Abstract
The safety of the cells used for Advanced Therapy Medicinal Products is crucial for patients. Reliable methods for the cell purification are very important for the commercialization of those new therapies. With the large production scale envisioned for commercialization, the cell isolation methods need to be efficient, robust, operationally simple and generic while ensuring cell biological functionality and safety. In this study, we used high magnetized magnetic agarose-based beads conjugated with protein A to develop a new method for cell separation. A high separation efficiency of 91% yield and consistent isolation performances were demonstrated using population mixtures of human mesenchymal stem cells and HER2+ SKBR3 cells (80:20, 70:30 and 30:70). Additionally, high robustness against mechanical stress and minimal unspecific binding obtained with the protein A base conjugated magnetic beads were significant advantages in comparison with the same magnetic microparticles where the antibodies were covalently conjugated. This study provided insights on features of large high magnetized microparticles, which is promising for the large-scale application of cell purification.
1. Introduction
Advanced Therapy Medicinal Products (ATMP), such as stem-cell-based therapies [1], have gained a rapidly growing interest for the treatment of severe, often incurable, diseases. The regeneration potential of stem cells is enormous and future therapies to repair damaged cell tissue [2,3,4], such as cardiac cells or neurons, have only begun to be exploited [5,6,7,8,9].
The advances in research and development for stem-cell-based therapies have created an increasing demand for reliable cell isolation methods that are efficient, robust, operationally simple and generic while ensuring the cell’s biological functionality and safety [4]. Another challenge is the scale-up for commercialization of allogenic therapies where large cell quantities are manufactured. Treatment safety is crucial, and a major concern is the elimination of unwanted cells prior to transplantation. For instance, cell products derived from pluripotent cells have to be imperatively free of undifferentiated cells before administration to the patient to annihilate the risk of teratoma formation [10,11,12,13,14,15].
Today, for targeted cell isolation, two main methods are used, immunomagnetic separation (IMS), e.g., Magnetic Activated Cell Sorting (Miltenyi Biotec) and Dynabeads (Thermo Fisher), and immunofluorescence-based isolation, such as Fluorescence Activated Cell Sorting (FACS) [16,17]. Both methods rely on the direct or indirect labelling of cellular biomarkers, for instance surface receptor proteins. While FACS utilizes fluorescence conjugated antibodies, immunomagnetic methods use antibodies conjugated with magnetic particles for the isolation. FACS usually enables the processing of multiple high purity subpopulations but suffers from substantial cell loss, long processing times and costly equipment [18,19].
In comparison, IMS processes larger cell quantities with rather high throughput rates [16], lower cell loss and faster processing time [19] and only requires a magnetic field for the isolation of subpopulations [20]. Unfortunately, a lower purity is typically obtained compared to FACS. While the operator can change the antibody used for the FACS, thus, providing high flexibility, the conjugation of the IMS beads is fixed to an antibody pre-selected to bind to the cells.
The IMS beads have a size much smaller than a cell, in the nm range, leading to the binding of multiple beads to a single cell. Today, the relatively small volumes handled in IMS or FACS systems might be unsuitable to solve the bottleneck of large scale allogenic manufacturing [21]. In this context, previous studies using magnetic beads (MAGicBeads) of ≈100 µm radius, have provided an excellent performance to process large volumes of cell suspension and high cell concentrations [22,23].
In the present study, a new process was developed for cell separation using agarose-based magnetic beads of a size of ≈100 µm and presenting antibodies against cell receptors. It was hypothesized that the cells could bind to these beads, which are much larger than the currently used beads, i.e., IMS beads, and that this interaction could advantageously be used for cell sorting. Large magnetic microparticles could bind the cells at multiple sites of surface receptors and offer simultaneously a high magnetization for efficient cell separation.
A cell mixture model of human mesenchymal stem cells derived from bone marrow (hMSCs) and human breast cancer cells SKBR3 expressing the human epidermal growth receptor 2 (HER2) at their surface, was used. Two kinds of conjugation methods of the antibody were investigated and compared, with antibodies either coupled via covalently binding or using an intermediate protein A. In this latter case, the beads could be manufactured only with the protein A and antibodies binding to cell surface receptors, which would be added by the operator prior to cell separation according to the cell sorting needs, thereby, providing high flexibility in terms of antibody selection.
