, A.; Akira, K.; Makio, N.; Masayuki, Y. Electrostatic Spray Deposition for Fabrication of Li-Ion Batteries. Trans. JWRI 2015, 44, 9–12. [Google Scholar]
- Cantillo, D. Synthesis of Active Pharmaceutical Ingredients Using Electrochemical Methods: Keys to Improve Sustainability. Chem. Commun. 2022, 58, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Francke, R. Concepts for Sustainable Organic Electrosynthesis. Curr. Opin. Electrochem. 2022, 36, 101111. [Google Scholar] [CrossRef]
- Thangavel, R.; Moorthy, M.; Ganesan, B.; Lee, W.; Yoon, W.; Lee, Y. Nanoengineered Organic Electrodes for Highly Durable and Ultrafast Cycling of Organic Sodium-Ion Batteries. Small 2020, 16, 2003688. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Kim, J.; Kim, M.; Song, T.; Paik, U. Sustainable and Cost-Effective Electrode Manufacturing for Advanced Lithium Batteries: The Roll-to-Roll Dry Coating Process. Chem. Sci. 2025, 16, 6598–6619. [Google Scholar] [CrossRef] [PubMed]
- Örüm Aydin, A.; Zajonz, F.; Günther, T.; Dermenci, K.B.; Berecibar, M.; Urrutia, L. Lithium-Ion Battery Manufacturing: Industrial View on Processing Challenges, Possible Solutions and Recent Advances. Batteries 2023, 9, 555. [Google Scholar] [CrossRef]
- Ludwig, B.; Zheng, Z.; Shou, W.; Wang, Y.; Pan, H. Solvent-Free Manufacturing of Electrodes for Lithium-Ion Batteries. Sci. Rep. 2016, 6, 23150. [Google Scholar] [CrossRef]
- Ryu, M.; Hong, Y.-K.; Lee, S.-Y.; Park, J.H. Ultrahigh Loading Dry-Process for Solvent-Free Lithium-Ion Battery Electrode Fabrication. Nat. Commun. 2023, 14, 1316. [Google Scholar] [CrossRef]
- Verdier, N.; Foran, G.; Lepage, D.; Prébé, A.; Aymé-Perrot, D.; Dollé, M. Challenges in Solvent-Free Methods for Manufacturing Electrodes and Electrolytes for Lithium-Based Batteries. Polymers 2021, 13, 323. [Google Scholar] [CrossRef]
- Sung, K.-E.; Hwang, I.; Choi, J.; Jung, S.-K.; Yoon, J. Enhanced Adhesion in PTFE-Based Dry Electrodes with Hydrogen Bonding Co-Binder Integration for Advanced Lithium-Ion Batteries. Chem. Eng. J. 2025, 511, 161789. [Google Scholar] [CrossRef]
- Hwang, I.; Sung, K.-E.; Hong, J.; Kang, G.-S.; Yoon, J. A Breakthrough in Dry Electrode Technology for High-Energy-Density Lithium-Ion Batteries with Spray-Dried SWCNT/NCM Composites. Chem. Eng. J. 2025, 506, 160159. [Google Scholar] [CrossRef]
- Oh, H.; Kim, G.-S.; Hwang, B.U.; Bang, J.; Kim, J.; Jeong, K.-M. Development of a Feasible and Scalable Manufacturing Method for PTFE-Based Solvent-Free Lithium-Ion Battery Electrodes. Chem. Eng. J. 2024, 491, 151957. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, S.; Lou, F.; Yu, Z. Solvent-Free Lithium Iron Phosphate Cathode Fabrication with Fibrillation of Polytetrafluoroethylene. Electrochim. Acta 2023, 456, 142469. [Google Scholar] [CrossRef]
- Kim, H.-M.; Yoo, B.-I.; Yi, J.-W.; Choi, M.-J.; Yoo, J.-K. Solvent-Free Fabrication of Thick Electrodes in Thermoplastic Binders for High Energy Density Lithium-Ion Batteries. Nanomaterials 2022, 12, 3320. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, X.; Ji, S.; Wang, Z.; Liu, Z.; Shen, J.; Hu, R.; Liu, J.; Zhu, M. Solvent-Free Method Prepared a Sandwich-like Nanofibrous Membrane-Reinforced Polymer Electrolyte for High-Performance All-Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2020, 12, 21586–21595. [Google Scholar] [CrossRef]
- Hu, L.; Ren, Y.; Wang, C.; Li, J.; Wang, Z.; Sun, F.; Ju, J.; Ma, J.; Han, P.; Dong, S.; et al. Fusion Bonding Technique for Solvent-Free Fabrication of All-Solid-State Battery with Ultrathin Sulfide Electrolyte. Adv. Mater. 2024, 36, 2401909. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, L.; Shi, Y.; Li, J.; Fu, Q.; Zhu, X.; Lu, Z.; Liao, Q. Biomass Waste-Derived Hierarchical Porous Composite Electrodes for High-Performance Thermally Regenerative Ammonia-Based Batteries. J. Power Sources 2022, 517, 230719. [Google Scholar] [CrossRef]
- Yu, J.; Lin, M.; Tan, Q.; Li, J. High-Value Utilization of Graphite Electrodes in Spent Lithium-Ion Batteries: From 3D Waste Graphite to 2D Graphene Oxide. J. Hazard. Mater. 2021, 401, 123715. [Google Scholar] [CrossRef]
- Zhu, Y.-H.; Yuan, S.; Bao, D.; Yin, Y.; Zhong, H.-X.; Zhang, X.-B.; Yan, J.-M.; Jiang, Q. Decorating Waste Cloth via Industrial Wastewater for Tube-Type Flexible and Wearable Sodium-Ion Batteries. Adv. Mater. 2017, 29, 1603719. [Google Scholar] [CrossRef]
- Ferdous, A.R.; Shah, S.S.; Shah, S.N.A.; Johan, B.A.; Al Bari, M.A.; Aziz, M.A. Transforming Waste into Wealth: Advanced Carbon-Based Electrodes Derived from Refinery and Coal By-Products for Next-Generation Energy Storage. Molecules 2024, 29, 2081. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liang, H.; Gu, Z.-Y.; Meng, Y.-F.; Yang, M.; Yu, M.; Zhao, C.; Wu, X.-L. Waste-to-Wealth: Low-Cost Hard Carbon Anode Derived from Unburned Charcoal with High Capacity and Long Cycle Life for Sodium-Ion/Lithium-Ion Batteries. Electrochim. Acta 2020, 361, 137041. [Google Scholar] [CrossRef]
- García-Rodríguez, M.; Cazorla-Amorós, D.; Morallón, E. Eco-Friendly Mechanochemical Synthesis of Bifunctional Metal Oxide Electrocatalysts for Zn-Air Batteries. ChemSusChem 2024, 17, e202401055. [Google Scholar] [CrossRef]
- Shi, X.; Liang, W.; Liu, G.; Chen, B.; Shao, L.; Sun, Z.; García, F.; Wu, Y. Electrode Materials for Li/Na Storage from Mechanochemically Synthesised MOFs/MXene Composites: A Solvent-Free Approach. Chem. Eng. J. 2023, 462, 142271. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Z.; Wang, G.; Hou, L.; Yuan, C. Eco-Friendly and Scalable Synthesis of Micro-/Mesoporous Carbon Sub-Microspheres as Competitive Electrodes for Supercapacitors and Sodium-Ion Batteries. Appl. Surf. Sci. 2020, 533, 147511. [Google Scholar] [CrossRef]
