Effect of Partial Cation Replacement on Anode Performance of Sodium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
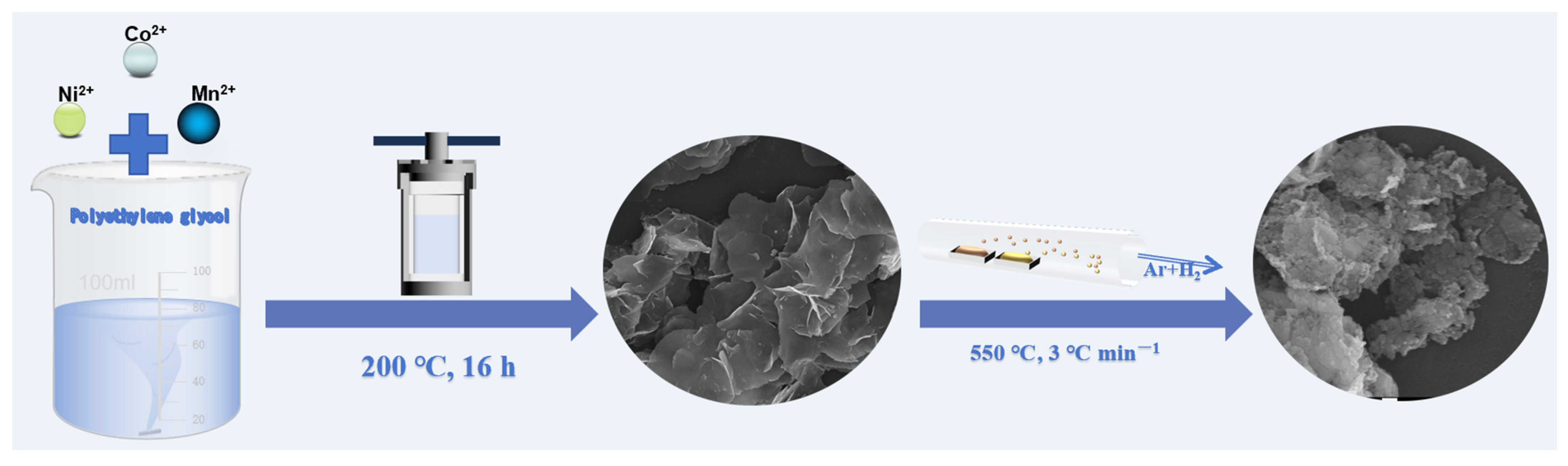
2.2. Synthesis of NCS Nanosheets
2.3. Synthesis of Mn-Replaced NCS Nanosheets
3. Results and Discussion
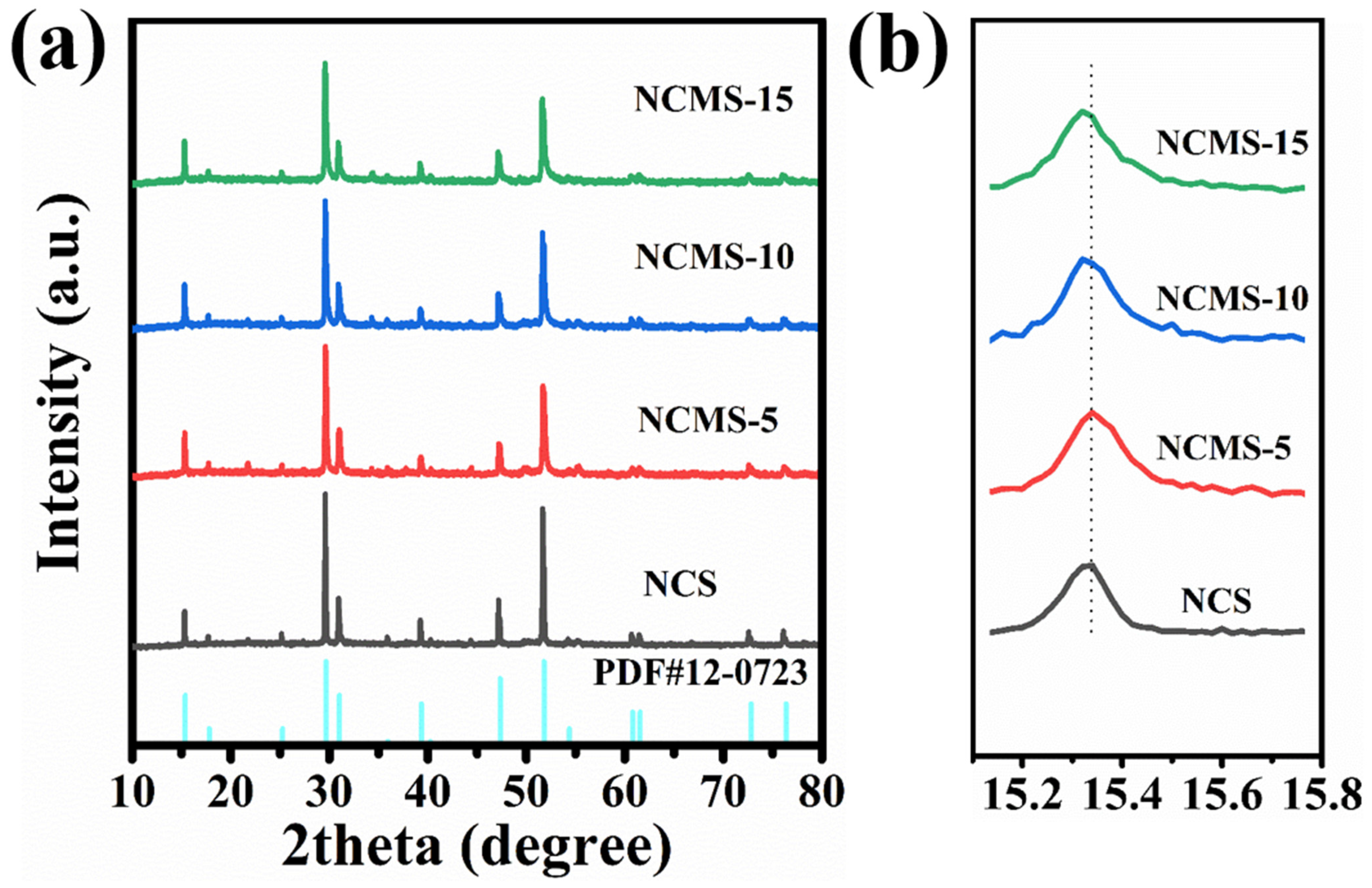
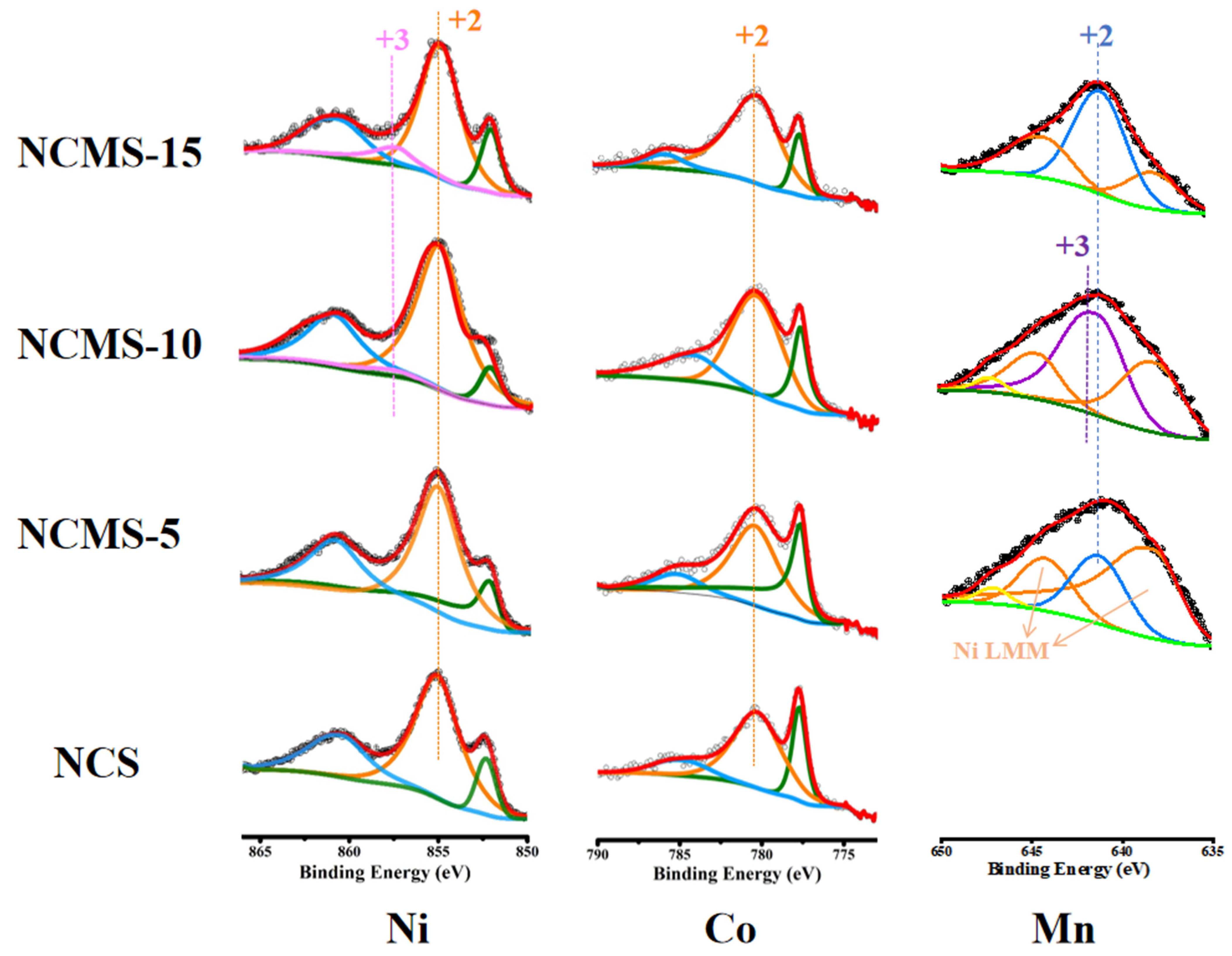
3.1. Material Characterization
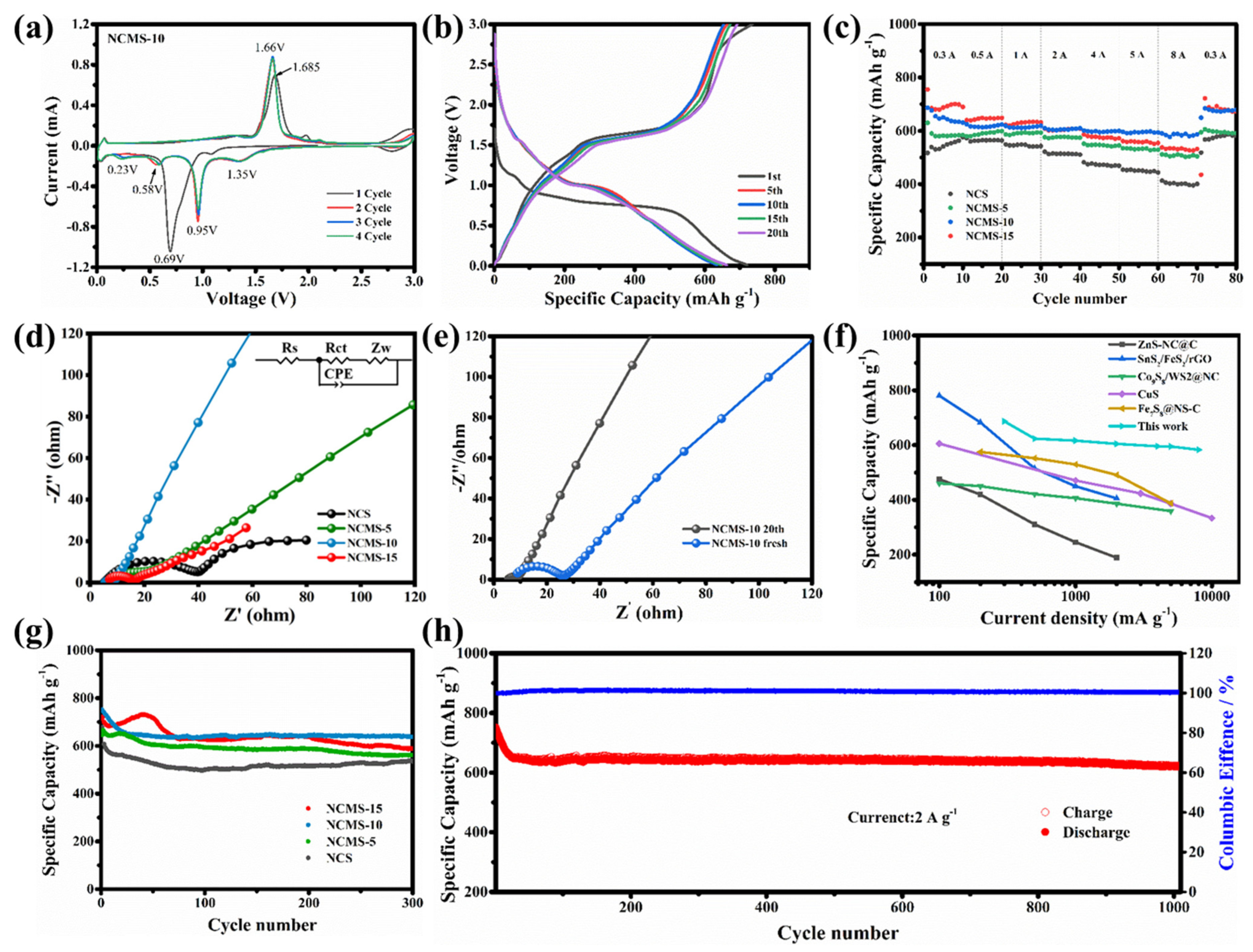
3.2. Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shang, C.; Li, X.L.; Wei, R.; Liu, X.Q.; Xu, S.Q.; Zhang, J.J. Research progress of metal oxide glass anode materials for lithium-ion batteries: A Review. J. Non. Cryst. Solids 2023, 618, 122547. [Google Scholar] [CrossRef]
- Majid, S.; Ali, A.S.G.; Taieb, S.; Mehran, A.G.; Asim, M.; Shayan, J.; Reza, R.; Meng, X.M.; Jin, Z.; Ge, Q. A Review of Nitrogen-Doped Carbon Materials for Lithium-Ion Battery Anodes. Carbon 2023, 209, 247–278. [Google Scholar] [CrossRef]
- Maia, B.A.; Magalhaes, N.; Cunha, E.; Braga, M.H.; Santos, R.M.; Correia, N. Designing Versatile Polymers for Lithium-Ion Battery Applications: A Review. Polymers 2022, 14, 403. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.B.; Sun, J.C.; Zheng, P.L.; Jiang, L.; Liu, H.Y.; Chai, J.C.; Liu, Q.Y.; Liu, Z.H.; Zheng, Y.; Rui, X.H. Recent advances of non-lithium metal anode materials for solid-state lithium-ion batteries. J. Mater. Chem. A 2022, 10, 16761–16778. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Li, H.Y.; Hu, Z.L.; Zhang, Q.B.; Zhao, J.B.; Zhang, L. Research Progresses on Structural Optimization and Interfacial Modification of Silicon Monoxide Anode for Lithium-Ion Battery. Acta Phys. Chim. Sin. 2022, 38, 210305. [Google Scholar] [CrossRef]
- Hayamizu, K.; Chiba, Y.; Haishi, T. Dynamic ionic radius of alkali metal ions in aqueous solution: A pulsed-field gradient NMR study. Rsc Adv. 2021, 11, 20252–20257. [Google Scholar] [CrossRef]
