Experimental Study of Magnetocaloric Effect in Tetraaquabis(Hydrogen Maleato)Nickel(II), [Ni(C4H3O4)2(H2O)4]—A Potential Realization of a Spin-1 Spatially Anisotropic Square Lattice with Ferromagnetic Interactions
Abstract
1. Introduction
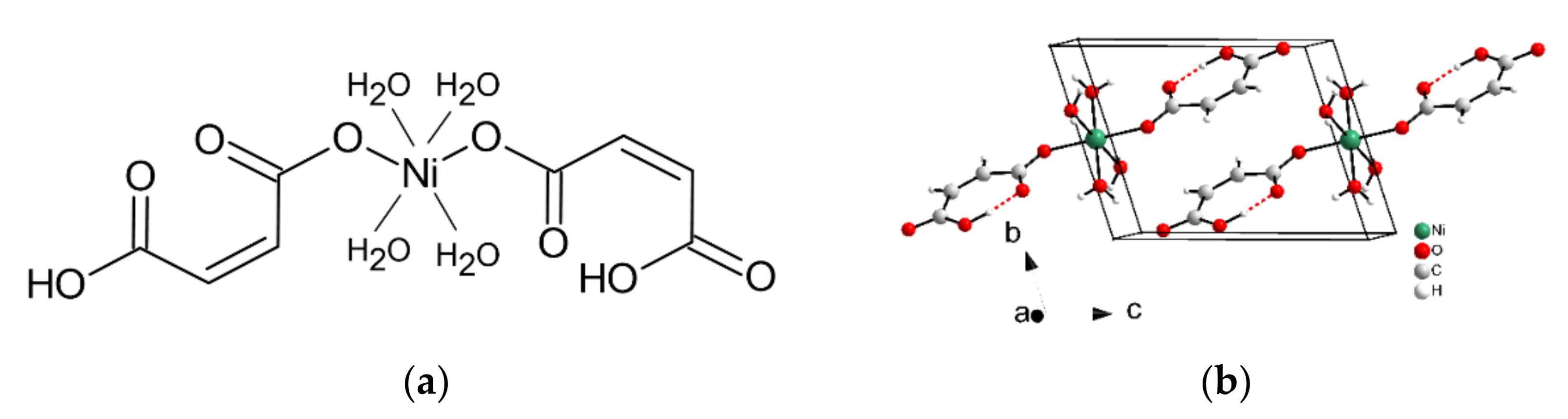
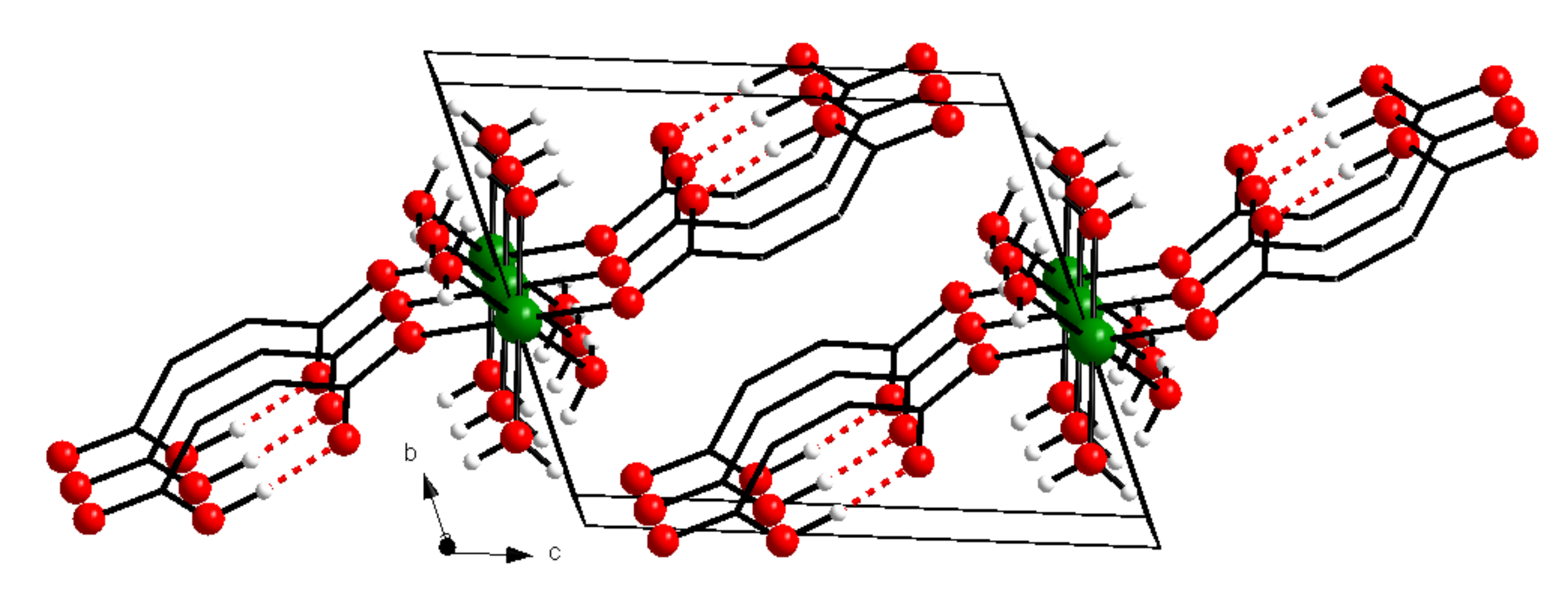
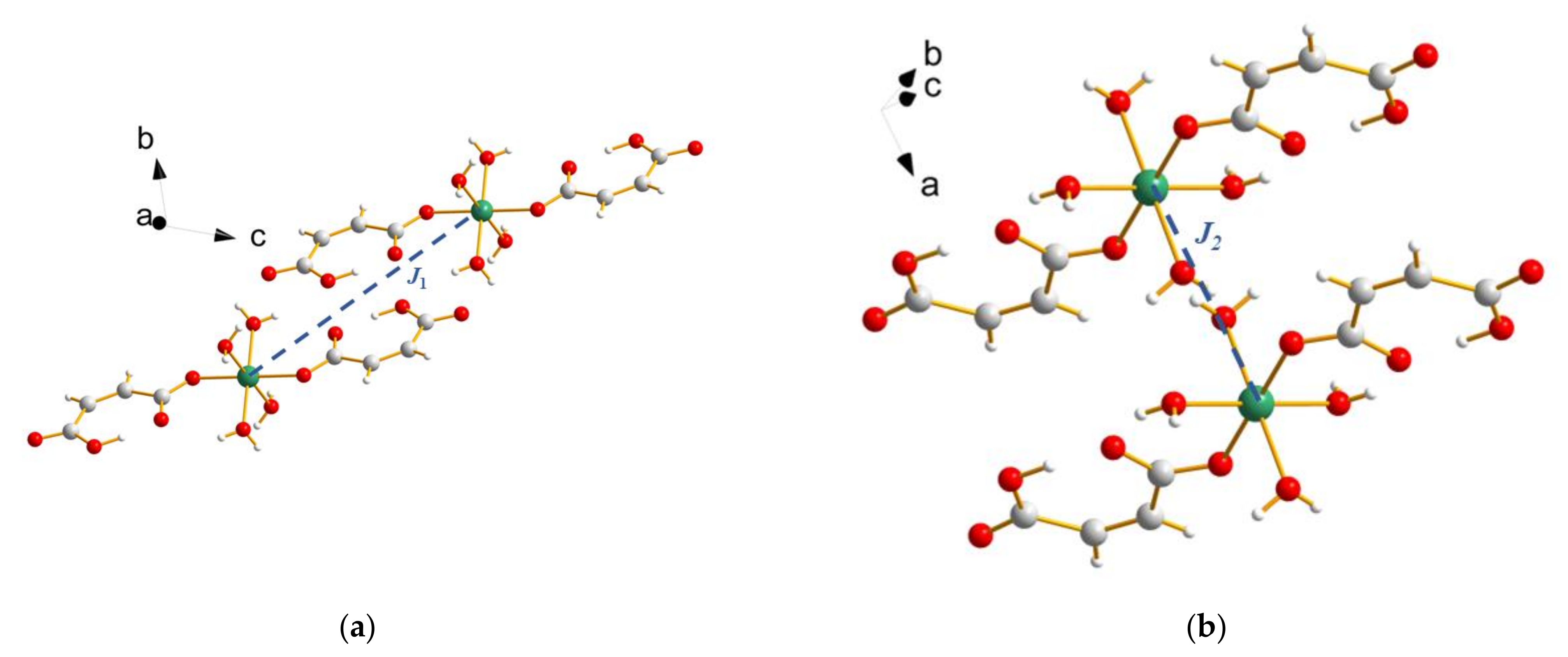
2. Materials and Methods
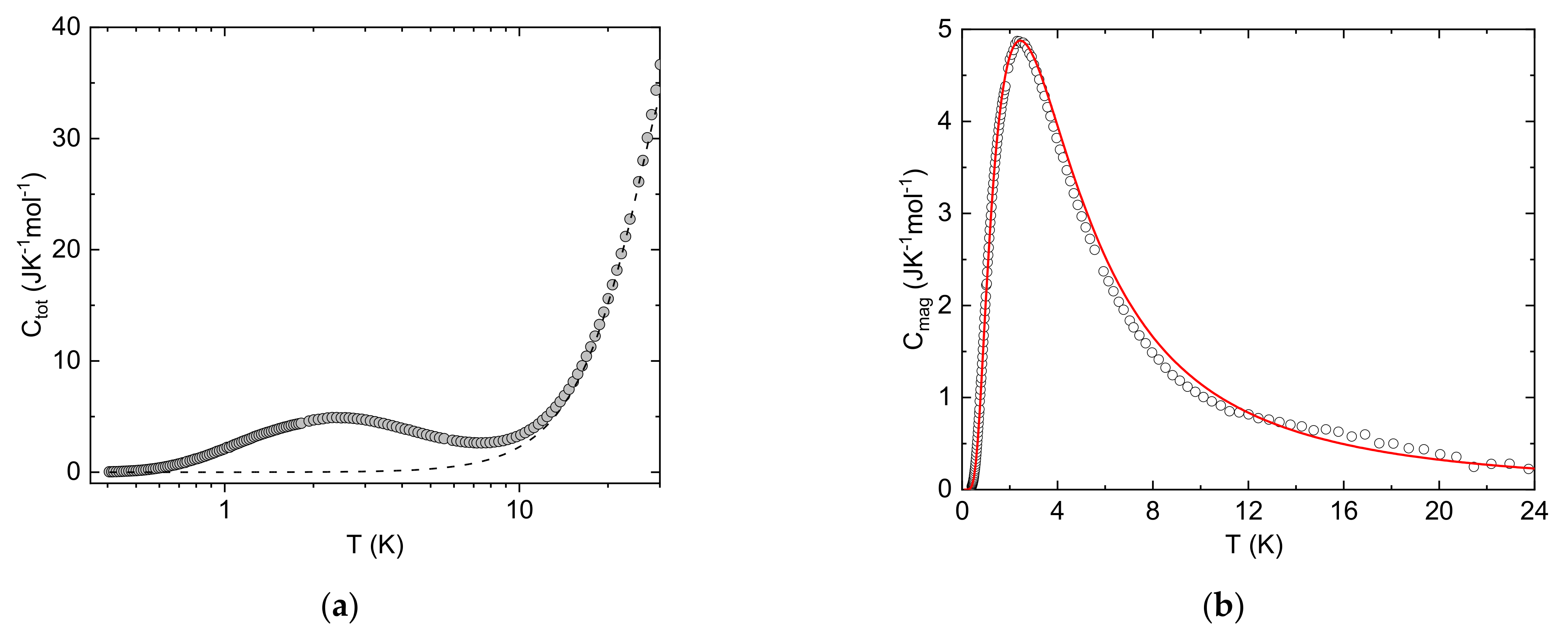
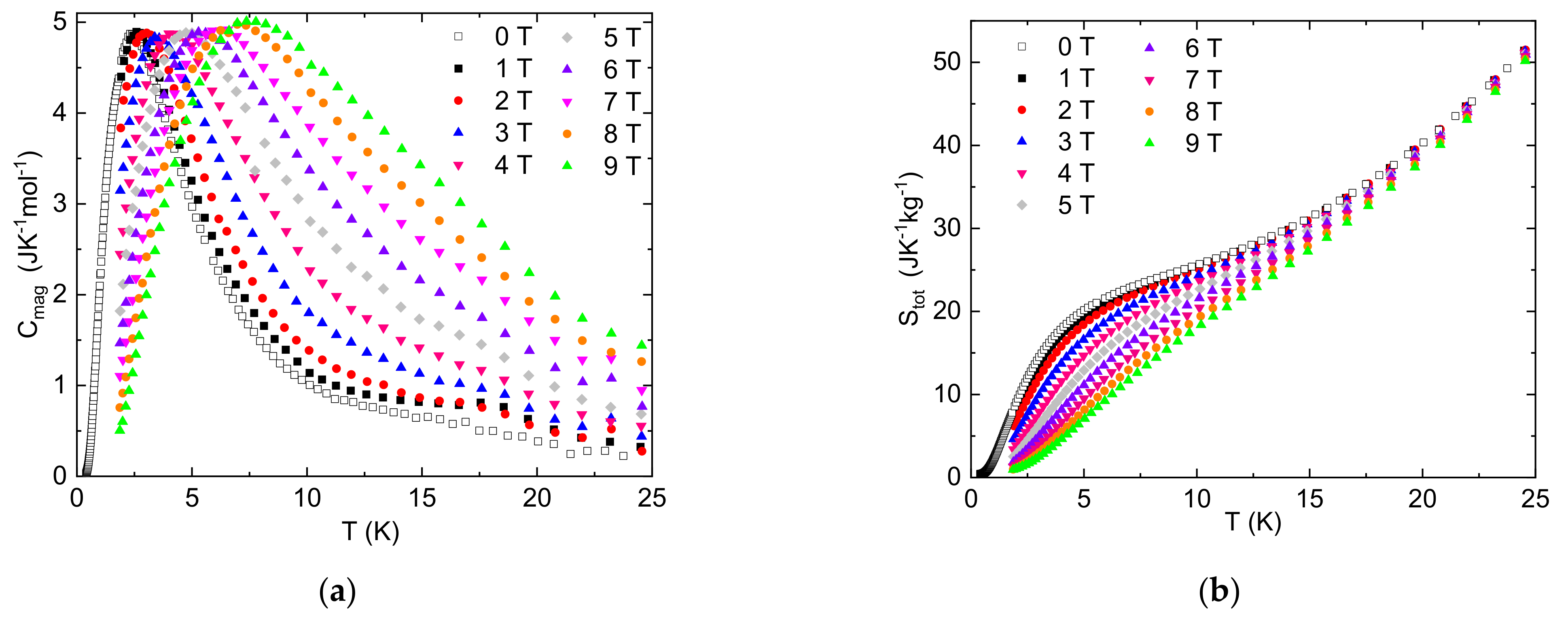
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sessoli, R. Chilling with Magnetic Molecules. Angew. Chem.-Int. Ed. 2012, 51, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Pęczkowski, P.; Łuszczek, M.; Szostak, E.; Muniraju, N.K.C.; Krztoń-Maziopa, A.; Gondek, Ł. Superconductivity and Appearance of Negative Magnetocaloric Effect in Ba1–XKxBiO3 Perovskites, Doped by Y, La and Pr. Acta Mater. 2022, 222, 117437. [Google Scholar] [CrossRef]
- Karl’ová, K.; Strečka, J.; Haniš, J.; Hagiwara, M. Insights into Nature of Magnetization Plateaus of a Nickel Complex [Ni4(μ-CO3)2(Aetpy)8](ClO4)4 from a Spin-1 Heisenberg Diamond Cluster. Magnetochemistry 2020, 6, 59. [Google Scholar] [CrossRef]
- Oyarzabal, I.; Echenique-Errandonea, E.; San Sebastián, E.; Rodríguez-Diéguez, A.; Seco, J.M.; Colacio, E. Synthesis, Structural Features and Physical Properties of a Family of Triply Bridged Dinuclear 3d-4f Complexes. Magnetochemistry 2021, 7, 22. [Google Scholar] [CrossRef]
- Spichkin, Y.I.; Zvezdin, A.K.; Gubin, S.P.; Mischenko, A.S.; Tishin, A.M. Magnetic Molecular Clusters as Promising Materials for Refrigeration in Low-Temperature Regions. J. Phys. D Appl. Phys. 2001, 34, 1162–1166. [Google Scholar] [CrossRef]
