One Stone, Three Birds: Innovations and Challenges of Layered Double Hydroxides in Batteries, Supercapacitors, and Hydrogen Production
Abstract
1. Introduction
2. Fundamentals of LDHs
3. Synthesis Methods of LDHs
3.1. Co-Precipitation
3.2. Hydrothermal Synthesis
3.3. Electrochemical Deposition
3.4. Other Advanced Synthesis Techniques (e.g., Sol-Gel, Ultrasonication, Exfoliation)
3.5. Impact of Synthesis Methods on LDH Properties
4. LDHs in Batteries
4.1. Lithium-Ion Batteries
4.2. Lithium–Sulfur Batteries
4.3. Sodium-Ion Batteries
4.4. Chloride-Ion Batteries
4.5. Zinc-Ion Batteries
4.6. Zinc–Air Batteries
5. LDHs in Supercapacitors
6. LDHs in Electrochemical Hydrogen Production
6.1. Fundamentals of Electrochemical Hydrogen Production
6.2. Mechanistic Role of LDHs Catalysts in Electrochemical HER
6.3. Challenges in Catalytic Activity, Stability, and Practical Deployment
7. Conclusions and Future Prospects
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, S.; Zhuo, Y.; Wang, X.; Li, S.; Lu, J.; Liu, D.; Pan, H.; Wang, Z. Advances of Layered Double Hydroxide Electrocatalysts for High-Current-Density Alkaline Water/Seawater Splitting. Coord. Chem. Rev. 2024, 510, 215832. [Google Scholar] [CrossRef]
- Long, X.; Wang, Z.; Xiao, S.; An, Y.; Yang, S. Transition Metal Based Layered Double Hydroxides Tailored for Energy Conversion and Storage. Mater. Today 2016, 19, 213–226. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, L. Layered Double Hydroxides as Electrode Materials for Flexible Energy Storage Devices. J. Semicond. 2023, 44, 041601. [Google Scholar] [CrossRef]
- Yu, W.; Deng, N.; Cheng, K.; Yan, J.; Cheng, B.; Kang, W. Advances in Preparation Methods and Mechanism Analysis of Layered Double Hydroxide for Lithium-Ion Batteries and Lithium-Sulfur Batteries. J. Energy Chem. 2021, 58, 472–499. [Google Scholar] [CrossRef]
- Sahoo, D.P.; Das, K.K.; Mansingh, S.; Sultana, S.; Parida, K. Recent Progress in First Row Transition Metal Layered Double Hydroxide (LDH) Based Electrocatalysts towards Water Splitting: A Review with Insights on Synthesis. Coord. Chem. Rev. 2022, 469, 214666. [Google Scholar] [CrossRef]
- Hu, X.; Zheng, W.; Wu, M.; He, Q.; Zhan, F.; Chen, L. Ternary Layered Double Hydroxide Cathode Materials for Electrochemical Energy Storage: A Review and Perspective. Sustain. Energy Fuels 2022, 6, 4551–4581. [Google Scholar] [CrossRef]
- Zhou, D.; Li, P.; Lin, X.; McKinley, A.; Kuang, Y.; Liu, W.; Lin, W.F.; Sun, X.; Duan, X. Layered Double Hydroxide-Based Electrocatalysts for the Oxygen Evolution Reaction: Identification and Tailoring of Active Sites, and Superaerophobic Nanoarray Electrode Assembly. Chem. Soc. Rev. 2021, 50, 8790–8817. [Google Scholar] [CrossRef]
- Jing, C.; Tao, S.; Fu, B.; Yao, L.; Ling, F.; Hu, X.; Zhang, Y. Layered Double Hydroxide-Based Nanomaterials for Supercapacitors and Batteries: Strategies and Mechanisms. Prog. Mater. Sci. 2024, 150, 101410. [Google Scholar] [CrossRef]
- Zhang, D.; Guo, X.; Tong, X.; Chen, Y.; Duan, M.; Shi, J.; Jiang, C.; Hu, L.; Kong, Q.; Zhang, J. High-Performance Battery-Type Supercapacitor Based on Porous Biocarbon and Biocarbon Supported Ni–Co Layered Double Hydroxide. J. Alloys Compd. 2020, 837, 155529. [Google Scholar] [CrossRef]
- Antil, B.; Olhan, S.; Vander Wal, R.L. Production of Graphitic Carbon from Renewable Lignocellulosic Biomass Source. Minerals 2025, 15, 262. [Google Scholar] [CrossRef]
- Chaudhuri, H.; Yun, Y.S. A Critical Review on the Properties and Energy Storage Applications of Graphene Oxide/Layered Double Hydroxides and Graphene Oxide/MXenes. J. Power Sources 2023, 564, 232870. [Google Scholar] [CrossRef]
- Tomboc, G.M.; Kim, J.; Wang, Y.; Son, Y.; Li, J.; Kim, J.Y.; Lee, K. Hybrid Layered Double Hydroxides as Multifunctional Nanomaterials for Overall Water Splitting and Supercapacitor Applications. J. Mater. Chem. A Mater. 2021, 9, 4528–4557. [Google Scholar] [CrossRef]
- Fan, K.; Xu, P.; Li, Z.; Shao, M.; Duan, X. Layered Double Hydroxides: Next Promising Materials for Energy Storage and Conversion. Next Mater. 2023, 1, 100040. [Google Scholar] [CrossRef]
- Sherryna, A.; Tahir, M. Recent Developments in Layered Double Hydroxide Structures with Their Role in Promoting Photocatalytic Hydrogen Production: A Comprehensive Review. Int. J. Energy Res. 2022, 46, 2093–2140. [Google Scholar] [CrossRef]
- Mohanty, U.A.; Sahoo, D.P.; Paramanik, L.; Parida, K. A Critical Review on Layered Double Hydroxide (LDH)-Derived Functional Nanomaterials as Potential and Sustainable Photocatalysts. Sustain. Energy Fuels 2023, 7, 1145–1186. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, P.; Liang, J.; Xia, X.; Ren, L.; Song, L.; Liu, W.; Sun, X. Unlocking Layered Double Hydroxide as a High-Performance Cathode Material for Aqueous Zinc-Ion Batteries. Adv. Mater. 2022, 34, 2204320. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.A.; Shah, S.S.; Mahnashi, Y.A.; Mahfoz, W.; Alzahrani, A.S.; Hakeem, A.S.; Shaikh, M.N. A High-Energy Asymmetric Supercapacitor Based on Tomato-Leaf-Derived Hierarchical Porous Activated Carbon and Electrochemically Deposited Polyaniline Electrodes for Battery-Free Heart-Pulse-Rate Monitoring. Small 2023, 19, 2300258. [Google Scholar] [CrossRef]
- Jing, C.; Dong, B.; Zhang, Y. Chemical Modifications of Layered Double Hydroxides in the Supercapacitor. Energy Environ. Mater. 2020, 3, 346–379. [Google Scholar] [CrossRef]
- Antil, B.; Elkasabi, Y.; Strahan, G.D.; Vander Wal, R.L. Development of Graphitic and Non-Graphitic Carbons Using Different Grade Biopitch Sources. Carbon 2025, 232, 119770. [Google Scholar] [CrossRef]
- Yuan, Z.; Bak, S.M.; Li, P.; Jia, Y.; Zheng, L.; Zhou, Y.; Bai, L.; Hu, E.; Yang, X.Q.; Cai, Z.; et al. Activating Layered Double Hydroxide with Multivacancies by Memory Effect for Energy-Efficient Hydrogen Production at Neutral pH. ACS Energy Lett. 2019, 4, 1412–1418. [Google Scholar] [CrossRef]
- Khorshidi, M.; Asadpour, S.; Sarmast, N.; Dinari, M. A Review of the Synthesis Methods, Properties, and Applications of Layered Double Hydroxides/Carbon Nanocomposites. J. Mol. Liq. 2022, 348, 118399. [Google Scholar] [CrossRef]
- Riaz, S.; Rehman, A.; Akhter, Z.; Najam, T.; Hossain, I.; Karim, M.R.; Assiri, M.A.; Shah, S.S.A.; Nazir, M.A. Recent Advancement in Synthesis and Applications of Layered Double Hydroxides (LDHs) Composites. Mater. Today Sustain. 2024, 27, 100897. [Google Scholar] [CrossRef]
- Jijoe, P.S.; Yashas, S.R.; Shivaraju, H.P. Fundamentals, Synthesis, Characterization and Environmental Applications of Layered Double Hydroxides: A Review. Environ. Chem. Lett. 2021, 19, 2643–2661. [Google Scholar] [CrossRef]
- Ali Khan, A.; Tahir, M.; Khan, N. Recent Developments in Titanium Carbide (Ti3C2)-Based Layered Double Hydroxide (LDH) Nanocomposites for Energy Storage and Conversion Applications: A Minireview and Perspectives. Energy Fuels 2022, 36, 9821–9843. [Google Scholar] [CrossRef]
- Parsapour, F.; Moradi, M.; Bahadoran, A. Metal-Organic Frameworks-Derived Layered Double Hydroxides: From Controllable Synthesis to Various Electrochemical Energy Storage/Conversion Applications. Adv. Colloid. Interface Sci. 2023, 313, 102865. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, J.; Xie, L.; Wu, J.; Ren, Q.; Wang, Y.; Li, G. Surface-Adsorbed Carboxylate Ligands on Layered Double Hydroxides/Metal–Organic Frameworks Promote the Electrocatalytic Oxygen Evolution Reaction. Angew. Chem. 2021, 133, 18277–18285. [Google Scholar] [CrossRef]
- Bukhtiyarova, M.V. A Review on Effect of Synthesis Conditions on the Formation of Layered Double Hydroxides. J. Solid. State Chem. 2019, 269, 494–506. [Google Scholar] [CrossRef]
- Wang, Q.; Ohare, D. Recent Advances in the Synthesis and Application of Layered Double Hydroxide (LDH) Nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef]
- Li, F.; Zhang, L.; Evans, D.G.; Duan, X. Structure and Surface Chemistry of Manganese-Doped Copper-Based Mixed Metal Oxides Derived from Layered Double Hydroxides. Colloids Surf. A Physicochem. Eng. Asp. 2004, 244, 169–177. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Zhou, Z.; Deng, L.; Liu, L.; Xu, M. Surface Defects Introduced by Metal Doping into Layered Double Hydroxide for CO2 Photoreduction: The Effect of Metal Species in Light Absorption, Charge Transfer and CO2 Reduction. Chem. Eng. J. 2022, 442, 136148. [Google Scholar] [CrossRef]
- Forano, C. Environmental Remediation Involving Layered Double Hydroxides. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2004; Volume 1, pp. 425–458. [Google Scholar]
- Perera-Solis, D.D.; Pimlott, M.; Fidment, E.; Whiting, A.; Greenwell, H.C. Adding Value to Waste Minerals in a Circular Economy Framework: Ochre-Derived Layered Double Hydroxide Catalysts in Fatty Acid Ketonisation. Minerals 2019, 9, 681. [Google Scholar] [CrossRef]
- Roy Chowdhury, P.; Medhi, H.; Bhattacharyya, K.G.; Mustansar Hussain, C. Layered Double Hydroxides Derived from Waste for Highly Efficient Electrocatalytic Water Splitting: Challenges and Implications towards Circular Economy Driven Green Energy. Coord. Chem. Rev. 2024, 501, 215547. [Google Scholar] [CrossRef]
- Chowdhury, P.R.; Medhi, H.; Bhattacharyya, K.G.; Hussain, C.M. Recent Developments in Ni-Based Layered Double Hydroxides Extracted from Waste for Oxygen Evolution Reactions: A Review on the Pursuit for Sustainable Green Hydrogen. Sustain. Energy Fuels 2025, 9, 1447–1463. [Google Scholar] [CrossRef]
- Jerome, M.P.; Alahmad, F.A.; Salem, M.T.; Tahir, M. Layered Double Hydroxide (LDH) Nanomaterials with Engineering Aspects for Photocatalytic CO2 Conversion to Energy Efficient Fuels: Fundamentals, Recent Advances, and Challenges. J. Environ. Chem. Eng. 2022, 10, 108151. [Google Scholar] [CrossRef]
- Singha Roy, A.; Kesavan Pillai, S.; Ray, S.S. Layered Double Hydroxides for Sustainable Agriculture and Environment: An Overview. ACS Omega 2022, 7, 20428–20440. [Google Scholar] [CrossRef]
- Zubair, M.; Daud, M.; McKay, G.; Shehzad, F.; Al-Harthi, M.A. Recent Progress in Layered Double Hydroxides (LDH)-Containing Hybrids as Adsorbents for Water Remediation. Appl. Clay Sci. 2017, 143, 279–292. [Google Scholar] [CrossRef]
- Li, L.; Soyhan, I.; Warszawik, E.; van Rijn, P. Layered Double Hydroxides: Recent Progress and Promising Perspectives Toward Biomedical Applications. Adv. Sci. 2024, 11, 2306035. [Google Scholar] [CrossRef]
- Nemček, L.; Hagarová, I.; Matúš, P. Layered Double Hydroxides as Next-Generation Adsorbents for the Removal of Selenium from Water. Appl. Sci. 2024, 14, 8513. [Google Scholar] [CrossRef]
- Altalhi, A.A.; Mohamed, E.A.; Negm, N.A. Recent Advances in Layered Double Hydroxide (LDH)-Based Materials: Fabrication, Modification Strategies, Characterization, Promising Environmental Catalytic Applications, and Prospective Aspects. Energy Adv. 2024, 3, 2136–2151. [Google Scholar] [CrossRef]
- Li, C.; Jing, H.; Wu, Z.; Jiang, D. Layered Double Hydroxides for Photo(Electro)Catalytic Applications: A Mini Review. Nanomaterials 2022, 12, 3525. [Google Scholar] [CrossRef]
- Gao, J.; Jin, B.; Shao, M. Layered Double Hydroxides Functionalization toward Rechargeable Batteries. Particuology 2024, 91, 138–154. [Google Scholar] [CrossRef]
- Lu, L.; Zheng, Y.; Yang, R.; Kakimov, A.; Li, X. Recent Advances of Layered Double Hydroxides–Based Bifunctional Electrocatalysts for ORR and OER. Mater. Today Chem. 2021, 21, 100488. [Google Scholar] [CrossRef]
- Gaur, A.; Sharma, J.; Lim, D.; Lee, H.I.; Han, H. Recent Advances in Electronic Structure Modifications of Layered Double Hydroxide (LDH) for the Water Splitting Application. ChemCatChem 2025, 17, e202401584. [Google Scholar] [CrossRef]
- Lin, Z.; Li, X.; Zhang, H.; Xu, B.B.; Wasnik, P.; Li, H.; Singh, M.V.; Ma, Y.; Li, T.; Guo, Z. Research Progress of MXenes and Layered Double Hydroxides for Supercapacitors. Inorg. Chem. Front. 2023, 10, 4358–4392. [Google Scholar] [CrossRef]
- Li, X.; Ren, J.; Sridhar, D.; Xu, B.B.; Algadi, H.; El-Bahy, Z.M.; Ma, Y.; Li, T.; Guo, Z. Progress of Layered Double Hydroxide-Based Materials for Supercapacitors. Mater. Chem. Front. 2023, 7, 1520–1561. [Google Scholar] [CrossRef]
- Luo, F.; San, X.; Wang, Y.; Meng, D.; Tao, K. Layered Double Hydroxide-Based Electrode Materials Derived from Metal–Organic Frameworks: Synthesis and Applications in Supercapacitors. Dalton Trans. 2024, 53, 10403–10415. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Ganjali, F.; Eivazzadeh-Keihan, R.; Maleki, A.; Geranmayeh, S. Recent Advances in Applications of Graphene-Layered Double Hydroxide Nanocomposites in Supercapacitors and Batteries. FlatChem 2024, 45, 100658. [Google Scholar] [CrossRef]