- Abdolrazzaghian, E.; Zhu, J.; Kim, J.; Yanilmaz, M. MoS2-Decorated Graphene@porous Carbon Nanofiber Anodes via Centrifugal Spinning. Nanomaterials 2022, 12, 2505. [Google Scholar] [CrossRef]
- Mikhaylov, A.; Medvedev, A.; Grishanov, D.; Edison, E.; Srinivasan, M.; Sladkevich, S.; Gun, J.; Prikhodchenko, P.; Lev, O. Green Synthesis of a Nanocrystalline Tin Disulfide-Reduced Graphene Oxide Anode from Ammonium Peroxostannate: A Highly Stable Sodium-Ion Battery Anode. ACS Sustain. Chem. Eng. 2020, 8, 5485–5494. [Google Scholar] [CrossRef]
- Nayak, S.; Kittur, A.A.; Nayak, S. Green Synthesis of Silver-Zirconia Composite Using Chitosan Biopolymer Binder for Fabrication of Electrode Materials in Supercapattery Application for Sustainable Energy Storage. Curr. Res. Green Sustain. Chem. 2022, 5, 100292. [Google Scholar] [CrossRef]
- Kim, H.; Jung, Y.; Lee, W.; Jeon, Y.-P.; Hong, J.-Y.; Lee, J.U. Sustainable MXene Synthesis via Molten Salt Method and Nano-Silicon Coating for Enhanced Lithium-Ion Battery Performance. Molecules 2025, 30, 812. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Yang, Q.; Liang, G.-J.; Huang, Z.; Ma, L.; Wang, D.; Mo, F.; Dong, B.; Huang, Q.; et al. In Situ Electrochemical Synthesis of MXenes without Acid/Alkali Usage in/for an Aqueous Zinc Ion Battery. Adv. Energy Mater. 2020, 10, 200179. [Google Scholar] [CrossRef]
- Ain, Q.U.; Irshad, M.; Butt, M.S.; Tabish, A.; Hanif, M.; Khalid, M.; Ghaffar, R.; Rafique, M.; Kazmi, S.D.S.; Siraj, K.; et al. Towards Sustainable Electrochemistry: Green Synthesis and Sintering Aid Modulations in the Development of BaZr0.87Y0.1M0.03O3−δ (M = Mn, Co, and Fe) IT-SOFC Electrolytes. Front. Chem. 2023, 11, 1322475. [Google Scholar] [CrossRef] [PubMed]
- Uzunoglu, G.Y.; Yuksel, R. Toward Green and Sustainable Zinc-Ion Batteries: The Potential of Natural Solvent-Based Electrolytes. Small 2025, 21, 2411478. [Google Scholar] [CrossRef] [PubMed]
- Rahmanudin, A.; Mohammadi, M.; Isacsson, P.; Li, Y.; Seufert, L.; Kim, N.; Mardi, S.; Engquist, I.; Crispin, R.; Tybrandt, K. Stretchable and Biodegradable Plant-Based Redox-Diffusion Batteries. Mater. Horiz. 2024, 11, 4400–4412. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Du, J.; Gao, Y.; Bu, F.; Tan, Y.; Wang, Y.; Fu, G.; Guan, C.; Xu, X.; Huang, W. Stereolithography of 3D Sustainable Metal Electrodes towards High-Performance Nickel Iron Battery. Adv. Funct. Mater. 2022, 32, 2205317. [Google Scholar] [CrossRef]
- Kang, W.; Kim, J.; Kim, D. Waste Glass Microfiber Filter-Derived Fabrication of Fibrous Yolk-Shell Structured Silicon/Carbon Composite Freestanding Electrodes for Lithium-Ion Battery Anodes. J. Power Sources 2020, 468, 228407. [Google Scholar] [CrossRef]
- Xi, F.; Zhang, Z.; Hu, Y.; Li, S.; Ma, W.; Chen, X.; Wan, X.; Chong, C.; Luo, B.; Wang, L. PSi@SiOx/Nano-Ag Composite Derived from Silicon Cutting Waste as High-Performance Anode Material for Li-Ion Batteries. J. Hazard. Mater. 2021, 414, 125480. [Google Scholar] [CrossRef]
- Allam, M.A.; Abdelkareem, M.A.; Alawadhi, H.; Olabi, A.; Fetyan, A. Upcycling Waste Cotton Cloth into a Carbon Textile: A Durable and Scalable Layer for Vanadium Redox Flow Battery Applications. Sustainability 2024, 16, 11289. [Google Scholar] [CrossRef]
- Egun, I.; Liu, Z.; Zheng, Y.; Wang, Z.; Song, J.; Hou, Y.; Lu, J.; Wang, Y.; Chen, Z. Turning Waste Tyres into Carbon Electrodes for Batteries: Exploring Conversion Methods, Material Traits, and Performance Factors. Carbon Energy 2024, 6, e571. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Zhou, X.; Mo, Y.; Zheng, Y.; Yuan, G.; Yang, M. All-Cellulose-Based Flexible Zinc-Ion Battery Enabled by Waste Pomelo Peel. J. Colloid Interface Sci. 2024, 678, 497–505. [Google Scholar] [CrossRef]
- Liu, T.; Tang, L.; Luo, H.; Cheng, S.; Liu, M. A Promising Water-in-Salt Electrolyte for Aqueous Based Electrochemical Energy Storage Cells with a Wide Potential Window: Highly Concentrated HCOOK. Chem. Commun. 2019, 55, 12817–12820. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, B.; Liu, X.; Liu, J.; Ding, J.; Zhong, C.; Hu, W. Water-in-Salt Electrolyte for Safe and High-Energy Aqueous Battery. Energy Storage Mater. 2021, 34, 461–474. [Google Scholar] [CrossRef]
- Hao, S.-M.; Zhu, J.; He, S.; Ma, L.; Liu, W.; Zhang, Y.; Xie, X.; Qin, X.; Fan, X.; Li, H.; et al. Water-in-Polymer Electrolyte with a Wide Electrochemical Window and Recyclability. Nat. Sustain. 2024, 7, 661–671. [Google Scholar] [CrossRef]
- Becker, M.; Kühnel, R.; Battaglia, C. Water-in-Salt Electrolytes for Aqueous Lithium-Ion Batteries with Liquidus Temperatures below −10 °C. Chem. Commun. 2019, 55, 12032–12035. [Google Scholar] [CrossRef]
- Ogawa, H.; Mori, H. Lithium Salt/Amide-Based Deep Eutectic Electrolytes for Lithium-Ion Batteries: Electrochemical, Thermal and Computational Study. Phys. Chem. Chem. Phys. 2020, 22, 8853–8863. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A. Indisputable Roles of Different Ionic Liquids, Deep Eutectic Solvents and Nanomaterials in Green Chemistry for Sustainable Organic Synthesis. J. Mol. Liq. 2024, 124469. [Google Scholar] [CrossRef]
- Yang, H.; Yu, F.; Li, S.; Geng, M.; Liu, J.; Chen, W.; Jin, X.; Peng, C. Acetamide-Based Hydrated Eutectic Electrolytes for Supercapacitors with High Voltage and Low Self-Discharge. Energy Storage Mater. 2025, 74, 103929. [Google Scholar] [CrossRef]
