Review of Flame Behavior and Its Suppression during Thermal Runaway in Lithium-Ion Batteries
Abstract
1. Introduction
2. Thermal Safety of Lithium-Ion Batteries
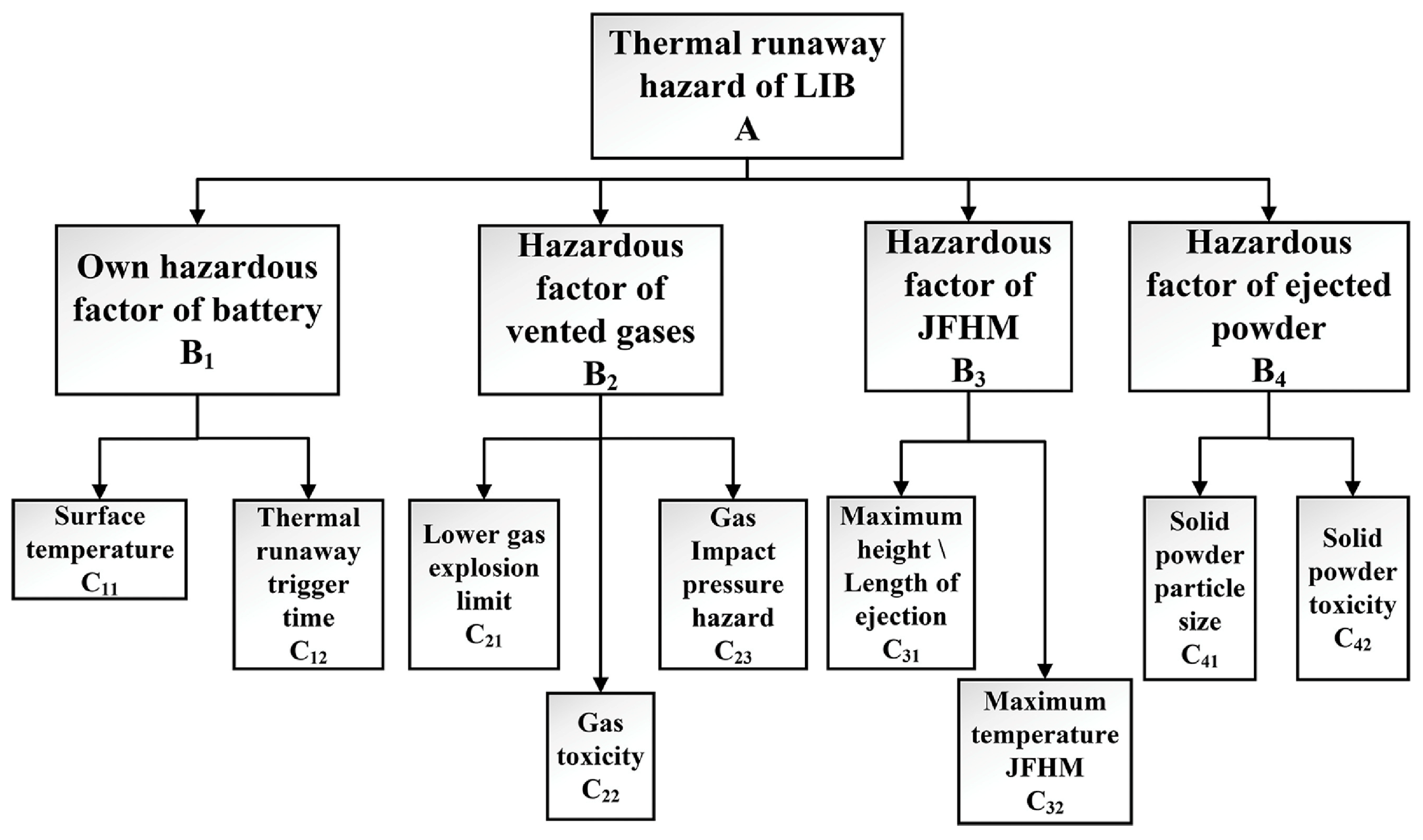
2.1. Flame Disaster of Lithium-Ion Batteries
2.2. The Necessity to Suppress TR Flames
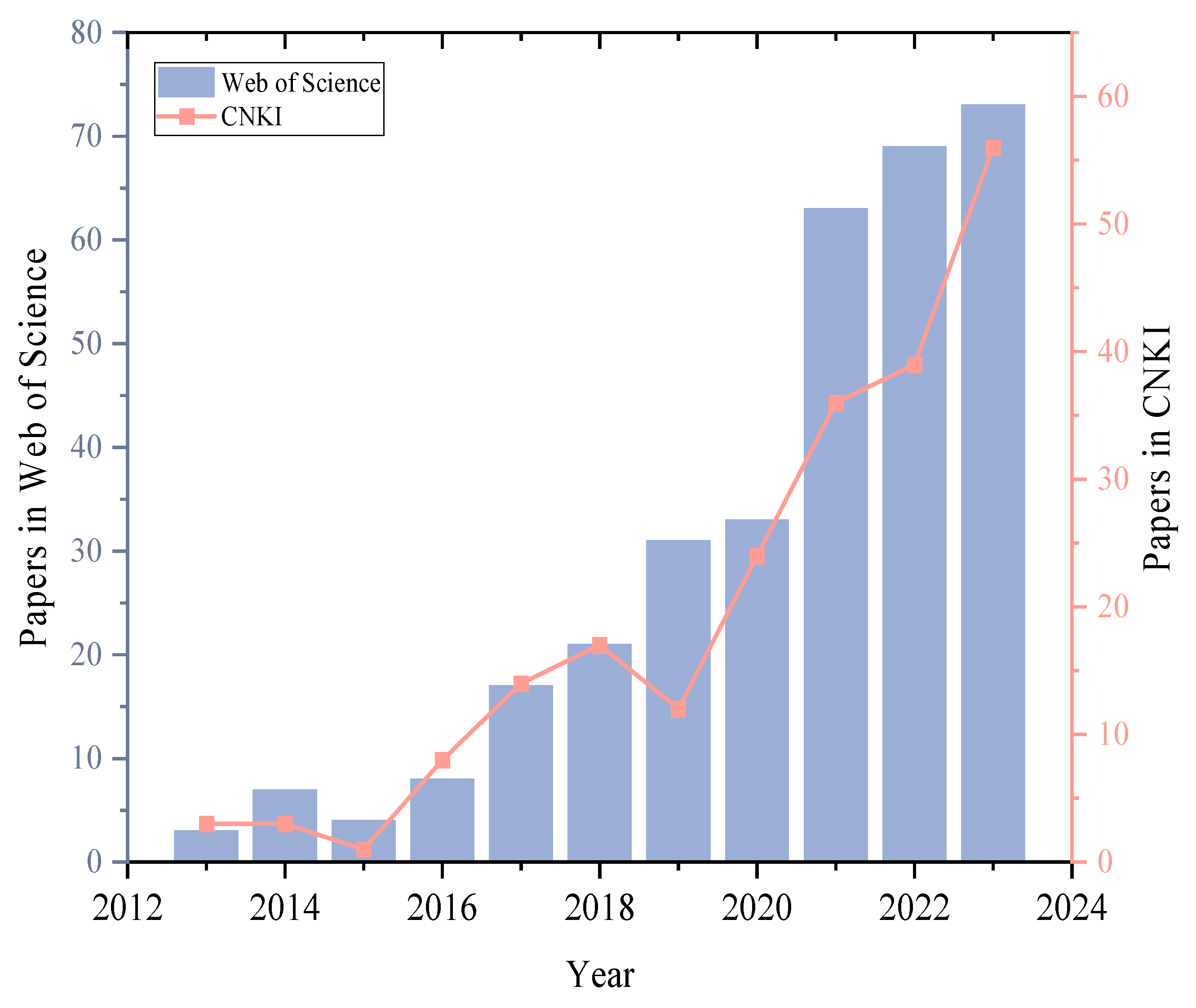
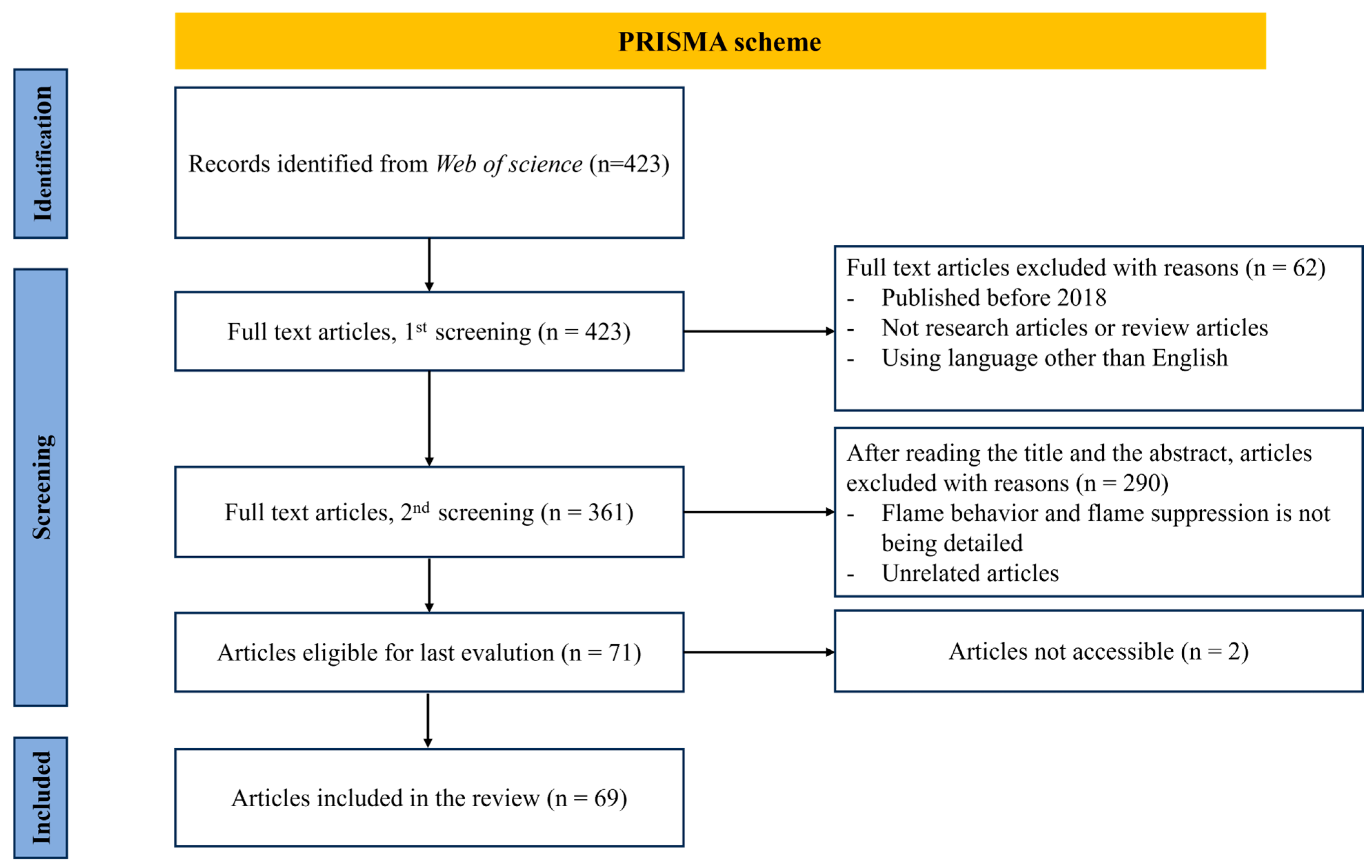
2.3. Literature Search
3. Flame Behavior during TR
3.1. Flame Behavior and TR in Single Batteries
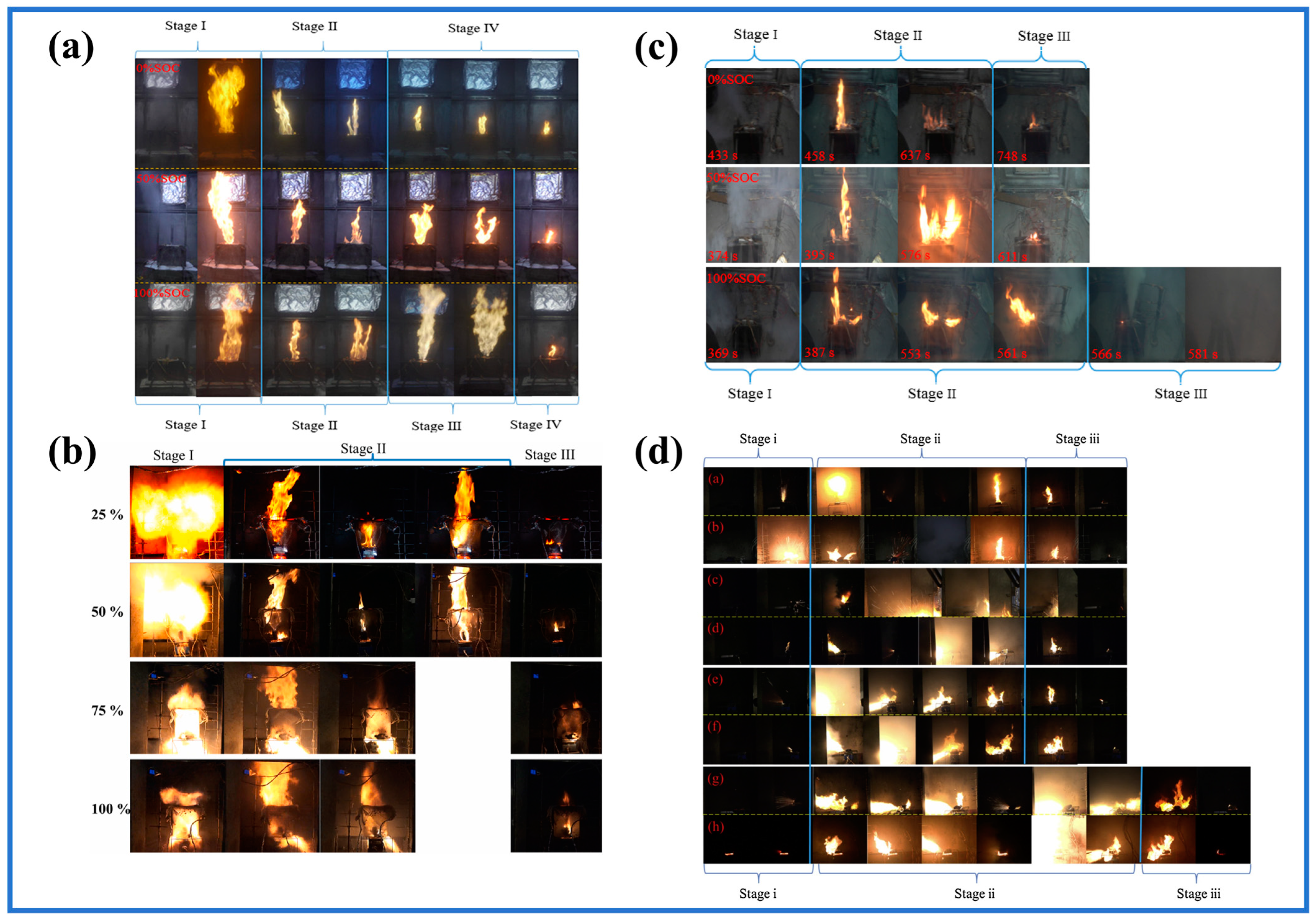
3.1.1. TR Progress
3.1.2. Influence of Internal Factors
3.1.3. Impact of External Factors

3.2. Discussion of Flame Behavior and Hazards for Different Types of Batteries
3.3. Flame Behavior and TPR in Battery Module
3.4. Flame Behavior and TPR in Confined Space
3.4.1. Difference between CS and OS
3.4.2. Flame Function
3.5. Relevant Flame Modeling
3.5.1. Development of Flame Modeling
3.5.2. Flame Modeling in a Confined Space
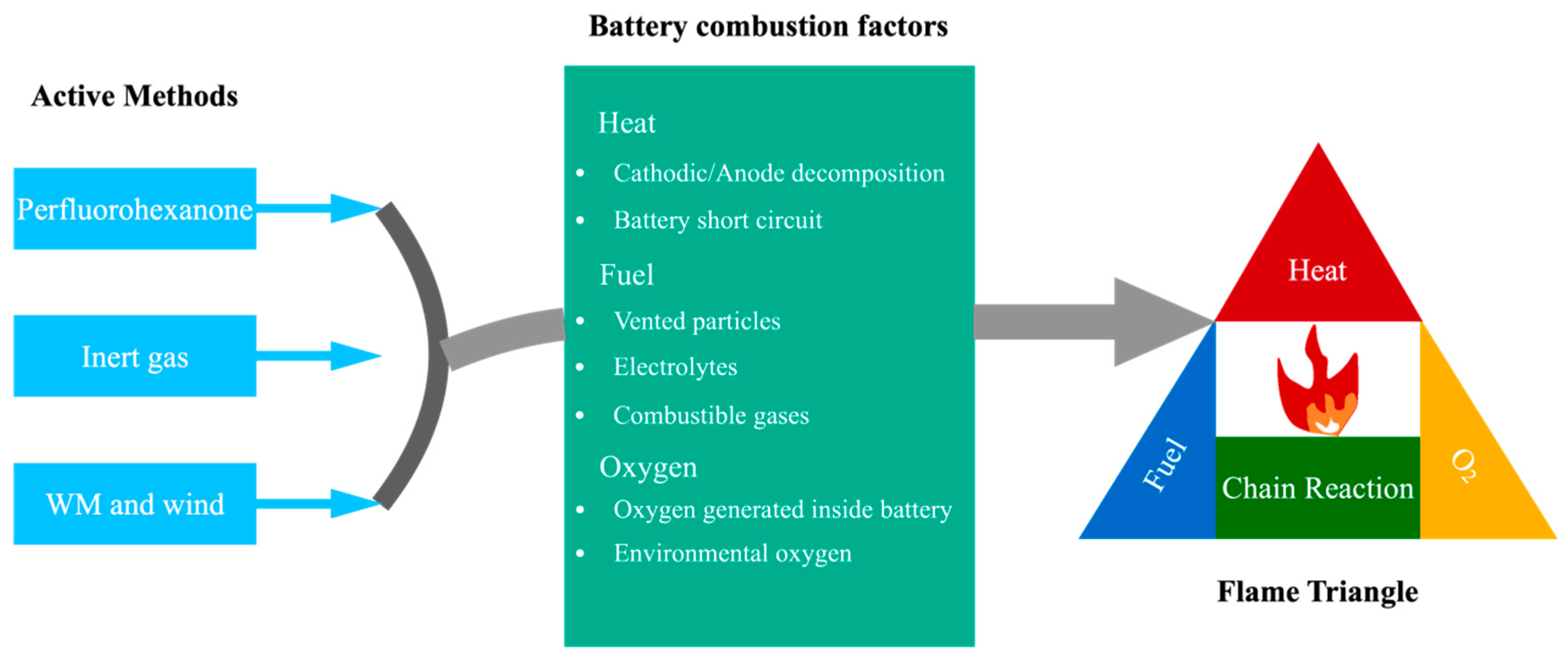
4. Methods of Suppressing Lithium-Ion Battery Flames
4.1. Active Suppression
4.2. Passive Suppression

5. Conclusions and Outlook
5.1. Conclusions
5.2. Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Whittingham, M.S. Electrical Energy Storage and Intercalation Chemistry. Science 1976, 192, 1126–1127. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wu, X.; Li, C.; Zhu, Y.; Fu, L.; Wu, Y.; Liu, X. Nanostructured positive electrode materials for post-lithium ion batteries. Energy Environ. Sci. 2016, 9, 3570–3611. [Google Scholar] [CrossRef]
- May, G.J.; Davidson, A.; Monahov, B. Lead batteries for utility energy storage: A review. J. Energy Storage 2018, 15, 145–157. [Google Scholar] [CrossRef]
- Zeng, X.; Li, M.; Abd El-Hady, D.; Alshitari, W.; Al-Bogami, A.S.; Lu, J.; Amine, K. Commercialization of Lithium Battery Technologies for Electric Vehicles. Adv. Energy Mater. 2019, 9, 1900161. [Google Scholar] [CrossRef]
- Noh, H.-J.; Youn, S.; Yoon, C.S.; Sun, Y.-K. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Sources 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Wang, Q.; Mao, B.; Stoliarov, S.I.; Sun, J. A review of lithium ion battery failure mechanisms and fire prevention strategies. Prog. Energy Combust. Sci. 2019, 73, 95–131. [Google Scholar] [CrossRef]
- Mao, B.; Chen, H.; Jiang, L.; Zhao, C.; Sun, J.; Wang, Q. Refined study on lithium ion battery combustion in open space and a combustion chamber. Process Saf. Environ. Prot. 2020, 139, 133–146. [Google Scholar] [CrossRef]
- Liu, P.; Sun, H.; Qiao, Y.; Sun, S.; Wang, C.; Jin, K.; Mao, B.; Wang, Q. Experimental study on the thermal runaway and fire behavior of LiNi0.8Co0.1Mn0.1O2 battery in open and confined spaces. Process Saf. Environ. Prot. 2022, 158, 711–726. [Google Scholar] [CrossRef]
- Qin, P.; Jia, Z.; Wu, J.; Jin, K.; Duan, Q.; Jiang, L.; Sun, J.; Ding, J.; Shi, C.; Wang, Q. The thermal runaway analysis on LiFePO4 electrical energy storage packs with different venting areas and void volumes. Appl. Energy 2022, 313, 118767. [Google Scholar] [CrossRef]
- Li, J.; Gao, P.; Tong, B.; Cheng, Z.; Cao, M.; Mei, W.; Wang, Q.; Sun, J.; Qin, P. Revealing the mechanism of pack ceiling failure induced by thermal runaway in NCM batteries: A coupled multiphase fluid-structure interaction model for electric vehicles. eTransportation 2024, 20, 100335. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, H.; Wang, G.; Gao, X.; Ping, P.; Kong, D.; Yin, X. Effect of flame heating on thermal runaway propagation of lithium-ion batteries in confined space. J. Energy Storage 2024, 78, 110052. [Google Scholar] [CrossRef]
- Feng, X.; Ren, D.; He, X.; Ouyang, M. Mitigating Thermal Runaway of Lithium-Ion Batteries. Joule 2020, 4, 743–770. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Z.; Jiang, C.; Ji, Z.; Zhu, Z. Internal temperature and flame jet characteristics during thermal runaway triggered by nail penetration for NCM811 lithium-ion battery. J. Therm. Anal. Calorim. 2022, 147, 14925–14938. [Google Scholar] [CrossRef]
- Mao, B.; Chen, H.; Cui, Z.; Wu, T.; Wang, Q. Failure mechanism of the lithium ion battery during nail penetration. Int. J. Heat Mass Transf. 2018, 122, 1103–1115. [Google Scholar] [CrossRef]
- Liu, P.; Liu, C.; Yang, K.; Zhang, M.; Gao, F.; Mao, B.; Li, H.; Duan, Q.; Wang, Q. Thermal runaway and fire behaviors of lithium iron phosphate battery induced by over heating. J. Energy Storage 2020, 31, 101714. [Google Scholar] [CrossRef]
