One-Step Hydrothermally Synthesized Ni11(HPO3)8(OH)6/Co3(HPO4)2(OH)2 Heterostructure with Enhanced Rate Performance for Hybrid Supercapacitor Applications
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals and Materials
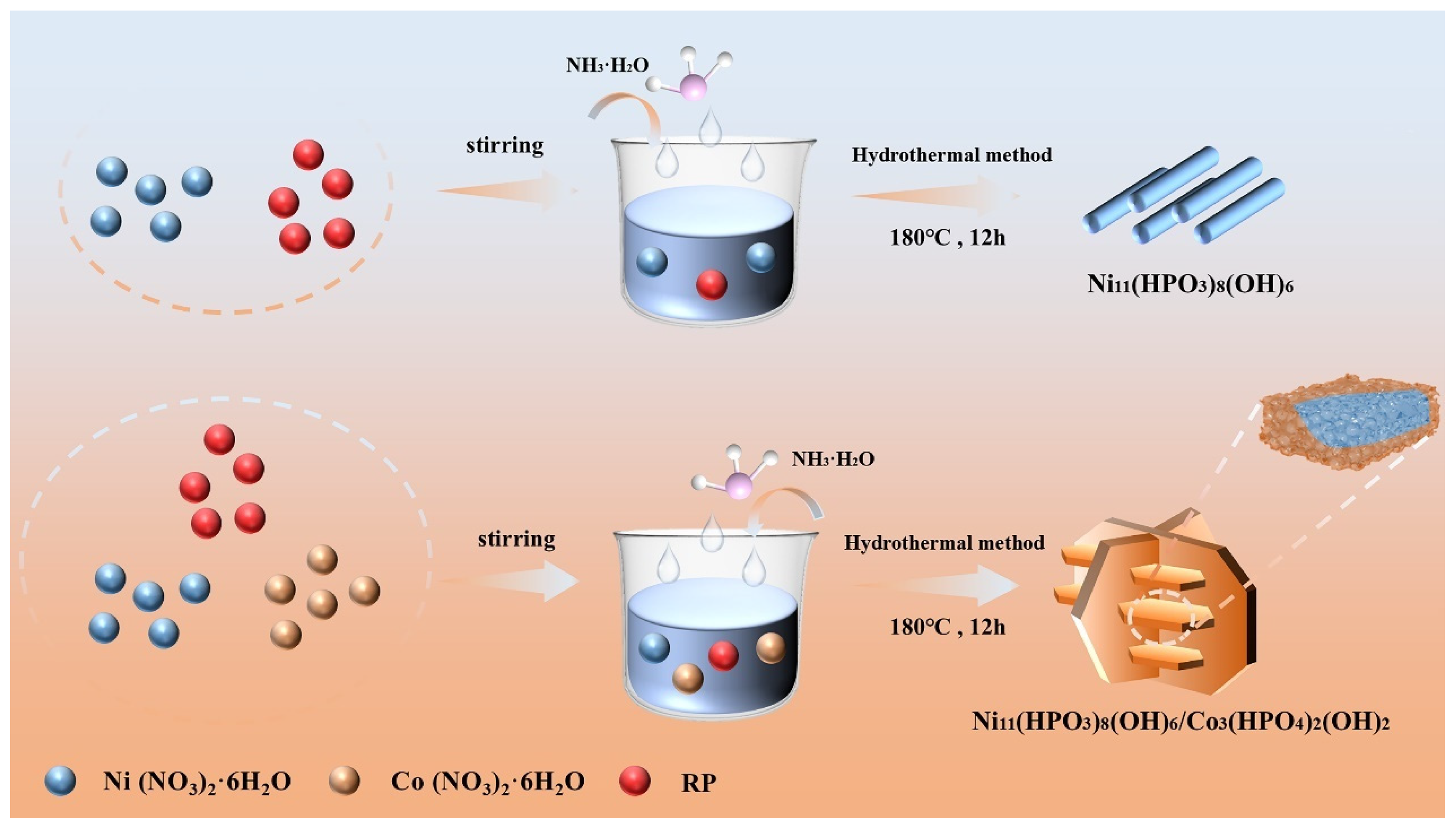
2.2. Synthesis of Ni11(HPO3)8(OH)6 Cathode Material
2.3. Synthesis of Ni11(HPO3)8(OH)6/Co3(HPO4)2(OH)2 Heterojunction Composites
2.4. Material Characteristics
2.5. Electrochemical Tests
3. Results and Discussion
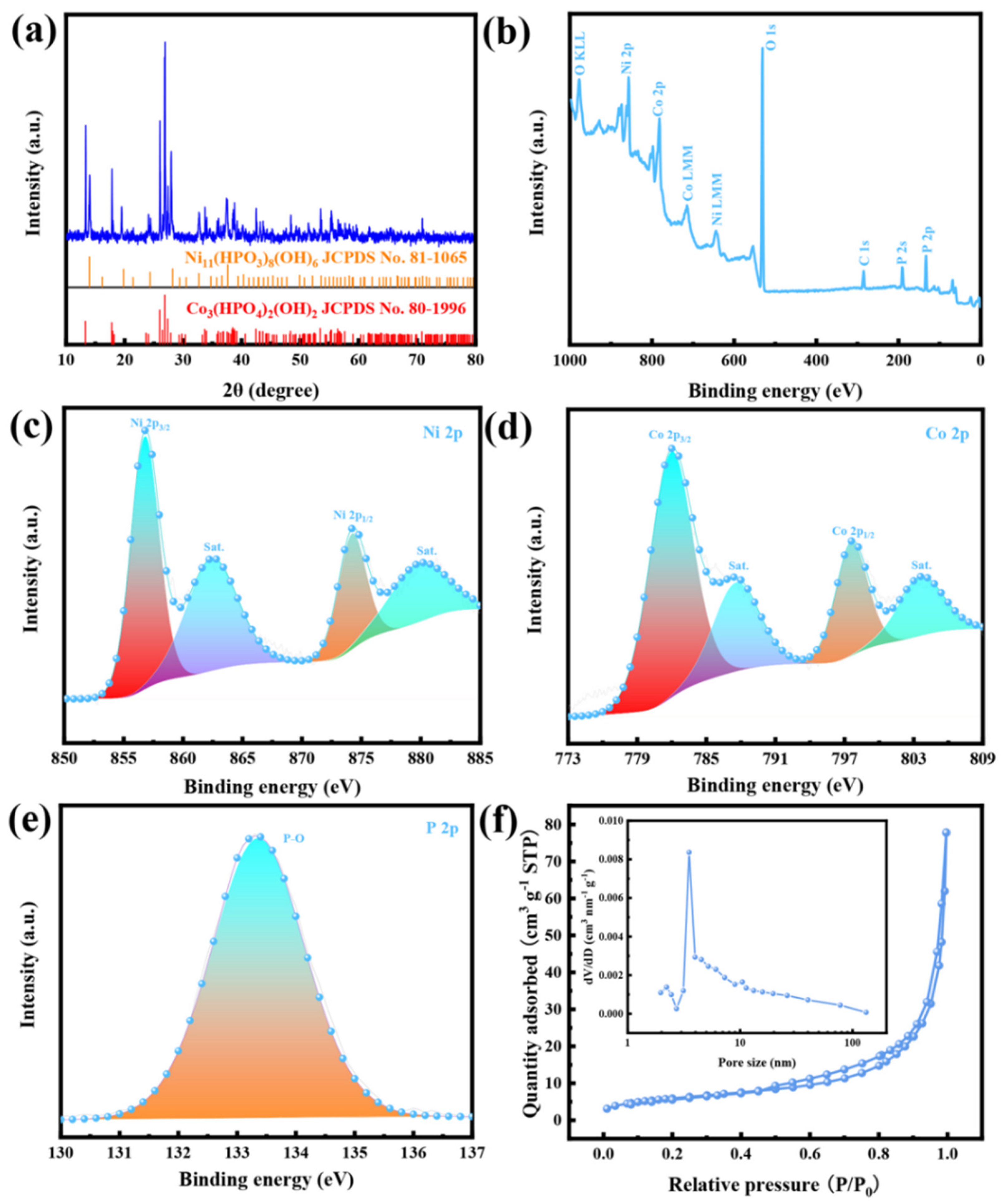
3.1. The Characterizations of Morphology, Composition, and Structure
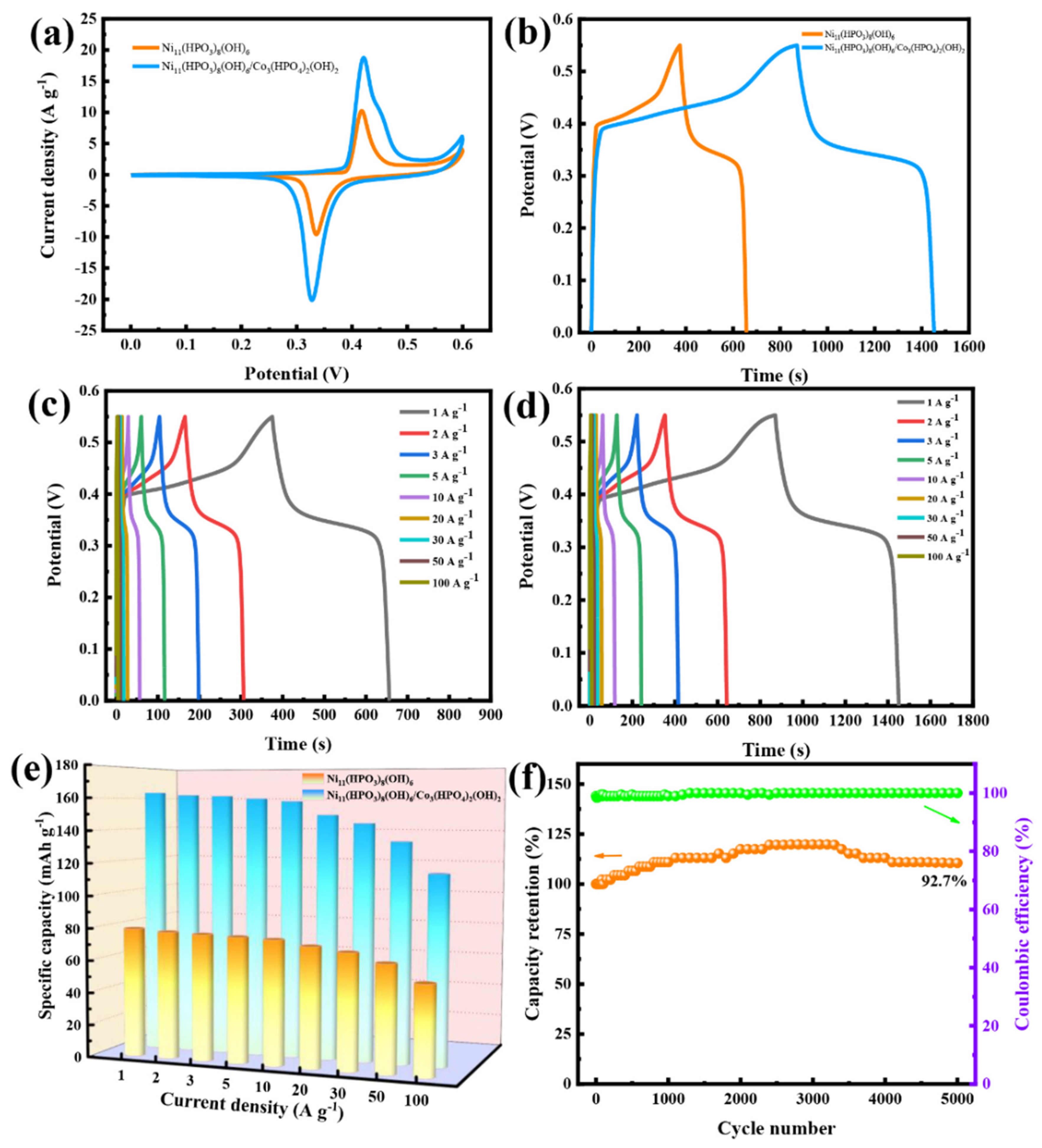
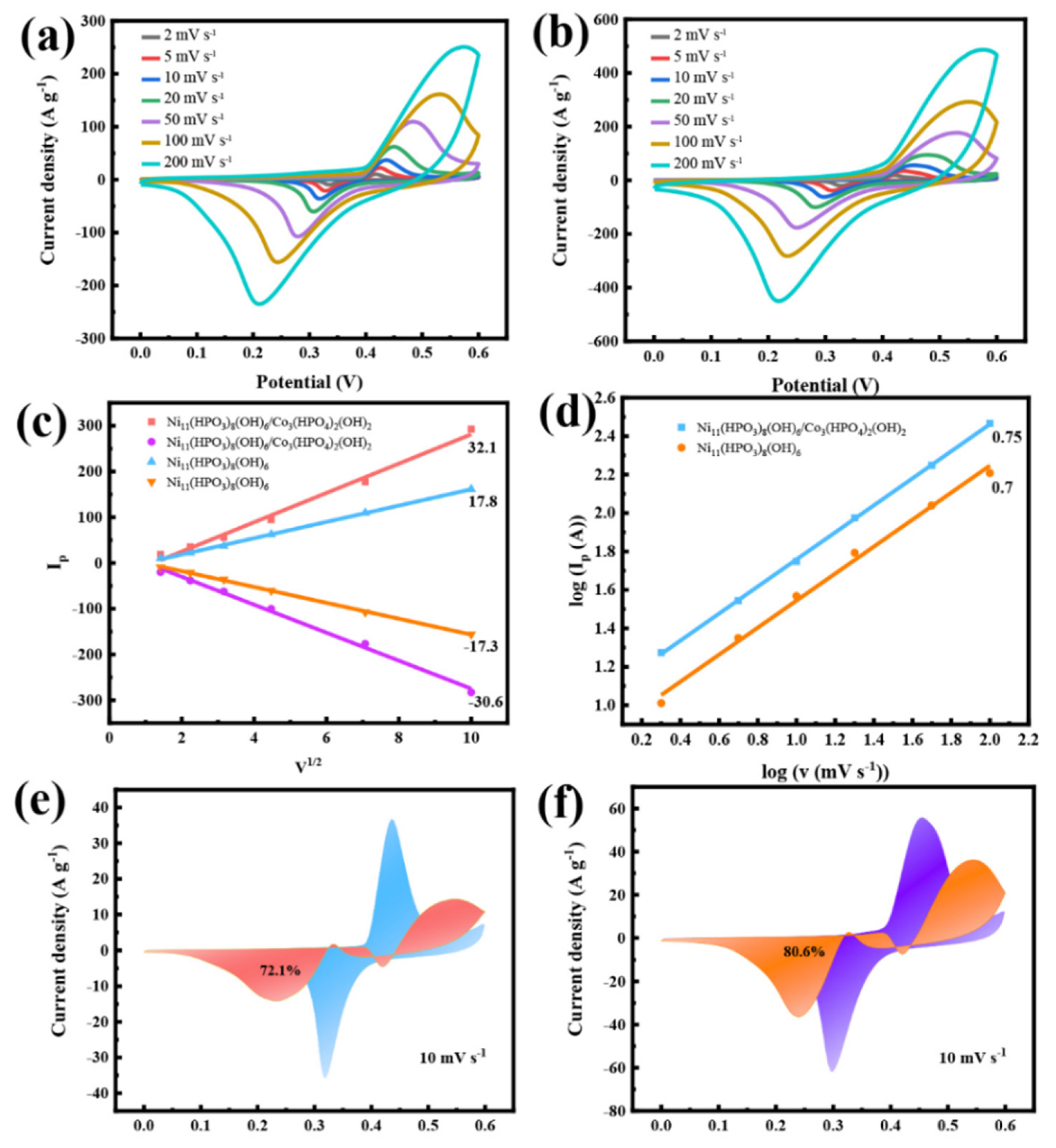
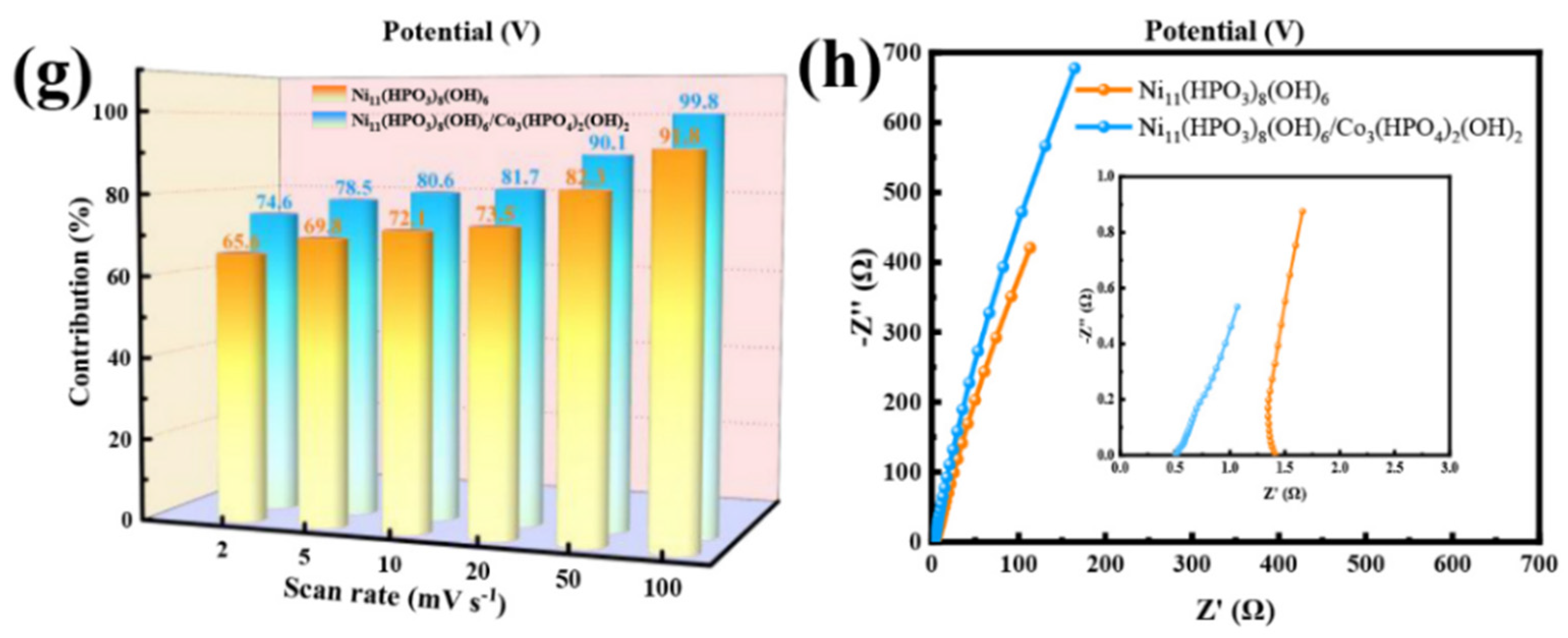
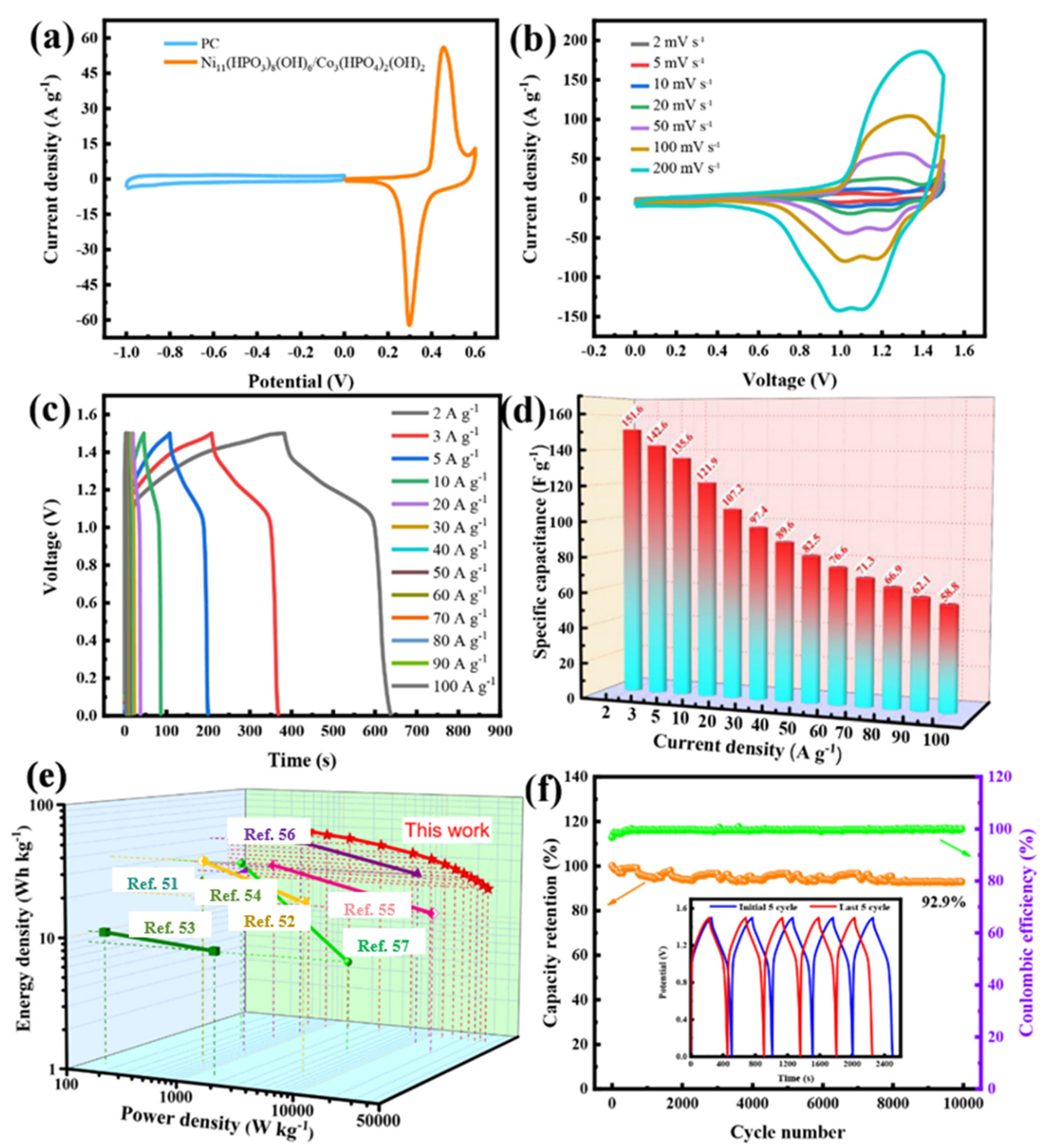
3.2. The Electrochemical Performances of Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.; Cui, Y.; Gogotsi, Y. Energy storage: The future enabled by nanomaterials. Science 2019, 366, eaan8285. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Banda, H.; Chen, M.; Niu, L.; Chen, M.; Wu, T.; Wang, J.; Wang, R.; Feng, J.; Chen, T.; et al. Molecular understanding of charge storage and charging dynamics in supercapacitors with MOF electrodes and ionic liquid electrolytes. Nat. Mater. 2020, 19, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Z.; Zhang, D.; Miao, Z.-C.; Zhang, X.-L.; Chou, S.-L. Research Progress in MnO2–Carbon Based Supercapacitor Electrode Materials. Small 2018, 14, 1702883. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Yang, G.; Kim, C.-H.; Mahajan, R.L.; Lee, S.-Y.; Park, S.-J. Flexible solid-state hybrid supercapacitors for the internet of everything (IoE). Energy Environ. Sci. 2022, 15, 2233–2258. [Google Scholar] [CrossRef]
- Zhao, Z.; Ye, Y.; Zhu, W.; Xiao, L.; Deng, B.; Liu, J. Bismuth oxide nanoflake@carbon film: A free-standing battery-type electrode for aqueous sodium ion hybrid supercapacitors. Chin. Chem. Lett. 2018, 29, 629–632. [Google Scholar] [CrossRef]
- Amiri, A.; Bruno, A.; Polycarpou, A.A. Configuration-dependent stretchable all-solid-state supercapacitors and hybrid supercapacitors. Carbon Energy 2023, 5, e320. [Google Scholar] [CrossRef]