- Gregorio, V.; Baur, C.; Jankowski, P.; Chang, J.H. Deep Eutectic Solvents as Electrolytes for Zn Batteries: Between Blocked Crystallization, Electrochemical Performance and Corrosion Issues. ChemSusChem 2025, 18, e202402494. [Google Scholar] [CrossRef]
- Xiao, D.; Zhang, L.; Li, Z.; Dou, H.; Zhang, X. Design Strategies and Research Progress for Water-in-Salt Electrolytes. Energy Storage Mater. 2022, 44, 10–28. [Google Scholar] [CrossRef]
- Han, J.; Mariani, A.; Zarrabeitia, M.; Jusys, Z.; Behm, R.; Varzi, A.; Passerini, S. Zinc-Ion Hybrid Supercapacitors Employing Acetate-Based Water-in-Salt Electrolytes. Small 2022, 18, 2201563. [Google Scholar] [CrossRef]
- Zhang, Y.; Lewis, N.; Mars, J.; Wan, G.; Weadock, N.; Takacs, C.; Lukatskaya, M.; Steinrück, H.; Toney, M.; Tokmakoff, A.; et al. Water-in-Salt LiTFSI Aqueous Electrolytes. 1. Liquid Structure from Combined Molecular Dynamics Simulation and Experimental Studies. J. Phys. Chem. B 2021, 125, 4501–4513. [Google Scholar] [CrossRef]
- Zhang, Y.; Maginn, E. Water-In-Salt LiTFSI Aqueous Electrolytes (2): Transport Properties and Li+ Dynamics Based on Molecular Dynamics Simulations. J. Phys. Chem. B 2021, 125, 13246–13254. [Google Scholar] [CrossRef]
- Khatoon, F.; Shabbir, M.; Annu. An Introduction to Regenerated Cellulose: Morphologies and Applications. In Regenerated Cellulose and Composites: Morphology-Property Relationship; Shabbir, M., Ed.; Springer Nature: Singapore, 2023; pp. 1–7. ISBN 978-981-99-1655-9. [Google Scholar]
- Gupta, M.; Sheikh, J.; Annu; Singh, A. An Eco-Friendly Route to Develop Cellulose-Based Multifunctional Finished Linen Fabric Using ZnO NPs and CS Network. J. Ind. Eng. Chem. 2021, 97, 383–389. [Google Scholar] [CrossRef]
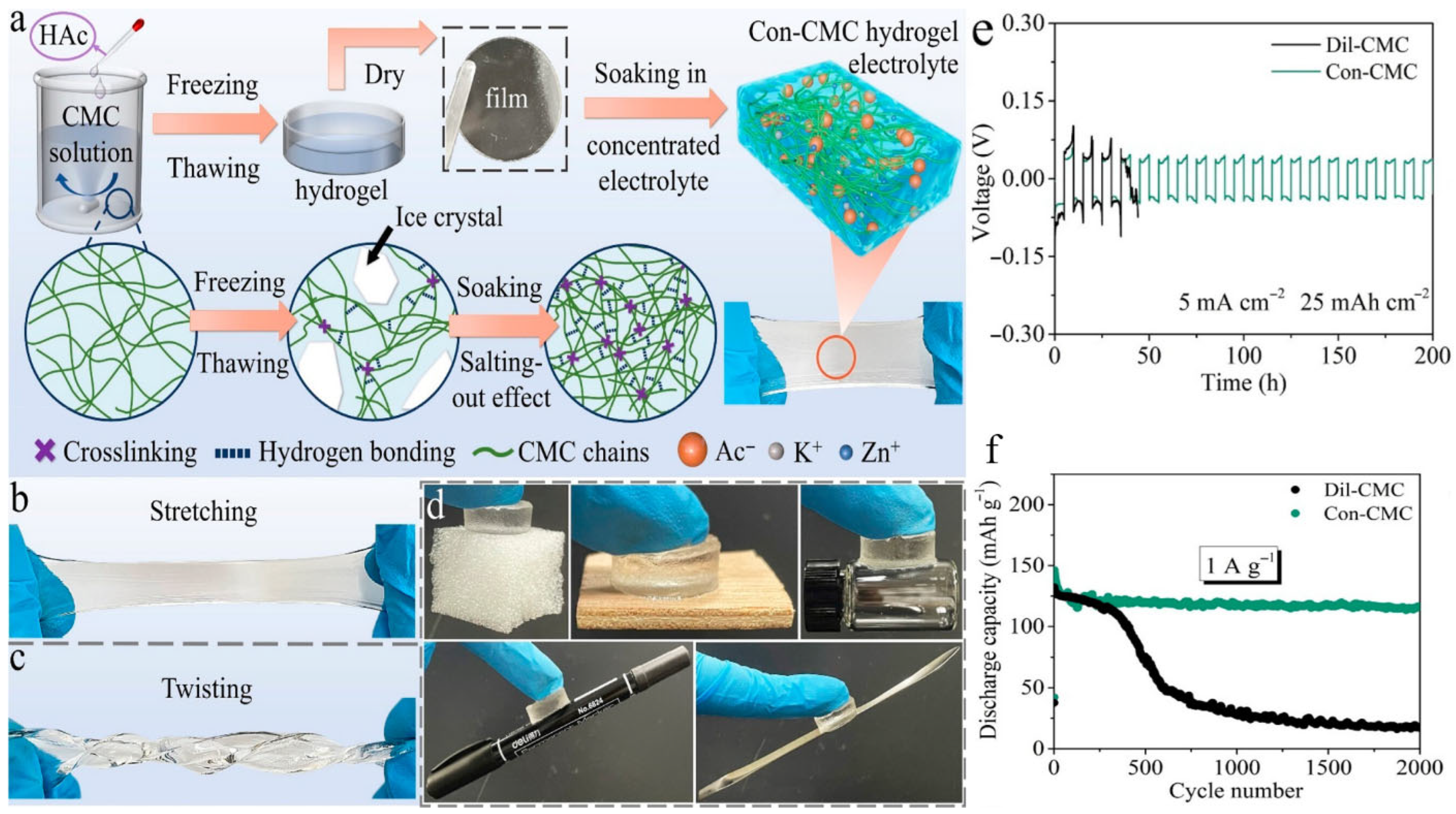
- Quan, Y.; Ma, H.; Chen, M.; Zhou, W.; Tian, Q.; Han, X.; Chen, J. Salting-Out Effect Realizing High-Strength and Dendrite-Inhibiting Cellulose Hydrogel Electrolyte for Durable Aqueous Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2023, 15, 44974–44983. [Google Scholar] [CrossRef]
- Mittal, N.; Tien, S.; Lizundia, E.; Niederberger, M. Hierarchical Nanocellulose-Based Gel Polymer Electrolytes for Stable Na Electrodeposition in Sodium Ion Batteries. Small 2022, 18, 2107183. [Google Scholar] [CrossRef] [PubMed]
- Rani, M.S.A.; Mohammad, M.; Sua’it, M.S.; Ahmad, A.; Mohamed, N.S. Novel Approach for the Utilization of Ionic Liquid-Based Cellulose Derivative Biosourced Polymer Electrolytes in Safe Sodium-Ion Batteries. Polym. Bull. 2021, 78, 5355–5377. [Google Scholar] [CrossRef]
- Tangthuam, P.; Kao-ian, W.; Sangsawang, J.; Rojviriya, C.; Chirawatkul, P.; Kasemchainan, J.; Mahlendorf, F.; Nguyen, M.T.; Yonezawa, T.; Kheawhom, S. Carboxymethyl Cellulose as an Artificial Solid Electrolyte Interphase for Stable Zinc-Based Anodes in Aqueous Electrolytes. Mater. Sci. Energy Technol. 2023, 6, 417–428. [Google Scholar] [CrossRef]
- Annu; Ahmed, S.; Ahmed, S.; Ikram, S. Chitin and Chitosan: History, Composition and Properties. In Chitosan; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 1–24. ISBN 9781119364849. [Google Scholar]
- Annu; Ahmed, S.; Ikram, S. Perspectives of Chitosan and Alginate Membranes for Biomedical Applications. In Natural Polymers: Derivatives, Blends and Composites; Ahmed, S., Ikram, S., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2017; Volume 2, pp. 157–182. ISBN 9781536104400. [Google Scholar]
- Lu, H.; Hu, J.; Wei, X.; Zhang, K.; Xiao, X.; Zhao, J.; Hu, Q.; Yu, J.; Zhou, G.; Xu, B. A Recyclable Biomass Electrolyte towards Green Zinc-Ion Batteries. Nat. Commun. 2023, 14, 4435. [Google Scholar] [CrossRef]
- Chopra, H.; Sharma, S.; Shabbir, M.; Annu; Shin, D.-K. Future Perspectives of Biopolymers for Biomedical Applications. In Biopolymers for Biomedical Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2024; pp. 509–530. ISBN 9781119865452. [Google Scholar]
- Kucinskis, G.; Kruze, B.; Korde, P.; Sarakovskis, A.; Viksna, A.; Hodakovska, J.; Bajars, G. Enhanced Electrochemical Properties of Na0.67MnO2 Cathode for Na-Ion Batteries Prepared with Novel Tetrabutylammonium Alginate Binder. Batteries 2022, 8, 6. [Google Scholar] [CrossRef]
- Ding, Y.; Zhong, X.; Yuan, C.; Duan, L.; Zhang, L.; Wang, Z.; Wang, C.; Shi, F. Sodium Alginate Binders for Bivalency Aqueous Batteries. ACS Appl. Mater. Interfaces 2021, 13, 20681–20688. [Google Scholar] [CrossRef]
- Shekh, M.I.; Annu; Ahmed, S. Chapter 1—Biopolymers: An Overview. In Advanced Applications of Biobased Materials; Ahmed, S., Annu, Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 3–21. ISBN 978-0-323-91677-6. [Google Scholar]
- Prasanna, K.; Subburaj, T.; Jo, Y.N.; Lee, W.J.; Lee, C.W. Environment-Friendly Cathodes Using Biopolymer Chitosan with Enhanced Electrochemical Behavior for Use in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 7884–7890. [Google Scholar] [CrossRef]
- Bargnesi, L.; Gigli, F.; Albanelli, N.; Toigo, C.; Arbizzani, C. Crosslinked Chitosan Binder for Sustainable Aqueous Batteries. Nanomaterials 2022, 12, 254. [Google Scholar] [CrossRef]
- Yu, L.; Tao, B.; Ma, L.; Zhao, F.; Wei, L.; Tang, G.; Wang, Y.; Guo, X. A Robust Network Sodium Carboxymethyl Cellulose-Epichlorohydrin Binder for Silicon Anodes in Lithium-Ion Batteries. Langmuir 2024, 40, 17930–17940. [Google Scholar] [CrossRef] [PubMed]
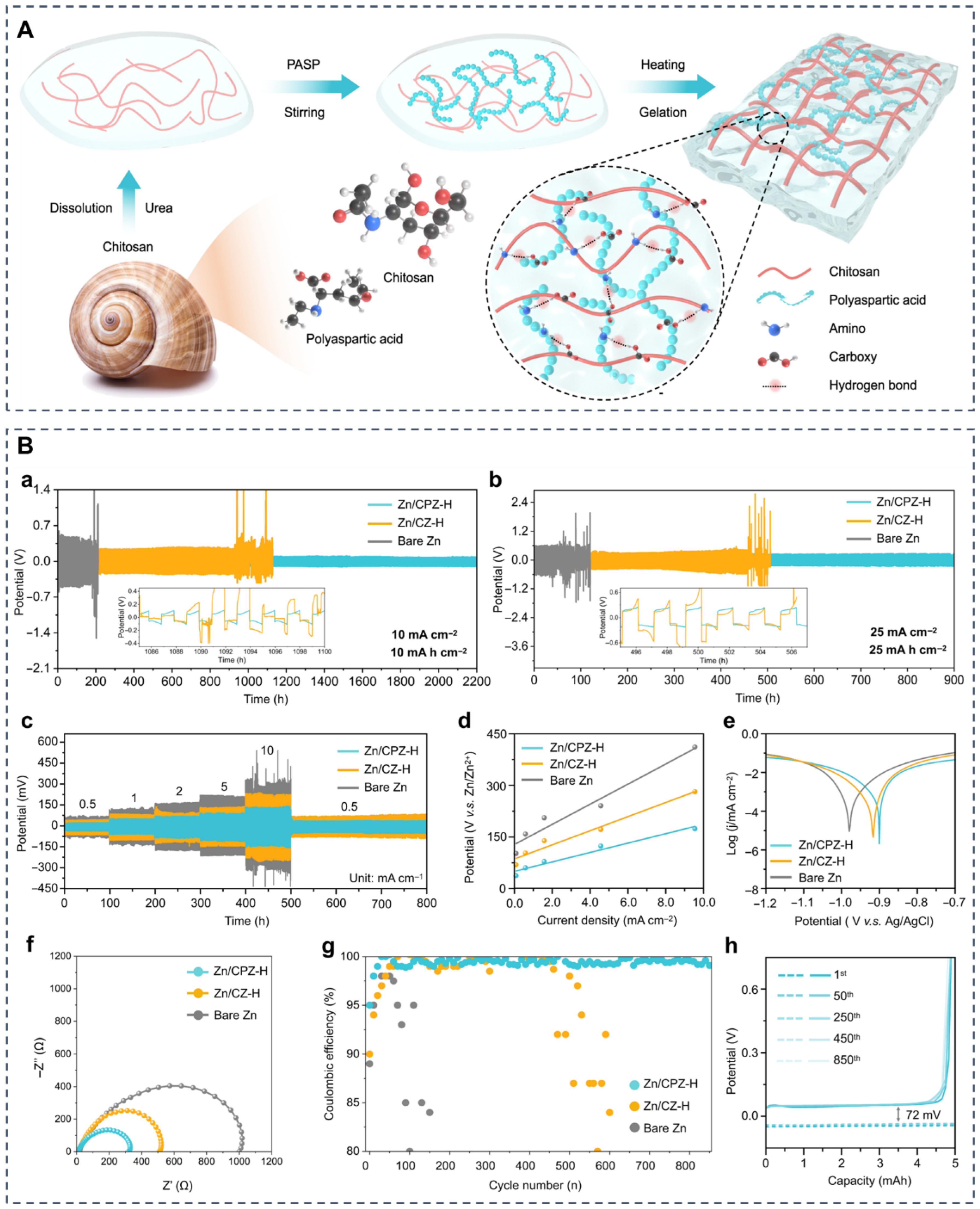