- Aman, M.; Konduparty, P.; Sharma, S.; Srivastava, R.P.; Bhattacharyya, S.; Sharma, V.; Balani, K.; Jha, S.K.; Omar, S. Layered Double Hydroxide Based Composites for Energy Storage Applications: Insights into Supercapacitors and Batteries. J. Energy Storage 2025, 116, 116093. [Google Scholar] [CrossRef]
- Fan, G.; Li, F.; Evans, D.G.; Duan, X. Catalytic Applications of Layered Double Hydroxides: Recent Advances and Perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066. [Google Scholar] [CrossRef]
- Cardinale, A.M.; Carbone, C.; Consani, S.; Fortunato, M.; Parodi, N. Layered Double Hydroxides for Remediation of Industrial Wastewater from a Galvanic Plant. Crystals 2020, 10, 443. [Google Scholar] [CrossRef]
- Tsuneishi, T.; Sakamoto, H.; Hayashi, K.; Kawamura, G.; Muto, H.; Matsuda, A. Preparation of Hydroxide Ion Conductive KOH–Layered Double Hydroxide Electrolytes for an All-Solid-State Iron–Air Secondary Battery. J. Asian Ceram. Soc. 2014, 2, 165–168. [Google Scholar] [CrossRef]
- Leung, D.W.J.; Laney, K.R.; Kenyon, P.; Rees, N.H.; Buffet, J.-C.; Chen, C.; O’Hare, D. Optimising the Acid–Base Ratio of Mg–Al Layered Double Oxides to Enhance CO2 Capture Performance: The Critical Role of Calcination Conditions. Dalton Trans. 2024, 53, 6200–6206. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-M.; Zhu, Y.-Q.; Chen, Z.-R.; Yang, J.-R.; Chen, X.; Yan, H. Theoretical Prediction of the Carrier Mobilities for MII2MIII-Cl-Layered Double Hydroxides in Three-Dimensional Directions. J. Mater. Chem. C Mater. 2022, 10, 9573–9585. [Google Scholar] [CrossRef]
- Xu, J.; Deng, H.; Song, J.; Zhao, J.; Zhang, L.; Hou, W. Synthesis of Hierarchical Flower-like Mg2Al-Cl Layered Double Hydroxide in a Surfactant-Free Reverse Microemulsion. J. Colloid. Interface Sci. 2017, 505, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Memon, J.; Sun, J.; Meng, D.; Ouyang, W.; Memon, M.A.; Huang, Y.; Yan, S.; Geng, J. Synthesis of Graphene/Ni–Al Layered Double Hydroxide Nanowires and Their Application as an Electrode Material for Supercapacitors. J. Mater. Chem. A Mater. 2014, 2, 5060–5067. [Google Scholar] [CrossRef]
- Palapa, N.R.; Taher, T.; Mohadi, R.; Lesbani, A. Removal of Anionic Direct Dye Using Zn/Al, Zn/Fe and Zn/Cr Layered Double Hydroxides Toward Interlayer Distance. Sci. Technol. Indones. 2019, 4, 70–76. [Google Scholar] [CrossRef]
- Ibrahimova, K.A.; Azizov, A.A.; Balayeva, O.O.; Alosmanov, R.M.; Abbasov, M.H. The sonochemical synthesis of PVA/Mg-Al-OH layered double hydroxide nanocomposite film. Azerbaijan Chem. J. 2023, 1, 141–148. [Google Scholar] [CrossRef]
- Harada, K.; Nguyen, T.K.N.; Matsui, Y.; Fujii, K.; Grasset, F.; Ohashi, N.; Matsuda, M.; Uchikoshi, T. Observation of Stacking Faults and Photoluminescence of Laurate Ion Intercalated Zn/Al Layered Double Hydroxide. Mater. Lett. 2018, 213, 323–325. [Google Scholar] [CrossRef]
- Kahl, M.; Golden, T.D. Corrosion Resistance of Electrochemically Synthesized Modified Zaccagnaite LDH-Type Films on Steel Substrates. Materials 2021, 14, 7389. [Google Scholar] [CrossRef]
- Sun, X.; Neuperger, E.; Dey, S.K. Insights into the Synthesis of Layered Double Hydroxide (LDH) Nanoparticles: Part 1. Optimization and Controlled Synthesis of Chloride-Intercalated LDH. J. Colloid. Interface Sci. 2015, 459, 264–272. [Google Scholar] [CrossRef]
- Geng, D.G. Removal of Nitrophenol from Water by Dodecyl Sulfate Modified Layered Double Hydroxides. Asian J. Chem. 2014, 26, 447–450. [Google Scholar] [CrossRef]
- Mohadi, R.; Amri, A.; Badaruddin, M.; Ahmad, N.; Lesbani, A. Adsorption of Phenol from Aqueous Solution Using Zn/Al Layered Double Hydroxides-Cellulose Composite. Sci. Technol. Indones. 2023, 8, 123–128. [Google Scholar] [CrossRef]
- Smalenskaite, A.; Sen, S.; Salak, A.N.; Ferreira, M.G.S.; Skaudzius, R.; Katelnikovas, A.; Kareiva, A. Sol-Gel Synthesis and Characterization of Non-Substituted and Europium- Substituted Layered Double Hydroxides Mg3/Al1−xEux. Curr. Inorg. Chem. 2017, 6, 149–154. [Google Scholar] [CrossRef]
- George, G.; Saravanakumar, M.P. Synthesising Methods of Layered Double Hydroxides and Its Use in the Fabrication of Dye Sensitised Solar Cell (DSSC): A Short Review. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032020. [Google Scholar] [CrossRef]
- Yang, B.; Yang, Z.; Wang, R.; Feng, Z. Silver Nanoparticle Deposited Layered Double Hydroxide Nanosheets as a Novel and High-Performing Anode Material for Enhanced Ni–Zn Secondary Batteries. J. Mater. Chem. A 2014, 2, 785–791. [Google Scholar] [CrossRef]
- Gong, M.; Li, Y.; Wang, H.; Liang, Y.; Wu, J.Z.; Zhou, J.; Wang, J.; Regier, T.; Wei, F.; Dai, H. An Advanced Ni–Fe Layered Double Hydroxide Electrocatalyst for Water Oxidation. J. Am. Chem. Soc. 2013, 135, 8452–8455. [Google Scholar] [CrossRef]
- Shao, M.; Ning, F.; Zhao, J.; Wei, M.; Evans, D.G.; Duan, X. Hierarchical Layered Double Hydroxide Microspheres with Largely Enhanced Performance for Ethanol Electrooxidation. Adv. Funct. Mater. 2013, 23, 3513–3518. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, Y.; Qi, T. Hierarchical Ni–Fe Layered Double Hydroxide/MnO2 Sphere Architecture as an Efficient Noble Metal-Free Electrocatalyst for Ethanol Electro-Oxidation in Alkaline Solution. RSC Adv. 2015, 5, 83314–83319. [Google Scholar] [CrossRef]
- Gao, X.; Lei, L.; Chen, L.; Wang, Y.; He, L.; Lian, Y. Synthesis and Controlled Release of Vitamin C Intercalated Zn/Al Layered Double Hydroxide. Asian J. Chem. 2014, 26, 3471–3476. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Sun, L.; Fedel, M. Synthesis of Novel Cone-Shaped CaAl-LDH Directly on Aluminum Alloy by a Facile Urea Hydrolysis Method. SN Appl. Sci. 2019, 1, 1415. [Google Scholar] [CrossRef]
- Liu, X.L. Chiral Recognition of Lysine by Cyclodextrin Intercalated Layered Double Hydroxides. Asian J. Chem. 2013, 25, 10277–10282. [Google Scholar] [CrossRef]
- ABD MALEK, A.H.; YASIN, Y. Use of Layered Double Hydroxides to Remove Sunset Yellow FCF Dye from Aqueous Solution. Chem. Sci. Trans. 2012, 1, 194–200. [Google Scholar] [CrossRef]
- Hanifa, Y.; Rahayu Palapa, N. Mg/Al Double Layer Hydroxides: Intercalation with H3[α-PW12O40]•nH2O. Sci. Technol. Indones. 2016, 1, 16–19. [Google Scholar] [CrossRef]
- Rasouli, N. Application of a Novel, Efficient and Recyclable Photo Redox Catalyst (Zn–Al Layered Double Hydroxide/Eosin) for the Synthesis of Substituted Pyridine Derivatives under Visible Light Irradiation. Appl. Organomet. Chem. 2018, 32, e4585. [Google Scholar] [CrossRef]
- Imron, M.; Said, M. Adsorption of Procion Red Using Layer Double Hydroxide Mg/Al. Sci. Technol. Indones. 2017, 2, 64–67. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, B.; Pandey, S. Layered Double Hydroxides: A Brief Review from Fundamentals to Application as Evolving Biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Shin, J.; Kim, K.; Hong, J. Zn-Al Layered Double Hydroxide Thin Film Fabricated by the Sputtering Method and Aqueous Solution Treatment. Coatings 2020, 10, 669. [Google Scholar] [CrossRef]
- Mattera, M.; Sorrenti, A.; De Gregorio Perpiñá, L.; Oestreicher, V.; Sevim, S.; Arteaga, O.; Chen, X.; Pané, S.; Abellán, G.; Puigmartí-Luis, J. “On-The-Fly” Synthesis of Self-Supported LDH Hollow Structures Through Controlled Microfluidic Reaction-Diffusion Conditions. Small 2024, 20, 2307621. [Google Scholar] [CrossRef]
- Wang, C.; Xu, J.; Zhou, Z. A Mini-Review on CO2 Photoreduction by MgAl-LDH Based Materials. Energies 2022, 15, 8117. [Google Scholar] [CrossRef]
- Wang, L.; Yuan, Z.; Zhang, Y.; Guo, W.; Sun, X.; Duan, X. Sandwich Layered Double Hydroxides with Graphene Oxide for Enhanced Water Desalination. Sci. China Mater. 2022, 65, 803–810. [Google Scholar] [CrossRef]
- Jaramillo-Hernández, C.; Oestreicher, V.; Mizrahi, M.; Abellán, G. Upscaling the Urea Method Synthesis of CoAl Layered Double Hydroxides. Beilstein J. Nanotechnol. 2023, 14, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Rosaiah, P.; Vadivel, S.; Prakash, N.G.; Dhananjaya, M.; Al-Asbahi, B.A.; Roy, S.; Chalapathi, U.; Park, S.-H. Fabrication of Porous Ni-Co LDH Nanocomposites as Efficient Electrodes for Supercapacitors. Int. J. Energy Res. 2023, 2023, 5793868. [Google Scholar] [CrossRef]
- Cao, Y.; Zheng, D.; Li, X.; Lin, J.; Wang, C.; Dong, S.; Lin, C. Enhanced Corrosion Resistance of Superhydrophobic Layered Double Hydroxide Films with Long-Term Stability on Al Substrate. ACS Appl. Mater. Interfaces 2018, 10, 15150–15162. [Google Scholar] [CrossRef]
- Kim, A.; Varga, I.; Adhikari, A.; Patel, R. Recent Advances in Layered Double Hydroxide-Based Electrochemical and Optical Sensors. Nanomaterials 2021, 11, 2809. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, F.; Zhao, B.; Zhang, S.; Zheng, X.; Wang, Y.; Jin, X.; Dai, C.; Wang, J.; Xie, J.; et al. Ultrafast Synthesis of Metal-Layered Hydroxides in a Dozen Seconds for High-Performance Aqueous Zn (Micro-) Battery. Nanomicro Lett. 2023, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, J.; Zhou, A.; Dong, S.; Shi, K.; Li, B.; Han, J.; O’Hare, D. High-Efficiency CO2 Separation Using Hybrid LDH-Polymer Membranes. Nat. Commun. 2021, 12, 3069. [Google Scholar] [CrossRef]
- Belskaya, O.B.; Terekhova, E.N.; Gorbunova, O.V.; Muromtsev, I.V.; Trenikhin, M.V.; Salanov, A.N.; Likholobov, V.A. Synthesis of CuAl-LDHs by Co-Precipitation and Mechanochemical Methods and Selective Hydrogenation Catalysts Based on Them. Inorganics 2023, 11, 247. [Google Scholar] [CrossRef]
- Shah, S.S.; Aziz, M.A.; Ali, M.; Hakeem, A.S.; Yamani, Z.H. Advanced High-Energy All-Solid-State Hybrid Supercapacitor with Nickel-Cobalt-Layered Double Hydroxide Nanoflowers Supported on Jute Stick-Derived Activated Carbon Nanosheets. Small 2024, 20, 2306665. [Google Scholar] [CrossRef]
- Wu, F.; Liang, J.; Peng, Z.; Liu, B. Electrochemical Deposition and Characterization of Zn-Al Layered Double Hydroxides (LDHs) Films on Magnesium Alloy. Appl. Surf. Sci. 2014, 313, 834–840. [Google Scholar] [CrossRef]
- Johnston, A.L.; Lester, E.; Williams, O.; Gomes, R.L. Understanding Layered Double Hydroxide Properties as Sorbent Materials for Removing Organic Pollutants from Environmental Waters. J. Environ. Chem. Eng. 2021, 9, 105197. [Google Scholar] [CrossRef]
- Ibrahimova, K.A. The synthesis methods and applications of layered double hydroxides—A brief review. NNC RK Bull. 2022, 4, 16–29. [Google Scholar] [CrossRef]
- Palapa, N.R.; Saria, Y.; Taher, T.; Mohadi, R.; Lesbani, A. Synthesis and Characterization of Zn/Al, Zn/Fe, and Zn/Cr Layered Double Hydroxides: Effect of M3+ Ions Toward Layer Formation. Sci. Technol. Indones. 2019, 4, 36–39. [Google Scholar] [CrossRef]
- Huang, X.; Yang, X.; Li, G.; Ezeh, C.I.; Sun, C.; Snape, C. Hybrid Two-Step Preparation of Nanosized MgAl Layered Double Hydroxides for CO2 Adsorption. In Sorption in 2020s; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- G.Fouad, H.; Sayed Amin, A.; S.Ahmed, I.; A.Ali, A. Zinc-Aluminium Layered Double Hydroxides: Fabrication, Study and Adsorption Application for Removal Organic Dye from Aqueous Media. Benha J. Appl. Sci. 2022, 7, 53–61. [Google Scholar] [CrossRef]
- Smalenskaite, A.; Kaba, M.M.; Grigoraviciute-Puroniene, I.; Mikoliunaite, L.; Zarkov, A.; Ramanauskas, R.; Morkan, I.A.; Kareiva, A. Sol–Gel Synthesis and Characterization of Coatings of Mg-Al Layered Double Hydroxides. Materials 2019, 12, 3738. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, T.; Li, Y.; Liu, Y.; Ge, F. Nanostructure Design and Catalytic Performance of Mo/ZnAl-LDH in Cationic Orchid X-BL Removal. Materials 2018, 11, 2390. [Google Scholar] [CrossRef]
- Wijitwongwan, R.P.; Saothayanun, T.K.; Ogawa, M. Synthesis of NiFe Layered Double Hydroxides with Varied Layer Charge Densities: The Templating Effect of Dioctyl Sulfosuccinate. Dalton Trans. 2023, 52, 4692–4699. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Li, M.-Y.; Li, X.; Bao, W.-W.; Jin, C.-Q.; Feng, X.-H.; Liu, G.; Yang, C.-M.; Zhang, N.-N. Chromium-Modified Ultrathin CoFe LDH as High-Efficiency Electrode for Hydrogen Evolution Reaction. Nanomaterials 2022, 12, 1227. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zheng, D.; Luo, J.; Zhang, F.; Dong, S.; Pan, J.; Lin, C. Insight into the Fabrication of ZnAl Layered Double Hydroxides Intercalated with Organic Anions and Their Corrosion Protection of Steel Reinforced Concrete. J. Electrochem. Soc. 2019, 166, C617–C623. [Google Scholar] [CrossRef]
- Fang, S.; Chen, K.; Yao, H.; Cao, Y.; Guo, S.; Wang, L.; Wang, Y.; Yu, S.; Wang, N. Preparation of Gallic Acid Intercalated Layered Double Hydroxide for Enhanced Corrosion Protection of Epoxy Coatings. Coatings 2023, 13, 128. [Google Scholar] [CrossRef]