- Zou, K.; Li, Q.; Lu, S. An experimental study on thermal runaway and fire behavior of large-format LiNi0.8Co0.1Mn0.1O2 pouch power cell. J. Energy Storage 2022, 49, 104138. [Google Scholar] [CrossRef]
- Liu, P.; Li, S.; Jin, K.; Fu, W.; Wang, C.; Jia, Z.; Jiang, L.; Wang, Q. Thermal Runaway and Fire Behaviors of Lithium Iron Phosphate Battery Induced by Overheating and Overcharging. Fire Technol. 2023, 59, 1051–1072. [Google Scholar] [CrossRef]
- Kim, S.W.; Park, S.G.; Lee, E.J. Assessment of the explosion risk during lithium-ion battery fires. J. Loss Prev. Process Ind. 2022, 80, 104851. [Google Scholar] [CrossRef]
- Tao, C.; Ye, Q.; Wang, C.; Qian, Y.; Wang, C.; Zhou, T.; Tang, Z. An experimental investigation on the burning behaviors of lithium ion batteries after different immersion times. J. Clean. Prod. 2020, 242, 118539. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Zhou, L.; Han, C.; He, T.; Wang, Z. Influencing factors of lithium-ion battery thermal runaway in confined space. J. Energy Storage 2023, 73, 109125. [Google Scholar] [CrossRef]
- Meng, D.; Wang, X.; Chen, M.; Wang, J. Effects of environmental temperature on the thermal runaway of lithium-ion batteries during charging process. J. Loss Prev. Process Ind. 2023, 83, 105084. [Google Scholar] [CrossRef]
- Chen, M.; Ouyang, D.; Weng, J.; Liu, J.; Wang, J. Environmental pressure effects on thermal runaway and fire behaviors of lithium-ion battery with different cathodes and state of charge. Process Saf. Environ. Prot. 2019, 130, 250–256. [Google Scholar] [CrossRef]
- Ding, C.; Zhu, N.; Yu, J.; Li, Y.; Sun, X.; Liu, C.; Huang, Q.; Wang, J. Experimental investigation of environmental pressure effects on thermal runaway properties of 21700 lithium-ion batteries with high energy density. Case Stud. Therm. Eng. 2022, 38, 102349. [Google Scholar] [CrossRef]
- Weng, J.; Ouyang, D.; Liu, Y.; Chen, M.; Li, Y.; Huang, X.; Wang, J. Alleviation on battery thermal runaway propagation: Effects of oxygen level and dilution gas. J. Power Sources 2021, 509, 230340. [Google Scholar] [CrossRef]
- Tao, C.; Zhu, Y.; Liu, Z.; Li, R.; Chen, Z.; Gong, L.; Liu, J. The experimental investigation of thermal runaway characteristics of lithium battery under different nitrogen concentrations. J. Therm. Anal. Calorim. 2023, 148, 12097–12107. [Google Scholar] [CrossRef]
- Ün, Ç.; Aydin, K.; Garo, J.P.; Coudour, B.; Gentilleau, M. Experimental study of fire suppression for li-ion electric batteries with H2O. Fresenius Environ. Bull. 2021, 30, 790–801. [Google Scholar]
- Meng, X.; Li, S.; Fu, W.; Chen, Y.; Duan, Q.; Wang, Q. Experimental study of intermittent spray cooling on suppression for lithium iron phosphate battery fires. eTransportation 2022, 11, 100142. [Google Scholar] [CrossRef]
- Huang, Q.; Li, X.; Zhang, G.; Weng, J.; Wang, Y.; Deng, J. Innovative thermal management and thermal runaway suppression for battery module with flame retardant flexible composite phase change material. J. Clean. Prod. 2022, 330, 129718. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, H.; Wu, D.; Yang, Y.; Yuan, C.; Li, G. Inhibition effect of inert gas jet on gas and hybrid explosions caused by thermal runaway of lithium-ion battery. J. Loss Prev. Process Ind. 2024, 90, 105336. [Google Scholar] [CrossRef]
- Zhao, L.; Li, W.; Luo, W.; Zheng, M.; Chen, M. Numerical study of critical conditions for thermal runaway of lithium-ion battery pack during storage. J. Energy Storage 2024, 84, 110901. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, N.; Jiang, L.; Wei, Z.; Zhang, Y.; Yu, Y.; Song, L.; Wang, L.; Duan, Q.; Sun, J.; et al. Assessment of the complete chain evolution process of LIBs from micro internal short circuit failure to thermal runaway under mechanical abuse conditions. Process Saf. Environ. Prot. 2024, 185, 296–306. [Google Scholar] [CrossRef]
- Liu, P.; Li, Y.; Mao, B.; Chen, M.; Huang, Z.; Wang, Q. Experimental study on thermal runaway and fire behaviors of large format lithium iron phosphate battery. Appl. Therm. Eng. 2021, 192, 116949. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Q.; Yin, B.; Zhai, H.; Wang, J.; An, W. Characterization of fire behaviors associated with a thermal runaway in large-scale commercial LiNi0.8Co0.1Mn0.1O2/graphite cells under external ignition. Case Stud. Therm. Eng. 2023, 47, 103126. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Li, Y.; Wang, G.; Wang, J. Thermal runaway and fire behaviors of large-scale lithium ion batteries with different heating methods. J. Hazard. Mater. 2019, 379, 120730. [Google Scholar] [CrossRef]
