A Review on Design Parameters for the Full-Cell Lithium-Ion Batteries
Abstract
1. Introduction
2. Working Principle of LIBs
3. Design Parameters for Full-Cell LIBs
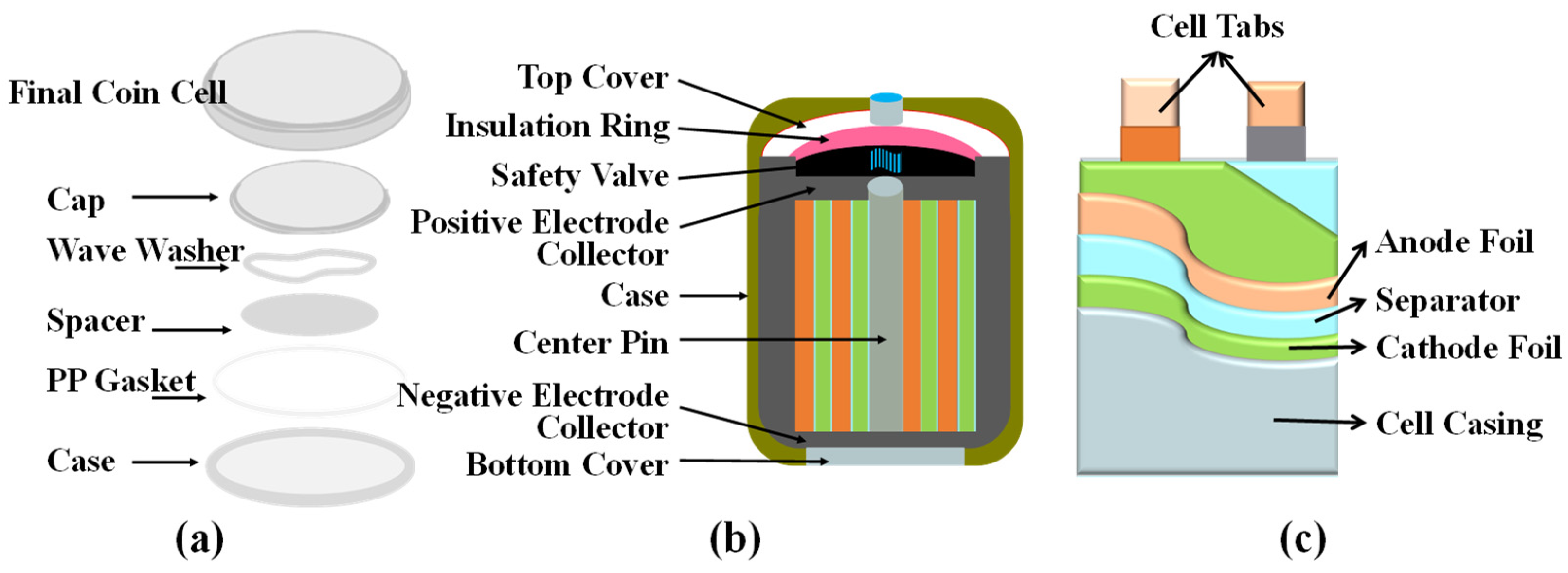
3.1. Form Factor
3.2. Choice and Types of Materials for Main Components
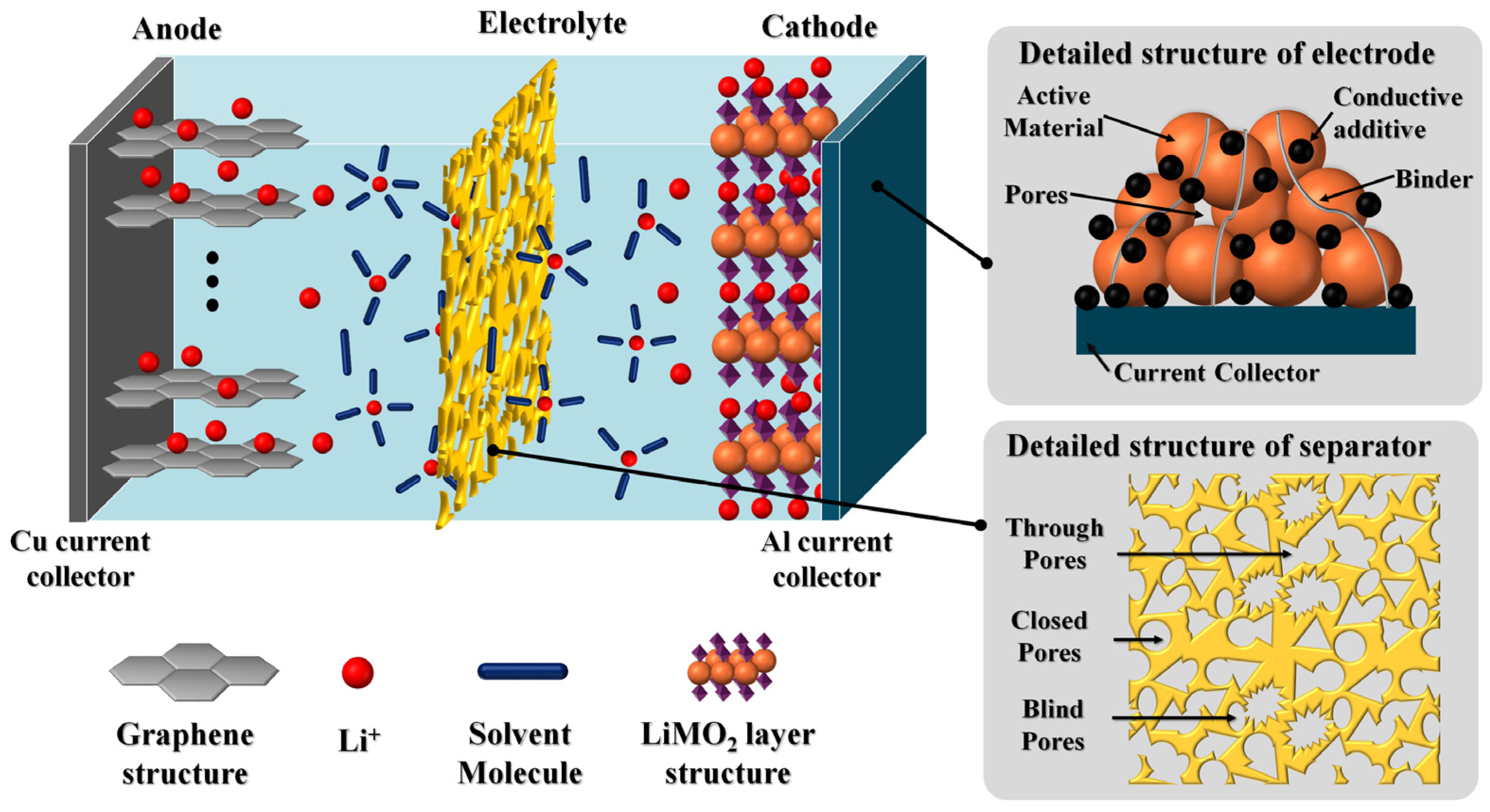
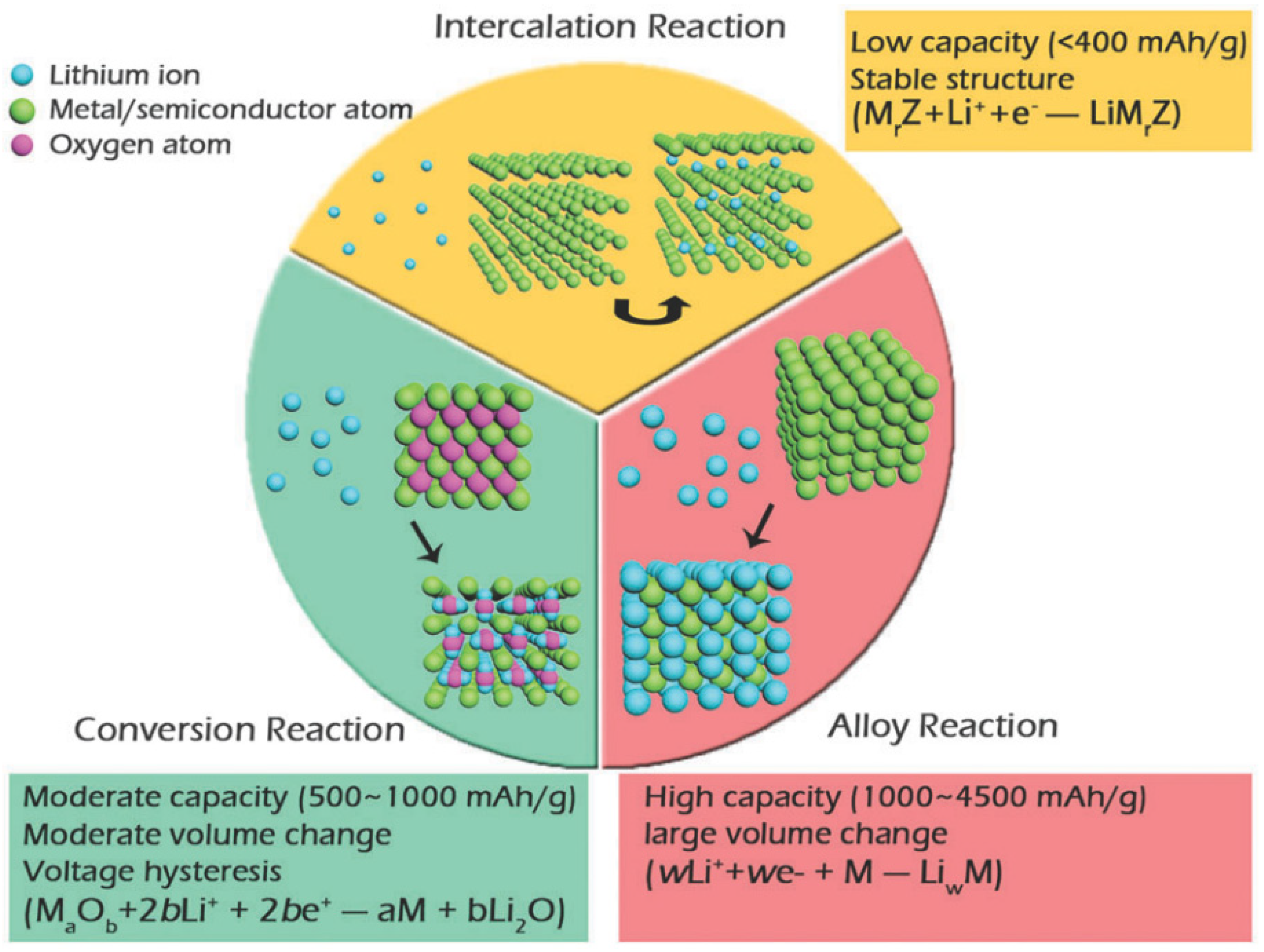
3.2.1. Electrode
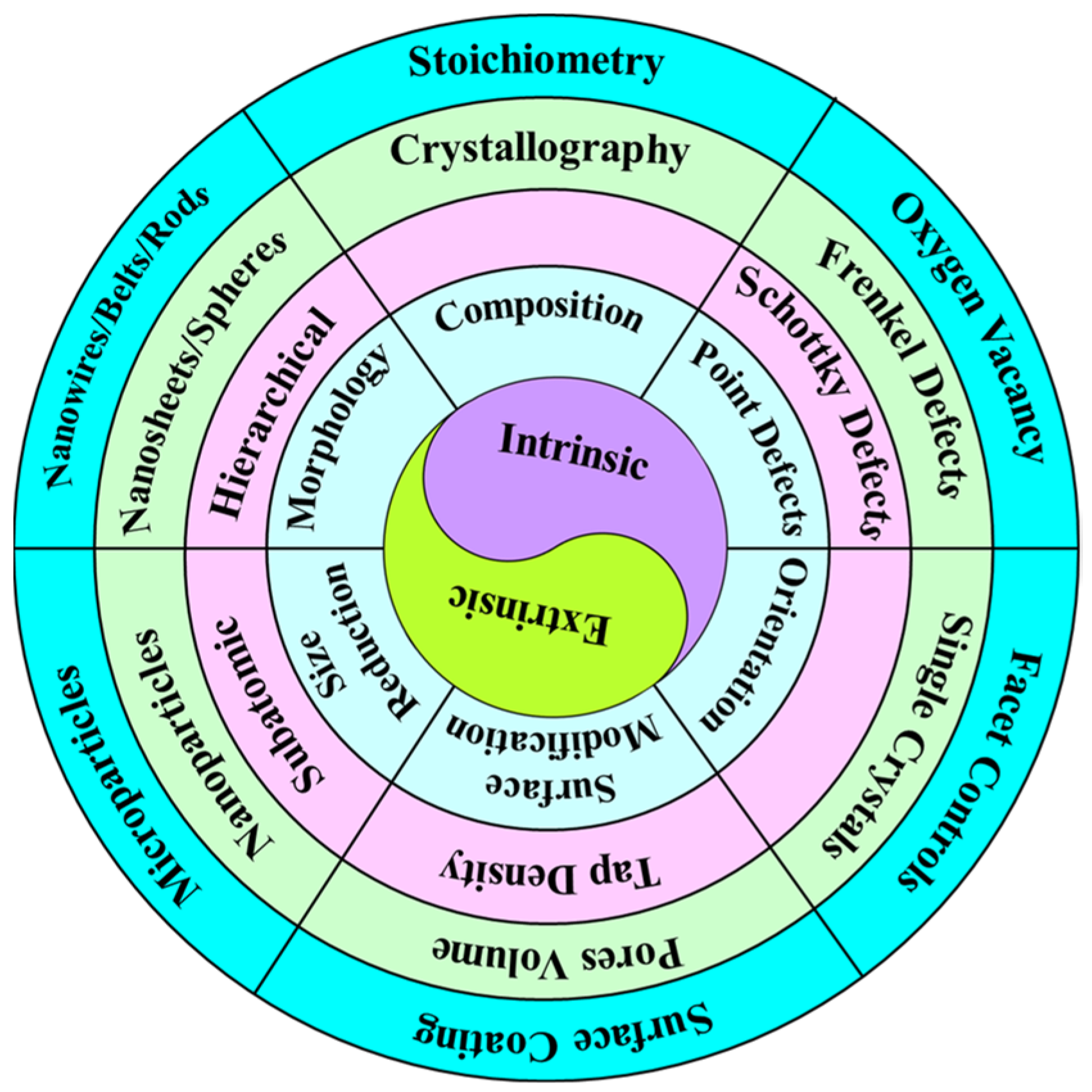
- Chemical compositions: The chemical composition defines the crystal structure and governs key properties such as mechanical strength (adhesion/cohesion), stability (structural, chemical, and thermal), phase transformation, and intrinsic conductivity (electrical and ionic). It also specifies the amount of Li+ ions that can be inserted or extracted from the crystal structure, directly impacting on the electrochemical properties of electrode materials [31,32,33,34]. Additionally, structural units, which represent the material’s ‘genes’, provide insights into local chemical coordination and molecular chemistry, establishing the physical and chemical properties of the electrodes. Understanding the correlation between structural units and these physical/chemical properties offers critical evidence about charge-transfer characteristics, which are essential for intrinsic properties like structural/thermal stability, electronic/ionic conductivities, and Li+ ion transport. These properties are crucial for enhancing the electrochemical performance of LIBs [35]. Therefore, it is essential to design and develop structurally tunable electrode materials that can accommodate additional Li+ ions, improve intrinsic conductivities, expand the voltage window, enhance diffusion kinetics, and provide excellent electrochemical performance for LIBs.
