Li3V2(PO4)3/Li3PO4 Cathode Materials for Li-Ion Batteries: Synthesis and Characterization
Abstract
:1. Introduction
2. Experimental Results
2.1. Sample Synthesis and Characterization
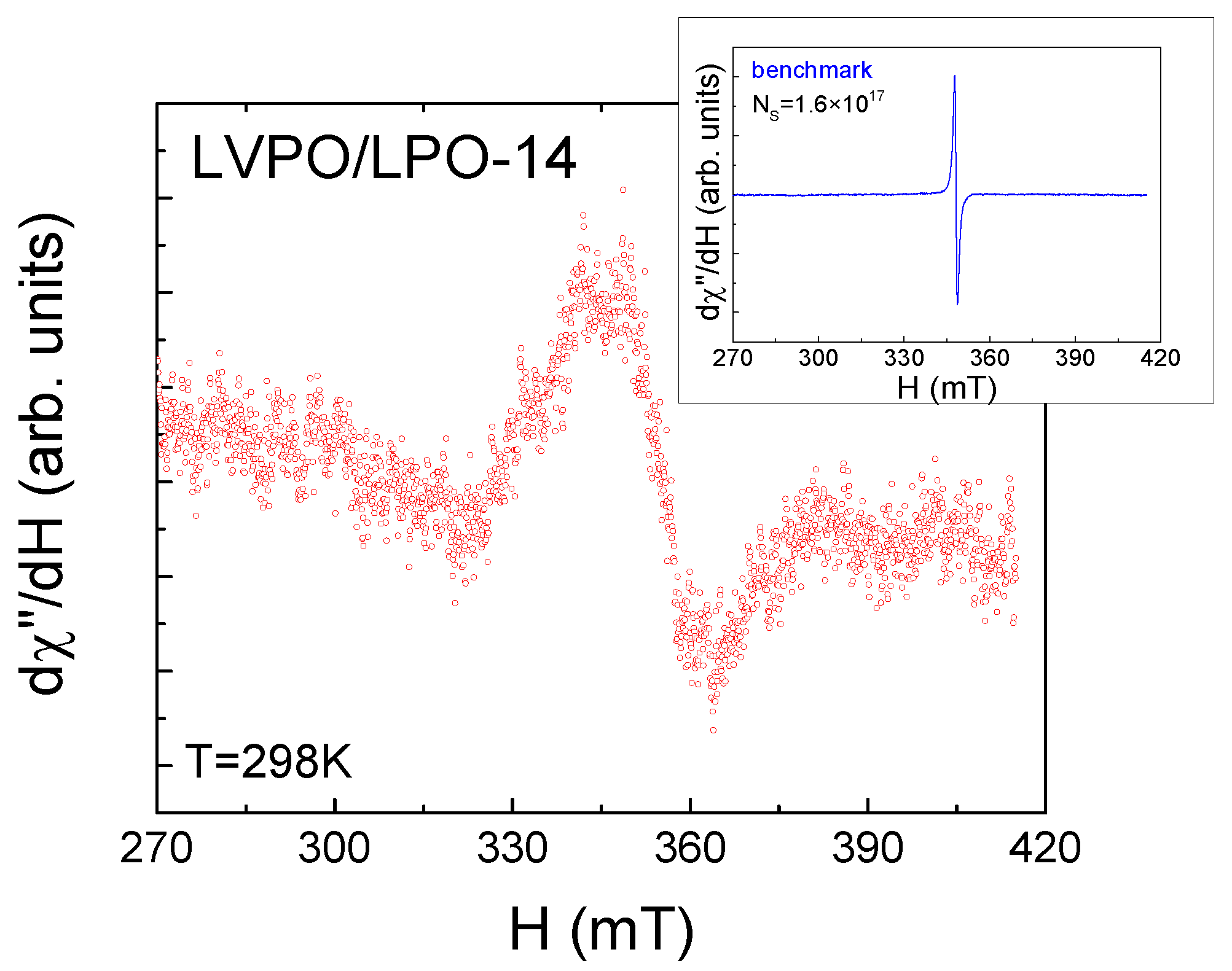
2.2. Electron Spin Resonance
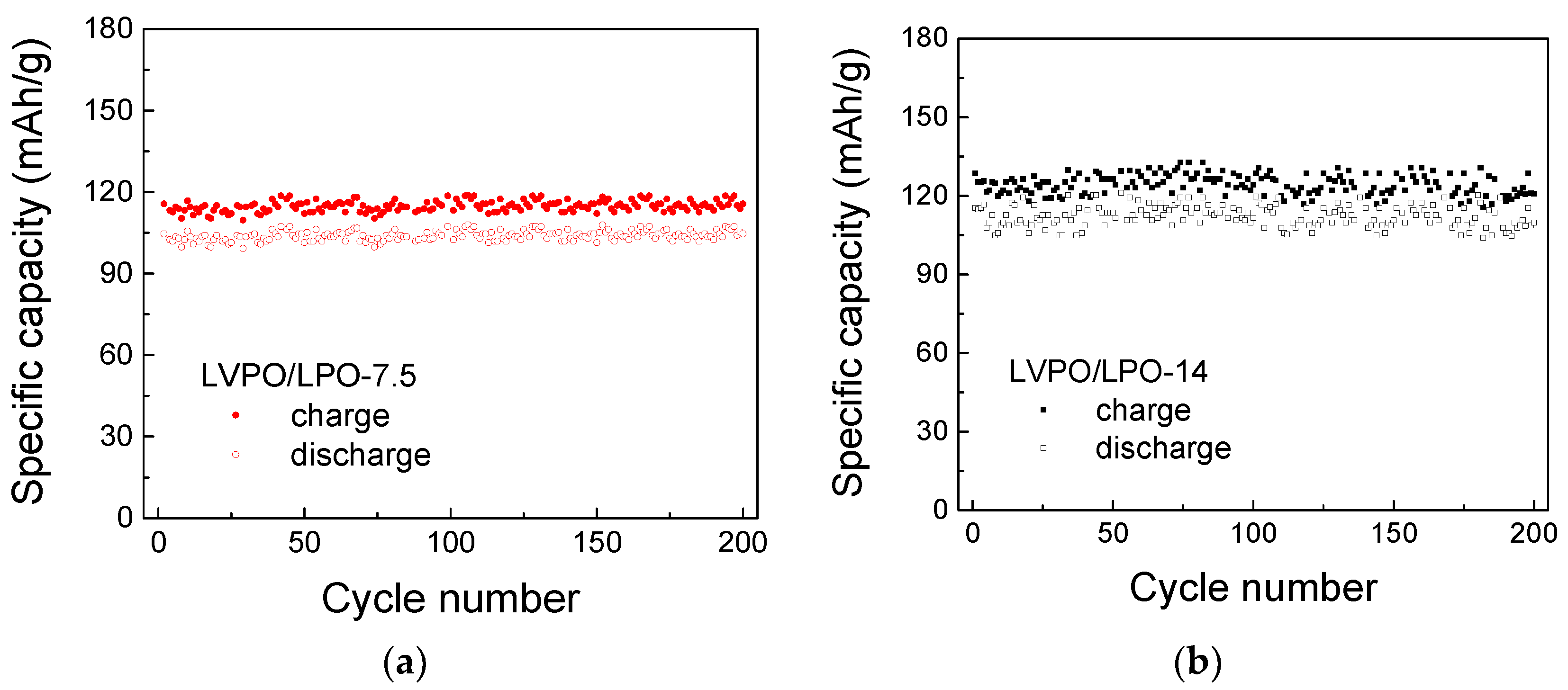
2.3. Electrochemical Performance
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef]
- Casimir, A.; Zhang, H.; Ogoke, O.; Amine, J.C.; Lu, J.; Wu, G. Silicon-based anode for lithium-ion batteries: Effectiveness of materials synthesis and electrode preparation. Nano Energy 2016, 27, 359–376. [Google Scholar] [CrossRef]
- Whittingham, M.S. Lithium batteries and cathode materials. Chem. Rev. 2004, 104, 4271–4301. [Google Scholar] [CrossRef]
- Liu, C.; Massé, R.; Nan, X.; Cao, G. A promising cathode for Li-ion batteries: Li3V2(PO4)3. Energy Storage Mater. 2016, 4, 15–58. [Google Scholar] [CrossRef]
- Rui, X.; Yan, Q.; Skyllas-Kazacos, M.; Lim, T.M. Li3V2(PO4)3 cathode materials for lithium-ion batteries: A review. J. Power Sources 2014, 258, 19–38. [Google Scholar] [CrossRef]
- Callegari, D.; Colombi, S.; Nitti, A.; Simari, C.; Nicotera, I.; Ferrara, C.; Mustarelli, P.; Pasini, D.; Quartarone, E. Autonomous self-healing strategy for stable sodium-ion battery: A case study of black phosphorus anodes. ACS Appl. Mater. Interfaces 2021, 13, 13170–13182. [Google Scholar] [CrossRef]
- Pan, A.; Liu, J.; Zhang, J.-G.; Xu, W.; Cao, G.; Nie, Z.; Arey, B.W.; Liang, S. Nano-structured Li3V2(PO4)3/carbon composite for high-rate lithium-ion batteries. Electrochem. Commun. 2010, 12, 1674–1677. [Google Scholar] [CrossRef]
