Assessment of Laser-Ablated Silicon Wafers as Lithium-Ion Battery Anodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Silicon Wafer Electrode Preparation
2.2. Cathode Preparation
2.3. Cell Fabrication
2.4. Characterization of Silicon Wafer Electrode
2.5. Electrochemical Evaluation
3. Results and Discussion
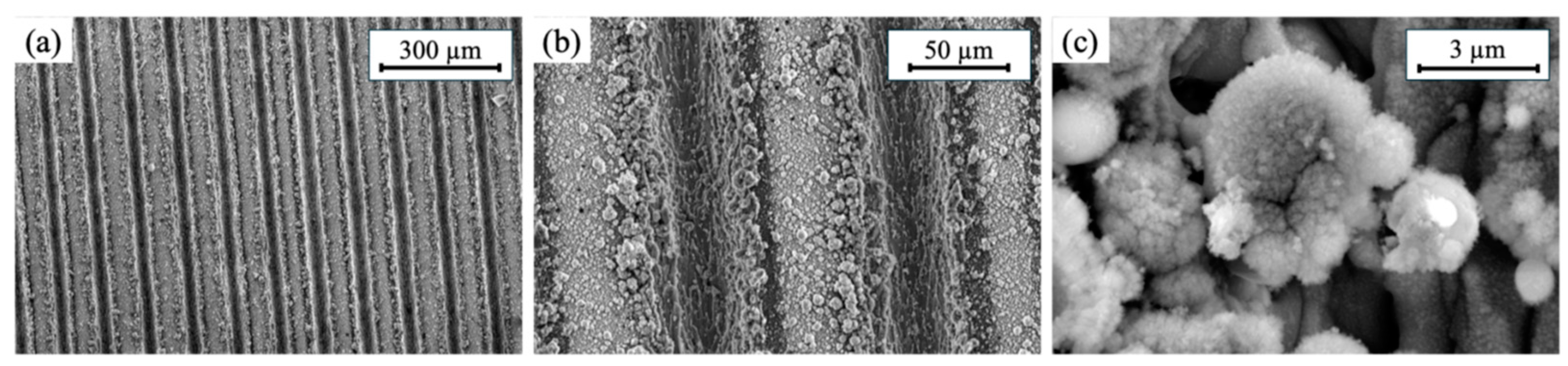
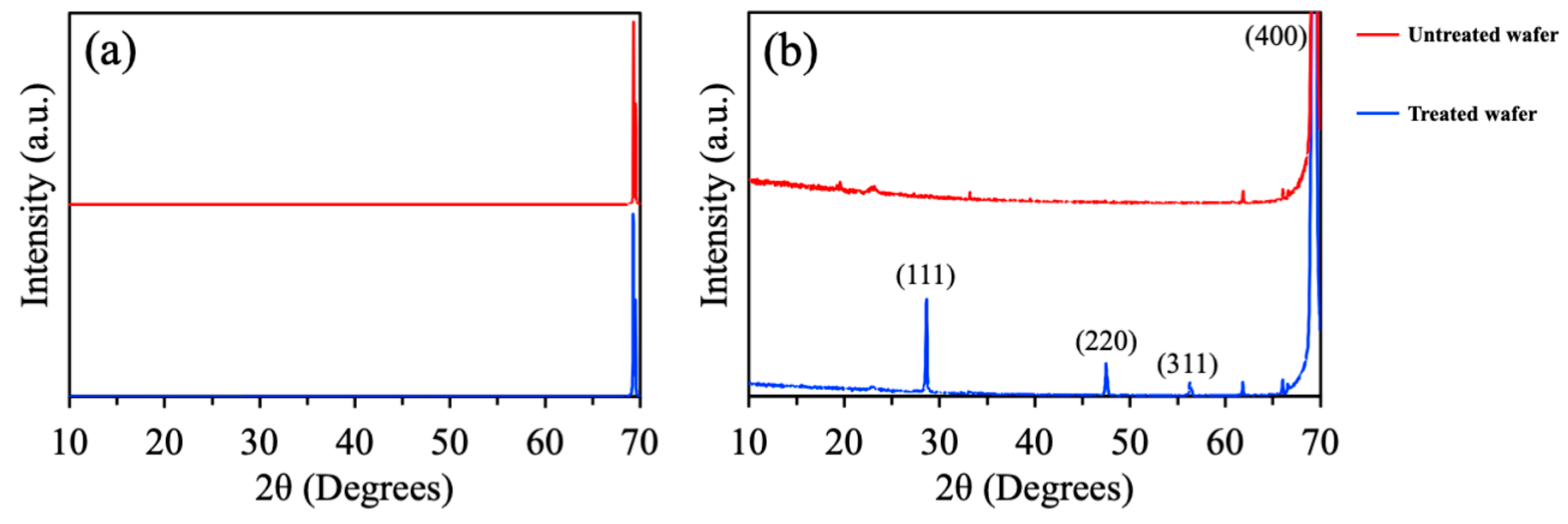
3.1. Material Characterization
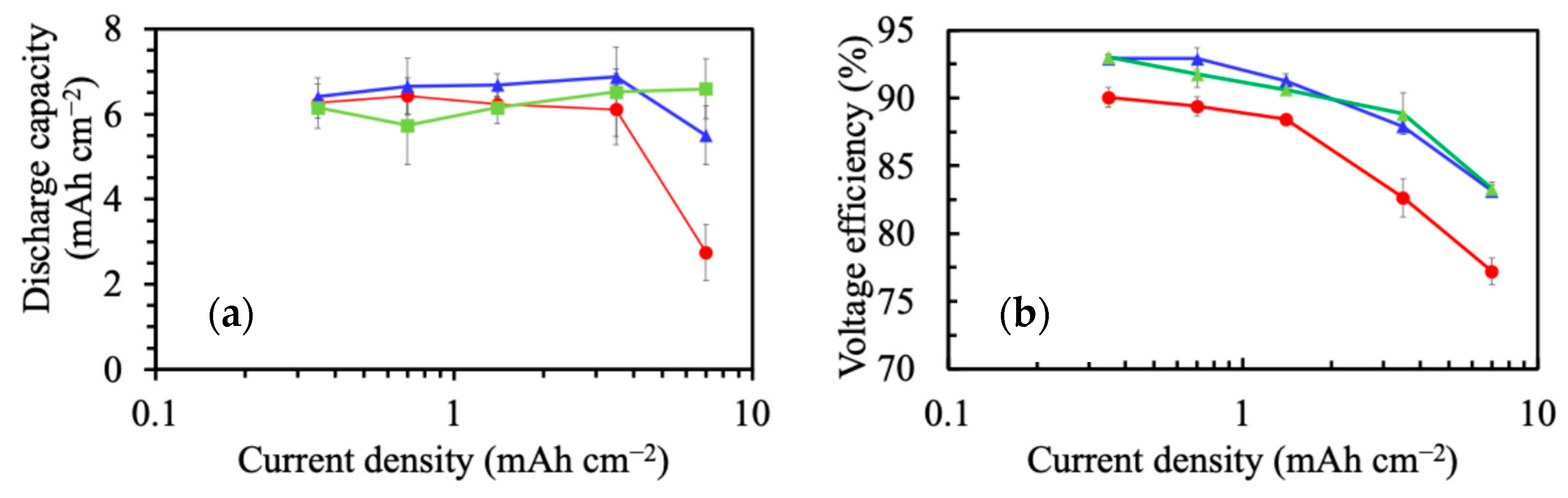
3.2. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PV | Photovoltaic |
| Li | Lithium |
| LCO | LiCoO2 |
| Si | Silicon |
| AAM | All-active material |
| UW | Untreated wafer |
| ST | Single-treated wafer |
| DT | Dual-treated wafer |
References
- Ghoniem, R.M.; Alahmer, A.; Rezk, H.; As’ad, S. Optimal Design and Sizing of Hybrid Photovoltaic/Fuel Cell Electrical Power System. Sustainability 2023, 15, 12026. [Google Scholar] [CrossRef]
- Esmaeili, M.; Ahmadi, A.A.; Nateghi, A.; Shafie-khah, M. Robust Power Management System with Generation and Demand Prediction and Critical Loads in DC Microgrid. J. Clean. Prod. 2023, 384, 135490. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelkareem, M.A. Renewable Energy and Climate Change. Renew. Sustain. Energy Rev. 2022, 158, 112111. [Google Scholar] [CrossRef]
- Ang, T.Z.; Salem, M.; Kamarol, M.; Das, H.S.; Nazari, M.A.; Prabaharan, N. A Comprehensive Study of Renewable Energy Sources: Classifications, Challenges and Suggestions. Energy Strat. Rev. 2022, 43, 100939. [Google Scholar] [CrossRef]
- Mariotti, N.; Bonomo, M.; Fagiolari, L.; Barbero, N.; Gerbaldi, C.; Bella, F.; Barolo, C. Recent Advances in Eco-Friendly and Cost-Effective Materials towards Sustainable Dye-Sensitized Solar Cells. Green Chem. 2020, 22, 7168–7218. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, D.; Kim, C.; Lee, C.; Kang, H.; Woo, H.Y.; Kim, B.J. Eco-Friendly Polymer Solar Cells: Advances in Green-Solvent Processing and Material Design. ACS Nano 2020, 14, 14493–14527. [Google Scholar] [CrossRef]
- Chowdhury, M.S.; Rahman, K.S.; Chowdhury, T.; Nuthammachot, N.; Techato, K.; Akhtaruzzaman, M.; Tiong, S.K.; Sopian, K.; Amin, N. An Overview of Solar Photovoltaic Panels’ End-of-Life Material Recycling. Energy Strat. Rev. 2020, 27, 100431. [Google Scholar] [CrossRef]
- Peplow, M. Solar Panels Face Recycling Challenge. ACS Cent. Sci. 2022, 8, 299–302. [Google Scholar] [CrossRef]
