Recent Progress of Urea-Based Deep Eutectic Solvents as Electrolytes in Battery Technology: A Critical Review
Abstract
1. Introduction
1.1. Ionic Liquids
1.2. Deep Eutectic Solvents
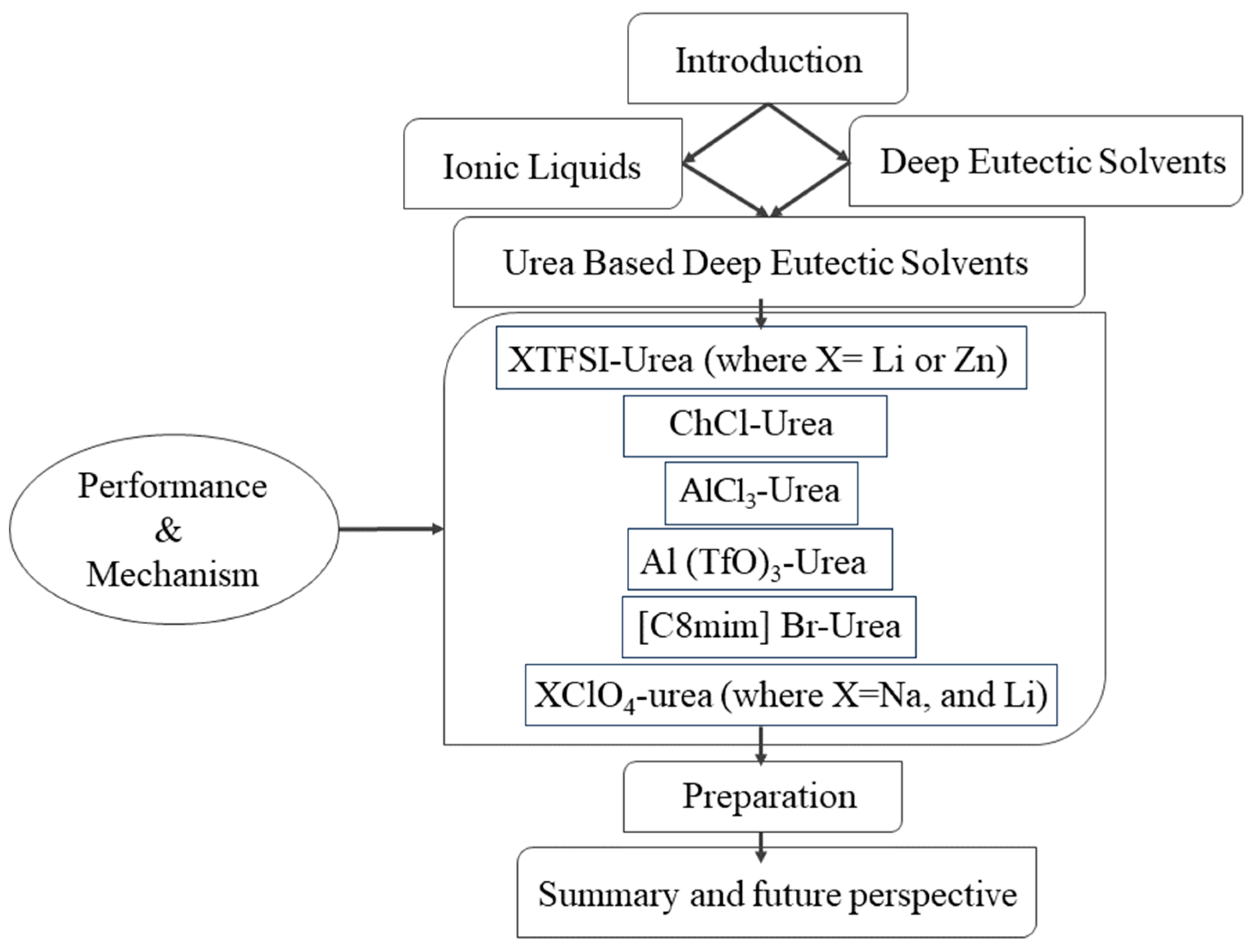
2. Urea-Based Deep Eutectic Solvents (DESs)
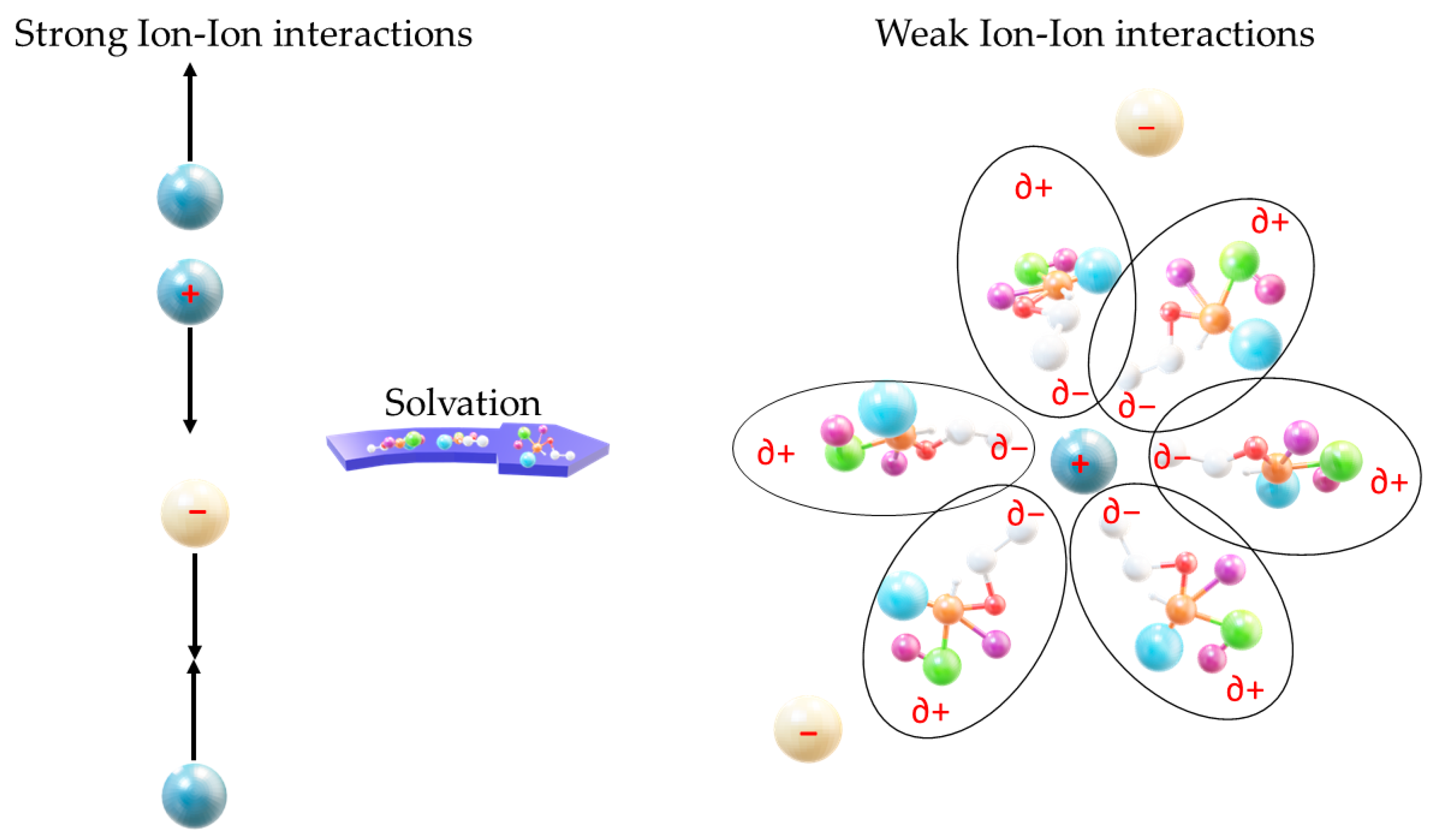
2.1. XTFSI-Urea (Where X = Li or Zn)
2.1.1. Performance
2.1.2. Mechanism
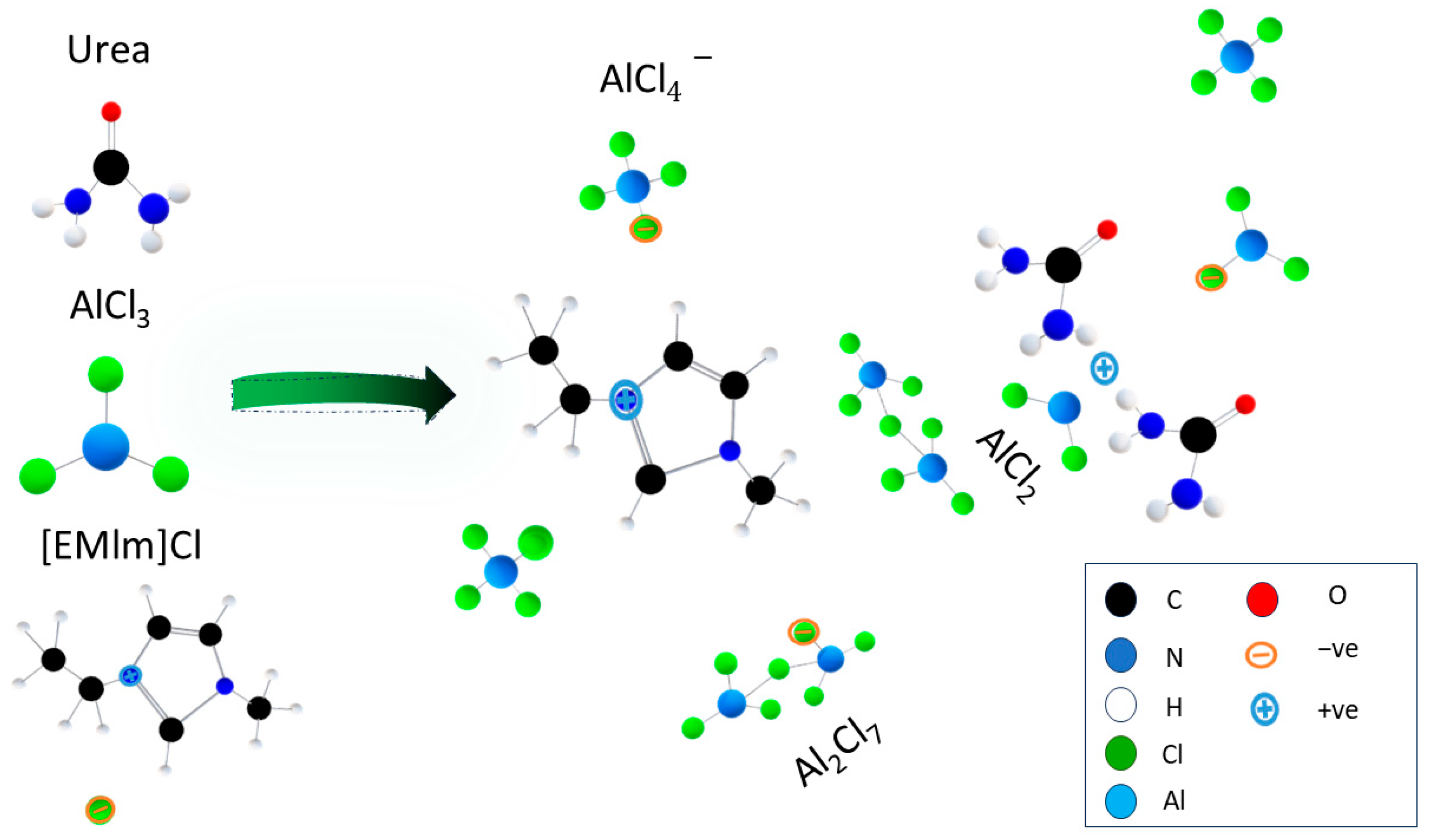
2.2. AlCl3-Urea
2.2.1. Performance
2.2.2. Mechanism of AlCl3-Urea Interactions
2.3. ChCl-Urea
2.3.1. Performance
2.3.2. Mechanism
2.4. [C8mim] Br-Urea
2.5. Al (TfO)3-Urea
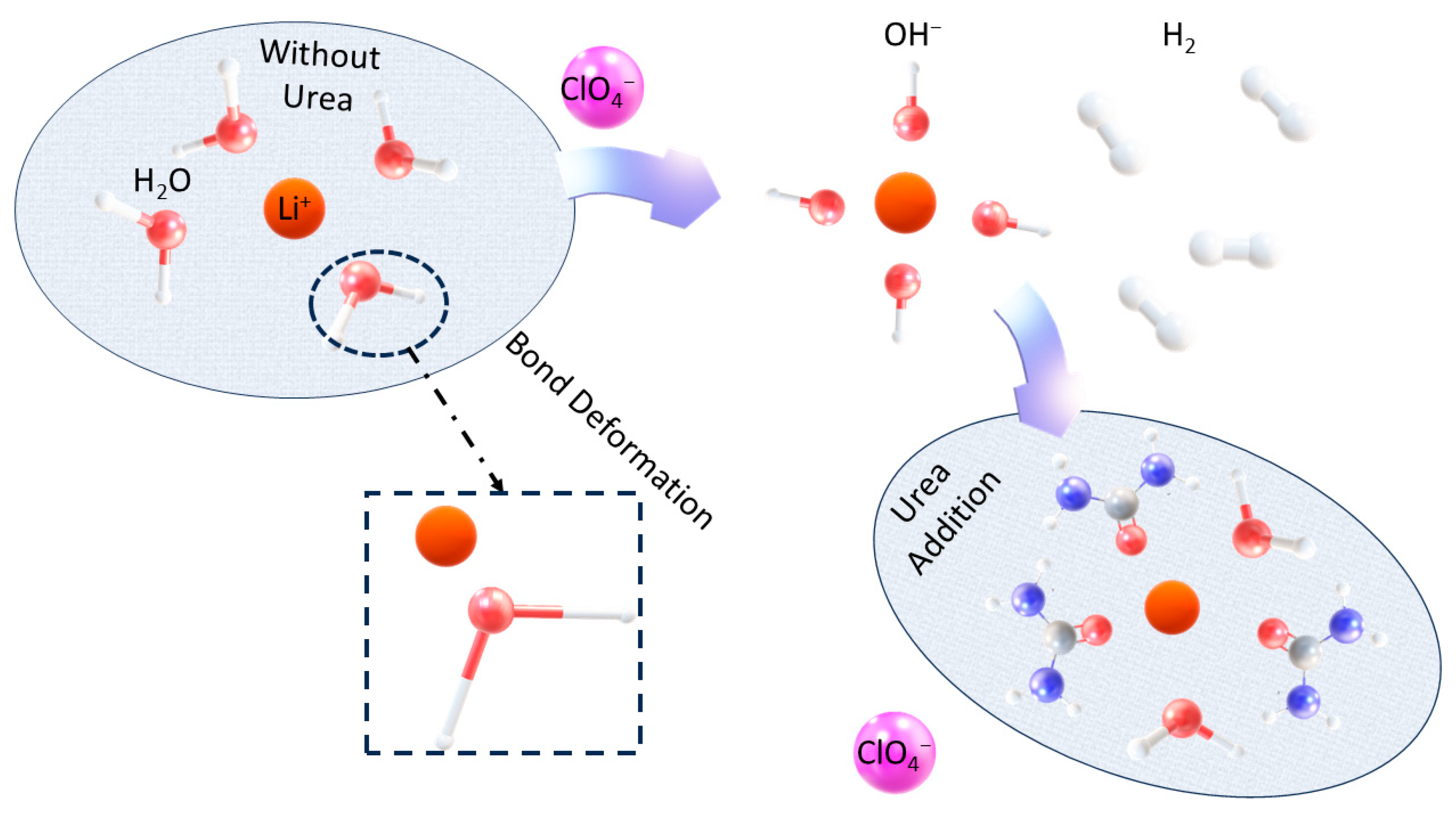
2.6. XClO4-Urea (Where X = Na, and Li)
2.6.1. Performance
2.6.2. Mechanism
3. Preparation
4. Summary and Future Perspective
- Urea is a well-known, widely used, and inexpensive compound that has several advantages including non-toxicity, low flammability, and high thermal stability, making it safer and more environmentally friendly than conventional electrolytes;
- Urea-based DESs have a relatively high electrochemical stability window (ESW) and ionic conductivity, which are crucial for the performance and efficiency of batteries. In addition, Urea-based DESs can support relatively high discharging voltage, high Coulombic efficiency, and high specific capacity of various battery types, such as lithium-ion, zinc-ion, or aluminum-ion batteries.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liang, Y.; Zhao, C.Z.; Yuan, H.; Chen, Y.; Zhang, W.; Huang, J.Q.; Yu, D.; Liu, Y.; Titirici, M.M.; Chueh, Y.L.; et al. A review of rechargeable batteries for portable electronic devices. InfoMat 2019, 1, 6–32. [Google Scholar] [CrossRef]
- Jan, W.; Khan, A.D.; Iftikhar, F.J.; Ali, G. Recent advancements and challenges in deploying lithium sulfur batteries as economical energy storage devices. J. Energy Storage 2023, 72, 108559. [Google Scholar] [CrossRef]
- Glushenkov, A.M. Recent commentaries on the expected performance, advantages and applications of sodium-ion batteries. Energy Mater. 2023, 3, 300010. [Google Scholar] [CrossRef]
- Chayambuka, K.; Mulder, G.; Danilov, D.L.; Notten, P.H.L. From Li-Ion Batteries toward Na-Ion Chemistries: Challenges and Opportunities. Adv. Energy Mater. 2020, 10, 2001310. [Google Scholar] [CrossRef]
- Tang, X.; Jia, Q.; Yang, L.; Bai, M.; Wu, W.; Wang, Z.; Gong, M.; Sa, S.; Tao, S.; Sun, M.; et al. Towards the high-energy-density battery with broader temperature adaptability: Self-discharge mitigation of quaternary nickel-rich cathode. Energy Storage Mater. 2020, 33, 239–249. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, M.; Xiong, Y.; Hou, Z.; Ao, H.; Liu, M.; Zhu, Y.; Qian, Y. Aqueous Rechargeable Li+/Na+ Hybrid Ion Battery with High Energy Density and Long Cycle Life. Small 2020, 16, e2003585. [Google Scholar] [CrossRef]
- Zha, C.; Wu, D.; Gu, X.; Chen, H. Triple-phase interfaces of graphene-like carbon clusters on antimony trisulfide nanowires enable high-loading and long-lasting liquid Li2S6-based lithium-sulfur batteries. J. Energy Chem. 2021, 59, 599–607. [Google Scholar] [CrossRef]
- Shi, C.; Yu, M. Flexible solid-state lithium-sulfur batteries based on structural designs. Energy Storage Mater. 2023, 57, 429–459. [Google Scholar] [CrossRef]
- Perveen, T.; Siddiq, M.; Shahzad, N.; Ihsan, R.; Ahmad, A.; Shahzad, M.I. Prospects in anode materials for sodium ion batteries—A review. Renew. Sustain. Energy Rev. 2020, 119, 109549. [Google Scholar] [CrossRef]
- Li, B.; Li, Q.; Wang, Q.; Yan, X.; Shi, M.; Wu, C. Deep eutectic solvent for spent lithium-ion battery recycling: Comparison with inorganic acid leaching. Phys. Chem. Chem. Phys. 2022, 24, 19029–19051. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Senanayake, G.; Sohn, J.; Shin, S.M. Recovery of cobalt sulfate from spent lithium ion batteries by reductive leaching and solvent extraction with Cyanex 272. Hydrometallurgy 2010, 100, 168–171. [Google Scholar] [CrossRef]