- Wu, M.; Zhang, Y.; Xu, L.; Yang, C.; Hong, M.; Cui, M.; Clifford, B.C.; He, S.; Jing, S.; Yao, Y.; et al. A Sustainable Chitosan-Zinc Electrolyte for High-Rate Zinc-Metal Batteries. Matter 2022, 5, 3402–3416. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, L.; Zhang, X.; Lu, X.; Peng, M.; Wang, C.; Liu, Y.; Zhang, X. Carboxymethyl Chitosan Modified Double-Skeleton Hydrogel Electrolyte Enables High Performance for Flexible Zinc-Air Batteries. Int. J. Biol. Macromol. 2025, 303, 140678. [Google Scholar] [CrossRef]
- Chi, B.-Y.; Wang, K.; Gao, X.-J.; Wang, K.-H.; Ren, W.-F.; Sun, R.-C. Carboxymethyl Chitosan Composited Poly (Ethylene Oxide) Electrolyte with High Ion Conductivity and Interfacial Stability for Lithium Metal Batteries. Int. J. Biol. Macromol. 2024, 273, 132993. [Google Scholar] [CrossRef] [PubMed]
- Asnawi, A.S.F.M.; Aziz, S.B.; Nofal, M.M.; Hamsan, M.H.; Brza, M.A.; Yusof, Y.M.; Abdilwahid, R.T.; Muzakir, S.K.; Kadir, M.F.Z. Glycerolized Li+ Ion Conducting Chitosan-Based Polymer Electrolyte for Energy Storage EDLC Device Applications with Relatively High Energy Density. Polymers 2020, 12, 1433. [Google Scholar] [CrossRef]
- Chen, C.; Lee, S.H.; Cho, M.; Kim, J.; Lee, Y. Cross-Linked Chitosan as an Efficient Binder for Si Anode of Li-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 2658–2665. [Google Scholar] [CrossRef]
- Künne, S.; Püttmann, F.; Linhorst, M.; Moerschbacher, B.M.; Winter, M.; Li, J.; Placke, T. Comparative Study on Chitosans as Green Binder Materials for LiMn2O4 Positive Electrodes in Lithium-Ion Batteries. ChemElectroChem 2022, 9, e202200600. [Google Scholar] [CrossRef]
- Lu, X.; Chen, Z.; Yu, Q.; Zhu, W.; Li, S.; Han, L.; Yuan, J.; Li, S.; Wu, Y.; Lv, Z.; et al. Electrochemical Properties of Chitosan-Modified PbO2 as Positive Electrode for Lead–Acid Batteries. Energy Technol. 2022, 10, 2200910. [Google Scholar] [CrossRef]
- Kazda, T.; Capková, D.; Jaššo, K.; Fedorková Straková, A.; Shembel, E.; Markevich, A.; Sedlaříková, M. Carrageenan as an Ecological Alternative of Polyvinylidene Difluoride Binder for Li-S Batteries. Materials 2021, 14, 5578. [Google Scholar] [CrossRef]
- Yi, R.; Shi, X.; Tang, Y.; Yang, Y.; Zhou, P.; Lu, B.; Zhou, J. Carboxymethyl Chitosan-Modified Zinc Anode for High-Performance Zinc–Iodine Battery with Narrow Operating Voltage. Small Struct. 2023, 4, 2300020. [Google Scholar] [CrossRef]
- Bósquez-Cáceres, M.F.; Bejar, J.; Álvarez-Contreras, L.; Tafur, J.P. Enhancing Electrochemical Performance of Zinc-Air Batteries Using Freeze Crosslinked Carboxymethylcellulose-Chitosan Hydrogels as Electrolytes. J. Electrochem. Soc. 2023, 170, 060502. [Google Scholar] [CrossRef]
- Ji, C.; Shi, N.; Li, Y.; Liu, D.; Wei, Q.; Ma, G.; Shi, X.; Hu, Z. A Processable and Recyclable Gelatin/Carboxymethyl Chitosan Hydrogel Electrolyte for High Performance Flexible Zinc-Ion Batteries. Carbohydr. Polym. 2025, 349, 122982. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, S.; Li, W.; Song, H.; Du, L.; Cui, Z. Highly Conductive Poly(ε-Caprolactone) and Chitosan Based Polymer Electrolyte for Lithium Metal Battery. J. Power Sources 2023, 553, 232271. [Google Scholar] [CrossRef]
- Mohapatra, S.; Halder, S.; Chaudhari, S.R.; Netz, R.R.; Mogurampelly, S. Insights into the Structure and Ion Transport of Pectin-[BMIM][PF6] Electrolytes. arXiv 2023, arXiv:2305.03959. [Google Scholar] [CrossRef]
- Harikumar, M.E.; Batabyal, S.K. Biopolymer Pectin with Calcium Ion Crosslinker as Biocompatiable Electrolyte for Energy Storage Applications. Electrochim. Acta 2024, 489, 144150. [Google Scholar] [CrossRef]
- Tian, P.; Zhong, X.; Gu, C.; Wang, Z.; Shi, F. SiO2-Alginate-Based Gel Polymer Electrolytes for Zinc-Ion Batteries. Batteries 2022, 8, 175. [Google Scholar] [CrossRef]
- Chikkatti, B.S.; Sajjan, A.M.; Banapurmath, N.R.; Ayachit, N.H.; Jazaa, Y.; Yunus Khan, T.M.; Umarfarooq, M.A. Novel Polymer Gel Electrolytes Comprising Montmorillonite Embedded in Sodium Alginate and Their Electrochemical Performance for Future Lead Acid Batteries. New J. Chem. 2025, 49, 3654–3660. [Google Scholar] [CrossRef]
- Oishi, A.; Tatara, R.; Togo, E.; Inoue, H.; Yasuno, S.; Komaba, S. Sulfated Alginate as an Effective Polymer Binder for High-Voltage LiNi0.5Mn1.5O4 Electrodes in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 51808–51818. [Google Scholar] [CrossRef]
- Jansi, R.; Vinay, B.; Revathy, M.S.; Sasikumar, P.; Marasamy, L.; Janani, A.; Haldhar, R.; Kim, S.-C.; Almarhoon, Z.M.; Hossain, M.K. Synergistic Blends of Sodium Alginate and Pectin Biopolymer Hosts as Conducting Electrolytes for Electrochemical Applications. ACS Omega 2024, 9, 13906–13916. [Google Scholar] [CrossRef]
- Petrus, H.T.B.M.; Daulay, A.; Astuti, W.; Supriyatna, Y.I.; Kurniawan, A.; Yuwono, A.H.; Maulana, F.A. Maleic Anhydride Esterified Potato Starch as the Binder in Silicon Nanoparticles Anode for Lithium-Ion Batteries. Int. J. Biol. Macromol. 2024, 281, 136407. [Google Scholar] [CrossRef] [PubMed]
- Abujayyab, M.; Hasan, S.W.; Lemaoui, T.; AlNashef, I.M.; Arafat, H.A.; Banat, F. High-Performance Functionalized Keratin for Efficient Lithium Recovery: Experimental and Statistical Physics Insights. Desalination 2025, 606, 118782. [Google Scholar] [CrossRef]
- Huo, P.; Ming, X.; Wang, Y.; Yu, Q.; Liang, R.; Sun, G. Stable Zinc Anode Facilitated by Regenerated Silk Fibroin-Modified Hydrogel Protective Layer. Small 2024, 20, 2400565. [Google Scholar] [CrossRef] [PubMed]
- Andonegi, M.; Serra, J.P.; Silva, M.M.; Gonçalves, R.; Costa, C.M.; Lanceros-Mendez, S.; de la Caba, K.; Guerrero, P. Lithium-Ion Battery Separators Based on Sustainable Collagen and Chitosan Hybrid Membranes. Electrochim. Acta 2025, 526, 146087. [Google Scholar] [CrossRef]