- Franco, V.; Blázquez, J.S.; Ipus, J.J.; Law, J.Y.; Moreno-Ramírez, L.M.; Conde, A. Magnetocaloric Effect: From Materials Research to Refrigeration Devices. Prog. Mater. Sci. 2018, 93, 112–232. [Google Scholar] [CrossRef]
- Tegus, O.; Brück, E.; Buschow, K.H.J.; De Boer, F.R. Transition-Metal-Based Magnetic Refrigerants for Room-Temperature Applications. Nature 2002, 415, 150–152. [Google Scholar] [CrossRef]
- Barclay, J.A.; Steyert, W.A. Materials for Magnetic Refrigeration between 2 K and 20 K. Cryogenics 1982, 22, 73–80. [Google Scholar] [CrossRef]
- Czaja, P.; Wróblewski, R.; Grzonka, J.; Przewoźnik, J.; Maziarz, W. Microstructure, Martensitic Transformation, and Inverse Magnetocaloric Effect in Ni48Mn39.5Sn12.5−xAlx Metamagnetic Shape Memory Alloys. Magnetochemistry 2018, 4, 19. [Google Scholar] [CrossRef]
- Balli, M.; Mansouri, S.; Jandl, S.; Fournier, P.; Dimitrov, D. Analysis of the Anisotropic Magnetocaloric Effect in RMn2O5 Single Crystals. Magnetochemistry 2017, 3, 36. [Google Scholar] [CrossRef]
- Zangana, K. Synthesis, Crystal Structures, and Magnetic Properties of Lanthanide (III) Amino-Phosphonate Complexes. Magnetochemistry 2018, 4, 29. [Google Scholar] [CrossRef]
- Castañeda, E.J.G.; Castro, R.E.B.; Briseño, A.C.; Arguijo, B.F.; Castillo, A.A.T.; Rodríguez, A.S.; Galindo, J.T.E.; Sánchez, S.A.P. Effect of Quenching and Normalizing on the Microstructure and Magnetocaloric Effect of a Cu–11Al–9Zn Alloy with 6.5 Wt % Ni–2.5 Wt % Fe. Magnetochemistry 2019, 5, 48. [Google Scholar] [CrossRef]
- Gschneidner, A.; Pecharsky, V.K.; Tsokol, A.O. Recent Developments in Magnetocaloric Materials. Rep. Prog. Phys. 2005, 68, 1479–1539. [Google Scholar] [CrossRef]
