Advances in Metal-Organic Frameworks (MOFs) for Rechargeable Batteries and Fuel Cells
Abstract
1. Introduction
2. Metal-Organic Frameworks (MOFs) in Batteries
2.1. Lithium-Ion Batteries
2.2. Zinc (Zn)-(Metal-Ion) Air Batteries
2.3. Zinc (Zn)-Air (Metal-Air) Batteries
2.4. Metal-Organic Framework (MOF)-Based Composite Materials
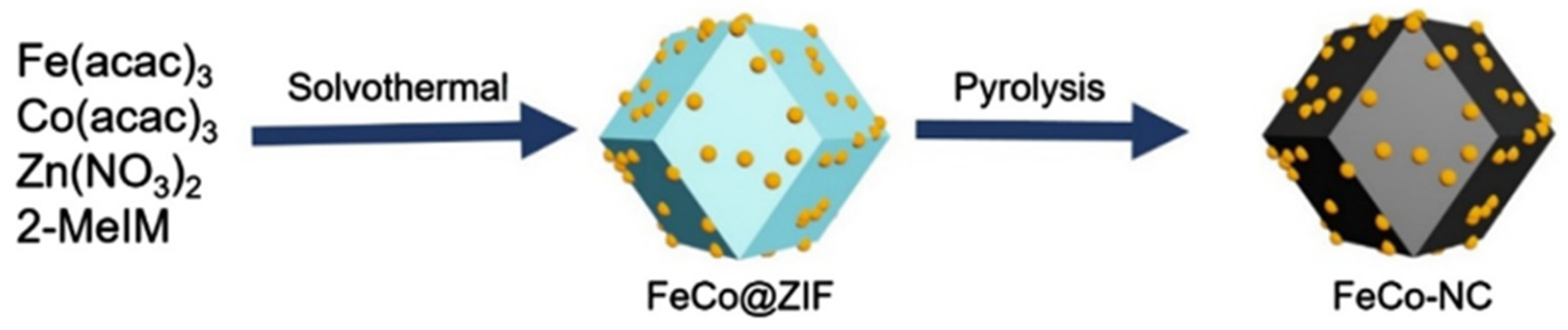
2.4.1. Metal-Air Batteries (MABs)
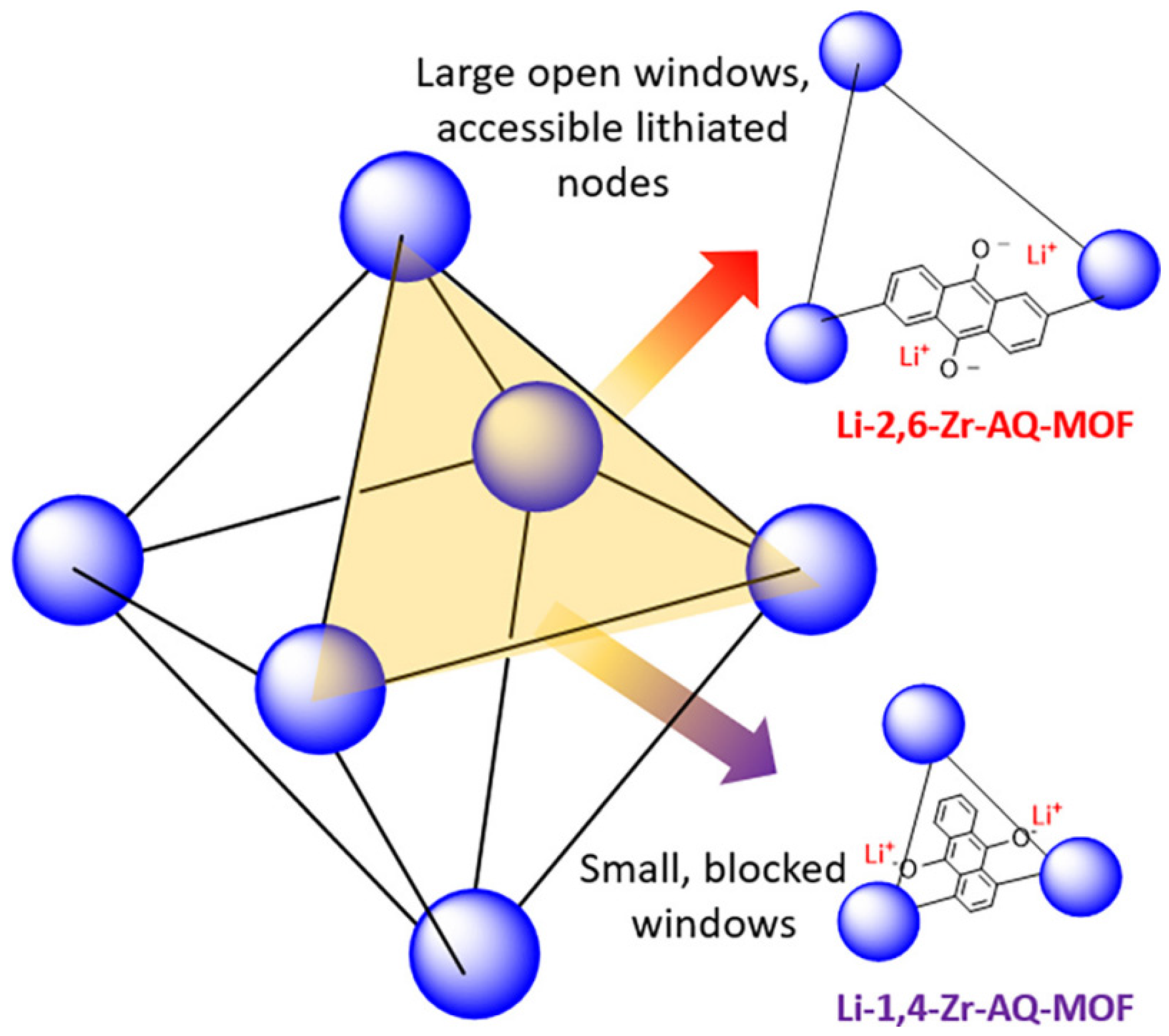
2.4.2. Lithium Batteries
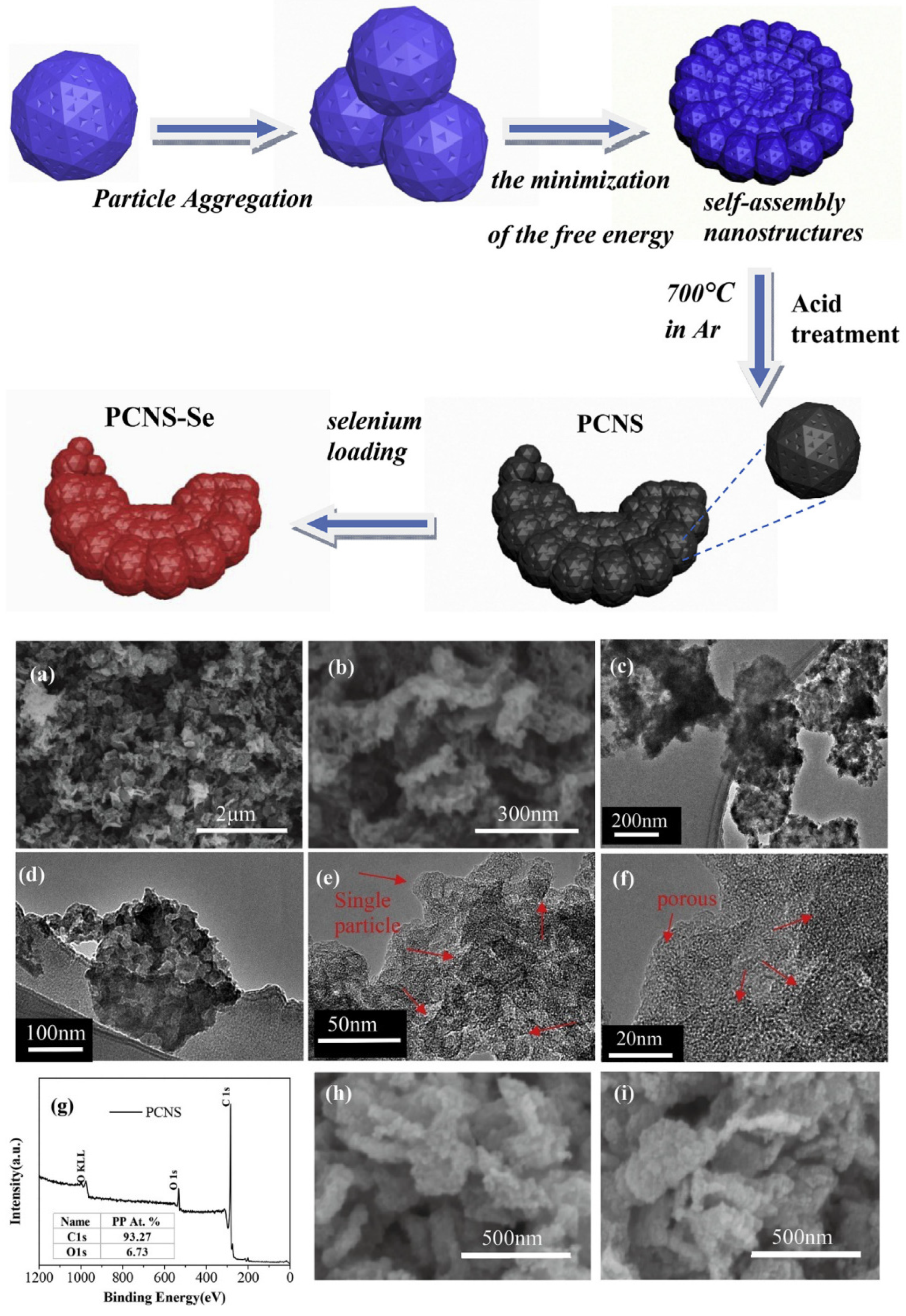
2.4.3. Solid-State Lithium Batteries
3. Metal-Organic Frameworks (MOFs) in Fuel Cells
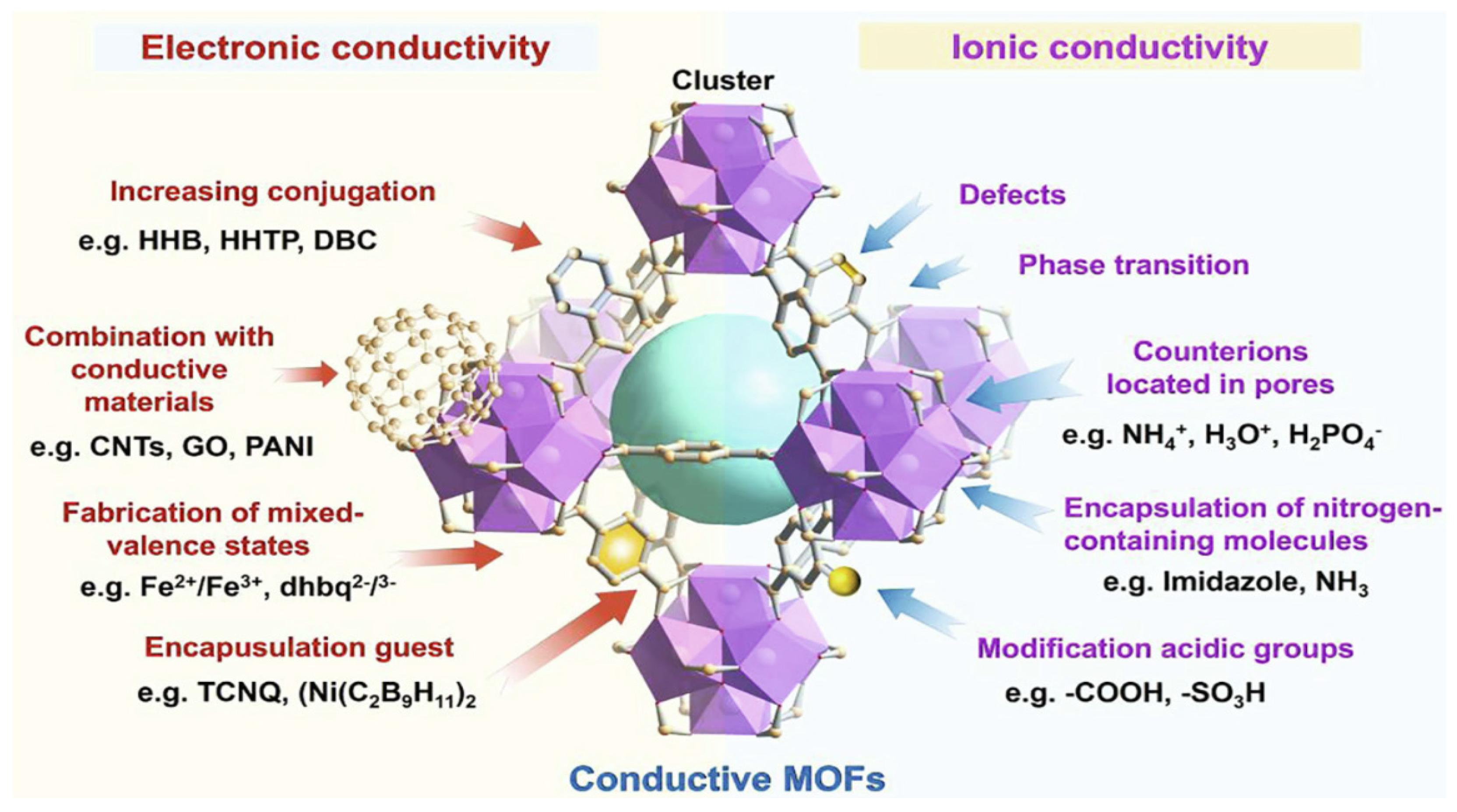
3.1. Metal-Organic Frameworks (MOFs) for Proton Exchange Membrane Fuel Cell (PEMFC) Electrolyte Membranes
3.2. Oxygen Reduction Reaction (ORR) Catalysts for Proton Exchange Membrane (PEM) Fuel Cells
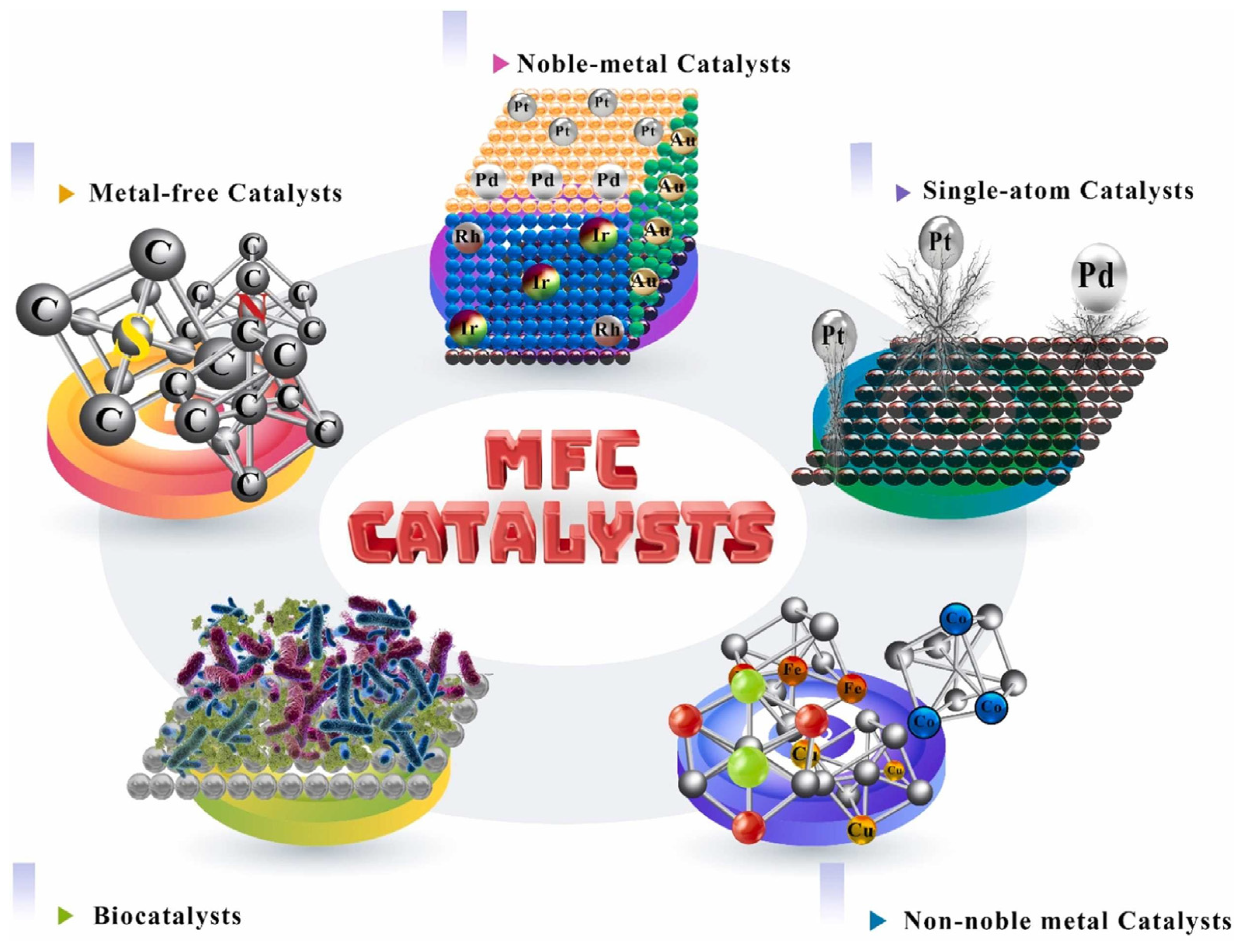
3.3. Oxygen Reduction Reaction (ORR) Catalysts for Microbial Fuel Cells
4. Limitations and Outlook
5. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- DNV As, Norway. Energy Transition Outlook 2024—A Global and Regional Forecast to 2050. Available online: www.dnv.com (accessed on 20 April 2025).
- Ahmed, S.; Ali, A.; Asif, M.; Shim, J.; Park, G. Exploring innovative trends and advancements in rechargeable zinc-air batteries. Inorg. Chem. Commun. 2024, 170, 113288. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Zhou, H.; He, Z.; Liang, H.; Ou, W.; Chung, L.-H.; He, J. Ligand engineering of metal-organic frameworks as efficient electrocatalysts for wide-temperature lithium-sulfur batteries. J. Power Sources 2025, 629, 236053. [Google Scholar] [CrossRef]
- Kim, D.; Park, Y.; Nam, K.W. Inhibiting polysulfide shuttle and enhancing polysulfide redox: Conductive 2D metal-organic framework coated separators for lithium-sulfur batteries. J. Alloys Compd. 2024, 1009, 176812. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Y.; Chen, Z.; Li, C.; Duan, C.; Kawi, S.; Li, Y. Construction of amino functional metal–organic framework modified aramid composite separators with high Li+ transport channels for dendrite-free lithium-ion batteries. J. Colloid Interface Sci. 2025, 683, 262–273. [Google Scholar] [CrossRef]
- Zou, M.; Dong, M.; Zhao, T. Advances in metal-organic frameworks MIL-101(Cr). Int. J. Mol. Sci. 2022, 23, 9396. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional Porous Coordination Polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Li, J.X.; Bao, T.; Zhang, C.; Song, H.; Zou, Y.Y.; Yuan, I.; Xi, Y.; Yu, C.Z.; Liu, C. A general strategy for direct growth of yolk-shell MOF-on-MOF hybrids. Chem. Eng. J. 2023, 472, 144926. [Google Scholar] [CrossRef]
- Arbulu, R.C.; Jiang, Y.B.; Peterson, E.J.; Qin, Y. Metal-organic framework (MOF) nanorods, nanotubes, and nanowires. Angew. Chem. Int. Ed. 2018, 57, 5813–5817. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 974. [Google Scholar] [CrossRef]
- Lei, L.; Cheng, Y.; Chen, C.; Kosari, M.; Jiang, Z.; He, C. Taming structure and modulating carbon dioxide (CO2) adsorption isosteric heat of nickel-based metal organic framework (MOF-74(Ni)) for remarkable CO2 capture. J. Colloid. Interface Sci. 2022, 612, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Bojdys, M.J.; Pinna, N. A universal synthesis strategy for tunable metal-organic framework nanohybrids. Angew. Chem. Int. Ed. 2023, 62, e202301021. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Chia, G.H.; Gao, Z. Metal—Organic frameworks in fuel cell technologies. Nano Today 2013, 8, 577–597. [Google Scholar] [CrossRef]
- Li, H.I.; Eddaoudi, M.; Keeffe, M.O.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Kumar, R.S.; Kumar, S.S.; Kulandainathan, M.A. Efficient electrosynthesis of highly active Cu3(BTC)2-MOF and its catalytic application to chemical reduction. Microporous Mesoporous Mater. 2013, 168, 57–64. [Google Scholar] [CrossRef]
- Kaur, G.; Kandwal Komal, P.; Sud, D. Sonochemically synthesized Zn (II) and Cd (II) based metal-organic frameworks as fluoroprobes for sensing of 2,6-dichlorophenol. J. Solid. State Chem. 2023, 319, 123833. [Google Scholar] [CrossRef]
- Glowniak, S.; Szczesniak, B.; Choma, J.; Jaroniec, M. Recent developments in sonochemical synthesis of nanoporous materials. Molecules 2023, 28, 2639. [Google Scholar] [CrossRef]
- Mendes, R.F.; Rocha, J.; Almeida Paz, F.A. Chapter 8—Microwave Synthesis of Metal-Organic Frameworks. In Metal-Organic Frameworks for Biomedical Applications; Mozafari, M.B.T., Ed.; Woodhead Publishing: Sawston, UK, 2020; pp. 159–176. [Google Scholar] [CrossRef]
- Kumari, P.; Kareem, A.; Jhariat, P.; Kumar, S.; Panda, T. Phase purity regulated by mechano-chemical synthesis of metal-organic frameworks for the electrocatalytic oxygen evolution reaction. Inorg. Chem. 2023, 62, 3457–3463. [Google Scholar] [CrossRef]
- Wang, W.P.; Chai, M.; Zulkifli, M.Y.B.; Xu, K.J.; Chen, Y.L.; Wang, L.Z.; Chen, V.; Hou, J.W. Metal-organic framework composites from a mechanochemical process. Mol. Syst. Des. Eng. 2023, 8, 560–579. [Google Scholar] [CrossRef]
- Vaitsis, C.; Kanellou, E.; Pandis, P.K.; Papamichael, I.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Sonochemical synthesis of zinc adipate Metal-Organic Framework (MOF) for the electrochemical reduction of CO2: MOF and circular economy potential. Sustain. Chem. Pharm. 2022, 29, 100786. [Google Scholar] [CrossRef]
- Muzaffar, N.; Afzal, A.M.; Hegazy, H.H.; Iqbal, M.W. Recent advances in two-dimensional metal-organic frameworks as an exotic candidate for the evaluation of redox-active sites in energy storage devices. J. Energy Storage 2023, 64, 107142. [Google Scholar] [CrossRef]
- Zhang, G.; Jin, L.; Zhang, R.; Bai, Y.; Zhu, R.; Pang, P. Recent advances in the development of electronically and ionically conductive metal-organic frameworks. Coord. Chem. Rev. 2021, 439, 213915. [Google Scholar] [CrossRef]
- Deng, F.; Zhang, Y.; Yu, Y. Conductive Metal–Organic Frameworks for Rechargeable Lithium Batteries. Batteries 2023, 9, 109. [Google Scholar] [CrossRef]
- Lee, S.J.; Telfer, S.G. Multicomponent metal-organic frameworks. Angew. Chem. Int. Ed. 2023, 62, e202306341. [Google Scholar] [CrossRef]
- Lin, Z.X.; Richardson, J.J.; Zhou, J.J.; Caruso, F. Direct synthesis of amorphous coordination polymers and metal-organic frameworks. Nat. Rev. Chem. 2023, 7, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, T.; Xu, J.; Shao, L.; Shi, X.; Sun, Z. Metal-organic frameworks based solid-state electrolytes for lithium metal batteries: Modifications and future prospects. Next Energy 2025, 6, 100191. [Google Scholar] [CrossRef]
- Ajpi, C.; Leiva, N.; Lundblad, A.; Lindbergh, G.; Cabrera, S. Synthesis and spectroscopic characterization of Fe3+-BDC metal organic framework as material for lithium ion batteries. J. Mol. Struct. 2023, 1272, 134127. [Google Scholar] [CrossRef]
- Guo, W.; Fu, Y. A perspective on energy densities of rechargeable Li-S batteries and alternative sulfur-based cathode materials. Energy Environ. Mater. 2018, 1, 20–27. [Google Scholar] [CrossRef]
- Ullah, A.; Majid, A.; Rani, N. A review on first principles based studies for improvement of cathode material of lithium ion batteries. J. Energy Chem. 2018, 27, 219–237. [Google Scholar] [CrossRef]
- Yuan, T.; Qi, S.; Ye, L.; Zhao, Y.; Jiang, Y.; Feng, Z.; Zhu, J.; Dai, L.; Wang, L.; He, Z. Metal-organic frameworks-based materials: A feasible path for redox flow battery. Coord. Chem. Rev. 2025, 531, 216503. [Google Scholar] [CrossRef]
- Naseer, M.; Siyal, S.H.; Najam, T.; Afzal, S.; Iqbal, R.; Ismail, M.A.; Rauf, A.; Ahmad Shah, S.S.; Nazir, M.A. Engineering of metal oxide integrated metal organic frameworks (MO@MOF) composites for energy and environment sector. Mater. Sci. Eng. B 2025, 313, 117909. [Google Scholar] [CrossRef]
- Boukayouht, K.; Bazzi, I.; Hankari, S.E. Sustainable synthesis of metal-organic frameworks and their derived materials from organic and inorganic wastes. Coord. Chem. Rev. 2023, 478, 214986. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, S.G.; Yan, X.T.; Lv, Y. Recent advances in metal-organic frameworks: Synthesis, application and toxicity. Sci. Total Environ. 2023, 902, 165944. [Google Scholar] [CrossRef]
- Connolly, B.M.; Mehta, J.P.; Moghadam, P.Z.; Wheatley, A.E.H.; Fairen-Jimenez, D. From synthesis to applications: Metal-organic frameworks for an environmentally sustainable future. Curr. Opin. Green Sustain. Chem. 2018, 12, 47–56. [Google Scholar] [CrossRef]