- Farrell, C.C.; Osman, A.I.; Doherty, R.; Saad, M.; Zhang, X.; Murphy, A.; Harrison, J.; Vennard, A.S.M.; Kumaravel, V.; Al-Muhtaseb, A.H.; et al. Technical Challenges and Opportunities in Realising a Circular Economy for Waste Photovoltaic Modules. Renew. Sustain. Energy Rev. 2020, 128, 109911. [Google Scholar] [CrossRef]
- Preet, S.; Smith, S.T. A Comprehensive Review on the Recycling Technology of Silicon Based Photovoltaic Solar Panels: Challenges and Future Outlook. J. Clean. Prod. 2024, 448, 141661. [Google Scholar] [CrossRef]
- Shin, J.; Park, J.; Park, N. A Method to Recycle Silicon Wafer from End-of-Life Photovoltaic Module and Solar Panels by Using Recycled Silicon Wafers. Sol. Energy Mater. Sol. Cells 2017, 162, 1–6. [Google Scholar] [CrossRef]
- Liu, T.; Dong, T.; Wang, M.; Du, X.; Sun, Y.; Xu, G.; Zhang, H.; Dong, S.; Cui, G. Recycled Micro-Sized Silicon Anode for High-Voltage Lithium-Ion Batteries. Nat. Sustain. 2024, 7, 1057–1066. [Google Scholar] [CrossRef]
- Sreenarayanan, B.; Vicencio, M.; Bai, S.; Lu, B.; Mao, O.; Adireddy, S.; Bao, W.; Meng, Y.S. Recycling Silicon Scrap for Spherical Si–C Composite as High-Performance Lithium-Ion Battery Anodes. J. Power Sources 2023, 578, 233245. [Google Scholar] [CrossRef]
- Pham, T.K.; Shin, J.H.; Karima, N.C.; Jun, Y.S.; Jeong, S.K.; Cho, N.; Lee, Y.W.; Cho, Y.; Lim, S.N.; Ahn, W. Application of Recycled Si from Industrial Waste towards Si/RGO Composite Material for Long Lifetime Lithium-Ion Battery. J. Power Sources 2021, 506, 230244. [Google Scholar] [CrossRef]
- Bao, Q.; Huang, Y.H.; Lan, C.K.; Chen, B.H.; Duh, J.G. Scalable Upcycling Silicon from Waste Slicing Sludge for High-Performance Lithium-Ion Battery Anodes. Electrochim. Acta 2015, 173, 82–90. [Google Scholar] [CrossRef]
- Rahman, M.M.; Mateti, S.; Sultana, I.; Hou, C.; Falin, A.; Cizek, P.; Glushenkov, A.M.; Chen, Y. End-of-Life Photovoltaic Recycled Silicon: A Sustainable Circular Materials Source for Electronic Industries. Adv. Energy Sustain. Res. 2021, 2, 202100081. [Google Scholar] [CrossRef]
- Shi, J.; Zu, L.; Gao, H.; Hu, G.; Zhang, Q. Silicon-Based Self-Assemblies for High Volumetric Capacity Li-Ion Batteries via Effective Stress Management. Adv. Funct. Mater. 2020, 30, 202002980. [Google Scholar] [CrossRef]