- Bani Hashemi, A.; Kasiri, G.; Glenneberg, J.; Langer, F.; Kun, R.; La Mantia, F. Electrochemical and Morphological Characterization of Zn−Al−Cu Layered Double Hydroxides as a Negative Electrode in Aqueous Zinc-Ion Batteries. ChemElectroChem 2018, 5, 2073–2079. [Google Scholar] [CrossRef]
- Neethu, P.P.; Venkatachalam, G.; Venkatesha, N.J.; Joseph, D.; Sakthivel, A. Cobalt-Based Hydrotalcite: A Potential Non-Noble Metal-Based Heterogeneous Catalyst for Selective Hydrogenation of Aromatic Aldehydes. Ind. Eng. Chem. Res. 2023, 62, 4976–4986. [Google Scholar] [CrossRef]
- Kitano, S.; Sato, Y.; Tagusari, R.; Zhu, R.; Kowalski, D.; Aoki, Y.; Habazaki, H. Facile Synthesis Approach of Bifunctional Co–Ni–Fe Oxyhydroxide and Spinel Oxide Composite Electrocatalysts from Hydroxide and Layered Double Hydroxide Composite Precursors. RSC Adv. 2023, 13, 10681–10692. [Google Scholar] [CrossRef]
- Huang, L.; Megías-Sayago, C.; Bingre, R.; Zheng, Q.; Wang, Q.; Louis, B. Catalytic Performance of Layered Double Hydroxides (LDHs) Derived Materials in Gas-Solid and Liquid-Solid Phase Reactions. ChemCatChem 2019, 11, 3279–3286. [Google Scholar] [CrossRef]
- Kovalenko, V.; Borysenko, A.; Kotok, V.; Nafeev, R.; Verbitskiy, V.; Melnyk, O. Determination of the Dependence of the Structure of Zn-Al Layered Double Hydroxides, as a Matrix for Functional Anions Intercalation, on Synthesis Conditions. East.-Eur. J. Enterp. Technol. 2022, 1, 12–20. [Google Scholar] [CrossRef]
- Nyaba, L.; Munonde, T.S.; Mpupa, A.; Nomngongo, P.N. Magnetic Fe3O4@Mg/Al-Layered Double Hydroxide Adsorbent for Preconcentration of Trace Metals in Water Matrices. Sci. Rep. 2021, 11, 2302. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yue, X.; Lu, X.; Guo, Y. Uptake Fluoride from Water by Starch Stabilized Layered Double Hydroxides. Water 2018, 10, 745. [Google Scholar] [CrossRef]
- Yang, K.; Huang, Y.; Wang, P.; Tang, Y.; Zhu, Y.; Zhu, X.; Xu, Y.; Jiang, W.; Pan, L.; Li, Q.; et al. LDH Nanocrystal@amorphousness Core–Shell Structure Derived from LDH→LDO Transformation: Synergistically Enhanced Energy Stored for LIBs Anode. Chem. Eng. J. 2024, 486, 150416. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Park, H.; Kim, H.; Kansara, S.; Sun, Y.K. Advanced Cathodes for Practical Lithium-Sulfur Batteries. Acc. Mater. Res. 2025, 6, 245–258. [Google Scholar] [CrossRef]
- Fortunato, M.; Sarapulova, A.; Schwarz, B.; Cardinale, A.M.; Dsoke, S. NiFe-NO3 Layered Double Hydroxide as a Novel Anode for Sodium Ion Batteries. Batter. Supercaps 2024, 8, 2400451. [Google Scholar] [CrossRef]
- Wu, Y.; Yuan, Q.; Wu, Z.; Zhao, Z.; Yin, Q.; Han, J. Interlayer-Spacing Regulation of NiFe LDH Nanosheets Cathode with High Rate Performance for Chloride Ion Battery. J. Ind. Eng. Chem. 2024, 143, 176–183. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Xu, S.; Weng, H. Chloride Ion Batteries-Excellent Candidates for New Energy Storage Batteries Following Lithium-Ion Batteries. Ionics 2024, 30, 27–38. [Google Scholar] [CrossRef]
- Song, Z.; Yin, Q.; Yang, S.; Miao, Y.; Wu, Y.; Li, Y.Z.; Ren, Y.; Sui, Y.; Qi, J.; Han, J. A High-Nickel Layered Double Hydroxides Cathode Boosting the Rate Capability for Chloride Ion Batteries with Ultralong Cycling Life. Small 2023, 19, 2302896. [Google Scholar] [CrossRef]
- Chen, K.; Chen, C.; Long, J.; Zhou, G. MOF/LDH Cross Composites Derived Heterojunction Nanospheres as Highly Efficient Catalysts for ORR-OER and Rechargeable Zn-Air Batteries. Appl. Surf. Sci. 2024, 657, 159803. [Google Scholar] [CrossRef]
- Allwyn, N.; Gokulnath, S.; Sathish, M. In-Situ Nanoarchitectonics of Fe/Co LDH over Cobalt-Enriched N-Doped Carbon Cookies as Facile Oxygen Redox Electrocatalysts for High-Rate Rechargeable Zinc–Air Batteries. ACS Appl. Mater. Interfaces 2024, 16, 20360–20374. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Asai, S. Hydrothermal Synthesis of Layered Double Hydroxide-Deoxycholate Intercalation Compounds. Chem. Mater. 2000, 12, 3253–3255. [Google Scholar] [CrossRef]
- Jiang, F.; Xie, Y.; Zhang, H.; Zhang, L.; Gao, X.; Bai, H.; Yao, F.; Yue, H. Hierarchical Core-Shelled CoMo Layered Double Hydroxide@CuCo2S4 Nanowire Arrays/Nickel Foam for Advanced Hybrid Supercapacitors. J. Colloid. Interface Sci. 2025, 677, 150–157. [Google Scholar] [CrossRef]
- Li, R.Y.; Shen, X.Y.; Li, J.; Zhao, D.P.; Zhao, R.D.; Wu, F.F. Enhanced Electrochemical Performance of NiCo-Layered Double Hydroxides: Optimal Synthesis Conditions and Supercapacitor Applications. Adv. Sustain. Syst. 2024, 9, 2400753. [Google Scholar] [CrossRef]
- Zhi, P.X.; Guo, Q.L. Hydrothermal Synthesis of Layered Double Hydroxides (LDHs) from Mixed MgO and Al2O3: LDH Formation Mechanism. Chem. Mater. 2005, 17, 1055–1062. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, C.; Meng, L. Adsorption of Methyl Orange from Aqueous Solution on Hydrothermal Synthesized Mg-Al Layered Double Hydroxide. J. Chem. Eng. Data 2011, 56, 4217–4225. [Google Scholar] [CrossRef]
- Knorpp, A.J.; Zawisza, A.; Huangfu, S.; Borzì, A.; Clark, A.H.; Kata, D.; Graule, T.; Stuer, M. Hydrothermal Synthesis of Multi-Cationic High-Entropy Layered Double Hydroxides. RSC Adv. 2022, 12, 26362–26371. [Google Scholar] [CrossRef]
- Rybka, K.; Matusik, J. Exploring Synthesis Approaches, Properties and Applications of Layered Double Hydroxides Derived from Alternative Sources: A Comprehensive Review. J. Clean. Prod. 2025, 498, 145215. [Google Scholar] [CrossRef]
- Sakai, M.; Imagawa, H.; Baba, N. Layered-Double-Hydroxide-Based Ni Catalyst for CO2 Capture and Methanation. Appl. Catal. A Gen. 2022, 647, 118904. [Google Scholar] [CrossRef]
- Liao, L.; Zhao, N.; Xia, Z. Hydrothermal Synthesis of Mg-Al Layered Double Hydroxides (LDHs) from Natural Brucite and Al(OH)3. Mater. Res. Bull. 2012, 47, 3897–3901. [Google Scholar] [CrossRef]
- Pan, Y.; Li, L.; Liu, D.; Wang, F.; Xia, M.; Wang, F. Macroscale Structures of Layered Double Hydroxides: A Review of Formation Strategies and Applications in Water Treatment. J. Environ. Chem. Eng. 2025, 13, 115284. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, L.; Li, L.; Wu, H.; Zheng, J.; Sun, J.; Li, G. One-Step Hydrothermal Synthesis of Glucose-Induced Low Crystallinity NiCo-Based Layered Double Hydroxides for High-Performance Asymmetric Supercapacitors. Chem. Eur. J. 2024, 31, e202403439. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiao, F.; Jiao, Q. Hydrothermal Synthesis of Ni/Al Layered Double Hydroxide Nanorods. J. Nanotechnol. 2011, 2011, 646409. [Google Scholar] [CrossRef]
- Zhang, F.; Du, N.; Song, S.; Liu, J.; Hou, W. Mechano-Hydrothermal Synthesis of Mg2Al-NO3 Layered Double Hydroxides. J. Solid. State Chem. 2013, 206, 45–50. [Google Scholar] [CrossRef]
- Clark, I.; Dunne, P.W.; Gomes, R.L.; Lester, E. Continuous Hydrothermal Synthesis of Ca2Al-NO3 Layered Double Hydroxides: The Impact of Reactor Temperature, Pressure and NaOH Concentration on Crystal Characteristics. J. Colloid. Interface Sci. 2017, 504, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, J.; Achari, G.; Yu, J.; Cai, W. Cr(VI) Removal from Aqueous Solutions by Hydrothermal Synthetic Layered Double Hydroxides: Adsorption Performance, Coexisting Anions and Regeneration Studies. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 33–40. [Google Scholar] [CrossRef]
- Tung, N.Q.; Van, D.T.C.; Thang, D.X.; An, N.T.K.; Trang, T.T.; Nhi, B.D.; Thao, N.P.; Son, L.T.; Huy, N.N.; Dung, N.T. Hydrothermal Synthesis of CuCoFe Layered Double Hydroxide and Its Performance in the Degradation of Antibiotics: Influencing Factors, Degradation Pathways, and Reaction Mechanism. J. Environ. Chem. Eng. 2023, 11, 110127. [Google Scholar] [CrossRef]
- Prevot, V.; Caperaa, N.; Taviot-Guého, C.; Forano, C. Glycine-Assisted Hydrothermal Synthesis of NiAl-Layered Double Hydroxide Nanostructures. Cryst. Growth Des. 2009, 9, 3646–3654. [Google Scholar] [CrossRef]
- Xiong, Q.; Li, X.; Zhou, M.; Chen, R.; Sun, C.; Zhou, Y.; Wang, S.; Qiu, X.; Song, M.; Wei, T. One-Pot Hydrothermal Synthesis of Flower-Shaped Zif-67@NiCo-LDH Heterostructure as Anode Materials for Lithium-Ion Batteries. Ionics 2023, 29, 1741–1749. [Google Scholar] [CrossRef]
- Zhu, L.; Han, T.; Ding, Y.; Long, J.; Lin, X.; Liu, J. A Metal–Organic-Framework Derived NiFe2O4@NiCo-LDH Nanocube as High-Performance Lithium-Ion Battery Anode under Different Temperatures. Appl. Surf. Sci. 2022, 599, 153953. [Google Scholar] [CrossRef]
- Luo, K.; Li, Y.; Yao, J.; Huang, B.; Zhu, Q.; Yang, J. Boosting the Lithium Storage Property of Nickel-Zinc Layered Double Hydroxides by Intercalation with Dodecyl Sulfate Anions. Appl. Surf. Sci. 2023, 620, 156850. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, X.; Chen, P.; Cao, X.; Liu, D.; Wang, R. Properties of Hollow Yolk-Shell NiS2/FeS2@NC@NiFe LDH/FeO(OH) Nanoflower Microspheres as Anode Materials for Lithium-Ion Batteries. J. Electroanal. Chem. 2023, 943, 117606. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, Z.; Ma, P.; Li, H. Constructing 1D/2D NiCo-LDH Nanowire/MXene Composites for Efficient and Stable Lithium Storage. Adv. Mater. Interfaces 2024, 11, 2300962. [Google Scholar] [CrossRef]
- Shah, S.S.; Aziz, M.A.; Rasool, P.I.; Mohmand, N.Z.K.; Khan, A.J.; Ullah, H.; Feng, X.; Oyama, M. Electrochemical Synergy and Future Prospects: Advancements and Challenges in MXene and MOFs Composites for Hybrid Supercapacitors. Sustain. Mater. Technol. 2024, 39, e00814. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhang, J.; Li, B.; Wu, Y.; Wu, Z.; Dou, Y.; Yin, Q.; Han, J. An MgAl Layered Double Hydroxide as a New Transition Metal-Free Anode for Lithium-Ion Batteries. Chem. Commun. 2023, 59, 13903–13906. [Google Scholar] [CrossRef]
- Zhang, D.; Hua, Y.; Fu, X.; Cheng, C.; Kong, D.; Zhang, M.; Lei, B.; Liu, Z. Hierarchical Flower Array NiCoOOH@CoLa-LDH Nanosheets for High-Performance Supercapacitor and Alkaline Zn Battery. Adv. Funct. Mater. 2025, 35, e202414686. [Google Scholar] [CrossRef]
- Yin, Q.; Song, Z.; Yang, S.; Zhao, Z.; Yuan, Q.; Qi, J.; Sui, Y.; Han, J. Heteroatoms Introduction via Temperature-Differential Doping Strategy Achieves Long-Cycle-Life NiCoMo-P LDH Cathode for Rechargeable Chloride-Ion Battery. J. Electroanal. Chem. 2023, 951, 117924. [Google Scholar] [CrossRef]
- Zhao, G.; Deng, Z.; Wan, G.; Zhao, J.; Wang, G. Array Structured NiAl-Layered Double Hydroxides Grown on Graphene by Atomic Layer Deposition as Chloride-Ion Battery Cathode. DeCarbon 2025, 8, 100106. [Google Scholar] [CrossRef]
- Yang, S.; Yin, Q.; Song, Z.; Xu, F.; Xie, Z.; Wu, Y.; Xu, S.; Li, Y.Z.; Zhao, D.; Xiao, B.; et al. Introducing High-Valence Molybdenum to Stimulate Lattice Oxygen in a NiCo LDH Cathode for Chloride Ion Batteries. Mater. Horiz. 2023, 10, 3429–3437. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wu, Y.; Yuan, Q.; Zhang, J.; Dou, Y.; Han, J. Aqueous Chloride-Ion Battery within a Neutral Electrolyte Based on a CoFe-Cl Layered Double Hydroxide Anode. ACS Appl. Mater. Interfaces 2023, 15, 38540–38549. [Google Scholar] [CrossRef]
- Li, J.; Qin, Y.; Bai, Z.; Li, S.; Li, L.; Ouyang, B.; Kan, E.; Zhang, W. Investigating the Role of 3D Hierarchical Ni-CAT/NiFe-LDH/CNFs in Enhancing the Oxygen Evolution Reaction and Zn-Air Battery Performance. Appl. Surf. Sci. 2024, 648, 159080. [Google Scholar] [CrossRef]
- Bhanuse, G.B.; Kumar, S.; Yu, C.C.; Fu, Y.P. Nanostructured ZnCo2O4@NiMn-LDH Electrodes for Supercapacitor and Zinc-Air Battery Application. ACS Appl. Nano Mater. 2024, 7, 13649–13663. [Google Scholar] [CrossRef]
- Jia, Z.; Shang, J.; Xue, K.; Yang, X.; Wang, S.; Xu, C.; Wang, Q. N-doped Nanocarbon Inserted NiCo-LDH Nanoplates on NF with High OER/ORR Performances for Zinc-Air Battery. ChemCatChem 2023, 15, e202201469. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Z.; Liu, Y.; Cheng, Z.; Zhang, J.; Zhang, Q.; Li, Y.; Hou, C.; Li, K.; Wang, H. Efficient Bifunctional NiFe-LDH@Co9S8 Nanoflower Electrocatalysts Anchored with Pt Nanocrystal for Flexible Quasi-Solid Rechargeable Zinc Air Battery. Energy Fuels 2024, 38, 10264–10274. [Google Scholar] [CrossRef]
- Liu, S.; Wan, R.; Lin, Z.; Liu, Z.; Liu, Y.; Tian, Y.; Qin, D.-D.; Tang, Z. Probing the Co Role in Promoting the OER and Zn–Air Battery Performance of NiFe-LDH: A Combined Experimental and Theoretical Study. J. Mater. Chem. A Mater. 2022, 10, 5244–5254. [Google Scholar] [CrossRef]
- Dighe, P.S.; Redekar, R.S.; Tarwal, N.L.; Sarawade, P.B. Design and Development of the High-Performance Aqueous Asymmetric Supercapacitor Based on the Hydrothermally Grown Binder-Less Ni-Co LDH Nanosheets. J. Energy Storage 2024, 88, 111467. [Google Scholar] [CrossRef]