- Vaitsis, C.; Sourkouni, G.; Argirusis, C. Metal organic frameworks (MOFs) and ultrasound: A review. Ultrason. Sonochem. 2019, 52, 106–119. [Google Scholar] [CrossRef]
- Esmaeili, M.S.; Mehrpooya, M.; Ganjali, M.R. Three-dimensional nanocomposites derived from combined metal organic frameworks doped with Ce, La, and Cu as a bifunctional electrocatalyst for supercapacitors and oxygen reduction reaction. Int. J. Hydrogen Energy 2025, 97, 483–500. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, L.; Chen, J.; Fu, M.; Diao, Z.; Liu, H.; Guo, H. Enhanced bioelectrochemical performance caused by porous metal-organic framework MIL-53(Fe) as the catalyst in microbial fuel cells. Process Biochem. 2020, 99, 147–153. [Google Scholar] [CrossRef]
- Li, X.; Zheng, S.; Jin, L.; Li, Y.; Geng, P.; Xue, H.; Pang, H.; Xu, Q. Metal-organic framework-derived carbons for battery applications. Adv. Energy Mater. 2018, 8, 1800716. [Google Scholar] [CrossRef]
- Wang, L.; Han, Y.; Feng, X.; Zhou, J.; Qi, P.; Wang, B. Metal-organic frameworks for energy storage: Batteries and supercapacitors. Coord. Chem. Rev. 2016, 307, 361–381. [Google Scholar] [CrossRef]
- Talukder, N.; Wang, Y.; Nunna, B.B.; Lee, E.S. N-Doped Graphene (N-G)/MOF(ZIF-8)-Based/ Derived Materials for Electrochemical Energy Applications: Synthesis, Characteristics, and Functionality. Batteries 2024, 10, 47. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Li, C.; Leng, X.; Prakash, N.G.; Ko, T.J. Functional La@ZIF-8-enhanced composite quasi-solid electrolyte for high-performance Li-metal batteries. Chem. Eng. J. 2025, 508, 160932. [Google Scholar] [CrossRef]
- Chen, G.; Jin, B.; Sun, S.; Jiang, Q. Surface-functionalized metal–organic framework with high specific surface area optimizing composite solid electrolyte for high-performance solid-state lithium metal batteries. Chem. Eng. J. 2024, 501, 157704. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, G.; Lin, Y.; Wang, Y.; Zhu, Q.; Dai, Z. MOF-derived hierarchical porous carbon octahedrons for aluminum-ion batteries. Carbon 2023, 202, 305–313. [Google Scholar] [CrossRef]
- Xu, K.; Bao, H.; Tang, C.; Maliutina, K.; Li, F.; Fan, L. Engineering hierarchical MOFs-derived Fe-N-C nanostructure with improved oxygen reduction activity for zinc-air battery: The role of iron oxide. Mater. Today Energy 2020, 18, 100500. [Google Scholar] [CrossRef]
- Kori, A.H.; Khan, M.; Soylak, M. Metal organic framework composite (Ti3AlC2 @ZIF-67) for vortex assisted solid phase extraction of lead from water and food samples. J. Food Compos. Anal. 2024, 125, 105810. [Google Scholar] [CrossRef]
- Javadian, S.; Moslemi, M.; Gharibi, H.; Parviz, Z.; Dalir, N.; Zeinodiny, A. Development and evaluation of a dual silica structure integrated with a metal-organic framework as a fast-charging anode for future lithium-ion batteries. J. Power Sources 2025, 629, 235974. [Google Scholar] [CrossRef]
- Han, H.; Chao, S.; Bai, Z.; Wang, X.; Yang, X.; Qiao, J.; Chen, Z.; Yang, L. Metal-organic-framework-derived Co nanoparticles deposited on N-doped bimodal mesoporous carbon nanorods as efficient bifunctional catalysts for rechargeable zinc-air batteries. Chemelectrochem 2018, 5, 1868–1873. [Google Scholar] [CrossRef]
- Mirandona-Olaeta, A.; Goikolea, E.; Lanceros-Mendez, S.; Fidalgo-Marijuan, A.; Ruiz de Larramendi, I. Ionic Liquid-Laden Zn-MOF-74-Based Solid-State Electrolyte for Sodium Batteries. Batteries 2023, 9, 588. [Google Scholar] [CrossRef]
- Hassan, H.; Sunny, M.A.; Iqbal, M.W.; Afzal, A.M.; Yaseen, T.; Mohammad, S.; Alotaibi, N.H.; Manzoor, M.; Ismayilova, N.A. Innovative Si@MOF-5@CNT hybrid: Tailoring energy storage and catalysis in batteries, supercapacitors, and hydrogen evolution. Diam. Relat. Mater. 2024, 148, 111507. [Google Scholar] [CrossRef]
- Zhou, Q.; Xia, C.; Li, J.; Cheng, A.; Liu, J.; Liu, H.; Li, L.; Wang, S. Sulfur-doped porous carbon derived by metal-organic framework (MOF-5) for high lithium/sodium storage performance. Solid. State Ion. 2024, 411, 116553. [Google Scholar] [CrossRef]
- Wu, D.; Guo, Z.; Yin, X.; Pang, Q.; Tu, B.; Zhang, L.; Wang, Y.G.; Li, Q. Metal-organic frameworks as cathode materials for Li-O2 batteries. Adv. Mater. 2014, 26, 3258–3262. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, J.; Dong, H.; Luo, Y.; Liu, C.; Huang, Y.; Lai, X.; Huang, X.; Yue, B. MIL-53(Fe) anode for lithium-ion batteries with the capacity self-activation effect based on electrochemically induced crystalline-to-amorphization transformation. Mater. Today Commun. 2024, 38, 108200. [Google Scholar] [CrossRef]
- Wang, Z.; Li, B.; Shao, F.; Zhang, S.; Shi, B.; Jing, Y.; Zou, X.; Jia, Y. Mechanistic insights into lithium-ion adsorption and isotope separation on ligand-tuned aluminum-based metal–organic frameworks. Chem. Eng. J. 2025, 504, 159061. [Google Scholar] [CrossRef]
- Zhou, J.; Li, R.; Fan, X.; Chen, Y.; Han, R.; Li, W.; Zheng, J.; Wang, B.; Li, X. Rational design of a metal-organic framework host for sulfur storage in fast, long-cycle Li-S batteries. Energy Environ. Sci. 2014, 7, 2715–2724. [Google Scholar] [CrossRef]
- Sondermann, L.; Smith, Q.; Strothmann, T.; Vollrath, A.; Beglau, T.H.J.; Janiak, C. Mechanochemical synthesis and application of mixed-metal copper–ruthenium HKUST-1 metal–organic frameworks in the electrocatalytic oxygen evolution reaction. RSC Mechanochem. 2024, 1, 296–307. [Google Scholar] [CrossRef]
- Baumann, A.E.; Aversa, G.E.; Roy, A.; Falk, M.L.; Bedford, N.M.; Thoi, V.S. Promoting sulfur adsorption using surface Cu sites in metal-organic frameworks for lithium sulfur batteries. J. Mater. Chem. 2018, 6, 4811–4821. [Google Scholar] [CrossRef]
- Liu, Z.; Kai Liu, K.; Zhi, K.; Luo, J.; Hu, Z.; Zhang, Y. A spontaneous spatial network structural metal-organic framework composite polymer electrolytes with excellent lithium transport performance for dendrite-suppressing lithium metal batteries. Chem. Eng. J. 2025, 504, 158820. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; He, L.; Sun, Y.; Huang, Q.; Xu, S.; Qiu, X.; Wei, T. A gel polymer electrolyte based on IL@NH2-MIL-53 (Al) for high-performance all-solid-state lithium metal batteries. Chin. J. Chem. Eng. 2024, 69, 47–55. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Luo, J.; Yu, Y. Application of metal-organic frameworks to the anode interface in metal batteries. Chin. Chem. Lett. 2024, 35, 109500. [Google Scholar] [CrossRef]
- Yan, J.; Zheng, X.; Wei, C.; Sun, Z.; Zeng, K.; Shen, L.; Sun, J.; Rümmeli, M.H.; Yang, R. Nitrogen-doped hollow carbon polyhedron derived from salt-encapsulated ZIF-8 for efficient oxygen reduction reaction. Carbon 2021, 171, 320–328. [Google Scholar] [CrossRef]
- Yamada, T.; Shiraishi, K.; Kitagawa, H.; Kimizuka, N. Applicability of MIL-101(Fe) as a cathode of lithium ion batteries. Chem. Commun. 2017, 53, 8215–8218. [Google Scholar] [CrossRef] [PubMed]
- Vallem, S.; Song, S.; Oh, Y.; Kim, J.; Li, M.; Li, Y.; Cheng, X.; Bae, J. Designing a Se-intercalated MOF/MXene-derived nanoarchitecture for advancing the performance and durability of lithium-selenium batteries. J. Colloid. Interface Sci. 2024, 665, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Ai, C.; Yang, Z.; Shi, G.; Xue, Y.; Dai, H.; Yi, Q.; Fang, C.; Han, J. Increment entropy promoted small-polaron breakdown in metal–organic frameworks for sodium-ion batteries. Chem. Eng. J. 2024, 500, 157006. [Google Scholar] [CrossRef]
- Chen, W.; Kang, T.; Lao, Z.; Cai, J.; Yang, W.; Zou, H.; Chen, S. Towards improved P2-type Na0.67Ni0.5Mn0.5O2 with fast Na ion diffusion via a metal organic framework coating for sodium ion batteries. J. Energy Storage 2025, 109, 115151. [Google Scholar] [CrossRef]
- Xie, X.; Zhai, Z.; Cao, W.; Dong, J.; Li, Y.; Hou, Q.; Du, G.; Wa, J.; Tian, L.; Zhang, J.; et al. Bifunctional ligand Co metal-organic framework derived heterostructured Co-based nanocomposites as oxygen electrocatalysts toward rechargeable zinc-air batteries. J. Colloid Interface Sci. 2024, 664, 319–328. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, Y.; Li, R.; Peng, X.; Ren, P.; Liu, A.; Wen, S.; Qin, J.; Zhang, J.; An, M.; et al. Metal–organic framework derived phosphide-hydroxide heterostructure as bifunctional oxygen electrocatalyst for zinc–air batteries. Alloys Compd. 2024, 976, 173237. [Google Scholar] [CrossRef]
- Vayenas, M.; Vaitsis, C.; Sourkouni, G.; Pandis, P.K.; Argirusis, C. Investigation of alternative materials as bifunctional catalysts for electrochemical applications. Chim. Techno Acta 2019, 6, 120–129. [Google Scholar] [CrossRef]