- Szczech, J.R.; Jin, S. Nanostructured Silicon for High Capacity Lithium Battery Anodes. Energy Env. Sci. 2011, 4, 56–72. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Yan, Z.; Cheng, F.; Chen, J. Structure Design and Mechanism Analysis of Silicon Anode for Lithium-Ion Batteries. Sci. China Mater. 2019, 62, 1515–1536. [Google Scholar] [CrossRef]
- Zuo, X.; Zhu, J.; Müller-Buschbaum, P.; Cheng, Y.J. Silicon Based Lithium-Ion Battery Anodes: A Chronicle Perspective Review. Nano Energy 2017, 31, 113–143. [Google Scholar] [CrossRef]
- Dou, F.; Weng, Y.; Wang, Q.; Chen, G.; Liu, H.; Shi, L.; Zhang, D. In Situ Imaging Analysis of the Inhibition Effect of Functional Coating on the Volume Expansion of Silicon Anodes. Chem. Eng. J. 2021, 417, 128122. [Google Scholar] [CrossRef]
- Ashuri, M.; He, Q.; Shaw, L.L. Silicon as a Potential Anode Material for Li-Ion Batteries: Where Size, Geometry and Structure Matter. Nanoscale 2016, 8, 74–103. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.; Yi, R.; Yang, H.; Zhao, Y.; Luo, L.; Gordin, M.L.; Sohn, H.; Chen, S.; Wang, C.; Zhang, S.; et al. Minimized Volume Expansion in Hierarchical Porous Silicon upon Lithiation. ACS Appl. Mater. Interfaces 2019, 11, 13257–13263. [Google Scholar] [CrossRef]
- Wu, F.; Dong, Y.; Su, Y.; Wei, C.; Chen, T.; Yan, W.; Ma, S.; Ma, L.; Wang, B.; Chen, L.; et al. Benchmarking the Effect of Particle Size on Silicon Anode Materials for Lithium-Ion Batteries. Small 2023, 19, e202301301. [Google Scholar] [CrossRef]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-Performance Lithium Battery Anodes Using Silicon Nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef]
- Wang, H.C.; Muruganantham, R.; Hsieh, C.T.; Liu, W.R. Electrochemical Elucidation of Phosphorus-Doped and 3D Graphene Aerogel Surface-Modified SiOx Porous Nanocomposite Electrode Material for High-Performance Lithium-Ion Batteries. Electrochim. Acta 2024, 477, 143775. [Google Scholar] [CrossRef]
- Muruganantham, R.; Yang, C.W.; Wang, H.J.; Huang, C.H.; Liu, W.R. Industrial Silicon-Wafer-Wastage-Derived Carbon-Enfolded Si/Si-C/C Nanocomposite Anode Material through Plasma-Assisted Discharge Process for Rechargeable Li-Ion Storage. Nanomaterials 2022, 12, 659. [Google Scholar] [CrossRef]
- Wei, D.; Mao, J.; Zheng, Z.; Fang, J.; Luo, Y.; Gao, X. Achieving a High Loading Si Anode via Employing a Triblock Copolymer Elastomer Binder, Metal Nanowires and a Laminated Conductive Structure. J. Mater. Chem. A Mater. 2018, 6, 20982–20991. [Google Scholar] [CrossRef]