- Chandra Sahoo, R.; Moolayadukkam, S.; Seok, J.H.; Lee, S.U.; Matte, H.S.S.R. Enhanced Charge Storage Capacity and High Rate Capabilities of Ni2Co-Layered Double Hydroxides/Expanded-Graphite Composites as Anodes for Li-Ion Batteries. J. Mater. Chem. A Mater. 2023, 11, 7142–7151. [Google Scholar] [CrossRef]
- Li, X.; Fortunato, M.; Cardinale, A.M.; Sarapulova, A.; Njel, C.; Dsoke, S. Electrochemical Study on Nickel Aluminum Layered Double Hydroxides as High-Performance Electrode Material for Lithium-Ion Batteries Based on Sodium Alginate Binder. J. Solid. State Electrochem. 2022, 26, 49–61. [Google Scholar] [CrossRef]
- Du, P.; Xi, W.; Zhang, Y.; Wang, R.; Gong, Y.; He, B.; Wang, H.; Jin, J. Laser Induction of Hierarchically Micro/Nanostructured CoNi-LDH/C Composite for High-Performance Lithium-Sulfur Batteries. J. Alloys Compd. 2024, 985, 174043. [Google Scholar] [CrossRef]
- Ye, Z.; Zhai, S.; Liu, R.; Liu, M.; Xu, Y.; Li, C.; Wang, X.; Mei, T. Sulfonate-Rich Polymer Intercalated LDH Artificial SEI Film to Enable High-Stability Li-S Batteries. Chem. Eng. J. 2024, 479, 147847. [Google Scholar] [CrossRef]
- Zhen, M.; Meng, X.; Wang, X.; Zhang, Z.; Guo, S.-Q.; Hu, Z.; Shen, B. Layered Double Hydroxides-MXene Heterointerfaces with Abundant Anion Vacancies Expediting Sulfur Redox Kinetics for High-Performance Lithium–Sulfur Batteries. Chem. Eng. J. 2024, 489, 151285. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Zhou, Y.; Wang, M.; Li, R.; Yue, W. Layered Double Hydroxides Used as the Sulfur Hosts for Lithium-Sulfur Batteries and the Influence of Metal Composition on Their Performance. J. Solid. State Electrochem. 2023, 27, 797–807. [Google Scholar] [CrossRef]
- Wang, H.Y.; Dai, Y.K.; Liao, K.M.; Deng, S.; Dai, G.P. Vertical Graphene Growth on LDH Nanosheets and Carbon Cloth Nanofibers with NiCo Nanoparticles as a Freestanding Host for High-Performance Lithium-Sulfur Batteries. J. Phys. Chem. Lett. 2025, 16, 1103–1113. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Ma, L.; Yang, M.; Zhao, X. NiCr-Cl LDH/RGO Composite as Anode Material for Sodium-Ion Batteries. J. Electron. Mater. 2022, 51, 6067–6075. [Google Scholar] [CrossRef]
- Li, B.; Li, J.; Li, P.; Zhang, N.; Shang, H.; Zhang, B. Sulfide Hollow ZIF-67 and NiCo-LDH Heterostructure for Superior Asymmetric Supercapacitors. J. Alloys Compd. 2025, 1017, 179128. [Google Scholar] [CrossRef]
- He, L.; Cai, P.; Lai, H.; Lu, K.; Xu, Z.; Zeng, R.; Hao, C.; Wang, Z.; Gan, W. Sandwich-like NiFe-LDH/MnCO3/MXene Ternary Nanocomposites Serve as Battery-Type Electrode for High-Performance Asymmetric Supercapacitor. Chem. Eng. J. 2025, 504, 159149. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; Sun, H.; Duan, L.; Yang, Z.; Wang, X.; Liu, J. Construction of Flexible MnCo2O4@FeCoNi-LDH Electrode Materials with Nanoflower-like and Hierarchical Structure for High-Performance Asymmetric Supercapacitor. J. Colloid. Interface Sci. 2025, 682, 1051–1061. [Google Scholar] [CrossRef]
- Tai, P.C.; Chung, R.J.; Wang, G.B.; Kongvarhodom, C.; Husain, S.; Yougbaré, S.; Chen, H.M.; Wu, Y.F.; Lin, L.Y. Polydopamine Derived Carbon Coated Cobalt Molybdenum Layered Double Hydroxide as Highly Stable Anode Materials of Sodium-Ion Battery. J. Energy Storage 2024, 101, 113961. [Google Scholar] [CrossRef]
- Lin, Q.; Yuan, Z.; Wang, D.; Wei, W.; Wang, X.; Han, W.; Wang, L. Co-Co LDH-Derived CoSe2 Anchored on N-Doped Carbon Nanospheres as High-Performance Anodes for Sodium-Ion Batteries. Electrochim. Acta 2022, 432, 141012. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, L.; Chen, T.; Sun, P.; Lv, X.; Yu, H.; Sun, X.; Leo Liu, T. High-Performance Aqueous Rechargeable NiCo//Zn Battery with Molybdate Anion Intercalated CoNi-LDH@CP Bilayered Cathode. J. Colloid. Interface Sci. 2024, 658, 728–738. [Google Scholar] [CrossRef]
- Wang, C.; Han, L.; Yang, S.; Liu, Z.; Liu, M.; Li, B. Nanosheet-Structured ZnCo-LDH Microsphere as Active Material for Rechargeable Zinc Batteries. J. Colloid. Interface Sci. 2024, 659, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, S.; Li, C.; Yan, X.; Zhang, Y.; Yin, R.; Wei, Y.; Gao, K.; Gao, H. Advanced Strategies for Enhancing Electrochemical Performance of NiAl LDH Electrodes in Supercapacitors. Coord. Chem. Rev. 2025, 531, 216497. [Google Scholar] [CrossRef]
- Jing, C.; Huang, L.; Tao, S.; Chen, Y.; Zhang, S.; Dong, W.; Ling, F.; Tang, X.; Li, Y.; Feng, L.; et al. Construction of MoB@LDH Heterojunction and Its Derivates through Phase and Interface Engineering for Advanced Supercapacitor Applications. J. Colloid. Interface Sci. 2024, 660, 10–20. [Google Scholar] [CrossRef]
- Tai, P.-Y.; Sakthivel, M.; Peng, Y.-J.; Kubendhiran, S.; Lin, L.-Y.; Ho, K.-C. In Situ Growing Te-Doped Ni–Mn Layered Double Hydroxide on a Cetyltrimethylammonium Bromide-Modified MXene Conductive Layer for Binder-Free Supercapacitors. ACS Appl. Energy Mater. 2025, 8, 4122–4133. [Google Scholar] [CrossRef]
- Han, X.; Li, J.; Lu, J.; Luo, S.; Wan, J.; Li, B.; Hu, C.; Cheng, X. High Mass-Loading NiCo-LDH Nanosheet Arrays Grown on Carbon Cloth by Electrodeposition for Excellent Electrochemical Energy Storage. Nano Energy 2021, 86, 106079. [Google Scholar] [CrossRef]
- He, T.-L.; Zhang, X.-R.; Ma, Q.-L.; Hua, W.-M.; Xiong, H.-M. Positive Carbon Dots Induced Electrodeposition of NiCo-LDH Nanosheets for High-Performance Supercapacitors. ACS Appl. Energy Mater. 2024, 7, 5508–5516. [Google Scholar] [CrossRef]
- Niu, Y.; Chang, L.; Huang, Y.; Sun, Q.; Lu, X.; Cheng, H. Electrochemical Constructing Versatile ZnAl-LDH Artificial Interface Layer Coated (002)-Textured Zn for Highly Reversible Zinc Anodes. Chem. Eng. J. 2024, 499, 155813. [Google Scholar] [CrossRef]
- Lashgari, M.; Yamini, Y. Fiber-in-tube Solid-phase Microextraction of Caffeine as a Molecular Tracer in Wastewater by Electrochemically Deposited Layered Double Hydroxide. J. Sep. Sci. 2018, 41, 2393–2400. [Google Scholar] [CrossRef]
- Seumo Tchekwagep, P.M.; Banks, C.E.; Crapnell, R.D.; Farsak, M.; Kardaş, G. Electrochemical Synthesis of NiCo Layered Double Hydroxides on Nickel-Coated Graphite for Water Splitting: Understanding the Electrochemical Experimental Parameters. RSC Adv. 2025, 15, 3969–3978. [Google Scholar] [CrossRef] [PubMed]
- Cysewska, K.; Zając, M.; Łapiński, M.; Karczewski, J.; Rybarczyk, M.K.; Kamecki, B.; Jasiński, P.; Molin, S. The Effect of Cobalt Incorporation into Nickel–Iron Oxide/(oxy)Hydroxide Catalyst on Electrocatalytic Performance Toward Oxygen Evolution Reaction. Energy Technol. 2021, 9, 2100688. [Google Scholar] [CrossRef]
- Wu, Q.; Li, F.; Sheng, H.; Qi, Y.; Yuan, J.; Bi, H.; Li, W.; Xie, E.; Lan, W. In Situ Fabrication of Hierarchical CuO@CoNi-LDH Composite Structures for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2024, 16, 23241–23252. [Google Scholar] [CrossRef]
- Kim, Y.; Jun, S.E.; Lee, G.; Nam, S.; Jang, H.W.; Park, S.H.; Kwon, K.C. Recent Advances in Water-Splitting Electrocatalysts Based on Electrodeposition. Materials 2023, 16, 3044. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.A.; Yu, S.; Ju, E.; Seo, H.; Yeu, J.; Kim, J.; Patil, D.S.; Shin, J.C. Zinc Cobalt Layered Double Hydroxide Electrode for High-Performance Supercapacitor. Appl. Sci. Converg. Technol. 2019, 28, 164–168. [Google Scholar] [CrossRef]
- Arbenin, A.Y.; Zemtsova, E.G.; Orekhov, E.V.; Sokolova, D.N.; Baburova, P.I.; Petrov, A.A.; Gaǐshun, V.E.; Smirnov, V.M. Features of Fabrication of Titanium Dioxide Based Coatings for Non-Lithographic Template Electrochemical Synthesis of Micron Metal Particle Arrays. Gels 2021, 7, 202. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Guan, Y.; Zhang, M.; Qin, J.; Guo, X.; Li, Z.; Zhang, B.; Tang, J. Dynamic Morphological Evolution of Co-Based Layered Double Hydroxide Nanosheets Investigated by In Situ Electrochemical-Atomic Force Microscopy. J. Phys. Chem. C 2023, 127, 5219–5229. [Google Scholar] [CrossRef]
- Wang, T.; Wang, W.; Shao, W.; Bai, M.; Zhou, M.; Li, S.; Ma, T.; Ma, L.; Cheng, C.; Liu, X. Synthesis and Electronic Modulation of Nanostructured Layered Double Hydroxides for Efficient Electrochemical Oxygen Evolution. ChemSusChem 2021, 14, 5112–5134. [Google Scholar] [CrossRef]
- Ramachandran, R.; Lan, Y.; Xu, Z.X.; Wang, F. Construction of NiCo-Layered Double Hydroxide Microspheres from Ni-MOFs for High-Performance Asymmetric Supercapacitors. ACS Appl. Energy Mater. 2020, 3, 6633–6643. [Google Scholar] [CrossRef]
- Shahid, N.F.; Jamal, A.; Haq, G.; Javed, M.; Saifullah, M.; Anjum, M.A.R. Cobalt–Iron Layered Double Hydroxide Nanosheet-Wrapped Nitrogen-Doped Graphite Felt as an Oxygen-Evolving Electrode. Energy Adv. 2023, 2, 2109–2118. [Google Scholar] [CrossRef]
- Ren, B.; Zhang, B.Y.; Zhang, H.; Jiang, X.; Yi, Q.; Xu, K.; Yu, H.; Hu, Z.; Jeerapan, I.; Ou, J.Z. CoNi Layered Double Hydroxide Nanosheets Vertically Grown on Electrodeposited Dendritic Copper Substrates for Supercapacitor Applications. ACS Appl. Nano Mater. 2022, 5, 2395–2404. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Hähnel, A.; Naumann, V.; Lin, C.; Azimi, S.; Schweizer, S.L.; Maijenburg, A.W.; Wehrspohn, R.B. Bifunctional Heterostructure Assembly of NiFe LDH Nanosheets on NiCoP Nanowires for Highly Efficient and Stable Overall Water Splitting. Adv. Funct. Mater. 2018, 28, 1706847. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Sun, L.; LaChance, A.M.; Ding, H.; Fedel, M. In Situ Growth of a CaAl-NO3− -Layered Double Hydroxide Film Directly on an Aluminum Alloy for Corrosion Resistance. Dalton Trans. 2020, 49, 3956–3964. [Google Scholar] [CrossRef] [PubMed]
- Gualandi, I.; Vlamidis, Y.; Mazzei, L.; Musella, E.; Giorgetti, M.; Christian, M.; Morandi, V.; Scavetta, E.; Tonelli, D. Ni/Al Layered Double Hydroxide and Carbon Nanomaterial Composites for Glucose Sensing. ACS Appl. Nano Mater. 2019, 2, 143–155. [Google Scholar] [CrossRef]
- Alsaç, E.P.; Zhou, K.; Rong, W.; Salamon, S.; Landers, J.; Wende, H.; Smith, R.D.L. Identification of Non-Traditional Coordination Environments for Iron Ions in Nickel Hydroxide Lattices. Energy Environ. Sci. 2022, 15, 2638–2652. [Google Scholar] [CrossRef]
- Shi, Z.; Yuan, Y.; Xiao, Q.; Li, Z.; Zhu, J. Carbonate Doped NiCo-LDH Modified with PANI for High Performance Asymmetric Supercapacitors. CrystEngComm 2022, 24, 3546–3555. [Google Scholar] [CrossRef]
- Tan, Z.; Luo, X.; Wei, T.; Zhang, F.; Lv, Y.; Chen, L.; Shi, Y. In Situ Formation of NiAl-Layered Double Hydroxide with a Tunable Interlayer Spacing in a Confined Impinging Jet Microreactor. Energy Fuels 2020, 34, 8939–8946. [Google Scholar] [CrossRef]
- Yan, Z.; Sun, H.; Chen, X.; Liu, H.; Zhao, Y.; Li, H.; Xie, W.; Cheng, F.; Chen, J. Anion Insertion Enhanced Electrodeposition of Robust Metal Hydroxide/Oxide Electrodes for Oxygen Evolution. Nat. Commun. 2018, 9, 2373. [Google Scholar] [CrossRef]
- Ghadimi, A.M.; Ghasemi, S.; Omrani, A.; Mousavi, F. Nickel Cobalt LDH/Graphene Film on Nickel-Foam-Supported Ternary Transition Metal Oxides for Supercapacitor Applications. Energy Fuels 2023, 37, 3121–3133. [Google Scholar] [CrossRef]
- Cysewska, K.; Rybarczyk, M.K.; Cempura, G.; Karczewski, J.; Łapiński, M.; Jasinski, P.; Molin, S. The Influence of the Electrodeposition Parameters on the Properties of Mn-Co-Based Nanofilms as Anode Materials for Alkaline Electrolysers. Materials 2020, 13, 2662. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Singh, R.P. Transforming Prussian Blue Analogues: The Future-Centric Pathway to Rise of Thin Layered Double Hydroxides for Superior Water Oxidation: An Overview. Int. J. Multidiscip. Res. 2024, 6, 240529213. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Q.; Long, Y.; Wang, F.; Wu, L.; Pan, J.; Han, J.; Lei, Y.; Shi, W.; Song, S. In Situ Formation of Co9S8 Quantum Dots in MOF-Derived Ternary Metal Layered Double Hydroxide Nanoarrays for High-Performance Hybrid Supercapacitors. Adv. Energy Mater. 2020, 10, 1903193. [Google Scholar] [CrossRef]
- Li, Z.; Han, F.; Li, C.; Jiao, X.; Chen, D. Multi-Anion Intercalated Layered Double Hydroxide Nanosheet-Assembled Hollow Nanoprisms with Improved Pseudocapacitive and Electrocatalytic Properties. Chem. Asian J. 2018, 13, 1129–1137. [Google Scholar] [CrossRef]
- Li, S.; Mo, T.; Chen, L.; Zhang, F.; Jamil, S.; Lu, Y.; Cai, Q. Hierarchical MgAl-Layered Double Hydroxide Growth on Porous MgO Template for Pollution Removal. Environ. Prog. Sustain. Energy 2022, 41, e13907. [Google Scholar] [CrossRef]
- Wei, M.; Huang, Q.; Zhou, Y.; Peng, Z.; Chu, W. Ultrathin Nanosheets of Cobalt-Nickel Hydroxides Hetero-Structure via Electrodeposition and Precursor Adjustment with Excellent Performance for Supercapacitor. J. Energy Chem. 2018, 27, 591–599. [Google Scholar] [CrossRef]