- Point defects: Similarly, point defects such as Frenkel defects (where an atom migrates from its lattice site to an interstitial site, creating an interstitial defect), Schottky defects (which involve the simultaneous presence of cation and anion vacancies), and oxygen vacancies (absence of oxygen atoms or presence of hydroxyl ions within the crystal structure) play a significant role in defining the local structure of electrode materials. These defects can enhance intrinsic conductivity, improve thermal and structural stability, facilitate pseudo-capacitive kinetics, limit volume expansion, and boost the electrochemical performance of LIBs. Generally, electrode materials with symmetric compositions tend to act as semiconductors, while non-stoichiometric materials (doped or defect-induced) behave like metals, which helps alleviate structural, chemical, and thermal changes [36,37,38,39]. However, the effect of oxygen vacancies, compared to Frenkel and Schottky defects, has been insufficiently studied and further investigations are required for the development of innovative LIBs.
- Crystal Orientation: Crystal orientation influences specific facets, crystal structures, and surface energies, which in turn affect thermodynamics and reaction kinetics at the surface/interfaces. In batteries, supercapacitors, and fuel cells, physical and chemical interactions at the interfaces play an important role in promoting electrochemical energy storage activities [40]. Additionally, single crystals, which offer advantages such as a small specific surface area, excellent structural stability, high mechanical and thermal stability, superior reaction homogeneity, and good crystallinity, have been studied for their impact on crystal orientation. These studies aim to significantly enhance the electrochemical performance of electrode materials for LIBs, including safety, capacity retention, and cycle life. Electrode materials with low activation energy and substantial adsorption kinetics of crystal facets are promising for achieving high energy density and rate performance in LIBs [41,42,43,44]. The interest in exploring single crystal electrodes and their potential applications continues to grow, highlighting the need for advanced research methodologies to address future energy challenges.
- Size reduction: The particle size, size distribution, and shape of particles influence the contact area, diffusion resistance, diffusion path, energy density, and overall electrochemical performance of LIBs. Reducing particle size shortens the transportation length of Li+ ions, decreases the Li+ ion diffusion barrier, enhances ionic diffusion, increases the contact area among electrode active materials, current collectors, and electrolytes, and ensures the electroactivity of the electrode materials. However, smaller particle sizes also increase the surface area, which can promote electrochemical activity and lead to more side reactions, potentially causing thermal issues and internal short circuits in LIBs [21,45,46]. Particle size distribution affects the physical and chemical properties and overall surface energy activity of electrode materials. A broad size distribution results in high energy density but poor cell homogeneity due to particle size variance and surface energy differences. In contrast, a uniform size distribution, although challenging to produce, offers stable electroactivity by reducing stress strains during the charging process, thereby improving the cycle performance of LIBs [47,48]. Additionally, particle shape directly affects the effective surface area and mass flow properties, particularly the tap density, which influences the Li+ ion diffusion channels and reaction kinetics, enhancing the cycle performance of LIBs. However, particles derived from single-crystal structures are expensive and difficult to manufacture and handle, requiring a highly regulated reaction environment [49,50,51].
- Morphological change: The shape and morphology of electrode materials affect various factors such as porosity, tap density, diffusion pathways, surface area, and interfacial contact area. These factors comprehensively lower the activation energy for electrochemical reactions, shorten the transportation length for Li+ ions, enhance diffusivity and electroactivity, and improve specific capacity and rate capability, ultimately determining the electrochemical performance for energy storage applications [52,53]. Several morphologies, including nanosheets, nanowires/rods/belts/tubes, hierarchical nanostructures, microcubes, microspheres, and micro-flowers, have been developed depending on synthesis and calcination conditions. Nanowires/rods/belts/tubes and nanosheets, with improved compact density, provide unidirectional diffusion pathways for Li+ ions. In contrast, microspheres/flowers, urchin-like structures, and 3D microspheres/microcubes with sizes around 5–10 μm increase electrode packing density, accommodate inactive components (binders and conductive additives used in slurry fabrication), offer extensive surface-active sites for electrolyte penetration, and promote Li+ ion diffusion, resulting in high energy density for LIBs [54,55,56]. However, micron-sized particles can limit the rate performance and power density of LIBs by extending the diffusion pathways for Li+ ions. Additionally, large cracks and deformations often appear between grain boundaries and at the electrode surface due to the accumulation of significant stresses during the charging process. These issues restrict electronic and ionic conductivity, leading to capacity fading, electrode detachment, and cell degradation [57,58].
- Surface modification: Surface modification is an accessible, cost-effective, and widely applied strategy and it is achieved through techniques such as surface coating, etching, and ion doping. These methods enhance ionic conductivity and create surface-active sites that facilitate electrolyte penetration, which is crucial for forming a solid electrolyte interface (SEI) layer. This layer helps buffer volume expansion and contraction, maintaining structural integrity and mitigating capacity fading during cycling [14,59,60]. Consequently, it is highly desirable to prepare electrodes with high voltage, high energy density, low cost, excellent intrinsic conductivity, and robust structural, chemical, and thermal stability. Additionally, electrodes should feature various morphologies with high surface area and porous characteristics. Surface modification techniques, including coating with carbonaceous materials or metal oxides, surface treatment (such as acid/base or metal oxide etching), and ion doping, are essential for enhancing electronic and ionic conductivity and developing coating layers. These modifications help alleviate volume changes, suppress microstrains in the crystal structure, and improve surface adsorption characteristics for additional Li+ ions, thus promoting the electrochemical performance of LIBs. Electrodes, whether designed intrinsically or extrinsically, are classified into various types based on the electrochemical reaction chemistry during the cycling process. Numerous reports detail the cathode and anode materials, synthesis methodologies, modifications, and investigations into electrochemical reaction mechanisms [24,61,62,63].
3.2.2. Binders
3.2.3. Separators
- Thickness: The thickness of a separator typically ranges from 20 to 50 μm, influencing the stability, mechanical properties, overall weight, and cell resistance of LIBs. For example, the commercially available Celgard 2400 separator has a thickness of 25 μm [114].
- Porosity: Porosity is a crucial factor in determining mass transport, as it ensures sufficient Li+ ion conductivity and helps inhibit the formation of dendritic lithium. Common separators in the battery market typically exhibit around 40% porosity, which is defined as the ratio of the volume of pores to the apparent total volume of the pores. Porosity is typically measured by calculating the weight difference of the separator before and after soaking it in liquid, as shown below [115,116].where ρm, ρp, W, Wo, ρL, and Vo represent the apparent density, separator material density, weight of void separator, weight of separator soaked in liquid, density of liquid, and geometric volume of separators, respectively.
- Mean pore size: The mean pore size is closely related to the size of Li+ ions, active ionic species in the electrolyte, and active mass components. Pore size controls the flow of Li+ ions, blocks lithium dendrites, and prevents short circuits. There exists a mean pore size of less than 1 μm for a commercially viable and safe separator to allow Li+ ion transportation and block other active species. They can be classified into closed, blind, and through pores, as shown in Figure 4. Closed pores are fully enclosed without void spaces, while blind pores open to a void space on one side but are blocked on the other, trapping Li+ ions and potentially leading to dendrite formation. Pores with open void spaces and high permeability allow effective Li+ ion transport.
- Geometric effect: The geometric effect of pore morphology on the conductivity of Li+ ions under certain pressure differences is known as tortuosity. It describes the morphological changes in the pores of the separator. Pores exhibit various morphologies, including interconnected, network-type hierarchical structures, circular shapes, and other microstructures. Tortuosity is the ratio of the mean path length that ions must travel through the pores to the direct straight-line distance as follows:where Ɛ is porosity, and Rs and Ro are resistivity of separators before and after soaking in liquid, respectively. Permeability is calculated using Darcy’s law, which describes the rate of fluid flow through a porous surface as follows:where is average velocity, and ∇P are viscosity and applied pressure gradient of the fluid, respectively, and κ is permeability of separators.
- Wettability: It is a key aspect of the separator that directly influences the capacity and cycle retention of LIBs. A separator must quickly absorb electrolytes and initiate uniform Li+ ion transportation to prevent uneven Li+ ion deposition on the electrodes. The wettability of the separator is measured through contact angle analysis and assesses its affinity for liquid electrolytes by determining the angle formed between the separator surface and the electrolyte droplet.
- Thermal stability: Thermal stability is a crucial factor for ensuring the safety of LIBs. The separator must remain thermodynamically stable and withstand rising heat flux during battery operation under extreme conditions. When the separator shrinks or wrinkles at high temperatures, it leads to poor interfacial contact with electrodes, resulting in significant energy loss. Excessive heat flow can trigger thermal runaway and internal short circuits in the LIBs. To prevent these issues, the thermal shrinkage of the separator must be kept below 5% after 60 min at 90 °C under vacuum and can be measured using the following equation:where Ai and Af indicate area of separators before and after heat treatment at a certain temperature, respectively [114,115,116]. Furthermore, the separator must possess a shutdown effect, where the pores are blocked once abnormal heat flow is detected. This feature prevents direct contact between electrodes, inhibiting thermal runaway and internal short circuits. In addition, the separator should be non-flammable, ideally flame retardant, because if thermal runaway occurs, a flammable separator could catch fire, potentially leading to a battery explosion [113,117].
- Mechanical properties: The mechanical properties (e.g., mechanical strength, strain percentages, compression percentages) play a crucial role in determining the stability of separators and LIBs. During cell assembly, the interaction between electrolytes and separators causes mechanical softening and swelling under compression. Battery operation induces volume expansion in the electrode’s active materials, exerting pressures of up to ~5 MPa. Under such stresses, the elastic modulus of the separator decreases, reducing its tolerance, altering its microstructure, hindering ionic conductivity, and leading to swelling. This compromises stability, promotes dendritic Li formation, and increases the risk of internal short circuits in LIBs [118,119].
3.2.4. Current Collector
- Electrochemical stability: It is essential to keep the stable reduction/oxidation environment during the battery operation as cathode and anode require high and low electrochemical potentials in LIBs, respectively. Any undesired reactions between the current collector and the electrolyte at these extremes can destabilize the system, leading to capacity fading and a shortened cycle life. Therefore, selecting a current collector with excellent electrochemical stability is crucial for achieving LIBs [123].
- Density: Current collectors with low densities are advantageous for reducing weight and cost, which can enhance the energy density of LIBs. Furthermore, high mechanical strength is crucial for preserving the integrity of the electrode materials and ensuring strong bonding with the current collector, electrodes, and polymer binder materials.
- Mechanical strength: A current collector with high mechanical strength helps suppress volume expansion, prevent electrode pulverization and delamination, and maintain the integrity of active components, thereby enhancing cycle stability and prolonging the cycle life of LIBs [94].
- Electrical conductivity: The electrical conductivity of the current collector and the interfaces between the electrode and current collector is crucial for LIB performance, as electrons generated at the electrodes travel through the current collector to the external circuits. A current collector with high electrical conductivity improves energy efficiency and minimizes heat generation, thus reducing the loss of chemical/electrical energy as heat during battery operation [124].
3.2.5. Electrolyte
- Ionic conductivity: The electrolyte must enable efficient ion transport, meaning it should be highly conductive to ions. High ionic conductivity within the electrolyte facilitates the rapid movement of ions between electrodes, promoting efficient charging and discharging of the LIB. This characteristic is crucial as it directly impacts the rate performance and power density of the LIB. Therefore, to achieve a high-rate and high-power density LIB, the electrolyte must exhibit excellent ionic conductivity.
