Enhanced Structural and Electrochemical Performance of LiNi0.5Mn1.5O4 Cathode Material by PO43−/Fe3+ Co-Doping
Abstract
1. Introduction
2. Materials and Methods
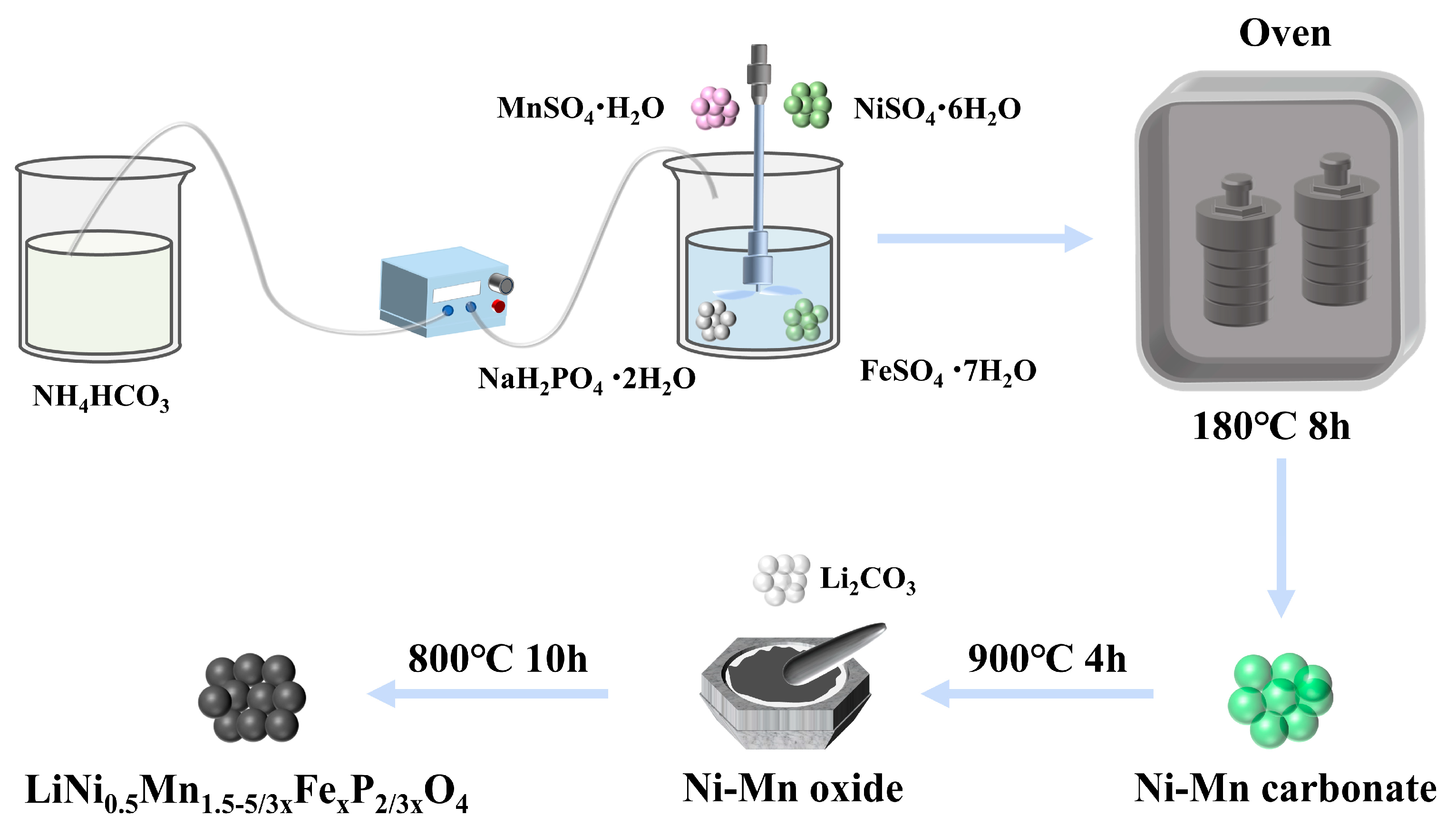
2.1. Material Synthesis
2.2. Material Characterization
2.3. Electrochemical Tests
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qian, H.; Ren, H.; Zhang, Y.; He, X.; Li, W.; Wang, J.; Hu, J.; Yang, H.; Sari, H.M.K.; Chen, Y.; et al. Surface doping vs. bulk doping of cathode materials for lithium-ion batteries: A review. Electrochem. Energy Rev. 2022, 5, 2. [Google Scholar] [CrossRef]
- Yi, H.; Liang, Y.; Qian, Y.; Feng, Y.; Li, Z.; Zhang, X. Low-cost Mn-based cathode materials for lithium-ion batteries. Batteries 2023, 9, 246. [Google Scholar] [CrossRef]
- Wu, W.; Zuo, S.; Zhang, X.; Feng, X. Two-step solid state synthesis of medium entropy LiNi0.5Mn1.5O4 cathode with enhanced electrochemical performance. Batteries 2023, 9, 91. [Google Scholar] [CrossRef]
- Wang, B.; Son, S.-B.; Badami, P.; Trask, S.E.; Abraham, D.; Qin, Y.; Yang, Z.; Wu, X.; Jansen, A.; Liao, C. Understanding and mitigating the dissolution and delamination issues encountered with high-voltage LiNi0.5Mn1.5O4. Batteries 2023, 9, 435. [Google Scholar] [CrossRef]
- Ma, C.; Wen, Y.; Qiao, Q.; He, P.; Ren, S.; Li, M.; Zhao, P.; Qiu, J.; Tang, G. Improving electrochemical performance of high-voltage spinel LiNi0.5Mn1.5O4 cathodes by silicon oxide surface modification. ACS Appl. Energy Mater. 2021, 4, 12201–12210. [Google Scholar] [CrossRef]
- Yu, C.; Dong, L.; Zhang, Y.; Du, K.; Gao, M.; Zhao, H.; Bai, Y. Promoting electrochemical performances of LiNi0.5Mn1.5O4 cathodes via YF3 surface coating. Solid State Ion. 2020, 357, 115464. [Google Scholar] [CrossRef]