- Chavan, H.S.; Lee, C.H.; Inamdar, A.I.; Han, J.; Park, S.; Cho, S.; Shreshta, N.K.; Lee, S.U.; Hou, B.; Im, H.; et al. Designing and Tuning the Electronic Structure of Nickel–Vanadium Layered Double Hydroxides for Highly Efficient Oxygen Evolution Electrocatalysis. ACS Catal. 2022, 12, 3821–3831. [Google Scholar] [CrossRef]
- Zhai, P.; Xia, M.; Wu, Y.; Zhang, G.; Gao, J.; Zhang, B.; Cao, S.; Zhang, Y.; Li, Z.; Fan, Z.; et al. Engineering Single-Atomic Ruthenium Catalytic Sites on Defective Nickel-Iron Layered Double Hydroxide for Overall Water Splitting. Nat. Commun. 2021, 12, 4587. [Google Scholar] [CrossRef]
- Wang, G.; Liu, G.; Jin, Z. In Situ Coupled Nickel-Based Layered Double Hydroxides with MXene to Enhance Supercapacitor Performance. J. Mater. Chem. C Mater. 2023, 11, 10547–10561. [Google Scholar] [CrossRef]
- Laipan, M.; Yu, J.; Zhu, R.; Zhu, J.; Smith, A.T.; He, H.; O’Hare, D.; Sun, L. Functionalized Layered Double Hydroxides for Innovative Applications. Mater. Horiz. 2020, 7, 715–745. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, J.; Yang, S.; Wei, Z.; Wang, Y.; Chen, A.; Huang, Z.; Zhi, C. MXene Supported Electrodeposition Engineering of Layer Double Hydroxide for Alkaline Zinc Batteries. Angew. Chem. Int. Ed. 2024, 63, e202411443. [Google Scholar] [CrossRef]
- Cheng, X.; Wu, D.; Gao, H.; Wang, Q.; Lv, P.; Yoon, S.S.; Wei, Q. A Mild, Configurable, Flexible CoNi-LDH(v)/Zn Battery Based on H-Vacancy-Induced Reversible Zn2+ Intercalation. J. Energy Chem. 2025, 100, 498–508. [Google Scholar] [CrossRef]
- Inamdar, A.I.; Chavan, H.S.; Jo, Y.; Im, H.; Kim, H. Nonprecious Bimetallic NiFe-layered Hydroxide Nanosheets as a Catalyst for Highly Efficient Electrochemical Water Splitting. Int. J. Energy Res. 2021, 45, 16963–16972. [Google Scholar] [CrossRef]
- Li, H.; Zhao, R.; Zhou, W.; Wang, L.; Li, W.; Zhao, D.; Chao, D. Trade-off between Zincophilicity and Zincophobicity: Toward Stable Zn-Based Aqueous Batteries. JACS Au 2023, 3, 2107–2116. [Google Scholar] [CrossRef]
- Meng, J.; Song, Y.; Qin, Z.; Wang, Z.; Mu, X.; Wang, J.; Liu, X.X. Cobalt–Nickel Double Hydroxide toward Mild Aqueous Zinc-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2204026. [Google Scholar] [CrossRef]
- Dai, L.; Peng, S.; Wang, X.; Chen, B.; Wu, Y.; Xie, Q.; Ruan, Y. Three-Dimensional NiCoS Nanotubes@NiCo-LDH Nanosheets Core–Shell Heterostructure for High-Rate Capability Alkaline Zinc-Based Batteries. RSC Adv. 2024, 14, 7999–8006. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Wang, S.; Zhong, G.; Chen, J.; Bao, Y.; Niu, L. Hierarchical Heterostructure Engineering of Layered Double Hydroxides on Nickel Sulfides Heteronanowire Arrays as Efficient Cathode for Alkaline Aqueous Zinc Batteries. Small 2022, 18, 2202799. [Google Scholar] [CrossRef]
- Cai, X.; Jiang, T.; Wu, M. Confined Growth of NiFe LDH with Hierarchical Structures on Copper Nanowires for Long-Term Stable Rechargeable Zn-Air Batteries. Appl. Surf. Sci. 2022, 577, 151911. [Google Scholar] [CrossRef]
- Zhou, P.; Ji, Y.; Zhang, B.; Zhang, P.; Zhang, S.; Wang, S. Rational Construction of Tremella-like CuCo LDH@Ni3S2 Nanocomposites as High-Performance Supercapacitor Electrode Materials. J. Alloys Compd. 2025, 1021, 179586. [Google Scholar] [CrossRef]
- Xing, H.; Deng, X.; Wang, X. Alkaline Capacity Decay Induced Vacancy-Rich LDH for High-Performance Magnesium Ions Hybrid Supercapacitor. J. Colloid. Interface Sci. 2025, 679, 43–53. [Google Scholar] [CrossRef]
- Khalafallah, D.; Ibrahim, M.A.; Hou, H.; Wang, J.; Liu, C.; Zhang, Q. Configuring Cations–Doped Cobalt Lanthanum LDH Nanoarray-on-Nanoarray Platforms for Supercapacitors. Sustain. Mater. Technol. 2025, 43, e01286. [Google Scholar] [CrossRef]
- Duddi, R.; Shivani; Dhiman, S.; Kumar Singh, A.; Kamboj, N.; Kumar, S. Superior Cycle Stability and Enhanced Pseudocapacitive Performance of NiAl-LDH Decorated Carbon Cloth for Supercapacitor Application. Appl. Surf. Sci. 2025, 679, 161298. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Mozaffari, S.A.; Rasche, B.; Ebrahimi, F. Bio-Inspired Coral Reef-like NiCo-LDH Nanostructure Fabricated on Carbon Felt for High Performance Flexible Supercapacitor. Sci. Rep. 2025, 15, 10542. [Google Scholar] [CrossRef] [PubMed]
- Klydziute, G.; Gliaudyte, L.; Sokol, D.; Vasiliauskiene, D.; Yang, T.C.-K.; Kareiva, A. Layered Double Hydroxides: Sol-Gel Synthesis, Characterization and Application. Preprint 2024. [Google Scholar] [CrossRef]
- Valente, J.S.; Lima, E.; Toledo-Antonio, J.A.; Cortes-Jacome, M.A.; Lartundo-Rojas, L.; Montiel, R.; Prince, J. Comprehending the Thermal Decomposition and Reconstruction Process of Sol−Gel MgAl Layered Double Hydroxides. J. Phys. Chem. C 2010, 114, 2089–2099. [Google Scholar] [CrossRef]
- Mallakpour, S.; Dinari, M.; Behranvand, V. Ultrasonic-Assisted Synthesis and Characterization of Layered Double Hydroxides Intercalated with Bioactive N,N′-(Pyromellitoyl)-Bis-L-α-Amino Acids. RSC Adv. 2013, 3, 23303–23308. [Google Scholar] [CrossRef]
- Sokol, D.; Vieira, D.E.L.; Zarkov, A.; Ferreira, M.G.S.; Beganskiene, A.; Rubanik, V.V.; Shilin, A.D.; Kareiva, A.; Salak, A.N. Sonication Accelerated Formation of Mg-Al-Phosphate Layered Double Hydroxide via Sol-Gel Prepared Mixed Metal Oxides. Sci. Rep. 2019, 9, 10419. [Google Scholar] [CrossRef]
- Butenko, E.; Malyshev, A.; Kapustin, A. LDHs as Adsorbents of Phenol and Their Environmental Applications. Am. J. Environ. Prot. 2014, 2, 11–15. [Google Scholar] [CrossRef][Green Version]
- Mahjoubi, F.Z.; Khalidi, A.; Abdennouri, M.; Barka, N. Zn–Al Layered Double Hydroxides Intercalated with Carbonate, Nitrate, Chloride and Sulphate Ions: Synthesis, Characterisation and Dye Removal Properties. J. Taibah Univ. Sci. 2017, 11, 90–100. [Google Scholar] [CrossRef]
- Shan, X.; Guo, Z.; Zhang, C.; Wang, W.; Zhao, L. Nickel Aerogel @ Ultra-Thin NiCo-LDH Nanosheets Integrated Freestanding Film as a Collaborative Adsorption and Accelerated Conversion Cathode to Improve the Rate Performance of Lithium Sulfur Batteries. Chem. Eng. J. 2024, 488, 151105. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, S.; Xia, M.; Guo, Y.; Zhang, Y.; Liu, L.; Zhou, C.; Chen, G.; Wang, X.; Wu, Q.; et al. Elaborately Converting Hierarchical NiCo–LDH to Rod–like LDH–Decorated MOF as Interlayer for High–Performance Lithium–Sulfur Battery. Mater. Today Phys. 2023, 35, 101112. [Google Scholar] [CrossRef]
- Li, J.; Qiu, W.; Liu, X.; Zhang, Y.; Zhao, Y. NiCo-Layered Double Hydroxide to Composite with Sulfur as Cathodes for High-Performance Lithium-Sulfur Batteries. ChemElectroChem 2022, 9, e202101211. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, J.; Zhu, Y.; Jin, C.; Zhang, J.; Wu, Y.; Tang, W. In Situ Transformation of LDH into NiCo2S4-NiS2 Nano-Heterostructures on Hollow Carbon Boxes to Promote Sulfur Electrochemistry for High-Performance Lithium-Sulfur Batteries. Energy Fuels 2023, 37, 4711–4719. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Cu, Q.; Li, Y.; Li, H.; Li, Z.; Li, M.; Liao, H.; Li, G.; Li, G.; et al. Hollow and Hierarchical CuCo-LDH Nanocatalyst for Boosting Sulfur Electrochemistry in Li-S Batteries. Energy Mater. Adv. 2023, 4, 0032. [Google Scholar] [CrossRef]
- Dong, H.; Qi, S.; Wang, L.; Chen, X.; Xiao, Y.; Wang, Y.; Sun, B.; Wang, G.; Chen, S. Conductive Polymer Coated Layered Double Hydroxide as a Novel Sulfur Reservoir for Flexible Lithium-Sulfur Batteries. Small 2023, 19, 2300843. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, S.; Liu, K.; Li, Q.; Wang, H.; Jiang, J. New Anode Materials for Sodium-Ion Batteries: NixCa2-xAl-Cl LDH and CoyCa2-yAl-Cl LDH Prepared from Dechlorination Wastes. J. Energy Storage 2024, 101, 113991. [Google Scholar] [CrossRef]
- Li, Y.; Xu, C.; Li, D.; Zhang, Y.; Ni, G.; Xu, S.; Liu, B.; Huo, P. Sodium-Ion Batteries Enabled by CoSn-LDH Microspheres Anchored on Few-Layer Ti3C2 MXene. ACS Appl. Nano Mater. 2024, 7, 20598–20608. [Google Scholar] [CrossRef]
- Yin, Q.; Wang, T.; Song, Z.; Yang, S.; Miao, Y.; Wu, Y.; Sui, Y.; Qi, J.; Li, Y.; Zhao, D.; et al. Computational High-Throughput Screening of Layered Double Hydroxides as Cathodes for Chloride Ion Batteries. Chem. Eng. J. 2023, 459, 141545. [Google Scholar] [CrossRef]
- Zhang, G.; Xing, J.; Zhao, Y.; Yang, F. Hierarchical N,P Co-Doped Graphene Aerogels Framework Assembling Vertically Grown CoMn-LDH Nanosheets as Efficient Bifunctional Electrocatalyst for Rechargeable Zinc-Air Battery. J. Colloid. Interface Sci. 2021, 590, 476–486. [Google Scholar] [CrossRef]
- Bao, E.; Ren, X.; Wang, Y.; Zhang, Z.; Luo, C.; Liu, X.; Xu, C.; Chen, H. Advanced Hybrid Supercapacitors Assembled with CoNi LDH Nanoflowers and Nanosheets as High-Performance Cathode Materials. J. Energy Storage 2024, 82, 110535. [Google Scholar] [CrossRef]
- Wu, S.; Cai, D.; Tian, Z.; Guo, L.; Wang, Y. One-Step Synthesis of NiCo-MOF@LDH Hybrid Nanosheets for High-Performance Supercapacitor. J. Energy Storage 2024, 89, 111670. [Google Scholar] [CrossRef]
- Owusu, K.A.; Wang, Z.; Saad, A.; Boakye, F.O.; Mushtaq, M.A.; Tahir, M.; Yasin, G.; Liu, D.; Peng, Z.; Cai, X. Room Temperature Synthesis of Vertically Aligned Amorphous Ultrathin NiCo-LDH Nanosheets Bifunctional Flexible Supercapacitor Electrodes. Energy Environ. Mater. 2024, 7, e12545. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, Y.; Ye, H.; Chen, J.; Wan, L.; Du, C.; Zhang, Y.; Xie, M. Gradually Sulfurized Co-Ni LDH as Electrode for Highly-Stable Asymmetric Supercapacitor. J. Energy Storage 2024, 91, 112117. [Google Scholar] [CrossRef]
- Hu, J.; Pan, Y.; Zhang, Q.; Dong, Z.; Han, S. Constructing Flower-Shaped NiCo2S4 @CoAl-LDH Heterojunction Nanosheets Exhibits Extraordinary Electrochemical Behavior for a Light-Assisted Asymmetric Supercapacitor. Energy Fuels 2024, 38, 6459–6470. [Google Scholar] [CrossRef]
- Zhao, W.; Deng, J.; Li, M.; Du, G.; Fan, M.; Gao, H.; Yuan, Z. Rational Synthesis of Sea Urchin-like NiCo-LDH/Tannin Carbon Microsphere Composites Using Microwave Hydrothermal Technique for High-Performance Asymmetric Supercapacitor. Adv. Compos. Hybrid. Mater. 2025, 8, 215. [Google Scholar] [CrossRef]
- Kovalenko, V.; Kotok, V.; Murashevych, B. Layered Double Hydroxides as the Unique Product of Target Ionic Construction for Energy, Chemical, Foods, Cosmetics, Medicine and Ecology Applications. Chem. Rec. 2024, 24, e202300260. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yang, Y.; Tian, Y.; Wu, Q.; Lin, W.-F.; Wen, M. Collaborative Reconstruction of FeOOH/FeNiCo-LDH Heterogeneous Nanosheets for Enhancing Anion Exchange Membrane Seawater Electrolysis. J. Mater. Chem. A Mater. 2025, 13, 7136–7148. [Google Scholar] [CrossRef]
- Yang, L.; Lin, Q.; Guo, D.; Wu, L.; Guan, Z.; Jin, H.; Fang, G.; Chen, X.; Wang, S. Nitrogen Plasma Activates CoMn-Layered Double Hydroxides for Superior Electrochemical Oxygen Evolution. Inorg. Chem. 2023, 62, 17565–17574. [Google Scholar] [CrossRef]
- Shankar Naik, S.; Theerthagiri, J.; Nogueira, F.S.; Lee, S.J.; Min, A.; Kim, G.-A.; Maia, G.; Pinto, L.M.C.; Choi, M.Y. Dual-Cation-Coordinated CoFe-Layered Double-Hydroxide Nanosheets Using the Pulsed Laser Ablation Technique for Efficient Electrochemical Water Splitting: Mechanistic Screening by In Situ/Operando Raman and Density Functional Theory Calculations. ACS Catal. 2023, 13, 1477–1491. [Google Scholar] [CrossRef]
- Wu, K.; Shi, L.; Wang, Z.; Zhu, Y.; Tong, X.; He, W.; Wang, J.; Zheng, L.; Kang, Y.; Shan, W.; et al. A General Strategy to Generate Oxygen Vacancies in Bimetallic Layered Double Hydroxides for Water Oxidation. Chem. Commun. 2023, 59, 3138–3141. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Y.; Chen, L.; Zhao, W.; Han, L. Precise Design and in Situ Synthesis of Hollow Co9S8 @CoNi-LDH Heterostructure for High-Performance Supercapacitors. Dalton Trans. 2023, 52, 12978–12987. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yang, T.; Huang, L.; Jiang, H.; Xiao, J.; Ma, X.; Lou, H.; Xie, C.; Yang, Y. Interfacial Coupling Engineering Boosting Electrocatalytic Performance of CoFe Layered Double Hydroxide Assembled on N-Doped Porous Carbon Nanosheets for Water Splitting and Flexible Zinc-Air Batteries. ACS Appl. Mater. Interfaces 2023, 15, 52530–52541. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ke, Q.; Qiu, X.; Chen, J.; Chen, P.; Wang, S.; Luo, X.; Rao, B. NiCo Layered Double Hydroxide Nanocages for High-Performance Asymmetric Supercapacitors. Inorg. Chem. Front. 2023, 10, 2154–2164. [Google Scholar] [CrossRef]