- Sadavar, S.; Wang, K.J.; Kang, T.; Hwang, M.; Saeed, G.; Yu, X.; Park, H.S. Anion storage for hybrid supercapacitor. Mater. Today Energy 2023, 37, 101388. [Google Scholar] [CrossRef]
- Wang, X.; Yan, C.; Yan, J.; Sumboja, A.; Lee, P.S. Orthorhombic niobium oxide nanowires for next generation hybrid supercapacitor device. Nano Energy 2015, 11, 765–772. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Y.; Yan, M.; Yang, Q.; Liu, X.; Shi, W. Lewis acid etched NixCo1-xSe2 derived from ZIF-L on CoO nanowires for hybrid-supercapacitors. Chem. Eng. J. 2022, 431, 133472. [Google Scholar] [CrossRef]
- Roy, A.; Inta, H.R.; Ghosh, S.; Koppisetti, H.V.S.R.M.; Mondal, A.; Verma, B.R.; Bag, S.; Mahalingam, V. Electrochemical surface reconstruction of nickel cobalt pyrophosphate to Ni/Co-hydroxide-(oxy)hydroxide: An efficient and highly durable battery-type supercapacitor electrode material. J. Mater. Chem. A 2024, 12, 4086–4098. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Kaneti, Y.V.; Yamauchi, Y. Metal–Organic Framework-Derived Nanoporous Metal Oxides toward Supercapacitor Applications: Progress and Prospects. ACS Nano 2017, 11, 5293–5308. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Y.; Lou, X.W. Mixed Metal Sulfides for Electrochemical Energy Storage and Conversion. Adv. Funct. Mater. 2018, 8, 1701592. [Google Scholar] [CrossRef]
- Zou, K.; Deng, W.; Silvester, D.S.; Zou, G.; Hou, H.; Banks, C.E.; Li, L.; Hu, J.; Ji, X. Carbonyl Chemistry for Advanced Electrochemical Energy Storage Systems. ACS Nano 2024, 18, 19950–20000. [Google Scholar] [CrossRef] [PubMed]
- Zan, G.; Li, S.; Chen, P.; Dong, K.; Wu, Q.; Wu, T. Mesoporous Cubic Nanocages Assembled by Coupled Monolayers With 100% Theoretical Capacity and Robust Cycling. ACS Cent. Sci. 2024, 10, 1283–1294. [Google Scholar] [CrossRef]
- Manikandan, R.; Savariraj, A.D.; Nagaraju, G.; Kale, A.M.; Puigdollers, J.; Park, H.; Kim, H.-S.; Oh, J.-M.; Raj, C.J.; Kim, B.C. Mixed-phase composites derived from cobalt terephthalate as efficient battery-type electrodes for high-performance supercapattery. J. Mater. Sci. Technol. 2023, 157, 220–233. [Google Scholar] [CrossRef]