- Liu, C.; Wu, B.; Liu, T.; Zhang, Y.; Cui, J.; Huang, L.; Tan, G.; Zhang, L.; Su, Y.; Wu, F. Metal-organic frameworks and their composites for advanced lithium-ion batteries: Synthesis, progress and prospects. J. Energy Chem. 2024, 89, 449–470. [Google Scholar] [CrossRef]
- Naskar, I.; Naskar, S.; Kaur, B.; Ghosal, P.; Deepa, M. MIL-53(Al) metal-organic framework/carbon nanofibers and Al-nanoflakes/carbon composites for a stable Al-battery. J. Energy Storage 2024, 77, 109956. [Google Scholar] [CrossRef]
- Mao, M.; Wu, X.; Hu, Y.; Yuan, Q.; He, Y.-B.; Kang, F. Charge storage mechanism of MOF-derived Mn2O3 as high performance cathode of aqueous zinc-ion batteries. J. Energy Chem. 2021, 52, 277–283. [Google Scholar] [CrossRef]
- Zhu, Q.; Sun, W.; Zhou, H.; Mao, D. A Review of Lithium–Sulfur Batteries Based on Metal–Organic Frameworks: Progress and Prospects. Batteries 2025, 11, 89. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, X.; Yin, D.; Mi, D.; Qu, C.; Feng, M. Metal-Organic Frameworks derived Pr2O2S-doped porous carbon nanosheets as efficient adsorption-catalytic separator coating materials for lithium-sulfur batteries. J. Power Sources 2025, 635, 236540. [Google Scholar] [CrossRef]
- Xiao, Y.; Qi, C.; Yang, D.; Ma, D.; Huang, S. Toward high performance Lithium-Sulfur batteries based on Metal-Organic Frameworks: Progress and prospects. Mater. Sci. Eng. B 2025, 313, 117983. [Google Scholar] [CrossRef]
- Zhao, T.; Chen, J.; Xiao, P.; Nie, S.; Luo, F.; Chen, Y. Exploring metal-organic frameworks in battery electrodes, separators, and electrolytes: A comprehensive review. Coord. Chem. Rev. 2025, 532, 216501. [Google Scholar] [CrossRef]
- Ngo, N.M.; Nguyen, M.N.; Song, S.-W.; Park, S. Bimetallic metal organic Framework-modified glass fiber as composite separator for lithium sulfur batteries. Mater. Lett. 2025, 382, 137835. [Google Scholar] [CrossRef]
- Wild, M.; O’Neill, L.; Zhang, T.; Purkayastha, R.; Minton, G.; Marinescu, M.; Offer, G.J. Lithium sulfur batteries, a mechanistic review. Energy Environ. Sci. 2015, 8, 3477–3494. [Google Scholar] [CrossRef]
- Wu, L.; Yuan, C.; Gao, F.; Qiang, X.; Hui, J.; Liu, Β. Self-assembly of polyoxometalate-based metal-organic framework with trefoil sign structural feature as efficient anode for lithium ion batteries. Solid State Sci. 2025, 159, 107774. [Google Scholar] [CrossRef]
- Ren, W.; Ma, W.; Zhang, S.; Tang, B. Recent advances in shuttle effect inhibition for lithium sulfur batteries. Energy Storage Mater. 2019, 23, 707–732. [Google Scholar] [CrossRef]
- Deng, W.; Phung, J.; Li, G.; Wang, X. Realizing high-performance lithium-sulfur batteries via rational design and engineering strategies. Nano Energy 2021, 82, 105761. [Google Scholar] [CrossRef]
- Liu, T.; Hu, H.; Ding, X.; Yuan, H.; Jin, C.; Nai, J.; Liu, Y.; Wang, Y.; Wan, Y.; Tao, X. 12 years roadmap of the sulfur cathode for lithium sulfur batteries (2009–2020). Energy Storage Mater. 2020, 30, 346–366. [Google Scholar] [CrossRef]
- Eftekhari, A.; Kim, D.-W. Cathode materials for lithium-sulfur batteries: A practical perspective. J. Mater. Chem. 2017, 5, 17734–17776. [Google Scholar] [CrossRef]
- Demir-Cakan, R.; Morcrette, M.; Nouar, F.; Davoisne, C.; Devic, T.; Gonbeau, D.; Dominko, R.; Serre, C.; Ferey, G.; Tarascon, J.M. Cathode composites for Li-S batteries via the use of oxygenated porous architectures. J. Am. Chem. Soc. 2011, 133, 16154–16160. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Mao, H.; Xu, L. MIL-100(V) and MIL-100(V)/rGO with various valence states of vanadium ions as sulfur cathode hosts for lithium-sulfur batteries. Nano Res. 2016, 10, 344–353. [Google Scholar] [CrossRef]
- Hong, X.J.; Tan, T.X.; Guo, Y.K.; Tang, X.Y.; Wang, J.Y.; Qin, W.; Cai, Y.P. Confinement of polysulfides within bi-functional metal-organic frameworks for high performance lithium-sulfur batteries. Nanoscale 2018, 10, 2774–2780. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Cui, Y.; Yang, Y.; Pan, H.; Wang, Z.; Wu, C.; Chen, B.; Qian, G. A metal-organic framework with open metal sites for enhanced confinement of sulfur and lithium-sulfur battery of long cycling life. Cryst. Growth Des. 2013, 13, 5116–5120. [Google Scholar] [CrossRef]
- Haro, J.; Benítez, A.; Caballero, Á.; Morales, J. Revisiting the HKUST-1/S composite as an electrode for Li-S batteries: Inherent problems that hinder its performance. Eur. J. Inorg. Chem. 2020, 2021, 177–185. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Du, G.; Zhang, J.; Liu, M.; Qu, X. Li2S-Embedded copper metal-organic framework cathode with superior electrochemical performance for Li-S batteries. New J. Chem. 2018, 42, 13775–13783. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Du, G.; Zhang, J.; Qu, X. Experimental and first-principles study of a metal-organic framework with sulfur embedding cathode for enhanced performance lithium-sulfur battery. Sustain. Energy Fuels 2018, 2, 1828–1836. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, X.; Fan, X.; Wang, X.; Li, H.; Zhang, Y.; Li, W.; Zheng, J.; Wang, B.; Li, X. The impact of the particle size of a metal-organic framework for sulfur storage in Li-S batteries. J. Mater. Chem. 2015, 3, 8272–8275. [Google Scholar] [CrossRef]
- Cui, G.; Li, G.; Luo, D.; Zhang, Y.; Zhao, Y.; Wang, D.; Wang, J.; Zhang, Z.; Wang, X.; Chen, Z. Three-dimensionally ordered macro-microporous metal organic frameworks with strong sulfur immobilization and catalyzation for high-performance lithium-sulfur batteries. Nano Energy 2020, 72, 104685. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, R.; Liu, Z.; Wang, X.; Han, C.; Liu, H.; Li, B. A 3D lithiophilic ZIF-8@RGO free-standing scaffold with dendrite-free behavior enabling high performance Li metal batteries. J. Mater. Chem. A 2023, 11, 12910. [Google Scholar] [CrossRef]
- Bai, L.; Chao, D.; Xing, P.; Tou, L.J.; Chen, Z.; Jana, A.; Shen, Z.X.; Zhao, Y. Refined sulfur nanoparticles immobilized in metal-organic polyhedron as stable cathodes for Li-S battery. ACS Appl. Mater. Interfaces 2016, 8, 14328–14333. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Li, C.; Li, Z.; Yin, L. Tannic acid tuned metal-organic framework as a high-efficiency chemical anchor of polysulfide for lithium-sulfur batteries. Electrochim. Acta 2018, 281, 700–709. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, L.; Chen, S.; Zhu, X.; Deng, Q.; Wang, J.; Zeng, Z.; Deng, S. Facile and low-temperature strategy to prepare hollow ZIF-8/CNT polyhedrons as high-performance lithium-sulfur cathodes. Chem. Eng. J. 2021, 404, 126579. [Google Scholar] [CrossRef]
- Kaye, S.S.; Dailly, A.; Yaghi, O.M.; Long, J.R. Impact of preparation and handling on the hydrogen storage properties of Zn4O(1,4-benzenedicarboxylate)3 (MOF-5). J. Am. Chem. Soc. 2007, 129, 14176–14177. [Google Scholar] [CrossRef]
- Shanthi, P.M.; Hanumantha, P.J.; Gattu, B.; Sweeney, M.; Datta, M.K.; Kumta, P.N. Understanding the origin of irreversible capacity loss in non-carbonized carbonate—Based metal organic framework (MOF) sulfur hosts for lithium—Sulfur battery. Electrochim. Acta 2017, 229, 208–218. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, B.; Yang, Y.; Cui, Y.; Wang, Z.; Chen, B.; Qian, G. Mixed-metal-organic framework with effective Lewis acidic sites for sulfur confinement in high-performance lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2015, 7, 20999–21004. [Google Scholar] [CrossRef]
- Liu, B.; Baumann, A.E.; Thoi, V.S. Modulating charge transport in MOFs with zirconium oxide nodes and redox-active linkers for lithium sulfur batteries. Polyhedron 2019, 170, 788–795. [Google Scholar] [CrossRef]
- Lai, M.; Zhang, D.; Chen, F.; Lin, X.; Qiu, A.; Lei, C.; Liang, J.; Liang, J.; Li, J.; Wang, Q.; et al. Advanced Metal-Organic Frameworks Based on Anthraquinone-2,3-Dicarboxylate Ligands as Cathode for Lithium-Ion Batteries. Batteries 2023, 9, 247. [Google Scholar] [CrossRef]
- Zhu, M.; Cai, C.; Quan, K.; Wei, Y.; Chen, H.; Zhang, S.; Yi, H.; Guo, Z.; Teng, X.; Zhang, J. Two-dimensional metal-organic frameworks with highly exposed active centers as a synergetic protective interlayer for shuttle-free lithium-sulfur batteries. Chem. Eng. Sci. 2025, 306, 121325. [Google Scholar] [CrossRef]
- Park, B. Pseudocapacitive charge storage in ultrathin NiCo-metal organic framework-derived NiCo2O4 Li-metal batteries. Mater. Lett. 2025, 394, 138654. [Google Scholar] [CrossRef]