- Zhao, X.; Lehto, V.P. Challenges and Prospects of Nanosized Silicon Anodes in Lithium-Ion Batteries. Nanotechnology 2021, 32, 042002. [Google Scholar] [CrossRef]
- Na, I.; Kim, H.; Kunze, S.; Nam, C.; Jo, S.; Choi, H.; Oh, S.; Choi, E.; Song, Y.B.; Jung, Y.S.; et al. Monolithic 100% Silicon Wafer Anode for All-Solid-State Batteries Achieving High Areal Capacity at Room Temperature. ACS Energy Lett. 2023, 8, 1936–1943. [Google Scholar] [CrossRef]
- Yang, C.; Chandran, K.S.R. Photolithographic Structuring of Ordered Silicon Micropillar Electrodes for Lithium-Ion Batteries and Electrochemical Performance. ACS Appl. Energy Mater. 2023, 6, 5930–5939. [Google Scholar] [CrossRef]
- Shi, F.; Song, Z.; Ross, P.N.; Somorjai, G.A.; Ritchie, R.O.; Komvopoulos, K. Failure Mechanisms of Single-Crystal Silicon Electrodes in Lithium-Ion Batteries. Nat. Commun. 2016, 7, 11886. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Noguchi, J.; Terashi, Y. Fabrication of Single-Crystal Silicon Micro Pillars on Copper Foils by Nanosecond Pulsed Laser Irradiation. CIRP Ann. Manuf. Technol. 2017, 66, 253–256. [Google Scholar] [CrossRef]
- Thakur, M.; Isaacson, M.; Sinsabaugh, S.L.; Wong, M.S.; Biswal, S.L. Gold-Coated Porous Silicon Films as Anodes for Lithium Ion Batteries. J. Power Sources 2012, 205, 426–432. [Google Scholar] [CrossRef]
- Park, J.; Suh, S.; Jeong, S.; Kim, H.J. New Approach for the High Electrochemical Performance of Silicon Anode in Lithium-Ion Battery: A Rapid and Large Surface Treatment Using a High-Energy Pulsed Laser. J. Power Sources 2021, 491, 229573. [Google Scholar] [CrossRef]
- Je, M.; Han, D.Y.; Ryu, J.; Park, S. Constructing Pure Si Anodes for Advanced Lithium Batteries. Acc. Chem Res. 2023, 56, 2213–2224. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Hwang, J.Y.; Sun, Y.K. Nano/Microstructured Silicon-Graphite Composite Anode for High-Energy-Density Li-Ion Battery. ACS Nano 2019, 13, 2624–2633. [Google Scholar] [CrossRef]
- Cai, C.; Hensley, D.; Koenig, G.M. High Capacity Rechargeable Coin Cells Using All Active Material Electrodes and Percolated Networks of LiCoO2 Blended with LiNi0.8Co0.15Al0.05O2. J. Alloys Compd. 2023, 968, 171965. [Google Scholar] [CrossRef]