- Pindar, S.; Dhawan, N. Microwave processing of spent coin cells for recycling of metallic values. J. Clean. Prod. 2021, 280, 124144. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, L.; Li, W.; Zhao, K.; Liu, M.; Wu, Q.; Yang, Y.; He, G.; Parkin, I.P.; Shearing, P.R.; et al. Sodium Superionic Conductors (NASICONs) as Cathode Materials for Sodium-Ion Batteries; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Jia, X.; Liu, C.; Neale, Z.G.; Yang, J.; Cao, G. Active Materials for Aqueous Zinc Ion Batteries: Synthesis, Crystal Structure, Morphology, and Electrochemistry. Chem. Rev. 2020, 120, 7795–7866. [Google Scholar] [CrossRef]
- He, X.; Kong, X.-Y.; Wen, L. A promising solution for highly reversible zinc metal anode chemistry: Functional gradient interphase. Nano Res. Energy 2023. [Google Scholar] [CrossRef]
- Liang, X.; Wen, Z.; Liu, Y.; Wu, M.; Jin, J.; Zhang, H.; Wu, X. Improved cycling performances of lithium sulfur batteries with LiNO 3-modified electrolyte. J. Power Sources 2011, 196, 9839–9843. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, B.; Liu, G.; Qian, D.; Yang, C.; Li, J. A systematically comparative study on LiNO3 and Li2SO4 aqueous electrolytes for electrochemical double-layer capacitors. Electrochim. Acta 2018, 274, 121–130. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Zakar, S.; Haider, S.S. Role of aqueous electrolytes on the performance of electrochemical energy storage device. J. Electroanal. Chem. 2020, 858, 113793. [Google Scholar] [CrossRef]
- Niu, H.; Wang, L.; Guan, P.; Zhang, N.; Yan, C.; Ding, M.; Guo, X.; Huang, T.; Hu, X. Recent Advances in Application of Ionic Liquids in Electrolyte of Lithium Ion Batteries. J. Energy Storage 2021, 40, 102659. [Google Scholar] [CrossRef]
- Yang, G.; Song, Y.; Wang, Q.; Zhang, L.; Deng, L. Review of ionic liquids containing, polymer/inorganic hybrid electrolytes for lithium metal batteries. Mater. Des. 2020, 190, 108563. [Google Scholar] [CrossRef]
- Shi, J.; Sun, T.; Bao, J.; Zheng, S.; Du, H.; Li, L.; Yuan, X.; Ma, T.; Tao, Z. “Water-in-Deep Eutectic Solvent” Electrolytes for High-Performance Aqueous Zn-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2102035. [Google Scholar] [CrossRef]
- Jaumaux, P.; Liu, Q.; Zhou, D.; Xu, X.; Wang, T.; Wang, Y.; Kang, F.; Li, B.; Wang, G. Deep-Eutectic-Solvent-Based Self-Healing Polymer Electrolyte for Safe and Long-Life Lithium-Metal Batteries. Angew. Chem. Int. Ed. 2020, 59, 9134–9142. [Google Scholar] [CrossRef]
- Dhananjay Jagtap, A.; Bharatrao Kondekar, N.; Sadani, A.A.; Chern, J.-W. Ureas: Applications in drug design. Curr. Med. Chem. 2017, 24, 622–651. [Google Scholar] [CrossRef]
- Wagle, D.V.; Zhao, H.; Baker, G.A. Deep eutectic solvents: Sustainable media for nanoscale and functional materials. Acc. Chem. Res. 2014, 47, 2299–2308. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liang, Q.; Yu, X.; Lü, Q.; Ma, L.; Qin, X.; Chen, G.; Li, B. Deep eutectic solvents for boosting electrochemical energy storage and conversion: A review and perspective. Adv. Funct. Mater. 2021, 31, 2011102. [Google Scholar] [CrossRef]
- Ru, J.; Hua, Y.; Li, J.; Xu, C.; Li, Y.; Wang, D.; Qi, C.; Jie, Y. Effects of existence form and concentration of PbO on the conductivity of choline chloride–urea deep eutectic solvent. J. Mol. Liq. 2014, 199, 208–214. [Google Scholar] [CrossRef]
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Mezzetta, A.; Perillo, V.; Guazzelli, L.; Chiappe, C. Thermal behavior analysis as a valuable tool for comparing ionic liquids of different classes. J. Therm. Anal. Calorim. 2019, 138, 3335–3345. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, H.; Singla, M. Diverse applications of ionic liquids: A comprehensive review. J. Mol. Liq. 2022, 351, 118556. [Google Scholar] [CrossRef]