- Manikandan, R.; Raj, C.J.; Goli, N.; Oh, J.-M.; Kim, B.C.; Periyasamy, S.; Lee, J. Interconnected Vanadyl Pyrophosphate Nanonetworks as a Flexible Electrode for High-Voltage and Long-Life Li-Ion Supercapacitors. Appl. Mater. Interfaces 2023, 15, 25452–25461. [Google Scholar] [CrossRef]
- Zhao, H.; Yuan, Z.-Y. Design Strategies of Transition-Metal Phosphate and Phosphonate Electrocatalysts for Energy-Related Reactions. Chem. Eng. Sci 2021, 14, 130–149. [Google Scholar] [CrossRef]
- Cheng, Q.; Zhao, X.; Yang, G.; Mao, L.; Liao, F.; Chen, L.; He, P.; Pan, D.; Chen, S. Recent advances of metal phosphates-based electrodes for high-performance metal ion batteries. Energy Storage Mater. 2021, 41, 842–882. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Fu, Z.; Xu, Y.; Yang, L.-X.; Wang, F.; Guo, X.; Sun, W.; Yang, Z.-L. Cobalt–Nickel Phosphate Composites for the All-Phosphate Asymmetric Supercapacitor and Oxygen Evolution Reaction. Appl. Mater. Interfaces 2021, 13, 34507–34517. [Google Scholar] [CrossRef]
- Xie, L.; Zong, Q.; Zhang, Q.; Sun, J.; Zhou, Z.; He, B.; Zhu, Z.; E, S.; Yao, Y. Hierarchical NiCoP nanosheet arrays with enhanced electrochemical properties for high-performance wearable hybrid capacitors. J. Alloys Compd. 2019, 781, 783–789. [Google Scholar] [CrossRef]
- Fan, J.; Wu, K.; Chen, A.; Wang, M.; Xie, X.; Fan, J. Fingerprint-like NiCoP electrode material with rapid charging/discharging performance under large current density. J. Alloys Compd. 2022, 902, 163866. [Google Scholar] [CrossRef]
- Liu, M.; Li, J.; Han, W.; Kang, L. Simple synthesis of novel phosphate electrode materials with unique microstructure and enhanced supercapacitive properties. J. Energy Chem. 2016, 25, 601–608. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, M.; Qu, M.; Sun, M.; Pang, H. Core–shell Co11(HPO3)8(OH)6–Co3O4 hybrids for high-performance flexible all-solid-state asymmetric supercapacitors. J. Alloys Compd. 2015, 651, 214–221. [Google Scholar] [CrossRef]