- Wu, Y.; Ben, L.; Zhan, Y.; Yu, H.; Qi, W.; Zhao, W.; Huang, X. Binding Li3PO4 to spinel LiNi0.5Mn1.5O4 via a surface Co-containing bridging layer to improve the electrochemical performance. Energy Technol. 2021, 9, 2100147. [Google Scholar] [CrossRef]
- Mou, J.R.; Deng, Y.L.; He, L.H.; Zheng, Q.J.; Jiang, N.; Lin, D.M. Critical roles of semi-conductive LaFeO3 coating in enhancing cycling stability and rate capability of 5 V LiNi0.5Mn1.5O4 cathode materials. Electrochim. Acta 2018, 260, 101–111. [Google Scholar] [CrossRef]
- Mereacre, V.; Stüble, P.; Trouillet, V.; Ahmed, S.; Volz, K.; Binder, J.R. Coating versus doping: Understanding the enhanced performance of high-voltage batteries by the coating of spinel LiNi0.5Mn1.5O4 with Li0.35La0.55TiO3. Adv. Mater. Interfaces 2023, 10, 2201324. [Google Scholar] [CrossRef]
- Wang, J.; Chen, D.; Wu, W.; Wang, L.; Liang, G. Effects of Na+ doping on crystalline structure and electrochemical performances of LiNi0.5Mn1.5O4 cathode material. Trans. Nonferrous Met. Soc. China 2017, 27, 2239–2248. [Google Scholar] [CrossRef]
- Liang, G.; Wu, Z.; Didier, C.; Zhang, W.; Cuan, J.; Li, B.; Ko, K.-Y.; Hung, P.-Y.; Lu, C.-Z.; Chen, Y.; et al. A long cycle-life high-voltage spinel lithium-ion battery electrode achieved by site-selective doping. Angew. Chem. Int. Ed. 2020, 59, 10594–10602. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.-S.; Huang, J.-H.; Cheng, T.-H.; Hu, C.-W.; Wu, N.-J.; Yen, C.-Y.; Hsu, S.-C.; Weng, H.C.; Chen, C.-P. Cr-doped LiNi0.5Mn1.5O4 derived from bimetallic Ni/Mn metal-organic framework as high-performance cathode for lithium-ion batteries. J. Energy Storage 2023, 68, 107686. [Google Scholar] [CrossRef]
- Zhou, D.; Li, J.; Chen, C.; Lin, F.; Wu, H.; Guo, J. A hydrothermal synthesis of Ru-doped LiMn1.5Ni0.5O4 cathode materials for enhanced electrochemical performance. RSC Adv. 2021, 11, 12549. [Google Scholar] [CrossRef]
