1. Introduction
Lithium-ion batteries (LIBs) have been at the forefront of the consumer application market for energy storage devices since their commercialization in 1991 [
1]. This has revolutionized the energy storage market since they are rechargeable, meaning that they can be reused in applications encompassing an electric grid [
2], in the medical field [
3], and in electric vehicles, which has seen a recent focus on LIBs as a power source for this emerging field [
4]. However, LIBs cannot be used infinitely because of the degradation buildup within the cell with use. This can be observed as capacity fade with each cycle over an LIB’s lifetime [
5]. This degradation within the cell is caused by lithium species’ increasing permanent bonds with the electrolyte and electrode in the LIB over its cycle life, observed as a change in the electrolyte solution and the formation of a solid electrolyte interface (SEI) around the electrode [
6]. Reduced availability of lithium species lowers the cell’s capacity; therefore, making predictions on the future degradation of an LIB will provide users with information on how to use the cell optimally to either prolong its lifetime or know when to replace it. One way of observing a battery’s degradation is through its state of health (SOH). One way of determining a battery’s SOH is by comparing its current capacity to the capacity it can initially store, expressed as a percentage. This is shown in Equation (1), where Q
max denotes the maximum available capacity, and
Qn is the nominal capacity [
7].
Different inputs can be used to predict the battery degradation of an LIB. Standard methods use information from voltage curves during cycling, such as voltage and/or capacity data, to perform battery prognosis [
8]. However, this avenue results in active cycling, where unwanted capacity fade occurs. A useful method to bypass this problem is by characterizing the battery using its impedance, which is called electrochemical impedance spectroscopy (EIS). This technique uses a small AC voltage (or current) signal applied to the cell to obtain its corresponding current (or voltage) response. This is performed over a range of frequencies, which results in a spectrum of impedance. Using the voltage as the catalyst, the impedance is calculated according to Equation (2), where
Eo is the voltage signal,
Io is the current response,
ωt is the angular frequency, and
∅ is the phase shift associated with the current response. This technique is non-destructive to the cell, which minimizes unwanted cycling to acquire the necessary data for battery prognosis studies [
9].
Potentiostats are the primary equipment used to acquire EIS spectra, which can measure the different kinetics within electrochemical devices ranging from under 0.001 Hz to over 1,000,000 Hz [
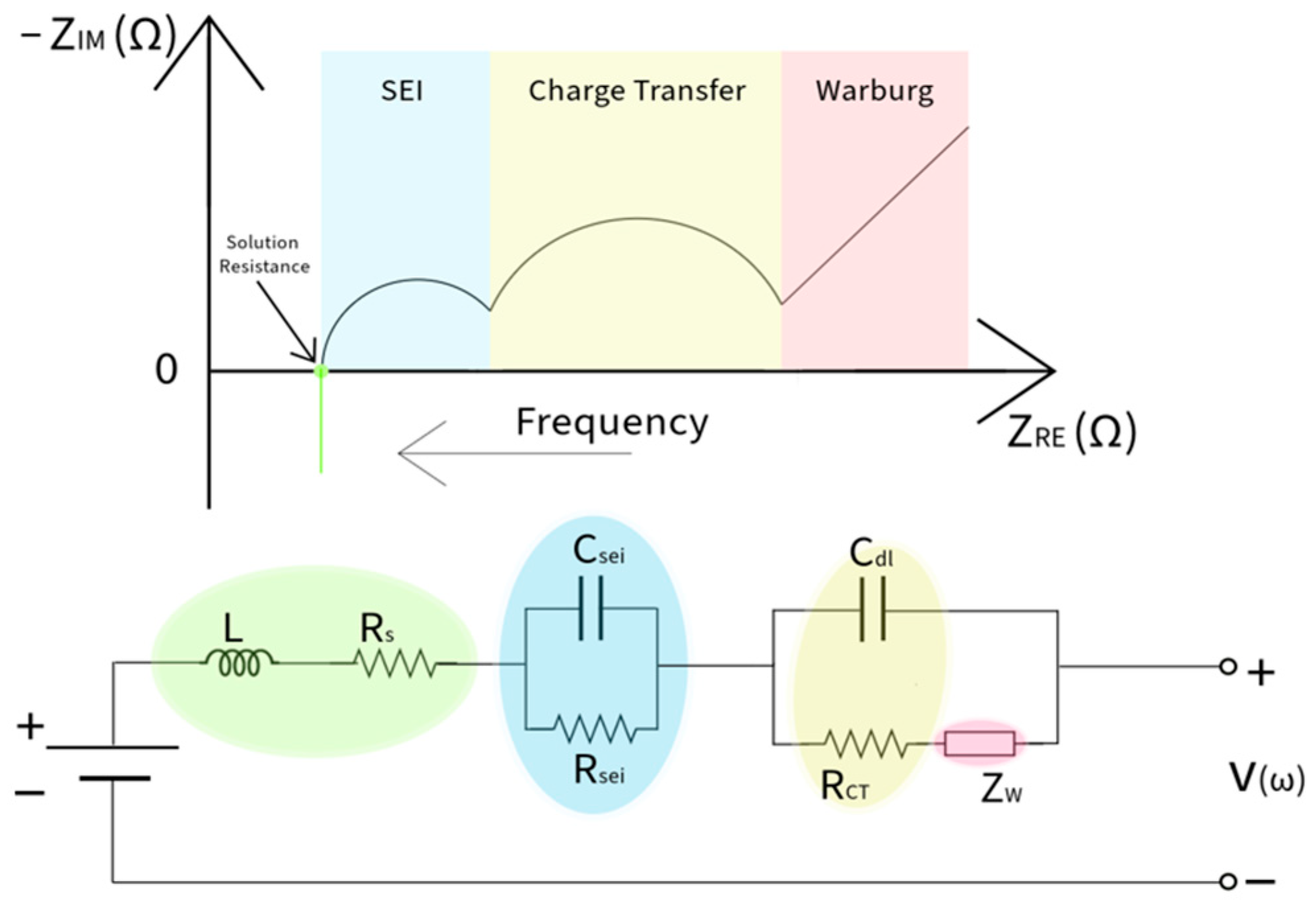
10]. These spectra are best shown on a Nyquist plot (NP), which graphs the imaginary impedance vs. real impedance. The impedance on the NP shows that the real impedance (resistance) gets smaller at higher frequencies. It also shows that the kinetics observed at different frequencies can be viewed as a region where that phenomenon is the dominant contributor to the overall impedance in that region. A battery model used to provide an accurate simulation of the Nyquist plot for LIBs is an equivalent circuit model (ECM). This method uses electrical elements such as resistors and capacitors to describe the electrochemical behavior of an LIB’s EIS [
11]. This method is helpful since it reduces the number of inputs to describe the EIS, making it less computationally expensive for battery health estimation. At very high frequencies where the impedance is below the Z
RE-axis, mutual inductance occurs between the connectors from the potentiostat and the cell’s connecting tabs [
12]. At the intersection of the Z
RE-axis is the bulk resistance [