- Li, X.; Elshahawy, A.M.; Guan, C.; Wang, J. Metal Phosphides and Phosphates-based Electrodes for Electrochemical Supercapacitors. Small 2017, 13, 1701530. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhai, X.; Lv, P.; Jiao, Y.; Wang, S.; Chi, J. Amorphous nickel phosphate as a high performance electrode material for supercapacitor. Synth. Met. 2023, 292, 117217. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Su, C.-Y.; Chen, J.-L.; Huang, W.-H.; Lou, R. Recent progress of transition metal-based biomass-derived carbon composites for supercapacitor. Rare Met. 2023, 42, 769–796. [Google Scholar] [CrossRef]
- Zhang, G.C.; Feng, M.; Li, Q.; Wang, Z.; Fang, Z.; Niu, Z.; Qu, N.; Fan, X.; Li, S.; Gu, J.; et al. High Energy Density in Combination with High Cycling Stability in Hybrid Supercapacitors. Appl. Mater. Interfaces 2022, 14, 2674–2682. [Google Scholar] [CrossRef]
- Mirghni, A.A.; Madito, M.J.; Oyedotun, K.O.; Masikhwa, T.M.; Ndiaye, N.M.; Ray, S.J.; Manyala, N. A high energy density asymmetric supercapacitor utilizing a nickel phosphate/graphene foam composite as the cathode and carbonized iron cations adsorbed onto polyaniline as the anode. RSC Adv. 2018, 8, 11608–11621. [Google Scholar] [CrossRef]
- Chen, G.; Yan, L.; Luo, H.; Guo, S. Nanoscale Engineering of Heterostructured Anode Materials for Boosting Lithium-Ion Storage. Adv. Mater. 2016, 28, 7580–7602. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, K.; Liu, S.; Chen, X.; Cheng, Y.; Cheong, W.-C.; Chen, Z.; Zheng, L.; Zhang, J.; Li, X.; et al. Construction of CoP/NiCoP Nanotadpoles Heterojunction Interface for Wide pH Hydrogen Evolution Electrocatalysis and Supercapacitor. Adv. Funct. Mater. 2019, 9, 1901213. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, Y.; Lu, Z.; Xiao, J.; Zou, G.; Hou, H.; Ji, X. Heterostructured flower-like NiO/Co3O4 microspheres modified by bifunctional carbon quantum dots as a battery-type cathode for high energy and power density hybrid supercapacitors. Carbon Neutralization 2023, 2, 721–737. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Chen, Q.; Xia, X.; Chen, M. Emerging of Heterostructure Materials in Energy Storage: A Review. Adv. Mater. 2021, 33, 2100855. [Google Scholar] [CrossRef] [PubMed]
- Yun, X.; Lu, T.; Zhou, R.; Lu, Z.; Li, J.; Zhu, Y. Heterostructured NiSe2/CoSe2 hollow microspheres as battery-type cathode for hybrid supercapacitors: Electrochemical kinetics and energy storage mechanism. Chem. Eng. J. 2021, 426, 131328. [Google Scholar] [CrossRef]