- Zhu, X.; Sun, Z.; Wang, Z.; Wang, H.; Lin, N.; Shan, C. Thermal runaway in commercial lithium-ion cells under overheating condition and the safety assessment method: Effects of SoCs, cathode materials and packaging forms. J. Energy Storage 2023, 68, 107768. [Google Scholar] [CrossRef]
- Zou, K.; Lu, S. Comparative study on the influence of incident heat flux on thermal runaway fire development of large-format lithium-ion batteries. Process Saf. Environ. Prot. 2023, 176, 831–840. [Google Scholar] [CrossRef]
- Zhang, Q.; Niu, J.; Zhao, Z.; Wang, Q. Research on the effect of thermal runaway gas components and explosion limits of lithium-ion batteries under different charge states. J. Energy Storage 2022, 45, 103759. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Fuchs, D.; Wagner, J.; Wiltsche, H.; Stangl, C.; Fauler, G.; Voitic, G.; Thaler, A.; Hacker, V. Thermal-runaway experiments on consumer Li-ion batteries with metal-oxide and olivin-type cathodes. RSC Adv. 2014, 4, 3633–3642. [Google Scholar] [CrossRef]
- Yuan, L.; Dubaniewicz, T.; Zlochower, I.; Thomas, R.; Rayyan, N. Experimental study on thermal runaway and vented gases of lithium-ion cells. Process Saf. Environ. Prot. 2020, 144, 186–192. [Google Scholar] [CrossRef]
- Yang, M.; Rong, M.; Pan, J.; Ye, Y.; Yang, A.; Chu, J.; Yuan, H.; Wang, X. Thermal runaway behavior analysis during overheating for commercial LiFePO4 batteries under various state of charges. Appl. Therm. Eng. 2023, 230, 120816. [Google Scholar] [CrossRef]
- Wang, S.; Song, L.; Li, C.; Tian, J.; Jin, K.; Duan, Q.; Wang, Q. Experimental study of gas production and flame behavior induced by the thermal runaway of 280 Ah lithium iron phosphate battery. J. Energy Storage 2023, 74, 109368. [Google Scholar] [CrossRef]
- Perea, A.; Paolella, A.; Dubé, J.; Champagne, D.; Mauger, A.; Zaghib, K. State of charge influence on thermal reactions and abuse tests in commercial lithium-ion cells. J. Power Sources 2018, 399, 392–397. [Google Scholar] [CrossRef]
- Doose, S.; Hahn, A.; Fischer, S.; Müller, J.; Haselrieder, W.; Kwade, A. Comparison of the consequences of state of charge and state of health on the thermal runaway behavior of lithium ion batteries. J. Energy Storage 2023, 62, 106837. [Google Scholar] [CrossRef]
- Wu, S.; Wang, C.; Luan, W.; Zhang, Y.; Chen, Y.; Chen, H. Thermal runaway behaviors of Li-ion batteries after low temperature aging: Experimental study and predictive modeling. J. Energy Storage 2023, 66, 107451. [Google Scholar] [CrossRef]
- Spotnitz, R.; Franklin, J. Abuse behavior of high-power, lithium-ion cells. J. Power Sources 2003, 113, 81–100. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Li, W.; Li, C.; Ouyang, M. Size distribution and elemental composition of vent particles from abused prismatic Ni-rich automotive lithium-ion batteries. J. Energy Storage 2019, 26, 100991. [Google Scholar] [CrossRef]
- Wang, J.-h.; Jiang, Z.; Mei, M.; Qiu, H.; Wang, Y. Numerical simulation study on two-phase flow of thermal runaway evolution and jet fire of 18650 lithium-ion battery under thermal abuse. Case Stud. Therm. Eng. 2024, 53, 103726. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, J.; Yang, K.; Chen, H.; Huang, Y. A 3D simulation model of thermal runaway in Li-ion batteries coupled particles ejection and jet flow. J. Power Sources 2023, 580, 233357. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, S.; He, X.; Wang, C.; Zhao, D. A multi-factor evaluation method for the thermal runaway risk of lithium-ion batteries. J. Energy Storage 2022, 45, 103767. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Bai, J. Influences of multi factors on thermal runaway induced by overcharging of lithium-ion battery. J. Energy Chem. 2022, 70, 531–541. [Google Scholar] [CrossRef]
- Said, A.O.; Lee, C.; Stoliarov, S.I. Experimental investigation of cascading failure in 18650 lithium ion cell arrays: Impact of cathode chemistry. J. Power Sources 2020, 446, 227347. [Google Scholar] [CrossRef]
- Liu, X.; Stoliarov, S.I.; Denlinger, M.; Masias, A.; Snyder, K. Comprehensive calorimetry of the thermally-induced failure of a lithium ion battery. J. Power Sources 2015, 280, 516–525. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Z.; Stoliarov, S.I.; Denlinger, M.; Masias, A.; Snyder, K. Heat release during thermally-induced failure of a lithium ion battery: Impact of cathode composition. Fire Saf. J. 2016, 85, 10–22. [Google Scholar] [CrossRef]