13], where ions behave as resistors to impede electrons from flowing from the working electrode to the reference electrode, and this property can be attributed to the electrolyte, separator, and contact. At high frequencies, the impedance can be observed from the formation of the SEI layer, which displays both resistive and capacitive characteristics [
14]. The mid-frequency region encompasses the overall charge transfer (CT) region, which includes the charge transfer resistance (R
CT) and double-layer capacitance (C
dl). The R
CT represents the opposition of Li
+ traveling between the electrolyte and the electrode [
15]. At the same time, when Li
+ is adsorbed on the electrode, a space is formed between the electrode and the electrolyte interface. The system has capacitive characteristics, showing charges on both the electrode and electrolyte with a space in between [
16]. The impedance at very low frequencies is observed through species moving from one electrode to the next, which is denoted as the Warburg diffusion [
17]. Several models are used to model EIS, the most basic of which is the Thevenin model [
18]. This model is used to capture the bulk resistance and the overall capacitive and resistive sections if the presence of the SEI is not apparent. However, it does not capture the diffusion spectra or consider the non-ideal capacitive behavior within the cell, so more advanced models for EIS modeling for LIBs are needed. One recent model useful for LIB approximation to obtain a global spectrum is the adaptive Randles ECM (AR-ECM) [
19]. The nuance of the AR-ECM is that the Warburg impedance is placed in series with the R
CT. This considers the mass transport effect that is influential in the mid-frequency region. This is shown in
Figure 1, which is used to simulate the EIS of an LIB. The associated parameters of ECMs are used in battery health studies as input to predict performance metrics, including state-of-charge estimation [
20], state-of-health estimation [
21], and remaining useful life estimation [
22].
The accuracy of the equivalent circuit parameters (ECPs) from the ECM is essential for battery health prediction. Merrouche et al. investigated ECP estimation using artificial intelligence with the weighted mean of vectors algorithm as its foundation [
23]. This method outperformed other state-of-the-art optimizers in convergence and speed metrics for ECP estimation and could predict unseen voltage data from its generated ECPs. Heydarzadeh et al. analyzed different modeling techniques to develop different ECPs depending on the battery’s chemistry [
24]. For battery prognosis studies, recent work showcased the different avenues for estimating ECPs that were used for data input. Shao et al. accurately predicted the capacity of batteries using parameters associated with the SEI, showcasing that the global impedance spectrum is unnecessary to predict battery degradation [
25]. Li et al. proposed a new ECM with three distinct time constants, with the Warburg parallel to a capacitor at the low-frequency spectrum [
26]. They could accurately predict SOH, with an average root mean square error (RMSE) of 1.77%. Rodriguez-Cea et al. used only the internal resistance (R
int) model as input for SOH prediction [
27]. The predictions of SOH fell between 2% and 5% of the measured SOH. Despite the diversity in methods using ECPs with various machine learning algorithms (MLAs) for battery prognosis, there is not much focus on using one LIB for model training and testing. Using one LIB for model building requires fewer resources for study in terms of experiment time and costs. This method also provides an alternative to conventional methods used in the literature by using simulated cells to predict their inputs and outputs based on EIS and capacity data, with uncertainties in measurement filtered by the equipment used to acquire EIS.