- Tian, Z.N.; Zou, Y.G.; Liu, G.; Wang, Y.Z.; Yin, J.; Ming, J.; Alshareef, H.N. Electrolyte Solvation Structure Design for Sodium Ion Batteries. Adv. Sci. 2022, 9, 2201207. [Google Scholar] [CrossRef]
- Lu, B.; Lin, C.J.; Xiong, H.J.; Zhang, C.; Fang, L.; Sun, J.Z.; Hu, Z.H.; Wu, Y.L.; Fan, X.H.; Li, G.F.; et al. Hard-Carbon Negative Electrodes from Biomasses for Sodium-Ion Batteries. Molecules 2023, 28, 4027. [Google Scholar] [CrossRef]
- Bai, X.; Wu, N.N.; Yu, G.C.; Li, T. Recent Advances in Anode Materials for Sodium-Ion Batteries. Inorganics 2023, 11, 289. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, J.; Guo, Z.Y.; Xie, F.; Liu, H.Y.; Yadegari, H.; Tebyetekerwa, M.; Ryan, M.P.; Hu, Y.S.; Titirici, M.M. The Role of Hydrothermal Carbonization in Sustainable Sodium-Ion Battery Anodes. Adv. Energy Mater. 2022, 12, 2200208. [Google Scholar] [CrossRef]
- dos Reis, G.S.; Molaiyan, P.; Subramaniyam, C.M.; García-Alvarado, F.; Paolella, A.; de Oliveira, H.P.; Lassi, U. Biomass-derived carbon-silicon composites (C@Si) as anodes for lithium-ion and sodium-ion batteries: A promising strategy towards long-term cycling stability: A mini review. Electrochem. Commun. 2023, 153, 107536. [Google Scholar] [CrossRef]
- Jaberi, S.Y.S.; Ghaffarinejad, A.; Khajehsaeidi, Z.; Sadeghi, A. The synthesis, properties, and potential applications of CoS2 as a transition metal dichalcogenide (TMD). Int. J. Hydrogen Energy 2023, 48, 15831–15878. [Google Scholar] [CrossRef]
- Yin, Z.; Hu, M.; Liu, J.; Fu, H.; Wang, Z.J.; Tang, A.W. Tunable crystal structure of Cu–Zn–Sn–S nanocrystals for improving photocatalytic hydrogen evolution enabled by copper element regulation. J. Semicond. 2022, 43, 032701. [Google Scholar] [CrossRef]
- Ying, H.J.; Han, W.Q. Metallic Sn-Based Anode Materials: Application in High-Performance Lithium-Ion and Sodium-Ion Batteries. Adv. Sci. 2017, 4, 1700298. [Google Scholar] [CrossRef]