- Yang, T.; Xiao, R.; Lu, F.; Ke, X.; Wang, M.; Wang, K. Conductivity enhancement mechanism and application of NiCo-MOF hollow sphere electrode materials in lithium-ion batteries. New J. Chem. 2025, 49, 6674–6683. [Google Scholar] [CrossRef]
- Wei, H.; Qin, F. Electrochemical Synthesis and Conductivity Fine Tuning of the 2D Iron-Quinoid Metal–Organic Framework. ACS Appl. Mater. Interfaces 2025, 17, 2010–2017. [Google Scholar] [CrossRef]
- Zanca, F.; Glasby, L.T.; Chong, S.; Chen, S.; Kim, J.; Fairen-Jimenez, D.; Monserrat, B.; Moghadam, P.Z. Computational techniques for characterisation of electrically conductive MOFs: Quantum calculations and machine learning approaches. J. Mater. Chem. C 2021, 9, 13584–13599. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Wang, T.; Fu, N.; Yang, Z. Manganese-based MOF interconnected carbon nanotubes as a high-performance cathode for rechargeable aqueous zinc-ion batteries. J. Energy Storage 2024, 76, 109873. [Google Scholar] [CrossRef]
- Li, X.; Cheng, F.; Zhang, S.; Chen, J. Shape-controlled synthesis and lithium-storage study of metal-organic frameworks Zn4O(1,3,5-benzenetribenzoate)2. J. Power Sources 2006, 160, 542–547. [Google Scholar] [CrossRef]
- de Combarieu, G.; Morcrette, M.; Millange, F.; Guillou, N.; Cabana, J.; Grey, C.P.; Margiolaki, I.; Férey, G.; Tarascon, J.M. Influence of the benzoquinone sorption on the structure and electrochemical performance of the MIL-53(Fe) hybrid porous material in a lithium-ion battery. Chem. Mater. 2009, 21, 1602–1611. [Google Scholar] [CrossRef]
- Fateeva, A.; Horcajada, P.; Devic, T.; Serre, C.; Marrot, J.; Grenèche, J.-M.; Morcrette, M.; Tarascon, J.-M.; Maurin, G.; Férey, G. Synthesis, structure, characterization, and redox properties of the porous MIL-68(Fe) solid. Eur. J. Inorg. Chem. 2010, 2010, 3789–3794. [Google Scholar] [CrossRef]
- Shin, J.; Kim, M.; Cirera, J.; Chen, S.; Halder, G.J.; Yersak, T.A.; Paesani, F.; Cohen, S.M.; Meng, Y.S. MIL-101(Fe) as a lithium-ion battery electrode material: A relaxation and intercalation mechanism during lithium insertion. J. Mater. Chem. 2015, 3, 4738–4744. [Google Scholar] [CrossRef]
- Deng, S.-Q.; Zhuang, Z.; Zhou, C.-A.; Zheng, H.; Zheng, S.-R.; Yan, W.; Zhang, J. Metal-organic framework derived FeNi alloy nanoparticles embedded in N-doped porous carbon as high-performance bifunctional air-cathode catalysts for rechargeable zinc-air battery. J. Colloid Interface Sci. 2023, 641, 265–276. [Google Scholar] [CrossRef]
- Li, C.; Zhang, C.; Xie, J.; Wang, K.; Li, J.; Zhang, Q. Ferrocene-based metal-organic framework as a promising cathode in lithium-ion battery. Chem. Eng. J. 2021, 404, 126463. [Google Scholar] [CrossRef]
- Chen, T.; Wang, F.; Cao, S.; Bai, Y.; Zheng, S.; Li, W.; Zhang, S.; Hu, S.X.; Pang, H. In situ synthesis of MOF-74 family for high areal energy density of aqueous nickel-zinc batteries. Adv. Mater. 2022, 34, e2201779. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Zhao, F.; Li, Y.; Yan, H.; Xu, B.; Sun, D. Moss-like two-dimensional MBene-Ni/Co bimetallic metal-organic framework composite as efficient cathode for alkaline zinc batteries. J. Power Sources 2025, 633, 236437. [Google Scholar] [CrossRef]
- Peng, Z.; Yi, X.; Liu, Z.; Shang, J.; Wang, D. Triphenylamine-based metal-organic frameworks as cathode materials in lithium-ion batteries with coexistence of redox active sites, high working voltage, and high rate stability. ACS Appl. Mater. Interfaces 2016, 8, 14578–14585. [Google Scholar] [CrossRef] [PubMed]
- Nagatomi, H.; Yanai, N.; Yamada, T.; Shiraishi, K.; Kimizuka, N. Synthesis and electric properties of a two-dimensional metal-organic framework based on phthalocyanine. Chemistry 2018, 24, 1806–1810. [Google Scholar] [CrossRef]
- Gu, S.; Bai, Z.; Majumder, S.; Huang, B.; Chen, G. Conductive metal-organic framework with redox metal center as cathode for high rate performance lithium ion battery. J. Power Sources 2019, 429, 22–29. [Google Scholar] [CrossRef]
- Spinner, N.; Zhang, L.; Mustain, W.E. Investigation of metal oxide anode degradation in lithium-ion batteries via identical-location TEM. J. Mater. Chem. A 2014, 2, 1627. [Google Scholar] [CrossRef]
- Xia, F.; Zeng, W.; Peng, H.; Wang, H.; Sun, C.; Zou, J.; Wu, J. Revealing structural degradation in layered structure oxides cathode of lithium ion batteries via in-situ transmission electron microscopy. J. Mater. Sci. Technol. 2023, 154, 189–201. [Google Scholar] [CrossRef]
- Tian, B.; Ning, G.H.; Gao, Q.; Tan, L.M.; Tang, W.; Chen, Z.; Su, C.; Loh, K.P. Crystal engineering of naphthalenediimide-based metal-organic frameworks: Structure-dependent lithium storage. ACS Appl. Mater. Interfaces 2016, 8, 31067–31075. [Google Scholar] [CrossRef]
- Bi, D.; Zhao, T.; Lai, Q.; Zhao, J.; Grigoriev, S.A.; Liang, Y.Y. In situ preparation of MOF-74 for compact zincophilic surfaces enhancing the stability of aqueous zinc-ion battery anodes. J. Alloys Compd. 2024, 1002, 175448. [Google Scholar] [CrossRef]
- Qiao, Q.-Q.; Li, G.-R.; Wang, Y.-L.; Gao, X.-P. To enhance the capacity of Li-rich layered oxides by surface modification with metal-organic frameworks (MOFs) as cathodes for advanced lithium-ion batteries. J. Mater. Chem. 2016, 4, 4440–4447. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Wang, X.; Weng, Q.; Sun, W. Engineering the conductive network of missing-linker metal-organic framework for improved performance in aqueous zinc-ion batteries. J. Power Sources 2024, 624, 235525. [Google Scholar] [CrossRef]
- Yu, T.; Cai, R.; Chen, Z. Zn-air batteries. In Metal-Air Batteries: Fundamentals and Applications; Zhang, X.-B., Ed.; Wiley-VCH: Hoboken, NJ, USA, 2018; pp. 265–291. ISBN 978-3-527-80766-6. [Google Scholar]
- Gilligan, G.E.; Qu, D. Zinc-air and other types of metal-air batteries. In Advances in Batteries for Medium and Large-Scale Energy Storage; Menictas, C., Skyllas-Kazacos, M., Lim, T.M., Eds.; Woodhead Publishing: Sawston, UK, 2015; pp. 441–461. [Google Scholar] [CrossRef]
- Li, H.; Ma, L.; Han, C.; Wang, Z.; Liu, Z.; Tang, Z.; Zhi, C. Advanced rechargeable zinc-based batteries: Recent progress and future perspectives. Nano Energy 2019, 62, 550–587. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Zhang, L.; Chen, J. Recent advances in isolated single-atom catalysts for zinc air batteries: A focus review. Nanomaterials 2019, 9, 1402. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Aasen, D.; Yu, H.; Labbe, M.; Ivey, D.G.; Veinot, J.G.C. Mn3O4 nanoparticle-decorated hollow mesoporous carbon spheres as an efficient catalyst for oxygen reduction reaction in Zn-air batteries. Nanoscale Adv. 2020, 2, 3367–3374. [Google Scholar] [CrossRef]
- Gu, Y.; Yan, G.; Lian, Y.; Qi, P.; Mu, Q.; Zhang, C.; Deng, Z.; Peng, Y. MnIII-enriched a-MnO2 nanowires as efficient bifunctional oxygen catalysts for rechargeable Zn-air batteries. Energy Storage Mater. 2019, 23, 252–260. [Google Scholar] [CrossRef]
- Yan, W.; Guo, Z.; Xu, H.; Lou, Y.; Chen, J.; Li, Q. Downsizing metal-organic frameworks with distinct morphologies as cathode materials for high-capacity LieO2 batteries. Mater. Chem. Front. 2017, 1, 1324–1330. [Google Scholar] [CrossRef]
- Lv, C.; Li, B.; Ren, Y.; Zhang, G.; Lu, Z.; Li, L.; Zhang, X.; Yang, X.; Yu, X. A “MOF-plus-MOF” strategy to synthesize Co-N3C1 single-atom catalyst for rechargeable Zn-air battery. Chem. Eng. J. 2024, 495, 153670. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, Y.J.; Kim, D.H.; Lee, Y.J. Bimetallic metal-organic frameworks as efficient cathode catalysts for Li-O2 batteries. ACS Appl. Mater. Interfaces 2018, 10, 660–667. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, Z.; Cheng, F.; Tao, Z.; Chen, J. Micro-nano structured Ni-MOFs as high-performance cathode catalyst for rechargeable Li-O2 batteries. Nanoscale 2015, 7, 11833–11840. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, L.; Xiao, Z.; Chen, R.; Jiang, Z.; Wang, S. 3D carbon electrocatalysts in situ constructed by defect-rich nanosheets and polyhedrons from NaCl-sealed zeolitic imidazolate frameworks. Adv. Funct. Mater. 2018, 28, 1705356. [Google Scholar] [CrossRef]