This study investigates the quality of ECPs for capacity prediction while providing a time-effective method to limit the time taken to acquire EIS and model training, showing usefulness in real-time SOH monitoring. A reduced equivalent circuit model is developed to estimate the ECPs in the high- and mid-frequency regions (R-ECM). These ECPs are input into a back propagation neural network (BPNN) to predict the simulated cells’ capacity. This algorithm is used over other algorithms, such as random forests and gradient boosting, that typically incur a long learning and running time for accurate results [
28]. An effective R-ECM is used to replace the CPE with an effective capacitor to compare its ability to predict the capacity of cells (ER-ECM). Further analysis is performed to evaluate the usefulness of using singular ECPs within the ECM for capacity prediction. This method is compared to using the ECPs for all frequencies in terms of capacity prediction and time taken for model training and testing.
Section 2 of this paper covers the methodology.
Section 3 of this paper covers the results and discussion.
Section 4 concludes this paper.
2. Methodology
The dataset used for this study includes EIS and its corresponding capacity data for every even cycle in the cycle life of four LCO coin cells [
29]. The dataset contains EIS for multiple stages during the charge/discharge cycle; however, the EIS data curated for this study were chosen 15 min after reaching 100% SOC. This was chosen so that the relaxation effect within the cell would slow to a negligible level so that it would not contribute to the overall EIS [
30]. The data for each cell range from its initial cycle to the cycle closest to its end of life (EOL), which is when the cell reaches 80% of its initial capacity. This ensures that predictions are taken within the operating lifetime.
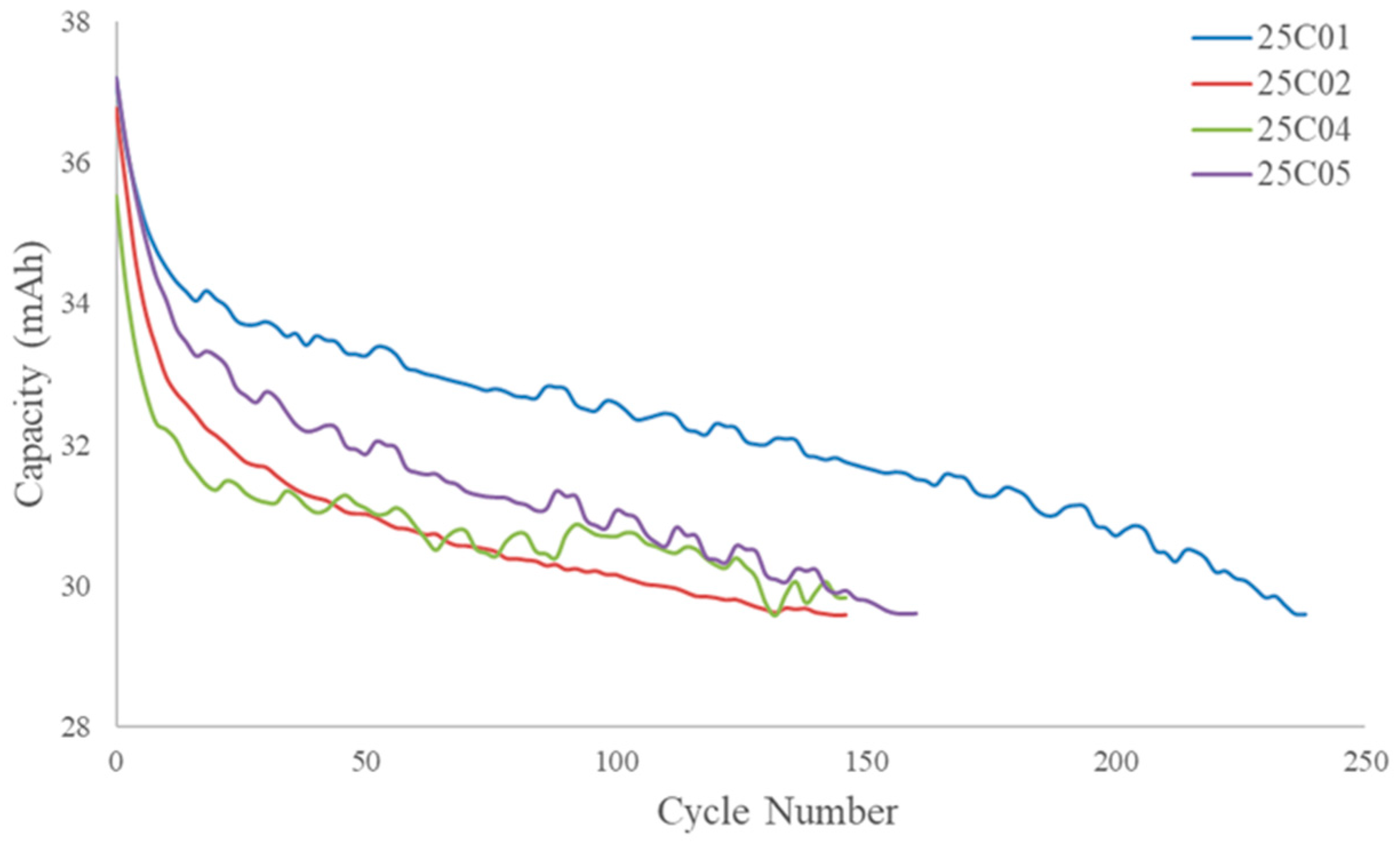
Figure 2 shows the capacity retention curves for the cells in this study, and the equations for SOC and EOL are presented below, where Q
curr is the current capacity of the cell.