2. Results
The work was initiated by assessing the biocompatibility of the commercial MAGicBeads with hMSCs. The cell isolation capability and the influence of the ligand density of these beads was then tested with the model of SKBR3 cells using their HER2 surface receptor binding to the anti-HER2 monoclonal antibody (mAb), trastuzumab. Two conjugation methods of the antibody were investigated and compared, with antibodies either coupled via covalently binding or using an intermediate protein A. To support the development of a method compatible with current applications and amenable to a commercial process, the effect of mechanical stress, the influence of EDTA and the bead-to-cell ratio were studied. The most efficient conjugation method was finally tested with mixtures of hMSCs and SKBR3 cells.
2.1. Biocompatibility Assay
To investigate the potential toxicity of the MAGicBeads coupled to anti-HER2 mAb on hMSCs, a biocompatibility assay was performed according to the Materials and Method section. For the assay, hMSCs were cultured in medium where MAGicBeads conjugated with anti-HER2 mAbs had been soaked for 24 h for condition 1, or in medium where MAGicBeads alone had been soaked for 24 h for condition 2, in comparison with positive control using untreated medium—for the negative control, cells were not included.
The cells were plated in untreated medium for 24 h, accounted as start of the biocompatibility assay, after which the medium was exchanged for the respective conditions. Figure 1 shows the cell morphology at start of the biocompatibility assay, i.e., 24 h after seeding, (Figure 1a) and after 72 h (Figure 1b) for the different conditions. After 72 h, the assay was terminated, and the metabolic activity was measured with alamarBlue® staining assay (Figure 1c).
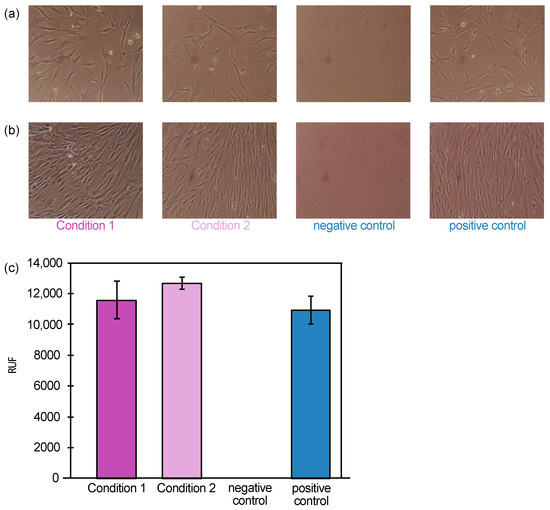
Figure 1.
Biocompatibility assay of the magnetic particles MAGicBeads with human mesenchymal stem cells. hMSCs were cultured in medium where MAGicBeads beads conjugated with anti-HER2 mAb’s had been soaked for 24 h for condition 1, or in medium where MAGicBeads alone had been soaked for 24 h for condition 2, in comparison with positive control using untreated medium—the negative control did not include cells. (a) Cells cultured in untreated medium for 24 h, accounted as start of the biocompatibility assay, after which the media were exchanged for conditions 1, 2 or positive/negative controls. (b) Cell cultures after 72 h. (c) Metabolic activity shown in relative fluorescence units (RFU) measured with alamarBlue® stain.
The cell growth and morphology for conditions 1, 2 and the positive control were highly similar (Figure 1b). This correlated well with the metabolic activity, where conditions 1, 2 and the positive control showed comparable fluorescence intensity of 11,588 ± 1219 RFU (relative fluorescence units), 12,658 ± 365 RFU and 10,921 ± 911, respectively (Figure 1c). The negative control did not include any cells and showed no metabolic activity.
2.2. Ligand Density
The MAGicBeads coupled with anti-HER2 antibodies via protein A, Pr A beads, were used for a first evaluation of the cell separation capacity of the beads and to study the effect of the ligand density on the cell isolation efficiency. The anti-HER2 antibody conjugation was based on the interaction of protein A, Pr A, bound to the bead interacting with the constant part of the antibody by amide bonds. Three densities of anti-HER2 antibodies conjugated to the magnetic particles were investigated, providing three densities of ligands for the cell attachment. The three densities were (1) full occupancy (100%) of the antibodies on the protein A binding sites of the magnetic beads, i.e., 42 mg mAb/mL beads (Pr A 42), (2) partial occupancy of 50% of the binding sites, i.e., 20.9 mAb/mL beads (Pr A 20.9) and (3) partial occupancy of 15%, i.e., 6.58 mg mAb/mL beads (Pr A 6.58).