- Zhai, H.; Wu, L.; Yu, L.; Li, L.; Wan, G.; Zhang, Y.; Yuan, X.; Wang, J.; Wang, G. Polypyrrole/NiFe-Layered Double Hydroxide Composite as an Anticorrosive Microwave Absorber. J. Mater. Chem. C Mater. 2024, 12, 11001–11011. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, B.; Wang, Y.; Cao, X.; Wang, J.; Lu, W.; Guo, Y. Gelatin-Based Hydrogel Functionalized with Dopamine and Layered Double Hydroxide for Wound Healing. Gels 2024, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi Zardkhoshoui, A.; Arian, R.; Hosseiny Davarani, S.S. Tunable Construction of CuS Nanosheets@flower-like ZnCo-Layered Double Hydroxide Nanostructures for Hybrid Supercapacitors. Ind. Chem. Mater. 2023, 1, 443–457. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Meng, Q.; Xiao, M.; Liu, C.; Xing, W.; Zhu, J. Facilitating Active NiOOH Formation via Mo Doping towards High-Efficiency Oxygen Evolution. Catal. Sci. Technol. 2024, 14, 4166–4173. [Google Scholar] [CrossRef]
- Lin, X.; Wang, Z.; Cao, S.; Hu, Y.; Liu, S.; Chen, X.; Chen, H.; Zhang, X.; Wei, S.; Xu, H.; et al. Bioinspired Trimesic Acid Anchored Electrocatalysts with Unique Static and Dynamic Compatibility for Enhanced Water Oxidation. Nat. Commun. 2023, 14, 6714. [Google Scholar] [CrossRef]
- Das, J.P.; Nardekar, S.S.; Kesavan, D.; Bhunia, K.; Ravichandran, V.; Kim, S.-J. Unveiling the Effect of Growth Time on Bifunctional Layered Hydroxide Electrodes for High-Performance Energy Storage and Green Energy Conversion. J. Mater. Chem. A Mater. 2024, 12, 20179–20190. [Google Scholar] [CrossRef]
- Chen, S.; Zheng, Z.; Li, Q.; Wan, H.; Chen, G.; Zhang, N.; Liu, X.; Ma, R. Boosting Electrocatalytic Water Oxidation of NiFe Layered Double Hydroxide via the Synergy of 3d–4f Electron Interaction and Citrate Intercalation. J. Mater. Chem. A Mater. 2023, 11, 1944–1953. [Google Scholar] [CrossRef]
- Matsuda, K.; Okuda, A.; Kawashimo, M.; Fukuzaki, R.; Iio, N.; Tarutani, N.; Katagiri, K.; Inumaru, K. Molecular-Level Pictures of Chemical and Structural Transformations of Mg–Al Layered Double Hydroxide Crystals (Mg/Al = 2) at Elevated Temperatures. J. Phys. Chem. C 2023, 127, 12599–12605. [Google Scholar] [CrossRef]
- Dillenburger, J.D.; Schulte, L.; Mahale, P.; Suleiman, M.; Mallouk, T.E. Anion-Dependent Structure, Dehydration, and Hydroxide Ion Conductivity of Magnesium Aluminum Layered Double Hydroxides. Chem. Mater. 2023, 35, 6437–6446. [Google Scholar] [CrossRef]
- Fang, J.; Wen, D.; Zhang, Z.; Zheng, D.; Meng, A.; Su, Y.; Zhou, S. CoNiMn-Layered Double Hydroxide/Ti3C2Tx Nanosheet Composites for Enhanced Pseudocapacitive Performance. J. Phys. Conf. Ser. 2023, 2566, 012004. [Google Scholar] [CrossRef]
- Zou, L.; Sun, S.; Zhang, C.; Zhao, X. NiTi-Layered Double Hydroxide@Carbon Nanotube as a Cathode Material for Chloride-Ion Batteries. Nanomaterials 2023, 13, 2779. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Liu, Q.; Lu, Z.; Liu, J.; Chen, R.; Li, R.; Song, D.; Jing, X.; Liu, P.; Wang, J. Rational Design of Sandwiched Ni-Co Layered Double Hydroxides Hollow Nanocages/Graphene Derived from Metal-Organic Framework for Sustainable Energy Storage. ACS Sustain. Chem. Eng. 2017, 5, 9923–9934. [Google Scholar] [CrossRef]
- Xu, J.; Tang, R.; Liu, M.; Xie, S.; Zhang, D.; Kong, X.; Jin, S.; Ji, H.; Zhang, T. Enhancing the Catalytic Activity of Layered Double Hydroxide Supported on Graphene for Lithium–Sulfur Redox Reactions. Batteries 2022, 8, 200. [Google Scholar] [CrossRef]
- Hu, J.; Yuan, C.; Zhi, L.; Zhang, H.; Yuan, Z.; Li, X. In Situ Defect-Free Vertically Aligned Layered Double Hydroxide Composite Membrane for High Areal Capacity and Long-Cycle Zinc-Based Flow Battery. Adv. Funct. Mater. 2021, 31, 2102167. [Google Scholar] [CrossRef]
- Zhang, D.; Cao, J.; Zhang, X.; Insin, N.; Wang, S.; Han, J.; Zhao, Y.; Qin, J.; Huang, Y. Inhibition of Manganese Dissolution in Mn2O3 Cathode with Controllable Ni2+ Incorporation for High-Performance Zinc Ion Battery. Adv. Funct. Mater. 2021, 31, 2009412. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, E.; Gao, J.; Yang, J.; Wu, C.; Jiang, L.; Zhu, M.; Sun, G. An Exceptionally Facile Synthesis of Highly Efficient Oxygen Evolution Electrodes for Zinc-Oxygen Batteries. ChemElectroChem 2017, 4, 2190–2195. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, J.; Li, Z.; Yang, W.; Wang, B.; Hou, M.; He, Y.; Liu, Q.; Mann, T.; Yang, P.; et al. Graphene Nanosheet/Ni2+ /Al3+ Layered Double-Hydroxide Composite as a Novel Electrode for a Supercapacitor. Chem. Mater. 2011, 23, 3509–3516. [Google Scholar] [CrossRef]
- Zhang, D.; Cao, J.; Zhang, X.; Zeng, Z.; Insin, N.; Qin, J.; Huang, Y. Modification Strategies of Layered Double Hydroxides for Superior Supercapacitors. Adv. Energy Sustain. Res. 2022, 3, 2100183. [Google Scholar] [CrossRef]
- Malak-Polaczyk, A.; Vix-Guterl, C.; Frackowiak, E. Carbon/Layered Double Hydroxide (LDH) Composites for Supercapacitor Application. Energy Fuels 2010, 24, 3346–3351. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, Z.; Zhang, Y.; Shao, W.; Wu, D.; Peng, G.; Liu, Z. Introduction of Bimetallic Oxide-Modified Carbon Nanotubes for Boosting the Energy Storage Performance of NiCo-LDH Based in-Plane Micro-Supercapacitors on Paper. Chem. Eng. J. 2024, 494, 153242. [Google Scholar] [CrossRef]
- Wang, J.; Ding, Q.; Bai, C.; Wang, F.; Sun, S.; Xu, Y.; Li, H. Synthesis of CNTs/CoNiFe-LDH Nanocomposite with High Specific Surface Area for Asymmetric Supercapacitor. Nanomaterials 2021, 11, 2155. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hu, L.; Chen, M.; Yan, Y.; Wu, L. Nickel–Cobalt Layered Double Hydroxide Nanosheets for High-performance Supercapacitor Electrode Materials. Adv. Funct. Mater. 2014, 24, 934–942. [Google Scholar] [CrossRef]
- Li, L.; Hui, K.S.; Hui, K.N.; Xia, Q.; Fu, J.; Cho, Y.-R. Facile Synthesis of NiAl Layered Double Hydroxide Nanoplates for High-Performance Asymmetric Supercapacitor. J. Alloys Compd. 2017, 721, 803–812. [Google Scholar] [CrossRef]
- Shah, S.S. Biomass-Derived Carbon Materials for Advanced Metal-Ion Hybrid Supercapacitors: A Step Towards More Sustainable Energy. Batteries 2024, 10, 168. [Google Scholar] [CrossRef]
- Jayababu, N.; Kim, D. CuCo LDHs Coated CuCoTe Honeycomb-Like Nanosheets as a Novel Anode Material for Hybrid Supercapacitors. Small 2021, 17, 2102369. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, M.; Zhao, D.; Li, H.; Wang, C.; Yin, L. Molecular-Level Heterostructures Assembled from Titanium Carbide MXene and Ni–Co–Al Layered Double-Hydroxide Nanosheets for All-Solid-State Flexible Asymmetric High-Energy Supercapacitors. ACS Energy Lett. 2018, 3, 132–140. [Google Scholar] [CrossRef]
- Yang, J.; Yu, C.; Fan, X.; Qiu, J. 3D Architecture Materials Made of NiCoAl-LDH Nanoplates Coupled with NiCo-Carbonate Hydroxide Nanowires Grown on Flexible Graphite Paper for Asymmetric Supercapacitors. Adv. Energy Mater. 2014, 4, 1400761. [Google Scholar] [CrossRef]
- Jia, H.; Wang, M.; Feng, M.; Li, G.; Li, L.; Liu, Y. Synergistic Enhancement of Supercapacitor Performance: Modish Designation of BPQD Modified NiCo-LDH/NiCo2S4 Hybrid Nanotube Arrays with Improved Conductivity and OH− Adsorption. Chem. Eng. J. 2024, 484, 149591. [Google Scholar] [CrossRef]
- Luo, C.-W.W.; Zhang, K.; Zeng, H.-Y.Y.; Yan, W.; Lv, S.-B.B.; Wu, G.-Z.Z. Engineering ZnNi-LDH with Improved Wettability by N-Direct-Doping for High-Performance Supercapacitor. J. Alloys Compd. 2025, 1010, 177406. [Google Scholar] [CrossRef]
- Wei, X.; Li, M.; Li, L.; Feng, W.; Wu, H. Black Phosphorus Quantum Dots Embedded NiCoCu-LDH with Porous Structure for High-Performance Hybrid Supercapacitor. Appl. Surf. Sci. 2025, 686, 162203. [Google Scholar] [CrossRef]
- Moradi, M.; Afkhami, A.; Madrakian, T.; Moazami, H.R.; Tirandaz, A. Partial Hydrothermal Sulfidation of Electrosynthesized Co-Mn Layered-Double-Hydroxide as an Active Material for Supercapacitor Applications. J. Power Sources 2025, 629, 235993. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Wang, F.; Zhang, X.; Wu, X.; Zhang, J.; Niu, S.; Chen, L. Introducing Ce into the Interface of NiCo LDH@PBAs to Build Multi Core–Shell Electrode with Superior Stability of Supercapacitor. Chem. Eng. J. 2024, 488, 150932. [Google Scholar] [CrossRef]
- Hu, W.; Hu, B.; Wu, Z.; Zuo, M.; Song, Y.; Zheng, Q.; Shan, G.; Du, M. Exceeding 50 000 Cycle Durability of Layered Hydroxide-Based Hybrid Supercapacitor Through Scandium Doping-Induced Superlong Activation Process. Small Struct. 2024, 5, 2300574. [Google Scholar] [CrossRef]
- Chen, R.X.; Cheng, L. Preparation of Flower-Like Layered Double Hydroxides Supercapacitor Cathode Based on Biomineralization Strategy. Key Eng. Mater. 2024, 993, 3–8. [Google Scholar] [CrossRef]
- Bhandari, R.; Shah, R.R. Hydrogen as Energy Carrier: Techno-Economic Assessment of Decentralized Hydrogen Production in Germany. Renew. Energy 2021, 177, 915–931. [Google Scholar] [CrossRef]
- Terlouw, T.; Bauer, C.; McKenna, R.; Mazzotti, M. Large-Scale Hydrogen Production via Water Electrolysis: A Techno-Economic and Environmental Assessment. Energy Environ. Sci. 2022, 15, 3583–3602. [Google Scholar] [CrossRef]
- Haque, M.A.; Kawawaki, T.; Negishi, Y. Navigating Challenges and Possibilities for Improving Polymer Electrolyte Membrane Fuel Cells via Pt Electrocatalyst, Support and Ionomer Advancements. Int. J. Hydrogen Energy 2024, 85, 30–47. [Google Scholar] [CrossRef]
- Zhang, B.; Ma, C.; Wang, J.; Zhang, X.; Tu, J. Layered Hydroxides as Electrocatalysts for Water Splitting. In Metal Oxides and Related Solids for Electrocatalytic Water Splitting; Elsevier: Amsterdam, The Netherlands, 2022; pp. 241–272. ISBN 9780323857352. [Google Scholar]
- Ye, S.; Xu, Y.; Bai, X.; Liang, Z.; Liu, Q.; Wei, Q.; Yang, D.; Yang, W.; Gao, F.; Shi, Q. Efficient Bifunctional Water Splitting Catalysts Enabled by Crystalline-Amorphous NixSy@NiFe LDH Heterojunctions. J. Mater. Chem. A Mater. 2025. [Google Scholar] [CrossRef]
- Xia, X.; Wang, S.; Liu, D.; Wang, F.; Zhang, X.; Zhang, H.; Yu, X.; Pang, Z.; Li, G.; Chen, C.; et al. Electronic Modulation in Cu Doped NiCo LDH/NiCo Heterostructure for Highly Efficient Overall Water Splitting. Small 2024, 20, 2311182. [Google Scholar] [CrossRef]
- Huo, J.M.; Ma, Z.L.; Wang, Y.; Cao, Y.J.; Jiang, Y.C.; Li, S.N.; Chen, Y.; Hu, M.C.; Zhai, Q.G. Monodispersed Pt Sites Supported on NiFe-LDH from Synchronous Anchoring and Reduction for High Efficiency Overall Water Splitting. Small 2023, 19, 2207044. [Google Scholar] [CrossRef] [PubMed]
- Tucker-Quiñónez, A.M.; Rivadeneira-Mendoza, B.F.; Gorozabel-Mendoza, M.L.; Pérez-Almeida, I.B.; García-Guerrero, A.J.; Dueñas-Rivadeneira, A.A.; Yadav, K.K.; Zambrano-Intriago, L.A.; Rodríguez-Díaz, J.M. Challenges and Potential of Layered Double Hydroxides as Electrocatalytic Materials for Hydrogen Production from Water: A Review of Recent Advances and Applications. Energy Nexus 2025, 17, 100399. [Google Scholar] [CrossRef]
- He, H.; Xiao, J.; Liu, Z.; Yang, B.; Wang, D.; Peng, X.; Zeng, L.; Li, Z.; Lei, L.; Qiu, M.; et al. Boosting the Hydrogen Evolution of Layered Double Hydroxide by Optimizing the Electronic Structure and Accelerating the Water Dissociation Kinetics. Chem. Eng. J. 2023, 453, 139751. [Google Scholar] [CrossRef]
- Jia, Q.; Liu, E.; Jiao, L.; Li, J.; Mukerjee, S. Current Understandings of the Sluggish Kinetics of the Hydrogen Evolution and Oxidation Reactions in Base. Curr. Opin. Electrochem. 2018, 12, 209–217. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhong, C.J. Hydrogen Production from Water Electrolysis: Role of Catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef]
- de Chialvo, M.R.G.; Chialvo, A.C. Hydrogen Evolution Reaction: Analysis of the Volmer-Heyrovsky-Tafel Mechanism with a Generalized Adsorption Model. J. Electroanal. Chem. 1994, 372, 209–223. [Google Scholar] [CrossRef]
- You, H.; Wu, D.; Si, D.; Cao, M.; Sun, F.; Zhang, H.; Wang, H.M.; Liu, T.F.; Cao, R. Monolayer NiIr-Layered Double Hydroxide as a Long-Lived Efficient Oxygen Evolution Catalyst for Seawater Splitting. J. Am. Chem. Soc. 2022, 144, 9254–9263. [Google Scholar] [CrossRef]
- Jadhav, H.S.; Roy, A.; Desalegan, B.Z.; Seo, J.G. An Advanced and Highly Efficient Ce Assisted NiFe-LDH Electrocatalyst for Overall Water Splitting. Sustain. Energy Fuels 2019, 4, 312–323. [Google Scholar] [CrossRef]
- Shivakumar, P.; Monika, M.N.; Deepu, M.; Manjunatha Kumara, K.S.; Budagumpi, S.; Nagaraju, D.H. Amorphous and Crystalline Heterostructure MoSe2/NiFe-LDH through p-n Junction Formation for Electrochemical Water Splitting-Synergistic Back Bonding Effect. Int. J. Hydrogen Energy 2024, 65, 74–82. [Google Scholar] [CrossRef]
- Liu, G.; Huang, C.; Yang, Z.; Su, J.; Zhang, W. Ultrathin NiMn-LDH Nanosheet Structured Electrocatalyst for Enhanced Electrocatalytic Urea Oxidation. Appl. Catal. A Gen. 2021, 614, 118049. [Google Scholar] [CrossRef]
- Cao, H.; Liu, B.; Bai, J.; Li, C.; Xu, G. Interfacial Engineering of Hierarchical Ultra-Thin NiCo-LDH Nanosheet Superstructures Nanofiber for Water Cracking Electrocatalysis. J. Alloys Compd. 2025, 1010, 178041. [Google Scholar] [CrossRef]