Table 1 gives an overview of each cell’s capacity and cycle life.
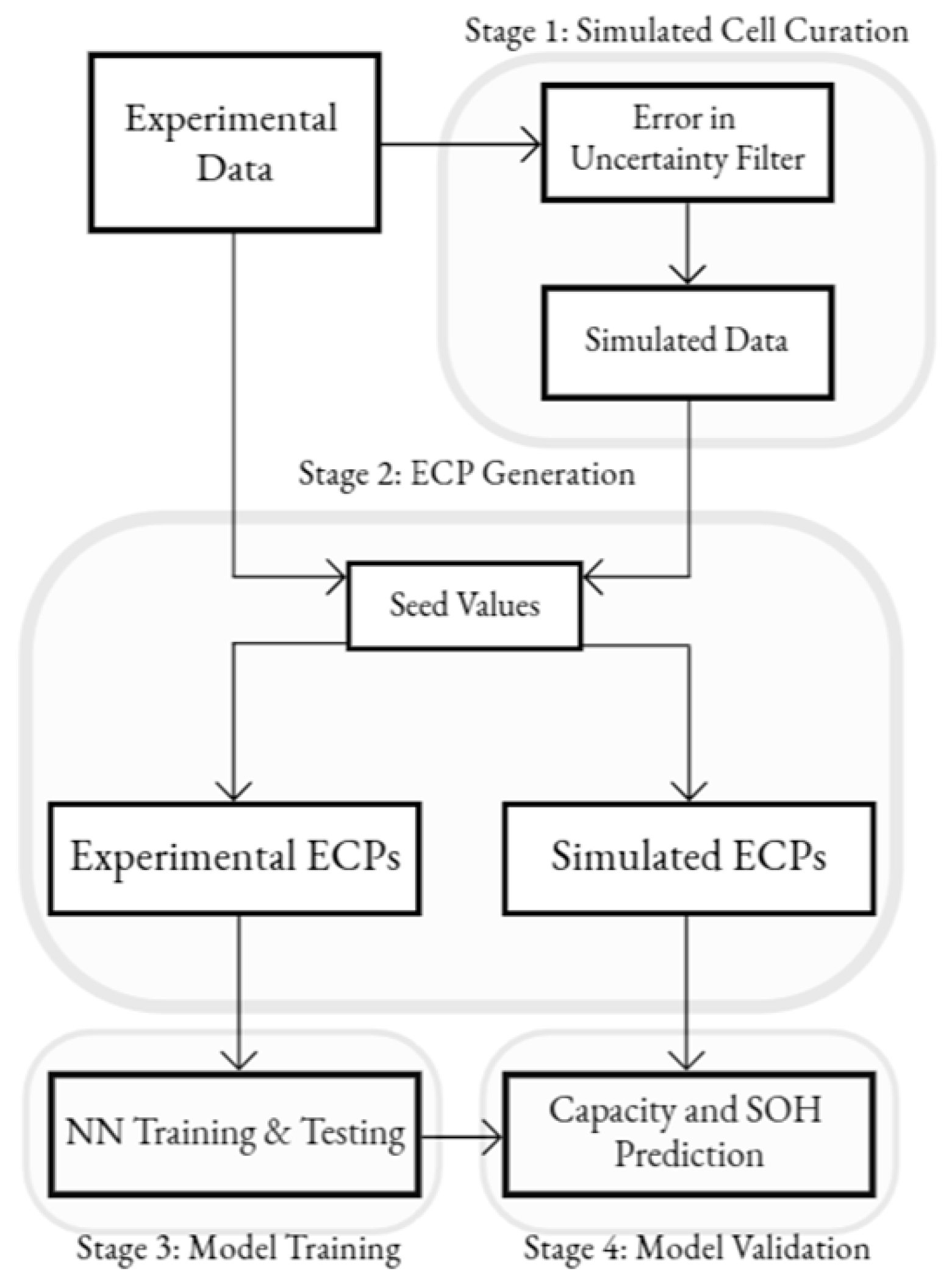
The overall framework of this study is shown in
Figure 3. The EIS and the corresponding capacity data are run through an error-in-uncertainty filter to provide simulated data to predict each cell. This filter is based on the errors in uncertainty in measurement from standard potentiostats. The Gamry 600 was used as the testbench, which is ±1%, given the range of EIS values in the dataset. The capacity filter was set to ±1.5% for this study. The ECPs were acquired from the ECM used in this study, using appropriate seed values for the experimental and simulated data. The experimental ECPs (E-ECPs) were used as input into the BPNN to train the model to be tested on the simulated ECPs (S-ECPs) to predict the capacity of the simulated cell. This study investigated the high and mid-frequency regions of the EIS using all ECPs in the model used. This was compared to the method of reducing the CPE to an equivalent capacitance (C
eff), an alternative method for characterizing the CPE using one variable instead of two. Lastly, analysis was performed using individual ECPs and their correlation to predict battery degradation.