- Liao, Y.; Li, C.; Lou, X.; Hu, X.; Ning, Y.; Yuan, F.; Chen, B.; Shen, M.; Hu, B. Carbon-coated Li3V2(PO4)3 derived from metal-organic framework as cathode for lithium-ion batteries with high stability. Electrochim. Acta 2018, 271, 608–616. [Google Scholar] [CrossRef]
- Ding, X.-K.; Zhang, L.-L.; Yang, X.-L.; Fang, H.; Zhou, Y.-X.; Wang, J.-Q.; Ma, D. Anthracite-Derived Dual-Phase Carbon-Coated Li3V2(PO4)3 as High-Performance Cathode Material for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 42788–42796. [Google Scholar] [CrossRef]
- Ren, M.M.; Zhou, Z.; Gao, X.P.; Peng, W.X.; Wei, J.P. Core-Shell Li3V2(PO4)3@C Composites as Cathode Materials for Lithium-Ion Batteries. J. Phys. Chem. C 2008, 112, 5689–5693. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Yang, G.; Zhang, X.; Yan, X.; Pan, X.; Wang, R. Electrochemical performance of Li3V2(PO4)3/C cathode material using a novel carbon source. Electrochim. Acta 2009, 54, 6451–6454. [Google Scholar] [CrossRef]
- Gavrilova, T.P.; Khantimerov, S.M.; Fatykhov, R.R.; Yatsyk, I.V.; Cherosov, M.A.; Lee, H.S.; Vishwanathan, R.; Saravanan, K.; Suleimanov, N.M. Magnetic properties and vanadium oxidation state in α-Li3V2(PO4)3/C composite: Magnetization and ESR measurements. Solid State Commun. 2021, 323, 114108. [Google Scholar] [CrossRef]
- Sun, C.; Rajasekhara, S.; Dong, Y.; Goodenough, J.B. Hydrothermal Synthesis and Electrochemical Properties of Li3V2(PO4)3/C-Based Composites for Lithium-Ion Batteries. ASC Appl. Mater. Interfaces 2011, 3, 3772–3776. [Google Scholar] [CrossRef]