- Zheng, X.M.; You, J.H.; Fan, J.J.; Tu, G.P.; Rong, W.Q.; Li, W.J.; Wang, Y.X.; Tao, S.; Zhang, P.Y.; Zhang, S.Y.; et al. Electrodeposited binder-free Sb/NiSb anode of sodium-ion batteries with excellent cycle stability and rate capability and new insights into its reaction mechanism by operando XRD analysis. Nano Energy 2020, 77, 105123. [Google Scholar] [CrossRef]
- Wang, Z.D.; Hong, P.; Zhao, H.P.; Lei, Y. Recent Developments and Future Prospects of Transition Metal Compounds as Electrode Materials for Potassium-Ion Hybrid Capacitors. Adv. Mater. Technol. 2023, 8, 2200515. [Google Scholar] [CrossRef]
- Li, Z.W.; Yang, Y.X.; Wen, B.; Liu, X.F.; Wang, Y.J.; Du, F.; Ma, M.M.; Li, L.; Yang, G.R.; Ding, S.J. Recovered cobalt-nickel sulfide from spent lithium-ion batteries as an advanced anode material toward sodium-ion batteries. J. Alloys Compd. 2023, 956, 170328. [Google Scholar] [CrossRef]
- Wu, Y.H.; Chen, G.B.; Wu, X.A.; Li, L.; Yue, J.Y.; Guan, Y.Y.; Hou, J.; Shi, F.N.; Liang, J.Y. Research progress on vanadium oxides for potassium-ion batteries. J. Semicond. 2023, 44, 041701. [Google Scholar] [CrossRef]
- Yin, H.; Jia, L.; Li, H.Y.; Liu, A.; Li, G.Y.; Zhu, Y.C.; Huang, J.L.; Cao, M.L.; Hou, Z.H. Point-cavity-like carbon layer coated SnS nanotubes with improved energy storage capacity for lithium/sodium ion batteries. J. Energy Storage 2023, 65, 107354. [Google Scholar] [CrossRef]
- Peng, B.; Liu, X.; Cui, Z.N.; Wang, Y.D.; Zhu, T.; Tan, Z.Q.; Li, M.F.; Wang, D. MOF-derived Ni3S2@C grown in situ on modified cotton textile as self-standing electrodes towards high performance sodium ion batteries. J. Alloys Compd. 2023, 967, 171743. [Google Scholar] [CrossRef]