- Cruz-Balaz, M.I.; Bósquez-Cáceres, M.F.; Delgado, A.D.; Arjona, N.; Morera Córdova, V.; Álvarez-Contreras, L.; Tafur, J.P. Green Energy Storage: Chitosan-Avocado Starch Hydrogels for a Novel Generation of Zinc Battery Electrolytes. Polymers 2023, 15, 4398. [Google Scholar] [CrossRef]
- Patra, J.; Rath, P.C.; Li, C.; Kao, H.-M.; Wang, F.-M.; Li, J.; Chang, J.-K. A Water-Soluble NaCMC/NaPAA Binder for Exceptional Improvement of Sodium-Ion Batteries with an SnO2-Ordered Mesoporous Carbon Anode. ChemSusChem 2018, 11, 3923–3931. [Google Scholar] [CrossRef]
- Hazaana, S.A.; Joseph, A.; Selvasekarapandian, S.; Naachiyar, R.M.; Vignesh, N.M. Performance of Solid-State Li-Ion Conducting Battery Using Biopolymer Electrolyte Based on Agar–Agar/Lithium Chloride. J. Solid State Electrochem. 2023, 27, 539–557. [Google Scholar] [CrossRef]
- Pan, R.; Cheung, O.; Wang, Z.; Tammela, P.; Huo, J.; Lindh, J.; Edström, K.; Strømme, M.; Nyholm, L. Mesoporous Cladophora Cellulose Separators for Lithium-Ion Batteries. J. Power Sources 2016, 321, 185–192. [Google Scholar] [CrossRef]
- Huang, Q.; Zhao, C.; Li, X. Enhanced Electrolyte Retention Capability of Separator for Lithium-Ion Battery Constructed by Decorating ZIF-67 on Bacterial Cellulose Nanofiber. Cellulose 2021, 28, 3097–3112. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, X.; Liang, J.; Chen, Q.; Huang, J. Environmentally Friendly Separators Based on Cellulose Diacetate-Based Crosslinked Networks for Lithium-Ion Batteries. Polymer 2024, 290, 126564. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, G.; Zhang, S.; Xie, C.; Yang, R.; Li, X. Chitosan Nanofiber Paper Used as Separator for High Performance and Sustainable Lithium-Ion Batteries. Carbohydr. Polym. 2024, 329, 121530. [Google Scholar] [CrossRef]
- Serra, J.P.; Fidalgo-Marijuan, A.; Teixeira, J.; Hilliou, L.; Gonçalves, R.; Urtiaga, K.; Gutiérrez-Pardo, A.; Aguesse, F.; Lanceros-Mendez, S.; Costa, C.M. Sustainable Lithium-Ion Battery Separator Membranes Based on Carrageenan Biopolymer. Adv. Sustain. Syst. 2022, 6, 2200279. [Google Scholar] [CrossRef]
- Tian, X.; Shi, H.; Wang, L.; Shao, L.; Tan, L. Calcium Alginate Fibers/Boron Nitride Composite Lithium-Ion Battery Separators with Excellent Thermal Stability and Cycling Performance. Molecules 2024, 29, 5311. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.V.; Loeffler, N.; Kim, G.-T.; Passerini, S. High Temperature Stable Separator for Lithium Batteries Based on SiO2 and Hydroxypropyl Guar Gum. Membranes 2015, 5, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.P.; Brito-Pereira, R.; Gonçalves, R.; Silva, M.P.; Costa, C.M.; Silva, M.M.; de Zea Bermudez, V.; Lanceros-Méndez, S. Silk Fibroin Separators: A Step Toward Lithium-Ion Batteries with Enhanced Sustainability. ACS Appl. Mater. Interfaces 2018, 10, 5385–5394. [Google Scholar] [CrossRef] [PubMed]
- Sapra, S.K.; Das, M.; Raja, M.W.; Chang, J.K.; Dhaka, R.S. Flexible Trilayer Cellulosic Paper Separators Engineered with BaTiO Ferroelectric Fillers for High Energy Density Sodium-Ion Batteries. arXiv 2024, arXiv:2409.06743. [Google Scholar]
- Hirai, Y.; Ohnishi, R.; Taminato, S.; Mori, D.; Eimura, H.; Ikoma, K.; Sawamoto, A.; Yamamoto, O.; Takeda, Y.; Imanishi, N. Microporous Polyethylene and Cellulose Composite Separators for Reversible Lithium Electrode in Lithium Rechargeable Batteries. Batter. Supercaps 2024, 7, e202400472. [Google Scholar] [CrossRef]
- Kim, J.; Lee, D.G.; Lee, J.H.; Kim, S.; Park, C.-Y.; Lee, J.; Kwon, H.; Cho, H.; Lee, J.; Son, D.; et al. Concurrent Electrode–Electrolyte Interfaces Engineering via Nano-Si3N4 Additive for High-Rate, High-Voltage Lithium Metal Batteries. Energy Environ. Sci. 2025, 18, 3148–3159. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, D.; Wen, J.; Fan, L.; Rao, A.M.; Lu, B. Sustainable Electrolytes: Design Principles and Recent Advances. Chem. A Eur. J. 2024, 30, e202400332. [Google Scholar] [CrossRef]
- Zhang, Q.; Han, A.; Zhang, X.; Tian, R.; Yang, S.; Xu, S.; Song, D.; Yang, Y. Green Synthesis for Battery Materials: A Case Study of Making Lithium Sulfide via Metathetic Precipitation. ACS Appl. Mater. Interfaces 2022, 15, 1358–1366. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Sasirajan Little Flower, S.R.; Hanamantrao, D.P.; Kasiviswanathan, K.; Sesu, D.C.; Muthu, K.; Elumalai, V.; Vediappan, K. Starch Gel Electrolyte and its Interaction with Trivalent Aluminum for Aqueous Aluminum-Ion Batteries: Enhanced Low Temperature Electrochemical Performance. Small 2024, 20, 2402245. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; He, Q.; Liu, X.; Jiang, Q.; Chu, D.; Wang, Q.; Yang, X.; Zhang, T.; Wang, H.; Li, Z. Cellulose/Sodium Alginate Gel Electrolyte Membranes with Synergistic Hydrogen Bonding and Metal Ion Coordination Networks for High Performance Aqueous Zinc-Ion Batteries. Carbohydr. Polym. 2025, 352, 123116. [Google Scholar] [CrossRef]
- Gao, J.; Chai, Y.; Ni, J.; Zeng, Y.; Zhang, G.; Liu, X.; Ning, D.; Jin, X.; Zhao, H.; Zhou, D.; et al. Fabrication of Flexible Polymer-MOF Composite Electrolyte for Solid-State Lithium Metal Batteries with High-Rate Performance. Chem. Eng. J. 2025, 512, 162738. [Google Scholar] [CrossRef]