- Li, M.; Li, M.; Zhao, M.; Wu, B.; Mourdikoudis, S.; Wei, S.; Oliveira, F.M.; He, J.; Děkanovský, L.; Luxa, J.; et al. Rational design of crystalline/amorphous nickel manganese phosphate octahydrate heterostructure for high-performance aqueous and all-solid-state asymmetric supercapacitors. Chem. Eng. J. 2024, 482, 148895. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, Y.; Du, C.; Xiao, T.; Wan, L. Two-step electrodeposition synthesis of iron cobalt selenide and nickel cobalt phosphate heterostructure for hybrid supercapacitors. J. Colloid Interface Sci. 2023, 629, 1049–1060. [Google Scholar] [CrossRef]
- Davidson, A.; Tempere, J.F.; Che, M.; Roulet, H.; Dufour, G. Spectroscopic Studies of Nickel(II) and Nickel(III) Species Generated upon Thermal Treatments of Nickel/Ceria-Supported Materials. J. Phys. Chem. 1996, 100, 4919–4929. [Google Scholar] [CrossRef]
- Casella, I.G.; Gatta, M. Electrochemical and XPS Characterization of Composite Modified Electrodes Obtained by Nickel Deposition on Noble Metals. Anal. Chem. 2000, 72, 2969–2975. [Google Scholar] [CrossRef]
- Huang, J.; Xiong, Y.; Peng, Z.; Chen, L.; Wang, L.; Xu, Y.; Tan, L.; Yuan, K.; Chen, Y. A General Electrodeposition Strategy for Fabricating Ultrathin Nickel Cobalt Phosphate Nanosheets with Ultrahigh Capacity and Rate Performance. ACS Nano 2020, 14, 14201–14211. [Google Scholar] [CrossRef]
- Dan, H.; Tao, K.; Zhou, Q.; Gong, Y.; Lin, J. Ni-Doped Cobalt Phosphite, Co11(HPO3)8(OH)6, with Different Morphologies Grown on Ni Foam Hydro(solvo)thermally for High-Performance Supercapacitor. Appl. Mater. Interfaces 2018, 10, 31340–31354. [Google Scholar] [CrossRef]
- Li, X.; Wu, H.; Elshahawy, A.M.; Wang, L.; Pennycook, S.J.; Guan, C.; Wang, J. Cactus-Like NiCoP/NiCo-OH 3D Architecture with Tunable Composition for High-Performance Electrochemical Capacitors. Adv. Funct. Mater. 2018, 28, 1800036. [Google Scholar] [CrossRef]
- Ding, R.; Li, X.; Shi, W.; Xu, Q.; Liu, E. One-pot solvothermal synthesis of ternary Ni-Co-P micro/nano-structured materials for high performance aqueous asymmetric supercapacitors. Chem. Eng. J. 2017, 320, 376–388. [Google Scholar] [CrossRef]