- Natarajan, J.; Lieuwen, T.; Seitzman, J. Laminar flame speeds of H2/CO mixtures: Effect of CO2 dilution, preheat temperature, and pressure. Combust. Flame 2007, 151, 104–119. [Google Scholar] [CrossRef]
- Qi, S.; Du, Y.; Zhang, P.; Li, G.; Zhou, Y.; Wang, B. Effects of concentration, temperature, humidity, and nitrogen inert dilution on the gasoline vapor explosion. J. Hazard. Mater. 2017, 323, 593–601. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, W.; Zhang, H.; Wu, Y.; Lu, J. Effects of inert dilution on the propagation and extinction of lean premixed syngas/air flames. Fuel 2015, 157, 115–121. [Google Scholar] [CrossRef]
- Hafiz, N.M.; Mansor, M.R.A.; Wan Mahmood, W.M.F. Simulation of the combustion process for a CI hydrogen engine in an argon-oxygen atmosphere. Int. J. Hydrogen Energy 2018, 43, 11286–11297. [Google Scholar] [CrossRef]
- Yan, W.; Wang, Z.; Chen, S. Quantitative analysis on the heat transfer modes in the process of thermal runaway propagation in lithium-ion battery pack under confined and semi-confined space. Int. J. Heat Mass Transf. 2021, 176, 121483. [Google Scholar] [CrossRef]
- Chen, M.; Liu, J.; He, Y.; Yuen, R.; Wang, J. Study of the fire hazards of lithium-ion batteries at different pressures. Appl. Therm. Eng. 2017, 125, 1061–1074. [Google Scholar] [CrossRef]
- Wang, H.; Du, Z.; Liu, L.; Zhang, Z.; Hao, J.; Wang, Q.; Wang, S. Study on the Thermal Runaway and Its Propagation of Lithium-Ion Batteries Under Low Pressure. Fire Technol. 2020, 56, 2427–2440. [Google Scholar] [CrossRef]
- Wang, H.; Xu, H.; Zhang, Z.; Wang, Q.; Jin, C.; Wu, C.; Xu, C.; Hao, J.; Sun, L.; Du, Z.; et al. Fire and explosion characteristics of vent gas from lithium-ion batteries after thermal runaway: A comparative study. eTransportation 2022, 13, 100190. [Google Scholar] [CrossRef]
- Xie, S.; Gong, Y.; Li, G.; Ping, X. Effect of low-pressure environment on thermal runaway behavior of NCM523/graphite pouch cells with different overcharge cycles. J. Energy Storage 2022, 55, 105444. [Google Scholar] [CrossRef]
- Wang, K.; Wu, D.; Chang, C.; Zhang, J.; Ouyang, D.; Qian, X. Charging rate effect on overcharge-induced thermal runaway characteristics and gas venting behaviors for commercial lithium iron phosphate batteries. J. Clean. Prod. 2024, 434, 139992. [Google Scholar] [CrossRef]
- Talele, V.; Moralı, U.; Patil, M.S.; Panchal, S.; Fraser, R.; Fowler, M.; Thorat, P.; Gokhale, Y.P. Computational modelling and statistical evaluation of thermal runaway safety regime response on lithium-ion battery with different cathodic chemistry and varying ambient condition. Int. Commun. Heat Mass Transf. 2023, 146, 106907. [Google Scholar] [CrossRef]
- Huang, P.; Ping, P.; Li, K.; Chen, H.; Wang, Q.; Wen, J.; Sun, J. Experimental and modeling analysis of thermal runaway propagation over the large format energy storage battery module with Li4Ti5O12 anode. Appl. Energy 2016, 183, 659–673. [Google Scholar] [CrossRef]
- Ping, P.; Wang, Q.; Huang, P.; Li, K.; Sun, J.; Kong, D.; Chen, C. Study of the fire behavior of high-energy lithium-ion batteries with full-scale burning test. J. Power Sources 2015, 285, 80–89. [Google Scholar] [CrossRef]
- Huang, P.; Wang, Q.; Li, K.; Ping, P.; Sun, J. The combustion behavior of large scale lithium titanate battery. Sci. Rep. 2015, 5, 7788. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, S.; Chen, Y.; Zhao, L.; Chen, M. Experimental and analytical investigation on the thermal runaway propagation characteristics of lithium-ion battery module with NCM pouch cells under various state of charge and spacing. J. Energy Storage 2023, 72, 108380. [Google Scholar] [CrossRef]
- An, Z.; Li, W.; Du, X.; Jia, L.; Li, Q.; Zhang, D. Experimental study on behaviors of lithium-ion cells experiencing internal short circuit and thermal runaway under nail penetration abuse condition. Appl. Therm. Eng. 2024, 247, 123058. [Google Scholar] [CrossRef]
- Kong, D.; Zhao, H.; Ping, P.; Zhang, Y.; Wang, G. Effect of low temperature on thermal runaway and fire behaviors of 18650 lithium-ion battery: A comprehensive experimental study. Process Saf. Environ. Prot. 2023, 174, 448–459. [Google Scholar] [CrossRef]