- Wilkes, J.S. A short history of ionic liquids—From molten salts to neoteric solvents. Green Chem. 2002, 4, 73–80. [Google Scholar] [CrossRef]
- Dupont, J. From molten salts to ionic liquids: A “nano” journey. Acc. Chem. Res. 2011, 44, 1223–1231. [Google Scholar] [CrossRef]
- Nasirpour, N.; Mohammadpourfard, M.; Zeinali Heris, S. Ionic liquids: Promising compounds for sustainable chemical processes and applications. Chem. Eng. Res. Des. 2020, 160, 264–300. [Google Scholar] [CrossRef]
- Macfarlane, D.R.; Tachikawa, N.; Forsyth, M.; Pringle, J.M.; Howlett, P.C.; Elliott, G.D.; Davis, J.H.; Watanabe, M.; Simon, P.; Angell, C.A. Energy applications of ionic liquids. Energy Environ. Sci. 2014, 7, 232–250. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Forsyth, M.; Howlett, P.C.; Kar, M.; Passerini, S.; Pringle, J.M.; Ohno, H.; Watanabe, M.; Yan, F.; Zheng, W.; et al. Ionic liquids and their solid-state analogues as materials for energy generation and storage. Nat. Rev. Mater. 2016, 1, 15005. [Google Scholar] [CrossRef]
- Yu, Q.; Wu, Y.; Li, D.M.; Cai, M.; Zhou, F.; Liu, W. Supramolecular ionogel lubricants with imidazolium-based ionic liquids bearing the urea group as gelator. J. Colloid. Interface Sci. 2017, 487, 130–140. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, A.S.; Lee, J.C.; Hong, S.M.; Hwang, S.S.; Koo, C.M. Hybrid ionogel electrolytes for high temperature lithium batteries. J. Mater. Chem. A Mater. 2015, 3, 2226–2233. [Google Scholar] [CrossRef]
- Peters, W.; Duong, H.T.; Lee, S.; Drillet, J.F. Investigation of Al(TfO)3-based deep eutectic solvent electrolytes for aluminium-ion batteries. Part I: Understanding the positively charged Al complex formation. Phys. Chem. Chem. Phys. 2021, 23, 21923–21933. [Google Scholar] [CrossRef] [PubMed]
- Dilasari, B.; Jung, Y.; Sohn, J.; Kim, S.; Kwon, K. Review on corrosion behavior of metallic materials in room temperature ionic liquids. Int. J. Electrochem. Sci. 2016, 11, 1482–1495. [Google Scholar] [CrossRef]
- Forsyth, M.; Howlett, P.C.; Somers, A.E.; MacFarlane, D.R.; Basile, A. Interphase engineering of reactive metal surfaces using ionic liquids and deep eutectic solvents—From corrosion control to next-generation batteries. NPJ Mater. Degrad. 2017, 1, 18. [Google Scholar] [CrossRef]
- Abbott, A.P.; Ahmed, E.I.; Harris, R.C.; Ryder, K.S. Evaluating water miscible deep eutectic solvents (DESs) and ionic liquids as potential lubricants. Green Chem. 2014, 16, 4156–4161. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; de la Guardia, M.; Andruch, V.; Vilková, M. Deep eutectic solvents vs ionic liquids: Similarities and differences. Microchem. J. 2020, 159, 105539. [Google Scholar] [CrossRef]
- Santana-Mayor, Á.; Rodríguez-Ramos, R.; Herrera-Herrera, A.V.; Socas-Rodríguez, B.; Rodríguez-Delgado, M.Á. Deep eutectic solvents. The new generation of green solvents in analytical chemistry. TrAC-Trends Anal. Chem. 2021, 134, 116108. [Google Scholar] [CrossRef]
- El Achkar, T.; Greige-Gerges, H.; Fourmentin, S. Basics and properties of deep eutectic solvents: A review. Environ. Chem. Lett. 2021, 19, 3397–3408. [Google Scholar] [CrossRef]
- Martins, M.A.R.; Pinho, S.P.; Coutinho, J.A.P. Insights into the Nature of Eutectic and Deep Eutectic Mixtures. J. Solut. Chem. 2019, 48, 962–982. [Google Scholar] [CrossRef]
- Cheng, H.; Qi, Z. Applications of deep eutectic solvents for hard-to-separate liquid systems. Sep. Purif. Technol. 2021, 274, 119027. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, R.; Thakur, R.C.; Singh, L. An overview of deep eutectic solvents: Alternative for organic electrolytes, aqueous systems & ionic liquids for electrochemical energy storage. J. Energy Chem. 2023, 82, 592–626. [Google Scholar] [CrossRef]