- Yan, F.; Guo, D.; Kang, J.; Liu, L.; Zhu, C.; Gao, P.; Zhang, X.; Chen, Y. Fast Fabrication of Ultrathin CoMn LDH Nanoarray as Flexible Electrode for Water Oxidation. Electrochim. Acta 2018, 283, 755–763. [Google Scholar] [CrossRef]
- Bodhankar, P.M.; Sarawade, P.B.; Singh, G.; Vinu, A.; Dhawale, D.S. Recent Advances in Highly Active Nanostructured NiFe LDH Catalyst for Electrochemical Water Splitting. J. Mater. Chem. A Mater. 2021, 9, 3180–3208. [Google Scholar] [CrossRef]
- Fu, X.; Liao, H.; Zhang, Z.; Zheng, Y.; Lu, J.; Cheng, S.; Jiang, Y.; Gao, Y. Medium-Entropy Heterostructure of Crystalline NiCoFeP @ Amorphous NiCoFe-LDH for Industrial-Current Density and Ultrastable Overall Water Splitting. Chem. Eng. J. 2025, 505, 159520. [Google Scholar] [CrossRef]
- Hong, C.; Ji, J.; Huang, J.; Zhang, Y.; Li, L. NiMo/NiFe-LDH Heterostructured Electrocatalyst for Hydrogen Production from Water Electrolysis. Mater. Lett. 2025, 379, 137664. [Google Scholar] [CrossRef]
- Hou, Y.; Lu, Q.; Guo, Z.; Zhang, Y.; Zhang, Y.; Wan, X.; Lei, L. One-Step Electrodeposition Synthesis of Composites of NiFeMn Alloy and Its Hydroxide as an Efficient Bifunctional Water Splitting Catalyst. J. Solid. State Chem. 2025, 348, 125356. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, P.; Zhong, W.; Zheng, X.; Cai, W. Active-Site-Enriched Cu-Doped Ni-Fe Layered Double Hydroxide Nanosheets for Boosting the Oxygen Evolution Reaction. New J. Chem. 2023, 47, 9536–9539. [Google Scholar] [CrossRef]
- Guo, J.; Wang, K.; Zhang, H.; Zhang, H. Enhanced Electrocatalytic Activity of Mo-Doped NiFe Layered Double Hydroxide Nanosheet Arrays for the Hydrogen Evolution Reaction. ACS Appl. Nano Mater. 2023, 6, 379–389. [Google Scholar] [CrossRef]
- Ding, L.; Li, K.; Xie, Z.; Yang, G.; Yu, S.; Wang, W.; Cullen, D.A.; Yu, H.; Zhang, F.Y. W-Induced Morphological Modification of NiFe Layered Double Hydroxides as Efficient Electrocatalysts for Overall Water Splitting. Electrochim. Acta 2021, 395, 139199. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.; Yu, X.; Li, J.; Wang, K.; Niu, J. Interfacial Interaction in NiFe LDH/NiS2/VS2 for Enhanced Electrocatalytic Water Splitting. Molecules 2024, 29, 951. [Google Scholar] [CrossRef] [PubMed]
- Udayakumar, A.; Dhandapani, P.; Ramasamy, S.; Angaiah, S. Layered Double Hydroxide (LDH)—MXene Nanocomposite for Electrocatalytic Water Splitting: Current Status and Perspective. ES Energy Environ. 2023, 20, 902. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, L.; Gao, G.; Chen, H.; Wang, B.; Zhou, J.; Soo, M.T.; Hong, M.; Yan, X.; Qian, G.; et al. A Heterostructure Coupling of Exfoliated Ni–Fe Hydroxide Nanosheet and Defective Graphene as a Bifunctional Electrocatalyst for Overall Water Splitting. Adv. Mater. 2017, 29, 1700017. [Google Scholar] [CrossRef]
- Yao, L.; Li, R.; Zhang, H.; Humayun, M.; Xu, X.; Fu, Y.; Nikiforov, A.; Wang, C. Interface Engineering of NiTe@CoFe LDH for Highly Efficient Overall Water-Splitting. Int. J. Hydrogen Energy 2022, 47, 32394–32404. [Google Scholar] [CrossRef]
- Wang, J.; Lv, G.; Wang, C. A Highly Efficient and Robust Hybrid Structure of CoNiN@NiFe LDH for Overall Water Splitting by Accelerating Hydrogen Evolution Kinetics on NiFe LDH. Appl. Surf. Sci. 2021, 570, 151182. [Google Scholar] [CrossRef]
- Kalusulingam, R.; Mariyaselvakumar, M.; Antonyraj, C.A.; Mathi, S.; Mikhailova, T.S.; Khubezhov, S.A.; Pankov, I.V.; Srinivasan, K.; Myasoedova, T.N. Highly Efficient Water Splitting with Pd-Integrated NiAl-LDH Nanosheets as Bifunctional Electrocatalysts. Energy Fuels 2023, 37, 13319–13330. [Google Scholar] [CrossRef]
- Zhai, P.; Zhang, Y.; Wu, Y.; Gao, J.; Zhang, B.; Cao, S.; Zhang, Y.; Li, Z.; Sun, L.; Hou, J. Engineering Active Sites on Hierarchical Transition Bimetal Oxides/Sulfides Heterostructure Array Enabling Robust Overall Water Splitting. Nat. Commun. 2020, 11, 5462. [Google Scholar] [CrossRef]
- Das, M.; Khan, Z.B.; Biswas, A.; Dey, R.S. Inter-Electronic Interaction between Ni and Mo in Electrodeposited Ni-Mo-P on 3D Copper Foam Enables Hydrogen Evolution Reaction at Low Overpotential. Inorg. Chem. 2022, 61, 18253–18259. [Google Scholar] [CrossRef]
- Govind Rajan, A.; Carter, E.A. Microkinetic Model for pH- And Potential-Dependent Oxygen Evolution during Water Splitting on Fe-Doped β-NiOOH. Energy Environ. Sci. 2020, 13, 4962–4976. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Liu, Y.; Zheng, Z.; Liu, B.; Chen, M.; Guan, G.; Yan, K. Recent Advances on Transition-Metal-Based Layered Double Hydroxides Nanosheets for Electrocatalytic Energy Conversion. Adv. Sci. 2023, 10, 2207519. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.Q.; Li, J.; Zhang, H.J.; Liu, C.; Che, G.; Liu, Z.Q. p–d Orbitals Coupling Heterosites of Ni2P/NiFe-LDH Interface Enable O–H Cleavage for Water Splitting. Adv. Funct. Mater. 2024, 34, 2411024. [Google Scholar] [CrossRef]
- Zheng, K.; Ren, J.; Li, X.; Li, G.; Jiao, L.; Xu, C. Engineering Crystalline CoMP-Decorated (M = Mn, Fe, Ni, Cu, Zn) Amorphous CoM LDH for High-Rate Alkaline Water Splitting. Chem. Eng. J. 2022, 441, 136031. [Google Scholar] [CrossRef]
- Feng, Y.; Smith, R.L.; Shen, F.; Qi, X. Structure-Oriented Electrochemical Synthesis of Layered Double Hydroxide Electrocatalytic Materials for 5-Hydroxymethylfurfural Oxidation. ACS Sustain. Chem. Eng. 2024, 12, 16905–16913. [Google Scholar] [CrossRef]
- Yi, W.; Yu, R.; Jiang, H.; Wu, J.; Cheng, G.J. Unveiling the Synergistic Potential of Laser Chemical Solid-Phase Deposition of Atomic Platinum-Metal Layer on 2D Materials for Bifunctional Catalysts. Adv. Funct. Mater. 2024, 34, 2308575. [Google Scholar] [CrossRef]
- Xiong, T.; Zhu, Z.; He, Y.; Balogun, M.-S.; Huang, Y. Phase Evolution on the Hydrogen Adsorption Kinetics of NiFe-Based Heterogeneous Catalysts for Efficient Water Electrolysis. Small Methods 2023, 7, 2201472. [Google Scholar] [CrossRef]
- Zhai, Z.; Li, H.; Zhou, C.; Zheng, H.; Liu, Y.; Yan, W.; Zhang, J. Anisotropic Strain Boosted Hydrogen Evolution Reaction Activity of F-NiCoMo LDH for Overall Water Splitting. J. Electrochem. Soc. 2023, 170, 036509. [Google Scholar] [CrossRef]
- Rosely, C.V.S.; John, H. Role of Surfactants on Electrocatalytic Activity of Co/Al Layered Double Hydroxides for Hydrogen and Oxygen Generation. ChemCatChem 2025, 17, 2401377. [Google Scholar] [CrossRef]
- Wang, J.; Wei, X.; Song, W.; Shi, X.; Wang, X.; Zhong, W.; Wang, M.; Ju, J.; Tang, Y. Plasmonic Enhancement in Water Splitting Performance for NiFe Layered Double Hydroxide-N10TC MXene Heterojunction. ChemSusChem 2021, 14, 1948–1954. [Google Scholar] [CrossRef]
- Xi, C.; Ding, W.; Jiang, J.; Wang, Y.; Wang, S.; Zhang, Z.; Han, S. Unveiling the CoFe LDH Generated by Partial in Situ Conversion Strategy: Synergistic Effect of Modified MXene and Cationic Vacancies for Overall Water Splitting. Fuel 2024, 370, 131844. [Google Scholar] [CrossRef]
- Shen, B.; Huang, H.; Jiang, Y.; Xue, Y.; He, H. 3D Interweaving MXene–Graphene Network–Confined Ni–Fe Layered Double Hydroxide Nanosheets for Enhanced Hydrogen Evolution. Electrochim. Acta 2022, 407, 139913. [Google Scholar] [CrossRef]
- Luo, H.; Liu, C.; Chen, N.; Xue, K.; Yu, L.; Zhu, H.; Zhang, Y. Metal-Organic Frameworks Derived NiFe-Hydroxides for Efficient Electrocatalytic Hydrogen Evolution. Adv. Comput. Mater. Sci. Res. 2024, 1, 192. [Google Scholar] [CrossRef]
- Lee, E.; Jeong, S.; Jeong, Y.; Kim, B.; Lee, K. Nanoscale-Confined Synthesis of 2D Metal Compounds for Electrochemical Applications. Small Methods 2025, 9, 2301782. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zheng, K.; Hua, Y.; Peng, L.; Meng, X.; Zhang, C.; Su, R.; Tao, X.; Hu, H.; Guo, Y.; et al. LDHs and Their Derivatives for Electrochemical Energy Storage and Conversion Systems: Design, Insights and Applications. ChemElectroChem 2024, 11, e202400156. [Google Scholar] [CrossRef]
- Yao, Y.; Zou, C.; Sun, S.; Guo, Y.; Hong, S.; Cai, Z.; Yang, C.; Zhuang, W.; Luo, F.; Hamdy, M.S.; et al. Ultrastable Seawater Oxidation at Ampere-level Current Densities with Corrosion-resistant CoCO3/CoFe Layered Double Hydroxide Electrocatalyst. Small 2025, 21, 2409627. [Google Scholar] [CrossRef]
- Ding, L.; Shen, Z.; Pan, H.; Chen, Y.; Hu, Y.; Zhao, G.; Hai, G.; Huang, X. Regulating Intermediate Adsorption and Promoting Charge Transfer of CoCr-LDHs by Ce Doping for Enhancing Electrooxidation of 5-Hydroxymethylfurfural. Small 2025, 21, 2409343. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Bai, X.; Zhao, J.; Yang, L.; Wang, X.; Wu, Q.; Hu, Z. Interlayer Engineering of Layered Double Hydroxides for Advanced Energy Storage and Conversion. FlatChem 2024, 48, 100775. [Google Scholar] [CrossRef]
- Wang, Y.; Ying, Z.; Gao, Y.; Shi, L. Layered Double Hydroxide Nanosheets: Synthesis Strategies and Applications in the Field of Energy Conversion. Chem. Eur. J. 2024, 30, e202303025. [Google Scholar] [CrossRef]
- Zhou, X.; Hou, Z.; Zhang, H.-Y.; Yu, J. Synergistic Coupling of NiVAl-Layered Double Hydroxide with Few-Layered Ti3C2Tx–MXene Nanosheets for Superior Asymmetric Supercapacitor Performance. J. Mater. Chem. C Mater. 2023, 11, 15571–15580. [Google Scholar] [CrossRef]
- Jaramillo Hernández, C.; Oestreicher, V.; Mizrahi, M.; Abellán, G. An In-Situ Study of the Transformation of Hybrid Layered Hydroxides into Metallic Nanocomposites with Interest in Energy Storage Systems. In Proceedings of the ECS Meeting Abstracts 2023, Boston, MA, USA, 28 May–2 June 2023; Volume MA2023-01, p. 1322. [Google Scholar] [CrossRef]
- Sun, X.; Chen, H.; Li, Y.; Zeng, D.; Qiu, P.; Zeng, H.; Ji, X.; Chen, L.; Shi, X. Efficient Harvesting Waste Heat by Zn-Ion Battery Under Thermally Regenerative Electrochemical Cycles. Adv. Mater. 2025, 37, 2418482. [Google Scholar] [CrossRef]
- Abu Nayem, S.M.; Hardianto, Y.P.; Shuaibu, A.D.; Shah, S.S.; Islam, S.; Jafar Mazumder, M.A.; Aziz, M.A.; Saleh Ahammad, A.J. Biomass Derived Amino Acid Assisted Synthesis of FeNi Layered Double Hydroxide for Efficient Oxygen Evolution Reaction. Inorg. Chem. Commun. 2025, 171, 113574. [Google Scholar] [CrossRef]
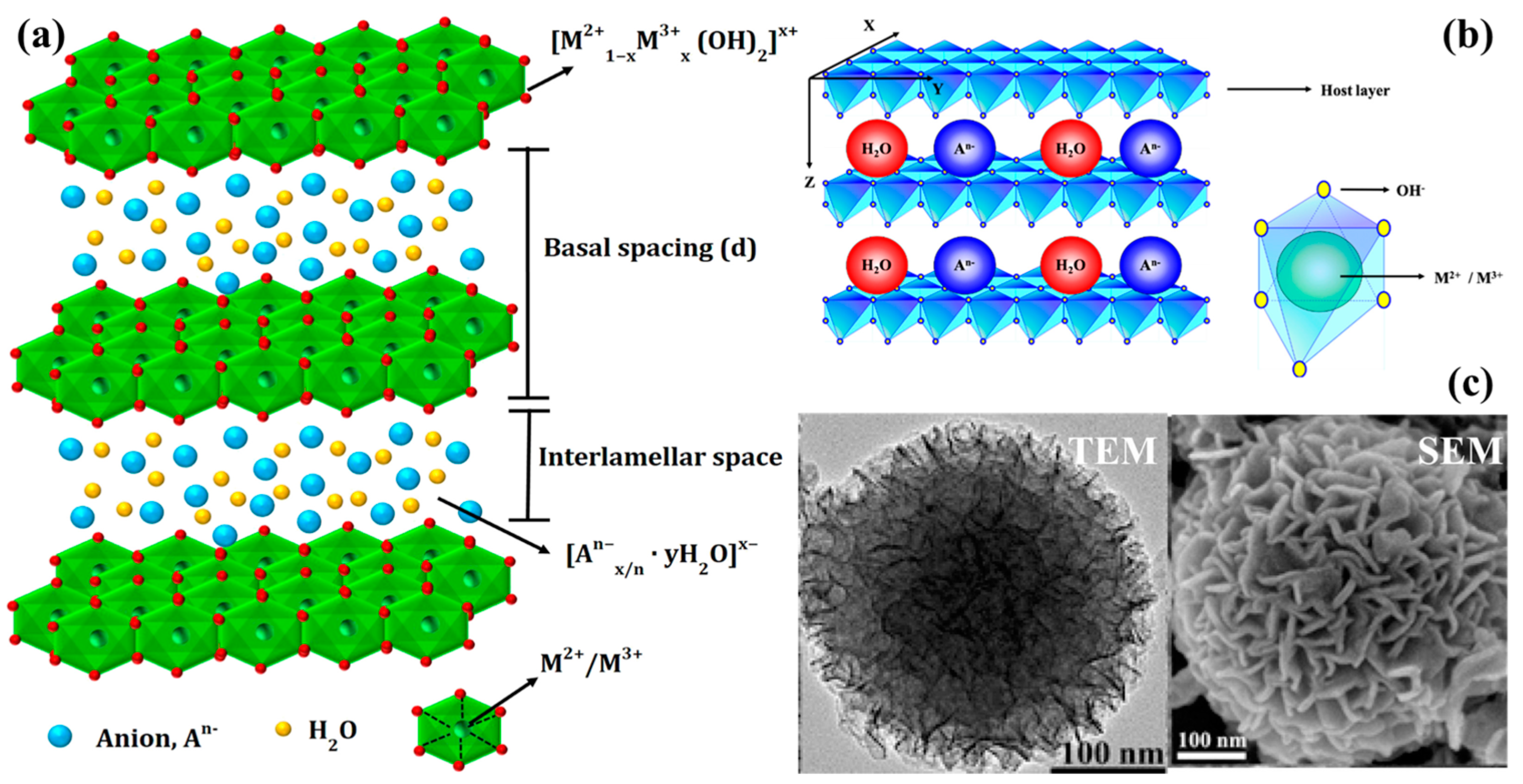

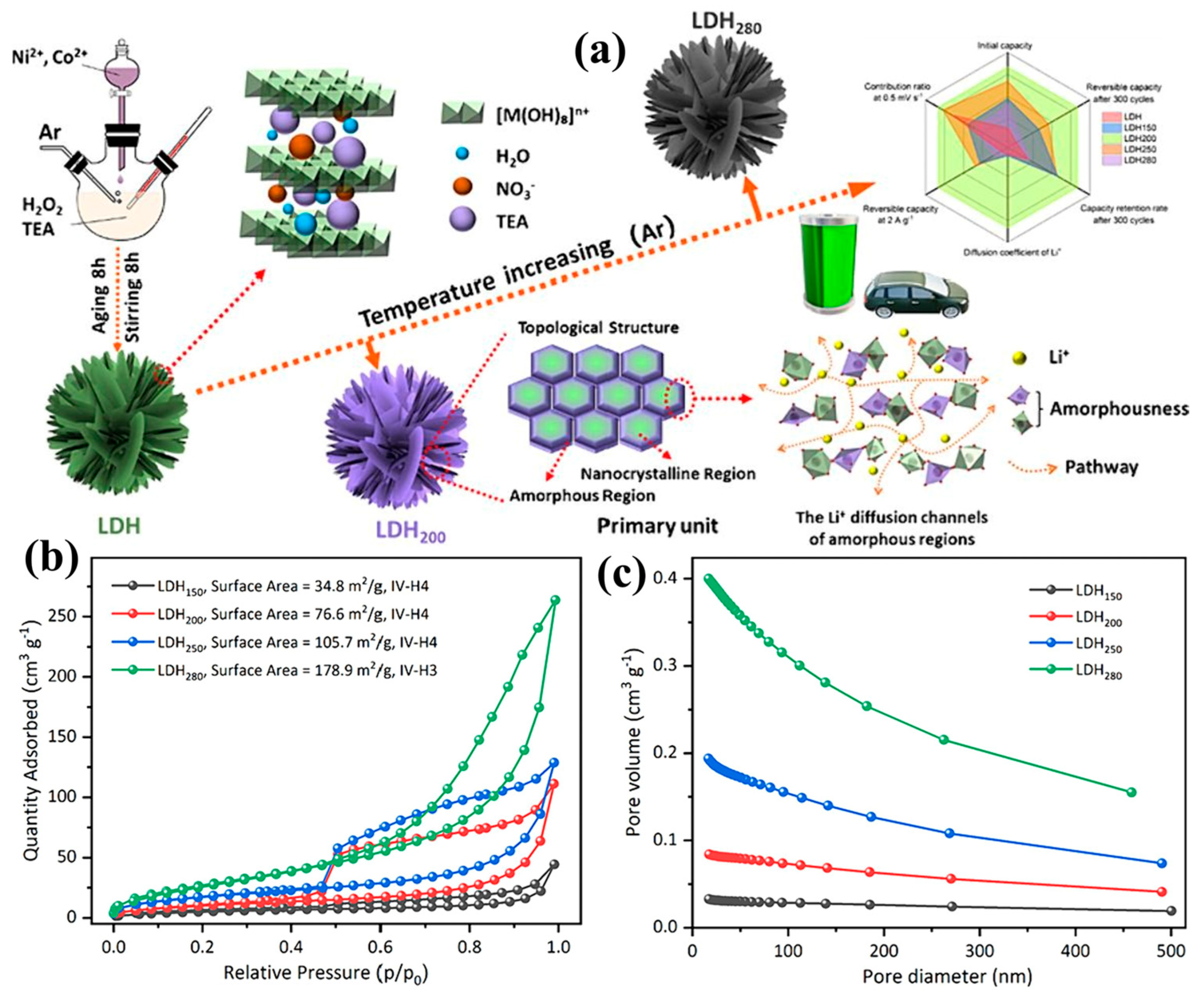
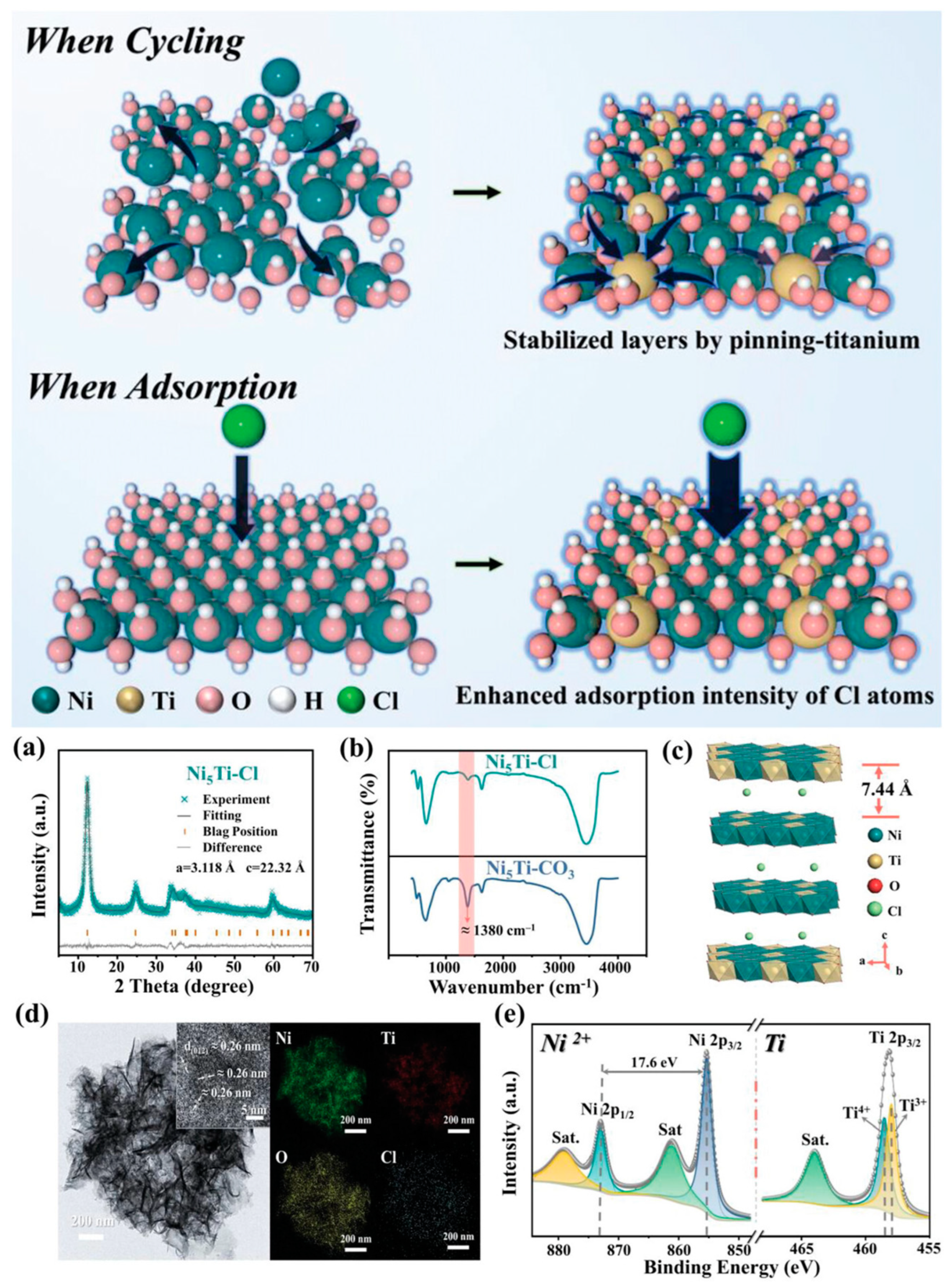

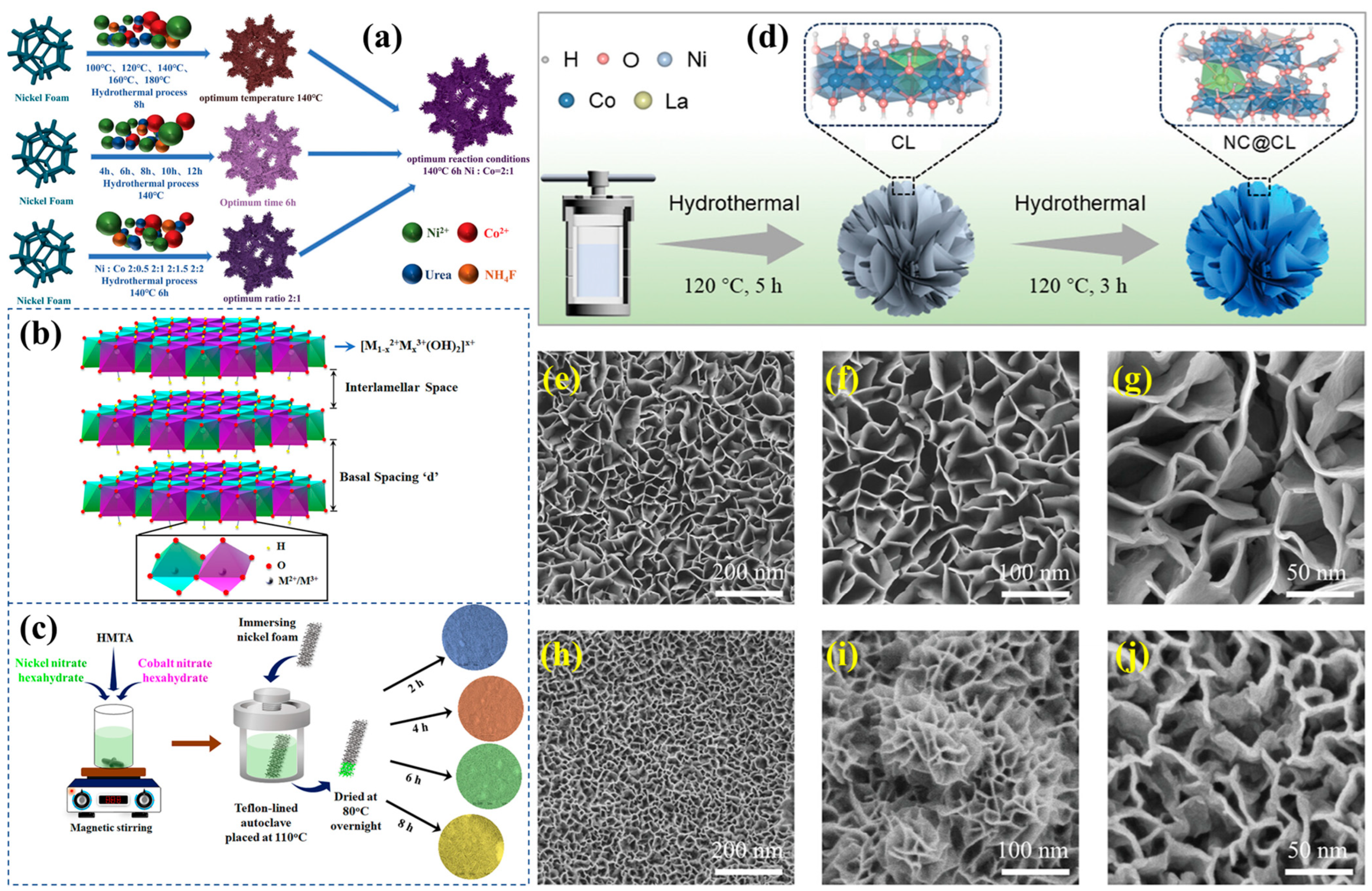
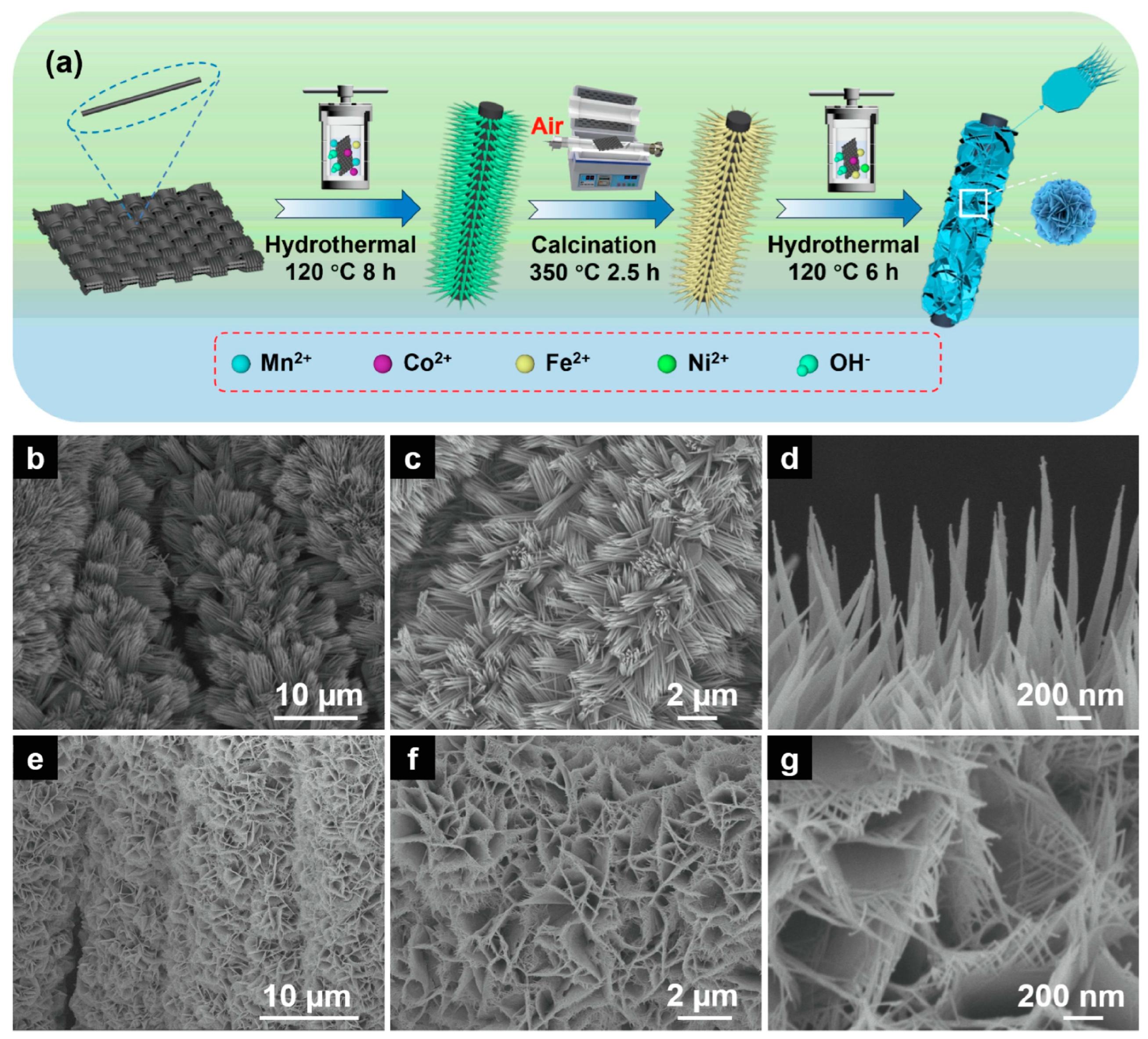
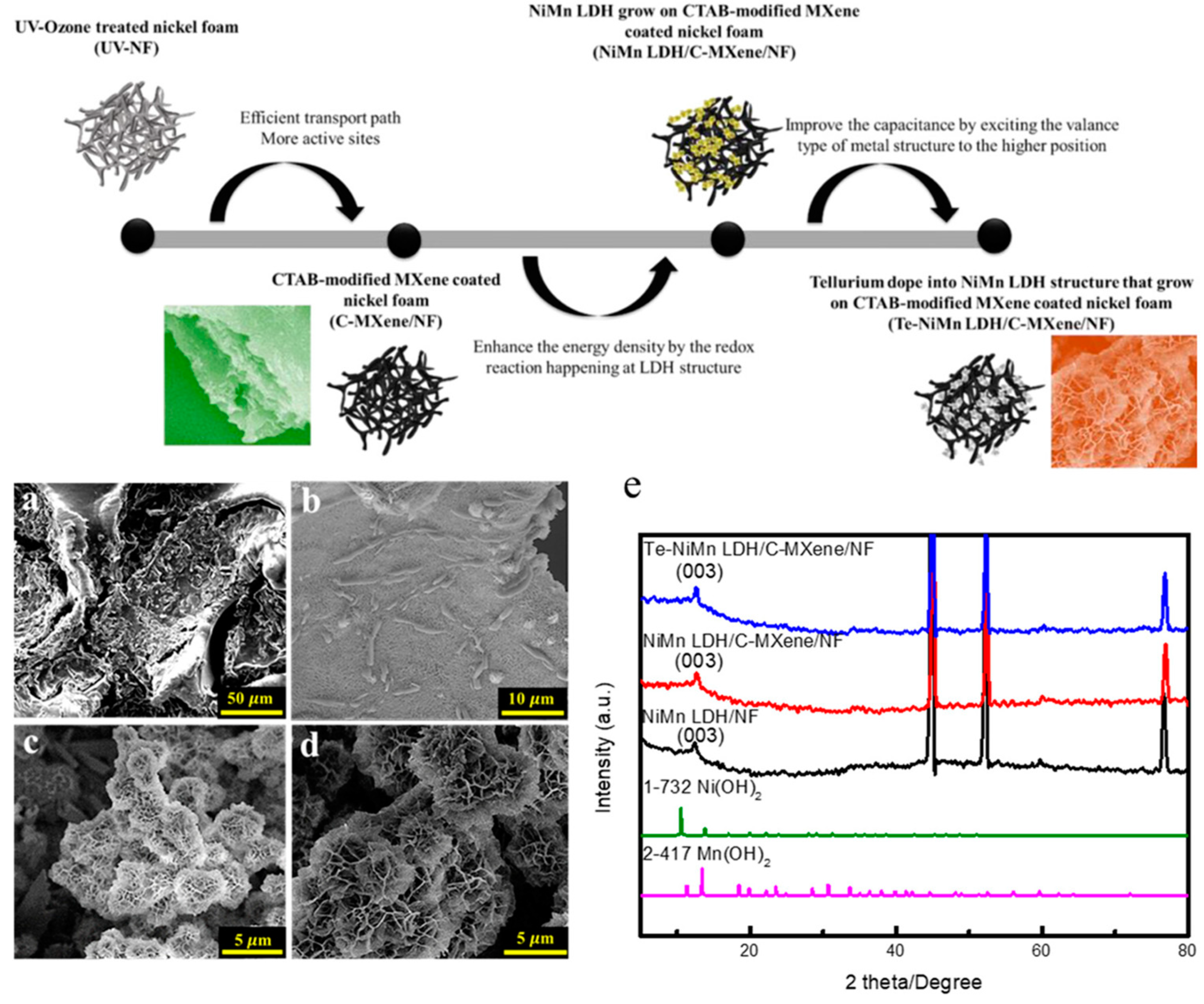
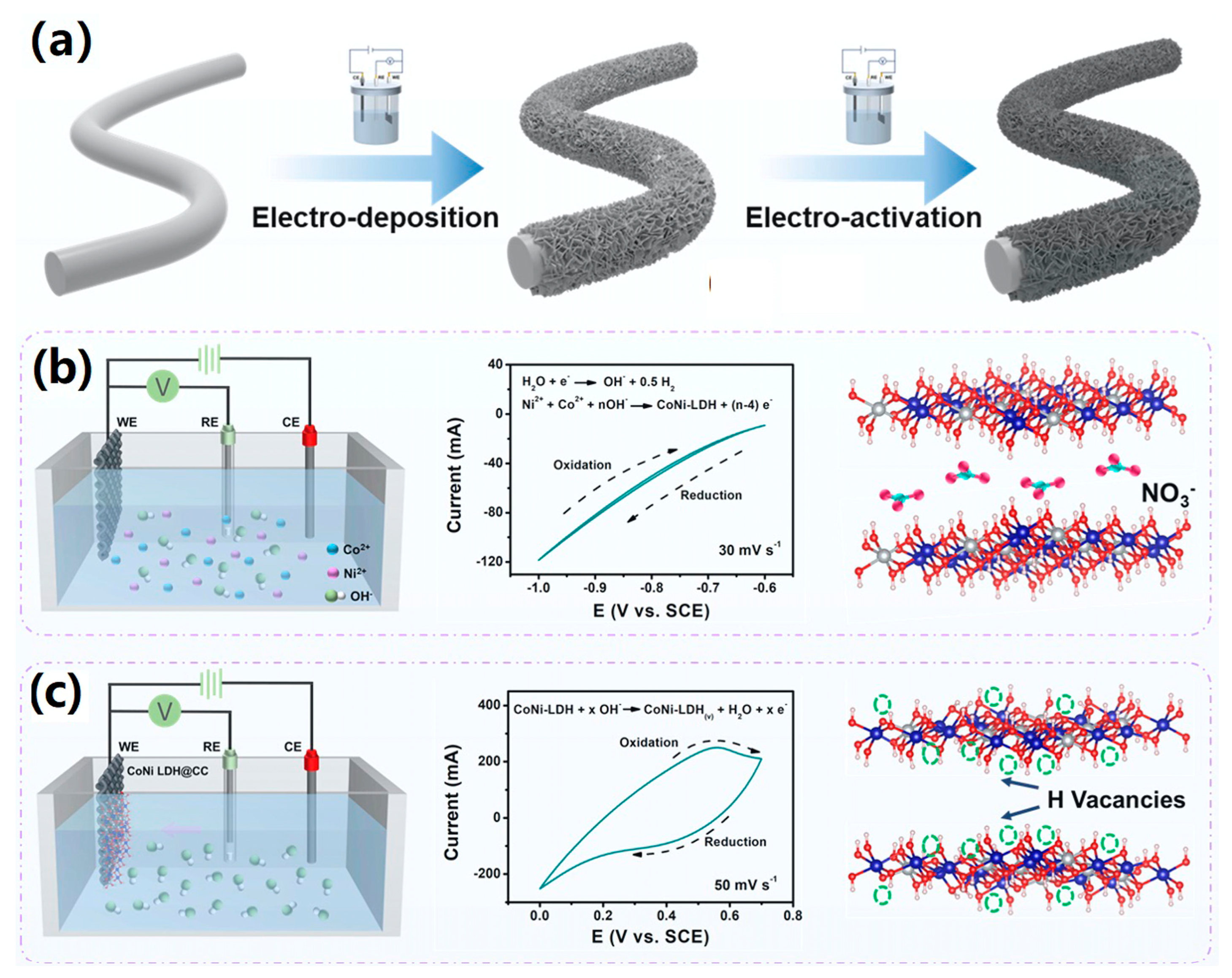




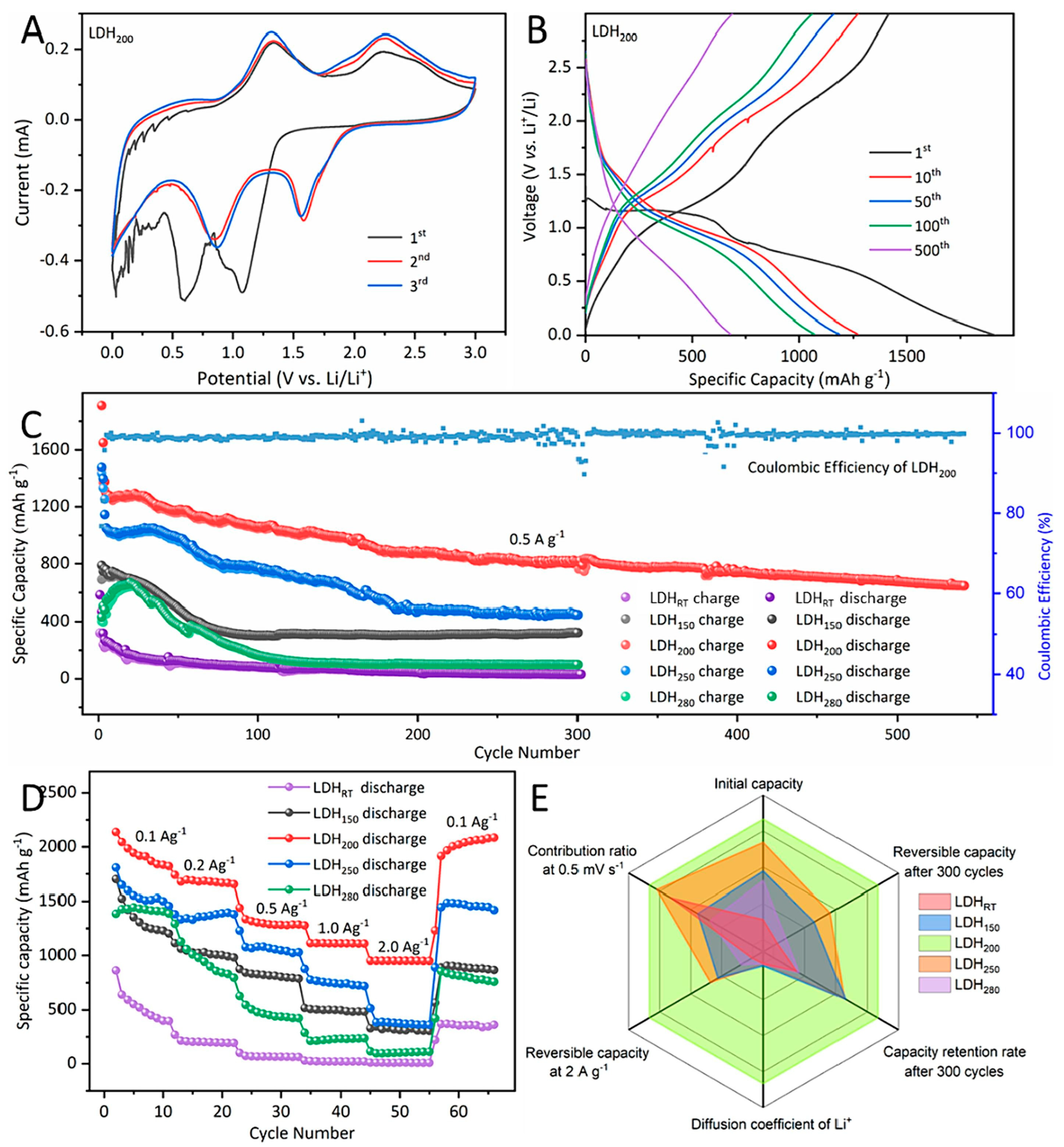
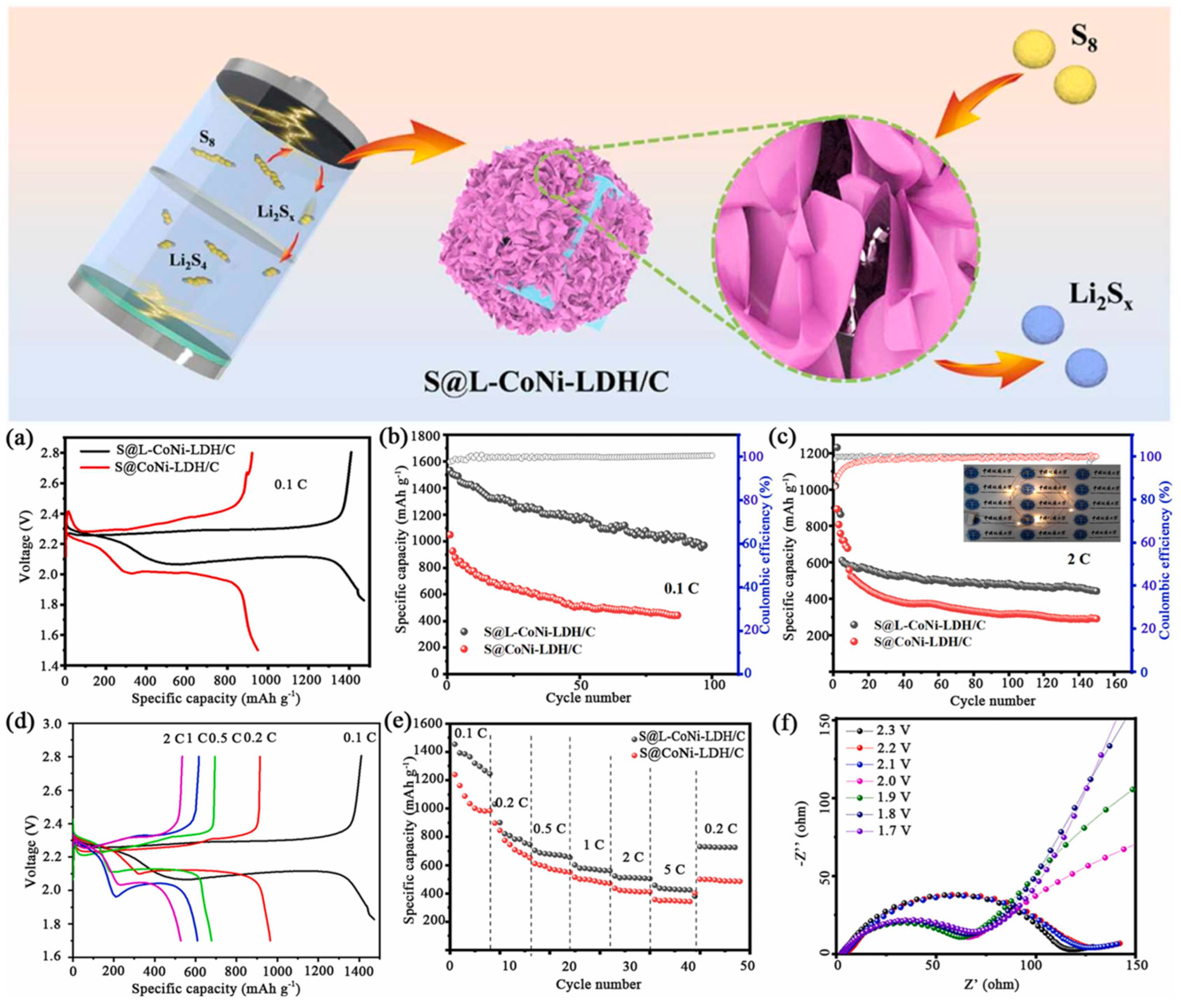
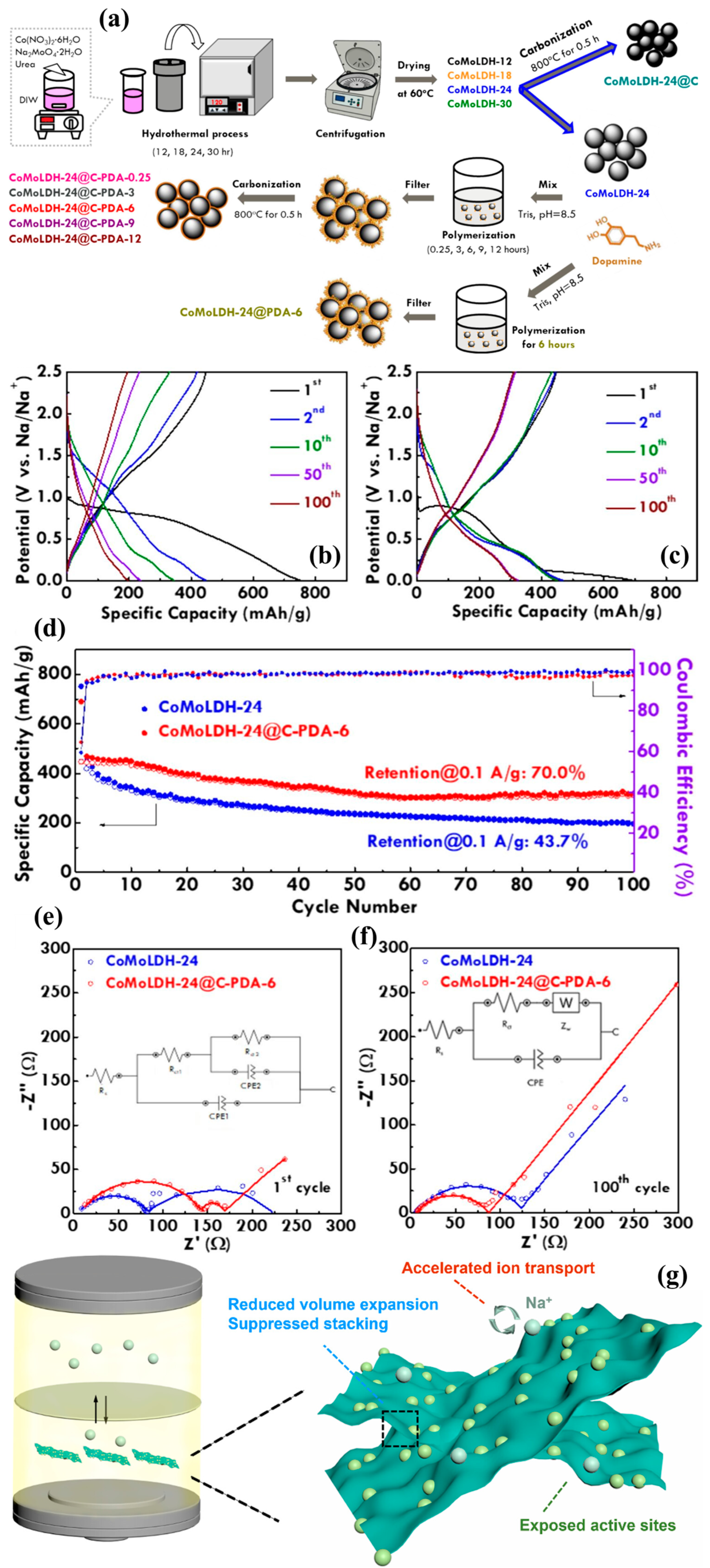

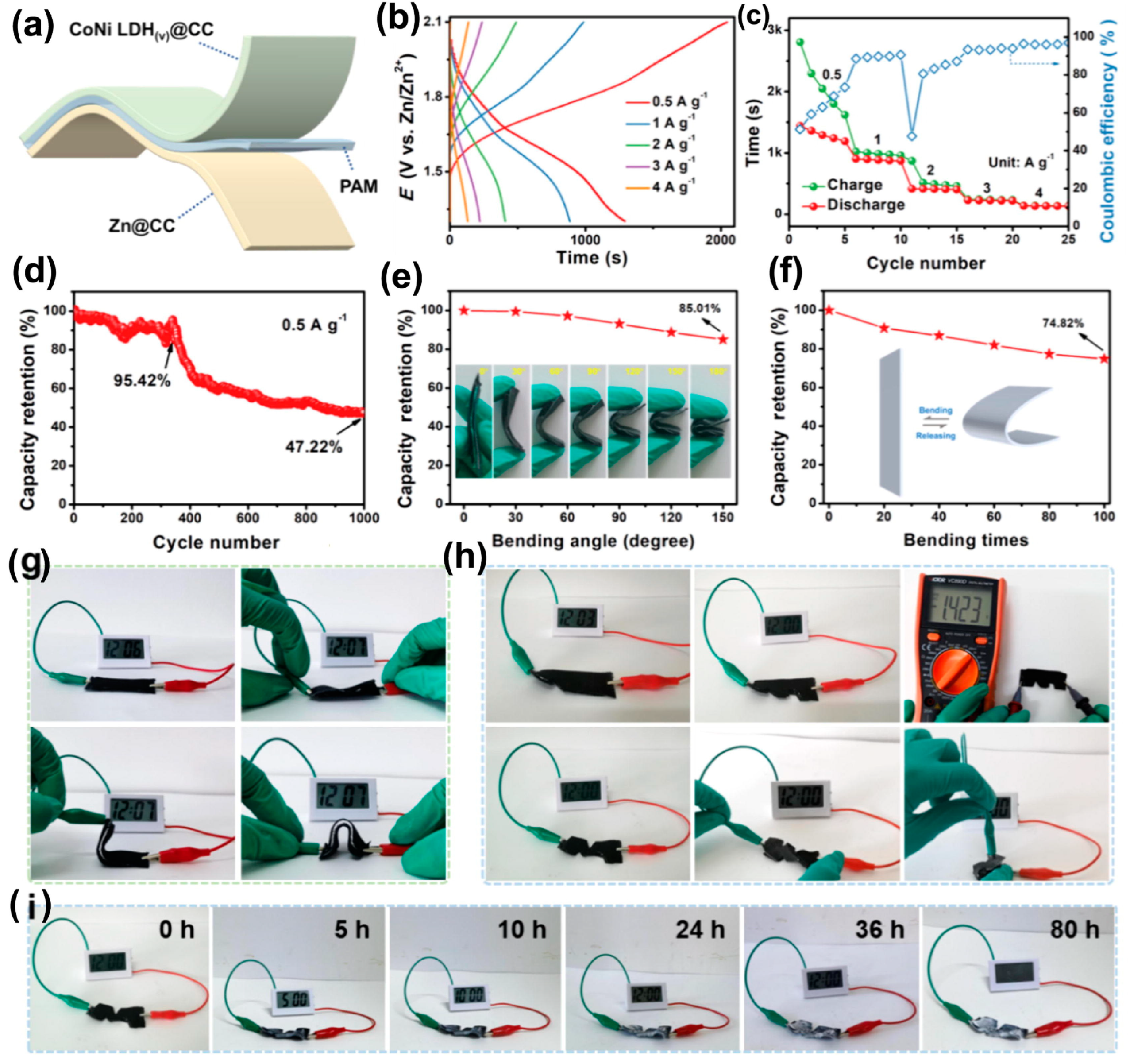
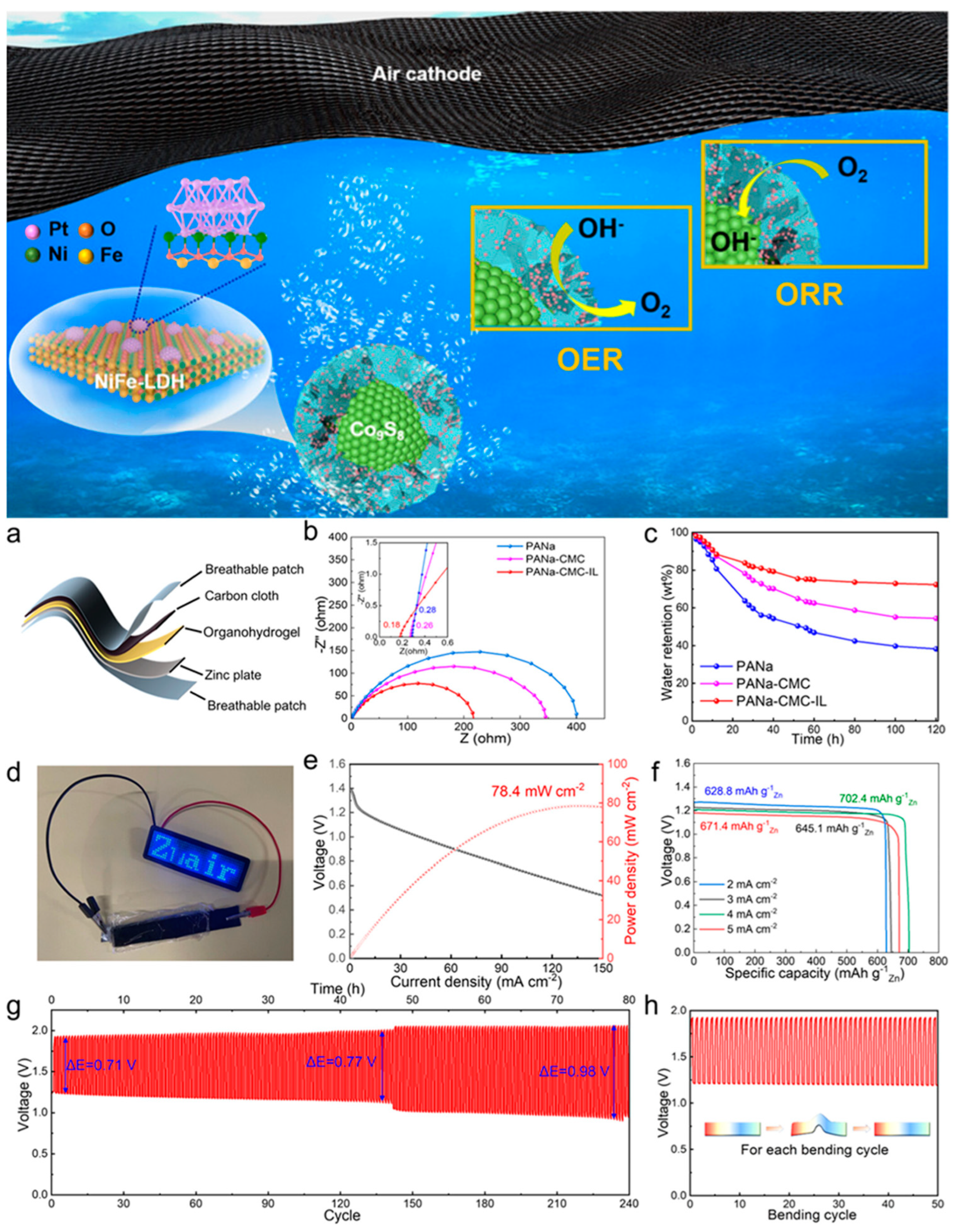

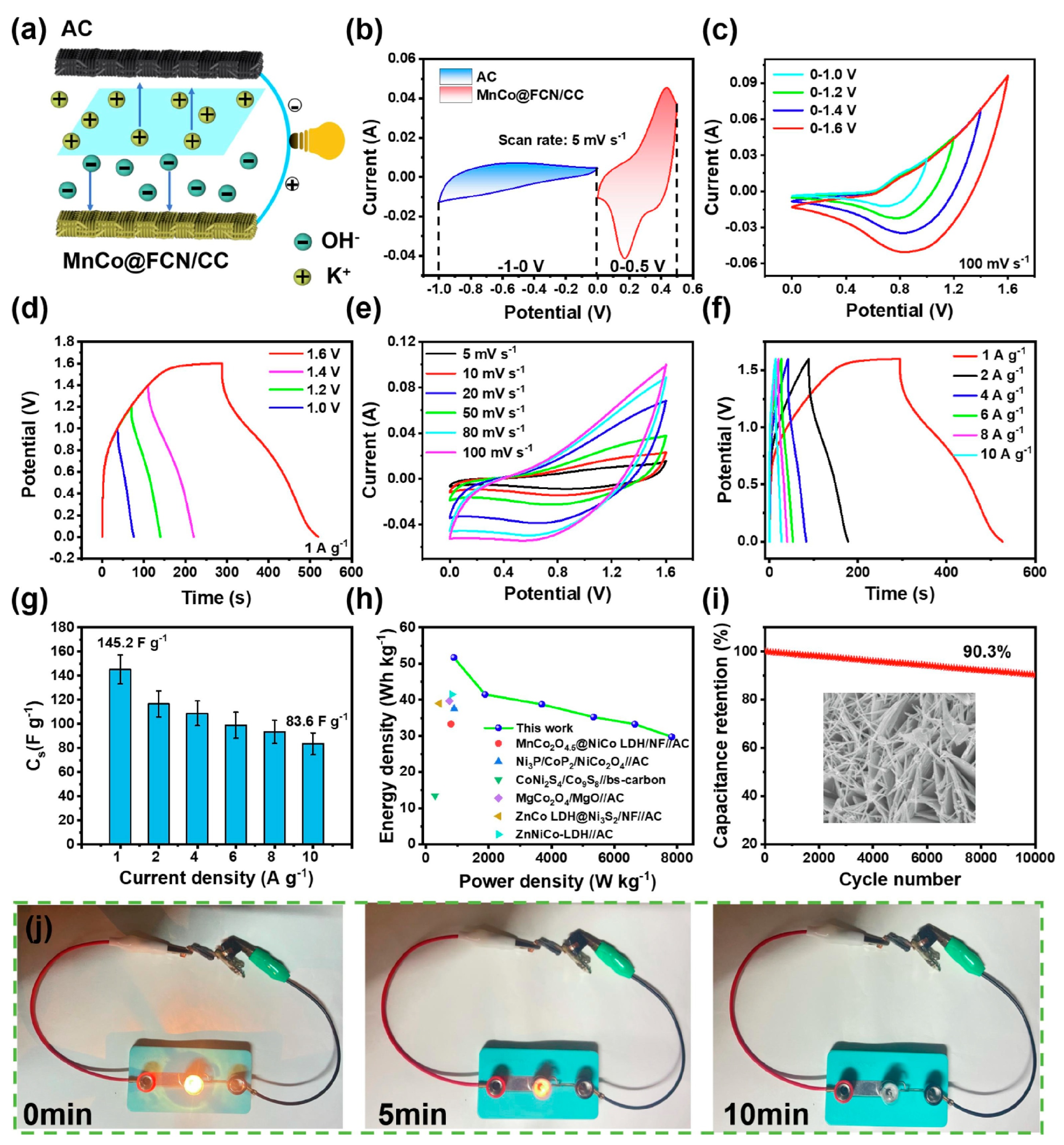
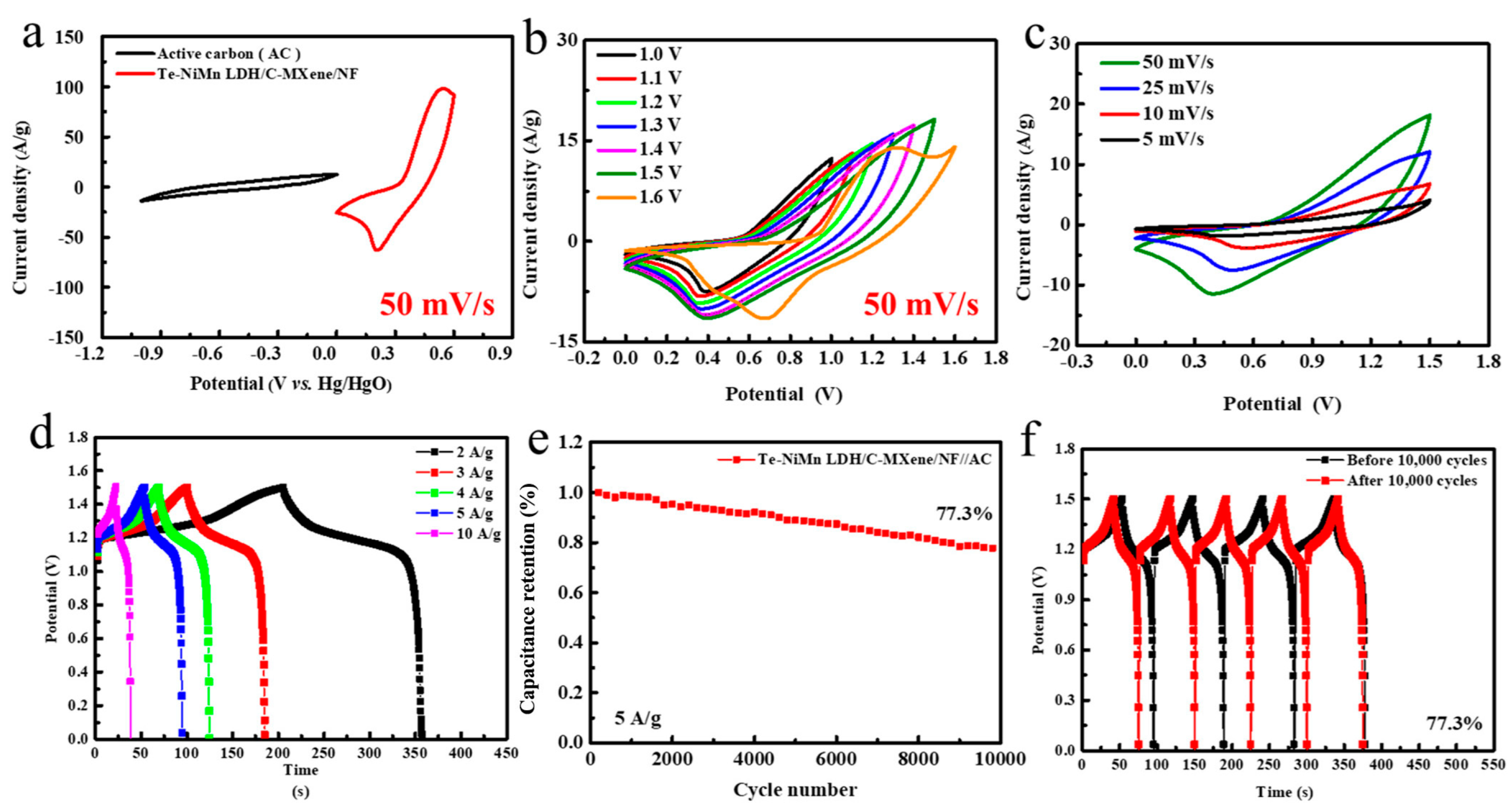


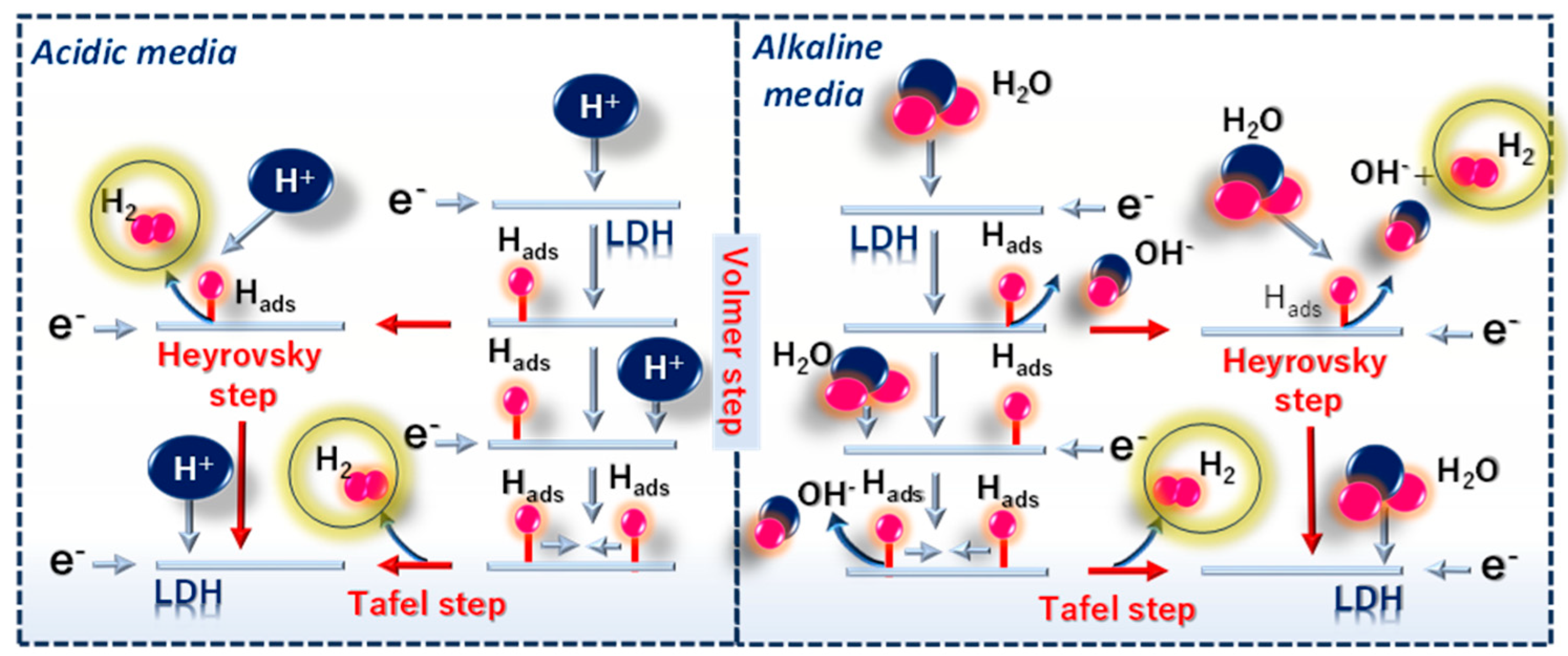
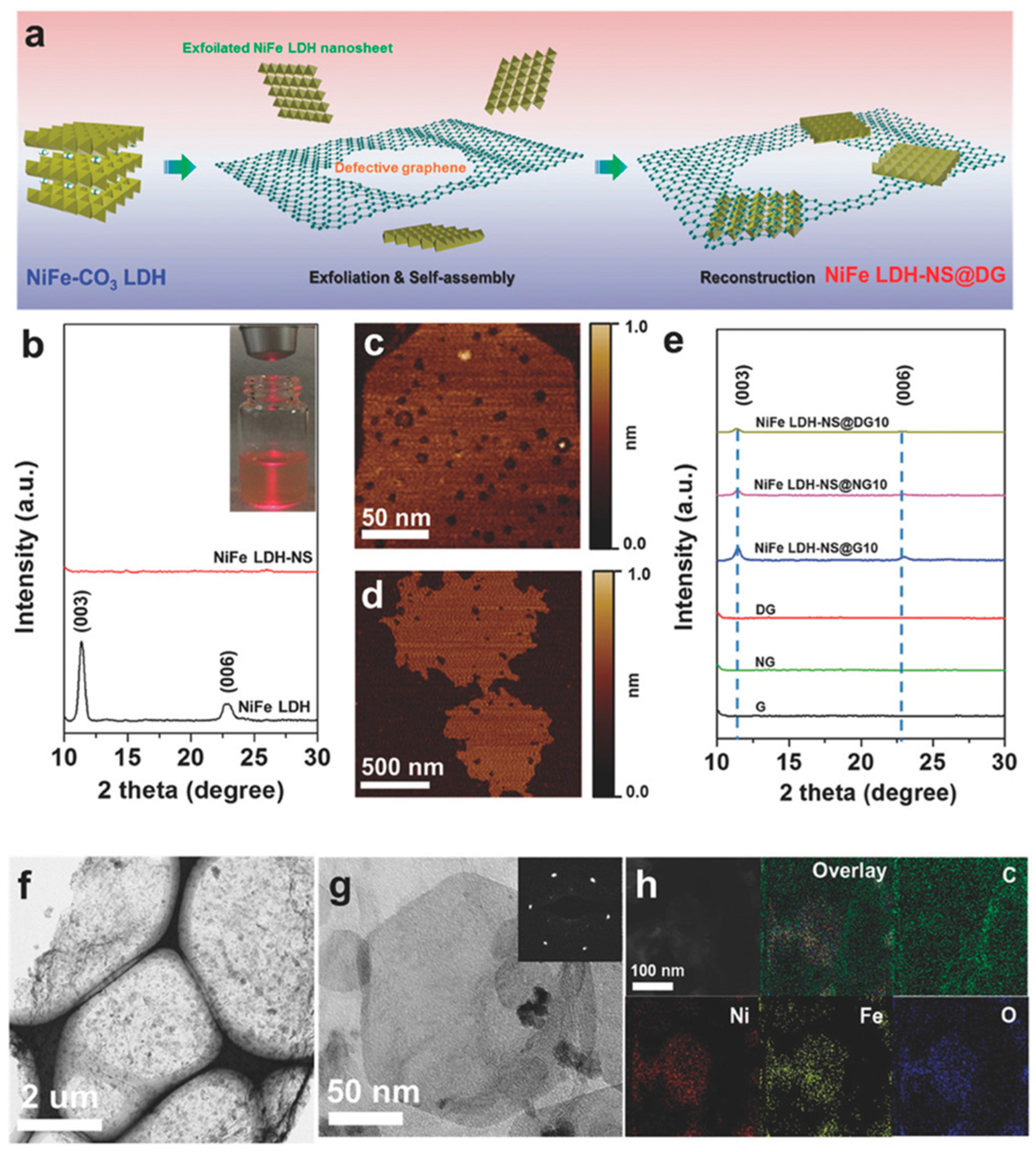


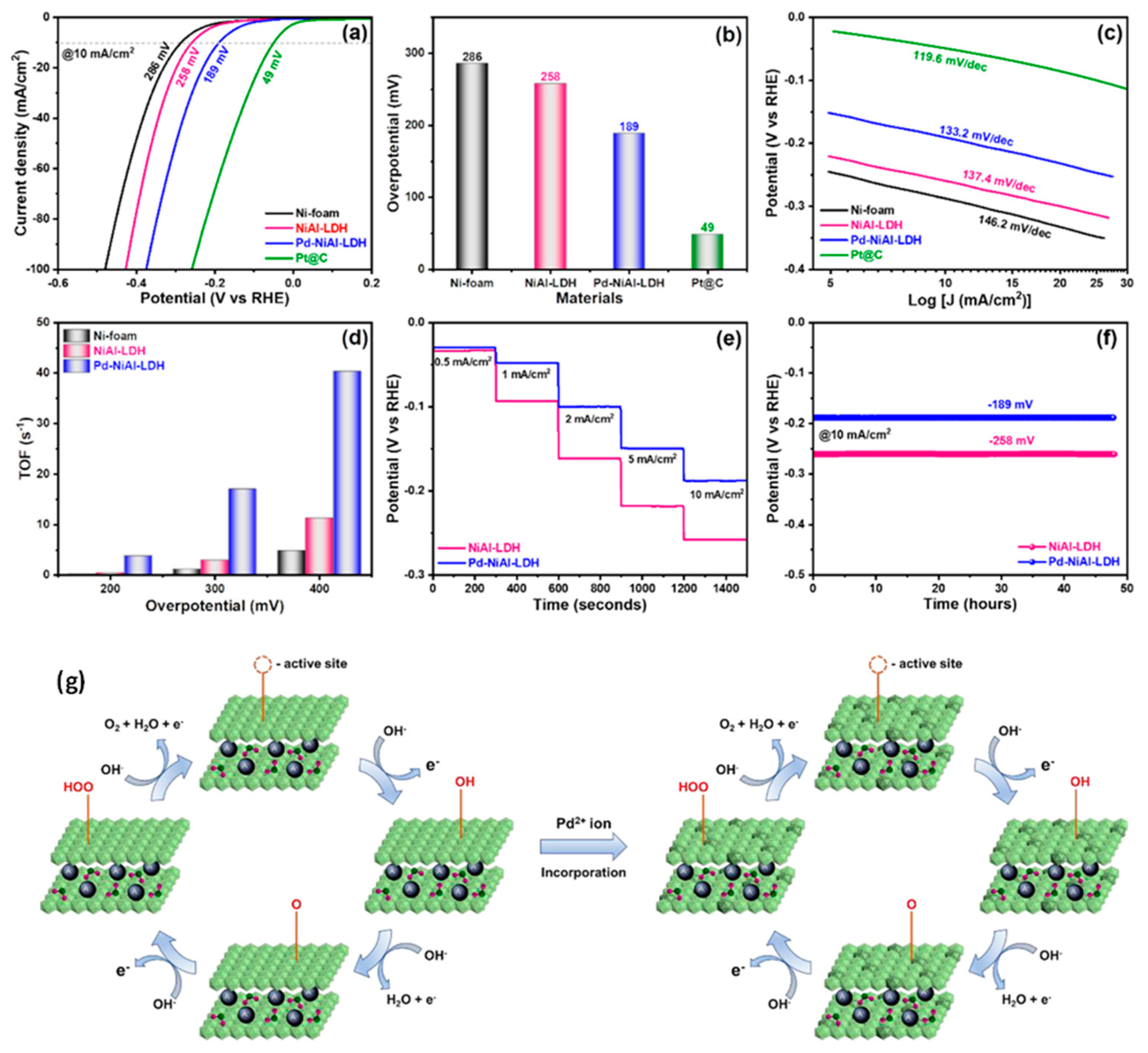
| LDH Name | Synthesis Procedure | Battery Type | Electrolyte | Specific Capacity (mAh/g) | Stability | Ref. |
|---|---|---|---|---|---|---|
| Hv-Ni3Mn0.7Fe0.3-LDH | Co-precipitation + Electrochemical Activation | ZIB | 0.2 M ZnSO4 | 328 | 85% capacity retention over 500 cycles at 1 A/g | [16] |
| NiCo-LDH nanocrystal@amorphousness core/shell structure (LDH200) | Co-precipitation followed by heat treatment at 200 °C | LIB | 1.0 M LiPF6 | 1821.3 (0.1 A/g) | ~687.7 mAh/g at 0.5 A/g after 500 cycles (attrition rate: 0.092%) | [109] |
| MgAl-LDH@CNT | Co-precipitation | LSB | Li6PS5Cl | … | Stable over 200 cycles at 1 C with 4.0 mg/cm2 S loading | [110] |
| NiFe-NO3 LDH | Co-precipitation | NIB | 1 M NaClO4 | 972 (initial); 388 (60 cycles at 50 mA/g); 122 (500 cycles at 500 mA/g) | 388 mAh/g after 60 cycles; 122 mAh/g after 500 cycles | [111] |
| NiFe-Cl LDH | Co-precipitation | CIB | 0.5 M Bpy14Cl/PC | 27.1 | 64.2mAh/g after 200 cycles | [112] |
| Ni5Ti-Cl LDH | Co-precipitation + anion exchange | CIB | 0.5 M Bpy14Cl | 257.8 | 127.9 mAh/g after 1000 cycles @1 A/g | [114] |
| CoFe/Fe3C@CN-900 | MOF-derived + Co-precipitation | ZAB | 0.1 M KOH | 795 | 96.5% current retention after 10 h discharge | [115] |
| Fe/Co LDH (CoL 2:1) | Co-precipitation | ZAB | 0.1 M KOH | 804 | 200 h (307 cycles) | [116] |
| ZIF-67@NiCo-LDH | One-pot hydrothermal synthesis | LIB | 1 M LiPF6 | 1997.1 (initial), 807.9 (after 100 cycles) | Stable up to 100 cycles, excellent rate capability | [134] |
| NiFe2O4@NiCo-LDH | Two-step hydrothermal method (MOF-derived) | LIB | 1 M LiPF6 | 636.9 | Stable after 100 cycles (636.9 mAh/g at 0.3 A/g), coulombic efficiency ~98% | [135] |
| Ni-Zn LDH intercalated with DS− | Hydrothermal synthesis | LIB | 1 M LiPF6 | 850 at 0.5 A/g | 850 mAh/g over 400 cycles | [136] |