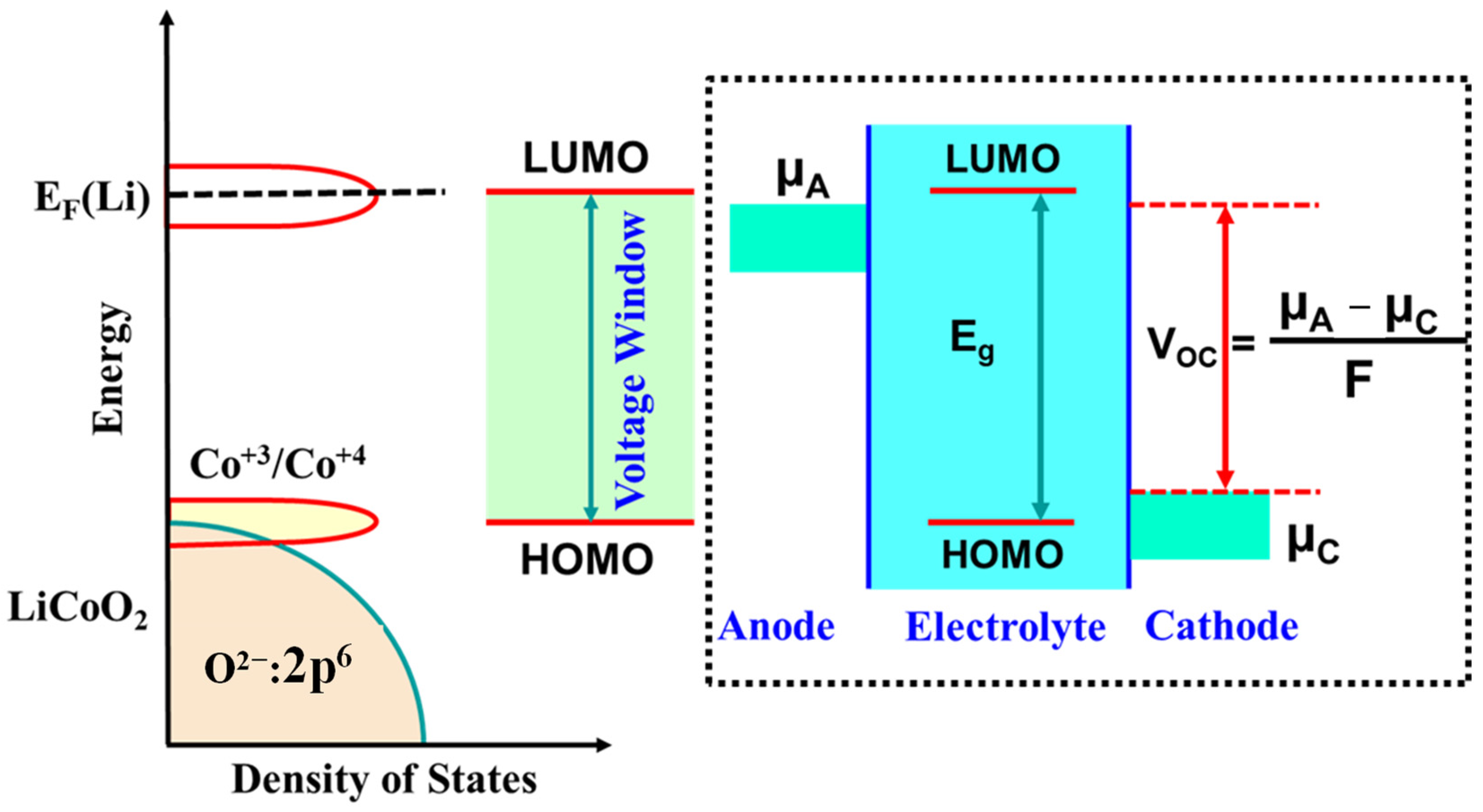
- Wide potential stability window: The potential window of the electrolyte defines the range within which ions can effectively move between the cathode and anode. To ensure optimal performance, the electrolyte must support ionic movement from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO) of the electrode materials. A broad potential window allows for a wider range of electrochemical reactions, enhancing the specific capacity, cycle life, and overall electrochemical performance of the LIB. Moreover, the potential window must remain stable during cycling to preserve the reaction chemistry, prevent thermal runaway, and maintain the structural and operational stability of the full-cell LIBs. Any fluctuation in this window can compromise the cell’s performance and safety. Therefore, a stable and wide potential window is essential for achieving high-performance, long-lasting, and safe LIBs.
- Chemically inert: Electrolytes must be electrochemically inert and should not participate in the electrochemical reaction of the full-cell LIBs.
- Low cost: The electrolyte should be cost-effective and have easy accessibility.
- Reducible: The electrolyte must undergo reduction during the electrochemical reaction so that Li+ ions can transfer under the migration, diffusion, and convection phenomena.
- Environment-friendly: The electrolyte materials should be non-toxic and environment-friendly and should not cause any harm to human beings, animals, or the environment.
- Electron insulator: The electrolyte must block electron flow while allowing uninterrupted ionic transport. In other words, it should act as an electrical insulator, preventing electron involvement in any reactions during the electrochemical process.
- High fluidity and low vapor pressure:
- (a)
- Aqueous electrolytes;
- (b)
- Non-aqueous electrolytes;
- (c)
- Ionic liquids;
- (d)
- Polymer electrolytes (gel polymer, solid polymer);
- (e)
- Hybrid electrolytes.
3.3. Design Parameters Directly Affecting Performance
3.3.1. Conductivity
3.3.2. Electrochemical Potential Window
3.3.3. Electrochemical Reaction Kinetics
3.3.4. Efficiency
3.4. Productivity and Cost of Full-Cell LIBs
3.4.1. Cell Fabrication Processes
3.4.2. Mass Loading of Active Material
4. Summary and Outlook
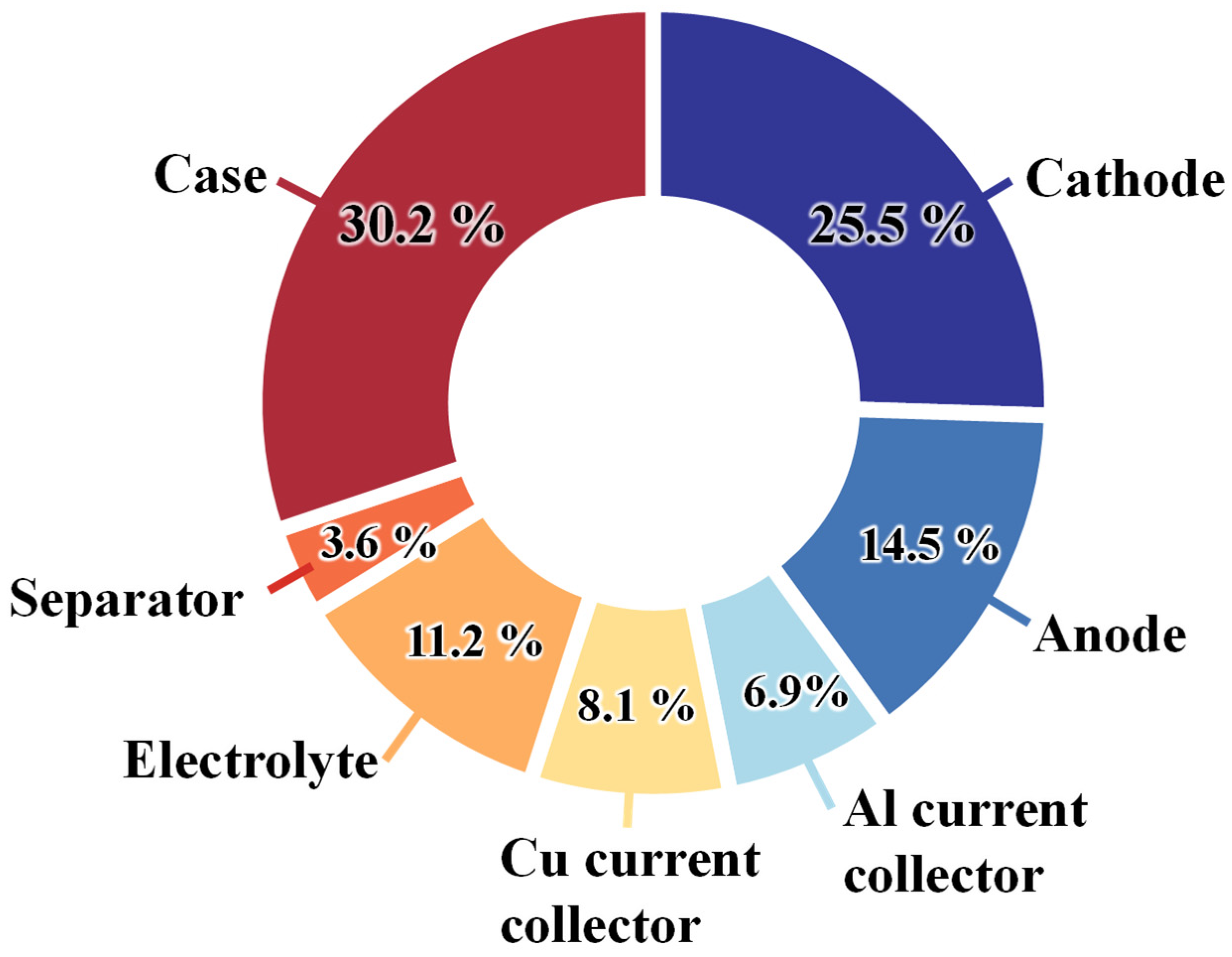
- The full-cell configuration of LIBs includes electrodes (cathodes, anodes), current collectors, a separator, and an electrolyte. The cathode functions as the positive electrode with a high oxidation potential, facilitating the delivery of Li⁺ ions to the battery system. On the other hand, the anode acts as the negative electrode with a low reduction potential, accepting incoming Li⁺ ions. Current collectors are typically metal foils, metal oxides, or carbon fibers. Commonly used commercial current collectors include copper and aluminum foils. PP sheets, glass fibers, and sodium alginates are commonly used separators that prevent the flow of electrons while allowing the conduction of Li⁺ ions within the electrolyte. The electrolyte manages the transportation of Li⁺ ions and supports the chemical reactions. The electrolyte is a mixture of lithium salts (LiClO4, LiPF6, LiTFSI, LiTf, LiAsF6, LiBF4) and solvents (aqueous solutions, organic solvents, ionic liquids, polymers, and gels). A commercially used electrolyte is 1.0 M LiPF6 in a solvent mixture of ethylene carbonate (EC) and diethyl carbonate (DEC) at a 1:1 volume ratio, or in EC and dimethyl carbonate (DMC) at a 3:7 volume ratio.
- The design of full-cell LIBs involves several critical factors, including form factors (such as length, width, height, shape, and volume), material selection, performance, and productivity/cost aspects. Obviously, the form factors must be carefully considered to meet the requirements of LIBs. Material selection is most fundamental and crucial since it defines the electrochemical reaction mechanism, performance, and cost of LIBs. Designing electrode materials requires careful consideration of both intrinsic and extrinsic approaches. Binders should be designed with robust adhesion and cohesion properties, optimal binder selection, excellent distribution, free radical quenching capabilities, strong chelation, and electrochemical compatibility. Current collectors are evaluated based on electrochemical stability, density, mechanical strength, electrical conductivity, sustainability, and cost. Separators should be designed with attention to thickness, porosity, mean pore size (typically less than 1 µm), pore morphology, wettability, thermal stability, and mechanical properties. The development of electrolytes involves considering characteristics such as ionic conductivity, wide potential stability window, temperature tolerance, mechanical and thermal stability, chemical stability, and the ability to support the reaction kinetics of LIBs.
- Performance in full-cell LIBs is determined by several factors: conductivity, electrochemical reaction mechanisms, voltage window, efficiency, and thermodynamics. Both electrical and ionic conductivities significantly impact the specific capacity, energy density, power density, and cycle stability of LIBs. Electrical conductivity is governed by the electronic structure of the electrode materials, whereas ionic conductivity is influenced by the crystal structure, physicochemical properties, morphology, and particle size of the electrode materials, as well as the porosity and geometry of the separator. These factors directly or indirectly influence Li⁺ ion diffusion, which in turn affects the rate performance and power density of LIBs. Thus, a thorough investigation is necessary to evaluate the performance of LIBs. The potential window indicates the range of electrochemical reactions that can occur within HOMO and LUMO of the electrolyte. It is influenced by factors such as the electronegativity of atoms, the nature of chemical bonds, lattice energy, crystal defects, and the crystal and electronic structures of both electrodes and electrolytes.