- De Oliveira Vigier, K.; Jérôme, F. Synthesis and Properties. In Deep Eutectic Solvents; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019; pp. 1–23. [Google Scholar] [CrossRef]
- Miguel, Á.; García, N.; Gregorio, V.; López-Cudero, A.; Tiemblo, P. Tough polymer gel electrolytes for aluminum secondary batteries based on urea: AlCl3, prepared by a new solvent-free and scalable procedure. Polymers 2020, 12, 1336. [Google Scholar] [CrossRef] [PubMed]
- Cvetković, V.S.; Vukićević, N.M.; Jovićević, N.; Stevanović, J.S.; Jovićević, J.N. Aluminium electrodeposition under novel conditions from AlCl3–urea deep eutectic solvent at room temperature. Trans. Nonferrous Met. Soc. China 2020, 30, 823–834. [Google Scholar] [CrossRef]
- Jiao, H.; Wang, C.; Tu, J.; Tian, D.; Jiao, S. A rechargeable Al-ion battery: Al/molten AlCl3-urea/graphite. Chem. Commun. 2017, 53, 2331–2334. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, J.; Jiao, H.; Tu, J.; Jiao, S. The electrochemical behavior of an aluminum alloy anode for rechargeable Al-ion batteries using an AlCl3–urea liquid electrolyte. RSC Adv. 2017, 7, 32288–32293. [Google Scholar] [CrossRef]
- Ao, H.; Chen, C.; Hou, Z.; Cai, W.; Liu, M.; Jin, Y.; Zhang, X.; Zhu, Y.; Qian, Y. Electrolyte solvation structure manipulation enables safe and stable aqueous sodium ion batteries. J. Mater. Chem. A Mater. 2020, 8, 14190–14197. [Google Scholar] [CrossRef]
- Hou, Z.; Dong, M.; Xiong, Y.; Zhang, X.; Zhu, Y.; Qian, Y. Formation of Solid–Electrolyte Interfaces in Aqueous Electrolytes by Altering Cation-Solvation Shell Structure. Adv. Energy Mater. 2020, 10, 1903665. [Google Scholar] [CrossRef]
- Suo, L.; Borodin, O.; Gao, T.; Olguin, M.; Ho, J.; Fan, X.; Luo, C.; Wang, C.; Xu, K. “Water-in-salt” electrolyte enables high-voltage aqueous lithium-ion chemistries. Science (1979) 2015, 350, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Azaceta, E.; Marcilla, R.; Mecerreyes, D.; Ungureanu, M.; Dev, A.; Voss, T.; Fantini, S.; Grande, H.J.; Cabañero, G.; Tena-Zaera, R. Electrochemical reduction of O2 in 1-butyl-1-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide ionic liquid containing Zn2+ cations: Deposition of non-polar oriented ZnO nanocrystalline films. Phys. Chem. Chem. Phys. 2011, 13, 13433–13440. [Google Scholar] [CrossRef]
- Kim, Y.N.; Jo, J.H.; Kim, J.; Kim, H.S.; Lee, W.I. Outstanding Thermal Stability of Perovskite Solar Cells Based on Zn(TFSI)2-Doped Spiro-MeOTAD. ACS Appl. Energy Mater. 2022, 6, 10225–10232. [Google Scholar] [CrossRef]
- Ou, J. Ionic Liquid Assisted Composite Ceramic-Polymer Electrolyte for Lithium Metal Batteries. Master’s Thesis, University of Waterloo, Waterloo, ON, Canada, 2020. [Google Scholar]
- Mendes-Felipe, C.; Barbosa, J.C.; Gonçalves, R.; Miranda, D.; Costa, C.M.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. Lithium bis(trifluoromethanesulfonyl)imide blended in polyurethane acrylate photocurable solid polymer electrolytes for lithium-ion batteries. J. Energy Chem. 2021, 62, 485–496. [Google Scholar] [CrossRef]
- Tran, K.T.T.; Le, L.T.M.; Phan, A.L.B.; Tran, P.H.; Vo, T.D.; Truong, T.T.T.; Nguyen, N.T.B.; Garg, A.; Le, P.M.L.; Tran, M.V. New deep eutectic solvents based on ethylene glycol—LiTFSI and their application as an electrolyte in electrochemical double layer capacitor (EDLC). J. Mol. Liq. 2020, 320, 114495. [Google Scholar] [CrossRef]
- Kufian, M.Z.; Ramesh, S.; Arof, A.K. PMMA-LiTFSI based gel polymer electrolyte for lithium-oxygen cell application. Opt. Mater. 2021, 120, 111418. [Google Scholar] [CrossRef]
- Shi, C.; Alexander, G.V.; O’Neill, J.; Duncan, K.; Godbey, G.; Wachsman, E.D. All-Solid-State Garnet Type Sulfurized Polyacrylonitrile/Lithium-Metal Battery Enabled by an Inorganic Lithium Conductive Salt and a Bilayer Electrolyte Architecture. ACS Energy Lett. 2023, 8, 1803–1810. [Google Scholar] [CrossRef]
