Honeycomb-like N-Doped Carbon Matrix-Encapsulated Co1−xS/Co(PO3)2 Heterostructures for Advanced Lithium-Ion Capacitors
Abstract
1. Introduction
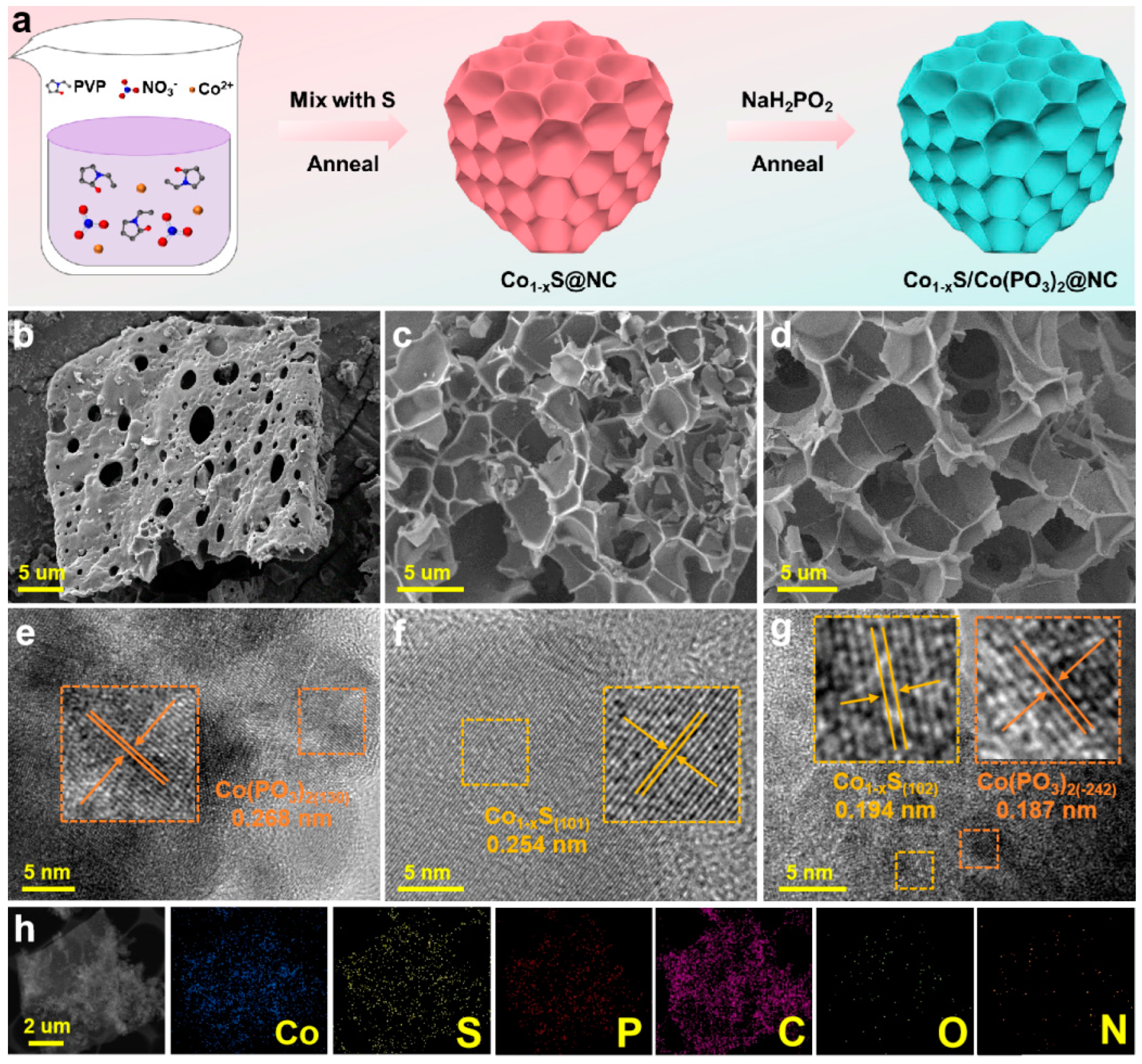
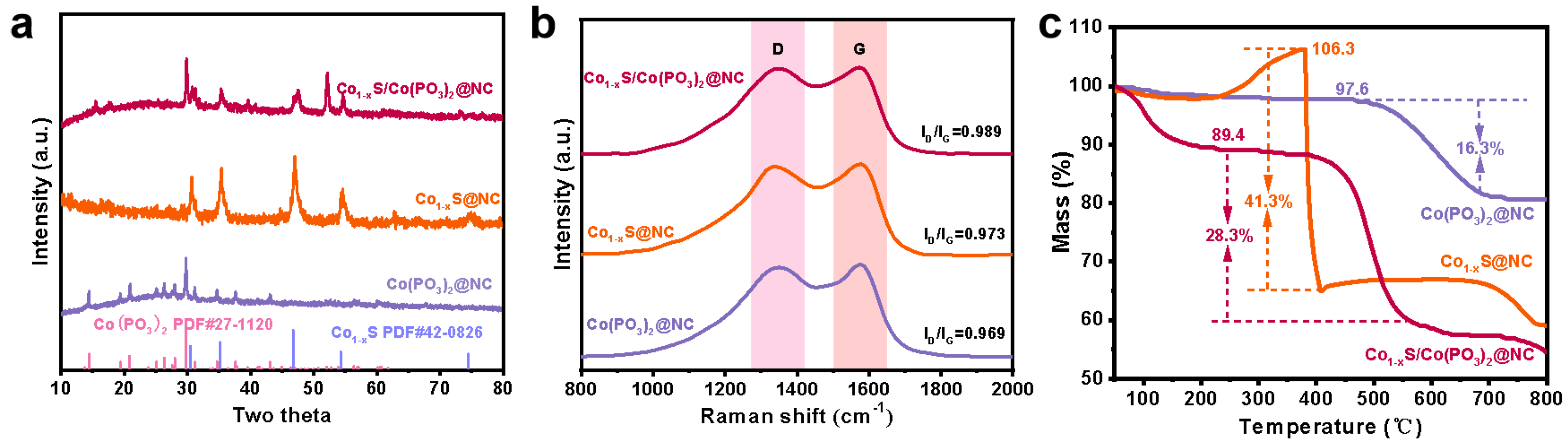
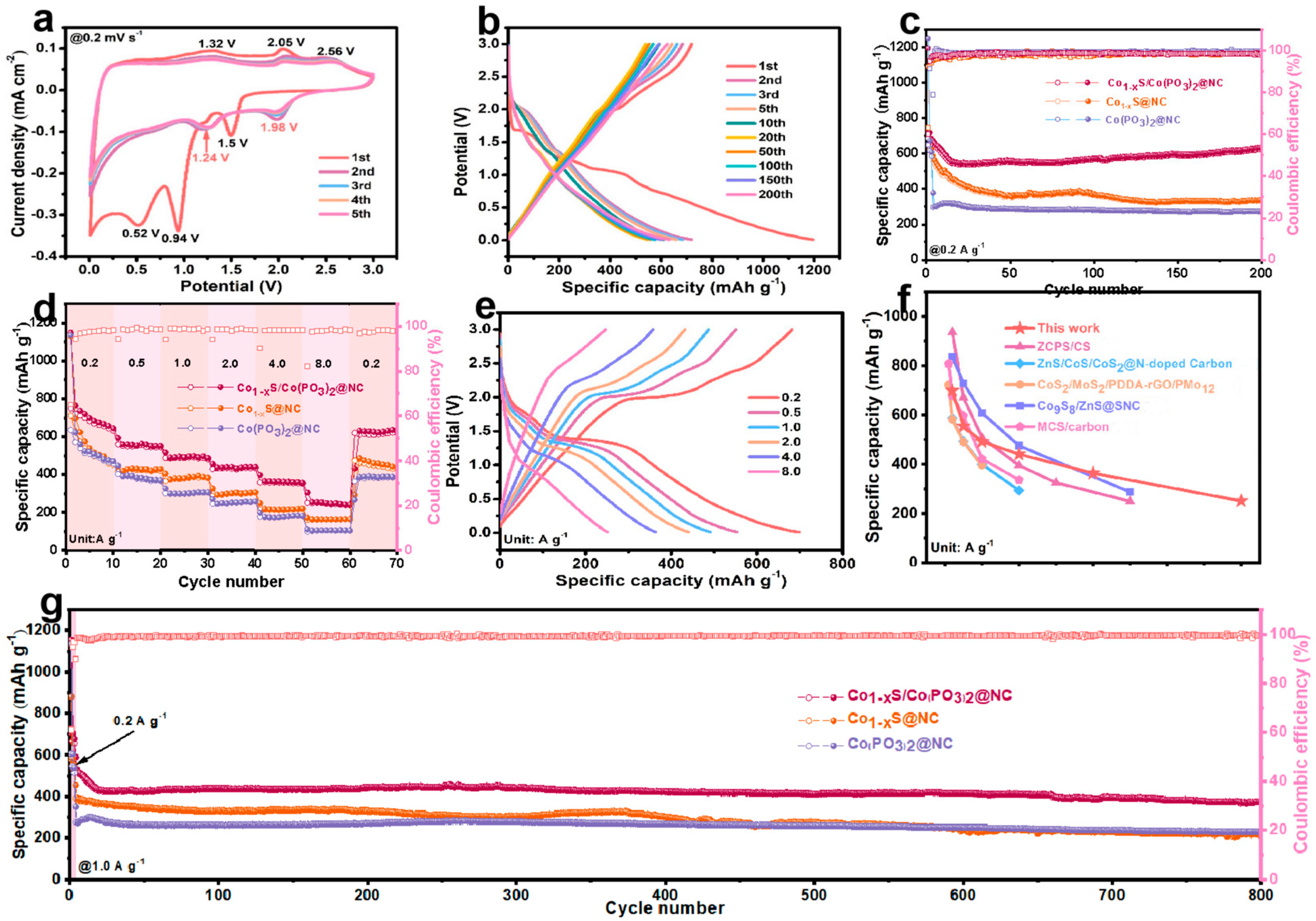
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, J.L.; Luo, B.C.; Li, H.X. A Review on the Conventional Capacitors, Supercapacitors, and Emerging Hybrid Ion Capacitors: Past, Present, and Future. Adv. Energy Sustain. Res. 2022, 3, 2000216–2100191. [Google Scholar] [CrossRef]
- Liang, J.; Wang, D.W. Design rationale and device configuration of lithium-ion capacitors. Adv. Energy Mater. 2022, 12, 2200920. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Li, C.; Sun, X.Z.; Wang, K.W.; Su, F.Y.; Liu, F.Y.; Ma, Y.W. Recent advances in transition metal chalcogenides for lithium-ion capacitors. Rare Met. 2022, 41, 2971–2984. [Google Scholar] [CrossRef]
- Yan, T.; Hu, H.; Duan, J.; Zhu, C.; Wang, Y.; Wen, J.; Li, L.; Xu, Z.; Wen, T.; Yang, P.; et al. Achieving superior sodium storage performance of sulfide heterostructures via copper-driven and electrochemical reconstruction strategy. Chem. Eng. J. 2024, 499, 155871. [Google Scholar] [CrossRef]
- Liang, L.P.; Li, J.C.; Zhu, M.Y.; Li, Y.; Chou, S.L.; Li, W.X. Cobalt Chalcogenides/Cobalt Phosphides/Cobaltates with Hierarchical Nanostructures for Anode Materials of Lithium-Ion Batteries: Improving the Lithiation Environment. Small 2021, 17, 1903418. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, K.; Lei, K.X.; Li, F.J.; Tao, Z.L.; Chen, J. Facile synthesis and electrochemical sodium storage of CoS2 micro/nano-structures. Nano Res. 2016, 9, 198–206. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Fang, Y.J.; Zhang, J.T.; Gao, S.Y.; Lou, X.W. Synthesis of cobalt sulfide multi-shelled nanoboxes with precisely controlled two to five shells for sodium-ion batteries. Angew. Chem. Int. Ed. 2019, 58, 2675–2679. [Google Scholar] [CrossRef]