- Guo, D.Q.; Chan, K.C.; Xia, L.; Yu, P. Magneto-Caloric Effect of FexZryB100−x−y Metallic Ribbons for Room Temperature Magnetic Refrigeration. J. Magn. Magn. Mater. 2017, 423, 379–385. [Google Scholar] [CrossRef]
- Pecharsky, V.K.; Gschneidner, K.A. Magnetocaloric Effect and Magnetic Refrigeration. J. Magn. Magn. Mater. 1999, 200, 44–56. [Google Scholar] [CrossRef]
- Tishin, A.M.; Spichkin, Y.I. The Magnetocaloric Effect and Its Applications; CRC Press: Boca Raton, FL, USA, 2003; p. 475. [Google Scholar]
- Wu, Y.D.; Duan, W.W.; Li, Q.Y.; Geng, W.; Zhang, C.; Lv, Q.Q.; He, L.; Chen, J.Q.; Hu, X.Y.; Qin, Y.L.; et al. Giant Conventional and Rotating Magnetocaloric Effects in TbScO3 Single Crystal. J. Alloys Compd. 2022, 894, 162447. [Google Scholar] [CrossRef]
- Wu, Y.D.; Qin, Y.L.; Ma, X.H.; Li, R.W.; Wei, Y.Y.; Zi, Z.F. Large Rotating Magnetocaloric Effect at Low Magnetic Fields in the Ising-like Antiferromagnet DyScO3 Single Crystal. J. Alloys Compd. 2019, 777, 673–678. [Google Scholar] [CrossRef]
- Ke, Y.J.; Zhang, X.Q.; Ma, Y.; Cheng, Z.H. Anisotropic Magnetic Entropy Change in RFeO3 Single Crystals(R = Tb, Tm, or Y). Sci. Rep. 2016, 6, 19775. [Google Scholar] [CrossRef]
- Balli, M.; Jandl, S.; Fournier, P.; Gospodinov, M.M. Anisotropy-Enhanced Giant Reversible Rotating Magnetocaloric Effect in HoMn2O5 Single Crystals. Appl. Phys. Lett. 2014, 104, 232402. [Google Scholar] [CrossRef]
- Balli, M.; Jandl, S.; Fournier, P.; Vermette, J.; Dimitrov, D.Z. Unusual Rotating Magnetocaloric Effect in the Hexagonal ErMn O3 Single Crystal. Phys. Rev. B 2018, 98, 184414. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Wu, Y.D.; Ma, Y.; Dong, Q.Y.; Ke, Y.J.; Cheng, Z.H. Large Rotating Magnetocaloric Effect in ErAlO3 Single Crystal. AIP Adv. 2017, 7, 056418. [Google Scholar] [CrossRef]