- Lux, S.F.; Terborg, L.; Hachmöller, O.; Placke, T.; Meyer, H.-W.; Passerini, S.; Winter, M.; Nowak, S. LiTFSI Stability in Water and Its Possible Use in Aqueous Lithium-Ion Batteries: pH Dependency, Electrochemical Window and Temperature Stability. J. Electrochem. Soc. 2013, 160, A1694–A1700. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, B.; Liu, X.; Liu, J.; Ding, J.; Zhong, C.; Hu, W. Water-in-salt electrolyte for safe and high-energy aqueous battery. Energy Storage Mater. 2021, 34, 461–474. [Google Scholar] [CrossRef]
- Liang, H.; Li, H.; Wang, Z.; Wu, F.; Chen, L.; Huang, X. New binary room-temperature molten salt electrolyte based on urea and LiTFSI. J. Phys. Chem. B 2001, 105, 9966–9969. [Google Scholar] [CrossRef]
- Lesch, V.; Heuer, A.; Rad, B.R.; Winter, M.; Smiatek, J. Atomistic insights into deep eutectic electrolytes: The influence of urea on the electrolyte salt LiTFSI in view of electrochemical applications. Phys. Chem. Chem. Phys. 2016, 18, 28403–28408. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, B.; Cai, H.; Huang, R.; Liu, Y.; Pan, H. Adjusting the local solvation structures and hydrogen bonding networks for stable aqueous batteries with reduced cost. J. Energy Chem. 2022, 68, 411–419. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, J.; Yang, W.; Chen, B.; Zhao, Z.; Qiu, H.; Dong, S.; Zhou, X.; Cui, G.; Chen, L. “Water-in-deep eutectic solvent” electrolytes enable zinc metal anodes for rechargeable aqueous batteries. Nano Energy 2019, 57, 625–634. [Google Scholar] [CrossRef]
- Sim, L.N.; Yahya, R.; Arof, A.K. Infrared studies of polyacrylonitrile-based polymer electrolytes incorporated with lithium bis(trifluoromethane)sulfonimide and urea as deep eutectic solvent. Opt. Mater. 2016, 56, 140–144. [Google Scholar] [CrossRef]
- Nandy, A.; Smiatek, J. Mixtures of LiTFSI and urea: Ideal thermodynamic behavior as key to the formation of deep eutectic solvents? Phys. Chem. Chem. Phys. 2019, 21, 12279–12287. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Mun, J.; Chae, O.B.; Kwon, O.M.; Kim, H.T.; Ryu, J.H.; Kim, Y.G.; Oh, S.M. Corrosion/passivation of aluminum current collector in bis(fluorosulfonyl) imide-based ionic liquid for lithium-ion batteries. Electrochem. Commun. 2012, 22, 1–3. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Ji, Y.; Ma, J.; Yu, H. Emerging Nonaqueous Aluminum-Ion Batteries: Challenges, Status, and Perspectives. Adv. Mater. 2018, 30, 1706310. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.T.; Patil, S.B.; An, C.Y.; Huang, S.K.; Lin, J.C.; Lee, T.S.; Lee, Y.C.; Chou, H.L.; Chen, C.W.; Chang, Y.J.; et al. A Quinone-Based Electrode for High-Performance Rechargeable Aluminum-Ion Batteries with a Low-Cost AlCl3/Urea Ionic Liquid Electrolyte. ACS Appl. Mater. Interfaces 2020, 12, 25853–25860. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tu, J.; Jiao, H.; Wang, C.; Jiao, S. Ternary AlCl3-Urea-[EMIm]Cl Ionic Liquid Electrolyte for Rechargeable Aluminum-Ion Batteries. J. Electrochem. Soc. 2017, 164, A3093–A3100. [Google Scholar] [CrossRef]
- Ng, K.L.; Malik, M.; Buch, E.; Glossmann, T.; Hintennach, A.; Azimi, G. A low-cost rechargeable aluminum/natural graphite battery utilizing urea-based ionic liquid analog. Electrochim. Acta 2019, 327, 135031. [Google Scholar] [CrossRef]
- Li, M.; Li, Y. Aluminum electrodeposition using AlCl3/urea ionic liquid. Int. J. Electrochem. Sci. 2020, 15, 8498–8505. [Google Scholar] [CrossRef]