The isolation of SKBR3 cells for the different ligand densities is given in Figure 2 showing the cell number before and after the cell isolation, as well as the percentage of sorted cells in relation to the input cell number. Full antibody occupancy of the magnetic beads, Pr A 42, showed a 70% uptake of the cells, while partial occupancies at 50% and 15%, Pr A 20.9 and Pr A 6.58, generated a separation efficiency of 94% (Figure 2). This demonstrated that cell separation was indeed achieved with the Pr A beads. Partial occupancy of 50% or 15% of the binding sites provided similar cell separation and was superior in comparison with full occupancy of the binding sites. Pr A 20.9 beads were then adopted for all the subsequent experiments performed using protein A beads.
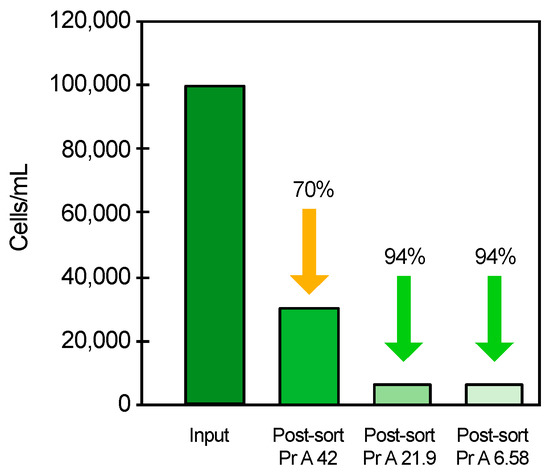
Figure 2.
Cell isolation efficiency for different densities of anti-HER2 antibody on the Pr A beads for HER2+ SKBR3 cell separation; full occupancy of the protein A binding sites with 42 mg mAb/mL beads (Pr 42), partial occupancy of 50% of the binding sites with 20.9 mg mAb/mL beads (Pr A 20.9) and partial occupancy of 15% of the binding sites with 6.58 mg mAb/mL beads (Pr A 6.58); the ratio of the post-sorting cell number over pre-sorting cell number is given in percent.
2.3. Antibody Conjugation to the Bead, Effect of Mechanical Stress and Influence of EDTA
Pr A beads were used for the previous experiment; however, a priori, a different type of conjugation of the anti-HER2 antibodies on the beads could potentially be valuable as well for the separation of HER2+ SKBR3 cells. In this experiment, three different beads varying in their conjugation methods and ligand coverages were studied. The conjugation was based either on the interaction of protein A, Pr A where 20.9 mg mAb/mL beads were used or on covalent conjugation. For the covalent conjugation, two ligand densities were used; a partial occupancy of 40% with 12 mg mAb/mL beads (cc12) and a partial occupancy of 4% with 1.2 mg mAb/mL beads (cc1.2).
The cc12 beads had concentrations of antibodies at the surface of the beads in the range studied for the protein A conjugation of 20.9 and 6.58 mg mAb/mL beads, which provided a comparable and efficient cell separation. Another aspect is that, beyond the present report, our long-term goal is to obtain a process supporting commercial manufacturing, for which a robust and scalable method will be required. In preparation for this, we investigated the impact of mechanical stress during homogenization by applying different types of fluid flows and shear scenarios; low shaking at 7 rpm, high shaking at 50 rpm and pipette mixing, considered to generate the highest shear stress. Furthermore, the effect of the EDTA concentrations 2 or 7 mM, commonly added in the buffer to prevent cell adhesion, was studied.
Figure 3 presents the results obtained in the separation of SKBR3 cells using beads conjugated with antibody in three different ways, Pr A, cc12 or cc1.2, each subjected to the three different mechanical conditions during the incubation and the wash steps, low shaking of 7 rpm, high shaking of 50 rpm and pipette mixing.