- Smith, D.M.; Pan, Q.; Cheng, S.; Wang, W.; Bunning, T.J.; Li, C.Y. Nanostructured, Highly Anisotropic, and Mechanically Robust Polymer Electrolyte Membranes via Holographic Polymerization. Adv. Mater. Interfaces 2018, 5, 1700861. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D. Composite Membrane Containing Titania Nanofibers for Battery Separators Used in Lithium-Ion Batteries. Membranes 2023, 13, 499. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wang, L.; Zhang, M.; Li, W.; Zhou, Q.; Zhong, L. Advanced Separators Based on Aramid Nanofiber (ANF) Membranes for Lithium-Ion Batteries: A Review of Recent Progress. J. Mater. Chem. A 2021, 9, 12923–12946. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kang, H.-J.; Lim, H.; Hwang, H.J.; Park, J.-W.; Lee, T.-G.; Cho, S.Y.; Jang, S.G.; Jun, Y.-S. Boron Nitride Nanotube-Based Separator for High-Performance Lithium-Sulfur Batteries. Nanomaterials 2022, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Narla, A.; Fu, W.; Kulaksizoglu, A.; Kume, A.; Johnson, B.R.; Raman, A.S.; Wang, F.; Magasinski, A.; Kim, D.; Kousa, M.; et al. Nanodiamond-Enhanced Nanofiber Separators for High-Energy Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2023, 15, 32678–32686. [Google Scholar] [CrossRef]
- Deng, Y.; Pan, Y.; Zhang, Z.; Fu, Y.; Gong, L.; Liu, C.; Yang, J.; Zhang, H.; Cheng, X. Novel Thermotolerant and Flexible Polyimide Aerogel Separator Achieving Advanced Lithium-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2106176. [Google Scholar] [CrossRef]
- Serra, J.P.; Fidalgo-Marijuan, A.; Martins, P.M.; Queirós, J.M.; Gonçalves, R.; Gutiérrez-Pardo, A.; Aguesse, F.; Costa, C.M.; Lanceros-Mendez, S. Porous Composite Bifunctional Membranes for Lithium-Ion Battery Separator and Photocatalytic Degradation Applications: Toward Multifunctionality for Circular Economy. Adv. Energy Sustain. Res. 2021, 2, 2100046. [Google Scholar] [CrossRef]
- Mohammad, I.; Barter, L.D.J.; Stolojan, V.; Crean, C.; Slade, R.C.T. Electrospun Polar-Nanofiber PVDF Separator for Lithium–Sulfur Batteries with Enhanced Charge Storage Capacity and Cycling Durability. Energy Adv. 2024, 3, 625–635. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Xia, Z.; Li, L.; Yuan, W. High Performance of Boehmite/Polyacrylonitrile Composite Nanofiber Membrane for Polymer Lithium-Ion Battery. RSC Adv. 2020, 10, 27492–27501. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Y.; Kang, Y.; Wang, L.; Yang, S.; Liang, Z.; Li, Y. Core-Shell Nanofiber Containing Large Amount of Flame Retardants via Coaxial Dual-Nozzle Electrospinning as Battery Separators. arXiv 2019, arXiv:1909.10671. [Google Scholar]
- Kim, H.; Guccini, V.; Lu, H.; Salazar-Alvarez, G.; Lindbergh, G.; Cornell, A. Lithium-Ion Battery Separators Based on Carboxylated Cellulose Nanofibers from Wood. ACS Appl. Energy Mater. 2019, 2, 1241–1250. [Google Scholar] [CrossRef]
- Yao, A.; Benson, S.M.; Chueh, W.C. Critically Assessing Sodium-Ion Technology Roadmaps and Scenarios for Techno-Economic Competitiveness against Lithium-Ion Batteries. Nat. Energy 2025, 10, 404–416. [Google Scholar] [CrossRef]
- Phogat, P.; Rawat, S.; Dey, S.; Wan, M. Advancements and Challenges in Sodium-Ion Batteries: A Comprehensive Review of Materials, Mechanisms, and Future Directions for Sustainable Energy Storage. J. Alloys Compd. 2025, 1020, 179544. [Google Scholar] [CrossRef]
- Dong, S.; Xie, G.; Xu, S.; Tan, X.; Chaudhary, M.; Zhang, Y.; Wu, R.; Wen, F.; Ayranci, C.; Michaelis, V.K.; et al. Cellulose-Encapsulated Composite Electrolyte Design: Toward Chemically and Mechanically Enhanced Solid-Sodium Batteries. ACS Nano 2024, 18, 16285–16296. [Google Scholar] [CrossRef]
- Gregorio, V.; Jankowski, P.; Garcia, N.; Garcia Lastra, J.M.; Tiemblo, P.; Chang, J.H. Electrolytes for Zn Batteries: Deep Eutectic Solvents in Polymer Gels. ChemSusChem 2023, 16, e202300256. [Google Scholar] [CrossRef]
- Shi, J.; Sun, T.; Bao, J.; Zheng, S.; Du, H.; Li, L.; Yuan, X.; Ma, T.; Tao, Z. “Water-in-Deep Eutectic Solvent” Electrolytes for High-Performance Aqueous Zn-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2102035. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, L.; Verma, A. Progress in Aluminum-Ion Battery: Evaluation of Deep Eutectic Solvent as Electrolyte. J. Energy Storage 2024, 98, 113039. [Google Scholar] [CrossRef]
- Molaiyan, P.; Bhattacharyya, S.; dos Reis, G.S.; Sliz, R.; Paolella, A.; Lassi, U. Towards Greener Batteries: Sustainable Components and Materials for Next-Generation Batteries. Green Chem. 2024, 26, 7508–7531. [Google Scholar] [CrossRef]
- Ma, H.; Chen, H.; Chen, M.; Li, A.; Han, X.; Ma, D.; Zhang, P.; Chen, J. Biomimetic and Biodegradable Separator with High Modulus and Large Ionic Conductivity Enables Dendrite-Free Zinc-Ion Batteries. Nat. Commun. 2025, 16, 1014. [Google Scholar] [CrossRef] [PubMed]
- FACT SHEET: Biden-Harris Administration Driving U.S. Battery Manufacturing and Good-Paying Jobs. Available online: https://bidenwhitehouse.archives.gov/briefing-room/statements-releases/2022/10/19/fact-sheet-biden-harris-administration-driving-u-s-battery-manufacturing-and-good-paying-jobs/ (accessed on 24 May 2025).
- Tesla Impact Reports 2020. Available online: https://www.tesla.com/ns_videos/2020-tesla-impact-report.pdf (accessed on 25 June 2025).
- ECHA Per- and Polyfluoroalkyl Substances (PFAS). Available online: https://echa.europa.eu/hot-topics/perfluoroalkyl-chemicals-pfas (accessed on 25 June 2025).
- EPA Per- and Polyfluoroalkyl Substances (PFAS). Available online: https://www.epa.gov/pfas (accessed on 25 June 2025).
- Maguire, G. Europe Eats into China’s Lead as Top EV Growth Market. Available online: https://www.reuters.com/markets/commodities/europe-eats-into-chinas-lead-top-ev-growth-market-2022-10-05/ (accessed on 24 May 2025).