- Productivity is determined by factors such as the electrode fabrication process, mass loading amount, and processability, all of which impact the cost and weight of the final product. Key factors influencing the final electrode’s properties include process parameters that affect the compact density, thickness, mass loading amount, and porosity of the electrode. The mass loading amount of the cathode and anode, determined by the thickness and mixing ratio of the electroactive materials, directly influences the specific capacity, energy, power density, and overall performance of the LIBs. Thus, each step in the fabrication process should be carefully managed to meet the requirements of full-cell LIBs. The final cost of full-cell LIBs is influenced by the costs of materials, cell components, and manufacturing processes, necessitating the optimization of cost analysis for each component to minimize the overall expense.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Behabtu, H.A.; Messagie, M.; Coosemans, T.; Berecibar, M.; Fante, K.A.; Kebede, A.A.; Mierlo, J.V. A review of energy storage technologies: Application potentials in renewable energy sources grid integration. Sustainability 2020, 12, 10511. [Google Scholar] [CrossRef]
- China Energy Storage Alliance (CNESA). CNESA Global Energy Storage Market Analysis-2019; Q4 (Summary); China Energy Storage Alliance (CNESA): Beijing, China, 2020. [Google Scholar]
- Whittingham, M.S. Electrical energy storage and intercalation chemistry. Science 1976, 192, 1126–1127. [Google Scholar] [CrossRef]
- Whittingham, M.S.; Jacobson, A.J. Intercalation Chemistry; Academic Press: New York, NY, USA, 1982. [Google Scholar]
- Nagaura, T.; Nagamine, M.; Tanabe, I.; Miyamoto, N. Solid-state batteries with sulfide-based electrolytes. Prog. Batter. Sol. Cells 1989, 8, 84–88. [Google Scholar]
- Yazami, R.; Touzain, P. A reversible graphit-lithium negative electrode for electrochemical generators. J. Power Sources 1983, 9, 365–371. [Google Scholar] [CrossRef]
- Sarre, G.; Blanchard, P.; Broussely, M. Aging of lithium-ion batteries. J. Power Sources 2004, 127, 65–71. [Google Scholar] [CrossRef]
- Guo, J.; Li, Y.; Pedersen, K.; Stroe, D.-I. Lithium-ion battery, operation, degradation, and aging mechanism in electric vehicles: An overview. Energies 2021, 14, 5220. [Google Scholar] [CrossRef]
- Zhang, X.; Li, P.; Huang, B.; Zhang, H. Numerical investigation on the thermal behavior of cylindrical lithium-ion batteries based on the electrochemical thermal coupling model. Int. J. Heat Mass Transf. 2022, 199, 123449. [Google Scholar] [CrossRef]
- Román-Ramírez, L.; Marco, J. Design of Experiments Applied to Lithium-Ion Batteries: A Literature Review. Appl. Energy 2022, 320, 119305. [Google Scholar] [CrossRef]
- Kim, H.-J.; Krishna, T.N.V.; Zeb, K.; Rajangam, V.; Gopi, C.V.V.M.; Sambasivam, S.; Raghavendra, K.V.G.; Obaidat, I.M. A comprehensive review of Li-ion battery materials and Their recycling techniques. Electronics 2020, 9, 1161. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Goncalves, R.; Costa, C.M.; Mendez, S.L. Recent advances on Materials for Lithium-ion batteries. Energies 2021, 14, 3145. [Google Scholar] [CrossRef]
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Chaudhary, M.; Tyagi, S.; Gupta, R.K.; Singh, B.P.; Singhal, R. Surface modification of cathode materials for energy storage devices: A review. Surf. Coat. Technol. 2021, 412, 127009. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, X.; Li, Q.; Pang, H. Synthesis and Progress of New Oxygen vacant electrode materials for high-energy rechargeable battery applications. Small 2018, 14, 1802193. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Xia, Q.; Xu, Y.; Feng, H.; Wang, P.; Tan, Q. A review on progress of lithium-rich manganese-based cathodes for lithium-ion batteries. J. Power Sources 2021, 487, 229362. [Google Scholar] [CrossRef]
- Fayaz, H.; Afzal, A.; Samee, A.D.M.; Soudagar, M.E.M.; Akram, N.; Mujtaba, M.A.; Jilte, M.; Islam, T.; Agbulut, U.; Saleel, C.A. Optimization of Thermal and Structural Design in Lithium-Ion Batteries to Obtain Energy Efficient Battery Thermal Management System (BTMS): A Critical Review. Arch. Comput. Methods Eng. 2022, 29, 129–194. [Google Scholar] [CrossRef]
- Du, S.; Lai, Y.; Ai, L.; Ai, L.; Cheng, Y.; Tang, Y.; Jia, M. An investigation of irreversible heat generation in lithium-ion batteries based on a thermo-electrochemical coupling method. Appl. Therm. Eng. 2017, 121, 501–510. [Google Scholar] [CrossRef]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Zaccaria, R.P.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef]
- Li, J.; Zhong, W.; Deng, Q.; Zhang, Q.; Yang, C. Recent progress in synthesis and surface modification of Nickle-rich layered oxide cathode materials for lithium-ion batteries. Int. J. Extrem. Manuf. 2022, 4, 042004. [Google Scholar] [CrossRef]
- Zhang, J.; Qiao, J.; Sun, K.; Wang, Z. Balancing particle properties for practical lithium-ion batteries. Particuology 2022, 61, 18–29. [Google Scholar] [CrossRef]
- Mayur, M.; DeCaluwe, S.C.; Kee, B.L.; Bessler, W.G. Modeling and simulation of the thermodynamics of lithium-ion battery intercalation materials in the open-source software Cantera. Electrochim. Acta 2019, 323, 134797. [Google Scholar] [CrossRef]
- Kim, J.-S.; Lee, D.-C.; Lee, J.-J.; Kim, C.-W. Optimization for the maximum specific energy density of a lithium-ion battery using progressive quadratic response surface method and design of experiments. Sci. Rep. 2020, 10, 15586. [Google Scholar] [CrossRef] [PubMed]
- Lain, M.J.; Brandon, J.; Kendrick, E. Design Strategies for High Power vs. High Energy Lithium-Ion Cells. Batteries 2019, 5, 64. [Google Scholar] [CrossRef]
- Tikekar, M.D.; Choudhury, S.; Tu, Z.; Archer, L.A. Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat. Energy 2016, 1, 16114. [Google Scholar] [CrossRef]
- Park, S.; Jeong, S.Y.; Lee, T.K.; Park, M.W.; Lim, H.Y.; Sung, J.; Cho, J.; Kwak, S.K.; Hong, S.Y.; Choi, N.-S. Replacing conventional battery electrolyte additives with dioxolone derivatives for high-energy-density lithium-ion batteries. Nat. Commun. 2021, 12, 838. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.N.U.; Rasul, M.G.; Sayem, A.S.M.; Mandal, N.K. Design and optimization of lithium-ion battery as an efficient energy storage device for electric vehicles: A comprehensive review. J. Energy Storage 2023, 71, 108033. [Google Scholar] [CrossRef]
- XU, J.; Cai, X.; Cai, S.; Shao, Y.; Hu, C.; Lu, S.; Ding, S. High-energy lithium-ion batteries: Recent progress and a promising future in applications. Energy Environ. Mater. 2023, 6, e12450. [Google Scholar] [CrossRef]
- Wang, Q.; O’Carroll, T.; Shi, F.; Huang, Y.; Chen, G.; Yang, X.; Nevar, A.; Dudko, N.; Tarasenko, N.; Xie, J.; et al. Designing Organic Material Electrodes for Lithium-Ion Batteries: Progress, Challenges, and Perspectives. Electrochem. Energy Rev. 2024, 7, 15. [Google Scholar] [CrossRef]
- Chawla, N.; Bharti, N.; Singh, S. Recent Advances in Non-flammable electrolytes for safer lithium-ion batteries. Batteries 2019, 5, 19. [Google Scholar] [CrossRef]
- Zhu, L.; Bao, C.; Xie, L.; Yang, X.; Cao, X. Review of synthesis and structural optimization of LiNi1/3Co1/3Mn1/3O2 cathode materials for lithium-ion batteries applications. J. Alloys Comp. 2020, 831, 154864. [Google Scholar] [CrossRef]
- Ren, D.; Shen, Y.; Yang, Y.; Shen, L.; Levin, B.D.A.; Yu, Y.; Muller, D.A.; Arbuna, H.D. Systematic optimization of battery materials: Key parameter optimization for the scalable synthesis of uniform high-energy, and high-stability LiNi0.6Mn0.2Co0.2O2 cathode materials for Lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 35811–35819. [Google Scholar] [CrossRef]
- Julien, C.; Mauger, A.; Zaghib, K.; Groult, H. Optimization of layered cathode materials for Lithium-ion batteries. Materials 2016, 9, 595. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A.; Chemelewski, K.; Lee, E.-S. A perspective on the high-voltage LiMn1.5Ni0.5O4 spinel cathode for lithium-ion batteries. Energy Environ. Sci. 2014, 7, 1339–1350. [Google Scholar] [CrossRef]
- Zheng, J.; Ye, Y.; Pan, F. Structure units as material genes in cathode materials for lithium-ion batteries. Natl. Sci. Rev. 2020, 7, 242–245. [Google Scholar] [CrossRef]
- Zheng, J.; Xiao, J.; Zhang, J.-G. The roles of oxygen non-stoichiometry on the electrochemical properties of oxide-based cathode materials. Nano Today 2016, 11, 678–694. [Google Scholar] [CrossRef]
- Kuganathan, N.; Ganeshalingham, S.; Chroneos, A. Defects, Dopants and Lithium mobility in Li9V3(P2O7)3(PO4)2. Sci. Rep. 2018, 8, 8140. [Google Scholar] [CrossRef]
- Kuganathan, N.; Solovjov, A.L.; Vovk, R.V.; Chroneos, A. Defects, diffusion, and dopants in Li8SnO6. Heliyon 2021, 7, e07460. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, H.; Zeng, Y.; Zhang, Y.; Guo, H. Oxygen-defective Co3O4 for pseudo-capacitive lithium storage. J. Power Sources 2019, 439, 227026. [Google Scholar] [CrossRef]
- Wang, F.; Wang, X.; Chang, Z.; Zhu, Y.; Fu, L.; Liu, X.; Wu, Y. Electrode materials with tailored facets for electrochemical energy storage. Nanoscale Horiz. 2016, 1, 272–289. [Google Scholar] [CrossRef]
- Guo, B.; Ruan, H.; Zheng, C.; Fei, H.; Wei, M. Hierarchical LiFePO4 with a controllable growth of the (010) facet for lithium-ion batteries. Sci. Rep. 2013, 3, 2788. [Google Scholar] [CrossRef]
- Zhang, F.; Lou, S.; Li, S.; Yu, Z.; Liu, Q.; Dai, A.; Cao, C.; Toney, M.F.; Ge, M.; Xiao, X.; et al. Surface regulation enables high stability of single-crystal lithium-ion cathodes at high voltage. Nat. Comm. 2020, 11, 3050. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, E.; Zhang, X.; Yu, H. High-Voltage Single-Crystal Cathode materials for Lithium-Ion Batteries. Energy Fuels 2021, 35, 1918–1932. [Google Scholar] [CrossRef]
- Langdon, J.; Manthiram, A. A perspective on single-crystal layered oxide cathodes for lithium-ion batteries. Energy Storage Mater. 2021, 37, 143–160. [Google Scholar] [CrossRef]
- Qian, G.; Zhang, Y.; Li, L.; Zhang, R.; Xu, J.; Cheng, Z.; Xie, S.; Wang, H.; Rao, Q.; He, Y.; et al. Single crystal Nickle-rich layered oxide battery cathode materials: Synthesis, electrochemistry, and intra-granular fracture. Energy Storage Mater. 2020, 27, 140–149. [Google Scholar] [CrossRef]
- Lu, C.-H.; Lin, S.-W. Influence of the particle size on the electrochemical properties of lithium manganese oxide. J. Power Sources 2001, 97–98, 458–460. [Google Scholar] [CrossRef]
- Majdabadi, M.M.; Farhad, S.; Farkhondeh, M.; Fraser, R.A.; Fowler, M. Simplified electrochemical multi-particle model for LiFePO4 cathodes in lithium-ion batteries. J. Power Sources 2015, 275, 633–643. [Google Scholar] [CrossRef]
- Wu, S.; Yu, B.; Wu, Z.; Fang, S.; Shi, B.; Yang, J. Effect of particle size distribution on the electrochemical performance of micro-sized silicon-based negative materials. RSC Adv. 2018, 8, 8544–8551. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, Y.; Yang, S.; Qu, Q.; Zheng, H. Correlation between the physical parameters and the electrochemical performance of a silicon anode in lithium-ion batteries. J. Mater. 2019, 5, 164–175. [Google Scholar] [CrossRef]