- Robinson, J.P.; Ruppert, J.J.; Dong, H.; Koenig, G.M. Sintered Electrode Full Cells for High Energy Density Lithium-Ion Batteries. J. Appl. Electrochem. 2018, 48, 1297–1304. [Google Scholar] [CrossRef]
- Cai, C.; Min, B.; Yost, D.; Koenig, G.M. Combining Ester Solvent-Containing Electrolytes with All-Active Material Electrodes for High Current Density Lithium-Ion Batteries. ACS Appl. Energy Mater. 2023, 6, 11226–11233. [Google Scholar] [CrossRef]
- Cai, C.; Koenig, G.M. Processing Temperature Impact on TiNb 2 O 7 Thick All Active Material Lithium-Ion Battery Electrodes. J. Electrochem. Soc. 2023, 170, 010529. [Google Scholar] [CrossRef]
- Thorat, I.V.; Stephenson, D.E.; Zacharias, N.A.; Zaghib, K.; Harb, J.N.; Wheeler, D.R. Quantifying Tortuosity in Porous Li-Ion Battery Materials. J. Power Sources 2009, 188, 592–600. [Google Scholar] [CrossRef]
- Khetri, M.; Gupta, M.C. Recycling of Silver from Silicon Solar Cells by Laser Debonding. Sol. Energy 2024, 270, 112381. [Google Scholar] [CrossRef]
- Gottumukkala, N.R.; Gupta, M.C. Laser Processing of Sapphire and Fabrication of Diffractive Optical Elements. Appl Opt. 2022, 61, 2391. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Gottumukkala, N.R.; Gupta, M.C. Superhydrophobic Surface by Laser Ablation of PDMS. Langmuir 2023, 39, 11259–11267. [Google Scholar] [CrossRef]
- Cai, C.; Koenig, G.M. Enhancing Low Electronic Conductivity Materials in All Active Material Electrodes through Multicomponent Architecture. Energy Adv. 2023, 2, 308–320. [Google Scholar] [CrossRef]
- Nie, Z.; Ong, S.; Hussey, D.S.; Lamanna, J.M.; Jacobson, D.L.; Koenig, G.M. Probing Transport Limitations in Thick Sintered Battery Electrodes with Neutron Imaging. Mol. Syst. Des. Eng. 2020, 5, 245–256. [Google Scholar] [CrossRef]
- Cai, C.; Hensley, D.; Koenig, G.M. Simulated Discharge Overpotential Distributions for Sintered Electrode Batteries in Rechargeable Coin Cell Form Factors. J. Energy Storage 2022, 54, 105218. [Google Scholar] [CrossRef]
- Nie, Z.; Parai, R.; Cai, C.; Michaelis, C.; LaManna, J.M.; Hussey, D.S.; Jacobson, D.L.; Ghosh, D.; Koenig, G.M. Pore Microstructure Impacts on Lithium Ion Transport and Rate Capability of Thick Sintered Electrodes. J. Electrochem. Soc. 2021, 168, 060550. [Google Scholar] [CrossRef]
- Yoon, T.; Nguyen, C.C.; Seo, D.M.; Lucht, B.L. Capacity Fading Mechanisms of Silicon Nanoparticle Negative Electrodes for Lithium Ion Batteries. J. Electrochem. Soc. 2015, 162, A2325–A2330. [Google Scholar] [CrossRef]