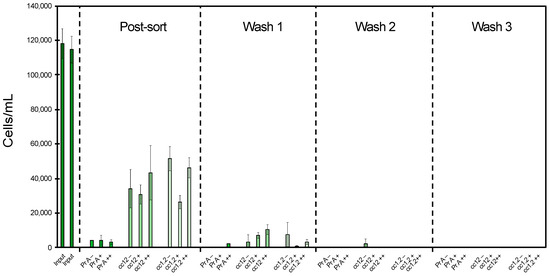
Figure 3.
Cell isolation efficiency for three bead conjugations, Pr A, cc12 and cc1.2, in the presence of different mechanical stress conditions during incubation and wash steps in relation to the HER2+ SKBR3 cell concentration prior to cell sorting (input); (–) low shaking at 7 rpm, (+) high shaking at 50 rpm and (++) pipette mixing. The first input cell density referrers to the pre-sort population for all low shaking and pipette mixing conditions and the second input for all high shaking conditions. The cells before isolation (input) are referred to as “pre-sort”, while “post-sort” refers to the cells after isolation.
Using the Pr A beads, a cell isolation of 96–97% was observed independently of the mechanical conditions (Figure 3). Furthermore, almost no cells were detected during the wash steps for these beads; in wash step 1, a small cell amount dissociated from the beads was observed only for the pipette mixing condition, and in the subsequent wash steps, no dissociated cells were observed. In comparison, both cc12 and cc1.2 covalently based conjugated beads generated lower cell isolations of 64–73% and 57–77%, respectively.
On average, the cell separation was slightly more efficient with the cc12 beads compared to the cc1.2 beads with 69% and 65% separation, respectively. Additionally, both cc12 and cc1.2 resulted in a higher cell dissociation from the beads during the first wash step for all the mechanical conditions with a tendency for higher cell loss for the more stressful conditions (Figure 3).
The low shaking 7 rpm was selected among the tested conditions for further study due to its mildest effect with maintained efficiency. The effect of EDTA concentration was then studied for the separation of SKBR3 cells, including the wash steps. Two conditions, i.e., 0.5% BSA + 7 mM EDTA and 0.5% BSA + 2 mM EDTA, were studied for the three bead types, Pr A, cc12 and cc1.2, with PBS (–/–) buffer as the control (Figure 3). In the presence of EDTA, the Pr A beads provided a slightly lower cell isolation of 84–85% (Figure 4) compared to the absence of EDTA with 96–97% isolation (Figure 3).
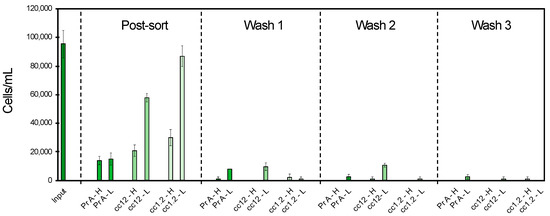
Figure 4.
The cell isolation efficiency in the presence of different EDTA concentrations during incubation and wash steps in relation to the HER2+ SKBR3 cell concentration prior cell sorting (input); (H) high EDTA concentration of 7 mM; (L) low EDTA concentration of 2 mM for three bead conjugations, Pr A, cc12 and cc1.2.
In this experiment, the formation of large aggregates of beads and cells was observed for all the conditions with covalently conjugated beads. These large aggregates trapped the majority of the cells, resulting in an apparent higher cell separation yield of 78% for cc12 and 68% for cc1.2 in the presence of 7 mM EDTA, while the real yield was much lower. In the presence of 2 mM EDTA, low cell isolations of 39% for cc12 and 9% for cc1.2 were observed. Cell–bead aggregates occurred, however, less severely than in the presence of 7 mM EDTA in comparison with 2 mM EDTA. Moreover, the number of cells dissociated from the beads was higher for all the conditions in the presence of EDTA during the wash steps 2 and 3, given in Figure 4, in comparison with the control wash buffer without EDTA, given in Figure 3.