- Garcia, J.C.; Bareno, J.; Yan, J.; Chen, G.; Hauser, A.; Croy, J.R.; Iddir, H. Surface structure, morphology, and stability of LiNi1/3Mn1/3Co1/3O2 cathode material. J. Phys. Chem. C 2017, 121, 8290–8299. [Google Scholar] [CrossRef]
- Li, H.; Ren, Y.; Yang, P.; Jian, Z.; Wang, W.; Xing, Y.; Zhang, S. Morphology, and size-controlled synthesis of the hierarchical structured Li1.2Mn0.54Ni0.13Co0.13O2 cathode materials for lithium-ion batteries. Electrochim. Acta 2019, 297, 406–416. [Google Scholar] [CrossRef]
- Wei, C.; He, W.; Zhang, X.; Xu, F.; Liu, Q.; Sun, C.; Song, X. Effect of morphology on the electrochemical performance Li3V2(PO4)3 cathode materials for lithium-ion batteries. RSC Adv. 2015, 5, 54225–54245. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; Jiang, Z.; Deb, A.; Yang, L.; Hirano, S.-I. Mesoporous Li3V2(PO4)3@CMK-3 nanocomposite cathode material for lithium-ion batteries. J. Power Sources 2014, 253, 294–299. [Google Scholar] [CrossRef]
- Muller, M.; Schneider, L.; Bohn, N.; Binder, J.R.; Bauer, W. Effect of nanostructured and open-porous particle morphology on electrodes processing and electrochemical performance of Li-ion batteries. ACS Appl. Energy Mater. 2021, 4, 1993–2003. [Google Scholar] [CrossRef]
- Seher, J.; Froba, M. Shape Matters: The effect of particle morphology on the fast-charging performance of LifePO4/C nanoparticle composite electrode. ACS Omega 2021, 6, 24062–24069. [Google Scholar] [CrossRef] [PubMed]
- Ghani, F.; Raza, A.; Kyung, D.; Kim, H.-S.; Lim, J.C.; Nah, I.W. Optimization of synthesis conditions of high-tap density FeVO4 hollow microspheres via spray pyrolysis for lithium-ion batteries. Appl. Surf. Sci. 2019, 497, 143718. [Google Scholar] [CrossRef]
- Birkl, C.R.; Roberts, M.R.; McTurk, E.; Bruce, P.G.; Howey, D.A. Degradation diagnostics for lithium-ion cells. J. Power Sources 2017, 341, 373–386. [Google Scholar] [CrossRef]
- Liu, H.; Wolfman, M.; Karki, K.; Yu, Y.-S.; Stach, E.A.; Cabana, J.; Chapman, K.W.; Chupas, P.J. Intergranular cracking as a major cause of long-term capacity fading of layered cathodes. Nano Lett. 2017, 17, 3452–3457. [Google Scholar] [CrossRef]
- Lei, Y.; Ni, J.; Hu, Z.; Wang, Z.; Gui, F.; Li, B.; Ming, P.; Zhang, C.; Elias, Y.; Aurbach, D.; et al. Surface modification of Li-rich Mn-based layered oxide cathodes: Challenges, materials, methods, and characterization. Adv. Energy Mater. 2020, 10, 2002506. [Google Scholar] [CrossRef]
- Raza, A.; Ghani, F.; Lim, J.C.; Nah, I.W.; Kim, H.-S. Eco-friendly prepared mesoporous carbon encapsulated SnO2 nanoparticles for high-reversible lithium-ion battery anodes. Microporous Mesoporous Mater. 2021, 314, 110853. [Google Scholar] [CrossRef]
- Palacin, M.R. Recent Advances in rechargeable battery materials: A chemist’s perspective. Chem. Soc. Rev. 2009, 38, 2565–2575. [Google Scholar] [CrossRef]
- Cheng, H.; Shapter, J.G.; Li, Y.; Gao, G. Recent progress of advanced anode materials of lithium-ion batteries. J. Energy Chem. 2021, 57, 451–468. [Google Scholar] [CrossRef]
- Bensalah, N.; Dawood, H. Review on synthesis, characterizations, and electrochemical properties of cathode materials for Lithium-ion batteries. J. Mater. Sci. Eng. 2016, 5, 4. [Google Scholar]
- Lyu, Y.; Wu, X.; Wang, K.; Feng, Z.; Cheng, T.; Liu, Y.; Wang, M.; Chen, R.; Xu, L.; Zhou, J.; et al. An overview on the advances of LiCoO2 cathodes for Lithium-Ion Batteries. Adv. Energy Mater. 2021, 11, 2000982. [Google Scholar] [CrossRef]
- Liu, L.; Li, M.; Chu, L.; Jiang, B.; Lin, R.; Zhu, X.; Cao, G. Layered ternary metal oxides: Performance degradation mechanisms as cathodes, and design strategies for high-performance batteries. Prog. Mater. Sci. 2020, 111, 100655. [Google Scholar] [CrossRef]
- Kim, K.H.; Jeong, H.; Lee, H.C.; Shon, J.K.; Park, J.; Park, H.-Y. Stable cycling of high-density three-dimensional sintered LiCoO2 plate cathodes. J. Power Sources 2022, 551, 232223. [Google Scholar] [CrossRef]
- Lv, Y.; Huang, S.; Zhao, Y.; Roy, S.; Lu, X.; Hou, Y.; Zhang, J. A review of nickel-rich layered oxides cathodes: Synthetic strategies, structural characteristics, failure mechanism, improvement approaches and prospects. Appl. Energy 2022, 305, 117849. [Google Scholar] [CrossRef]
- Aishova, A.; Park, G.-T.; Yoon, C.S.; Sun, Y.-K. Cobalt-free high-capacity Ni-rich layered Li [Ni0.9Mn0.1] O2 cathode. Adv. Energy Mater. 2020, 10, 1903179. [Google Scholar] [CrossRef]
- Zhang, Q.; Su, Y.; Chen, L.; Lu, Y.; Bao, L.; He, T. Pre-oxidizing the precursors of Nickel-rich cathode materials to regulate their Li+/Ni2+ cation ordering towards cyclability improvements. J. Power Sources 2018, 396, 734–741. [Google Scholar] [CrossRef]
- Sun, J.; Cao, X.; Zhou, H. Advanced single-crystal layered Ni-rich cathode materials for next-generation high-energy density and long-life Li-ion batteries. Phys. Rev. Mater. 2022, 6, 070201. [Google Scholar] [CrossRef]
- Zuo, W.; Luo, M.; Liu, X.; Wu, J.; Liu, H.; Li, J.; Winter, M.; Fu, R.; Yang, W.; Yang, Y. Li-rich cathodes for rechargeable Li-based batteries: Reaction mechanisms and advanced characterization techniques. Energy Environ. Sci. 2020, 13, 4450–4497. [Google Scholar] [CrossRef]
- Zheng, H.; Han, X.; Guo, W.; Lin, L.; Xie, Q.; Liu, P.; He, W.; Wang, L.; Peng, D.-L. Recent developments and challenges of Li-rich Mn-based cathode materials for high-energy lithium-ion batteries. Mater. Today Energy 2020, 18, 100518. [Google Scholar] [CrossRef]
- Li, Q.; Ning, D.; Wong, D.; An, K.; Tang, Y.; Zhou, D.; Schuck, G.; Chen, Z.; Zhang, N.; Liu, X. Improving the oxygen redox reversibility of Li-rich battery cathode materials via coulombic repulsive interactions strategy. Nat. Commun. 2022, 13, 1123. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Fan, X.; Zhou, X.; Chen, J.; Wang, Q.; Ma, L.; Yang, C.; Hu, E.; Yang, X.-Q.; Wang, C. Structure and interface design enable stable Li-rich cathode. J. Am. Chem. Soc. 2020, 142, 8918–8927. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.T.; Rui, X.; Sun, W.; Yan, Q.; Lim, T.M. Vanadium-based nanostructure materials for secondary lithium battery applications. Nanoscale 2015, 7, 14595. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Rui, X.; Hng, H.H.; Yan, Q. Vanadium pentaoxide-based cathode materials for Lithium-Ion Batteries: Morphology control, carbon hybridization, and cation doping. Part. Part. Syst. Charact. 2015, 32, 276–294. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, S.; Chen, D.; Jiang, Y.; Ang, E.H.; Liu, W.; Feng, Y.; Rui, X.; Yu, Y. Vanadate-based electrodes for rechargeable batteries. Mater. Chem. Front. 2021, 5, 1585. [Google Scholar] [CrossRef]
- Radzi, Z.I.; Arifin, K.H.; Kufian, M.Z.; Balakrishnan, V.; Raihan, S.R.S.; Rahim, N.A.; Subramanium, R. Review of spinal LiMn2O4 cathode materials under high cut-off voltage in lithium-ion batteries: Challenges and strategies. J. Electroanal. Chem. 2022, 920, 116623. [Google Scholar] [CrossRef]
- Ma, J.; Hu, P.; Cui, G.; Chen, L. Surface and Interface Issues in spinel LiNi0.5Mn1.5O4: Insights into a potential cathode material for High-energy density lithium-ion batteries. Chem. Mater. 2016, 28, 3578–3606. [Google Scholar] [CrossRef]
- Xu, X.; Deng, S.; Wang, H.; Liu, J.; Yan, H. Research progress in improving the cycling stability of high-voltage LiNi0.5Mn1.5O4 cathode in Lithium-Ion Battery. Nano-Micro Lett. 2017, 9, 22. [Google Scholar] [CrossRef]
- Ling, J.; Karuppiah, C.; Krishnan, S.G.; Reddy, M.V.; Misnon, I.I.; Rahim, M.H.A.; Yang, C.-C.; Jose, R. Phosphate polyanion materials as high-voltage lithium-ion battery cathodes: A Review. Energy Fuels 2021, 35, 10428–10450. [Google Scholar] [CrossRef]
- Peng, Y.; Tan, R.; Ma, J.; Li, Q.; Wang, T.; Duan, X. Electrospun Li3V2(PO4)3 nanocubes/carbon nanofibers as free-standing cathodes for high-performance lithium-ion batteries. J. Mater. Chem. A 2019, 7, 14681–14688. [Google Scholar] [CrossRef]
- Hu, J.; Huang, W.; Yang, L.; Pan, F. Structure and performance of the LiFePO4 cathode material: From the bulk to the surface. Nanoscale 2020, 12, 15036–15044. [Google Scholar] [CrossRef] [PubMed]
- Ramsubramanian, B.; Sundarrajan, S.; Chellappan, V.; Reddy, M.V.; Ramakrishna, S.; Zaghib, K. Recent development in carbon- LiFePO4 cathode for Lithium-Ion Batteries: A Mini-Review. Batteries 2022, 8, 133. [Google Scholar] [CrossRef]
- Islam, M.S.; Dominko, R.; Masquelier, C.; Sirisopanaporn, C.; Armstrong, A.R.; Bruce, P.G. Silicate cathodes for lithium batteries: Alternatives to phosphates? J. Mater. Chem. 2011, 21, 9811–9818. [Google Scholar] [CrossRef]
- Bao, L.; Gao, W.; Su, Y.; Wang, Z.; Li, N.; Chen, S.; Wu, F. Progression of the silicate cathode materials used in lithium-ion batteries. Chin. Sci. Bull. 2013, 58, 575–584. [Google Scholar] [CrossRef]
- Yang, S.-H.; Xue, H.; Guo, S.-P. Borates as promising electrode materials for rechargeable batteries. Coord. Chem. Rev. 2021, 427, 213551. [Google Scholar] [CrossRef]
- Ma, T.; Muslim, A.; Su, Z. Microwave synthesis and electrochemical properties of lithium manganese borate as cathode for lithium-ion batteries. J. Power Sources 2015, 282, 95–99. [Google Scholar] [CrossRef]
- Daniel, C.; Mohanty, D.; Li, J.; Wood, D.L. Review on Electrochemical Storage Materials and Technology. AIP Conf. Proc. 2014, 1597, 26–43. [Google Scholar]
- Ghosh, S.; Bhattacharjee, U.; Bhowmik, S.; Martha, S.K. A Review on High-Capacity and High-Voltage Cathodes for Next-Generation Lithium-ion Batteries. J. Energy Power Technol. 2022, 1, 2. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material-fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387. [Google Scholar] [CrossRef]
- Chang, H.; Wu, Y.-R.; Han, X.; Yi, T.-F. Recent developments in advanced anode materials for lithium-ion batteries. Energy Mater. 2021, 1, 100003. [Google Scholar] [CrossRef]
- Zhao, W.; Choi, W.; Yoon, W.-S. Nanostructured Electrode materials for rechargeable Lithium-Ion batteries. J. Electrochem. Sci. Technol. 2020, 11, 195–219. [Google Scholar] [CrossRef]
- Ma, D.; Cao, Z.; Hu, A. Si-based Anode materials for Li-ion batteries: A mini review. Nano-Micro Lett. 2014, 6, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Bresser, D.; Passerini, S. Transition metal oxide anodes for electrochemical energy storage in Lithium- and Sodium-Ion Batteries. Adv. Energy Mater. 2020, 10, 1902485. [Google Scholar] [CrossRef]
- Nzereogu, P.U.; Omah, A.D.; Ezema, F.I.; Iwuoha, E.I.; Nwanya, A.C. Anode materials for lithium-ion batteries: A review. Appl. Surf. Sci. Adv. 2022, 9, 100233. [Google Scholar] [CrossRef]
- Parikh, P.; Sina, M.; Banerjee, A.; Wang, X.; D’Souza, M.S.; Doux, J.-M.; Wu, E.A.; Trieu, O.Y.; Gong, Y.; Zhou, Q.; et al. Role of polyacrylic acid (PAA) Binder on the solid electrolyte interphase in silicon anodes. Chem. Mater. 2019, 31, 2535–2544. [Google Scholar] [CrossRef]