- Yang, K.; Fu, H.; Duan, Y.X.; Wang, M.X.; Tran, M.X.; Lee, J.K.; Yang, W.; Liu, G.C. Uniform Metal Sulfide@N-doped Carbon Nanospheres for Sodium Storage: Universal Synthesis Strategy and Superior Performance. Energy Environ. Mater. 2023, 6, e12380. [Google Scholar] [CrossRef]
- Chao, X.; Yan, C.Z.; Zhao, H.P.; Wang, Z.J.; Lei, Y. Micro-nano structural electrode architecture for high power energy storage. J. Semicond. 2023, 44, 050201. [Google Scholar] [CrossRef]
- Hu, P.; Dong, Y.L.; Yang, G.W.; Chao, X.; He, S.J.; Zhao, H.P.; Fu, Q.; Lei, Y. Hollow CuSbSy Coated by Nitrogen-Doped Carbon as Anode Electrode for High-Performance Potassium-Ion Storage. Batteries 2023, 9, 238. [Google Scholar] [CrossRef]
- Dong, Y.L.; Xu, C.F.; Li, Y.L.; Zhang, C.L.; Zhao, H.P.; Kaiser, U.; Lei, Y. Ultrahigh-Rate and Ultralong-Duration Sodium Storage Enabled by Sodiation-Driven Reconfiguration. Adv. Energy Mater. 2023, 13, 2204324. [Google Scholar] [CrossRef]
- Li, C.W.; Hou, J.C.; Zhang, J.Y.; Li, X.Y.; Jiang, S.Q.; Zhang, G.Q.; Yao, Z.J.; Liu, T.C.; Shen, S.H.; Liu, Z.Q.; et al. Heterostructured NiS@SnS hollow spheres as superior high-rate and durable anodes for sodium-ion batteries. Sci. China Chem. 2022, 65, 1420–1432. [Google Scholar] [CrossRef]
- Sun, G.; Lin, H.Z.; Tian, R.Y.; Wei, Z.X.; Wang, X.Q.; Jin, X.; Yao, S.Y.; Chen, G.; Shen, Z.X.; Du, F. Rational design and synthesis of nanosheets self-assembled hierarchical flower-ball-like CuFeS for boosted wide temperature sodium-ion batteries. Nano Res. 2023, 16, 9407–9415. [Google Scholar] [CrossRef]
- He, S.J.; Wang, Z.D.; Wang, Z.J.; Lei, Y. Recent progress and future prospect of novel multi-ion storage devices. J. Semicond. 2023, 44, 040201. [Google Scholar] [CrossRef]
- Gao, Y.S.; Peng, B.X.; Lv, Z.R.; Han, Z.; Hu, K.Y.; Huang, F.Q. Bifunctional structure modulation of Sb-based sulfide for boosting fast and high-capacity sodium storage. Inorg. Chem. Front. 2023, 10, 2466–2473. [Google Scholar] [CrossRef]