- Kuang, Q.; Zhao, Y.; An, X.; Liu, J.; Dong, Y.; Chena, L. Synthesis and electrochemical properties of Co-doped Li3V2(PO4)3 cathode materials for lithium-ion batteries. Electrochimica Acta 2010, 55, 1575–1581. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, G. Developments in nanostructured cathode materials for high-performance lithium-ion batteries. Adv. Mater. 2008, 20, 2251–2269. [Google Scholar] [CrossRef]
- Yin, S.-C.; Grondey, H.; Strobel, P.; Anne, M.; Nazar, L.F. Electrochemical Property: Structure Relationships in Monoclinic Li3-yV2(PO4)3. J. Am. Chem. Soc. 2003, 125, 10402–10411. [Google Scholar] [CrossRef]
- Yin, S.-C.; Strobel, P.S.; Grondey, H.; Nazar, L.F. Li2.5V2(PO4)3: A Room-Temperature Analogue to the Fast-Ion Conducting High-Temperature γ-Phase of Li3V2(PO4)3. Chem. Mater. 2004, 16, 1456–1465. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; He, W.; Wei, C.; Cheng, Q. A review for the synthesis methods of lithium vanadium phosphate cathode materials. J. Mater. Sci. Mater. Electron. 2017, 28, 18269–18295. [Google Scholar] [CrossRef]
- Tai, Z.; Zhu, W.; Shi, M.; Xin, Y.; Guo, S.; Wu, Y.; Chen, Y.; Liu, Y. Improving electrochemical performances of Lithium-rich oxide by cooperatively doping Cr and coating Li3PO4 as cathode material for Lithium-ion batteries. J. Colloid Interface Sci. 2020, 576, 468–475. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, L.; Zhao, F.; Liu, Y.; Hou, L.; Yuan, C. Ni-rich LiNi0.8Co0.1Mn0.1O2 coated with Li-ion conductive Li3PO4 as competitive cathodes for high-energy-density lithium ion batteries. Electrochim. Acta 2020, 340, 135871. [Google Scholar] [CrossRef]
- Available online: https://patentscope.wipo.int/search/ru/detail.jsf?docId=CN277613493&_cid=P21-L6W3RH-06136-1 (accessed on 15 November 2019).