- Shahid, S.; Agelin-Chaab, M. A review of thermal runaway prevention and mitigation strategies for lithium-ion batteries. Energy Convers. Manag. X 2022, 16, 100310. [Google Scholar] [CrossRef]
- Song, L.; Huang, Z.; Mei, W.; Jia, Z.; Yu, Y.; Wang, Q.; Jin, K. Thermal runaway propagation behavior and energy flow distribution analysis of 280 Ah LiFePO4 battery. Process Saf. Environ. Prot. 2023, 170, 1066–1078. [Google Scholar] [CrossRef]
- Mishra, D.; Tummala, R.; Jain, A. Investigation of propagation of thermal runaway during large-scale storage and transportation of Li-ion batteries. J. Energy Storage 2023, 72, 108315. [Google Scholar] [CrossRef]
- Wang, Z.; He, T.; Bian, H.; Jiang, F.; Yang, Y. Characteristics of and factors influencing thermal runaway propagation in lithium-ion battery packs. J. Energy Storage 2021, 41, 102956. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, M.; Zhou, X.; Ju, X.; Yang, L. Investigating thermal runaway characteristics and trigger mechanism of the parallel lithium-ion battery. Appl. Energy 2023, 349, 121690. [Google Scholar] [CrossRef]
- Jin, C.; Sun, Y.; Wang, H.; Zheng, Y.; Wang, S.; Rui, X.; Xu, C.; Feng, X.; Wang, H.; Ouyang, M. Heating power and heating energy effect on the thermal runaway propagation characteristics of lithium-ion battery module: Experiments and modeling. Appl. Energy 2022, 312, 118760. [Google Scholar] [CrossRef]
- Wang, H.; Xu, H.; Zhao, Z.; Wang, Q.; Jin, C.; Li, Y.; Sheng, J.; Li, K.; Du, Z.; Xu, C.; et al. An experimental analysis on thermal runaway and its propagation in Cell-to-Pack lithium-ion batteries. Appl. Therm. Eng. 2022, 211, 118418. [Google Scholar] [CrossRef]
- Said, A.O.; Lee, C.; Stoliarov, S.I.; Marshall, A.W. Comprehensive analysis of dynamics and hazards associated with cascading failure in 18650 lithium ion cell arrays. Appl. Energy 2019, 248, 415–428. [Google Scholar] [CrossRef]
- Kim, J.T.; Choi, J.Y.; Kang, S.; Han, N.G.; Kim, D.K. Development of thermal runaway propagation model considering vent gas combustion for electric vehicles. J. Energy Storage 2023, 60, 106535. [Google Scholar] [CrossRef]
- Wang, G.; Ping, P.; Zhang, Y.; Zhao, H.; Lv, H.; Gao, X.; Gao, W.; Kong, D. Modeling thermal runaway propagation of lithium-ion batteries under impacts of ceiling jet fire. Process Saf. Environ. Prot. 2023, 175, 524–540. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, D.; Ping, P.; Zhao, H.; Dai, X.; Chen, X. Effect of a plate obstacle on fire behavior of 18650 lithium ion battery: An experimental study. J. Energy Storage 2022, 54, 105283. [Google Scholar] [CrossRef]
- Liu, P.; Wang, C.; Sun, S.; Zhao, G.; Yu, X.; Hu, Y.; Mei, W.; Jin, K.; Wang, Q. Understanding the influence of the confined cabinet on thermal runaway of large format batteries with different chemistries: A comparison and safety assessment study. J. Energy Storage 2023, 74, 109337. [Google Scholar] [CrossRef]
- Wang, G.; Ping, P.; Peng, R.; Lv, H.; Zhao, H.; Gao, W.; Kong, D. A semi reduced-order model for multi-scale simulation of fire propagation of lithium-ion batteries in energy storage system. Renew. Sustain. Energy Rev. 2023, 186, 113672. [Google Scholar] [CrossRef]
- You, H.Z.; Faeth, G.M. Ceiling heat transfer during fire plume and fire impingement. Fire Mater. 1979, 3, 140–147. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, L.; Zhu, W.; Zhang, X.; Yang, L. Flame extension length and temperature profile in thermal impinging flow of buoyant round jet upon a horizontal plate. Appl. Therm. Eng. 2014, 73, 15–22. [Google Scholar] [CrossRef]
- Gao, Z.; Jie, J.; Wan, H.; Zhu, J.; Sun, J. Experimental investigation on transverse ceiling flame length and temperature distribution of sidewall confined tunnel fire. Fire Saf. J. 2017, 91, 371–379. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, K.; Zhang, L.; Nie, X.; Wu, Y.; Jiang, J.; Dederichs, A.S.; He, L. Flame extension area and temperature profile of horizontal jet fire impinging on a vertical plate. Process Saf. Environ. Prot. 2021, 147, 547–558. [Google Scholar] [CrossRef]
- Zhai, H.; Chi, M.; Li, J.; Li, D.; Huang, Z.; Jia, Z.; Sun, J.; Wang, Q. Thermal runaway propagation in large format lithium ion battery modules under inclined ceilings. J. Energy Storage 2022, 51, 104477. [Google Scholar] [CrossRef]