- Hao, Z.Q.; Dimov, N.; Chang, J.K.; Okada, S. Synthesis of bimetallic sulfide FeCoS@carbon nanotube graphene hybrid as a high-performance anode material for sodium-ion batteries. Chem. Eng. J. 2021, 423, 130070. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Jin, Y.H.; Song, Y.Y.; Wang, H.; Jia, M.Q. Induced bimetallic sulfide growth with reduced graphene oxide for high-performance sodium storage. J. Colloid Interface Sci. 2023, 642, 554–564. [Google Scholar] [CrossRef]
- Krengel, M.; Adelhelm, P.; Klein, F.; Bensch, W. FeVS as a high-capacity electrode material for sodium-ion batteries. Chem. Commun. 2015, 51, 13500–13503. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, J.; Zhang, Y.; Chen, X.; Sun, G.; Yang, X.; Zeng, Y.; Tian, R.Y.; Du, F. Entropy-Change Driven Highly Reversible Sodium Storage for Conversion-Type Sulfide. Adv. Funct. Mater. 2022, 32, 2206531. [Google Scholar] [CrossRef]
- Zhang, X.M.; Zhao, Q.H.; Ma, T.H.; Li, Z.Q.; Tan, X.F.; Bateer, B. Design of thin-layer porous nickel cobalt sulfide for high-performance asymmetric supercapacitors. J. Alloys Compd. 2023, 945, 168902. [Google Scholar] [CrossRef]
- Wang, K.; Zhuo, H.X.; Wang, J.T.; Poon, F.; Sun, X.L.; Xiao, B.W. Recent Advances in Mn-Rich Layered Materials for Sodium-Ion Batteries. Adv. Funct. Mater. 2023, 33, 2212607. [Google Scholar] [CrossRef]
- Ma, J.Y.; Guo, E.Y.; Yin, L.W. Porous hierarchical spinel Mn-doped NiCoO nanosheet architectures as high-performance anodes for lithium-ion batteries and electrochemical reaction mechanism. J. Mater. Sci. Mater. Electron. 2019, 30, 8555–8567. [Google Scholar] [CrossRef]
- Chen, H.Y.; Yang, X.T.; Lv, P.F.; Tian, P.F.; Wan, S.Y.; Liu, Q.M. Mn-doped FeS with larger lattice spacing as advance anode for sodium ion half/full battery. Chem. Eng. J. 2022, 450, 137960. [Google Scholar] [CrossRef]