- Gavrilova, T.; Khantimerov, S.; Cherosov, M.; Batulin, R.; Lyadov, N.; Yatsyk, I.; Deeva, Y.; Turkin, D.; Chupakhina, T.; Suleimanov, N. Magnetic properties of Li3V2(PO4)3/Li3PO4 composite. Magnetochemistry 2021, 7, 64. [Google Scholar] [CrossRef]
- Yang, G.; Ji, H.; Liu, H.; Qian, B.; Jiang, X. Crystal structure and electrochemical performance of Li3V2(PO4)3 synthesized by optimized microwave solid-state synthesis route. Electrochim. Acta 2010, 55, 3669–3680. [Google Scholar] [CrossRef]
- Patoux, S.; Wurm, C.; Morcrette, M.; Rousse, G.; Masquelier, C. A comparative structural and electrochemical study of monoclinic Li3Fe2(PO4)3 and Li3V2(PO4)3. J. Power Sources 2003, 119–121, 278–284. [Google Scholar] [CrossRef]
- Liu, H.; Yang, G.; Zhang, X.; Gao, P.; Wang, L.; Fang, J.; Pinto, J.; Jiang, X. Kinetics of conventional carbon coated-Li3V2(PO4)3 and nanocomposite Li3V2(PO4)3/graphene as cathode materials for lithium ion batteries. J. Mater. Chem. 2012, 22, 11039–11047. [Google Scholar] [CrossRef]