- Zhang, H.; Xing, C.; Zhou, H.; Zheng, X.; Miao, X.; He, L.; Chen, J.; Lu, H.; Liu, E.; Han, W.; et al. Giant Anisotropic Magnetocaloric Effect by Coherent Orientation of Crystallographic Texture and Rare-Earth Ion Moments in HoNiSi Ploycrystal. Acta Mater. 2020, 193, 210–220. [Google Scholar] [CrossRef]
- Orendáč, M.; Gabáni, S.; Gažo, E.; Pristáš, G.; Shitsevalova, N.; Siemensmeyer, K.; Flachbart, K. Rotating Magnetocaloric Effect and Unusual Magnetic Features in Metallic Strongly Anisotropic Geometrically Frustrated TmB4. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pęczkowski, P.; Zachariasz, P.; Jastrzębski, C.; Piętosa, J.; Drzymała, E.; Gondek, Ł. On the Superconductivity Suppression in Eu1−xPrxBa2Cu3O7−δ. Materials 2021, 14, 3503. [Google Scholar] [CrossRef]
- Synoradzki, K.; Nowotny, P.; Skokowski, P.; Toliński, T. Magnetocaloric Effect in Gd5(Si,Ge)4 Based Alloys and Composites. J. Rare Earths 2019, 37, 1218–1223. [Google Scholar] [CrossRef]
- Gębara, P. Magnetocaloric Effect of LaFe11.35Co0.6Si1.05 Alloy. Rare Met. 2022, 41, 1563–1569. [Google Scholar] [CrossRef]
- Pecharsky, A.O.; Gschneidner, K.A.; Pecharsky, V.K. The Giant Magnetocaloric Effect of Optimally Prepared Gd5Si2Ge2. J. Appl. Phys. 2003, 93, 4722. [Google Scholar] [CrossRef]
- Alahmer, A.; Al-Amayreh, M.; Mostafa, A.O.; Al-Dabbas, M.; Rezk, H. Magnetic Refrigeration Design Technologies: State of the Art and General Perspectives. Energies 2021, 14, 4662. [Google Scholar] [CrossRef]
- Tarasenko, R.; Danylchenko, P.; Tkáč, V.; Orendáčová, A.; Čižmár, E.; Orendáč, M.; Feher, A. Experimental Study of the Magnetocaloric Effect in [Ni(fum)(phen)]—The Ferromagnetic Dimer with Spin 1. Phys. B Condens. Matter 2020, 576, 411671. [Google Scholar] [CrossRef]