- Meng, X.R.; Chai, C.C.; Li, F.X.; Sun, Y.; Yang, Y.T. High-power microwaves response characteristics of silicon and GaAs solar cells. J. Semicond. 2022, 43, 112701. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, Z.X.; Zhang, Z.C.; Niu, W.X.; Li, C.L.; Yang, N.L.; Chen, B.; Zhang, H. Two-Dimensional Metal Nanomaterials: Synthesis, Properties, and Applications. Chem. Rev. 2018, 118, 6409–6455. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Zhao, X.; Wang, H.Y.; Xu, H.; Xie, L.; Zhao, C.L.; Chen, L.Y. Novel Two-dimensional Porous Materials for Electrochemical Energy Storage: A Minireview. Chem. Rec. 2020, 20, 922–935. [Google Scholar] [CrossRef]
- Jiang, N.; Tang, Q.; Sheng, M.L.; You, B.; Jiang, D.E.; Sun, Y.J. Nickel sulfides for electrocatalytic hydrogen evolution under alkaline conditions: A case study of crystalline NiS, NiS, and NiS nanoparticles. Catal. Sci. Technol. 2016, 6, 1077–1084. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Legrand, D.; Bancroft, G.M. Interpretation of Ni 2p XPS spectra of Ni conductors and Ni insulators. Phys. Chem. Miner. 2000, 27, 357–366. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Wei, S.Q.; Xing, P.X.; Dai, L.Y.; Wang, Y.Y. Iron-doped nickel sulfide nanoparticles grown on N-doped reduced graphene oxide as efficient electrocatalysts for oxygen evolution reaction. J. Electroanal. Chem. 2023, 936, 117323. [Google Scholar] [CrossRef]
- Zhao, W.W.; Bothra, P.; Lu, Z.Y.; Li, Y.B.; Mei, L.P.; Liu, K.; Zhao, Z.H.; Chen, G.X.; Back, S.; Siahrostami, S.; et al. Improved Oxygen Reduction Reaction Activity of Nanostructured CoS through Electrochemical Tuning. ACS Appl. Energy Mater. 2019, 2, 8605–8614. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Xiao, W.; Xi, P.X.; Xi, S.B.; Du, Y.H.; Gao, D.Q.; Ding, J. Activating and Optimizing Activity of CoS for Hydrogen Evolution Reaction through the Synergic Effect of N Dopants and S Vacancies. ACS Energy Lett. 2017, 2, 1022–1028. [Google Scholar] [CrossRef]