- Xiao, Y.; Hwang, J.Y.; Belharouak, I.; Sun, Y.K. Superior Li/Na-storage capability of a carbon-free hierarchical CoSx hollow nanostructure. Nano Energy 2017, 32, 320–328. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; He, L.; Kong, X.R.; Song, Y.; Zhao, Y. Three-dimensional porous N-doped graphite carbon with embedded CoS2 nanoparticles as advanced anode for sodium-ion batteries. Appl. Surf. Sci. 2022, 603, 154481. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Kong, Y.Y.; Li, C.; An, Y.B.; Sun, X.Z.; Wang, K.; Ma, Y.W. Metal-organic framework-derived CoSe2@ N-doped carbon nanocubes for high-performance lithium-ion capacitors. Rare Met. 2024, 43, 2150–2160. [Google Scholar] [CrossRef]
- Li, Q.; Jiao, Q.Z.; Yan, Y.; Li, H.J.; Zhou, W.; Gu, T.T.; Shen, X.R.; Lu, C.X.; Zhao, Y.; Zhang, Y.Y.; et al. Optimized Co-S bonds energy and confinement effect of hollow MXene@CoS2/NC for enhanced sodium storage kinetics and stability. Chem. Eng. J. 2022, 450, 137922. [Google Scholar] [CrossRef]
- Lei, D.; Gao, Y.; Hou, Z.; Ren, L.; Jiang, M.; Cao, Y.; Zhang, Y.; Wang, J.-G. A Superior Lithium-Ion Capacitor Based on Ultrafine MnO/Dual N-Doped Carbon Anode and Porous Carbon Cathode. Batteries 2023, 9, 241. [Google Scholar] [CrossRef]
- Lin, Y.; Qiu, Z.; Li, D.; Ullah, S.; Hai, Y.; Xin, H.; Liao, W.; Yang, B.; Fan, H.; Xu, J.; et al. NiS2@CoS2 nanocrystals encapsulated in N-doped carbon nanocubes for high performance lithium/sodium ion batteries. Energy Storage Mater. 2018, 11, 67–74. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Y.G.; Kong, L.B. Cleverly embedded CoS2/NiS2 on two-dimensional graphene nanosheets as high-performance anode material for improved sodium ion batteries and sodium ion capacitors. J. Mater. Sci. Mater. Electron. 2020, 31, 9946–9959. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, S.S.; Zhang, X.; Xu, Y.N.; An, Y.B.; Li, C.; Yi, S.; Liu, C.; Wang, K.; Sun, X.Z.; et al. In Situ Construction of Bimetallic Selenides Heterogeneous Interface on Oxidation-Stable Ti3C2Tx MXene Toward Lithium Storage with Ultrafast Charge Transfer Kinetics. Small 2024, 2403078. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.F.; Fan, F.F.; Zhu, Z.C.; Zhang, Z.G.; Gu, Y.F.; Li, Q.H. MoS2/CoS heterostructures grown on carbon cloth as free-standing anodes for high-performance sodium-ion batteries. Nanoscale 2023, 15, 6822–6829. [Google Scholar] [CrossRef]
- Cai, J.M.; Zhou, Y.L.; Tao, S.S.; Liu, Y.C.; Deng, W.T.; Hou, H.H.; Zou, G.Q.; Ji, X.B. Nanocrystalline Heterostructure with Low Voltage Hysteresis for Ultrahigh-Power Sodium-Ion Capacitors. Energy Storage Mater. 2024, 71, 103582. [Google Scholar] [CrossRef]
- Xiao, Y.H.; Kong, Y.; Wang, X.Z.; Luo, H.R.; Yuan, G.Z.; Zhang, S.W.; Zhang, A.Q.; Zhou, J.; Fan, Y.Y.; Xin, L.; et al. Synergetic interface engineering and space-confined effect in CoSe2@Ti3C2Tx heterostructure for high power and long life sodium ion capacitors. J. Colloid Interface Sci. 2025, 677, 577–586. [Google Scholar] [CrossRef]
- Dong, C.F.; Guo, L.J.; Li, H.B.; Zhang, B.; Gao, X.; Tian, F.; Qian, Y.T.; Wang, D.B.; Xu, L.Q. Rational fabrication of CoS2/Co4S3@N-doped carbon microspheres as excellent cycling performance anode for half/full sodium ion batteries. Energy Storage Mater. 2020, 25, 679–686. [Google Scholar] [CrossRef]
- Wan, S.Y.; Cheng, M.; Chen, H.Y.; Zhu, H.J.; Liu, Q.M. Nanoconfined bimetallic sulfides (CoSn)S heterostructure in carbon microsphere as a high-performance anode for half/full sodium-ion batteries. J. Colloid Interface Sci. 2022, 609, 403–413. [Google Scholar] [CrossRef]
- Zhao, M.T.; Lu, Q.P.; Ma, Q.L.; Zhang, H. Two-Dimensional Metal-Organic Framework Nanosheets. Small Methods 2017, 1, 1600030. [Google Scholar] [CrossRef]