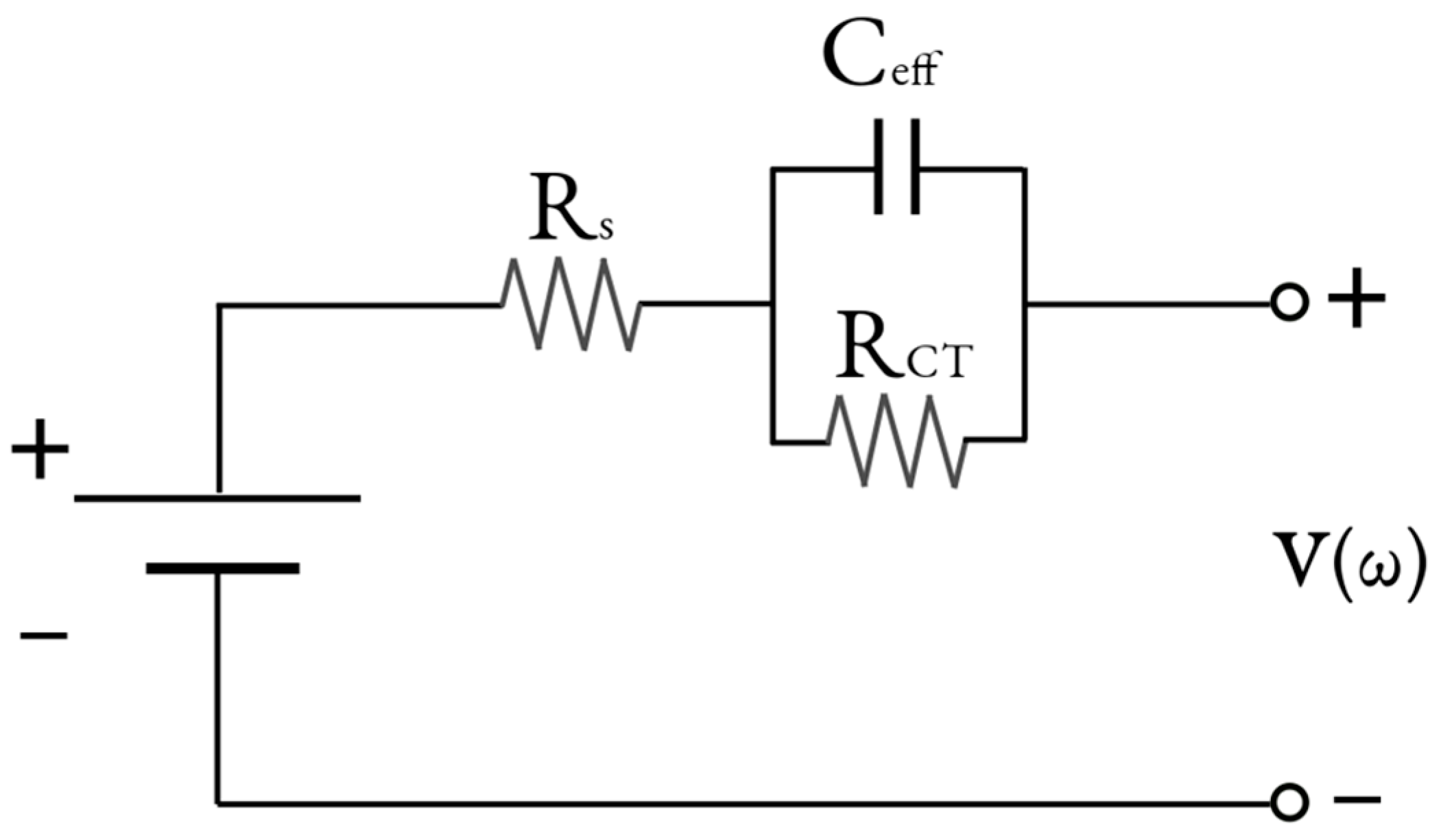
The ECM used to obtain the ECPs was a one-time-constant (OTC) Thevenin model using the constant phase element to describe the double-layer capacitance (C
dl). This choice was made due to the non-ideal observation of the capacitance in EIS. It is equated below with ω being the angular frequency,
Y0, and
n being the characteristic parameters of the impedance [
31].