2.4. Bead-to-Cell Ratio
This experiment investigated the bead-to-cell ratio suitable for the cell isolation. Four conditions were studied from 20 to 0.75 µL settled magnetic beads coupled with anti-HER2 antibodies via Pr A, added to 1 × 105 SKBR3 cells (Figure 5). In Figure 5, the isolation obtained for the different bead-to-cell ratios is represented. There was a trend of cell isolation decreasing with the bead-to-cell ratio, with the highest, 99.5%, and lowest isolation, 95.9%, obtained using 20 and 0.75 µL beads, respectively. This difference was significant, while the other conditions were not. For all the further experiments, 20 µL beads to 1 × 105 cells were used to avoid binding limitations, except cases where only the preliminary feasibility was tested.
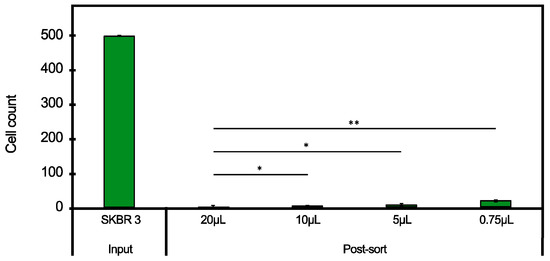
Figure 5.
Effect of bead-to-cell ratio investigated with 20, 10, 5 and 0.75 µL settled Pr A beads applied to 1 × 105 SKBR3 cells where the input is the cell number pre-sorting and the output is the cell number post-sorting—the differences between the different conditions were not significant (* p value > 0.05), except for the comparison of 20 to 0.75 µL (** actual p value = 0.03).
2.5. Cell Separation from Heterogenous Populations
After studying different parameters for the isolation of a single cell population (SKBR3 cells), the cell separation from mixed populations of hMSCs and SKBR3 cells was studied, where SKBR3 cells were separated from the mixture using beads conjugated with anti-HER2 antibodies. The first experiment aimed at confirming that no unspecific binding of hMSCs occurred. hMSCs express a variety of surface makers, including CD73, CD90 and CD105 [24] but not HER2. All three types of conjugated beads, Pr A, cc12 and cc1.2, were tested for unspecific isolation of hMSCs (Figure 6). Pr A beads provided very low unspecific cell isolation with roughly 1% cell binding and an error bar within the variation of the analytical method. For both covalently conjugated beads, the unspecific binding was higher compared to the Pr A beads with 14% and 15% cell separation, respectively.
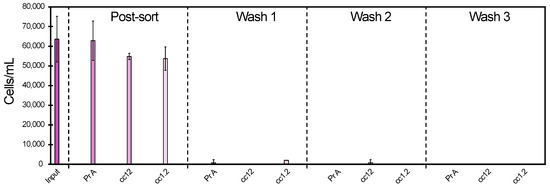
Figure 6.
Unspecific binding assay for three different bead conjugations with anti-HER2 antibodies, protein A (Pr A) covalently coupled with 40% saturation (cc12) and covalently coupled with 4% saturation (cc1.2) after cell sorting and wash steps in relation to the hMSCs cell number (input).
The model system of hMSCs and SKBR3 cells was used for negative cell selection of SKBR3 cells based on their affinity for anti-HER2 antibodies. Several population ratios of 80:20, 70:30 and 30:70 hMSCs:SKBR3 cells were considered using Pr A beads at 50% ligand coverage with 20 µL settled bead added to 1 × 105 cell and shaking at 7 rpm. Figure 7a presents the depletion of HER2+ cells in 80:20 (hMSCs:SKBR3) heterogenous population measured by flowcytometry (FC), showing a reduction of 91.6% of HER2+ cells, i.e., removing the majority of the SKBR3 cells from the mixture.
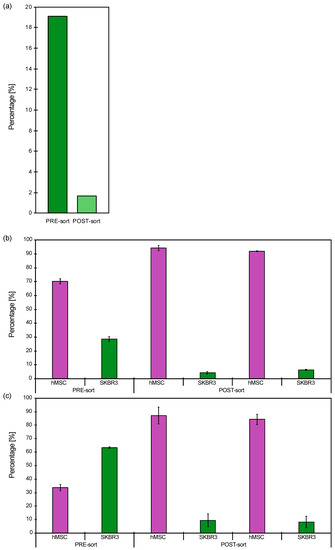
Figure 7.