- Jiang, S.; Hu, B.; Shi, Z.; Chen, W.; Zhang, Z.; Zhang, L. Re-Engineering Poly (Acrylic Acid) Binder toward optimized electrochemical performance for silicon lithium-ion batteries: Branching Architecture leads to balanced properties of polymeric binders. Adv. Funct. Mater. 2020, 30, 1908558. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Qi, Y.; Sun, T.; Li, X. Unveiling the roles of binder in the mechanical integrity of electrodes for Lithium-ion batteries. J. Electrochem. Soc. 2013, 160, A1502–A1509. [Google Scholar] [CrossRef]
- Dong, T.; Mu, P.; Zhang, S.; Zhang, H.; Liu, W.; Cui, G. How Do Polymer Binders Assist Transition Metal Oxide Cathodes to Address the Challenge of High-Voltage Lithium Battery Applications? Electrochem. Energy Rev. 2021, 4, 545–565. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, X.; Yu, G. Material and Structural Design of Novel Binder Systems for High-Energy, High-power Lithium-ion batteries. Acc. Chem. Res. 2017, 50, 2642–2652. [Google Scholar] [CrossRef]
- Kazzazi, A.; Bresser, D.; Birrozzi, A.; van Zamory, J.; Hekmatfar, M.; Passerini, S. Comparative analysis of aqueous binders for high-energy Li-rich NMC as a lithium-ion cathode and the impact of adding phosphoric acid. ACS Appl. Mater. Interfaces 2018, 10, 17214–17222. [Google Scholar] [CrossRef]
- He, J.; Wei, Y.; Hu, L.; Li, H.; Zhai, T. Aqueous binders enhanced high-performance GeP5 anode for lithium-ion batteries. Front. Chem. 2018, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Bresser, D.; Buchholz, D.; Moretti, A.; Varzi, A.; Passerini, S. Alternative binders for sustainable electrochemical energy storage-the transition to aqueous electrode processing and bio-derived polymers. Energy Environ. Sci. 2018, 11, 3096–3127. [Google Scholar] [CrossRef]
- Fitz, O.; Ingenhoven, S.; Bischoff, C.; Gentischer, H.; Birke, K.P.; Saracsan, D.; Biro, D. Comparison of aqueous and non-aqueous based binder polymers and the mixing ratio of Zn//MnO2 batteries with mildly acidic aqueous electrolytes. Batteries 2021, 7, 40. [Google Scholar] [CrossRef]
- Wang, R.; Feng, L.; Yang, W.; Zhang, Y.; Zhang, Y.; Bai, W.; Liu, B.; Zhang, W.; Chuan, Y.; Zheng, Z.; et al. Effect of different binders on the electrochemical performance of metal oxide anode for lithium-ion batteries. Nanoscale Res Lett. 2017, 12, 575. [Google Scholar] [CrossRef]
- Xing, J.; Bliznakov, S.; Bonville, L.; Oljace, M.; Maric, R. A review of non-aqueous electrolytes, binders, and separators for lithium-ion batteries. Electrochem. Energy Rev. 2022, 5, 14. [Google Scholar] [CrossRef]
- Cholewinski, A.; Si, P.; Uceda, M.; Pope, M.; Zhao, B. Polymer Binders: Characterization and development towards aqueous electrode fabrication for sustainability. Polymers 2021, 13, 631. [Google Scholar] [CrossRef]
- Yim, T.; Choi, S.J.; Jo, Y.N.; Kim, T.-H.; Kim, K.J.; Jeong, G.; Kim, Y.-J. Effect of binder properties on electrochemical performance for silicon-graphite anode: Method and application of binder screening. Electrochim. Acta 2014, 136, 112–120. [Google Scholar] [CrossRef]
- Rapisarda, M.; Marken, F.; Meo, M. Graphene oxide and starch gel as a hybrid binder for environmentally friendly high-performance supercapacitors. Commun. Chem. 2021, 4, 169. [Google Scholar] [CrossRef] [PubMed]
- Kannan, D.R.R.; Terala, P.K.; Moss, P.L.; Weatherspoon, M.H. Analysis of the separator thickness and porosity on the performance of lithium-ion batteries. Intl. J. Electrochem. 2018, 2018, 1925708. [Google Scholar]
- Kim, P.J. Surface-functionalized separator for stable and reliable lithium-ion batteries: A review. Nanomaterials 2021, 11, 2275. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, K. Rational design on separators and liquid electrolytes for safer lithium-ion batteries. J. Energy Chem. 2020, 43, 58–70. [Google Scholar] [CrossRef]
- Chen, K.; Li, Y.; Zhan, H. Advanced separators for lithium-ion batteries. Earth Environ. Sci. 2022, 1011, 012009. [Google Scholar] [CrossRef]
- Ding, L.; Yan, N.; Zhang, S.; Xu, R.; Wu, T.; Wang, F.; Cao, Y.; Xiang, M. Facile manufacture technique for lithium-ion batteries composite separator via online construction of fumed SiO2 coating. Mater. Des. 2022, 215, 110476. [Google Scholar] [CrossRef]
- Kim, G.; Noh, J.H.; Lee, H.; Shin, J.; Lee, D. Roll-to-Roll Gravure Coating of PVDF on a Battery Separator for the Enhancement of Thermal Stability. Polymers 2023, 15, 4108. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiang, Y.; Hu, Q.; Guo, S.; Yu, L.; Li, Q.; Liu, Q.; Hu, X. Safer lithium-ion batteries from separator aspect: Development and future perspective. Energy Environ. Mater. 2021, 4, 336–362. [Google Scholar] [CrossRef]
- Huang, X.; He, R.; Li, M.; Chee, M.O.L.; Dong, P.; Lu, J. Functionalized separator for next generation batteries. Mat. Today 2020, 41, 143–155. [Google Scholar] [CrossRef]
- Lagadec, M.F.; Zahn, R.; Wood, V. Designing Polyolefin separators to minimize the impact of local compressive stresses on lithium-ion battery performance. J. Electrochem. Soc. 2018, 165, A1829–A1836. [Google Scholar] [CrossRef]
- Zhu, P.; Gastol, D.; Marshall, J.; Sommerville, R.; Goodship, V.; Kendrick, E. A review of current collectors for lithium-ion batteries. J. Power Sources 2021, 485, 229321. [Google Scholar] [CrossRef]
- Yamada, M.; Watanbe, T.; Gunji, T.; Wu, J.; Matsumoto, F. Review of the design of current collectors for improving the battery performance in lithium-ion and post lithium-ion batteries. Electrochem 2020, 1, 124–159. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef]
- Zhang, S.; Jow, T. Aluminum corrosion in electrolyte of Li-ion battery. J. Power Sources 2002, 109, 458–464. [Google Scholar] [CrossRef]
- Kumar, P.S.; Ayyasamy, S.; Tok, E.S.; Adams, S.; Reddy, M. Impact of electrical conductivity on the electrochemical performance of layered structured Lithium Trivanadate (LiV3-xMxO8, M = Zn/Co/Fe/Sn/Ti/Zr/Nb/Mo, x = 0.01–0.1) as cathode material for energy storage. ACS Omega 2018, 3, 3036–3044. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Okano, S.; Yaguma, N.; Morinaga, Y.; Takahara, H.; Yamada, Y. Electrochemical performance of cathodes prepared on current collector with different surface morphologies. J. Power Sources 2013, 244, 532–537. [Google Scholar] [CrossRef]
- Doberdo, I.; Loffler, N.; Laszczynski, N.; Cericola, D.; Penazzi, N.; Bodoardo, S.; Kim, G.-T.; Passerini, S. Enabling aqueous binder for lithium battery cathodes-carbon coating of aluminum current collector. J. Power Sources 2014, 248, 1000–1006. [Google Scholar] [CrossRef]
- Boz, B.; Dev, T.; Salvadori, A.; Schaefer, J.L. Review-Electrolyte and Electrode Designs for enhanced ion transport properties to enable high performance lithium batteries. J. Electrochem. Soc. 2021, 168, 090501. [Google Scholar] [CrossRef]
- Meutzner, F.; de Vivanco, M.U. Electrolytes-Technology review. AIP Conf. Proc. 2014, 1597, 185–195. [Google Scholar]
- Chagnes, A.; Swiatowska, J. Lithium Process Chemistry: Resources, Extractions, Batteries and Recycling, 1st ed.; Elsevier: Amsterdam, The Netherland, 2015; Volume 5, pp. 167–189. [Google Scholar]
- Zhang, H.; Liu, X.; Li, H.; Hasa, I.; Passerini, S. Challenges and Strategies for high-energy Aqueous Electrolytes rechargeable batteries. Angew. Chem. Int. Ed. 2021, 60, 598–616. [Google Scholar] [CrossRef]
- Luo, J.-Y.; Cui, W.-J.; He, P.; Xia, Y.-Y. Raising the cycling stability of aqueous lithium-ion batteries by eliminating oxygen in the electrolyte. Nat. Chem. 2010, 2, 760–765. [Google Scholar] [CrossRef]
- He, P.; Zhang, X.; Wang, Y.-G.; Cheng, L.; Xia, Y.-Y. Lithium-ion intercalation behavior of LiFePO4 in aqueous and nonaqueous electrolyte solutions. J. Electrochem. Soc. 2007, 155, A144. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Jiang, N.; Li, X.; Pasupath, S.; Fang, Y.; Liu, Q.; Dang, D. Rational Design of an Ionic Liquid-based electrolyte with High ionic conductivity towards safe Lithium/Lithium-Ion batteries. Chem. Asian J. 2019, 14, 2810–2814. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-Liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Chen, B.; Koo, Y.-M.; MacFarlane, D.R. Introduction: Ionic Liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Jiang, K.; Yu, G.; Chen, X.; Zhang, C.; Yao, Y.; Jiang, B.; Long, H. Recent progress of composite solid polymer electrolytes for all solid-state lithium metal batteries. Chin. Chem. Lett. 2021, 32, 2659–2678. [Google Scholar] [CrossRef]
- Sasikumar, M.; Krishna, R.H.; Raja, M.; Therese, H.A.; Balakrishna, N.T.M.; Raghavan, P.; Sivakumar, P. Titanium dioxide nano-ceramic filler in solid polymer electrolytes: Strategy towards suppressed dendrite formation and enhanced electrochemical performance for safe lithium-ion batteries. J. Alloys Compd. 2021, 882, 160709. [Google Scholar] [CrossRef]
- Suo, L.; Borodin, O.; Gao, T.; Olguin, M.; Ho, J.; Fan, X.; Luo, C.; Wang, C.; Xu, K. Water in salt electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 2015, 350, 938–943. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, M.; Zou, P.; Sun, B.; Tao, S. Historical development, and novel concepts on electrolytes for aqueous rechargeable batteries. Energy Environ. Sci. 2022, 15, 1805–1839. [Google Scholar] [CrossRef]
- Suo, L.; Oh, D.; Lin, Y.; Zhuo, Z.; Borodin, O.; Gao, T.; Wang, F.; Kushima, A.; Wang, Z.; Kim, H.-C.; et al. How solid-electrolyte interphase forms in aqueous electrolytes. J. Am. Chem. Soc. 2017, 139, 18670–18680. [Google Scholar] [CrossRef]
- Yue, F.; Tie, Z.; Deng, S.; Wang, S.; Yang, M.; Niu, Z. An Ultralow Temperature Aqueous Battery with Proton Chemistry. Angew. Chem. Int. Ed. 2021, 60, 13882–13886. [Google Scholar] [CrossRef]
- Sun, T.; Du, H.; Zheng, S.; Shi, J.; Tao, Z. High Power and Energy Density Aqueous Proton Battery Operated at −90 °C. Adv. Funct. Mater. 2021, 31, 2010127. [Google Scholar] [CrossRef]
- Wang, F.; Borodin, O.; Ding, M.S.; Gobet, M.; Vatamanu, J.; Fan, X.; Gao, T.; Eidson, N.; Liang, Y.; Sun, W.; et al. Aqueous/Non-aqueous electrolyte for safe and high-energy Li-Ion batteries. Joule 2018, 2, 927–937. [Google Scholar] [CrossRef]
- Chagnes, A.; Swiatowska, J. New Developments in Lithium-Ion Batteries, 1st ed.; Belharouak, I., Ed.; InTech: Vienna, Austria, 2012; Volume 6, pp. 145–172. [Google Scholar]
- Hu, L.; Zhang, Z.; Amine, K. Electrochemical investigation of carbonate-based electrolytes for high voltage lithium-ion cells. J. Power Sources 2013, 236, 175–180. [Google Scholar] [CrossRef]
- Yang, L.; Ravdel, B.; Lucht, B.L. Electrolyte Reaction with the surface of high voltage LiNi0.5Mn1.5O4 cathodes for Lithium-ion batteries. Electrochem. Solid State Lett. 2010, 13, A95–A97. [Google Scholar] [CrossRef]
- Flamme, B.; Garcia, G.R.; Weil, M.; Haddad, M.; Phansavath, P.; Vidal, V.R.; Chagnes, A. Guidelines to design organic electrolytes for lithium-ion batteries: Environmental impact, physicochemical and electrochemical properties. Green Chem. 2017, 19, 1828–1849. [Google Scholar] [CrossRef]
- Abe, K.; Ushigoe, Y.; Yoshitake, H.; Yoshio, M. Functional electrolytes: Novel type additives for cathode materials, providing high cycleability performance. J. Power Sources 2006, 153, 328–335. [Google Scholar] [CrossRef]