- Yang, M.; Yan, Z.H.; Zhang, H.; Li, J.W.; Zhu, Z.Q.; Liu, L.; Jiao, L.F. Controllable Synthesis of Sub-10 nm ZnS Nanograins Confined in Micro-Size Carbon Skeleton for Aqueous Zn-S Batteries. Adv. Funct. Mater. 2024, 2406077–2406085. [Google Scholar] [CrossRef]
- Wen, F.; Zhu, C.; Li, L.; Zeng, G.; Duan, J.; Chen, Z. Enhanced pseudocapacitive behaviors of Sb-based anodes for lithium ion batteries via dual modification approach of Fe doping combined with double carbon coatings. J. Alloys Compd. 2021, 889, 161658. [Google Scholar] [CrossRef]
- Wu, Y.L.; Zhua, C.; Shu, L.; Duan, J.F.; Wei, D.H.; Xu, J.X.; Zhu, Z.Y.; Li, L.J.; Peng, Z.Y.; Chen, Z.Y. Co9S8 confined in bifunctional N/S co-doped carbon/carbon with high electrochemical performance for lithium-ion batteries. Appl. Surf. Sci. 2019, 489, 528–537. [Google Scholar] [CrossRef]
- Yan, T.C.; Wen, F.; Duan, J.F.; Zhu, C.; Wen, J.H.; Wang, Y.X.; Tong, J.T.; Chen, Z.Y. Fabricating tunable metal sulfides embedded with honeycomb-structured N-doped carbon matrices for high-performance lithium-ion capacitors. Chem. Eng. J. 2023, 474, 145839–145850. [Google Scholar] [CrossRef]
- Huang, G.; Hu, M.; Xu, X.T.; Alothman, A.A.; Mushab, M.S.S.; Ma, S.J.; Shen, P.K.; Zhu, J.L.; Yamauchi, Y. Optimizing Heterointerface of Co2P-CoxOy Nanoparticles within a Porous Carbon Network for Deciphering Superior Water Splitting. Small Struct. 2023, 4, 2200235–2200244. [Google Scholar] [CrossRef]
- Tan, L.; Huang, X.; Yin, T.; Guo, Y.; Ning, T.; Mei, Y.; Zou, K.; Li, L.; Ji, X.; Zou, G. A 5 V ultrahigh energy density lithium metal capacitor enabled by the fluorinated electrolyte. Energy Storage Mater. 2024, 71, 103692. [Google Scholar] [CrossRef]
- Cheng, Q.; Liu, X.Z.; Deng, Q.; Chen, C.D.; Zhong, W.T.; Yang, C.H. Heterostructured Ni3S4/Co9S8 Encapsulated in Nitrogen-Doped Carbon Nanocubes for Advanced Potassium Storage. Chem. Eng. J. 2022, 446, 136829–136836. [Google Scholar] [CrossRef]