- Yang, M.; Hu, X.; Fang, Z.; Sun, L.; Yuan, Z.; Wang, S.; Hong, W.; Chen, X.; Yu, D. Bifunctional MOF-derived carbon photonic crystal architectures for advanced Zn-air and Li-S batteries: Highly exposed graphitic nitrogen matters. Adv. Funct. Mater. 2017, 27, 1701971. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Wu, H.B.; Shen, G.; Le, Z.; Chen, G.; Lu, Y. Iron-decorated nitrogen-rich carbons as efficient oxygen reduction electrocatalysts for Zn-air batteries. Nanoscale 2018, 10, 16996–17001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Han, X.; Jiang, Z.; Xu, J.; Chen, L.; Xue, Y.; Nie, A.; Xie, Z.; Kuang, Q.; Zheng, L. Atomically dispersed hierarchically ordered porous Fe-N-C electrocatalyst for high performance electrocatalytic oxygen reduction in Zn-Air battery. Nano Energy 2020, 71, 104547. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, H.; Zhu, J.; Li, W.; Zhang, C.; Zhao, J.; Luo, F.; Sun, Z.; Mu, S. 3D flower-like ZnFe-ZIF derived hierarchical Fe, N-Codoped carbon architecture for enhanced oxygen reduction in both alkaline and acidic media, and zinc-air battery performance. Carbon 2020, 161, 502–509. [Google Scholar] [CrossRef]
- Jin, H.; Zhou, H.; Li, W.; Wang, Z.; Yang, J.; Xiong, Y.; He, D.; Chen, L.; Mu, S. In situ derived Fe/N/S-codoped carbon nanotubes from ZIF-8 crystals as efficient electrocatalysts for the oxygen reduction reaction and zinc-air batteries. J. Mater. Chem. 2018, 6, 20093–20099. [Google Scholar] [CrossRef]
- Chen, K.; Ci, S.; Xu, Q.; Cai, P.; Li, M.; Xiang, L.; Hu, X.; Wen, Z. Iron-incorporated nitrogen-doped carbon materials as oxygen reduction electrocatalysts for zinc-air batteries. Chin. J. Catal. 2020, 41, 858–867. [Google Scholar] [CrossRef]
- Chen, D.; Ji, J.; Jiang, Z.; Ling, M.; Jiang, Z.; Peng, X. Molecular-confinement synthesis of sub-nano Fe/N/C catalysts with high oxygen reduction reaction activity and excellent durability for rechargeable Zn-Air batteries. J. Power Sources 2020, 450, 227660. [Google Scholar] [CrossRef]
- Li, J.-C.; Wu, X.-T.; Chen, L.-J.; Li, N.; Liu, Z.-Q. Bifunctional MOF-derived Co-N-doped carbon electro-catalysts for high-performance zinc-air batteries and MFCs. Energy 2018, 156, 95–102. [Google Scholar] [CrossRef]
- Amiinu, I.S.; Liu, X.; Pu, Z.; Li, W.; Li, Q.; Zhang, J.; Tang, H.; Zhang, H.; Mu, S. From 3D ZIF nanocrystals to Co-Nx/C nanorod array electrocatalysts for ORR, OER, and Zn-air batteries. Adv. Funct. Mater. 2018, 28, 1704638. [Google Scholar] [CrossRef]
- Zhang, M.; Dai, Q.; Zheng, H.; Chen, M.; Dai, L. Novel MOF-derived Co@N-C bifunctional catalysts for highly efficient Zn-air batteries and water splitting. Adv. Mater. 2018, 30, 1705431. [Google Scholar] [CrossRef] [PubMed]
- Zang, W.; Sumboja, A.; Ma, Y.; Zhang, H.; Wu, Y.; Wu, S.; Wu, H.; Liu, Z.; Guan, C.; Wang, J.; et al. Single Co atoms anchored in porous N-doped carbon for efficient zinc-air battery cathodes. ACS Catal. 2018, 8, 8961–8969. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, J.; Lin, H.-F.; Tang, P.; Morante, J.R.; Arbiol, J.; Wan, K.; Mao, B.-W.; Liu, L.-M.; Fransaer, J. Tailor-made metal-nitrogen-carbon bifunctional electrocatalysts for rechargeable Zn-air batteries via controllable MOF units. Energy Storage Mater. 2019, 17, 46–61. [Google Scholar] [CrossRef]
- Zhong, B.; Zhang, L.; Yu, J.; Fan, K. Ultrafine iron-cobalt nanoparticles embedded in nitrogen-doped porous carbon matrix for oxygen reduction reaction and zinc-air batteries. J. Colloid Interface Sci. 2019, 546, 113–121. [Google Scholar] [CrossRef]
- Duan, X.; Ren, S.; Pan, N.; Zhang, M.; Zheng, H. MOF-derived Fe,Co@NeC bifunctional oxygen electro-catalysts for Zn-air batteries. J. Mater. Chem. 2020, 8, 9355–9363. [Google Scholar] [CrossRef]
- Shin, S.; Yoon, H.; Yoon, Y.; Park, S.; Shin, M.W. Porosity tailoring of the Zn-MOF-5 derived carbon materials and its effects on the performance as a cathode for lithium-air batteries. Microporous Mesoporous Mater. 2021, 311, 110726. [Google Scholar] [CrossRef]
- Ge, Q.; Chen, M.; Lou, X.; Zhang, W.; Shen, M.; Yang, Q.; Hu, B. Centrifugal field guided dual templating synthesis of functional macro-microporous carbon. Part. Part. Syst. Char. 2018, 35, 1800262. [Google Scholar] [CrossRef]
- Yu, H.; Dinh, K.N.; Sun, Y.; Fan, H.; Wang, Y.; Jing, Y.; Li, S.; Srinivasan, M.; Yan, Q. Performance-improved Li-O2 batteries by tailoring the phases of MoxC porous nanorods as an efficient cathode. Nanoscale 2018, 10, 14877–14884. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; He, H. Integrated 3D foam-like porous Ni3S2 as improved deposition support for high-performance LieO2 battery. J. Power Sources 2020, 448, 227397. [Google Scholar] [CrossRef]
- Zhang, P.; Hui, X.; Wang, H.; Gao, X.; Yin, L. Porous hollow ZnCo2S4 nanosheet arrays derived from metal-organic framework as efficient cathode for lithium oxygen batteries. J. Alloys Compd. 2021, 860, 157656. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, W.Q.; Zou, M.C.; Wang, Y.S.; Chen, Y.J.; Xu, L.; Wu, H.S.; Cao, A.Y. 3D, mutually embedded MOF@carbon nanotube hybrid networks for high-performance lithium-sulfur batteries. Adv. Energy Mater. 2018, 8, 1800013. [Google Scholar] [CrossRef]
- Ji, X.; Li, Q.; Yu, H.; Hu, X.; Luo, Y.; Li, B. Three-dimensional ordered macroporous ZIF-8 nanoparticle-derived nitrogen-doped hierarchical porous carbons for high-performance lithium-sulfur batteries. RSC Adv. 2020, 10, 41983–41992. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, M.; Xi, B.; Mi, K.; Yuan, A.; Xiong, S. Systematic study of effect on enhancing specific capacity and electrochemical behaviors of lithium-sulfur batteries. Adv. Energy Mater. 2018, 8, 1701330. [Google Scholar] [CrossRef]
- Li, Z.; Yin, L. Nitrogen-doped MOF-derived micropores carbon as immobilizer for small sulfur molecules as a cathode for lithium sulfur batteries with excellent electrochemical performance. ACS Appl. Mater. Interfaces 2015, 7, 4029–4038. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, H.; Tan, X.; Guo, L.; Zhang, J.; Liu, S.; Guo, Y.; Zhang, J.; Wang, H.; Chu, W. Monoclinic ZIF-8 nanosheet-derived 2D carbon nanosheets as sulfur immobilizer for high-performance lithium sulfur batteries. ACS Appl. Mater. Interfaces 2017, 9, 25239–25249. [Google Scholar] [CrossRef]
- Hao, G.P.; Tang, C.; Zhang, E.; Zhai, P.; Yin, J.; Zhu, W.; Zhang, Q.; Kaskel, S. Thermal exfoliation of layered metal-organic frameworks into ultrahydrophilic graphene stacks and their applications in Li-S batteries. Adv. Mater. 2017, 29, 1702829. [Google Scholar] [CrossRef]
- Kim, D.; Kim, G.; Oh, S.; Park, J.; Lee, S.; Yoon, S.; Lee, J.; Lee, W.; Jeon, T.-Y.; Cho, E.; et al. Dual-doping of sulfur on mesoporous carbon as a cathode for the oxygen reduction reaction and lithium-sulfur batteries. ACS Sustain. Chem. Eng. 2020, 8, 8537–8548. [Google Scholar] [CrossRef]
- Liu, Y.; Si, L.; Zhou, X.; Liu, X.; Xu, Y.; Bao, J.; Dai, Z. A selenium-confined microporous carbon cathode for ultrastable lithium-selenium batteries. J. Mater. Chem. A 2014, 2, 17735–17739. [Google Scholar] [CrossRef]
- Li, Z.; Yin, L. MOF-derived, N-doped, hierarchically porous carbon sponges as immobilizers to confine selenium as cathodes for Li-Se batteries with superior storage capacity and perfect cycling stability. Nanoscale 2015, 7, 9597–9606. [Google Scholar] [CrossRef]
- Jin, W.-W.; Li, H.-J.; Zou, J.-Z.; Inguva, S.; Zhang, Q.; Zeng, S.-Z.; Xu, G.-Z.; Zeng, X.-R. Metal organic framework-derived carbon nanosheets with fish-scale surface morphology as cathode materials for lithium-selenium batteries. J. Alloys Compd. 2020, 820, 153084. [Google Scholar] [CrossRef]
- Yin, C.; Zhou, H.; Yang, Z.; Li, J. Synthesis and electrochemical properties of LiNi0.5Mn1.5O4 for Li-ion batteries by the metal-organic framework method. ACS Appl. Mater. Interfaces 2018, 10, 13625–13634. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Peng, Y.; Chen, W.; Niu, Y.; Wu, S.; Zhang, X.; Hu, L. V-MOF derived porous V2O5 nanoplates for high performance aqueous zinc ion battery. Appl. Surf. Sci. 2019, 493, 368–374. [Google Scholar] [CrossRef]
- Bates, A.M.; Preger, Y.; Torres-Castro, L.; Harrison, K.L.; Harris, S.J.; Hewson, J. Are solid-state batteries safer than lithium-ion batteries? Joule 2022, 6, 742–755. [Google Scholar] [CrossRef]
- Ye, L.; Li, X. A dynamic stability design strategy for lithium metal solid state batteries. Nature 2021, 593, 218–222. [Google Scholar] [CrossRef]
- Lou, S.; Zhang, F.; Fu, C.; Chen, M.; Ma, Y.; Yin, G.; Wang, J. Interface Issues and Challenges in All-Solid-State Batteries: Lithium, Sodium, and Beyond. Adv. Mater. 2021, 33, 2000721. [Google Scholar] [CrossRef]
- Hatzell, K.B.; Chen, X.C.; Cobb, C.L.; Dasgupta, N.P.; Dixit, M.B.; Marbella, L.E.; McDowell, M.T.; Mukherjee, P.P.; Verma, A.; Viswanathan, V.; et al. Challenges in Lithium Metal Anodes for Solid-State Batteries. ACS Energy Lett. 2020, 5, 922–934. [Google Scholar] [CrossRef]