- Cao, X.; Wu, H.; Ge, P.; Zhao, Y.; Zhu, L.; Liu, F.; Wang, J. Synthesis of Li3V2(PO4)3/C Composites as Cathode Materials for Lithium Ion Batteries via a Sol-Gel Method. Int. J. Electrochem. Sci. 2015, 10, 2997–3009. [Google Scholar]
- Chen, Y.; Zhang, D.; Bian, X.; Bie, X.; Wang, C.; Du, F.; Jang, M.; Chen, G.; Wei, Y. Characterizations of the electrode/electrolyte interfacial properties of carbon coated Li3V2(PO4)3 cathode material in LiPF6 based electrolyte. Electrochim. Acta 2012, 79, 95–101. [Google Scholar] [CrossRef]
- Saïdi, M.Y.; Barker, J.; Huang, H.; Swoyer, J.L.; Adamson, G. Electrochemical properties of lithium vanadium phosphate as a cathode material for lithium-ion batteries. Electrochemical and Solid-State Letters 2002, 5, A149–A151. [Google Scholar] [CrossRef]
- Ferdi, C.A.; Belaiche, M.; Iffer, E. Structural, electrochemical, electronic, and magnetic properties of monoclinic LixV2(PO4)3 for x = 3, 2, 1 using first-principles calculations. J. Solid State Electrochem. 2021, 25, 301–313. [Google Scholar] [CrossRef]
- Lee, H.S.; Vishwanathan, R.; Saravanan, K.; Nagarathinam, M.; Law, M.; Wang, C.; Tripathi, A.; Palani, B. Key design considerations for synthesis of mesoporous α-Li3V2(PO4)3/C for high power lithium batteries. Electrochim. Acta 2021, 372, 137831. [Google Scholar] [CrossRef]
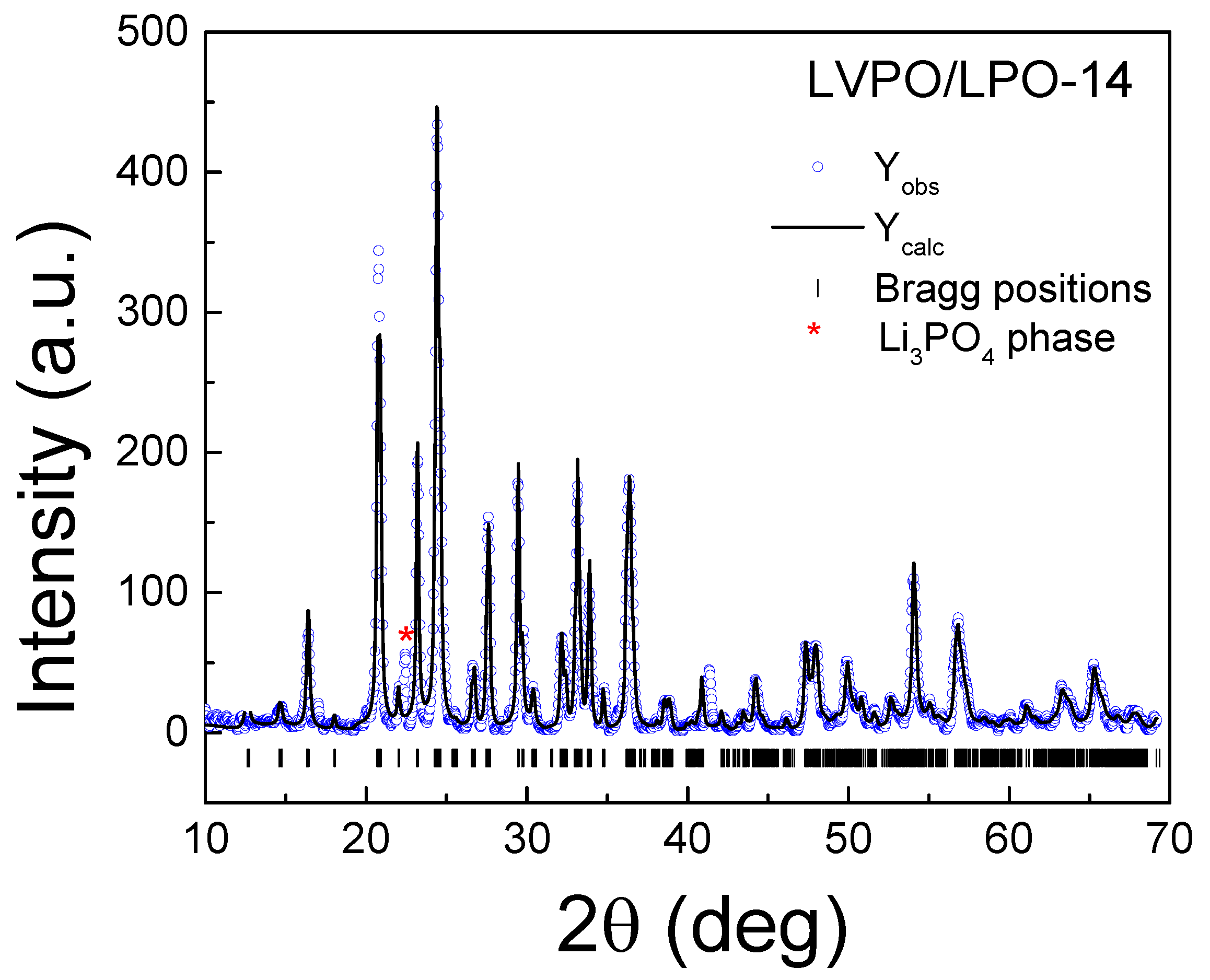
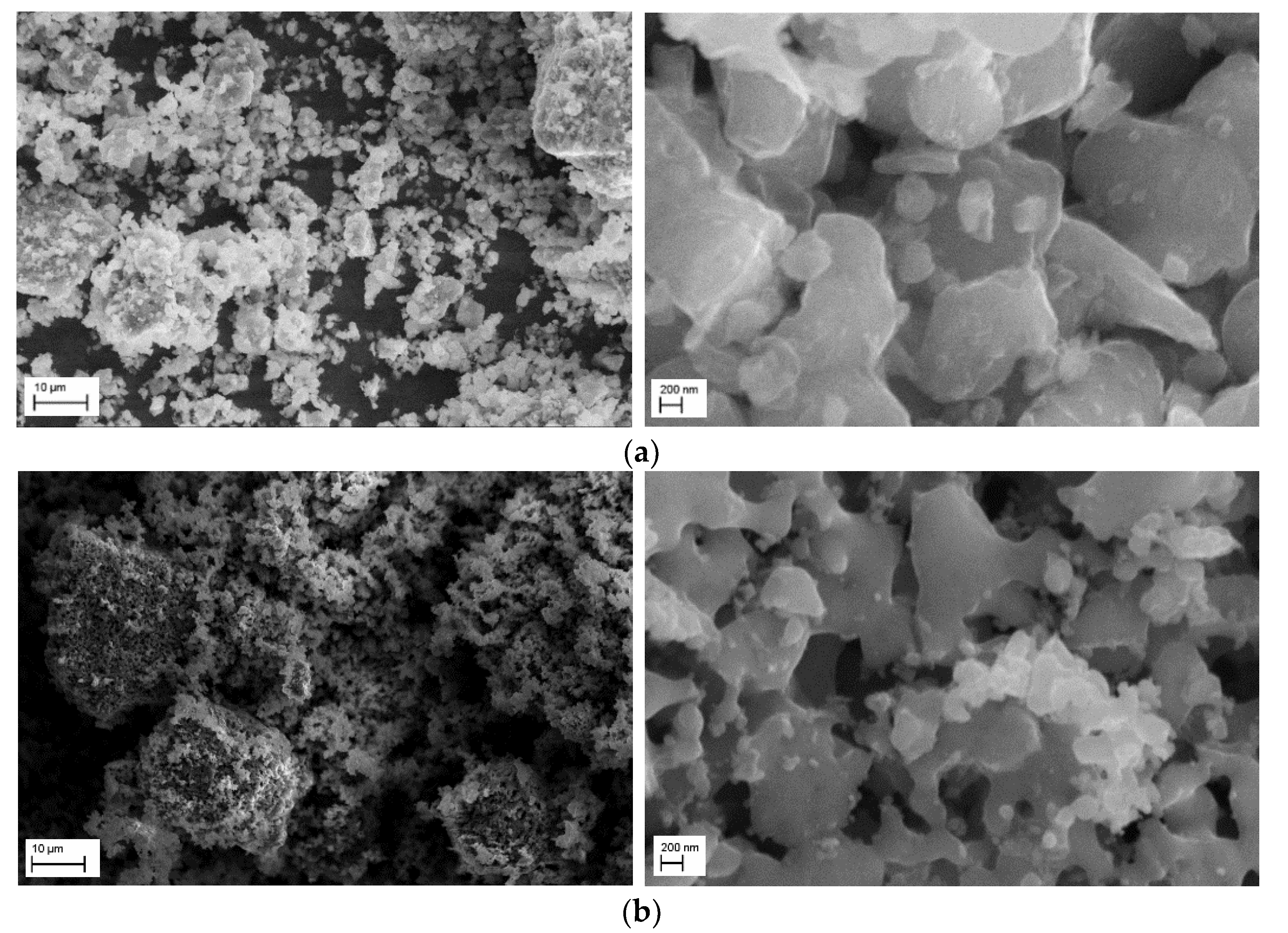
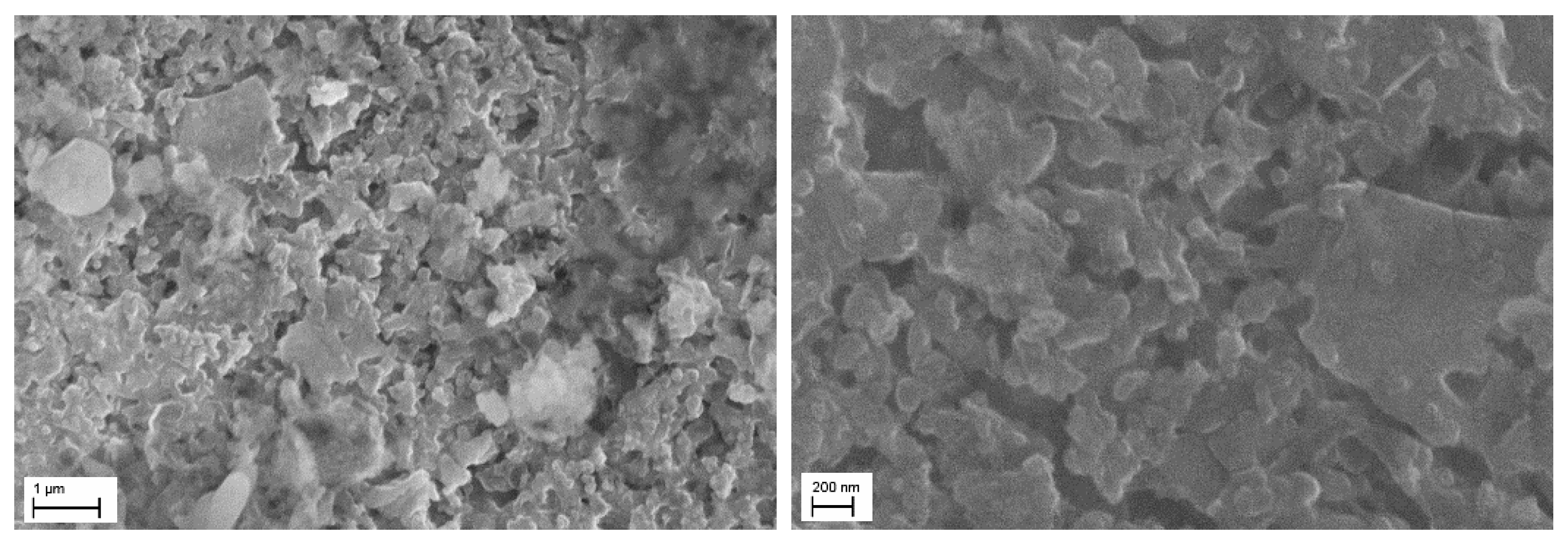
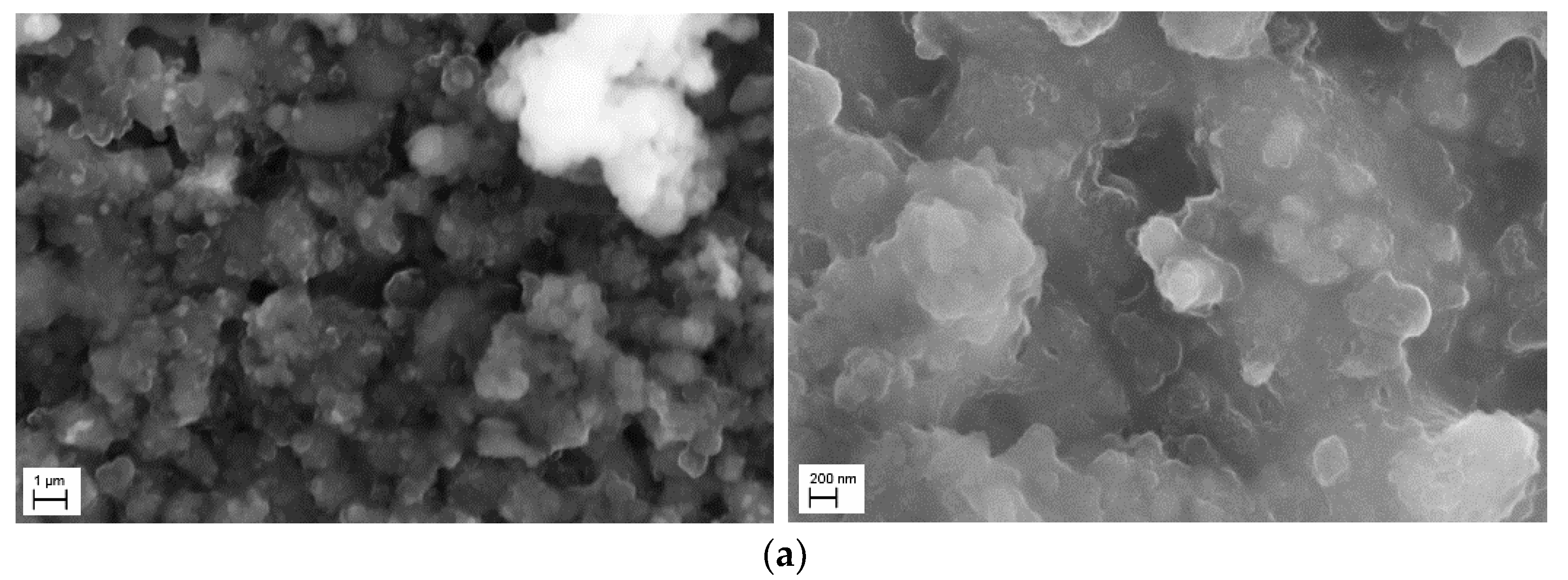


| Sample | LVPO/LPO-7.5 | LVPO/LPO-14 |
|---|---|---|
| main phase | Li3V2(PO4)3, 92.5 wt% | Li3V2(PO4)3, 86 wt% |
| syngony | monoclinic | monoclinic |
| space group | P21/n (#14) | P21/n (#14) |
| a, Å | 8.606(1) | 8.614(2) |
| b, Å | 8.587(4) | 8.595(3) |
| c, Å | 12.032(1) | 12.040(3) |