- Orendáč, M.; Tarasenko, R.; Tkáč, V.; Orendáčová, A.; Sechovský, V. Specific Heat Study of the Magnetocaloric Effect in the Haldane-Gap S = 1 Spin-Chain Material [Ni(C2H8N2)2NO2](BF4). Phys. Rev. B 2017, 96, 094425. [Google Scholar] [CrossRef]
- Ráczová, K.; Cižmár, E.; Feher, A. Magnetocaloric Effect in NiCl2(bipy) at Low Temperatures. Acta Phys. Pol. A 2017, 131, 922–924. [Google Scholar] [CrossRef]
- Tarasenko, R.; Orendáčová, A.; Čižmár, E.; Orendáč, M.; Potočňák, I.; Feher, A. Experimental Study of the Magnetocaloric Effect in Ni(en)(H2O)4SO4·2H2O—An S = 1 Molecular Magnet with Easy-Plane Anisotropy. Acta Phys. Pol. A 2017, 131, 904–906. [Google Scholar] [CrossRef]
- Gupta, M.P.; Geise, H.J.; Lenstra, A.T.H. IUCr Tetraaquabis(Hydrogen Maleato)Nickel(II), [Ni(C4H3O4)2(H2O)4]. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1984, 40, 1152–1154. [Google Scholar] [CrossRef]
- Sequeira, A.; Rajagopal, H.; Gupta, M.P.; Vanhouteghem, F.; Lenstra, A.T.H.; Geise, H.J. IUCr Tetraaquabis(Hydrogen Maleato)Zinc(II) by Neutron Diffraction and Tetraaquabis(Hydrogen Maleato)Nickel(II) by High-Order X-Ray Diffraction. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1992, 48, 1192–1197. [Google Scholar] [CrossRef]
- Neese, F. Software Update: The ORCA Program System, Version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Atanasov, M.; Ganyushin, D.; Pantazis, D.A.; Sivalingam, K.; Neese, F. Detailed Ab Initio First-Principles Study of the Magnetic Anisotropy in a Family of Trigonal Pyramidal Iron(II) Pyrrolide Complexes. Inorg. Chem. 2011, 50, 7460–7477. [Google Scholar] [CrossRef] [PubMed]
- Angeli, C.; Cimiraglia, R.; Evangelisti, S.; Leininger, T.; Malrieu, J.P. Introduction of N-Electron Valence States for Multireference Perturbation Theory. J. Chem. Phys. 2001, 114, 10252. [Google Scholar] [CrossRef]