- Luo, Y.; Li, H.; Lu, T.; Zhang, Y.; Mao, S.S.; Liu, Z.; Wen, W.; Xie, J.; Yan, L. Fluorine gradient-doped LiNi0.5Mn1.5O4 spinel with improved high voltage stability for Li-ion batteries. Electrochim. Acta 2017, 238, 237–245. [Google Scholar] [CrossRef]
- Kim, D.-W.; Zettsu, N.; Shiiba, H.; Sánchez-Santolino, G.; Ishikawa, R.; Ikuhara, Y.; Teshima, K. Metastable oxysulfide surface formation on LiNi0.5Mn1.5O4 single crystal particles by carbothermal reaction with sulfur-doped heterocarbon nanoparticles: New insight into their structural and electrochemical characteristics, and their potential applications. J. Mater. Chem. A 2020, 8, 22302–22314. [Google Scholar]
- Kim, W.-K.; Han, D.-W.; Ryu, W.-H.; Lim, S.-J.; Eom, J.-Y.; Kwon, H.-S. Effects of Cl doping on the structural and electrochemical properties of high voltage LiMn1.5Ni0.5O4 cathode materials for Li-ion batteries. J. Alloys Compd. 2014, 592, 48–52. [Google Scholar] [CrossRef]
- Chen, T.; Li, X.; Wang, H.; Yan, X.; Wang, L.; Deng, B.; Ge, W.; Qu, M. The effect of gradient boracic polyanion-doping on structure, morphology, and cycling performance of Ni-rich LiNi0.8Co0.15Al0.05O2 cathode material. J. Power Sources 2018, 374, 1–11. [Google Scholar] [CrossRef]
- Yang, G.; Pan, K.; Lai, F.; Wang, Z.; Chu, Y.; Yang, S.; Han, J.; Wang, H.; Zhang, X.; Li, Q. Integrated co-modification of PO43- polyanion doping and Li2TiO3 coating for Ni-rich layered LiNi0.6Co0.2Mn0.2O2 cathode material of lithium-ion batteries. Chem. Eng. J. 2021, 421, 129964. [Google Scholar] [CrossRef]
- Zhang, H.-Z.; Li, F.; Pan, G.-L.; Li, G.-R.; Gao, X.-P. The effect of polyanion-doping on the structure and electrochemical performance of Li-rich layered oxides as cathode for lithium-ion batteries. J. Electrochem. Soc. 2015, 162, A1899–A1904. [Google Scholar] [CrossRef]
- Fu, S.; Zhang, Y.; Bian, Y.; Xu, J.; Wang, L.; Liang, G. Effect of Fe3+ and/or PO43− doping on the electrochemical performance of LiNi0.5Mn1.5O4 cathode material for Li-ion batteries. Ind. Eng. Chem. Res. 2023, 62, 1016–1028. [Google Scholar] [CrossRef]