Negative selection of HER2+ SKBR3 cells from heterogenous populations of hMSCs and SKBR3 cells (hMSCs:SKBR3) measured by FC showing the pre- and post-sort population percentage: (a) 80:20, (b) 70:30 and (c) 30:70.
This was followed by two biological replicates of cell separation of a 70:30 hMSCs:SKBR3 cells population, showing an average cell depletion of 85% HER2+ cells (Figure 7b). The hMSCs enrichment coincided with the SKBR3 cell depletion, achieving a cell enrichment of >90%. The cell separation of a mixture 30:70 hMSCs:SKBR3 (Figure 7c), i.e., opposite proportion in comparison with Figure 7b, generated a similar depletion with an average of 86% SKBR3 isolation and hMSCs enrichment ≈ 90%.
3. Discussion
Cell separation based on magnetic beads has become a powerful technique and has been a well-established standard procedure for several decades [25]. Its ease of use and gentleness to the cells compared to FACS has made it attractive for many applications [25].
Magnetic particles have to be biocompatible, as any toxic effects might compromise the cell isolation as well as the cell quality and the therapeutic efficiency of the cell-based treatment [26]. The MAGicBeads consist of biodegradable material, such as agarose and iron oxide, and these materials are known to be not harmful to cells [27]. To ensure the MAGicBeads biocompatibility for stem cells, a toxicity test was performed with hMSCs.
The cell morphology after the toxicity assay was comparable to the control (Figure 1a,b). The cell attachment, growth and metabolic activity were similar to the positive control. (Figure 1c). The toxicity was tested in a 72-h culture with medium soaked during 24 h with the MAGicBeads, and thus the contact time was much longer than the normal time for the cell separation procedure. The biocompatibility assay did not show any signs of release of cytotoxic compounds from the beads providing a non-toxic environment for the hMSCs.
Currently, the techniques of cell isolation commercially available are based either on magnetic nanoparticles ranging from 20 to 500 nm or magnetic beads in the very low microparticle range (1–3 µm) [25,28]. In the present study, larger microparticles were used with an average diameter of 100 µm. When the IMS technology was established in the early 1990s, the advantages of smaller nanoparticles were thoroughly discussed [20]; however, we believe that large microparticles can be advantageous. As a matter of fact, due to the high magnetization of the MAGicBeads, no additional column is needed as required for other techniques [25].
Furthermore, possible multiple attachment points for the cells can provide a more robust system for a potential scale-up. Additionally, studies have shown that the cells can retain the magnetic properties of the IMS up to 2 weeks after initial isolation [27] providing a potential safety risk. In the same study, it was also shown that these small magnetic beads can be taken up by the cells and have been found on the cell surface and in the intra-cellular spaces [27]. In comparison with IMS nanoparticles, larger microparticles with a high magnetization are easier to retain and eliminate, significantly reducing the risk of residual magnetic beads in the cell product used for therapeutic purpose and thus offering a high safety.
The present proof-of-concept study aimed at establishing if large magnetic beads, such as the MAGicBeads, could be used for cell isolation and understand the effects of several parameters on the cell separation. First, the influence of the ligand densities on the cell adhesion was studied by varying the concentration of anti-HER2 antibodies on the protein A conjugated beads. A lower bead coverage of 50% or 15% provided significantly better cell separation compared to full ligand saturation of 100%. To understand this phenomenon, the ligand distribution needs to be put in perspective.
The MAGicBeads are porous with protein A present on their surface. In the porous cavities, however, the cells are too large to penetrate. When in the presence of anti-HER2 antibodies, the outer binding sides of the Pr A beads will be first occupied by the antibodies, followed by the occupation of the binding sides inside the pores. Only the ligands displayed on the outer bead surface are responsible for the cell isolation while the majority of the binding sites are in the bead cavities, and therefore the full saturation by antibodies will not improve the cell isolation. This, however, does not fully explain why the cell isolation is lower in the case of full antibody coverage.
One explanation could be that the anti-HER2 antibody binding site might be less accessible in case of full occupancy of the antibodies on the protein A. Since one protein A can bind up to two antibodies, the anti-HER2 binding sites might be less accessible by the volumetric hinderance of the antibodies to each other, if these are coupled to the same protein A. Therefore, a lower occupancy of the antibodies on the protein A will reduce this hinderance.