- Hu, Y.-M.; Liu, M.-C.; Hu, Y.-X.; Yang, Q.-Q.; Kong, L.-B.; Kang, L. One-pot hydrothermal synthesis of porous nickel cobalt phosphides with high conductivity for advanced energy conversion and storage. Electrochim. Acta 2016, 215, 114–125. [Google Scholar] [CrossRef]
- Li, B.; Gu, P.; Feng, Y.; Zhang, G.; Huang, K.; Xue, H.; Pang, H. Ultrathin Nickel–Cobalt Phosphate 2D Nanosheets for Electrochemical Energy Storage under Aqueous/Solid-State Electrolyte. Adv. Funct. Mater. 2017, 27, 1605784. [Google Scholar] [CrossRef]
- Liang, B.; Chen, Y.; He, J.; Chen, C.; Liu, W.; He, Y.; Liu, X.; Zhang, N.; Roy, V.A.L. Controllable Fabrication and Tuned Electrochemical Performance of Potassium Co–Ni Phosphate Microplates as Electrodes in Supercapacitors. Appl. Mater. Interfaces 2018, 10, 3506–3514. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Y.; Zhang, Z.; Xu, X.; Wang, X. Rapid synthesis of mesoporous NixCo3−x(PO4)2 hollow shells showing enhanced electrocatalytic and supercapacitor performance. J. Mater. Chem. A 2014, 2, 20182–20188. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, N.; He, Y.; Liang, B.; Ma, R.; Liu, X. Controllable Fabrication of Amorphous Co—Ni Pyrophosphates for Tuning Electrochemical Performance in Supercapacitors. Appl. Mater. Interfaces 2016, 8, 23114–23121. [Google Scholar] [CrossRef]
- Li, B.; Shi, Y.; Huang, K.; Zhao, M.; Qiu, J.; Xue, H.; Pang, H. Cobalt-Doped Nickel Phosphite for High Performance of Electrochemical Energy Storage. Small 2018, 14, 1703811. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Z.; Xiong, D.-B.; Qiao, Y.; Tang, Y.; Gao, F. Hybridized Phosphate with Ultrathin Nanoslices and Single Crystal Microplatelets for High Performance Supercapacitors. Sci. Rep. 2016, 6, 17613. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Z.; Guo, W.; Chen, T.; Qiao, Y.; Mu, S.; Zhao, Y.; Gao, F. Honeycomb-like mesoporous cobalt nickel phosphate nanospheres as novel materials for high performance supercapacitor. Electrochim. Acta 2016, 190, 118–125. [Google Scholar] [CrossRef]
- Elshahawy, A.M.; Guan, C.; Li, X.; Zhang, H.; Hu, Y.; Wu, H.; Pennycook, S.J.; Wang, J. Sulfur-doped cobalt phosphide nanotube arrays for highly stable hybrid supercapacitor. Nano Energy 2017, 39, 162–171. [Google Scholar] [CrossRef]