| β, ° | 90.554(1) | 90.55(1) |
| V, Å3 | 889.1(2) | 881.6(2) |
| additional phase | Li3PO4, 7.5 wt% | Li3PO4, 14 wt% |
| syngony | orthorhombic | orthorhombic |
| space group | Pnma (#62) | Pnma (#62) |
| a, Å | 6.146 | 6.146 |
| b, Å | 10.453 | 10.453 |
| c, Å | 4.913 | 4.913 |
| V, Å3 | 315.64 | 315.64 |
| No | Sample | LVPO/LPO-7.5 | LVPO/LPO-14 |
|---|---|---|---|
| 1 | mass (mg) | 4.7 | 2.5 |
| 2 | ILVPO/LPO/I0 | 4.813 | 8.355 |
| 3 | 7.7 × 1017 | 1 × 1018 | |
| 4 | 12.83 × 1018 | 6.34 × 1018 | |
| 5 | 6% | 15.8% | |
| 6 | lithium deficiency | 4% | 10.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavrilova, T.; Deeva, Y.; Chupakhina, T.; Yatsyk, I.; Lyadov, N.; Garipov, R.; Suleimanov, N.; Khrizanforov, M.; Khantimerov, S. Li3V2(PO4)3/Li3PO4 Cathode Materials for Li-Ion Batteries: Synthesis and Characterization. Magnetochemistry 2022, 8, 105. https://doi.org/10.3390/magnetochemistry8090105