Furthermore, the similar cell separation observed with 50% or 15% bead coverage by the antibodies could be due to the fact, that, due to its size, one cell is likely bound to a bead through several HER2 molecules binding to several anti-HER2 antibodies due to its size, so that binding thanks to additional HER2—anti-HER2 mAb in the case of 50% might not be advantageous in comparison with 15%.
The next step in the study was to investigate different conditions of mechanical stress and EDTA concentrations for different types of binding of the anti-HER2 antibody on the beads, using protein A (Pr A) or covalent (cc12 and cc1.2) interactions. It is important to understand the impact of mechanical shear on the bead–cell construct to develop a process suitable to potential application at large scale. Compared to the covalently bound beads cc12 and cc1.2, the Pr A beads resulted in superior cell separation, retaining more than 96% of the cells (Figure 3). The cell separation by cc12 was slightly better than cc1.2, however, not significantly.
Protein A interaction occurs at the Fc part of the antibody; therefore, the antibody binding site is always presented outwards. On the contrary, covalent binding generates random presentation of the antibody, and thus the binding site is potentially not accessible. This had a clear impact on the cell–bead binding. However, once the assembly of cell and bead, Pr A, cc1.2 or cc12, was formed, the cells did not dissociate from the beads anymore as indicated by almost no cells present in wash 2 and 3 (Figure 3). The Pr A beads showed a high resistance towards mechanical stress, a parameter that is very important for the process scale-up.
EDTA is a usual component of the buffer for cell separation based on magnetic beads to prevent cell adhesion and aggregates. The presence of proteins in the form of FCS or BSA is also systematically included in these procedures as a cell protectant. In the present experiment, the supplementations of EDTA at 2 or 7 mM together with 0.5% BSA deteriorated the cell separation performances, severely for cc1.2, significantly for cc12 and slightly for Pr A (Figure 4) compared to PBS buffer only.
The formation of large cell aggregates was observed for all these conditions however to a lesser extend for Pr A beads compared to cc1.2 or cc12 beads. This was also observed with another adherent cell type (data not shown). The reasons for the cell aggregation and why aggregation is influenced by the type of beads, are not clear. One potential factor could have been the fact that the Pr A beads provided a superior cell binding in comparison with the covalently bound beads, likely associated to faster binding to the Pr A beads, which partially prevented them from forming aggregates.
As illustrated in Figure 5, it was observed that, potentially, the bead-to-cell ratio could be significantly reduced if desired. Down to 5 µL settled beads for the separation of 1 × 105 SKBR3 cells provided almost the same separation as 20 µL. This aspect can be very important for the economical aspect of the process when it is scaled-out since the antibodies are expensive.
The last part of the study was dedicated to the evaluation of sorting a cell mixture of hMSCs and HER2+ SKBR3 cells. All the bead types presented negligible unspecific binding of HER2- cells (Figure 6), confirming that the cells were binding to the antibody and not to the beads. Finally, a good reduction of the HER2+ cell subpopulation was obtained for the different tested mixtures, 80:20, 30:70 and 70:30 (Figure 7), confirming that the present Pr A beads method was excellent for cell separation in this proof-of-concept study.
In the present investigation, the Pr A beads were revealed to be the most promising approach. These presented a good resistance to mechanical stress, which is also an advantage for scale-up. Importantly, the Pr A beads offer a high flexibility since the beads are supplied with protein A ligand, on which any antibody specific to the targeted cells can be added for the separation process. Interestingly, the technology can easily be applied to magnetic beads conjugated to Protein G, which broadens the application to antibodies of other subtypes or species.
The technology can also be applied to Protein L; however, this latter primarily binds to the κ-Fab fragment, so that it would potentially block the binding site of the cell-surface receptor and would therefore not be adequate for cell separation. Furthermore, the present study was a proof-of-concept exploiting our know-how of magnetic bead development and knowledge from a previous application for antibody purification process [22,23]. Potentially, other manufacturing processes, such as magnetic nanoparticles incorporated in hydrogel beads [29] could be used to manufacture such large magnetic particles.