- Wei, Y.; Zhou, H.; Deng, H.; Ji, W.; Tia, K.; Ma, Z.; Zhang, K.; Fu, Q. “Toolbox” for the Processing of Functional Polymer Composites. Nano Micro Lett. 2022, 14, 35. [Google Scholar] [CrossRef]
- Lin, R.; Jin, Y.; Li, Y.; Zhang, X.; Xiong, Y. Recent Advances in Ionic Liquids—MOF Hybrid Electrolytes for Solid-State Electrolyte of Lithium Battery. Batteries 2023, 9, 314. [Google Scholar] [CrossRef]
- Xu, L.; Xiao, X.; Tu, H.; Zhu, F.; Wang, J.; Liu, H.; Huang, W.; Deng, W.; Hou, H.; Liu, T.; et al. Engineering Functionalized 2D Metal-Organic Frameworks Nanosheets with Fast Li+ Conduction for Advanced Solid Li Batteries. Adv. Mater. 2023, 35, 2303193. [Google Scholar] [CrossRef]
- Liu, C.; Fu, X.; Liao, S.; Zou, G.; Yang, H. Interface Engineering Enables High-Performance Sb Anode for Sodium Storage. Nanomaterials 2023, 13, 254. [Google Scholar] [CrossRef]
- Deng, X.; Tian, Y.; Zou, K.; Chen, J.; Xiao, X.; Tao, S.; Song, Z.; Deng, W.; Hou, H.; Zou, G.; et al. KxCy phase induced expanded interlayer in ultra-thin carbon toward full potassium-ion capacitors. Carbon Energy 2022, 4, 1151. [Google Scholar] [CrossRef]
- Wu, J.-F.; Guo, X. Nanostructured Metal–Organic Framework (MOF)-Derived Solid Electrolytes Realizing Fast Lithium Ion Transportation Kinetics in Solid-State Batteries. Small 2019, 15, 1804413. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tan, R.; Wang, H.; Yang, L.; Hu, J.; Chen, H.; Pan, F. A Metal–Organic-Framework-Based Electrolyte with Nanowetted Interfaces for High-Energy-Density Solid-State Lithium Battery. Adv. Mater. 2018, 30, 1704436. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, H.; Meng, C.; Xiong, W.; Cai, Y.; Hu, P.; Pang, H.; Yuan, A. Enhancing Ion Transport: Function of Ionic Liquid Decorated MOFs in Polymer Electrolytes for All-Solid-State Lithium Batteries. ACS Appl. Energy Mater. 2020, 3, 4265–4274. [Google Scholar] [CrossRef]
- Yu, J.; Guo, T.; Wang, C.; Shen, Z.; Dong, X.; Li, S.; Zhang, H.; Lu, Z. Engineering Two-Dimensional Metal−Organic Framework on Molecular Basis for Fast Li + Conduction. Nano Lett. 2021, 21, 5805–5812. [Google Scholar] [CrossRef]
- Pettinari, C.; Tombesi, A. MOFs for electrochemical energy conversion and storage. Inorganics 2023, 11, 65. [Google Scholar] [CrossRef]
- Vielstich, W.; Lamm, A.; Gasteiger, H.A. (Eds.) Handbook of Fuel Cells; John Wiley and Sons: New York, NY, USA, 2009; ISBN 978-0-470-74151-1. [Google Scholar]
- Wong, C.Y.; Wong, W.Y.; Sudarsono, W.; Loh, K.S.; Lim, K.L.; Bo, W. Tuning the functionality of metal–organic frameworks (MOFs) for fuel cells and hydrogen storage applications. J. Mater. Sci. 2023, 58, 8637–8677. [Google Scholar] [CrossRef]
- Sudarsono, W.; Tan, S.Y.; Wong, W.Y.; Omar, F.S.; Ramya, K.; Mehmood, S.; Numan, A.; Walvekar, R.; Khalid, M. From catalyst structure design to electrode fabrication of platinum-free electrocatalysts in proton exchange membrane fuel cells: A review. J. Ind. Eng. Chem. 2023, 122, 1–26. [Google Scholar] [CrossRef]
- Chen, Z.; Kirlikovali, K.O.; Idrees, K.B.; Wasson, M.C.; Farha, O.K. Porous materials for hydrogen storage. Chem 2022, 8, 693–716. [Google Scholar] [CrossRef]
- Jiao, L.; Jiang, H.-L. Metal–organic-framework-based single-atom catalysts for energy applications. Chem 2019, 5, 786–804. [Google Scholar] [CrossRef]
- Tang, J.; Yamauchi, Y. Carbon materials: MOF morphologies in control. Nat. Chem. 2016, 8, 638–639. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.Y.; Rani, M.A.A.A.; Loh, K.S.; Lim, K.L.; Jeffery Minggu, L. Current progress on rational design of porous MOF-derived transition metal–nitrogen–carbon as oxygen reduction reaction catalysts for proton exchange membrane fuel cells. Curr. Opin. Green. Sustain. Chem. 2025, 52, 101001. [Google Scholar] [CrossRef]
- Choi, I.; Lim, J.; dos Reis, R.; Kim, E.; Lim, S.Y.; Dravid, V.P.; Kim, H.; Oh, K.-H.; Nam, K.W. Metal–organic framework for high-performance catalyst layers in proton-exchange membrane fuel cells. J. Mater. Chem. A 2023, 11, 20583. [Google Scholar] [CrossRef]
- Yin, R.; Ma, S.; Ying, J.; Lu, Z.; Niu, X.; Feng, J.; Xu, F.; Zheng, Y.; Liu, W.; Cao, X. MOF–Derived N–Doped C @ CoO/MoC Heterojunction Composite for Efficient Oxygen Reduction Reaction and Long-Life Zn–Air Battery. Batteries 2023, 9, 306. [Google Scholar] [CrossRef]
- Dhanabalan, K.; Perumalsamy, M.; Sriram, G.; Murugan, N.; Shalu; Sadhasivam, T.; Oh, T.H. Metal–Organic Framework (MOF)-Derived Catalyst for Oxygen Reduction Reaction (ORR) Applications in Fuel Cell Systems: A Review of Current Advancements and Perspectives. Energies 2023, 16, 4950. [Google Scholar] [CrossRef]
- Lu, X.F.; Fang, Y.; Luan, D.; Lou, X.W.D. Metal–organic frameworks derived functional materials for electrochemical energy storage and conversion: A mini review. Nano Lett. 2021, 21, 1555–1565. [Google Scholar] [CrossRef]
- Radwan, A.; Jin, H.; He, D.; Mu, S. Design engineering, synthesis protocols, and energy applications of MOF-derived electrocatalysts. Nano-Micro Lett. 2021, 13, 132. [Google Scholar] [CrossRef]
- Li, L.; He, J.; Wang, Y.; Lv, X.; Gu, X.; Dai, P.; Liu, D.; Zhao, X. Metal–organic frameworks: A promising platform for constructing non-noble electro-catalysts for the oxygen-reduction reaction. J. Mater. Chem. A 2019, 7, 1964–1988. [Google Scholar] [CrossRef]
- Kang, I.; Lee, S.; Choi, W.-J.; Lee, S.; So, S.; Yu, D.M.; Yoon, S.J.; Kim, D.-W.; Nam, K.W.; Oh, K.-W. Effect of metal–organic framework on hydrogen volume fraction in the oxygen-rich anode catalyst layer of proton exchange membrane water electrolyzer. Chem. Eng. J. 2025, 508, 161094. [Google Scholar] [CrossRef]
- Yu, S.; Jing, G.; Li, S.; Li, Z.; Ju, X. Tuning the hydrogen storage properties of MOF-650: A combined DFT and GCMC simulations study. Int. J. Hydrogen Energy 2020, 45, 6757–6764. [Google Scholar] [CrossRef]
- Sule, R.; Mishra, A.K.; Nkambule, T.T. Recent advancement in consolidation of MOFs as absorbents for hydrogen storage. Int. J. Energy Res. 2021, 45, 12481–12499. [Google Scholar] [CrossRef]
- Suresh, K.; Aulakh, D.; Purewal, J.; Siegel, D.J.; Veenstra, M.; Matzger, A.J. Optimizing hydrogen storage in MOFs through engineering of crystal morphology and control of crystal size. J. Am. Chem. Soc. 2021, 143, 10727–10734. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, A.; Pushpara, H. Synthesis, Characterization, and Fabrication of Nickel Metal−Organic Framework-Incorporated Polymer Electrolyte Membranes for Fuel-Cell Applications. ACS Appl. Mater. Interfaces 2024, 16, 31145–31157. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Shen, J.; Zhang, W.; Wang, Q.; Wang, J.; Deng, Y.; Wang, C. Exploring porous zeolitic imidazolate framework-8 (ZIF-8) as an efficient filler for high-performance poly(ethyleneoxide)-based solid polymer electrolytes. Nano Res. 2020, 13, 2259–2267. [Google Scholar] [CrossRef]
- Zhu, B.; Sui, Y.; Wei, P.; Wen, J.; Cao, H.; Cong, C.; Meng, X.; Zhou, Q. NH2-UiO-66 coated fibers to balance the excellent proton conduction efficiency and significant dimensional stability of proton exchange membrane. J. Membr. Sci. 2021, 628, 119214. [Google Scholar] [CrossRef]
- Zhao, G.; Xu, X.; Zhao, H.; Shi, L.; Zhuang, X.; Cheng, B.; Yin, Y. Zeolitic imidazolate framework decorated on 3D nanofiber network towards superior proton conduction for proton exchange membrane. J. Membr. Sci. 2020, 601, 117914. [Google Scholar] [CrossRef]
- Pathak, A.; Watanabe, H.; Manna, B.; Hatakeyama, K.; Ida, S. Hydrogen-Bonded Metal–Organic Framework Nanosheet as a Proton Conducting Membrane for an H2/O2 Fuel Cell. Small 2024, 20, 2400222. [Google Scholar] [CrossRef]
- Li, Q.; Gao, W.; Zhang, N.; Gao, X.; Wu, D.; Che, Q. Preparation of high temperature proton exchange membranes with multilayered structures through alternate deposition of carbon dots@Metal organic framework and Sulfonated Poly(Ether Ketone). J. Membr. Sci. 2025, 713, 123306. [Google Scholar] [CrossRef]
- Divya, K.; Liu, Q.; Murali, R.; Asghar, M.R.; Liu, H.; Zhang, W.; Xu, Q.; Ren, J.; Su, H. Integration of phosphorylated MOF/COF core-shell structures into hybrid membrane for high-temperature fuel cell application. Appl. Surf. Sci. 2025, 681, 161544. [Google Scholar] [CrossRef]
- Ye, Y.; Gong, L.; Xiang, S.; Zhang, Z.; Chen, B. Metal-Organic Frameworks as a Versatile Platform for Proton Conductors. Adv. Mater. 2020, 32, e1907090. [Google Scholar] [CrossRef]