- Häkkinen, R.; Willberg-Keyriläinen, P.; Ropponen, J.; Virtanen, T. Effect of composition and water content on physicochemical properties of choline chloride-boric acid low-melting mixtures. J. Mol. Liq. 2019, 280, 104–110. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Radošević, K.; Cvjetko Bubalo, M.; Gaurina Srček, V.; Grgas, D.; Landeka Dragičević, T.; Redovniković, R.I. Evaluation of toxicity and biodegradability of choline chloride based deep eutectic solvents. Ecotoxicol. Environ. Saf. 2015, 112, 46–53. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, Z.; Qiu, Z.; Tang, D.; Shu, J. Insight into the Molecular Structure, Interaction, and Dynamics of Aqueous Reline Deep Eutectic Solvent: A Nuclear Magnetic Resonance Investigation. J. Phys. Chem. B 2023, 127, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Agieienko, V.; Buchner, R. Densities, Viscosities, and Electrical Conductivities of Pure Anhydrous Reline and Its Mixtures with Water in the Temperature Range (293.15 to 338.15) K. J. Chem. Eng. Data 2019, 64, 4763–4774. [Google Scholar] [CrossRef]
- Umecky, T.; Goto, A.; Hayashi, N.; Eguchi, K. Fine-tuning the Basicity of Deep Eutectic Solvents by Substituting Guanidine for Urea in Reline. ACS Omega 2023, 8, 14694–14698. [Google Scholar] [CrossRef]
- Kao-ian, W.; Pornprasertsuk, R.; Thamyongkit, P.; Maiyalagan, T.; Kheawhom, S. Rechargeable Zinc-Ion Battery Based on Choline Chloride-Urea Deep Eutectic Solvent. J. Electrochem. Soc. 2019, 166, A1063–A1069. [Google Scholar] [CrossRef]
- Tang, J.; Xu, C.; Zhu, X.; Liu, H.; Wang, X.; Huang, M.; Hua, Y.; Zhang, Q.; Li, Y. Anodic Dissolution of Copper in Choline Chloride-Urea Deep Eutectic Solvent. J. Electrochem. Soc. 2018, 165, E406–E411. [Google Scholar] [CrossRef]
- Yue, D.; Jia, Y.; Yao, Y.; Sun, J.; Jing, Y. Structure and electrochemical behavior of ionic liquid analogue based on choline chloride and urea. Electrochim. Acta 2012, 65, 30–36. [Google Scholar] [CrossRef]
- Cao, X.; Xu, L.; Shi, Y.; Wang, Y.; Xue, X. Electrochemical behavior and electrodeposition of cobalt from choline chloride-urea deep eutectic solvent. Electrochim. Acta 2019, 295, 550–557. [Google Scholar] [CrossRef]
- Wang, X.; Xu, C.; Liu, H.; Huang, M.; Ren, X.; Wang, S.; Hua, Y.; Zhang, Q.B.; Ru, J. Influence of chloride ion on zinc electrodeposition from choline chloride based deep eutectic solvent. Ionics 2020, 26, 1483–1490. [Google Scholar] [CrossRef]
- Zhu, F.; Deng, R.X.; Jiang, Q.H. Effects of Water on Electrochemical Behavior of ZnCl2 and FeCl3 in Deep Eutectic Solvent Composed of Choline Chloride and Urea. Russ. J. Electrochem. 2022, 58, 617–625. [Google Scholar] [CrossRef]
- Ogawa, H.; Mori, H. Lithium salt/amide-based deep eutectic electrolytes for lithium-ion batteries: Electrochemical, thermal and computational study. Phys. Chem. Chem. Phys. 2020, 22, 8853–8863. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Zhao, B.; Chen, X.B.; Birbilis, N.; Yang, H. Effect of water presence on choline chloride-2urea ionic liquid and coating platings from the hydrated ionic liquid. Sci. Rep. 2016, 6, 29225. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, M.; Huang, Y.; Yang, H.; Hang, T. An electrolyte for electrodeposition of hard gold based on choline chloride–urea ionic liquid. Electrochem. Commun. 2023, 148, 107454. [Google Scholar] [CrossRef]
- Di Pietro, M.E.; Goloviznina, K.; van den Bruinhorst, A.; de Araujo Lima e Souza, G.; Costa Gomes, M.; Padua, A.A.H.; Mele, A. Lithium Salt Effects on the Liquid Structure of Choline Chloride-Urea Deep Eutectic Solvent. ACS Sustain. Chem. Eng. 2022, 10, 11835–11845. [Google Scholar] [CrossRef]
- Nanda, R.; Rai, G.; Kumar, A. Interesting viscosity changes in the aqueous urea-ionic liquid system: Effect of alkyl chain length attached to the cationic ring of an ionic liquid. J. Solut. Chem. 2015, 44, 742–753. [Google Scholar] [CrossRef]
- Mandai, T.; Johansson, P. Al conductive haloaluminate-free non-aqueous room-temperature electrolytes. J. Mater. Chem. A Mater. 2015, 3, 12230–12239. [Google Scholar] [CrossRef]