- Dahn, J.; Richard, M. Accelerating Rate Calorimetry Study on the Thermal Stability of Lithium Intercalated Graphite in Electrolyte. II. Modeling the Results and Predicting Differential Scanning Calorimeter Curves. Fuel Energy Abstr. 1999, 146, 2078–2084. [Google Scholar]
- Hatchard, T.D.; Macneil, D.D.; Basu, A.; Dahn, J.R. Thermal Model of Cylindrical and Prismatic Lithium-Ion Cells. J. Electrochem. Soc. 2001, 148, A755–A761. [Google Scholar] [CrossRef]
- Kim, G.H.; Pesaran, A.; Spotnitz, R. Three-Dimensional Thermal Abuse Model for Lithium-Ion Cells. J. Power Sources 2007, 170, 476–489. [Google Scholar] [CrossRef]
- Coman, P.T.; Rayman, S.; White, R.E. A lumped model of venting during thermal runaway in a cylindrical Lithium Cobalt Oxide lithium-ion cell. J. Power Sources 2016, 307, 56–62. [Google Scholar] [CrossRef]
- Feng, X.; Lu, L.; Ouyang, M.; Li, J.; He, X. A 3D thermal runaway propagation model for a large format lithium ion battery module. Energy 2016, 115, 194–208. [Google Scholar] [CrossRef]
- Coman, P.; Matefi-Tempfli, S.; Veje, C.; White, R. Modeling Vaporization, Gas Generation and Venting in Li-Ion Battery Cells with a Dimethyl Carbonate Electrolyte. J. Electrochem. Soc. 2017, 164, A1858–A1865. [Google Scholar] [CrossRef]
- Bugryniec, P.J.; Davidson, D.J.N.; Brown, D.S.F. Advanced abuse modelling of Li-ion cells—A novel description of cell pressurisation and simmering reactions. J. Power Sources 2020, 474, 228396. [Google Scholar] [CrossRef]
- Wang, G.; Kong, D.; Ping, P.; Wen, J.; He, X.; Zhao, H.; He, X.; Peng, R.; Zhang, Y.; Dai, X. Revealing particle venting of lithium-ion batteries during thermal runaway: A multi-scale model toward multiphase process. eTransportation 2023, 16, 100237. [Google Scholar] [CrossRef]
- Kong, D.; Wang, G.; Ping, P.; Wen, J. A coupled conjugate heat transfer and CFD model for the thermal runaway evolution and jet fire of 18650 lithium-ion battery under thermal abuse. eTransportation 2022, 12, 100157. [Google Scholar] [CrossRef]
- Voigt, S.; Sträubig, F.; Kwade, A.; Zehfuß, J.; Knaust, C. An empirical model for lithium-ion battery fires for CFD applications. Fire Saf. J. 2023, 135, 103725. [Google Scholar] [CrossRef]
- Azizighalehsari, S.; Venugopal, P.; Singh, D.; Batista, T.; Rietveld, G. Empowering Electric Vehicles Batteries: A Comprehensive Look at the Application and Challenges of Second-Life Batteries. Batteries 2024, 10, 161. [Google Scholar] [CrossRef]
- Placke, T.; Heckmann, A.; Schmuch, R.; Meister, P.; Beltrop, K.; Winter, M. Perspective on Performance, Cost, and Technical Challenges for Practical Dual-Ion Batteries. Joule 2018, 2, 2528–2550. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, W.; Chen, L.; Li, Y. Inhibition and enhancement of hydrogen explosion by perfluorohexanone. Int. J. Hydrogen Energy 2024, 53, 522–534. [Google Scholar] [CrossRef]
- Wang, Z.; He, C.; Geng, Z.; Li, G.; Zhang, Y.; Shi, X.; Yao, B. Experimental study of thermal runaway propagation suppression of lithium-ion battery module in electric vehicle power packs. Process Saf. Environ. Prot. 2024, 182, 692–702. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Duan, Q.; Chen, M.; Xu, J.; Zhao, C.; Sun, J.; Wang, Q. Experimental study on the synergistic effect of gas extinguishing agents and water mist on suppressing lithium-ion battery fires. J. Energy Storage 2020, 32, 101801. [Google Scholar] [CrossRef]
- Prathap, C.; Ray, A.; Ravi, M.R. Investigation of nitrogen dilution effects on the laminar burning velocity and flame stability of syngas fuel at atmospheric condition. Combust. Flame 2008, 155, 145–160. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Qiu, X.; Li, B.; Zhang, H. Effects of Inert Dilution and Preheating Temperature on Lean Flammability Limit of Syngas. Energy Fuels 2014, 28, 3442–3452. [Google Scholar] [CrossRef]
- Lin, C.; Yan, H.; Qi, C.; Liu, Z.; Liu, D.; Liu, X.; Lao, L.; Li, Z.; Sun, Y. Thermal runaway and gas production characteristics of semi-solid electrolyte and liquid electrolyte lithium-Ion batteries: A comparative study. Process Saf. Environ. Prot. 2024, 189, 577–586. [Google Scholar] [CrossRef]
- Miao, B.; Lv, J.; Wang, Q.; Zhu, G.; Guo, C.; An, G.; Ou, J. The Suppression Effect of Water Mist Released at Different Stages on Lithium-Ion Battery Flame Temperature, Heat Release, and Heat Radiation. Batteries 2024, 10, 232. [Google Scholar] [CrossRef]