| NiS2/FeS2@NC@NiFe LDH/FeO(OH) | Solvothermal followed by hydrothermal synthesis | LIB | 1 M LiPF6 | 709.9 | 709.9 mAh/g at 0.2 A/g after 200 cycles; 403.3 mAh/g at 1.0 A/g after 500 cycles | [137] |
| NiCo-LDH/MXene | Hydrothermal | LIB | 1 M LiPF6 | 1081 (at 100 mA/g) | 100 cycles at 0.1 A/g with excellent capacity retention | [138] |
| Mg2Al1–CO3-LDH | Hydrothermal synthesis | LIB | 1 M LiPF6 | 814 | 203.8 mAh/g after 300 cycles | [140] |
| NiCoOOH@CoLa-LDH (NC@CL) | Hydrothermal synthesis (2-step) | ZIB | 1 M KOH + 5 mM ZnO | 381.1 | 98% capacity retention after 2000 cycles | [141] |
| NiCoMo-P150 LDH | Hydrothermal + Anion exchange + Temperature-differential phosphorus doping | CIB | 0.5 M Bpy14Cl | 363.4 (initial), 150.2 (stable) | 800 cycles at 300 mA/g | [142] |
| NiAl-LDH@G | Atomic layer deposition + Hydrothermal | CIB | 0.5 M Bpy14Cl | 223.3 | 107 mAh/g after 500 cycles; 72 mAh/g after 120 days | [143] |
| Mo0.3NiCo2-Cl LDH | Hydrothermal synthesis + Ion exchange | CIB | 0.5 M Bpy14Cl | 352.5 | 159.7 mAh/g (after 300 cycles) | [144] |
| CoFe–Cl-LDH/CNT | Co-precipitation + Hydrothermal synthesis | CIB | 1.0 M NaCl | 190 | 125 mAh/g after 200 cycles | [145] |
| Ni-CAT/NiFe-LDH/CNFs | Hydrothermal synthesis + in situ MOF growth | ZAB | 6 M KOH | … | 66 h (liquid)/30 h (solid) | [146] |
| ZnCo2O4@NiMn-LDH (2:1) | Two-step hydrothermal | ZAB | 6 M KOH | 639 | 200 h cycling | [147] |
| NiCo-LDH/NCM@NF | Hydrothermal synthesis | ZAB | 1 M KOH | 685 | Stable over 500 cycles | [148] |
| Pt-NiFe-LDH@Co9S8 | Hydrothermal + Spontaneous-redox strategy | ZAB | 6.0 M KOH/0.2 M Zn(OAc)2 | 796.6 | 240 cycles (80 h, flex) | [149] |
| Co@NiFe-LDH | Two-step hydrothermal | ZAB | 0.2 M Zn(OAC)2 + 6 M KOH | 652 | 90 h cycling with minor degradation | [150] |
| Ni2Co-LDH/EG composites | Co-precipitation and Microwave irradiation | LIB | 1 M LiPF6 | 1880 (0.05 A/g); 919 (1 A/g) | ~973 mAh/g after 100 cycles at 1 A/g | [152] |
| NiAl LDH | Hydrothermal | LIB | 1 M LiPF6 | 2586 (initial), 697 (400th cycle @ 0.5 A/g) | 27.6% after 1400 cycles @ 1.0 A/g | [153] |
| L-CoNi-LDH/C | Laser-induced + Hydrothermal synthesis | LSB | 1.0 M LiTFSI | 1574 (initial), 1097 (100th) | 503 mAh/g after 200 cycles @ 2 C | [154] |
| LDH@PSS (1:1.5) | Hydrothermal + Exfoliation + Self-assembly | LSB | 1 M LiTFSI | 1247.2 (initial), 1032.6 (after 200 cycles @ 0.2 C) | 0.086% capacity decay per cycle over 200 cycles (0.2 C); 65.2% retention after 1000 cycles (1 C) | [155] |
| Vo-LDHs-MXenes | Self-growth aging | LSB | 1.0 M LiTFSI | 1549 (initial), 701 (after 300 cycles at 1 C) | 300 cycles at 1.0 C, 0.084% decay/cycle | [156] |
| V2O5/Cys/FeNi-LDH | Hydrothermal + Reflux | LSB | 1.0 M LiTFSI | 1035.2 (initial), 920.1 (after 300 cycles) | 88.9% retention after 300 cycles; 0.039% decay per cycle | [156] |
| NiFe-LDH@S | Hydrothermal | LSB | 1 M LiTFSI | 676 (initial), 386 (after 500 cycles at 2 C) | 386 mAh/g after 500 cycles @2 C | [157] |
| NiAl-LDH@S | Hydrothermal | LSB | 1 M LiTFSI | 432 (initial), 238 (after 500 cycles at 2 C) | 238 mAh/g after 500 cycles @2 C | [157] |
| ZnAl-LDH@S | Hydrothermal | LSB | 1 M LiTFSI | 357 (initial), 198 (after 500 cycles at 2 C) | 198 mAh/g after 500 cycles @2 C | [157] |
| NiCoAl-LDH | Hydrothermal + PECVD (for VG growth) | LSB | 1.0 M LiTFSI with 1 wt.% LiNO3 | 1182.4 (initial), 441.3 (after 750 cycles at 0.5 C) | 0.0755% capacity decay per cycle (750 cycles at 0.5 C) | [158] |
| NiCr-Cl LDH/rGO | Hydrothermal + Ion Exchange | NIB | 1 M NaCF3SO3 | 218 (200 cycles @ 100 mA/g) | 34.5% capacity retention after 200 cycles | [159] |
| CoMoLDH@C-PDA-6 | Hydrothermal + Polymerization + Carbonization | NIB | 1 M NaClO4 | 779.9 (initial); 310.9 (after 100 cycles) | 70% retention after 100 cycles at 0.1 A/g | [163] |
| Co-Co LDH-derived CoSe2@NHCNS@C | Hydrothermal synthesis + selenization + carbon coating | NIB | 1 M NaClO4 | 465.6 (initial), 373.8 (after 100 cycles) | 373.8 mAh/g after 100 cycles; 325 mAh/g after 1000 cycles at 0.5 A/g | [164] |
| MCN-LDH@CP | Two-step hydrothermal | ZIB | 3.0 M KOH | 1.74 mAh/cm2 | 97.8% @ 7000 cycles | [165] |
| ZnCo-LDH | Hydrothermal | Zn-ZnCo/Hybrid Zn | 6 M KOH + 0.2 M Zn(AC)2 | 2.5 mAh/cm2 | 86% after 5000 cycles | [166] |
| NiCo LDH/MXene@NF | Electrodeposition | ZIB | 1 M KOH | 311.7 | capacity retention of 88.6% after 10,000 cycles at 2 A/g | [203] |
| CoNi-LDH(v) | Electrochemical deposition + Electrochemical activation (ECA) | Aqueous and Solid-state ZIB | 3 M ZnSO4 | 225 | 53.9% after 900 cycles (aqueous); 47.22% after 1000 cycles (solid-state) | [204] |
| ZnAl-LDH@Zn | Electrochemical deposition | ZIB | 3 M ZnSO4 | 251.45 | Stable after 1500 cycles | [172] |
| CoNi LDH(v) | Electrochemical deposition + CV activation | ZIB | 1 M ZnSO4 | 185 | 80% retention after 1000 cycles | [207] |
| NiCoS@NiCo-LDH | Hydrothermal + Electrodeposition | ZIB | 6 M KOH | 312 | 95.9% after 3000 cycles @ 20 mA/cm2 | [208] |
| Ni3S2/NiS@NiCo-LDH | Hydrothermal + Electrochemical deposition | ZIB | 6 M KOH | 434.5 | 116.7% after 5000 cycles | [209] |
| NiFe LDH on Cu NWs | Electrochemical deposition | ZAB | 6 M KOH and 0.2 M zinc acetate | … | >250 h @ 10 mA/cm2 | [210] |
| NiCo-LDH | Sol–gel (Ni aerogel) + Solvothermal | LSB | 1.0 M LITFSI | 1238.4 (0.1 C); 805.8 (5.0 C) | 647.1 mAh/g after 700 cycles at 5.0 C (0.018% decay/cycle) | [222] |
| NiCo–MOF/LDH | Solvothermal synthesis | LSB | 1 M LITFSI | 950 (after 200 cycles @ 1 C) | 0.033% capacity decay per cycle; 633 mAh/g after 1000 cycles @ 1 C | [223] |
| NiCo-LDH | ZIF-67 template etching + melt-diffusion with sulfur | LSB | 1.0 M LiTFSI | 1540 @ 0.1 C, 485 @ 5 C | 475 mAh/g after 500 cycles @ 1 C (78%) | [224] |
| NiCo2S4–NiS2 NH@C | Ion exchange + in situ transformation | LSB | 5 mM Li2S6 | 1207 at 0.2 C; 766 at 2 C | 60.23% retention after 450 cycles at 1 C; 6.09 mAh/cm2 at 5 mg/cm2 sulfur loading | [225] |
| CuCo-LDH | Chemical etching (ZIF-67 template) | LSB | 1 M LiTFSI | 1262.8 (initial), 697.0 (after 500 cycles at 1 C) | 500 cycles, 0.049% capacity decay per cycle | [226] |
| PPy@LDH-S | Ion exchange + solid-state melting + polymerization | LSB | 1 M LiTFSI with 1% LiNO3 | 907.2 (initial, 1 C); 633.4 after 500 cycles | 0.06% capacity fading per cycle over 500 cycles | [227] |
| Ni0.8Ca1.2Al-Cl LDH | Ion exchange | NIB | 1.0 M NaPF6 | 256.9 (200 cycles at 0.2 A/g) | 102 mAh/g after 600 cycles at 2 A/g | [228] |
| Co0.6Ca1.4Al-Cl LDH | Ion exchange | NIB | 1.0 M NaPF6 | 292.8 (200 cycles at 0.2 A/g) | 118.1 mAh/g after 600 cycles at 2 A/g | [228] |
| CoSn-LDH@MXene | Solvothermal + Ultrasonic | NIB | 1 M NaClO4 | 976.1 (0.1 A/g) | 87.6% retention after 1000 cycles | [229] |
| Ni3Ti-Cl LDH | Urea precipitation + anion exchange | CIB | 0.5 M PP14Cl | 346.4 | 131.8 mAh/g over 200 cycles | [230] |
| CoMn-LDH/NPGA | Cross-linking gelation + Hydrothermal self-assembly + Freeze drying | ZAB | 1 M KOH | … | 72 h (~432 cycles) stable operation | [231] |
| LDH Synthesis Procedure | Positive Electrode | Negative Electrode | Electrolyte | Specific Capacitance (F/g) | Energy Density (Wh/kg) | Power Density (W/kg) | Stability (%, No. of Cycles) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Hydrothermal synthesis | NiCoLDH-1@JAC-2 | JAC-2 | PVA/KOH | 750 | 100 | 250 | 95%, 10,000 | [89] |
| Hydrothermal synthesis | NC@CL nanosheets | AC | 1 M KOH | 213 | 66.56 | 148.83 | 88%, 20,000 | [141] |
| Hydrothermal synthesis | NiCo-LDH | AC | PVA/KOH | 400.2 C/g | 51.59 μWh/cm2 | 1.125 mW/cm2 | 70%, 10,000 | [119] |
| Hydrothermal synthesis | Ni–Co LDH/NF (NCL3) | AC | 1 M KOH | 107 | 38 | 1702 | 76%, 2000 | [151] |
| Hydrothermal + TA etching + sulfurization | S-NCCO (NiCo-LDH on S-ZIF-67) | AC | PVA/KOH | 295 | 92.3 | 750 | 87%, 5000 | [160] |
| Hydrothermal | NiFe-LDH/MnCO3/MXene | AC | 6 M KOH | 215 | 67.3 | 750.9 | 89%, 5000 | [161] |
| Hydrothermal synthesis | MnCo2O4@FeCoNi-LDH/CC | AC/CC | 6 M KOH | 145.2 | 51.66 | 890.81 | 90.3%, 10,000 | [162] |
| Electrodeposition | NiAl LDH-rGO | Fe2O3-rGO | 2 M KOH | 214.4 | 76.23 | 800 | 95%, 5000 | [167] |
| Hydrothermal synthesis + Phosphorization + NaBH4 reduction | MP2 (MoB@NiCoP) | AC | 3 M KOH | 112.52 | 39.91 | 948.25 | 78.76%, 5000 | [168] |
| Hydrothermal + Te soaking | Te-NiMn LDH/C-MXene/NF | AC | 6 M KOH | 202.6 | 52.3 | 6452 | 77.3%, 10,000 | [169] |
| Two-step electrodeposition | CuCo LDH@Ni3S2 | AC | 3 M KOH | 11.24 F/cm2 | 0.62 mWh/cm2 | 8 mW/cm2 | 72.2%, 6000 | [211] |
| Electrodeposition + Electrochemical Activation | Hv-rich NiCo-LDH | VS2 | 1 M KOH | 238.5 F/g | 48.44 | 937.49 | 42%, 500 | [212] |
| Electrodeposition of CoLa LDH nanoarrays followed by Zn2+ doping | Zn/CoLa LDH | AC | 3 M KOH | 269.4 | 59.9 | 800 | 86.4%, 12,000 | [213] |
| Electrochemical deposition | NiAl-LDH@CC | CC | 1 M KOH | 310 | 51.67 | 913.84 | 90%, 10,000 | [214] |
| Electrochemical deposition | CF/CRBI-NiCo-LDH | CF/AC | 6 M KOH | 167.85 | 57.2 | 820 | 89%, 3000 | [215] |
| Solvothermal synthesis | CoNi LDH nanoflowers | AC | 2 M KOH | 768.3 C/g @ 1 A/g | 37.1 | 748.0 | 93.7%, 4000 | [232] |
| Solvothermal | NiCo-MOF@LDH-2 nanosheets | AC | 2 M KOH | 1873.9 | 49.8 | 422.4 | 83%, 10,000 | [233] |
| Ion exchange | NiCo-LDH/ACC | NiCo-LDH/ACC | 3 M KOH | 876 mF/cm2 | 0.352 mWh/cm2 | 559.5 mW/cm3 | 94.9%, 15,000 | [234] |
| Cation/anion exchange | CoNiS-50 | AC | 6 M KOH | 150 | 37.8 | 750 | 92.2%, 10,000 | [235] |
| Two-step solvothermal | NCS@CoAl-LDH | AC | 3 M KOH | 112.2 | 35.1 | 751.2 | 97.7%, 10,000 | [236] |
| Microwave hydrothermal synthesis | NiCo-LDH@TAC600-0 | TAC600-2 | 6 M KOH | 1250 (3-electrode) | 30.8 | 800 | 72.5%, 5000 | [237] |
| Solvothermal synthesis followed by BPQD modification and hydrothermal sulfidation | NiCo-LDH/NiCo2S4/BPQD | AC | 6 M KOH | 376.1 | 133.7 | 800 | 76.5%, 10,000 | [273] |
| Hydrothermal synthesis using 2-methylimidazole for N-doping | N-doped ZnNi-LDH | AC | 3 M KOH | 351.6 | 64.6 | 850 | 85.6%, 10,000 | [274] |
| Precipitation and hydrothermal method with BPQD anchoring on CC | NiCoCu-LDH/BPQD | AC | 6 M KOH | 568.7 | 202.2 | 800 | 81.4%, 10,000 | [275] |
| Electrochemical deposition followed by partial hydrothermal sulfidation | CoMn-LDH/CoMn-S | AC | 3 M KOH | 201 | 82.63 | 985 | 94%, 6000 | [276] |
| Core/shell formation using Ce bridging between NiCo LDH and PBAs | NiCo-Ce@PBAs | AC | 1 M KOH | 222.3 | 99.8 | 1283 | 95.7%, 10,000 | [277] |
| LDH Name | Synthesis Procedure | Electrolyte | HER Overpotential (η/mV, j/mA/cm2) | Stability (Retention/%, Time/h) | Tafel Slope (mV/dec), FE (%) | Ref. |
|---|---|---|---|---|---|---|
| NiFe LDH-NS@DG10 | Exfoliation method | 1 M KOH | 115, 10 | ~100%, 5.5 h | 110, 97.3% | [307] |
| NiTe@CoFe-LDH | Hydrothermal and electrodeposition | 1 M KOH | 103, 10 | ~100%, 50 h | 20, 100% | [308] |
| Cu-NiCo LDH/NiCo@CC | Electrodeposition process | 1 M KOH | 73, 10 | …, 100 h | 78.5, … | [285] |
| CoNiN@NiFe | Co-deposition | 1 M KOH | 150, 10 | …, 60 h | 167, 100% | [309] |
| Pt@LDH-4h | Hydrolysis reaction | 1 M KOH | 58, 10 | ~100%, 24 h | 43.6, 95% | [286] |
| Pd-NiAl-LDH | Chemical precipitation | 1 M KOH | 189, 10 | ~100%, 48 h | 133.2, … | [310] |
| Ni2P/NiFe-LDH | Hydrothermal | 1 M KOH | 230, 100 | ~100%, 32 h | 47.43, … | [315] |
| c-CoMnP/a-CoMn LDH/NF | Chemical and thermal route | 1 M KOH | 170, 100 | 99%, 60 h | 73.7, … | [316] |
| MOF-d-CoFe LDH@N-MXene | Hydrothermal and electrophoretic deposition | 1 M KOH | 206, 10 | …, 60 h | 113, 93% | [323] |
| LDH(60%)/MX-RGO | Hydrothermal co-assembly | 1 M KOH | 326, 10 | …, 40 h | 100, … | [324] |
| LDH: Layered double hydroxide, HER: Hydrogen evolution reaction, FE: Faradaic efficiency | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, S.S.; Das, M.; Ogawa, T. One Stone, Three Birds: Innovations and Challenges of Layered Double Hydroxides in Batteries, Supercapacitors, and Hydrogen Production. Batteries 2025, 11, 193. https://doi.org/10.3390/batteries11050193
Shah SS, Das M, Ogawa T. One Stone, Three Birds: Innovations and Challenges of Layered Double Hydroxides in Batteries, Supercapacitors, and Hydrogen Production. Batteries. 2025; 11(5):193. https://doi.org/10.3390/batteries11050193
Chicago/Turabian StyleShah, Syed Shaheen, Manisha Das, and Takaya Ogawa. 2025. "One Stone, Three Birds: Innovations and Challenges of Layered Double Hydroxides in Batteries, Supercapacitors, and Hydrogen Production" Batteries 11, no. 5: 193. https://doi.org/10.3390/batteries11050193
APA StyleShah, S. S., Das, M., & Ogawa, T. (2025). One Stone, Three Birds: Innovations and Challenges of Layered Double Hydroxides in Batteries, Supercapacitors, and Hydrogen Production. Batteries, 11(5), 193. https://doi.org/10.3390/batteries11050193