- Chae, S.; Ko, M.; Kim, K.; Ahn, K.; Cho, J. Confronting Issues of the Practical Implementation of Si Anode in High-Energy Lithium-Ion Batteries. Joule 2017, 1, 47–60. [Google Scholar] [CrossRef]
- Zheng, H.; Li, J.; Song, X.; Liu, G.; Battaglia, V.S. A Comprehensive Understanding of Electrode Thickness Effects on the Electrochemical Performances of Li-Ion Battery Cathodes. Electrochim. Acta 2012, 71, 258–265. [Google Scholar] [CrossRef]
- Singh, M.; Kaiser, J.; Hahn, H. Thick Electrodes for High Energy Lithium Ion Batteries. J. Electrochem. Soc. 2015, 162, A1196–A1201. [Google Scholar] [CrossRef]
- Hwang, B.-H. Calculation and Measurement of All (002) Multiple Diffraction Peaks from a (001) Silicon Wafer. J. Phys. D Appl. Phys. 2001, 34, 2469–2474. [Google Scholar] [CrossRef]
- Wahab, R.; Ahmad, N.; Alam, M. Silicon Nanoparticles: A New and Enhanced Operational Material for Nitrophenol Sensing. J. Mater. Sci. Mater. Electron. 2020, 31, 17084–17099. [Google Scholar] [CrossRef]
- Delpuech, N.; Dupre, N.; Moreau, P.; Bridel, J.S.; Gaubicher, J.; Lestriez, B.; Guyomard, D. Mechanism of Silicon Electrode Aging upon Cycling in Full Lithium-Ion Batteries. ChemSusChem 2016, 9, 841–848. [Google Scholar] [CrossRef]
- Ezzedine, M.; Jardali, F.; Florea, I.; Zamfir, M.R.; Cojocaru, C.S. Nanostructuring Strategies for Silicon-Based Anodes in Lithium-Ion Batteries: Tuning Areal Silicon Loading, SEI Formation/Irreversible Capacity Loss, Rate Capability Retention and Electrode Durability. Batter. Supercaps 2023, 6, 202200451. [Google Scholar] [CrossRef]
- Chew, H.B.; Hou, B.; Wang, X.; Xia, S. Cracking Mechanisms in Lithiated Silicon Thin Film Electrodes. Int. J. Solids Struct. 2014, 51, 4176–4187. [Google Scholar] [CrossRef]
- Peng, K.; Jie, J.; Zhang, W.; Lee, S.T. Silicon Nanowires for Rechargeable Lithium-Ion Battery Anodes. Appl. Phys. Lett. 2008, 93, 033105. [Google Scholar] [CrossRef]
- Farid, G.; Amade-Rovira, R.; Ospina, R.; Bertran-Serra, E. Surface Modification of Silicon Nanowires via Drop-Casting for High-Performance Li-Ion Battery Electrodes: SiNWs Decorated with Molybdenum Oxide Nanoparticles. J. Energy Storage 2024, 78, 110104. [Google Scholar] [CrossRef]
- Lau, D.; Hall, C.A.; Lim, S.; Yuwono, J.A.; Burr, P.A.; Song, N.; Lennon, A. Reduced Silicon Fragmentation in Lithium Ion Battery Anodes Using Electronic Doping Strategies. ACS Appl. Energy Mater. 2020, 3, 1730–1741. [Google Scholar] [CrossRef]