- New EU Regulatory Framework for Batteries: Setting Sustainability Requirements. Available online: https://www.europarl.europa.eu/thinktank/en/document/EPRS_BRI(2021)689337 (accessed on 24 May 2025).
- India Significantly Scales Up Electric Vehicle Adoption. Available online: https://www.wri.org/outcomes/india-significantly-scales-electric-vehicle-adoption (accessed on 24 May 2025).
- Kannan, S. Sustainability of Electric Vehicle Batteries. Available online: https://www.cag.org.in/blogs/sustainability-electric-vehicle-batteries (accessed on 24 May 2025).
- Delhi’s EV Policy 2.0 to Phase out CNG Autos, Target 95% EVs by 2027: Details. Available online: https://timesofindia.indiatimes.com/auto/news/delhis-ev-policy-2-0-to-phase-out-cng-autos-target-95-evs-by-2027-details/articleshow/118876259.cms (accessed on 24 May 2025).
- South Korean: Strategic Crossroad & Critical Transformation. Available online: https://www.globalfleet.com/en/safety-environment/asia-pacific/analysis/south-korean-strategic-crossroad-critical-transformation?a=YHE11&t%5B0%5D=Korea&t%5B1%5D=Electrification&t%5B2%5D=Incentives&curl=1 (accessed on 24 May 2025).
- Busch, J. South Korea Introduces Government-Led EV Battery Certification and Tracking. Available online: https://www.korea-certification.com/en/south-korea-introduces-government-led-ev-battery-certification-and-tracking/ (accessed on 24 May 2025).
- Lee, Y.; Kim, M.; Kim, K. Revisiting Actors’ Role in Circular Economy Governance: A Case of Electric Vehicle Waste Batteries in South Korea. Environ. Eng. Res. 2024, 29, 230350–230351. [Google Scholar] [CrossRef]
- EV Adoption, Republic of Korea. Available online: https://evtcp.org/country/republic-of-korea/?utm (accessed on 24 May 2025).
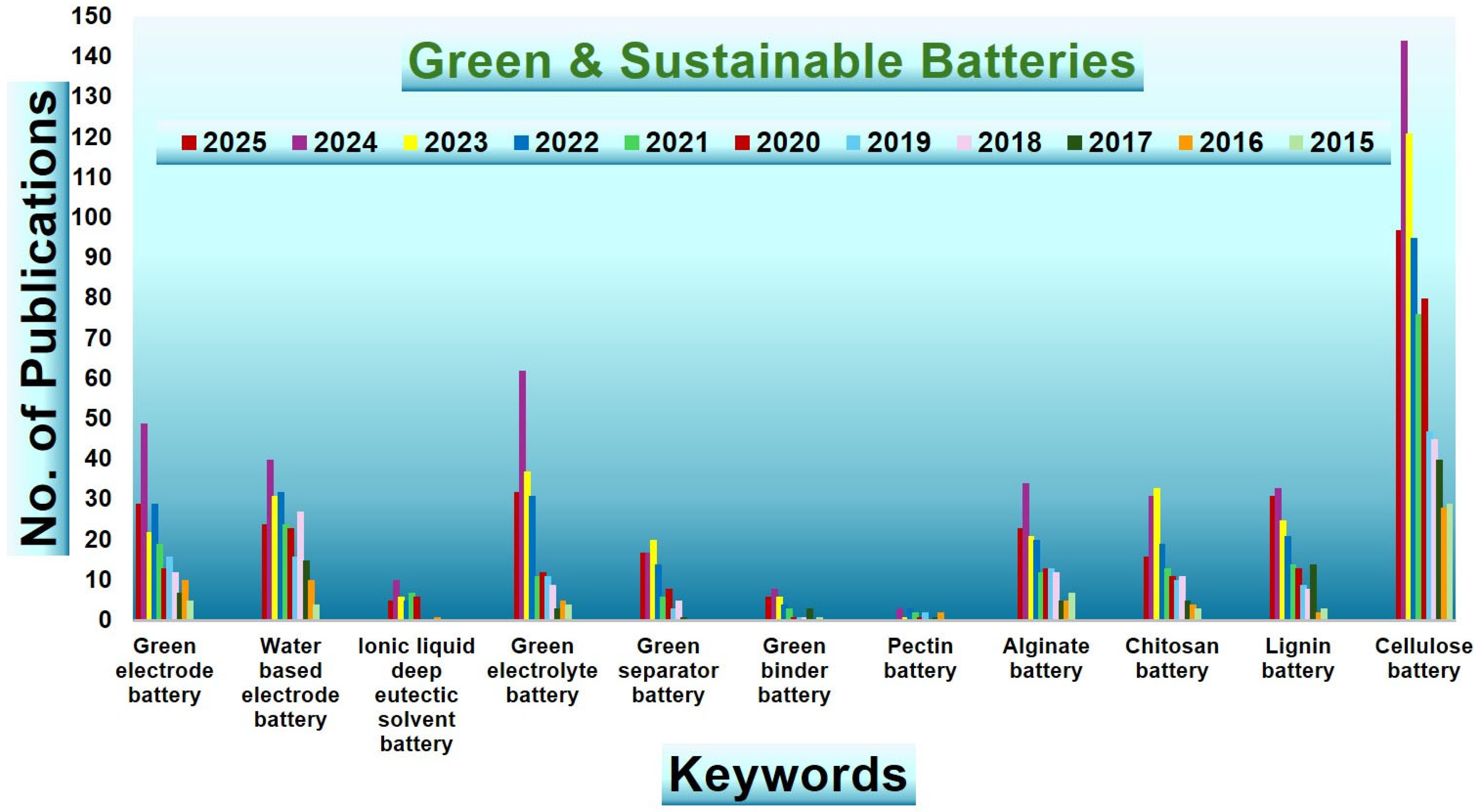



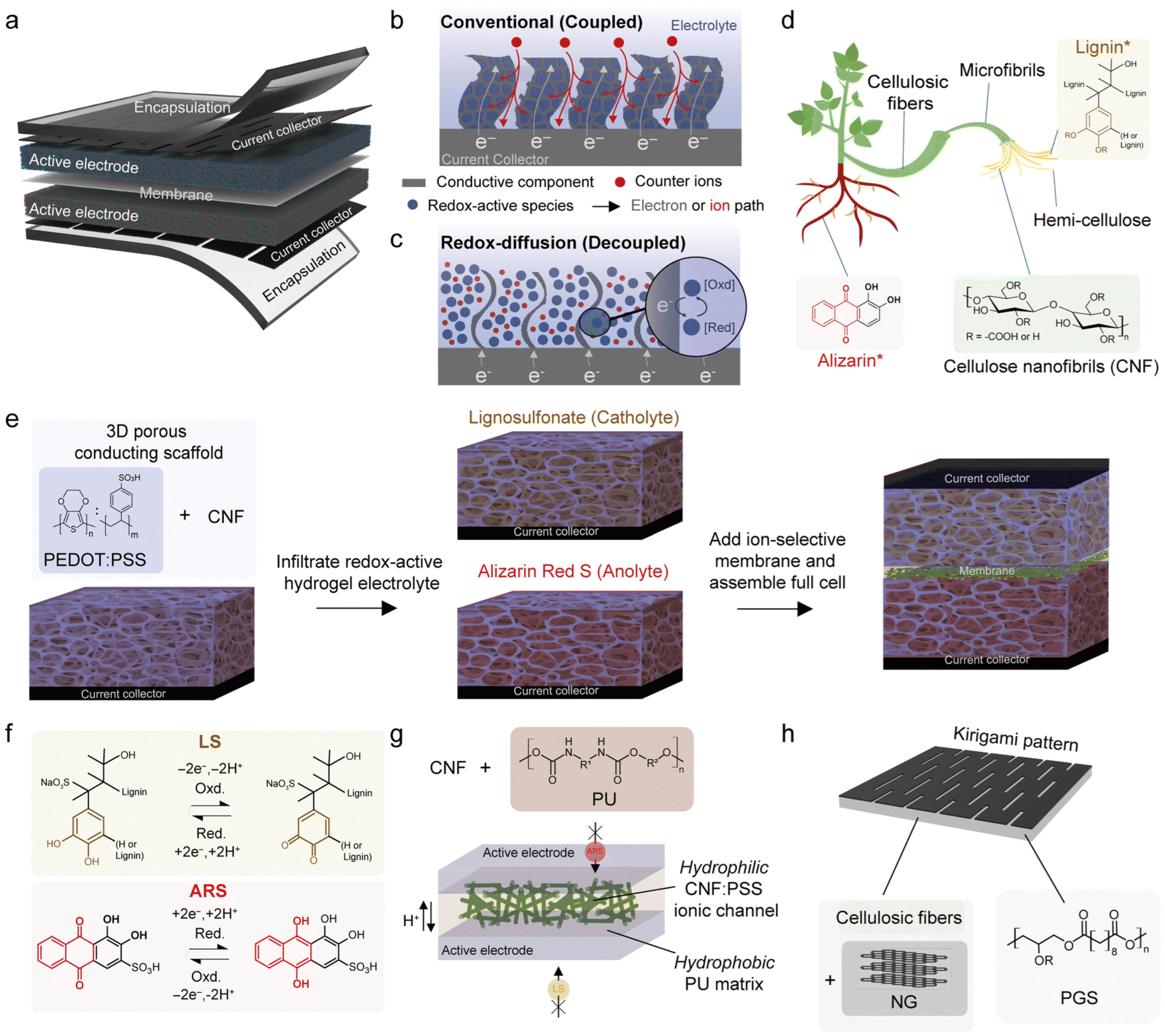
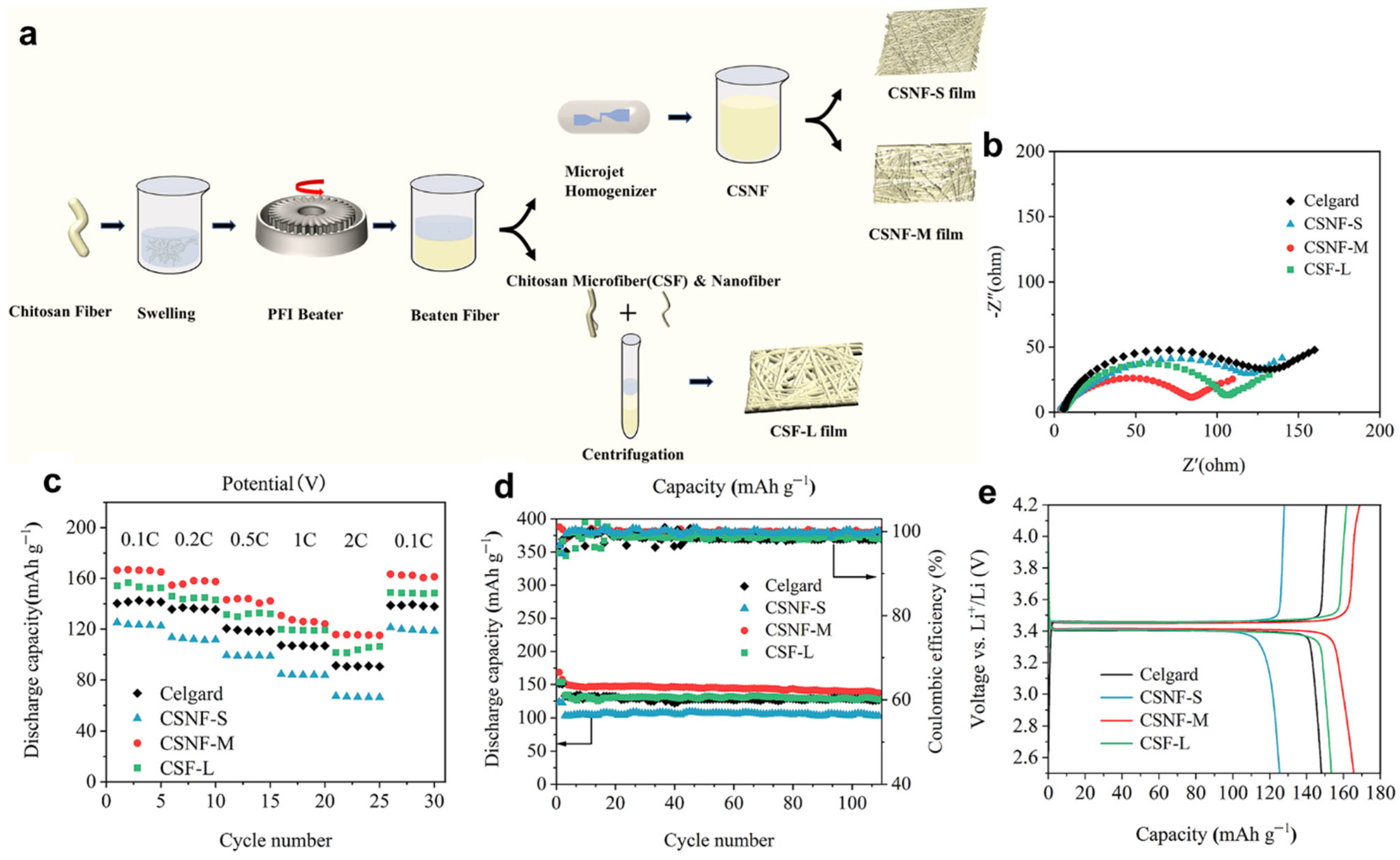
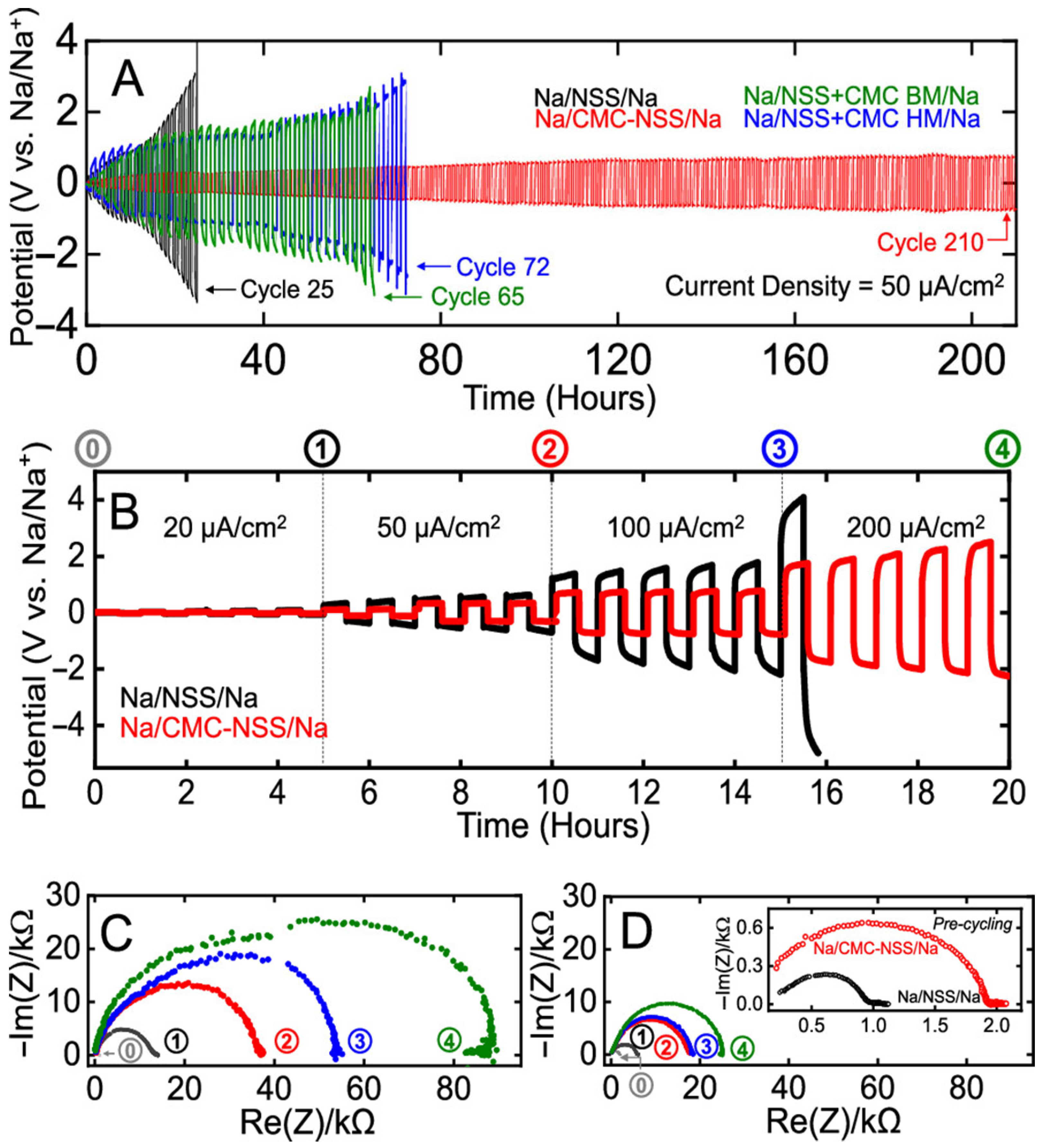
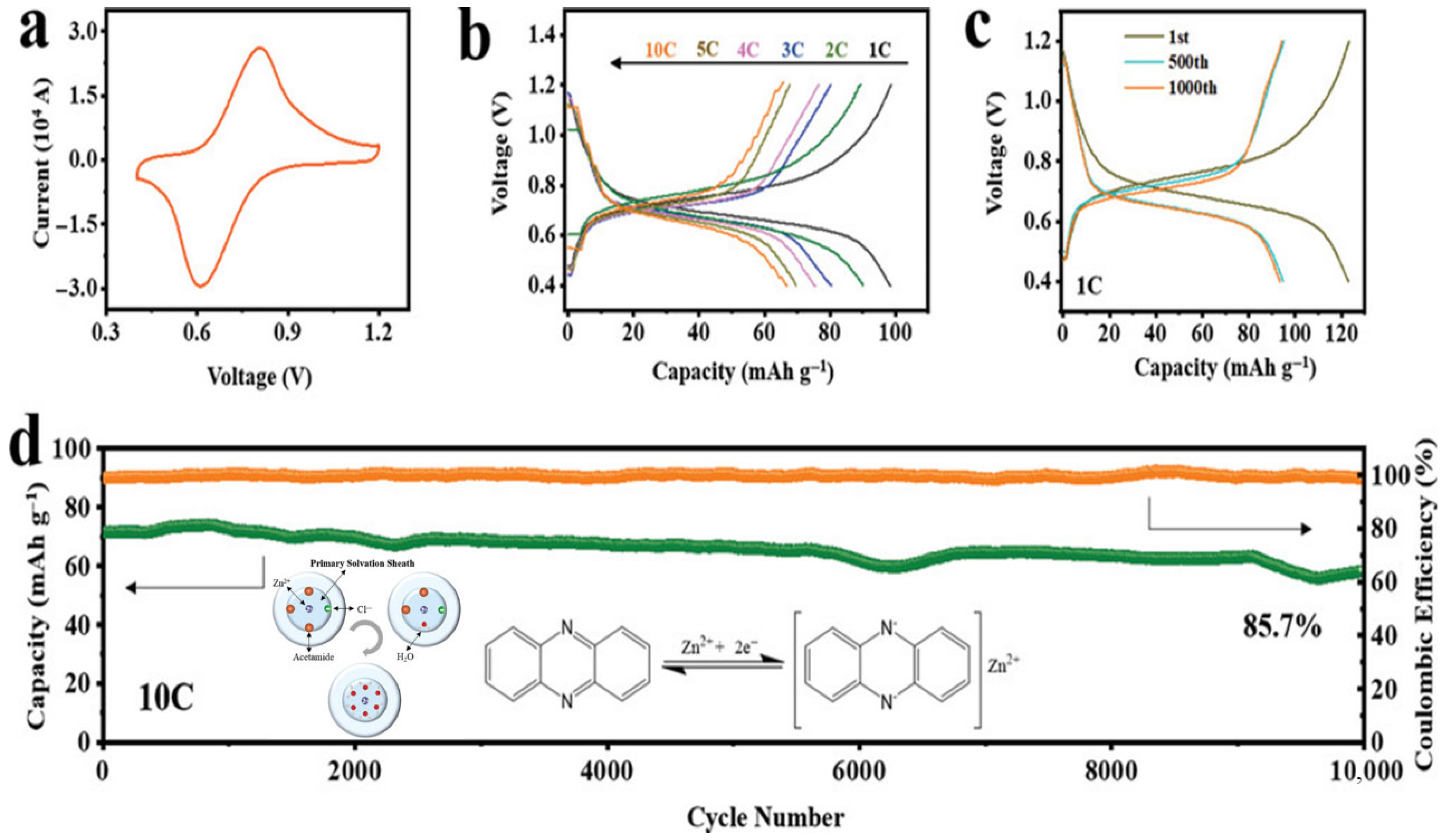


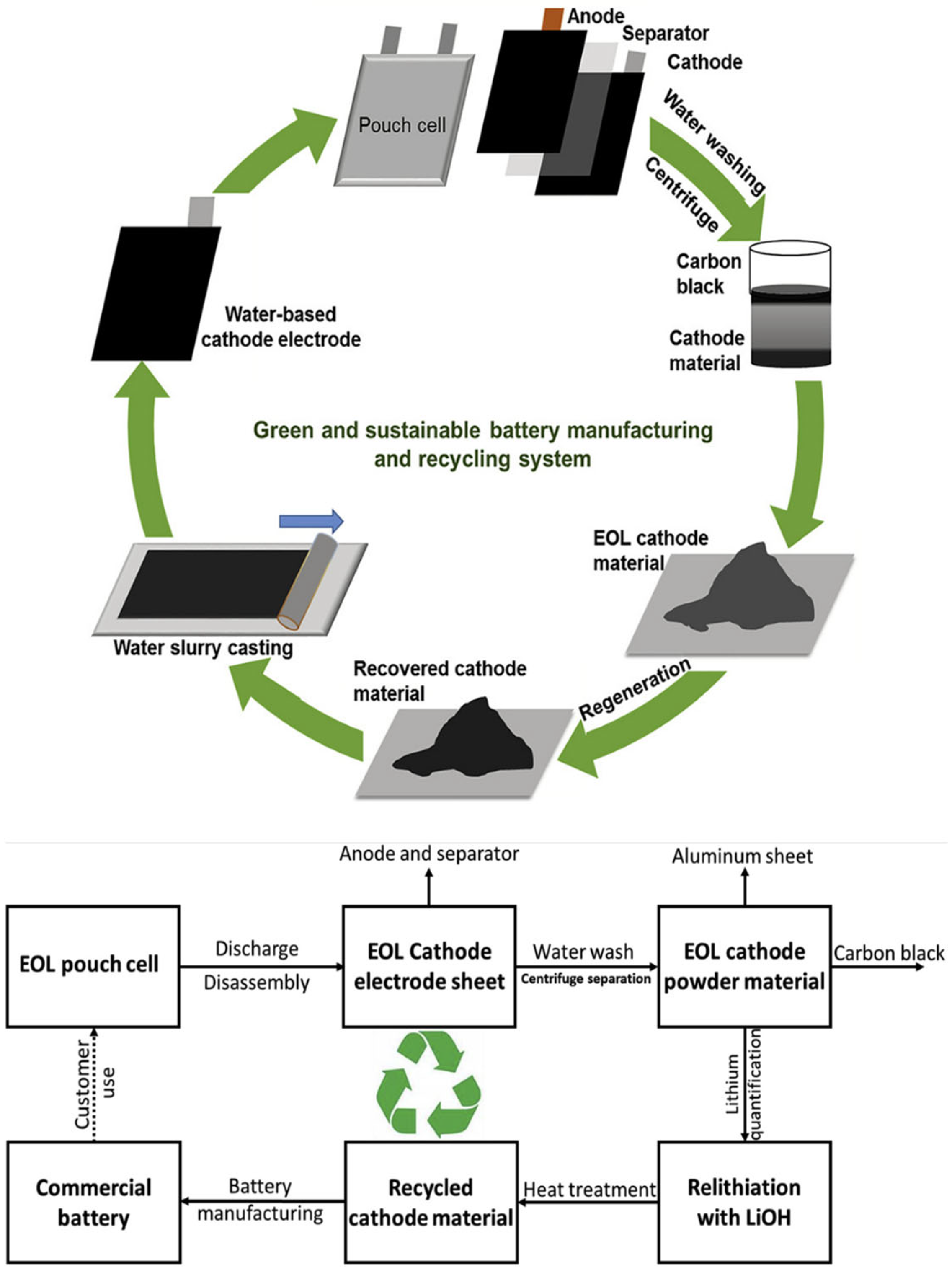
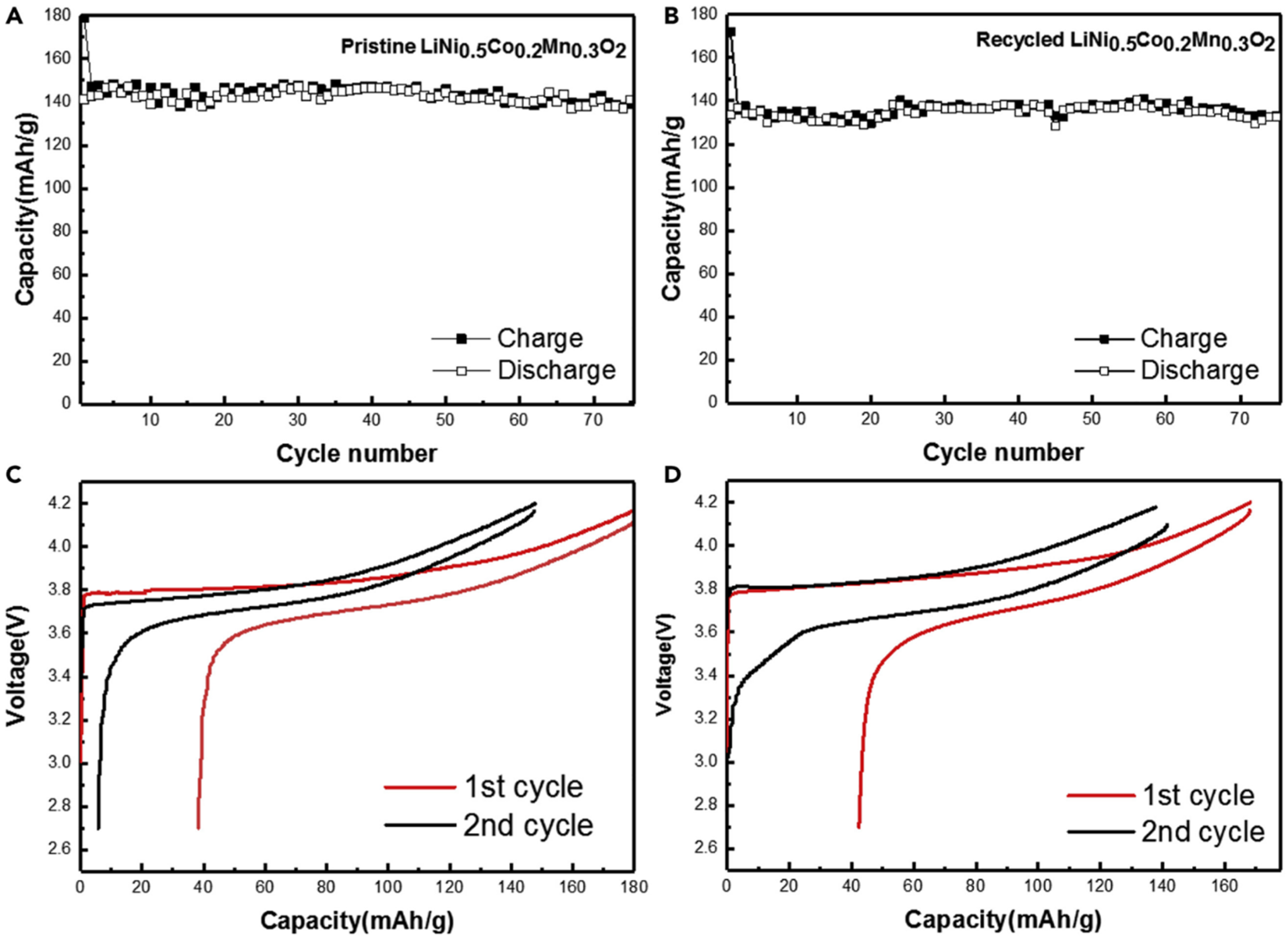

| Biopolymer | Material Type | Battery Type | Key Electrochemical Performance | Ref. |
|---|---|---|---|---|
| Chitosan | Binder | Li-ion | Discharge capacity: 159.4 mAh/g; Capacity retention: 98.38% | [76] |
| Crosslinked Chitosan | Binder | Li-ion | Initial capacity: 133 mAh/g; Final capacity after 15 cycles: ~40 mAh/g | [77] |
| Carboxymethyl cellulose-epichlorohydrin | Binder | Li-ion | Specific capacity of 1054.2 mAh/g at 0.2 C following 200 cycles, capacity retention is 65.6% | [78] |
| Chitosan-Zn | Electrolyte | Zn-metal battery | Coulombic efficiency: 99.7%; >1000 cycles at 50 mA/cm2; High-rate performance up to 20 C | [79] |
| Carboxymethyl chitosan | Electrolyte | Zn-air | Impressive discharge capacity 805.3 mAh g−1, long cycle life 118 h, power density 91.04 mW cm−2 | [80] |
| Carboxymethyl chitosan-poly(ethylene oxide) | Electrolyte | Li-metal | High ionic conductivity and interface stability, long-term cycling life of 2500 h, 135.6 mAh/g capacity retention of 83.5% after 400 cycles at 0.2 C. | [81] |
| Glycerolized Chitosan | Electrolyte | EDLC (Supercapacitor) | Specific capacitance: 105.5 F/g (1st cycle) to 136.8 F/g (700th cycle); Energy density: up to 19.1 Wh/kg | [82] |
| Crosslinked Chitosan | Binder | Li-ion | Initial capacity: 2782 mAh/g; Capacity after 100 cycles: 1969 mAh/g at 500 mA/g | [83] |
| Chitosan | Binder | Li-ion | Discharge capacity at 10 C: ~80 mAh/g; Superior to PVdF (~55 mAh/g) | [84] |
| Chitosan | Modifier | Lead–Acid | First discharge capacity: 4257 mAh; After 500 cycles: 196 mAh/g | [85] |
| Carrageenan | Binder | Li-S | Capacity drop of ~37% after 50 cycles at 0.2–2 C | [86] |
| Carboxymethyl Chitosan | Electrolyte | Zn-iodine | Over 28,000 cycles with narrow voltage window of 0.23 V | [87] |
| Carboxymethylcellulose-Chitosan | Electrolyte | Zn-Air | Power density of 117 mW cm−2 and a specific capacitance of 1899 mAh g−1. | [88] |
| Gelatin/Carboxymethyl chitosan | Electrolyte | Zn-ion | High specific capacity and superior cyclic performance (2200 h at 0.1 A g−1) without the formation of zinc dendrites, high recycling rate (above 80%) | [89] |
| Poly(ε-caprolactone)-chitosan | Electrolyte | Li-metal | Excellent stability and compatibility, ionic conductivity of 7.7 × 10−4 S cm−1, and long cycling for over 1850 h | [90] |
| Xanthan Gum | Binder | Li-ion | Improved electrode stability and capacity retention | |
| Pectin | Electrolyte | Li-ion | Enhanced ion transport properties with [BMIM][PF6] ionic liquid | [91] |
| Pectin | Electrolyte | Supercapacitor | Specific capacitance of 879 mF cm−2 at 15 mA cm−2 | [92] |
| Carrageenan | Binder | Li-S | Enhanced stability and capacity retention compared to PVDF binder | [86] |
| Alginate | Electrolyte | Zn-ion | SiO2-alginate-based gel polymer electrolytes with enhanced performance | [93] |
| Sodium Alginate | Electrolyte | Lead–Acid | Discharge capacity of 138 μAh cm−2 at 0.55 mA cm−2; 89% capacity retention after 500 cycles | [94] |
| Sulfated Alginate | Binder | Li-ion | Enhanced performance for high-voltage LiNi0.5Mn1.5O4 electrodes | [95] |
| Sodium Alginate–Pectin | Electrolyte | Li-ion | Ionic conductivity of 1.264 × 10−7 S cm−1; breakdown voltage at 0.66 V | [96] |
| Potato starch (maleic anhydride esterified | Binder | Li-ion | Cycling performance capacity of 1950 mAh/g. | [97] |
| Keratin–Phosphoric acid | Biosorbent | Li-ion recovery | High separation factors ranging from 11.3 to 22.0 | [98] |