- Hong, Y.; Jin, C.; Chen, S.; Xu, C.; Wang, H.; Wu, H.; Huang, S.; Wang, Q.; Li, H.; Zheng, Y.; et al. Experimental study of the suppressing effect of the primary fire and thermal runaway propagation for electric bicycle batteries using flood cooling. J. Clean. Prod. 2024, 435, 140392. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Q.; Yin, B.; Shi, B.; Wang, J.; An, W. Influence of longitudinal wind on thermal runaway and fire behaviors of 18650 lithium-ion batteries in a restricted channel. J. Power Sources 2023, 567, 232974. [Google Scholar] [CrossRef]
- Jiang, X.; Han, H.; Liu, X.; Zhang, P. The synergistic effect of wind and two-phase flow water mist on thermal runaway and its propagation of lithium-ion battery module within battery case. J. Energy Storage 2024, 89, 111701. [Google Scholar] [CrossRef]
- Bhavsar, S.; Kant, K.; Pitchumani, R. Robust model-predictive thermal control of lithium-ion batteries under drive cycle uncertainty. J. Power Sources 2023, 557, 232496. [Google Scholar] [CrossRef]
- Vikram, S.; Vashisht, S.; Rakshit, D. Performance analysis of liquid-based battery thermal management system for Electric Vehicles during discharge under drive cycles. J. Energy Storage 2022, 55, 105737. [Google Scholar] [CrossRef]
- Nicholls, R.A.; Moghimi, M.A.; Sehhat, S. Thermal performance analysis of battery modules with passive cooling under different cycling loads in electric vehicles. J. Energy Storage 2024, 94, 112349. [Google Scholar] [CrossRef]
- Qin, M.; Zeng, Z.; Wu, Q.; Liu, X.; Liu, Q.; Cheng, S.; Xie, J. 1,3,5-Trifluorobenzene endorsed EC-free electrolyte for high-voltage and wide-temperature lithium-ion batteries. J. Energy Chem. 2023, 85, 49–57. [Google Scholar] [CrossRef]
- Wu, F.; Shi, Q.; Chen, L.; Dong, J.; Zhao, J.; Wang, H.; Gao, F.; Liu, J.; Zhang, H.; Li, N.; et al. New insights into dry-coating-processed surface engineering enabling structurally and thermally stable high-performance Ni-rich cathode materials for lithium ion batteries. Chem. Eng. J. 2023, 470, 144045. [Google Scholar] [CrossRef]
- Roh, Y.; Jin, D.; Kim, E.; Byun, S.; Lee, Y.-S.; Ryou, M.-H.; Lee, Y.M. Highly improved thermal stability of the ceramic coating layer on the polyethylene separator via chemical crosslinking between ceramic particles and polymeric binders. Chem. Eng. J. 2022, 433, 134501. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, Z.; Zhong, W.; Ge, Z.; Li, L.; Lei, S.; Wu, Q.; Zhang, H.; Cheng, S.; Xie, J. Non-flammable fluorobenzene-diluted highly concentrated electrolytes enable high-performance Li-metal and Li-ion batteries. J. Colloid Interface Sci. 2022, 619, 399–406. [Google Scholar] [CrossRef]
- Shao, D.; Yang, L.; Luo, K.; Chen, M.; Zeng, P.; Liu, H.; Liu, L.; Chang, B.; Luo, Z.; Wang, X. Preparation and performances of the modified gel composite electrolyte for application of quasi-solid-state lithium sulfur battery. Chem. Eng. J. 2020, 389, 124300. [Google Scholar] [CrossRef]
- Lv, J.; Ye, J.; Dai, G.; Niu, Z.; Sun, Y.; Zhang, X.; Zhao, Y. Flame-retarding battery cathode materials based on reversible multi-electron redox chemistry of phenothiazine-based polymer. J. Energy Chem. 2020, 47, 256–262. [Google Scholar] [CrossRef]
- Huang, B.; Hua, H.; Peng, L.; Wang, X.; Shen, X.; Li, R.; Zhang, P.; Zhao, J. The functional separator for lithium-ion batteries based on phosphonate modified nano-scale silica ceramic particles. J. Power Sources 2021, 498, 229908. [Google Scholar] [CrossRef]
- Du, X.; Jin, L.; Deng, S.; Zhou, M.; Du, Z.; Cheng, X.; Wang, H. Recyclable, Self-Healing, and Flame-Retardant Solid–Solid Phase Change Materials Based on Thermally Reversible Cross-Links for Sustainable Thermal Energy Storage. ACS Appl. Mater. Interfaces 2021, 13, 42991–43001. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, W.; Wu, T.; Wang, C.; Liang, Z. Thermal management system study of flame retardant solid–solid phase change material battery. Surf. Interfaces 2023, 36, 102558. [Google Scholar] [CrossRef]
- Weng, J.; Xiao, C.; Ouyang, D.; Yang, X.; Chen, M.; Zhang, G.; Yuen, R.K.K.; Wang, J. Mitigation effects on thermal runaway propagation of structure-enhanced phase change material modules with flame retardant additives. Energy 2022, 239, 122087. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, M.; Zhao, L.; Chen, Y. Study on thermal runaway propagation inhibition of battery module by flame-retardant phase change material combined with aerogel felt. Appl. Energy 2024, 367, 123394. [Google Scholar] [CrossRef]