- Nugroho, A.P.; Hawari, N.H.; Prakoso, B.; Refino, A.D.; Yulianto, N.; Iskandar, F.; Kartini, E.; Peiner, E.; Wasisto, H.S.; Sumboja, A. Vertically Aligned N-Type Silicon Nanowire Array as a Free-Standing Anode for Lithium-Ion Batteries. Nanomaterials 2021, 11, 3137. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Koenig, G.M. High-Performance LiCoO2 Sub-Micrometer Materials from Scalable Microparticle Template Processing. ChemistrySelect 2016, 1, 3992–3999. [Google Scholar] [CrossRef]
- Lee, P.-K.; Li, Y.; Yu, D.Y.W. Insights from Studying the Origins of Reversible and Irreversible Capacities on Silicon Electrodes. J. Electrochem. Soc. 2017, 164, A6206–A6212. [Google Scholar] [CrossRef]
- Marinaro, M.; Weinberger, M.; Wohlfahrt-Mehrens, M. Toward Pre-Lithiatied High Areal Capacity Silicon Anodes for Lithium-Ion Batteries. Electrochim. Acta 2016, 206, 99–107. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, C.; Lopez, J.; Lu, Z.; Cui, Y.; Bao, Z. High-Areal-Capacity Silicon Electrodes with Low-Cost Silicon Particles Based on Spatial Control of Self-Healing Binder. Adv. Energy Mater. 2015, 5, 201401826. [Google Scholar] [CrossRef]
- Lee, G.; Schweizer, S.L.; Wehrspohn, R.B. Electrochemical Characteristics of Plasma-Etched Black Silicon as Anodes for Li-Ion Batteries. J. Vac. Sci. Technol. A Vac. Surf. Film. 2014, 32, 4897609. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, B.; Chakraborty, A.; Cai, C.; Gupta, M.C.; Koenig, G.M., Jr. Assessment of Laser-Ablated Silicon Wafers as Lithium-Ion Battery Anodes. Batteries 2025, 11, 121. https://doi.org/10.3390/batteries11040121
Min B, Chakraborty A, Cai C, Gupta MC, Koenig GM Jr. Assessment of Laser-Ablated Silicon Wafers as Lithium-Ion Battery Anodes. Batteries. 2025; 11(4):121. https://doi.org/10.3390/batteries11040121
Chicago/Turabian StyleMin, Byeongcheol, Anustup Chakraborty, Chen Cai, Mool C. Gupta, and Gary M. Koenig, Jr. 2025. "Assessment of Laser-Ablated Silicon Wafers as Lithium-Ion Battery Anodes" Batteries 11, no. 4: 121. https://doi.org/10.3390/batteries11040121
APA StyleMin, B., Chakraborty, A., Cai, C., Gupta, M. C., & Koenig, G. M., Jr. (2025). Assessment of Laser-Ablated Silicon Wafers as Lithium-Ion Battery Anodes. Batteries, 11(4), 121. https://doi.org/10.3390/batteries11040121