Figure 4 provides a visual representation of the ECM used to acquire the ECPs in this study. The OTC was used in this study because of the nature of the cells selected. The presence of the SEI was not apparent in the NPs, and the bode plot, which shows the phase angle of the impedance (arg(Z)) vs. the frequency, only shows one Gaussian bell curve, which supports using only one OTC for the overall charge transfer region.
Figure 5 shows the bode plots for all four cells, highlighting the one Gaussian bell curve for each cell.
To obtain ECPs from the ECM, appropriate initial values are needed to attain a good fit for the ECM. These are called seed values, and they generally need to be within two decades of the best-fit values to obtain a good fit [
32]. Since EIS is non-linear, a non-linear least-squares fitting technique is required to obtain the ECPs. Modeling programs for fitting an ECM to EIS data use the Levenberg–Marquardt technique [
33]. The average values of the ECPs obtained for the four cells are shown in
Table 2, along with their associated standard deviation errors.
Table 3 provides the ECPs for the simulated data. These errors were obtained at the 95% confidence level. The std. errors obtained are considered a good fit since they are less than the estimated parameter value, i.e., less than 100%. The root mean squared error (RMSE) of the initial values of each cell provides insight into the accuracy of the ECPs’ ability to predict the EIS spectra. They range between 0.0118 and 0.0173 Ω, showing negligible deviation from the impedance of the cells.
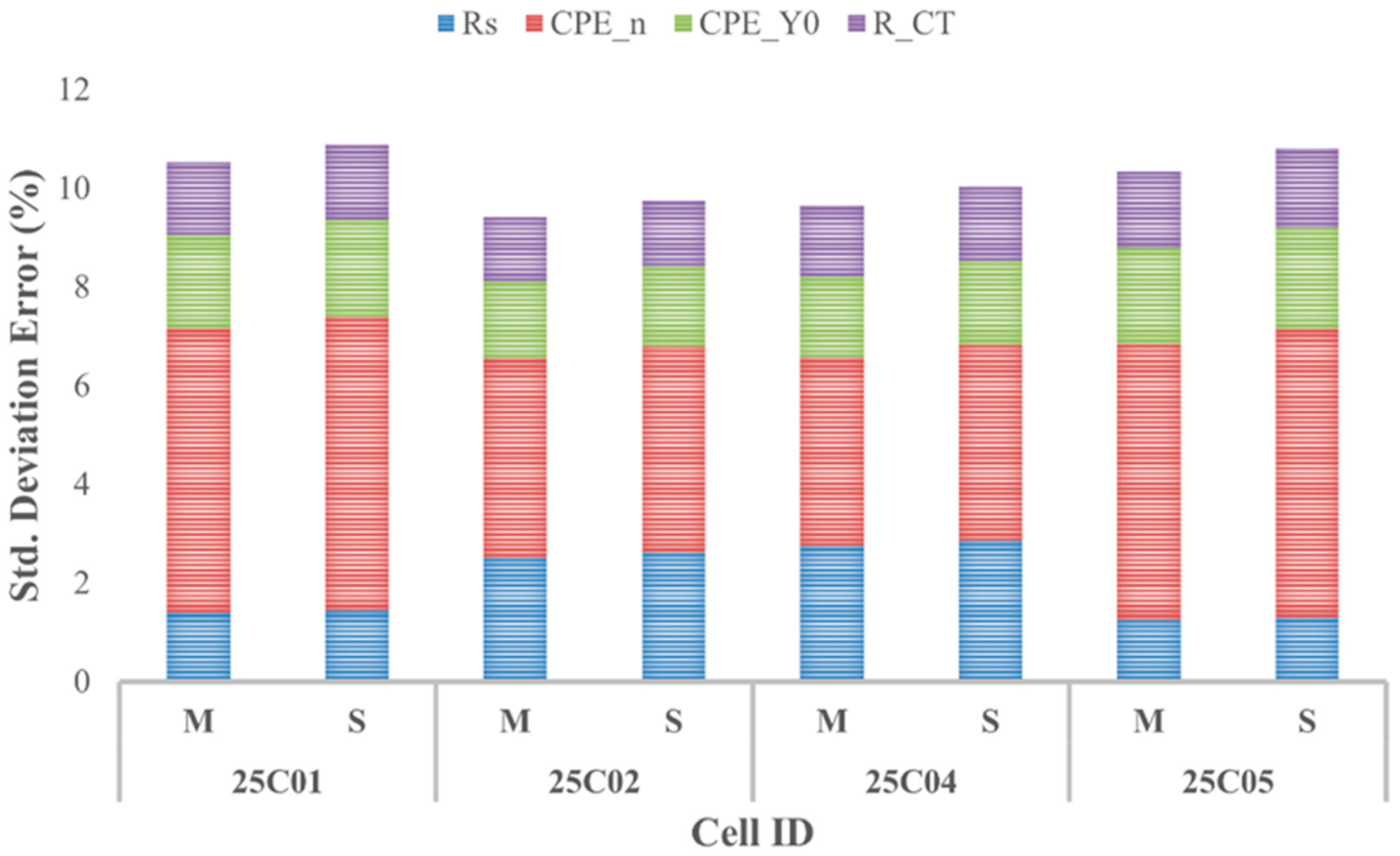
Figure 6 shows all the cells’ average cumulative E-ECP and S-ECP deviation errors. The cumulative errors show that the ECPs acquired are of good fit and are usable for further study in predicting the capacity of the simulated cells.