| Silk Fibroin | Binder | Zn-ion | Coulombic efficiency of 99.04% under a large plating capacity for 800 cycles, full cells 20,000 cycles, >80% capacity retention at 5 and 10 A g−1 | [99] |
| Collagen–chitosan | Separator | Li-ion | Ionic conductivity is >0.49 mS·cm−1, Discharge capacity 140 mAh·g−1 at C/8 rate, | [100] |
| Chitosan–Avocado starch | Electrolyte | Zn-air | Improved conductivity value of 0.61 S·cm−1, specific capacity value 1618 mA h·g−1 | [101] |
| Carboxymethylcellulose | Binder | Na-Ion | Capacity retention of 90% after 300 cycles, electrode capacities of 850 and 425 mAh g−1 at charge–discharge rates of 20 and 2000 mA g−1 | [102] |
| Agar-Agar | Electrolyte | Li-Ion | High ionic conductivity, 3.12 ± 0.11 × 10−2 S/cm, 20 mol% of AA:80 mol% of LiCl | [103] |
| Cellulose (Cladophora) | Separator | Lithium-ion | Ionic conductivity: 0.4 mS/cm; 99.5% capacity retention after 50 cycles at 0.2 C | [104] |
| Cellulose (Bacterial)/ZIF-67 | Separator | Lithium-ion | Ionic conductivity: 0.096 mS cm−1, discharge capacity retention 91.41% after 100 cycles (0.2 C), discharge capacity 156 mAh g−1 | [105] |
| Modified Cellulose (M-CDA/PEO) | Separator | Lithium-ion | Ionic conductivity: 2.83 mS/cm; 170.3 mAh/g after 100 cycles at 0.5 C; no shrinkage at 200 °C | [106] |
| Chitosan Nanofiber | Separator | Lithium-ion | Electrolyte uptake: 281%; superior thermal stability; higher discharge capacity over 100 cycles | [107] |
| Carrageenan (Iota) | Separator | Lithium-ion | Ionic conductivity: 1.34 mS/cm; discharge capacity: 145 mAh/g at C/10; 25 mAh/g at 1 C | [108] |
| Alginate (Calcium Alginate) | Separator | Lithium-ion | Ionic conductivity: 2.80 mS/cm at 65 °C; lithium-ion transference number: 0.55; over 1000 cycles at 60 °C with 97% CE | [109] |
| Guar Gum (Hydroxypropyl) | Separator | Lithium-ion | High thermal stability; no dimensional shrinkage at elevated temperatures; good electrochemical stability | [110] |
| Silk Fibroin (Lyophilized) | Separator | Lithium-ion | Ionic conductivity: 1.00 mS/cm; discharge capacity: 126 mAh/g at C/2; 99 mAh/g at 2 C; 72% capacity retention over 50 cycles | [111] |
| Cellulose (Trilayer with BaTiO3) | Separator | Sodium-ion | Energy density: ~376 Wh/kg; 62% capacity retention over 240 cycles; nearly 100% Coulombic efficiency | [112] |
| Cellulose (Composite with PE) | Separator | Lithium-metal | Improved Coulombic efficiency; enhanced lithium deposition and stripping performance | [113] |
| Nanostructured Membrane Type | Battery Type | Key Electrochemical Performance | Ref. |
|---|---|---|---|
| Titania nanofiber composite membrane | Li-ion | Enhanced wettability and thermal stability; lower interfacial resistance; higher ionic conductivity leading to improved discharge capacity and cycling performance. | [121] |
| Aramid nanofiber (ANF) membrane | Li-ion | Superior strength and modulus; excellent thermal stability; improved safety and reliability in battery operations. | [122] |
| Boron nitride nanotube-based separator | Li-ion | High thermal conductivity; chemical stability; improved cycle life and capacity retention in lithium–sulfur batteries. | [123] |
| Nanodiamond-enhanced nanofiber separator | Li-ion | Improved thermal stability and mechanical strength; enhanced electrolyte wettability; better cycling performance. | [124] |
| Polyimide aerogel separator | Li-ion | High porosity (78.35%); electrolyte absorption (321.66%); maintained capacity of 118 mAh/g after 1000 cycles at 1 C; stable performance at 90 °C over 300 cycles. | [125] |
| TiO2/PVDF-TrFE composite membrane | Li-ion | High ionic conductivity and lithium transference number; discharge capacity retention of 83% at 2 C after 100 cycles; improved rate capability. | [126] |
| Electrospun PVDF nanofiber separator | Li-ion | Enhanced charge storage capacity; improved cycling durability; reduced polarization voltage difference (η = 0.13 V) compared to PP separator (η = 0.32 V). | [127] |
| Boehmite/polyacrylonitrile (PAN) composite | Li-ion | Ionic conductivity up to 2.85 mS/cm with 30 wt% boehmite; improved mechanical strength; better electrolyte uptake compared to pure PAN and PP membranes. | [128] |
| Core-shell nanofiber with flame retardants | Li-ion | Incorporation of over 60 wt% flame retardants; enhanced safety without compromising cycling stability or rate performance; potential for coating on commercial separators. | [129] |
| Carboxylated cellulose nanofibers | Li-ion | Hierarchical porous TOCN structure; improved high-temperature 94.5% of discharge capacity maintained after 100 cycles at 1 C | [130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Annu; Harisha, B.S.; Yewale, M.; Akkinepally, B.; Shin, D.K. Green Batteries: A Sustainable Approach Towards Next-Generation Batteries. Batteries 2025, 11, 258. https://doi.org/10.3390/batteries11070258
Annu, Harisha BS, Yewale M, Akkinepally B, Shin DK. Green Batteries: A Sustainable Approach Towards Next-Generation Batteries. Batteries. 2025; 11(7):258. https://doi.org/10.3390/batteries11070258
Chicago/Turabian StyleAnnu, Bairi Sri Harisha, Manesh Yewale, Bhargav Akkinepally, and Dong Kil Shin. 2025. "Green Batteries: A Sustainable Approach Towards Next-Generation Batteries" Batteries 11, no. 7: 258. https://doi.org/10.3390/batteries11070258
APA StyleAnnu, Harisha, B. S., Yewale, M., Akkinepally, B., & Shin, D. K. (2025). Green Batteries: A Sustainable Approach Towards Next-Generation Batteries. Batteries, 11(7), 258. https://doi.org/10.3390/batteries11070258