- Zhang, J.F.; Sun, C.H.; Qu, S.Q.; Qian, M.M.; Zhan, W.; Su, A.Q.; Zhang, K.; Liu, Q.; Shao, R.W.; Wang, J.; et al. Paradigm metallothermic-sulfidation-carbonization constructing ZIFs-derived TMSs@Graphene/CNx heterostructures for high-capacity and long-life energy storage. Nano Energy 2023, 111, 108401–108410. [Google Scholar] [CrossRef]
- Shen, M.; Ma, H. Metal-organic frameworks (MOFs) and their derivative as electrode materials for lithium-ion batteries, Coordination. Chem. Rev. 2022, 470, 214715. [Google Scholar]
- Shi, X.; Li, G.; Liu, X.; Li, J.; Zhang, X.; Guo, J. Carbon coated Co(PO3)2/CoSe2 heterostructure as high performance sodium storage anode. J. Alloys Compd. 2023, 951, 169989. [Google Scholar] [CrossRef]
- Zhong, H.Y.; Lu, X.; Zhong, Y.; Zhao, Y.; Liu, X.M.; Cheng, D.H.; Huang, X.Y.; Du, K.Z.; Wu, X.H. Lithium Storage Performance Boosted via Delocalizing Charge in ZnxCo1−xPS3/CoS2 of 2D/3D Heterostructure. Small 2022, 18, 2104295. [Google Scholar] [CrossRef]
- Cheng, W.; Di, H.F.; Shi, Z.; Zhang, D. Synthesis of ZnS/CoS/CoS2@N-doped carbon nanoparticles derived from metal-organic frameworks via spray pyrolysis as anode for lithium-ion battery. J. Alloys Compd. 2020, 831, 154607. [Google Scholar] [CrossRef]
- Li, Q.; Xu, M.Q.; Wang, T.; Wang, H.J.; Sun, J.W.; Sha, J.Q. Nanohybridization of CoS2/MoS2 Heterostructure with Polyoxometalate on Functionalized Reduced Graphene Oxide for High-Performance LIBs. Chem. A Eur. J. 2022, 28, e202200207. [Google Scholar] [CrossRef] [PubMed]
- Nong, Y.T.; Zhang, M.; Li, Q.Q.; Pan, Q.C.; Huang, Y.G.; Wang, H.Q.; Zheng, F.H.; Li, Q.Y. Carbon coated bimetallic sulfides Co9S8/ZnS heterostructures microrods as advanced anode materials for lithium ion batteries. J. Taiwan Inst. Chem. Eng. 2022, 141, 104601. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, H.Y.; Xiao, F.P.; Yao, T.H.; Liu, T.; Li, F.; Wang, J.K.; Han, X.G.; Cheng, Y.H.; Wang, H.K. Flower-like Mn/Co Glycerolate-Derived ɑ-MnS/Co9S8/Carbon Heterostructures for High-Performance Lithium-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 10215. [Google Scholar] [CrossRef]