- Du, J.; Yu, G.; Lin, H.; Jie, P.; Zhang, F.; Qu, F.; Wen, C.; Feng, L.; Liang, X. Enhanced proton conductivity of metal organic framework at low humidity by improvement in water retention. J. Colloid. Interface Sci. 2020, 573, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Ojha, R.; Pradhan, D. The potential of microbial fuel cell for converting waste to energy: An overview. Sustain. Chem. Environ. 2025, 9, 100196. [Google Scholar] [CrossRef]
- Tremouli, A.; Kamperidis, T.; Pandis, P.K.; Argirusis, C.; Lyberatos, G. Exploitation of Digestate from Thermophilic and Mesophilic Anaerobic Digesters Fed with Fermentable Food Waste Using the MFC Technology. Waste Biomass Valorization 2021, 12, 5361–5370. [Google Scholar] [CrossRef]
- Kamperidis, T.; Pandis, P.K.; Argirusis, C.; Lyberatos, G.; Tremouli, A. Effect of Food Waste Condensate Concentration on the Performance of Microbial Fuel Cells with Different Cathode Assemblies. Sustainability 2022, 14, 2625. [Google Scholar] [CrossRef]
- Logan, B.E. Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Microbiol. 2009, 7, 375–381. [Google Scholar] [CrossRef]
- Jiang, N.; Su, C.; Li, J.; Zhang, Y.; Wang, B.; Jiang, B.; Gao, W. Mesoporous silica-modified metal organic frameworks derived bimetallic electrocatalysts for oxygen reduction reaction in microbial fuel cells. Int. J. Hydrogen Energy 2025, 97, 571–580. [Google Scholar] [CrossRef]
- Yoon, H.S.; Park, H.-Y.; Jung, W.S. Synergistic effects of fluorine and nitrogen dopants in fluorine/nitrogen-coordinated iron-co-doped carbon catalysts for enhanced oxygen reduction in alkaline media. Int. J. Hydrogen Energy 2024, 60, 1092–1100. [Google Scholar] [CrossRef]
- Chu, C.; Liu, J.; Wei, L.; Feng, J.; Li, H.; Shen, J. Iron carbide and iron phosphide embedded N-doped porous carbon derived from biomass as oxygen reduction reaction catalyst for microbial fuel cell. Int. J. Hydrogen Energy 2023, 48, 4492–4502. [Google Scholar] [CrossRef]
- Koo, B.; Jung, S.P. Improvement of air cathode performance in microbial fuel cells by using catalysts made by binding metal-organic framework and activated carbon through ultrasonication and solution precipitation. Chem. Eng. J. 2021, 424, 130388. [Google Scholar] [CrossRef]
- Koo, B.; Lee, S.-M.; Oh, S.-E.; Kim, E.J.; Hwang, Y.; Seo, D.; Kim, J.Y.; Kahng, Y.H.; Lee, Y.W.; Chung, S.-Y.; et al. Addition of reduced graphene oxide to an activated-carbon cathode increases electrical power generation of a microbial fuel cell by enhancing cathodic performance. Electrochim. Acta 2018, 297, 613–622. [Google Scholar] [CrossRef]
- Santoro, C.; Kodali, M.; Kabir, S.; Soavi, F.; Serov, A.; Atanassov, P. Three-dimensional graphene nanosheets as cathode catalysts in standard and supercapacitive microbial fuel cell. J. Power Sources 2017, 356, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Choi, S.-J.; Kim, H.J. Enhanced Proton Conductivity of a Zn(II)-Based MOF/Aquivion Composite Membrane for PEMFC Applications. Energy Fuels 2020, 34, 10067–10077. [Google Scholar] [CrossRef]
- Cao, J.; Su, C.; Ji, Y.; Yang, G.; Shao, Z. Recent advances and perspectives of fluorite and perovskite-based dual-ion conducting solid oxide fuel cells. J. Energy Chem. 2021, 57, 406–427. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Shao, Z.; Xu, W.; Wu, Q.; Ding, X.; Hou, H. Proton conduction of Nafion hybrid membranes promoted by NH3-modified Zn-MOF with host-guest collaborative hydrogen bonds for H2/O2 fuel cell applications. ACS Appl. Mater. Interfaces 2021, 13, 7485–7497. [Google Scholar] [CrossRef]
- Yoo, D.K.; Lee, G.; Mondol, M.H.; Lee, H.J.; Kim, C.M.; Jhung, S.H. Preparation and applications of metal–organic frameworks composed of sulfonic acid. Coord. Chem. Rev. 2023, 474, 214868. [Google Scholar] [CrossRef]
- Li, X.-M.; Gao, J. Recent advances of metal–organic frameworks-based proton exchange membranes in fuel cell applications. SusMat. 2022, 2, 504–534. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Das, A.; Jana, T.; Das, S.K. Fabricating a MOF Material with Polybenzimidazole into an Efficient Proton Exchange Membrane. ACS Appl. Energy Mater. 2020, 3, 7964–7977. [Google Scholar] [CrossRef]
- Li, D.; Zhang, L.; Kuang, L.; Qin, H.; Hu, X.; He, J.; Ni, H.; He, Y. Carbothermal shock synthesis of CoO/N/C nanoparticles with superior durability for oxygen reduction reaction. J. Power Sources 2023, 587, 233699. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, Y.; Yan, Y.; Larissa, T.Y.P.; Zhang, X.; Wuu, D.; Zhang, H.; Yang, Y.; Wang, X. Core-shell carbon materials derived from metal-organic frameworks as an efficient oxygen bifunctional electrocatalyst. Nano Energy 2016, 30, 368–378. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Li, D.; Qin, H.; Ni, H.; Chi, H.; He, J.; He, Y. Metal-organic framework derived Co@N/C with enhanced oxygen reduction reaction in direct borohydride fuel cells. Chem. Eng. Sci. 2025, 303, 120953. [Google Scholar] [CrossRef]
- Parsa, S.M.; Chen, Z.; Feng, S.; Yang, Y.; Luo, L.; Ngo, H.H.; Wei, W.; Ni, B.-J.; Guo, W. Metal-free nitrogen-doped carbon-based electrocatalysts for oxygen reduction reaction in microbial fuel cells: Advances, challenges, and future directions. Nano Energy 2025, 134, 110537. [Google Scholar] [CrossRef]
- Li, C.; Song, Z.; Liu, H. Octahedral copper metal-organic framework grafted with MoS2 nanoflowers for high-performance oxygen reduction reaction. J. Environ. Chem. Eng. 2025, 13, 115856. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, G.; Lan, F.; Liu, Y.; Wang, R.; Yang, Y.; Chen, J. Boosting oxygen reduction in microbial fuel cells with zeolitic imidazolate frameworks-coated molybdenum disulfide as cathode catalyst. J. Power Sources 2025, 632, 236386. [Google Scholar] [CrossRef]
- Zhu, L.; Han, T.; Ding, Y.; Long, J.; Lin, X.; Liu, J. A Metal-Organic-Framework Derived NiFe2O4@NiCo-LDH Nanocube as High-Performance Lithium-Ion Battery Anode Under Different Temperatures. Appl. Surf. Sci. 2022, 599, 153953. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, H.; Zhu, M.; Yuan, A.; Shen, X. Metal-organic framework-derived Co3O4 covered by MoS2 nanosheets for high-performance lithium-ion batteries. J. Alloys Compd. 2018, 744, 220–227. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, M.; Liu, Z.; Lin, C.; Zeng, Y. Morella-rubra-like metal–organic-framework-derived multilayered Co3O4/NiO/C hybrids as high-performance anodes for lithium storage. J. Mater. Chem. A 2017, 5, 24269–24274. [Google Scholar] [CrossRef]
- Peng, H.-J.; Hao, G.-X.; Chu, Z.-H.; Lin, Y.-W.; Lin, X.-M.; Cai, Y.-P. Porous carbon with large surface area derived from a metal–organic framework as a lithium-ion battery anode material. RSC Adv. 2017, 7, 34104–34109. [Google Scholar] [CrossRef]
- Sun, C.; Yang, J.; Rui, X.; Zhang, W.; Yan, Q.; Chen, P.; Huo, F.; Huang, W.; Dong, X. MOF-directed templating synthesis of a porous multicomponent dodecahedron with hollow interiors for enhanced lithium-ion battery anodes. J. Mater. Chem. A 2015, 3, 8483–8488. [Google Scholar] [CrossRef]
- Leng, W.; Cui, L.; Liu, Y.; Gong, Y. MOF-derived MnV2O4/C microparticles with graphene coating anchored on graphite sheets: Oxygen defect engaged high perfromance aqueous Zn-ion battery. Adv. Mater. Interfaces 2022, 9, 2101705. [Google Scholar] [CrossRef]
- Zhang, X.; Zi, W.; Zhou, J.; Zhou, L.; Zhang, J.; Xuan, X. Li-Mn Bimetallic Metal-Organic Framework and Its Derivative as a Cathode for Lithium-Ion Batteries. Inorg. Chem. 2023, 62, 9501–9507. [Google Scholar] [CrossRef]
- Li, H.; Dong, W.; Li, C.; Wang, Y.; Sun, M.-H.; Barakat, T.; Li, Y.; Su, B.-L. Boosting Reaction Kinetics and Shuttle Effect Suppression by Single Crystal MOF-Derived N-Doped Ordered Hierarchically Porous Carbon for High Performance Li-Se Battery. Sci. China Mater. 2022, 65, 2975–2988. [Google Scholar] [CrossRef]
- Shen, C.; Li, Y.; Gong, M. Ultrathin Cobalt Phthalocyanine@Graphene Oxide Layer-Modified Separator for Stable Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2021, 13, 60046–60053. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Du, X.; Wu, F.; Wu, L.; Li, J.; Liu, G. Conductive Al-Doped Zno Framework Embedded With Catalytic Nanocages as a Multistage-Porous Sulfur Host in Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2021, 13, 44389–44400. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, C.; Ren, J.; Zhang, K.; Wang, L. Synergistic Effect of Organic-Inorganic Hybrid Electrolyte for Ultra-Long Zn–I2 Batteries. Int. J. Hydrogen Energy 2023, 48, 21985–21995. [Google Scholar] [CrossRef]