- Angeli, C.; Cimiraglia, R.; Malrieu, J.P. N-Electron Valence State Perturbation Theory: A Spinless Formulation and an Efficient Implementation of the Strongly Contracted and of the Partially Contracted Variants. J. Chem. Phys. 2002, 117, 9138. [Google Scholar] [CrossRef]
- Angeli, C.; Borini, S.; Cestari, M.; Cimiraglia, R. A Quasidegenerate Formulation of the Second Order N-Electron Valence State Perturbation Theory Approach. J. Chem. Phys. 2004, 121, 4043. [Google Scholar] [CrossRef]
- Neese, F. Calculation of the Zero-Field Splitting Tensor on the Basis of Hybrid Density Functional and Hartree-Fock Theory. J. Chem. Phys. 2007, 127, 164112. [Google Scholar] [CrossRef]
- Maurice, R.; Bastardis, R.; de Graaf, C.; Suaud, N.; Mallah, T.; Guihéry, N. Universal Theoretical Approach to Extract Anisotropic Spin Hamiltonians. J. Chem. Theory Comput. 2009, 5, 2977–2984. [Google Scholar] [CrossRef]
- Van Lenthe, E.; Baerends, E.J.; Snijders, J.G. Relativistic Regular Two-component Hamiltonians. J. Chem. Phys. 1998, 99, 4597. [Google Scholar] [CrossRef]
- Van Wüllen, C. Molecular Density Functional Calculations in the Regular Relativistic Approximation: Method, Application to Coinage Metal Diatomics, Hydrides, Fluorides and Chlorides, and Comparison with First-Order Relativistic Calculations. J. Chem. Phys. 1998, 109, 392. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Pantazis, D.A.; Chen, X.Y.; Landis, C.R.; Neese, F. All-Electron Scalar Relativistic Basis Sets for Third-Row Transition Metal Atoms. J. Chem. Theory Comput. 