- Mei, J.; Shi, G.; Liu, H.; Wang, Z.; Chen, M. Experimental study on the effect of passive retardation method for thermal runaway mitigation of lithium-ion battery. Appl. Therm. Eng. 2023, 230, 120861. [Google Scholar] [CrossRef]

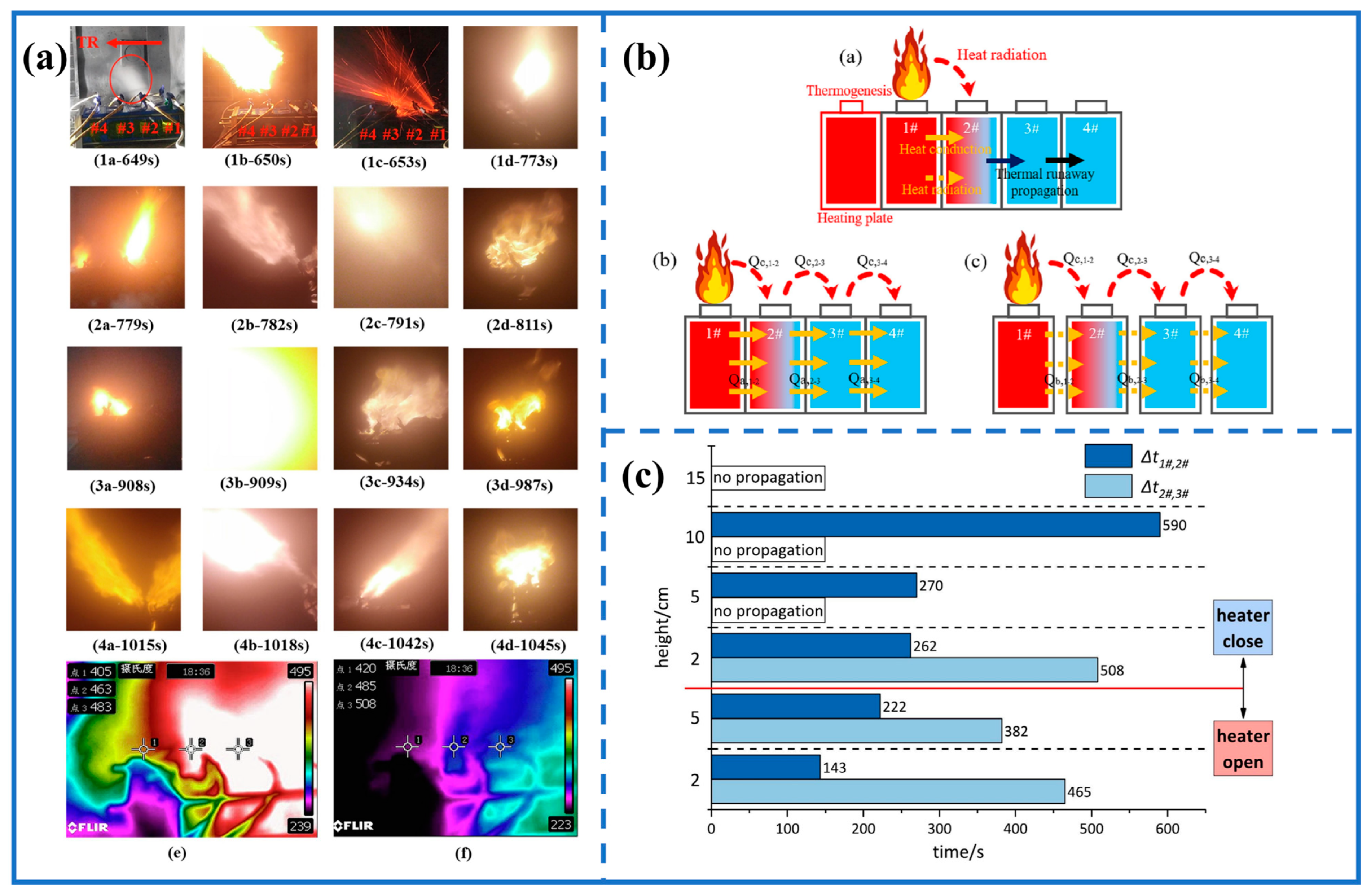
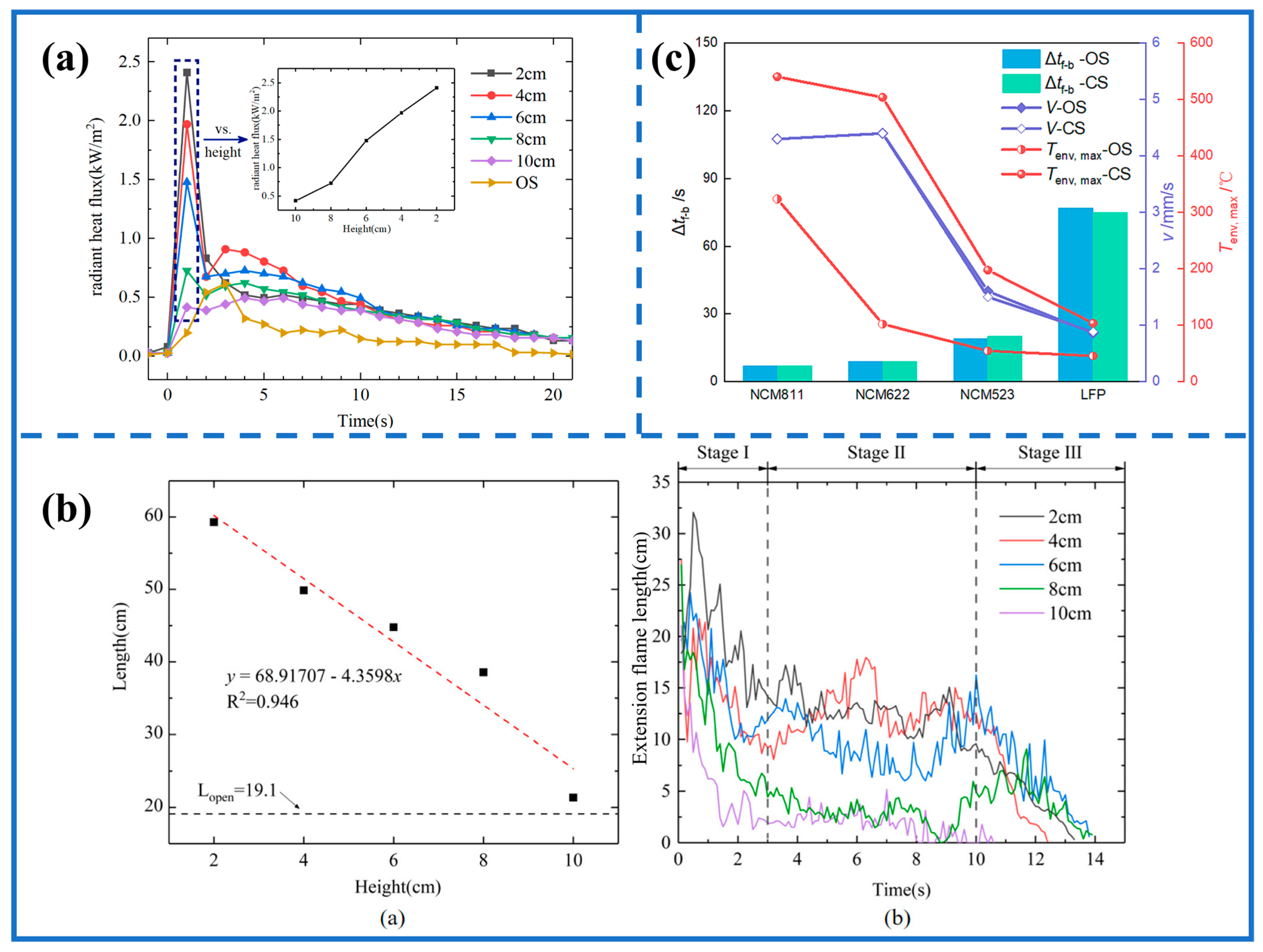
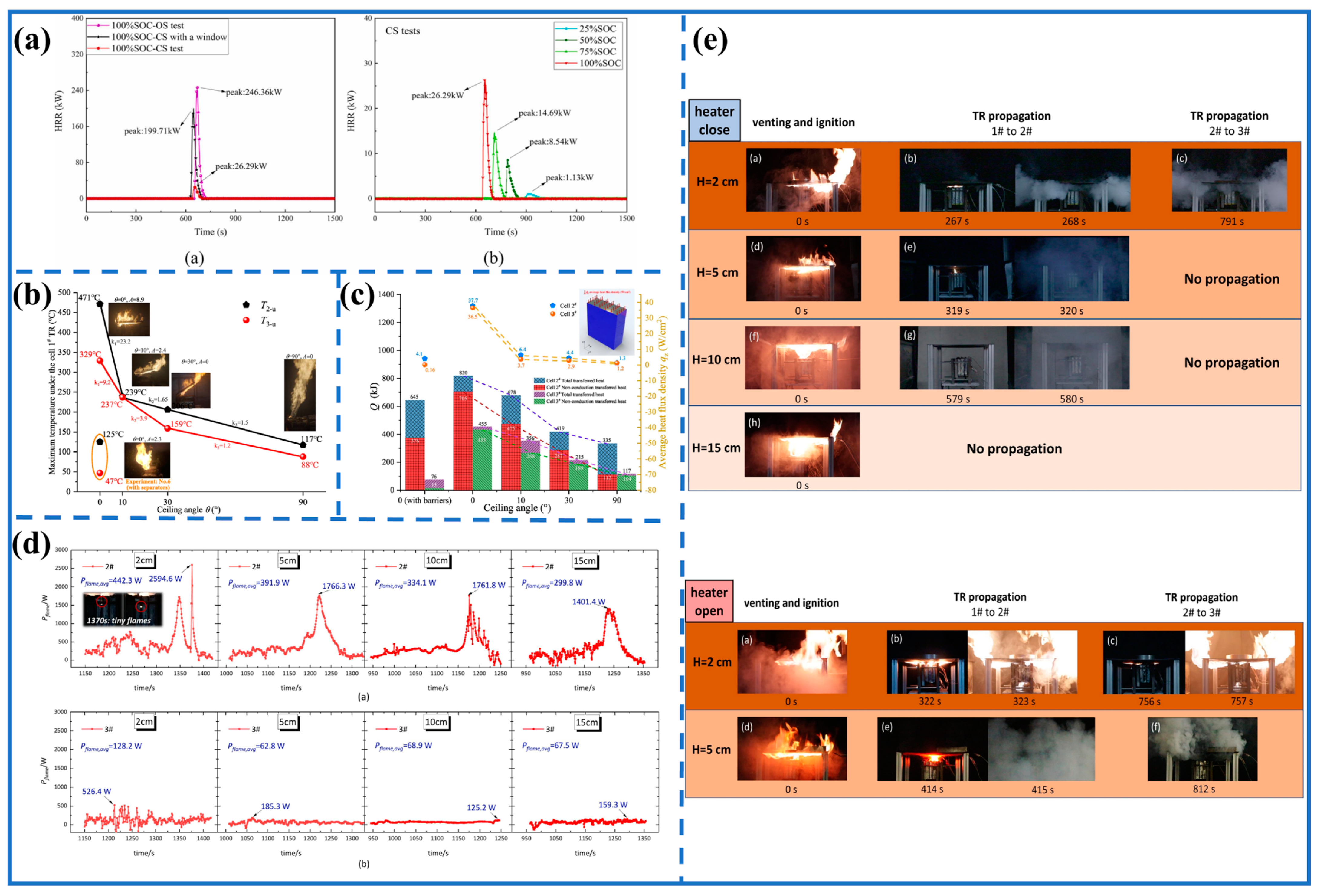

| Type | Materials | Trigger Mode | Flame Pattern | Refs. |
|---|---|---|---|---|
| Cylindrical LIBs | LFP/graphite | Overheat | Venting, sparks, combustion | [22,41] |
| LFP/graphite | Penetration | Venting | [43] | |
| LCO/graphite | Overheat | Venting, sparks, combustion | [22,60] | |
| NCM/graphite | Penetration | Venting, sparks, combustion | [13,14] | |
| Prismatic LIBs | LFP/graphite | Overheat | Venting, jet fire | [33,42] |
| LFP/carbon | Overheat | Venting, jet fire | [15] | |
| LFP/graphite | Overcharge | Venting, jet fire | [17] | |
| NCM/graphite | Overheat | Venting, jet fire | [35,37] | |
| Pouch | NCM/graphite | Overheat | Venting, jet fire | [34,69] |
| NCM/graphite | Penetration | Venting, combustion | [70] | |
| LFP/graphite | Penetration | No combustion | [70] | |
| LCO/graphite | Penetration | Venting, combustion | [70] |
| Heating Mode | Height (cm) | Qtransfer | Qtr | Qflame |
|---|---|---|---|---|
| Non-continuous heating | 2 | 2.7% | 22.1% | 75.2% |
| 5 | 5.2% | 20.2% | 74.7% | |
| 10 | 29.4% | 17.1% | 53.5% | |
| 15 | / | / | / | |
| Continuous heating | 2 | 3.7% | 20.6% | 75.7% |
| 5 | 16.5% | 17.4% | 66.1% |
| Year | Aim | Methods | Simulations | Highlights | Ref. |
|---|---|---|---|---|---|
| 1999 | Calculate self-heating rate profiles | ARC | Mathematical model | A mathematical model was used to study the thermal stability | Dahn [90] |
| 2001 | Predict the response of a new battery | ARC Fortran | One-dimensional predictive model | A one-dimensional predictive model was established | Hatchard [91] |
| 2007 | Illustrate abuse behaviors | Fluent | Thermal–fluid dynamics | A three-dimensional thermal abuse model was established | Kim [92] |
| 2016 | Analyze thermal runaway during venting | Comsol | Mathematical and multi-physics model | Flow equations were used to better understand thermal runaway | Coman [93] |
| 2016 | Find solutions for preventing TPR | Comsol | Mathematical and multi-physics model | A 3D TPR model for a battery pack was built | Feng [94] |
| 2017 | Predict the temperature–pressure behavior in battery packs | Comsol | Mathematical and multi-physics model | The pressure inside the battery was predicted | Coman [95] |
| 2020 | Investigate cell pressurization and abuse reaction of LIBS | Comsol | AAM (advanced abuse model) | Capable of predicting pressure accumulation | Bugryniec [96] |
| 2022 | Predict the thermal abuse reactions and jet dynamics | CFD | A coupled numerical model | Combined many sub-models that are necessary for prediction | Kong [98] |
| 2022 | Predict the hazards induced by heat and gas release of battery fire | CFD | Thermal–fluid dynamics | It can determine the release of heat and gases from the battery | Voigt [99] |
| 2022 | Simulate the particle ejection process | CFD | Multi-scale and multiphase modeling framework | The complete evolution of the ejected particles was simulated | Wang [97] |
| 2023 | Analyze the effect of particles’ radiation | CFD | A coupled numerical model | Combined the effect of particle radiation into TR innovatively | Zhang [49] |
| 2023 | Analyze the influence of particles on the jet flame | CFD | A coupled numerical model | The connection of the particles and characteristics of the jet flame was revealed | Wang [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, Y.; Chen, Y.; Chen, M. Review of Flame Behavior and Its Suppression during Thermal Runaway in Lithium-Ion Batteries. Batteries 2024, 10, 307. https://doi.org/10.3390/batteries10090307
Mao Y, Chen Y, Chen M. Review of Flame Behavior and Its Suppression during Thermal Runaway in Lithium-Ion Batteries. Batteries. 2024; 10(9):307. https://doi.org/10.3390/batteries10090307
Chicago/Turabian StyleMao, Yikai, Yin Chen, and Mingyi Chen. 2024. "Review of Flame Behavior and Its Suppression during Thermal Runaway in Lithium-Ion Batteries" Batteries 10, no. 9: 307. https://doi.org/10.3390/batteries10090307
APA StyleMao, Y., Chen, Y., & Chen, M. (2024). Review of Flame Behavior and Its Suppression during Thermal Runaway in Lithium-Ion Batteries. Batteries, 10(9), 307. https://doi.org/10.3390/batteries10090307