- Mao, H.; Zhang, F.; Liu, X.; Qiu, J.; Li, B.; Jin, Z. Synthesis of cobalt phosphate nanoflakes for high-performance flexible symmetric supercapacitors. J. Mater. Sci. Mater. Electron. 2018, 29, 16721–16729. [Google Scholar] [CrossRef]
- Pang, H.; Wang, S.; Shao, W.; Zhao, S.; Yan, B.; Li, X.; Li, S.; Chen, J.; Du, W. Few-layered CoHPO4·3H2O ultrathin nanosheets for high performance of electrode materials for supercapacitors. Nanoscale 2013, 5, 5752–5757. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Yan, Z.; Wang, W.; Chen, J.; Zhang, J.; Zheng, H. Facile fabrication of NH4CoPO4·H2O nano/microstructures and their primarily application as electrochemical supercapacitor. Nanoscale 2012, 4, 5946–5953. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Zhao, H.; Zong, Y.; Li, X.; Sun, Y.; Feng, J.; Wang, Y.; Zheng, X.; Du, Y. Phosphorization boosts the capacitance of mixed metal nanosheet arrays for high performance supercapacitor electrodes. Nanoscale 2018, 10, 11775–11781. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Jiang, S.; Xu, B.; Huang, C.; Hu, Y.; Qin, Y.; He, M.; Cao, H. Sea-urchin-like nickel–cobalt phosphide/phosphate composites as advanced battery materials for hybrid supercapacitors. J. Mater. Chem. A 2019, 7, 6241–6249. [Google Scholar] [CrossRef]
- Ning, W.-W.; Chen, L.-B.; Wei, W.-F.; Chen, Y.-J.; Zhang, X.-Y. NiCoO2/NiCoP@Ni nanowire arrays: Tunable composition and unique structure design for high-performance winding asymmetric hybrid supercapacitors. Rare Met. 2020, 39, 1034–1044. [Google Scholar] [CrossRef]
- Li, H.; Yu, H.; Zhai, J.; Sun, L.; Yang, H.; Xie, S. Self-assembled 3D cobalt phosphate octahydrate architecture for supercapacitor electrodes. Mater. Lett. 2015, 152, 25–28. [Google Scholar] [CrossRef]

| Material | Specific Capacity (mAh g−1) | Rate Retention | Cyclic Property | Ref. |
|---|---|---|---|---|
| NiCoP/NiCo-OH | 152.8 at 1 A g−1 | 60% (1–10 A g−1) | 88% after 1000 cycles | [40] |
| NiCoP | 160.8 at 1 A g−1 | 81% (1–16 A g−1) | / | [41] |
| Porous NiCoP | 158.6 at 1 A g−1 | 72.8% (1–20 A g−1) | 72% after 3000 cycles | [42] |