- Ou, G.; Chen, J.; Lu, M.; Liu, J.; Zhang, X.; Lin, X.; Wu, Y.; Zeb, A.; Reddy, R.C.K.; Xu, Z. A Metal-Organic Framework Approach to Engineer ZnO/Co3ZnC/N-doped Carbon Composite as Anode Material for Boosting Lithium Storage. J. Alloys Compd. 2022, 923, 166436. [Google Scholar] [CrossRef]
- Hou, J.; Guo, Y.; Zhang, Y.; Li, J.; Xu, Y.; Fang, Z.; Yang, J.; Wu, M. Facile Green and Sustainable Synthesis of MnO@rGO as Electrochemically Stable Anode for Lithium-Ion Batteries. Mater. Lett. 2022, 325, 132761. [Google Scholar] [CrossRef]
- Chu, K.; Li, Z.; Xu, S.; Yao, G.; Xu, Y.; Niu, P.; Zheng, F. MOF-Derived Hollow NiCo2O4 Nanowires as Stable Li-Ion Battery Anodes. Dalton Trans. 2020, 49, 10808–10815. [Google Scholar] [CrossRef]
- Zhai, S.; Liu, W.; Hu, Y.; Chen, Z.; Xu, S.; Wu, L.; Ye, Z.; Wang, X.; Mei, T. Kinetic Acceleration of Lithium Polysulfide Conversion via a Copper-Iridium Alloying Catalytic Strategy in Li-S Batteries. ACS Appl. Mater. Interfaces 2022, 14, 50932–50946. [Google Scholar] [CrossRef]
- Zhou, F.; Qiao, Z.; Zhang, Y.; Xu, W.; Zheng, H.; Xie, Q.; Luo, Q.; Wang, L.; Qu, B.; Peng, D.-L. Bimetallic MOF-Derived CNTs- Grafted Carbon Nanocages as Sulfur Host for High-Performance Lithium–Sulfur Batteries. Electrochim. Acta 2020, 349, 136378. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Wang, F.; Qin, Z.; Zhang, Y.; Zhang, N.; Liu, X.; Chen, G. Anionic Metal-Organic Framework Modified Separator Boosting Efficient Li-Ion Transport. Chem. Eng. J. 2023, 451, 138536. [Google Scholar] [CrossRef]
- Zuo, L.; Ma, Q.; Xiao, P.; Guo, Q.; Xie, W.; Lu, D.; Yun, X.; Zheng, C.; Chen, Y. Upgrading the Separators Integrated with Desolvation and Selective Deposition Toward the Stable Lithium Metal Batteries. Adv. Mater. 2024, 36, 2311529. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tian, Y.; Ge, P.; Zhao, G.; Pu, T.; Yang, Y.; Zou, G.; Hou, H.; Huang, L.; Ji, X. Perovskite ABO3-Type MOF-Derived Carbon Decorated Fe3O4 with Enhanced Lithium Storage Performance. ChemElectroChem 2018, 5, 3426–3436. [Google Scholar] [CrossRef]
- Li, A.; Zhong, M.; Shuang, W.; Wang, C.; Liu, J.; Chang, Z.; Bu, X.-H. Facile synthesis of Co3O4 nanosheets from MOF nanoplates for high performance anodes of lithium-ion batteries. Inorg. Chem. Front. 2018, 5, 1602–1608. [Google Scholar] [CrossRef]
- Zheng, F.; Zhu, D.; Shia, X.; Chen, Q. Metal–organic framework-derived porous Mn1.8Fe1.2O4 nanocubes with an interconnected channel structure as high-performance anodes for lithium ion batteries. J. Mater. Chem. A 2015, 3, 2815–2824. [Google Scholar] [CrossRef]
- Yan, Z.; Sun, Z.; Li, A.; Liu, H.; Guo, Z.; Zhao, L.; Feng, J.; Qian, L. Vacancy and architecture engineering of porous FeP nanorods for achieving superior Li+ storage. Chem. Eng. J. 2022, 429, 132249. [Google Scholar] [CrossRef]
- Cao, L.; Lv, F.; Liu, Y.; Wang, W.; Huo, Y.; Fu, X.; Sun, R.; Lu, Z. A high performance O2 selective membrane based on CAU-L-NH2@ polydopamine and the pmma polymer for Li–air batteries. Chem. Commun. 2015, 51, 4364–4367. [Google Scholar] [CrossRef]
- Ding, J.; Du, T.; Thomsen, E.H.; Andresen, D.; Fischer, M.R.; Moller, A.K.; Petersen, A.R.; Pedersen, A.K.; Jensen, L.R.; Wang, S.; et al. Metal-organic framework glass as a functional filler enables enhanced performance of solid-state polymer electrolytes for lithium metal batteries. Adv. Sci. 2024, 11, e2306698. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, H.; Li, H.; Zhao, Z.; Wang, K.; Zhou, Y.; Zhao, X.; Dubal, D.P. CxNy-based materials as gas sensors: Structure, performance, mechanism and perspective. Coord. Chem. Rev. 2024, 503, 215653. [Google Scholar] [CrossRef]
- Wang, G.; Hu, Y.; Yan, H.; Ren, X.; Yang, L.; Huang, Z.; Wu, X. Effects of F substitution on crystal structure, band characteristics and microwave dielectric properties of Li3Mg2NbO6 ceramics for high frequency applications. Ceram. Int. 2024, 50, 17097–17105. [Google Scholar] [CrossRef]
- Cheng, X.; Pan, Q.; Tan, H.; Chen, K.; Liu, W.; Shi, Y.; Dua, S.; Zhu, B. The construction of an efficient magnesium–lithium separation thin film composite membrane with dual aqueous-phase monomers (PIP and MPD). RSC Adv. 2023, 13, 22113–22121. [Google Scholar] [CrossRef]
- Wu, W.; Xia, R.; Qian, G.; Liu, Z.; Razavi, N.; Berto, F.; Gao, H. Mechanostructures: Rational mechanical design, fabrication, performance evaluation, and industrial application of advanced structures. Prog. Mater. Sci. 2023, 131, 101021. [Google Scholar] [CrossRef]
- Saapi, S.S.Y.; Andrianisa, H.A.; Zorom, M.; Mounirou, L.A.; Kouassi, H.A.A.; Ahossouhe, M.S. New developments on vermifiltration as a bio-ecological wastewater treatment technology: Mechanism, application, performance, modelling, optimization, and sustainability. Heliyon 2024, 10, e25795. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Yuan, J.; Sundén, B. Review on modeling development for multiscale chemical reactions coupled transport phenomena in solid oxide fuel cells. Appl. Energy 2010, 87, 1461–1476. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, Y.; Huang, H.; Lu, J. Recent advances in shape–memory polymers: Structure, mechanism, functionality, modeling and applications. Prog. Polym. Sci. 2012, 37, 1720–1763. [Google Scholar] [CrossRef]
- Le, T.; Epa, V.C.; Burden, F.R.; Winkler, D.A. Quantitative Structure Property Relationship Modeling of Diverse Materials Properties. Chem. Rev. 2012, 112, 2889–2919. [Google Scholar] [CrossRef]
- Ji, C.; Dai, J.; Zhai, C.; Wang, J.; Tian, Y.; Sun, W. A Review on Lithium-Ion Battery Modeling from Mechanism-Based and Data-Driven Perspectives. Processes 2024, 12, 1871. [Google Scholar] [CrossRef]




| MOFs | Derivatives/Devices | Discharge Capacity [mAh g−1 @ mA g−1 or @ C] | Cycle Performance [mAh g−1 @ Cycles] | Ref. |
|---|---|---|---|---|
| Ni/Co-MOF | NiFe2O@NiCo-LDH | 891.2 @ 500 | 636.9 @ 100 | [229] |
| Ni-Co-BTC MOF | NixCo3−xO4 | 1619.2 @ 1000 | 832 @ 673 | [230] |
| Zn-Co PBA | Co3O4/NiO-C | 864 @ 1000 | 776 @ 1000 | [231] |
| ZIF-67, red P | CoxP-NC-800 | 2450 @ 1000 | 550 @ 100 | [232] |
| MOF-74 | MnCo-MOF | 11,150 @ 200 | 1000 @ 44 | [133] |
| ZIF-67 | NiCo2O4/NiO | 1535 @ 200 | 1492 @ 100 | [233] |
| [Mn(phen)H2O] [V2O6] | MnV2O4/C | 400 @ 100 | 377 @ 1000 | [234] |
| MIL-125(Ti) | LiNi0.5Co0.2Mn0.3O2 | 186.7 @ 1000 | 145.3 @ 100 | [235] |
| ZIF-8 | S@S-NOHPC | 927.7 @ 200 | 367 @ 200 | [236] |
| CoPC | CoPC@GO | 938 @ 1 C | 919 @ 250 | [237] |
| ZIF-8 | Co-NC@AZO/S | 1206.2 @ 200 | 950.03 @ 500 | [238] |
| ZIF-8@ZIF-67 | ZnSe@CoSe@CN | 2739 @ 100 | 1500 @ 100 | [239] |
| Zn-Co PBA | ZnO/Co3ZnC | 1163.9 @ 200 | 1162.4 @ 300 | [240] |
| Mn-MOF | MnO@rGO | 1412.2 @ 2000 | 920 @ 160 | [241] |
| Ni-Co NTA | NiCo2O4 | 3158 @ 100 | 1310 @ 100 | [242] |
| ZIF-8 | S@CuIr/NC | 1288 @ 0.2 C | 689 @ 500 | [243] |
| ZIF-8 | CNT-NC@GC/S | 1498 @ 0.1 C | 743 @ 500 | [244] |
| UiOSOL | LiFePO4/Li metal | 161 @ 0.1 C | 155 @ 600 | [245] |
| MOF-801 | NCM811/Li metal | 179 @ 0.5 C | 113 @ 400 | [246] |
| [NH2 (CH3)2] [FeIII FeII(HCOO)6] | Fe3O4@C | 1714 @ 100 | 1041 @ 50 | [247] |
| Co-BDC nanoplates | Co3O4 nanosheets | 961 @ 1000 | 775 @ 200 | [248] |
| Fe-Ni-BDC MOF | NixFe3−xO4 nanotubes | 1466 @ 250 | 1184 @ 200 | [249] |
| Fe-NTA | V-FeP | 1228.3 @ 100 | 590.7 @1000 | [250] |
| CAU-1-NH2 | CAU-1-NH2-PMMA | 1480 @ 200 | 450 @ 66 | [251] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argirusis, C.; Alizadeh, N.; Katsanou, M.-Ε.; Argirusis, N.; Sourkouni, G. Advances in Metal-Organic Frameworks (MOFs) for Rechargeable Batteries and Fuel Cells. Batteries 2025, 11, 192. https://doi.org/10.3390/batteries11050192
Argirusis C, Alizadeh N, Katsanou M-Ε, Argirusis N, Sourkouni G. Advances in Metal-Organic Frameworks (MOFs) for Rechargeable Batteries and Fuel Cells. Batteries. 2025; 11(5):192. https://doi.org/10.3390/batteries11050192
Chicago/Turabian StyleArgirusis, Christos, Niyaz Alizadeh, Maria-Εleni Katsanou, Nikolaos Argirusis, and Georgia Sourkouni. 2025. "Advances in Metal-Organic Frameworks (MOFs) for Rechargeable Batteries and Fuel Cells" Batteries 11, no. 5: 192. https://doi.org/10.3390/batteries11050192
APA StyleArgirusis, C., Alizadeh, N., Katsanou, M.-Ε., Argirusis, N., & Sourkouni, G. (2025). Advances in Metal-Organic Frameworks (MOFs) for Rechargeable Batteries and Fuel Cells. Batteries, 11(5), 192. https://doi.org/10.3390/batteries11050192