- Shcherbakov, V.V.; Artemkina, Y.M.; Akimova, I.A.; Artemkina, I.M. Dielectric characteristics, electrical conductivity and solvation of ions in electrolyte solutions. Materials 2021, 14, 5617. [Google Scholar] [CrossRef]
- Yao, N.; Chen, X.; Shen, X.; Zhang, R.; Fu, Z.; Ma, X.; Zhang, X.; Li, B.; Zhang, Q. An Atomic Insight into the Chemical Origin and Variation of the Dielectric Constant in Liquid Electrolytes. Angew. Chem. 2021, 133, 21643–21648. [Google Scholar] [CrossRef]
- Li, M.; Li, Y. Direct electrochemical reduction of solid bismuth oxide in urea–1-methylimidazolium trifluoromethylsulfonate low-temperature molten salt. Electrochem. Commun. 2020, 118, 106794. [Google Scholar] [CrossRef]
- Tsai, H.Y.; Kumar, M.S.; Vedhanarayanan, B.; Shen, H.H.; Lin, T.W. Urea-Based Deep Eutectic Solvent with Magnesium/Lithium Dual Ions as an Aqueous Electrolyte for High-Performance Battery-Supercapacitor Hybrid Devices. Batteries 2023, 9, 69. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Xu, Z.; Dong, Q.; Ao, H.; Hou, Z.; Qian, Y. Aqueous electrolyte with moderate concentration enables high-energy aqueous rechargeable lithium ion battery for large scale energy storage. Energy Storage Mater. 2022, 46, 147–154. [Google Scholar] [CrossRef]
- Chen, X.; Xu, C.; Xiang, Q.; Wang, S.; Li, J.; Hua, Y. The effect of the zinc salt on the electrochemical behaviors of Zn in ChCl-urea deep eutectic solvent. Ionics 2023, 29, 1255–1265. [Google Scholar] [CrossRef]
- Wang, H.; Li, M.; Garg, S.; Wu, Y.; Nazmi Idros, M.; Hocking, R.; Duan, H.; Gao, S.; Yago, A.J.; Zhuang, L.; et al. Cobalt Electrochemical Recovery from Lithium Cobalt Oxides in Deep Eutectic Choline Chloride+Urea Solvents. ChemSusChem 2021, 14, 2972–2983. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.; Wong, C.; Walvekar, R.; Khalid, M. Choline chloride: Urea-based deep eutectic solvent as additive to proton conducting chitosan films. J. Eng. Sci. Technol. 2018, 13, 2995–3006. [Google Scholar]
- Bučko, M.; Roy, S.; Valverde-Armas, P.; Onjia, A.; Bastos, A.C.; Bajat, J.B. Voltammetric Response of Water in Deep Eutectic Solvent Based on Choline Chloride and Urea. J. Electrochem. Soc. 2018, 165, H1059. [Google Scholar] [CrossRef]
- Zhu, J.; Shao, H.; Feng, L.; Lu, Y.; Meng, H.; Li, C. Absorptive separation of HCl gas by choline chloride-based deep eutectic solvents. J. Mol. Liq. 2021, 341, 116928. [Google Scholar] [CrossRef]










| Anode//Cathode | Electrolyte | Specific Capacity (m·A·h·g−1) | Cycle Number | Discharge Voltage (V) | Charge Voltage (V) | Current Rate (* mA·g−1)/ (** C) | Coulombic Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Al//natural graphite | /Urea | 50 | 100 | 1.0 | 2.1 | 600 * | 99 | [74] |
| 48 | 500 | 1.0 | 2.1 | 600 * | 98 | |||
| 47 | 1000 | 1.0 | 2.1 | 600 * | 95 | |||
| 91 | 10 | 0.5 | 2.2 | 100 * | 99 | |||
| 89 | 50 | 0.5 | 2.2 | 100 * | 99 | |||
| 87 | 1000 | 0.5 | 2.2 | 100 * | - | |||
| NVP | Na-H2O-urea-DMF | 110 | 1 | 0 | 0.9 | 0.2 ** | - | [52] |
| 138 | 1 | −1.1 | −0.2 | 0.2 ** | - | |||
| 98 | 2 | 0 | 0.9 | 0.2 ** | - | |||
| 125 | 2 | −1.1 | −0.2 | 0.2 ** | - | |||
| NVP//NTP | 80 | 100 | 0.6 | 1.6 | 0.2 ** | - | ||
| 65 | 1 | 0.6 | 1.6 | 1 ** | - | |||
| 55 | 1 | 0.6 | 1.6 | 5 ** | - | |||
| 60 | 100 | 0.6 | 1.6 | 10 ** | 99 | |||
| Li4Ti5O12(LTO)//LiMn2O4 (LMO) | 160 | 1 | 1 | 3.1 | 1 ** | - | [66] | |
| 95 | 50 | 1 | 3.1 | 1 ** | 92 | |||
| 60 | 100 | 1 | 3.1 | 1 ** | 92 | |||
| 40 | 200 | 1 | 3.1 | 1 ** | 92 | |||
| Zn//LMO | LZ-DES/2H2O | 70 | 18 | 1.5 | 2.2 | 2 ** | - | [67] |
| 80 | 25 | 1.5 | 2.2 | 1 ** | - | |||
| 90 | 10 | 1.5 | 2.2 | 0.5 ** | - | |||
| 120 | 30 | 1.5 | 2.2 | 0.06 ** | - | |||
| LZ-DES | 35 | 1 | 1.5 | 2.2 | 2 ** | - | ||
| 45 | 1 | 1.5 | 2.2 | 1 ** | - | |||
| 55 | 1 | 1.5 | 2.2 | 0.5 ** | - | |||
| 80 | 1 | 1.5 | 2.2 | 0.06 ** | - | |||