- Hao, J.H.; Yang, W.S.; Peng, Z.; Zhang, C.; Huang, Z.P.; Shi, W.D. A Nitrogen Doping Method for CoS Electrocatalysts with Enhanced Water Oxidation Performance. ACS Catal. 2017, 7, 4214–4220. [Google Scholar] [CrossRef]
- Yang, L.J.; Zhang, A.; Zhang, L. Light-Driven Fuel Cell with a 2D/3D Hierarchical CuS@MnS Z-Scheme Catalyst for H2O2 Generation. ACS Appl. Mater. Interfaces 2023, 15, 18951–18961. [Google Scholar] [CrossRef] [PubMed]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. The role of the Auger parameter in XPS studies of nickel metal, halides and oxides. Phys. Chem. Chem. Phys. 2012, 14, 2434–2442. [Google Scholar] [CrossRef] [PubMed]
- Brandt, T.G.; Tuokkola, A.R.; Yu, M.J.; Laine, R.M. Liquid-feed flame spray pyrolysis enabled synthesis of Co- and Cr-free, high-entropy spinel oxides as Li-ion anodes. Chem. Eng. J. 2023, 474, 145495. [Google Scholar] [CrossRef]
- Kowalik, M.; Zalecki, R.; Kolodziejczyk, A. Electronic States of Collosal Magnetoresistive Manganites La0.67Pb0.33Mn1-X FexO3 from Photoemission Spectroscopy. Acta Phys. Pol. A 2010, 117, 277–280. [Google Scholar] [CrossRef]
- Guan, B.L.; Sheng, L.; Zhang, N.; Sun, T.; Zhu, Y.R.; Zhang, J.H.; Yi, T.F. Bimetallic metal-organic framework derived transition metal sulfide microspheres as high-performance lithium/sodium storage materials. Chem. Eng. J. 2022, 446, 137154. [Google Scholar] [CrossRef]
- Cheng, A.; Hu, Y.N.; Yue, M.; Fu, J.X.; Wu, H.M.; Wang, S.Q.; Liu, J.W. Hollow N,C-co-doped carbon-coated ZnS nanospheres derived from metal organic framework (ZIF-8) with improved lithium and sodium storage performance. J. Phys. Mater. 2021, 4, 044003. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.B.; Guo, X.L.; Zhu, S.P.; Zhao, Y.; Iikubo, S.; Ma, T.L. Bimetallic Sulfide SnS/FeS Nanosheets as High-Performance Anode Materials for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 39248–39256. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.K.; Kang, C.X.; Xiong, W.Q.; Tian, P.F.; Cao, S.Y.; Wan, S.Y.; Chen, H.Y.; Zhou, C.B.; Liu, Q.M. WS nanosheets@ZIF-67-derived N-doped carbon composite as sodium ion battery anode with superior rate capability. J. Colloid Interfaces Sci. 2021, 595, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.F.; Hu, Y.; Liu, Y.; Bai, J.X.; Ruan, H.; Guo, S.W. Tunable CuS nanocables with hierarchical nanosheet-assembly for ultrafast and long-cycle life sodium-ion storage. Ceram. Int. 2021, 47, 14138–14145. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Fan, R.Z.; Dai, J.J.; Dong, Y.T.; Wu, J.; Gao, J.K.; Cai, Y.R. Cu triggered phase transitions in FeS@NS-C anode: A neglected factor affecting the electrochemical performance of sodium storage. Appl. Surf. Sci. 2023, 609, 155407. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Wang, Z.; Qiu, W.; Zhao, H.; Lei, Y. Effect of Partial Cation Replacement on Anode Performance of Sodium-Ion Batteries. Batteries 2024, 10, 44. https://doi.org/10.3390/batteries10020044
He S, Wang Z, Qiu W, Zhao H, Lei Y. Effect of Partial Cation Replacement on Anode Performance of Sodium-Ion Batteries. Batteries. 2024; 10(2):44. https://doi.org/10.3390/batteries10020044
Chicago/Turabian StyleHe, Shijiang, Zidong Wang, Wenbo Qiu, Huaping Zhao, and Yong Lei. 2024. "Effect of Partial Cation Replacement on Anode Performance of Sodium-Ion Batteries" Batteries 10, no. 2: 44. https://doi.org/10.3390/batteries10020044
APA StyleHe, S., Wang, Z., Qiu, W., Zhao, H., & Lei, Y. (2024). Effect of Partial Cation Replacement on Anode Performance of Sodium-Ion Batteries. Batteries, 10(2), 44. https://doi.org/10.3390/batteries10020044