2008, 4, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Nagao, H.; Nishino, M.; Shigeta, Y.; Soda, T.; Kitagawa, Y.; Onishi, T.; Yoshioka, Y.; Yamaguchi, K. Theoretical Studies on Effective Spin Interactions, Spin Alignments and Macroscopic Spin Tunneling in Polynuclear Manganese and Related Complexes and Their Mesoscopic Clusters. Coord. Chem. Rev. 2000, 198, 265–295. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1998, 98, 5648. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158. [Google Scholar] [CrossRef]
- Staroverov, V.N.; Scuseria, G.E.; Tao, J.; Perdew, J.P. Comparative Assessment of a New Nonempirical Density Functional: Molecules and Hydrogen-Bonded Complexes. J. Chem. Phys. 2003, 119, 12129. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Takahara, Y.; Fueno, T. Ab-Initio Molecular Orbital Studies of Structure and Reactivity of Transition Metal-OXO Compounds. In Applied Quantum Chemistry; Reidel: Dordecht, The Netherlands, 1986; pp. 155–184. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Hansen, A.; Becker, U. Efficient, Approximate and Parallel Hartree–Fock and Hybrid DFT Calculations. A ‘Chain-of-Spheres’ Algorithm for the Hartree–Fock Exchange. Chem. Phys. 2009, 356, 98–109. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-Fitting Basis Sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Hellweg, A.; Hättig, C.; Höfener, S.; Klopper, W. Optimized Accurate Auxiliary Basis Sets for RI-MP2 and RI-CC2 Calculations for the Atoms Rb to Rn. Theor. Chem. Acc. 2007, 117, 587–597. [Google Scholar] [CrossRef]
- Liu, J.L.; Chen, Y.C.; Guo, F.S.; Tong, M.L. Recent Advances in the Design of Magnetic Molecules for Use as Cryogenic Magnetic Coolants. Coord. Chem. Rev. 2014, 281, 26–49. [Google Scholar] [CrossRef]


| B3LYP | TPSSh | PBE0 | |
|---|---|---|---|