| LTO//LMO | LiClO4-urea | 125 | 1 | 1.1 | 2.5 | 0.5 ** | - | [99] |
| 100 | 10 | 1.1 | 2.5 | 0.5 ** | - | |||
| 95 | 50 | 1.1 | 2.5 | 0.5 ** | - | |||
| 90 | 100 | 1.1 | 2.5 | 0.5 ** | - | |||
| 95 | 1 | 1.5 | 2.5 | 1 ** | - | |||
| 65 | 1 | 1.1 | 2.5 | 10 ** | 99 | |||
| 55 | 1 | 1.1 | 2.5 | 20 ** | - | |||
| 62 | 500 | 1.1 | 2.5 | 10 ** | 99 | |||
| 60 | 1000 | 1.1 | 2.5 | 10 ** | 99 | |||
| Mo6S8//LMO | LiClO4-H2O-urea | 50 | 1 | 0.8 | 2.2 | 0.1 ** | 98 | [53] |
| 40 | 100 | 0.8 | 2.2 | 0.1 ** | 98 | |||
| 37 | 500 | 0.8 | 2.2 | 0.1 ** | 98 | |||
| 40 | 30 | 0.8 | 2.2 | 1 ** | 98 | |||
| 35 | 50 | 0.8 | 2.2 | 10 ** | 98 | |||
| 30 | 70 | 0.8 | 2.2 | 30 ** | 98 | |||
| Na3V2(PO4)3//Na3V2(PO4)3 | 35 | 200 | - | - | 0.1 ** | 99 | [53] | |
| 35 | 100 | |||||||
| 20 | 1000 | - | - | 5 ** | 99 | |||
| 25 | 100 | |||||||
| Zn//MnO2 | ChCl-urea-ZnCl2 | 90 | 1 | 0.4 | 1.9 | 50 * | - | [83] |
| 70 | 1 | 0.4 | 1.9 | 100 * | - | |||
| 55 | 1 | 0.4 | 1.9 | 200 * | - | |||
| 200 | 1 | - | - | 50 * | 85 | |||
| 80 | 50 | - | - | 50 * | 85 | |||
| 60 | 50 | - | - | 100 * | 45 | |||
| 40 | 140 | - | - | 100 * | 35 | |||
| PCDI-rGO//LMO | LiClO4-urea-MgCl2-water | 45 | 0 | 2.5 | 30 * | - | [98] | |
| 44 | 1 | 50 * | - | |||||
| 43 | 1 | 100 * | - | |||||
| 32 | 1 | 500 * | - | |||||
| 25 | 1 | 1000 * | - | |||||
| 17 | 50 | - | - | 200 * | 95 | |||
| 14 | 100 | 200 * | 95 | |||||
| 10 | 200 | 200 * | 95 |
| Composition | Scan Rate (mV·s−1) | E Oxidation (V) | E Reduction (V) | Jox (mA·cm−2) | Jred (mA·cm−2) | |Eox − Ered| | Reference | Refs. |
|---|---|---|---|---|---|---|---|---|
| KCL-FeCl3 | 20 | 0.8 | 0.3 | 20 | −60 | 0.5 | Ag/AgCl | [88] |
| ChCl-urea-Zn (OTf)2 | 20 | −0.6 | −1.3 | −30 | 8 | 0.7 | [100] | |
| ChCl-urea-ZnCl2 | 20 | −0.7 | −1.6 | −8 | 8 | 0.9 | ||
| ChCl-urea-ZnSO4 | 20 | −0.7 | −1.6 | −30 | 11 | 0.9 | ||
| ChCl-urea-Zn (OTf)2 | 100 | −0.4 | −1.5 | −10 | 15 | 1.1 | ||
| ChCl-urea-ZnCl2 | 100 | −0.4 | −1.8 | −20 | 15 | 1.4 | ||
| ChCl-urea-ZnSO4 | 100 | −0.6 | −1.7 | −30 | 25 | 1.1 | ||
| ChCl-urea-CoCl2 | 20 | −0.1 | −0.9 | −5 | 5 | 0.8 | Ag wire | [101] |
| 100 | 0 | −1.1 | −10 | 8 | 1.1 | |||
| ChCl-urea-H2O | 20 | 0.7 | 0.5 | 20 | −60 | 0.2 | [88] | |
| ChCl-urea-H2O-FeCl3 | 100 | 0.8 | 0.3 | 40 | −100 | 0.5 | ||
| ChCl-urea | 20 | 1.3 | −1 | - | - | 2.3 | [85] | |
| ChCl-urea-EG-ZnO | 10 | −0.9 | −1.4 | 10 | −5 | 0.5 | Ag/AgCl | [87] |
| 90 | −0.7 | −1.6 | 25 | −15 | 0.9 | |||
| Urea-MIMTfO | 50 | 2.5 | −1.0 | - | - | 3.5 | Ag | [97] |
| Urea-MIMTfO + graphite electrode filled with Bi2O3 | 50 | 0 | −0.25 | 3 | −4 | 0.25 | ||
| LiClO4-urea-water | 0.5 | 1.5 | −1.5 | - | - | 3 | SHE | [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ammar, M.; Ashraf, S.; Gonzalez-casamachin, D.A.; Awotoye, D.T.; Baltrusaitis, J. Recent Progress of Urea-Based Deep Eutectic Solvents as Electrolytes in Battery Technology: A Critical Review. Batteries 2024, 10, 45. https://doi.org/10.3390/batteries10020045
Ammar M, Ashraf S, Gonzalez-casamachin DA, Awotoye DT, Baltrusaitis J. Recent Progress of Urea-Based Deep Eutectic Solvents as Electrolytes in Battery Technology: A Critical Review. Batteries. 2024; 10(2):45. https://doi.org/10.3390/batteries10020045
Chicago/Turabian StyleAmmar, Mohamed, Sherif Ashraf, Diego Alexander Gonzalez-casamachin, Damilola Tomi Awotoye, and Jonas Baltrusaitis. 2024. "Recent Progress of Urea-Based Deep Eutectic Solvents as Electrolytes in Battery Technology: A Critical Review" Batteries 10, no. 2: 45. https://doi.org/10.3390/batteries10020045
APA StyleAmmar, M., Ashraf, S., Gonzalez-casamachin, D. A., Awotoye, D. T., & Baltrusaitis, J. (2024). Recent Progress of Urea-Based Deep Eutectic Solvents as Electrolytes in Battery Technology: A Critical Review. Batteries, 10(2), 45. https://doi.org/10.3390/batteries10020045