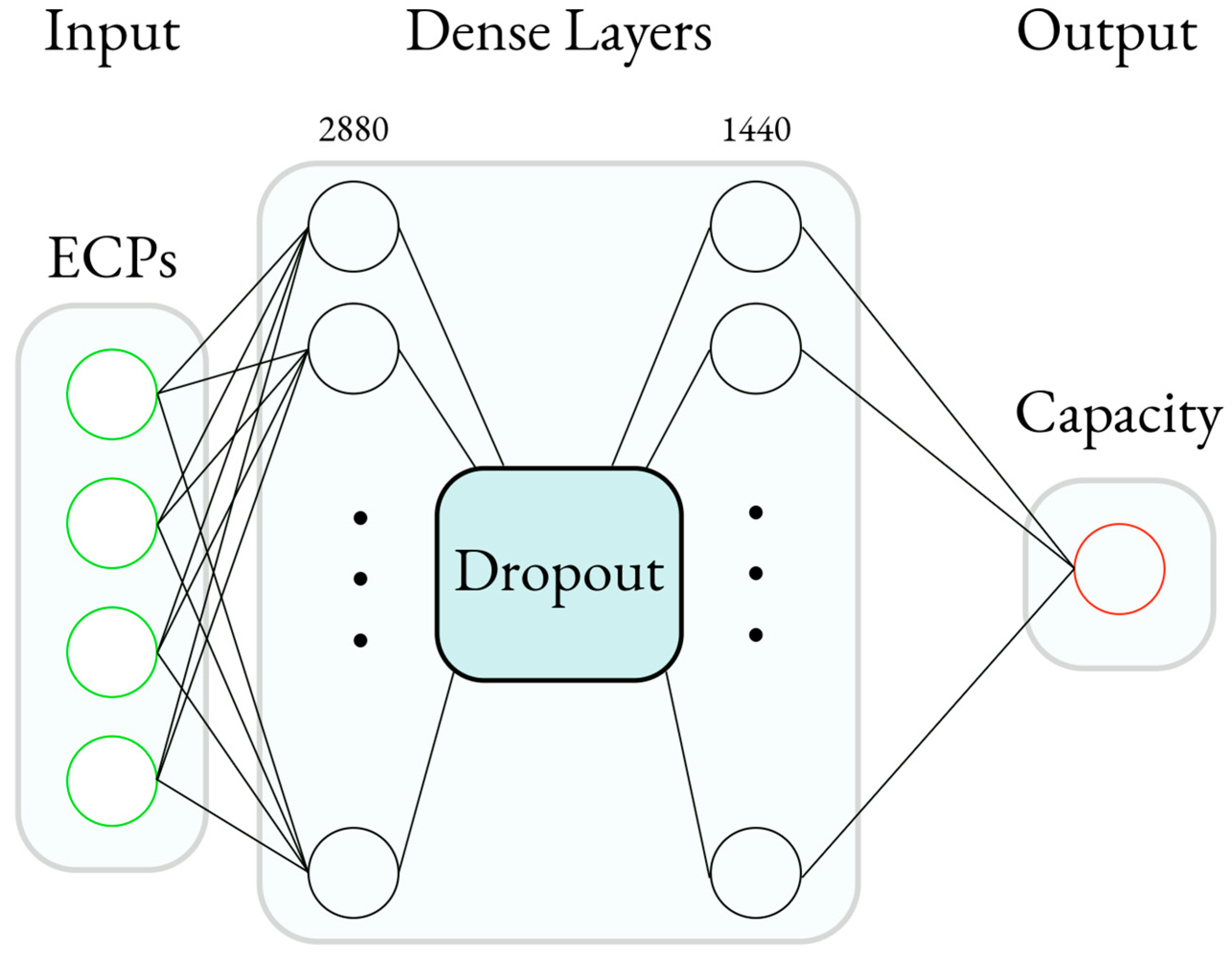
This study used a BPNN model as the machine learning algorithm due to its scalability and fast implementation. After acquiring the E-ECPs and S-ECPs, the E-ECPs were used for training and testing, with the E-ECPs as the input and the capacity as the output. The model was then used to predict the simulated capacity using the S-ECPs.
Figure 7 gives an overview of the BPNN used, and
Table 4 shows the hyperparameters used for the BPNN.