| J1/kB | 1.50 K | 1.18 K | 0.80 K |
| J2/kB | 1.44 K | 1.04 K | 0.92 K |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danylchenko, P.; Tarasenko, R.; Čižmár, E.; Tkáč, V.; Uhrinová, A.; Orendáčová, A.; Orendáč, M. Experimental Study of Magnetocaloric Effect in Tetraaquabis(Hydrogen Maleato)Nickel(II), [Ni(C4H3O4)2(H2O)4]—A Potential Realization of a Spin-1 Spatially Anisotropic Square Lattice with Ferromagnetic Interactions. Magnetochemistry 2022, 8, 106. https://doi.org/10.3390/magnetochemistry8090106
Danylchenko P, Tarasenko R, Čižmár E, Tkáč V, Uhrinová A, Orendáčová A, Orendáč M. Experimental Study of Magnetocaloric Effect in Tetraaquabis(Hydrogen Maleato)Nickel(II), [Ni(C4H3O4)2(H2O)4]—A Potential Realization of a Spin-1 Spatially Anisotropic Square Lattice with Ferromagnetic Interactions. Magnetochemistry. 2022; 8(9):106. https://doi.org/10.3390/magnetochemistry8090106
Chicago/Turabian StyleDanylchenko, Petro, Róbert Tarasenko, Erik Čižmár, Vladimír Tkáč, Anna Uhrinová, Alžbeta Orendáčová, and Martin Orendáč. 2022. "Experimental Study of Magnetocaloric Effect in Tetraaquabis(Hydrogen Maleato)Nickel(II), [Ni(C4H3O4)2(H2O)4]—A Potential Realization of a Spin-1 Spatially Anisotropic Square Lattice with Ferromagnetic Interactions" Magnetochemistry 8, no. 9: 106. https://doi.org/10.3390/magnetochemistry8090106
APA StyleDanylchenko, P., Tarasenko, R., Čižmár, E., Tkáč, V., Uhrinová, A., Orendáčová, A., & Orendáč, M. (2022). Experimental Study of Magnetocaloric Effect in Tetraaquabis(Hydrogen Maleato)Nickel(II), [Ni(C4H3O4)2(H2O)4]—A Potential Realization of a Spin-1 Spatially Anisotropic Square Lattice with Ferromagnetic Interactions. Magnetochemistry, 8(9), 106. https://doi.org/10.3390/magnetochemistry8090106