| Ni-Co phosphate | 125.8 at 1 A g−1 | 63.4% (1–10 A g−1) | 93% after 8000 cycles | [43] |
| NH4Co0.33Ni0.67PO4·H2O | 158 at 1.5 A g−1 | 66% (1.5–30 A g−1) | 57% after 1000 cycles | [44] |
| NixCo3−x(PO4)2 | 94.2 at 1 A g−1 | 81.4% (1–10 A g−1) | 85% after 1000 cycles | [45] |
| Co0.5Ni0.5 pyrophosphate | 161 at 1.5 A g−1 | / | / | [46] |
| Cobalt-doped Ni phosphite | 83.6 at 0.5 A g−1 | 85% (0.5–5 A g−1) | 93% after 8000 cycles | [47] |
| (Ni,Co)3(PO4)2·8H2O/(NH4)(Ni, Co)PO4·0.67H2O | 141 at 0.5 A g−1 | 88% (0.5–24 A g−1) | / | [48] |
| Ni-Co phosphate | 147 at 0.2 A g−1 | 85% (0.2–10 A g−1) | / | [49] |
| Co(P, S)/CC | 101.6 at 1 A g−1 | 56% (1–20 A g−1) | / | [50] |
| Ni11(HPO3)8(OH)6/Co3(HPO4)2(OH)2 | 163.2 at 1 A g−1 | 96.9% (1–10 A g−1) 91.9% (1–20 A g−1) 71% (1–100 A g−1) | 92.7% after 5000 cycles | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, M.; Long, K.; Liu, R.; Wang, X.; Wu, T.; Zhu, Y.; Liu, L.; Zhang, S.; Zhang, Y.; Liu, C. One-Step Hydrothermally Synthesized Ni11(HPO3)8(OH)6/Co3(HPO4)2(OH)2 Heterostructure with Enhanced Rate Performance for Hybrid Supercapacitor Applications. Batteries 2024, 10, 339. https://doi.org/10.3390/batteries10100339
Jing M, Long K, Liu R, Wang X, Wu T, Zhu Y, Liu L, Zhang S, Zhang Y, Liu C. One-Step Hydrothermally Synthesized Ni11(HPO3)8(OH)6/Co3(HPO4)2(OH)2 Heterostructure with Enhanced Rate Performance for Hybrid Supercapacitor Applications. Batteries. 2024; 10(10):339. https://doi.org/10.3390/batteries10100339
Chicago/Turabian StyleJing, Mingjun, Kaige Long, Rui Liu, Xingyu Wang, Tianjing Wu, Yirong Zhu, Lijie Liu, Sheng Zhang, Yang Zhang, and Cheng Liu. 2024. "One-Step Hydrothermally Synthesized Ni11(HPO3)8(OH)6/Co3(HPO4)2(OH)2 Heterostructure with Enhanced Rate Performance for Hybrid Supercapacitor Applications" Batteries 10, no. 10: 339. https://doi.org/10.3390/batteries10100339
APA StyleJing, M., Long, K., Liu, R., Wang, X., Wu, T., Zhu, Y., Liu, L., Zhang, S., Zhang, Y., & Liu, C. (2024). One-Step Hydrothermally Synthesized Ni11(HPO3)8(OH)6/Co3(HPO4)2(OH)2 Heterostructure with Enhanced Rate Performance for Hybrid Supercapacitor Applications. Batteries, 10(10), 339. https://doi.org/10.3390/batteries10100339