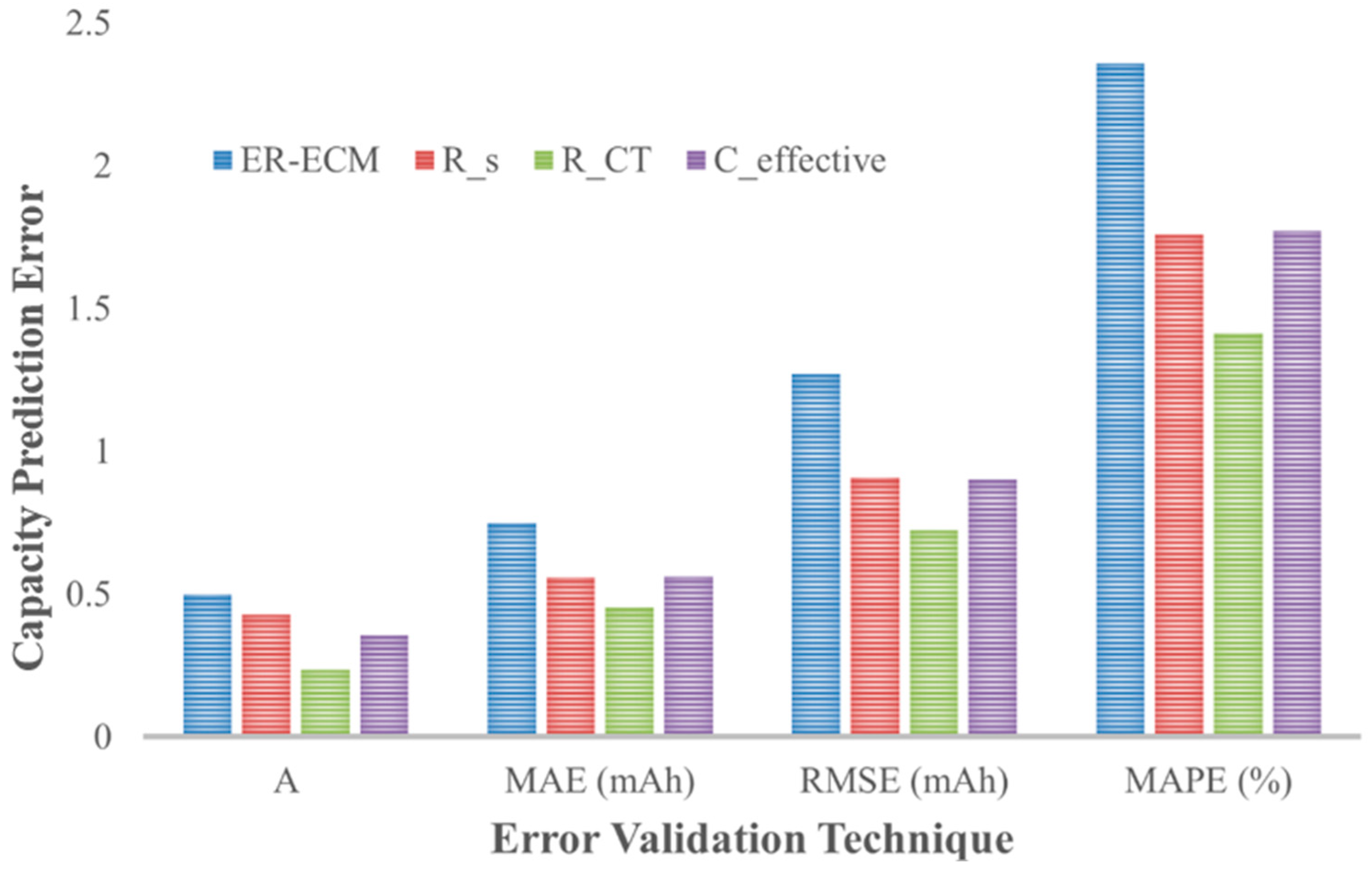
The simulated cell’s capacity prediction was evaluated using several error validation techniques, namely, the mean absolute error (MAE), the coefficient of determination (R
2), the RMSE, and the mean absolute percentage error (MAPE). The MAE, R
2, and MAPE formulae are presented below, where
y is the simulated capacity and
ŷ is the predicted capacity from the S-ECPs. This combination of error validation techniques provides an exhaustive understanding of the robustness of the BPNN in predicting the capacity, where one technique may provide insight that others may not.
To observe individual ECPs and their contribution to cell prediction, the CPE was converted into an effective capacitance, C
eff [
34]. This was calculated according to Equation (10), where Y
0 is the admittance of the CPE, and n is a parameter used to describe the partial rotation of the depressed semicircle, with n = 1 indicating no depression, and a reduction in n describes an increase in rotation when it gets closer to 0. This reduced model is shown in
Figure 8. This method reduces the CPE, a function of two parameters, into one, providing a pathway for measuring the C
dl using one parameter.