- Hou, J.B.; Shao, Y.Y.; Ellis, M.W.; Moore, R.B.; Yi, B.L. Graphene-based electrochemical energy conversion and storage: Fuel cells, supercapacitors and lithium ion batteries. Phys. Chem. Chem. Phys. 2011, 13, 15384–15402. [Google Scholar] [CrossRef]
- Tao, S.S.; Momen, R.; Luo, Z.; Zhu, Y.R.; Xiao, X.H.; Cao, Z.W.; Xiong, D.Y.; Deng, W.T.; Liu, Y.C.; Hou, H.S.; et al. Trapping Lithium Selenides with Evolving Heterogeneous Interfaces for High-Power Lithium-Ion Capacitors. Small 2023, 19, 2207975. [Google Scholar] [CrossRef]
- Shi, Z.C.; Wei, S.; Zuo, H.; Huang, M.H.; Shi, J.; Wang, H.L. Boosting capacitance and energy density by construction NiCoO2/CoS2 nanocomposites arrays as pseudocapacitor. J. Alloys Compd. 2021, 881, 160627. [Google Scholar] [CrossRef]
- Wang, S.Q.; Song, Y.P.; Ma, Y.; Zhu, Z.Q.; Zhao, C.H.; Zhao, C.J. Attaining a high energy density of 106 Wh kg−1 for aqueous supercapacitor based on VS4/rGO/CoS2@Co electrode. Chem. Eng. J. 2019, 365, 88. [Google Scholar] [CrossRef]
- Sonia, Y.K.; Paliwal, M.K.; Meher, S.K. The rational design of hierarchical CoS2/CuCo2S4 for three-dimensional all-solid-state hybrid supercapacitors with high energy density, rate efficiency, and operational stability. Sustain. Energy Energy Fuels 2021, 5, 973. [Google Scholar] [CrossRef]
- Li, X.N.; Zhang, W.Y.; Kang, H.W.; Liu, H.L.; Yang, B.C.; Li, Z.J.; Li, Z.K. Self-assembled CoS2/NiCo2S4/RGO nanohybrids as advanced electrode for hybrid supercapacitor with enhanced energy density and ultra-long durability. J. Energy Storage 2023, 67, 107528. [Google Scholar] [CrossRef]