- Assary, R.S.; Curtiss, L.A.; Redfern, P.C.; Zhang, Z.; Amine, K. Computational Studies of Polysiloxanes: Oxidation Potentials and Decomposition Reactions. J. Phys. Chem. C 2011, 115, 12216–12223. [Google Scholar] [CrossRef]
- Nanbu, N.; Suzuki, Y.; Ohtsuki, K.; Meguro, T.; Takehara, M.; Ue, M.; Sasaki, Y. Physical and Electrochemical Properties of Monofluorinated Ethyl Acetates for Lithium Rechargeable Batteries. Electrochemistry 2010, 78, 446–449. [Google Scholar] [CrossRef]
- McElroy, P.J.; Gellen, A.T.; Kolahi, S.S. Excess second virial coefficients and critical temperatures of methyl acetate and diethyl sulfide. J. Chem. Eng. Data 1990, 35, 38. [Google Scholar] [CrossRef]
- Laurence, C.; Nicolet, P.; Dalati, M.T.; Abboud, J.-L.M.; Notario, R. The empirical treatment of solvent-solute interactions: 15 years of π*. J. Phys. Chem. 1994, 98, 5807–5816. [Google Scholar] [CrossRef]
- Obama, M.; Oodera, Y.; Kohama, N.; Yanase, T.; Saito, Y.; Kusano, K. Densities, molar volumes, and cubic expansion coefficients of 78 aliphatic ethers. J. Chem. Eng. Data 1985, 30, 1–5. [Google Scholar] [CrossRef]
- Dutt, H.D.P.H.; Jeewan, R. Dielectric relaxation studies of oligether of ethylene glycol at microwave frequencies. Bull. Chem. Soc. Jpn. 1991, 64, 2030–2031. [Google Scholar]
- Tobishima, S.-I.; Okada, T. Lithium cycling efficiency and conductivity for high dielectric solvent/low viscosity solvent mixed systems. Electrochim. Acta 1985, 30, 17151722. [Google Scholar] [CrossRef]
- Mozhzhukhina, N.; Méndez De Leo, L.P.; Calvo, E.J. Infrared spectroscopy studies on stability of dimethyl sulfoxide for application in a Li-Air Battery. J. Phys. Chem. C 2013, 117, 18375–18380. [Google Scholar] [CrossRef]
- Bennion, D.N.; Tiedemann, W.H. Density, viscosity, and conductivity of lithium trifluoromethanesulfonate solutions in dimethylsulfite. J. Chem. Eng. Data 1971, 16, 368. [Google Scholar] [CrossRef]
- Svirbely, W.J.; Lander, J.J. The dipole moments of diethyl sulfite, triethyl phosphate and tetraethyl silicate. J. Am. Chem. Soc. 1948, 70, 4121. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Vatamanu, J.; Xing, L.; Borodin, O.; Chen, H.; Guan, X.; Liu, X.; Xu, K.; Li, W. Improving electrochemical stability and low-temperature performance with water/acetonitrile hybrid electrolyte. Adv. Energy Mater. 2020, 10, 1902654. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Fan, L.; Kong, X.; Lu, Y. Progress in electrolytes for rechargeable Li-based batteries and beyond. Green Energy Environ. 2016, 1, 18–42. [Google Scholar] [CrossRef]
- Pan, S.; Yao, M.; Zhang, J.; Li, B.; Xing, C.; Song, X.; Su, P.; Zhang, H. Recognition of ionic liquids as high-voltage electrolytes for supercapacitors. Front. Chem. 2020, 8, 261. [Google Scholar] [CrossRef]
- Ahmad, S. Polymer Electrolyte: Characteristics and peculiarities. Ionics 2009, 15, 309–321. [Google Scholar] [CrossRef]
- Xi, G.; Xiao, M.; Wang, S.; Han, D.; Li, Y.; Meng, Y. Polymer-based Solid Electrolytes: Material Selection, design, and Application. Adv. Funct. Mater. 2021, 31, 2007598. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Zhou, Y.; Liang, Z.; Tavajohi, N.; Li, B.; Li, T. Solid Polymer Electrolytes with High Conductivity, and transference number of Li ions for Li-based rechargeable Batteries. Adv. Sci. 2021, 8, 2003675. [Google Scholar] [CrossRef] [PubMed]
- Mackanic, D.G.; Yan, X.; Zhang, Q.; Matsuhisa, N.; Yu, Z.; Jiang, Y.; Manika, T.; Lopez, J.; Yan, H.; Chen, X.; et al. Decoupling of mechanical properties and ionic conductivity in supramolecular lithium-ion conductors. Nat. Commun. 2019, 10, 5384. [Google Scholar] [CrossRef]
- An, Y.; Han, X.; Liu, Y.; Azhar, A.; Na, J.; Nanjundan, A.K.; Wang, S.; Yu, J.; Yamauchi, Y. Progress in Solid polymer electrolytes for lithium-ion batteries and beyond. Small 2022, 18, 2103617. [Google Scholar] [CrossRef]
- Aravindan, V.; Gnanaraj, J.; Madhavi, S.; Liu, H.-K. Lithium-ion conducting electrolyte salts for lithium batteries. Chem.-Eur. J. 2011, 17, 14326. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.C.; Goncalves, R.; Costa, C.M.; Bermudez, V.D.; Marijuan, A.F.; Zhang, Q.; Mendez, S.L. Metal-organic framework, and zeolite materials as active fillers for lithium-ion battery solid polymer electrolytes. Mater. Adv. 2021, 2, 3790–3805. [Google Scholar] [CrossRef]
- Mazzapioda, L.; Sgambetterra, M.; Tsurumaki, A.; Navarra, M.A. Different approaches to obtain functionalized alumina as additive in polymer electrolyte membranes, Different approaches to obtain functionalized alumina as additive in polymer electrolyte membranes. J. Solid State Electrochem. 2022, 26, 17–27. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, Y.; Li, H.; He, P. The Development of a new type of rechargeable batteries based on Hybrid electrolytes. ChemSusChem 2010, 3, 1009–1019. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Han, L.; Lehmann, M.L.; Zhu, J.; Liu, T.; Zhou, Z.; Tang, X.; Heish, C.-T.; Sokolov, A.P.; Cao, P.; Chen, X.C.; et al. Recent developments, and challenges in hybrid solid electrolytes for Lithium-ion batteries. Front. Energy Res. 2020, 8, 202. [Google Scholar] [CrossRef]
- Kwon, T.; Choi, I.; Park, M.J. Highly Conductive Solid-state hybrid electrolytes operating at subzero temperature. ACS Appl. Mater. Interfaces 2017, 9, 24250–24258. [Google Scholar] [CrossRef]
- Janek, J.; Zeier, W.G. A solid future battery development. Nat. Energy 2016, 1, 16141. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Q.; Zhang, H.; Cheng, J.; Li, Y.; Zeng, Z.; Zhang, S.; Xu, X.; Ji, F.; Li, D.; et al. The application road of silicon-based anode in lithium-ion batteries: From liquid electrolyte to solid-state electrolyte. Energy Storage Mater. 2023, 55, 244–263. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast Lithium Ion Conduction in Garnet-Type Li7La3Zr2O12. Angew. Chem. Int. Ed. 2007, 46, 7778–7781. [Google Scholar] [CrossRef]
- Kim, K.H.; Iriyama, Y.; Yamamoto, K.; Kumazaki, S.; Asaka, T.; Tanabe, K.; Fisher, C.A.J.; Hirayama, T.; Murugan, R.; Ogumi, Z. Characterization of the interface between LiCoO2 and Li7La3Zr2O12 in an all-solid-state rechargeable lithium battery. J. Power Sources 2011, 196, 764–767. [Google Scholar] [CrossRef]
- Ihrig, M.; Kuo, L.-Y.; Lobe, S.; Laptev, A.M.; Lin, C.-A.; Tu, C.-H.; Ye, R.; Kaghazchi, P.; Cressa, L.; Eswara, S.; et al. Thermal Recovery of the Electrochemically Degraded LiCoO2/Li7La3Zr2O12:Al,Ta Interface in an All-Solid-State Lithium Battery. ACS Appl. Mater. Interfaces 2023, 15, 4101–4112. [Google Scholar] [CrossRef]
- Teng, Y.; Liu, H.; Wang, Q.; He, Y.; Hua, Y.; Li, C.; Bai, J. In-doped Li7La3Zr2O12 nanofibers enhances electrochemical properties and conductivity of PEO-based composite electrolyte in all-solid-state lithium battery. J. Energy Storage 2024, 76, 109784. [Google Scholar] [CrossRef]
- Morimoto, H.; Yamashita, H.; Tatsumisago, M.; Minami, T. Mechanochemical Synthesis of New Amorphous Materials of 60Li2S·40SiS2 with High Lithium Ion Conductivity. J. Am. Ceram. Soc. 1999, 82, 1352–1354. [Google Scholar] [CrossRef]
- Wheaton, J.; Martin, S.W. Impact of impurities on the thermal properties of a Li2S–SiS2–LiPO3 glass. Appl. Glass Sci. 2024, 15, 317–328. [Google Scholar] [CrossRef]
- Olson, M.; Kmiec, S.; Riley, N.; Oldham, N.; Krupp, K.; Manthiram, A.; Martin, S.W. Structure and Properties of Na2S−SiS2−P2S5−NaPO3 Glassy Solid Electrolytes. Inorg. Chem. 2024, 63, 9129–9144. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Xue, C.; Xin, C.; Lin, Y.; Shen, Y.; Li, L.; Nan, C.-W. Self-Suppression of Lithium Dendrite in All-Solid-State Lithium Metal Batteries with Poly(vinylidene difluoride)-Based Solid Electrolytes. Adv. Mater. 2019, 31, 186082. [Google Scholar] [CrossRef]
- Tang, Y.; Xiong, Y.; Wu, L.; Xiong, X.; Me, T.; Wang, X. A Solid-State Lithium Battery with PVDF−HFP-Modified Fireproof Ionogel Polymer Electrolyte. ACS Appl. Energy Mater. 2023, 6, 4016–4026. [Google Scholar] [CrossRef]
- Tran, H.K.; Truong, B.T.; Zhang, B.-R.; Jose, R.; Chang, J.-K.; Yang, C.-C. Sandwich-Structured Composite Polymer Electrolyte Based on PVDF-HFP/PPC/Al-Doped LLZO for High-Voltage Solid-State Lithium Batteries. ACS Appl. Energy Mater. 2023, 6, 1475–1487. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Jiao, X.; Kapitanova, O.O.; Song, Z.; Xiong, S. Role of Interfacial Defects on Electro–Chemo–Mechanical Failure of Solid-State Electrolyte. Adv. Mater. 2023, 35, 2301152. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Xu, X.; Jiao, X.; Wang, Y.; Kapitanova, O.O.; Song, Z.; Liu, Y. Mechanical Failure of Solid-State Electrolyte Rooted in Synergy of Interfacial and Internal Defects. Adv. Energy Mater. 2023, 13, 2203614. [Google Scholar] [CrossRef]
- Akhtar, S.; Lee, W.; Kim, M.; Park, M.S.; Yoon, W.-S. Conduction Mechanism of charge carriers in electrodes and design factors for the improvement of charge conduction in Li-Ion batteries. J. Electrochem. Sci. Technol. 2021, 12, 1–20. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, L.; Xie, C.; Wang, D.; Zou, Y.; Chen, R.; Wang, Y.; Jia, C.; Wang, S. Defect Engineering on Electrode Materials for rechargeable batteries. Adv. Mater. 2020, 32, 1905923. [Google Scholar] [CrossRef]
- Guo, W.; Meng, Y.; Hu, Y.; Wu, X.; Ju, Z.; Zhuang, Q. Surface, and Interface modification of electrode materials for lithium-ion batteries with organic liquid electrolytes. Front. Energy Res. 2020, 8, 170. [Google Scholar] [CrossRef]
- Nasir, U.; Muralidharan, N.; Essehli, R.; Amin, R.; Belharouak, I. Valuation of Surface Coatings in High-Energy Density Lithium-Ion battery cathode Materials. Energy Storage Mater. 2021, 38, 309–328. [Google Scholar]
- Vu, A.; Qian, Y.; Stein, A. Porous Electrode Materials for Lithium-Ion Batteries How to Prepare Them and What makes them Special. Adv. Energy Mater. 2012, 2, 1056–1085. [Google Scholar] [CrossRef]
- Du, H.-L.; Jeong, M.-G.; Lee, Y.-S.; Choi, W.; Lee, J.K.; Oh, I.-H.; Jung, H.-G. Coating lithium titanate with nitrogen-doped carbon by simple refluxing for high-power lithium-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 10250–10257. [Google Scholar] [CrossRef]
- Xu, K.; Wang, C. Batteries: Widening voltages windows. Nat. Energy 2016, 1, 16161. [Google Scholar] [CrossRef]
- Gao, J.; Shi, S.-Q.; Li, H. Brief overview of electrochemical potential in lithium-ion batteries. Chin. Phys. B 2016, 25, 018210. [Google Scholar] [CrossRef]
- Ven, A.V.D.; Bhattacharya, J.; Belak, A.A. Understanding Li Diffusion in Li-ion Intercalation compounds. Acc. Chem. Res. 2013, 46, 1216–1225. [Google Scholar]
- Dong, C.; Xu, F.; Chen, L.; Chen, Z.; Cao, Y. Design Strategies for High-Voltage Aqueous batteries. Small Struct. 2021, 2, 2100001. [Google Scholar] [CrossRef]
- Melot, B.C.; Tarascon, J.-M. Design and Preparation of Materials for Advanced Electrochemical Storage. Acc. Chem. Res. 2013, 46, 1226–1238. [Google Scholar] [CrossRef] [PubMed]
- Masquelier, C.; Croguennec, L. Polyanionic (Phosphates, Silicates, Sulfates) Frameworks as Electrode materials for rechargeable Li (or Na) Batteries. Chem. Rev. 2013, 113, 6552–6591. [Google Scholar] [CrossRef]