Gavrilova T, Deeva Y, Chupakhina T, Yatsyk I, Lyadov N, Garipov R, Suleimanov N, Khrizanforov M, Khantimerov S. Li3V2(PO4)3/Li3PO4 Cathode Materials for Li-Ion Batteries: Synthesis and Characterization. Magnetochemistry. 2022; 8(9):105. https://doi.org/10.3390/magnetochemistry8090105
Chicago/Turabian StyleGavrilova, Tatiana, Yulia Deeva, Tatiana Chupakhina, Ivan Yatsyk, Nikolay Lyadov, Ranis Garipov, Nail Suleimanov, Mikhail Khrizanforov, and Sergey Khantimerov. 2022. "Li3V2(PO4)3/Li3PO4 Cathode Materials for Li-Ion Batteries: Synthesis and Characterization" Magnetochemistry 8, no. 9: 105. https://doi.org/10.3390/magnetochemistry8090105
APA StyleGavrilova, T., Deeva, Y., Chupakhina, T., Yatsyk, I., Lyadov, N., Garipov, R., Suleimanov, N., Khrizanforov, M., & Khantimerov, S. (2022). Li3V2(PO4)3/Li3PO4 Cathode Materials for Li-Ion Batteries: Synthesis and Characterization. Magnetochemistry, 8(9), 105. https://doi.org/10.3390/magnetochemistry8090105