- Lan, L.; Li, S.; Li, J.; Lu, L.; Lu, Y.; Huang, S.; Xu, S.; Pan, C.; Zhao, F. Enhancement of the electrochemical performance of the spinel structure LiNi0.5−xGaxMn1.5O4 cathode material by Ga doping. Nanoscale Res. Lett. 2018, 13, 251. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Fu, S.; Zhang, Y.; Yan, S.; Lang, Y.; Guo, J.; Wang, L.; Liang, G. Enhanced electrochemical performance of 5V LiNi0.5Mn1.5−xZrxO4 cathode material for lithium-ion batteries. ChemistrySelect 2021, 6, 7202–7212. [Google Scholar] [CrossRef]
- Liu, D.; Hamel-Paquet, J.; Trottier, J.; Barray, F.; Gariépy, V.; Hovington, P.; Guerfi, A.; Mauger, A.; Julien, C.M.; Goodenough, J.B.; et al. Synthesis of pure phase disordered LiMn1.45Cr0.1Ni0.45O4 by a post-annealing method. J. Power Sources 2012, 217, 400–406. [Google Scholar] [CrossRef]
- Kunduraci, M.; Amatucci, G.G. Synthesis and characterization of nanostructured 4.7 V LixMn1.5Ni0.5O4 spinels for high-power lithium-ion batteries. J. Electrochem. Soc. 2006, 153, A1345–A1352. [Google Scholar] [CrossRef]
- Kim, J.-H.; Myung, S.-T.; Yoon, C.S.; Kang, S.G.; Sun, Y.-K. Comparative study of LiNi0.5Mn1.5O4−δ and LiNi0.5Mn1.5O4 cathodes having two crystallographic structures: Fd3m and P4332. Chem. Mater. 2004, 16, 906–914. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, W.; Qu, Q.; Zheng, H.; Huang, Y. Bi-functions of titanium and lanthanum co-doping to enhance the electrochemical performance of spinel LiNi0.5Mn1.5O4 cathode. J. Mater. 2019, 5, 156–163. [Google Scholar] [CrossRef]