- Liu, C.; Neale, Z.G.; Cao, G. Understanding electrochemical potentials of cathode materials in rechargeable batteries. Mat. Today 2016, 19, 109–123. [Google Scholar] [CrossRef]
- Yazami, R. Nanomaterials for Lithium-Ion Batteries: Fundamentals and Applications; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2013. [Google Scholar]
- Park, J.-K. Principles, and Applications of Lithium Secondary Batteries; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Hahn, B.P.; Long, J.W.; Rolison, D.R. Something from Nothing: Enhancing Electrochemical Charge Storage with Cation Vacancies. Acc. Chem. Res. 2013, 46, 1181–1191. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Y.; Li, W.; Ma, B.; Chen, X. Rational material design for ultrafast rechargeable lithium-ion batteries. Chem. Soc. Rev. 2015, 44, 5926–5940. [Google Scholar] [CrossRef]
- Kim, I.G.; Ghani, F.; Lee, K.-Y.; Park, S.; Kwak, S.; Kim, H.-S.; Nah, I.W.; Lim, J.C. Electrochemical performance of Mn2O3 nanorods by N-doped reduced graphene oxide using ultrasonic spray pyrolysis for lithium storage. Intl. J. Energy Res. 2020, 44, 11171–11184. [Google Scholar] [CrossRef]
- Cao, K.; Jin, T.; Yang, L.; Jiao, L. Recent progress in Conversion reaction metal oxide anodes for Li-ion batteries. Mater. Chem. Front. 2017, 1, 2213–2242. [Google Scholar] [CrossRef]
- Ghani, F.; Nah, I.W.; Kim, H.-S.; Lim, J.C.; Marium, A.; Ijaz, M.F.; Rana, A.H.S. Facile one-step hydrothermal synthesis of rGO@Ni3V2O8 interconnected hollow microspheres composite for lithium-ion batteries. Nanomaterials 2020, 10, 2389. [Google Scholar] [CrossRef] [PubMed]
- He, L.-P.; Sun, S.-Y.; Song, X.-F.; Yu, J.-G. Recovery of cathode materials and Al from spent lithium-ion batteries by ultrasonic cleaning. Waste Manag. 2015, 46, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cheng, X.; Chong, Y.; Yuan, H.; Huang, J.-Q.; Zhang, Q. Advanced electrode processing of lithium-ion batteries: A review of powder technology in battery fabrication. Particuology 2021, 57, 56–71. [Google Scholar] [CrossRef]
- Goncalves, R.; Mendez, S.L.; Costa, C.M. Electrode fabrication process and its influence in lithium-ion battery performance: State of the art and future trends. Electrochem. Comm. 2022, 135, 107210. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, R.; Wang, J.; Wang, Y. Current and future lithium-ion battery manufacturing. iScience 2021, 24, 102332. [Google Scholar] [CrossRef]
- Gonçalves, R.; Dias, P.; Hilliou, L.; Costa, P.; Silva, M.M.; Costa, C.M.; Galvan, S.C.; Mendez, S.L. Optimized printed cathode electrodes for high performance batteries. Energy Technol. 2021, 9, 2000805. [Google Scholar] [CrossRef]
- Lalau, C.C.; Low, C.T.J. Electrophoretic deposition for lithium-ion battery electrode manufacture. Batter. Supercaps 2019, 2, 551–559. [Google Scholar] [CrossRef]
- Goren, A.; Juarez, D.C.; Martins, P.; Ferdov, S.; Silva, M.M.; Tirado, J.L.; Costa, C.M.; Mendez, S.L. Influence of solvent evaporation rate in the preparation of carbon-coated lithium iron phosphate cathode films on battery performance. Energy Technol. 2016, 4, 573–582. [Google Scholar] [CrossRef]
- Meyer, C.; Bockholt, H.; Haselrieder, W.; Kwade, A. Characterization of the calendaring process for compaction of electrodes for lithium-ion batteries. J. Mater. Process. Technol. 2017, 249, 172–178. [Google Scholar] [CrossRef]
- Jeon, D.H. Wettability in electrodes and its impact on the performance of lithium-ion batteries. Energy Storage Mater. 2019, 18, 139–147. [Google Scholar] [CrossRef]
- Robles, D.J.; Vyas, A.A.; Fear, C.; Jeevarajan, J.A.; Mukherjee, P.P. Overcharge and Aging Analytics of Li-Ion Cells. J. Electrochem. Soc. 2020, 167, 090547. [Google Scholar] [CrossRef]
- Qian, J.; Liu, L.; Yang, J.; Li, S.; Wang, X.; Zhuang, H.L.; Lu, Y. Electrochemical surface passivation of LiCoO2 particles at ultrahigh voltage and its applications in lithium-based batteries. Nat. Commun. 2018, 9, 4918. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Y.; Guo, C.; Liu, H.; Bao, W.; Li, J.; Zhang, P.; Wang, F. Spinal LiMn2O4 cathode materials in wide voltage window: Single-Crystalline versus Polycrystalline. Crystals 2022, 12, 317. [Google Scholar] [CrossRef]
- Hu, L.-H.; Wu, F.-Y.; Lin, C.; Khlobystov, A.N.; Li, L.-J. Graphene-modified LiFePO4 cathode for lithium-ion battery beyond theoretical capacity. Nat. Commun. 2013, 4, 1687. [Google Scholar] [CrossRef]
- Song, H.; Li, J.; Luo, M.; Zhao, Q.; Liu, F. Ultra-Thin mesoporous LiV3O8 Nanosheet with exceptionally large specific area for fast and reversible Li storage in Lithium-ion battery cathode. J. Electrochem. Soc. 2021, 168, 050515. [Google Scholar] [CrossRef]
- Wu, X.; Song, K.; Zhang, X.; Hu, N.; Li, L.; Li, W.; Zhang, L.; Zhang, H. Safety Issues in Lithium-Ion Batteries: Materials and cell Design. Front. Energy Res. 2019, 7, 65. [Google Scholar] [CrossRef]
- Kim, C.-S.; Jeong, K.M.; Kim, K.; Yi, C.-W. Effects of Capacity ratios between anode and cathode on electrochemical properties for lithium polymer batteries. Electrochim. Acta 2015, 155, 431–436. [Google Scholar] [CrossRef]
- Fanfeng, L.; Cheng, C.; Zhiyuan, Z.; Weikang, Z.; Zhengzhong, L.Y.U. The influence of N/P ratio on the performance of lithium iron phosphate batteries. Energy Storage Sci. Technol. 2021, 10, 1325–1329. [Google Scholar]
- Angelopoulou, P.; Avgouropoulos, G. Effect of electrode loading on the electrochemical performance of LiAl0.1Mn1.9O4 Cathode for lithium-ion batteries. Mater. Res. Bull. 2019, 119, 110562. [Google Scholar] [CrossRef]




| Types of LIBs | Properties |
|---|---|
| Coin/Button Cell |
|
| Cylindrical Cell |
|
| Pouch-Type Cell |
|
| Class | Types | Advantages | Disadvantages | Refs |
|---|---|---|---|---|
| Layered Oxides | Co-based oxides |
|
| [64,65,66] |
| Ni-rich oxides |
|
| [67,68,69,70] | |
| Li-rich oxides |
|
| [71,72,73,74] | |
| V-based oxides |
|
| [75,76,77] | |
| Spinal Oxides | LiM2O4 (M=Co, Mn, Ni) |
|
| [78,79,80] |
| Polyanionic | Phosphate oxides |
|
| [81,82,83,84] |
| Silicate oxide |
|
| [85,86] | |
| Borate oxides |
|
| [87,88] | |
| Tavorites |
|
| [89,90] |
| Class | Types | Advantage | Disadvantage | Refs |
|---|---|---|---|---|
| Insertion/ Extraction | Carbonaceous |
|
| [19,61,91,92,93,94] |
| Ti oxides |
|
| [19,61,92,93,94] | |
| Alloy/de-Alloy | Si, Ge, Sn, Sb, SnO, SiO, Zn, etc. |
|
| [19,61,92,93,94] |
| Conversion | Metal oxides |
|
| [12,16,61,92,93,94,95,96] |
| Chalcogenides |
|
| [19,61,92,93,94,95,96] |
| Types | Materials | General Properties | Refs |
|---|---|---|---|
| Aqueous | Na based CMC, SBR, Chitosan, Alginate, etc. |
| [102,103,104] |
| Non-aqueous | PVDF, SBR, NBR, CMC, PAN, CA, etc. |
| [105,106] |
| Polymer | CMC, SBR, PVA, PVD, PVDF, SA, FPI, AR/CMC, Lignin, Sericin protein, etc. |
| [100,107,108] |
| Hybrid | PAA-PAI, GO-StC, β-CDp, Natural polymer, WS-PS, etc. |
| [109,110] |
| Types | Advantages | Disadvantages | Refs |
|---|---|---|---|
| Aqueous |
|
| [130,131] |
| Non-aqueous |
|
| [107,132,133] |
| Ionic Liquids |
|
| [134,135,136] |
| Polymers (GPEs, SPEs) |
|
| [106,107,137,138] |
| Hybrids |
|
| [100,108,109] |
| Types | Aqueous | Non-Aqueous | Ionic Liquids | Polymer (Gels, Hybrid) |
|---|---|---|---|---|
| Mechanical strength | Poor | Good | Good | Medium |
| Ionic conductivity | >10−3 Scm−1 | >10−3 Scm−1 | 10−3~102 Scm−1 | >10−4 Scm−1 |
| Thermal stability | Poor | Medium | Good | Medium |
| Electrochemical stability | Poor | Good | Good | Poor |
| Safety | Poor | Medium | Medium | Medium |
| Interfacial properties | Good | Medium | Good | Medium |
| Electrolytes | Strategies | General Properties | Refs |
|---|---|---|---|
| Aqueous | Enriching salt concentration | Cut anions at anode surface Enhance anion/cation interaction Break hydrogen bonding to reduce O2 solubility and H2 evolution Develop eutectic system for better ORR kinetics to improve the potential window (1.23 to ~3.0 V) Improve the ionic conductivity (≈102) Affect the rate performance and overpotential | [130,131,139] |
| Incorporating additive | Suppresses OER at the cathode surface Prevent corrosion/dendrite formation Modify interfaces (electrodes/current collectors/electrolyte/separator) Change solvation sheath to widen potential window for high temperature and freezing temperature | [139] | |
| Tuning interfaces \ (electrodes/current collectors/electrolyte) | Suppresses free radicals, reactive anions/cations to control side reactions Affects interfaces stability and reactivity To achieve thermodynamics (chemical, thermal) and kinetic (charge/mass transportation activity) stability | [140,141] | |
| Addition of decoupling gel/polymer material | Solidifies water (lower fluidity) Develop anti-freezing function at low temperature Stabilize/widen the potential window and working temperature range Lowers the production cost | [142,143] | |
| Solvent-hybrid electrolyte | Efficiently reduce the cost and environmental problems Improve interfacial chemistry Enhance performance of LIBs | [144] |
| Solvents | Names | m.p | b.p | η (20 °C) | Ɛr | µ | ρ (Vm) | κ | Eox vs. Li+/Li | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| Carbonates | EC | 36.4 | 248 | 1.90 | 89.8 | 4.61 | 1.32 (66.71) | 8.3 | 6.7 | [149] |
| PC | −48.8 | 242 | 2.53 | 64.9 | 4.81 | 1.20 (85.08) | 5.6 | 6.0 | [150] | |
| DMC | 4.6 | 96 | 0.59 | 3.1 | 0.76 | 1.06 (84.98) | 6.0 | 5.5 | [148] | |
| DEC | −74.3 | 126 | 0.75 | 2.8 | 0.96 | 0.97 (121.78) | 2.4 | 5.2 | [133] | |
| Esters | EA | −84 | 77 | 0.45 | 6.0 | 1.83 | 0.90 (97.90) | 11.5 | 5.4 | [151] |
| MA | −98.2 | 57 | 0.37 | 6.7 | 1.70 | 0.93 (79.66) | 14.8 | 5.2 | [152] | |
| MB | −84 | 102 | 0.60 | 5.5 | 1.71 | 0.90 (113.48) | 4.2 | 4.6 | [153] | |
| Ethers/Acetals | EPE | −126.7 | 63 | 0.31 | - | 1.16 | 0.73 (120.75) | 4.5 | 5.5 | [154] |
| DEE | −74 | 121 | 0.56 | 5.1 | 1.76 | 0.84 (140.69) | 5.8 | 4.5 | [155] | |
| THF | −109 | 66 | 0.46 | 7.4 | 1.70 | 0.88 (81.94) | 9.1 | 3.5 | [156] | |
| Sulfur Compounds | DMSO | 18.5 | 189 | 1.90 | 46.6 | 3.90 | 1.09 (71.68) | 8.6 | 4.1 | [157] |
| DMS | −141 | 126 | 0.87 | 22.5 | 2.90 | 1.20 (91.78) | 13.6 | 4.2 | [158] | |
| DES | −112 | 156 | 0.83 | 15.6 | 2.96 | 1.08 (127.94) | 10.2 | 2.9 | [159] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghani, F.; An, K.; Lee, D. A Review on Design Parameters for the Full-Cell Lithium-Ion Batteries. Batteries 2024, 10, 340. https://doi.org/10.3390/batteries10100340
Ghani F, An K, Lee D. A Review on Design Parameters for the Full-Cell Lithium-Ion Batteries. Batteries. 2024; 10(10):340. https://doi.org/10.3390/batteries10100340
Chicago/Turabian StyleGhani, Faizan, Kunsik An, and Dongjin Lee. 2024. "A Review on Design Parameters for the Full-Cell Lithium-Ion Batteries" Batteries 10, no. 10: 340. https://doi.org/10.3390/batteries10100340
APA StyleGhani, F., An, K., & Lee, D. (2024). A Review on Design Parameters for the Full-Cell Lithium-Ion Batteries. Batteries, 10(10), 340. https://doi.org/10.3390/batteries10100340