- Wang, Y.K.; Liu, M.C.; Cao, J.Y.; Zhang, H.J.; Kong, L.B.; Trudgeon, D.P.; Li, X.H.; Frank, C.W. 3D Hierarchically Structured CoS Nanosheets: Li+ Storage Mechanism and Application of the High-Performance Lithium-Ion Capacitors. ACS Appl. Mater. Interfaces 2020, 12, 3709. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Tian, L.H.; Zhao, X.; Ali, M.; Feng, H.M.; Han, S.Y.; Xing, Z.C.; Kumar, S.; Ding, J. Synthesis of MoS2/CoS composite electrode and its application for supercapacitors. J. Alloys Compd. 2023, 960, 170835. [Google Scholar] [CrossRef]
- Reddy, P.A.K.; Han, H.; Kim, K.C.; Bae, S. Synthesis of ZIF-67-derived CoS2@graphitic carbon/reduced graphene oxide for supercapacitor application. Chem. Eng. J. 2023, 471, 144608. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, X.; Yao, F.; Xie, Y.Q.; Bai, H.; Sun, Y.J.; Liu, R.R.; Yue, H.Y. Construction of hierarchical MOF-derived CoS2 microsheet arrays@NiMo2S4 nanoflakes on Ni foam as a high-performance supercapacitor electrode. J. Colloid Interface Sci. 2023, 650, 105. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Xie, X.; Wu, Z.; Wen, T.; Zhao, F.; He, H.; Duan, J.; Wang, W. Honeycomb-like N-Doped Carbon Matrix-Encapsulated Co1−xS/Co(PO3)2 Heterostructures for Advanced Lithium-Ion Capacitors. Batteries 2024, 10, 346. https://doi.org/10.3390/batteries10100346
Liu Y, Xie X, Wu Z, Wen T, Zhao F, He H, Duan J, Wang W. Honeycomb-like N-Doped Carbon Matrix-Encapsulated Co1−xS/Co(PO3)2 Heterostructures for Advanced Lithium-Ion Capacitors. Batteries. 2024; 10(10):346. https://doi.org/10.3390/batteries10100346
Chicago/Turabian StyleLiu, Yutao, Xiaopeng Xie, Zhaojia Wu, Tao Wen, Fang Zhao, Hao He, Junfei Duan, and Wen Wang. 2024. "Honeycomb-like N-Doped Carbon Matrix-Encapsulated Co1−xS/Co(PO3)2 Heterostructures for Advanced Lithium-Ion Capacitors" Batteries 10, no. 10: 346. https://doi.org/10.3390/batteries10100346
APA StyleLiu, Y., Xie, X., Wu, Z., Wen, T., Zhao, F., He, H., Duan, J., & Wang, W. (2024). Honeycomb-like N-Doped Carbon Matrix-Encapsulated Co1−xS/Co(PO3)2 Heterostructures for Advanced Lithium-Ion Capacitors. Batteries, 10(10), 346. https://doi.org/10.3390/batteries10100346