- Zeng, Y.-P.; Wu, X.-l.; Mei, P.; Cong, L.-N.; Yao, C.; Wang, R.-S.; Xie, H.-M.; Sun, L.-Q. Effect of cationic and anionic substitutions on the electrochemical properties of LiNi0.5Mn1.5O4 spinel cathode materials. Electrochim. Acta 2014, 138, 493–500. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Zhang, X.; Zhou, D.; Qi, X.; Qiu, B.; Fang, J.; Kloepsch, R.; Schumacher, G.; Liu, Z.; et al. Morphological evolution of high-voltage spinel LiNi0.5Mn1.5O4 cathode materials for lithium-ion batteries: The critical effects of surface orientations and particle size. ACS Appl. Mater. Interfaces 2016, 8, 4661–4675. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, R.; Du, P.; Hu, H.; Wang, T.; Zhu, L.; Chen, H. Polyhedral LiNi0.5Mn1.5O4 with excellent electrochemical properties for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 12835–12848. [Google Scholar] [CrossRef]
- Liu, W.; Li, X.; Xiong, D.; Hao, Y.; Li, J.; Kou, H.; Yan, B.; Li, D.; Lu, S.; Koo, A.; et al. Significantly improving cycling performance of cathodes in lithium ion batteries: The effect of Al2O3 and LiAlO2 coatings on LiNi0.6Co0.2Mn0.2O2. Nano Energy 2018, 44, 111–120. [Google Scholar] [CrossRef]
- Liu, J.; Manthiram, A. Understanding the improved electrochemical performances of Fe-substituted 5 V spinel cathode LiMn1.5Ni0.5O4. J. Phys. Chem. C 2009, 113, 15073–15079. [Google Scholar] [CrossRef]
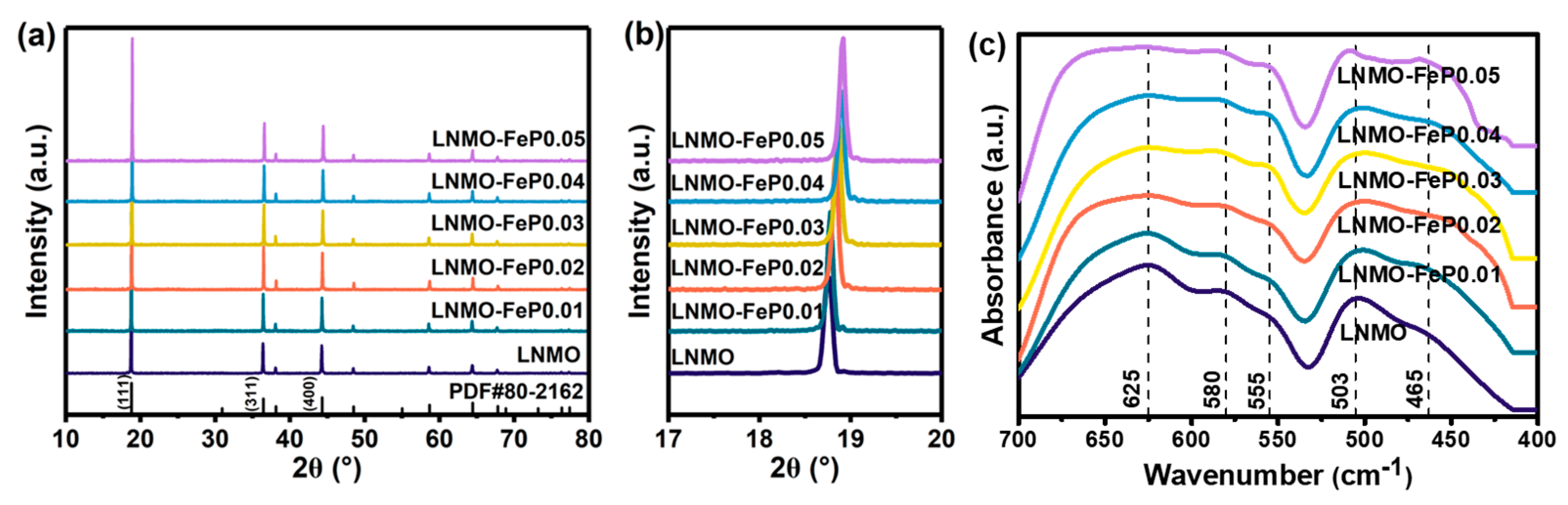
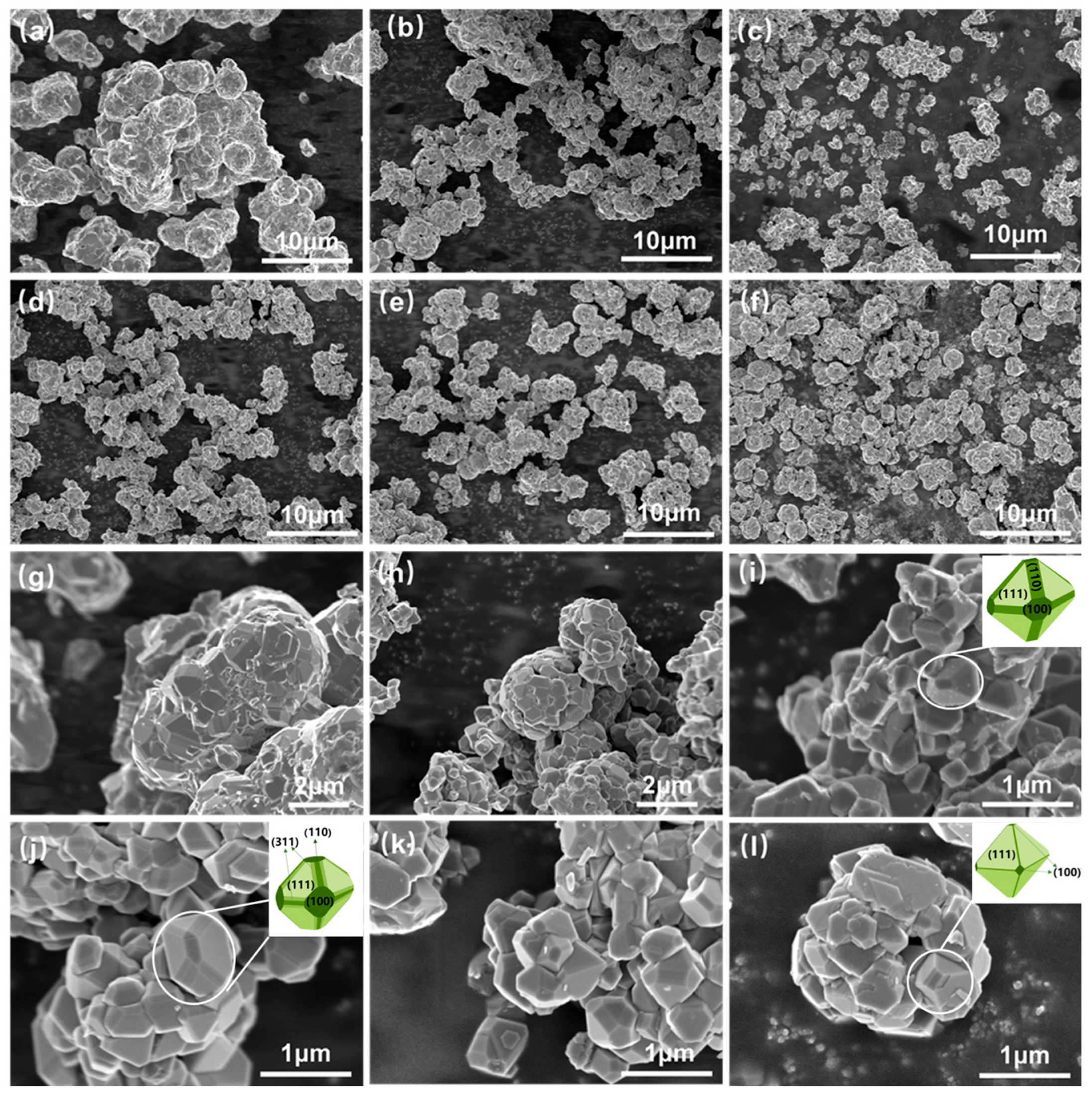



| Sample | I440/I111 | I311/I111 |
|---|---|---|
| LNMO | 0.0857 | 0.312 |
| LNMO-FeP0.01 | 0.0862 | 0.313 |
| LNMO-FeP0.02 | 0.0871 | 0.314 |
| LNMO-FeP0.03 | 0.0895 | 0.321 |
| LNMO-FeP0.04 | 0.0967 | 0.328 |
| LNMO-FeP0.05 | 0.0870 | 0.309 |
| Sample | DLi (×10−10 cm2 s−1) | Da (×10−10 cm2 s−1) | |||
|---|---|---|---|---|---|
| O1 | R1 | O2 | R2 | ||
| LNMO | 0.497 | 1.694 | 1.327 | 0.817 | 1.084 |
| LNMO-FeP0.01 | 1.947 | 3.933 | 2.027 | 1.098 | 2.251 |
| LNMO-FeP0.02 | 2.039 | 2.959 | 6.136 | 3.302 | 3.609 |
| LNMO-FeP0.03 | 3.665 | 6.502 | 2.606 | 3.269 | 4.011 |
| LNMO-FeP0.04 | 1.694 | 6.058 | 6.850 | 3.725 | 4.582 |
| LNMO-FeP0.05 | 1.565 | 4.745 | 1.410 | 2.657 | 2.594 |
| Sample | Resistance (Ω) | After 50 Cycles | After 100 Cycles | After 150 Cycles | After 200 Cycles |
|---|---|---|---|---|---|
| LNMO | Rsf | 38.56 | 68.16 | 79.70 | 91.11 |
| Rct | 65.22 | 72.16 | 123.70 | 136.20 | |
| LNMO-FeP0.01 | Rsf | 30.60 | 49.26 | 72.25 | 97.14 |
| Rct | 49.37 | 68.34 | 78.01 | 92.70 | |
| LNMO-FeP0.02 | Rsf | 21.75 | 36.36 | 44.34 | 46.70 |
| Rct | 34.54 | 36.04 | 43.86 | 48.64 | |
| LNMO-FeP0.03 | Rsf | 12.48 | 18.84 | 24.09 | 25.71 |
| Rct | 15.60 | 24.38 | 41.57 | 43.21 | |
| LNMO-FeP0.04 | Rsf | 38.74 | 57.52 | 66.39 | 74.19 |
| Rct | 26.65 | 45.79 | 52.78 | 65.52 | |
| LNMO-FeP0.05 | Rsf | 39.10 | 44.73 | 72.28 | 78.66 |
| Rct | 70.32 | 77.62 | 84.31 | 114.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Fu, S.; Du, X.; Wei, D.; Zhang, J.; Wang, L.; Liang, G. Enhanced Structural and Electrochemical Performance of LiNi0.5Mn1.5O4 Cathode Material by PO43−/Fe3+ Co-Doping. Batteries 2024, 10, 341. https://doi.org/10.3390/batteries10100341
Wang Y, Fu S, Du X, Wei D, Zhang J, Wang L, Liang G. Enhanced Structural and Electrochemical Performance of LiNi0.5Mn1.5O4 Cathode Material by PO43−/Fe3+ Co-Doping. Batteries. 2024; 10(10):341. https://doi.org/10.3390/batteries10100341
Chicago/Turabian StyleWang, Yong, Shaoxiong Fu, Xianzhen Du, Dong Wei, Jingpeng Zhang, Li Wang, and Guangchuan Liang. 2024. "Enhanced Structural and Electrochemical Performance of LiNi0.5Mn1.5O4 Cathode Material by PO43−/Fe3+ Co-Doping" Batteries 10, no. 10: 341. https://doi.org/10.3390/batteries10100341
APA StyleWang, Y., Fu, S., Du, X., Wei, D., Zhang, J., Wang, L., & Liang, G. (2024). Enhanced Structural and Electrochemical Performance of LiNi0.5Mn1.5O4 Cathode Material by PO43−/Fe3+ Co-Doping. Batteries, 10(10), 341. https://doi.org/10.3390/batteries10100341