Overall, the Pr A method offers not only flexibility during the process development but it is also highly advantageous for the manufacturing. As a matter of fact, the Pr A beads can be produced in a large amount bringing down their manufacturing costs. Furthermore, the antibody production is then not strictly depending on the bead manufacturer but can be acquired from another source, potentially presenting economical and regulatory advantages, as well as reducing a situation of monopoly. This presents the flexibility of the FACS system and the robustness and efficiency of a magnetic bead-based system.
4. Materials and Methods
4.1. Cells
The cells used for all the experiments performed in this study were human mesenchymal stem cells (hMSCs) derived from bone marrow and human breast cancer cells SKBR3. The hMSCs were purchased from ScienCell TM (catalogue number: 7500, Carlsbad, CA, USA) and characterized as mesenchymal stem cells with positive expression of CD73, CD90 and CD105 according to the International Society for Cellular Therapy standard criteria [24]. SKBR3 cell line, a human breast cancer cell line expressing human epidermal growth receptor 2 (HER2/neu), was purchased from American Type Culture Collection (ATCC, via LGC Promochem, Borås, Sweden).
4.2. Cell Cultures
Both cell types, hMSCs and SKBR3 cells, were cultured with Dulbecco’s Modified Eagle’s Medium low glucose (DMEM) (Sigma-Aldrich, St. Louis, MI, USA) supplemented with 10% Fetal Bovine Serum (FBS origin: United States, Gibco, Waltham, MA, USA), as adherent cells at 37 °C and 5% CO2. Culture flask were coated prior to cell seeding with Poly L-lysine (PLL) 0.01% solution (Sigma-Aldrich, St. Louis, MI, USA) with 1 mL PLL solution per 25 cm2.
Cryopreserved cells were thawed and expanded in T-75 culture flasks with seeding density around 5 × 103 cells/cm2 for hMSCs and 1.5 × 104 cells/cm2 for SKBR3 cells. The cell expansion and collection for cell separation assays were performed when 80–90% cell confluency was reached. The cell detachment was conducted by 5 min incubation of 1× TrypLETM Express Enzyme (Thermo Fisher Scientific, Waltham, MA, USA) at 37 °C. Cell counts were performed with a Bürker hemocytometer for the determination of the seeding densities and accurate mix of heterogenous cell populations.
4.3. Magnetic Beads
The cell separation experiments were performed with commercial MAGicBeads from MAGic Bioprocessing (Uppsala, Sweden), a superparamagnetic 4% agarose resin with an average diameter of 100 µm. Sterilization by gamma radiation was performed when needed. The MAGicBeads provide a high magnetic saturation of 40 emu/g beads. A humanized monoclonal antibody IgG1, trastuzumab, recognizing the human epidermal growth factor receptor 2 (HER2/neu), was used for the separation of HER2+ cells. This antibody had been produced in house, in studies previously published [22,23].
In short, it had been produced in cultures using a recombinant Chinese Hamster cell line CHO-M producing trastuzumab (Selexis, Switzerland) with chemically defined medium BalanCD CHO and supplements (FudjiFilm Irvine Scientific, Santa Ana, CA, USA) and purified by protein A capture. Two types of mAb binding were used, via protein A, PrA, or covalently, cc. The Pr A MAGicBeads were covalently coupled to recombinant protein A (Sino Biological, Beijing, China). Prior to cell separation, these beads were conjugated with trastuzumab antibodies using protein A interaction with the constant part of the antibody, providing a binding with an orientation of the variable region of the antibody outwards. For cc MAGicBeads, the trastuzumab antibodies were covalently coupled to the beads, providing a random binding.
4.4. Biocompatibility Assay
hMSCs were used for the biocompatibility study of the magnetic beads, investigating the cell growth, cell morphology and metabolic activity using alamarBlue® cell viability stain (Invitrogen, Waltham, MA, USA). For the preparation of the biocompatibility, two different conditions were prepared. In condition 1, Pr A magnetic microparticles conjugated with anti-HER2 mAb (Section 2.3) were soaked in DMEM + 10% FBS cell culture medium for 24 h. For condition 2, unconjugated Pr A magnetic microparticles were used and soaked in DMEM + 10% FBS cell culture medium for 24 h. Additionally, non-seeded culture wells and non-treated cell medium culture wells were used as negative and positive controls.
For the assay, the cells were seeded one day prior to the start of the experiment in a 6-well culture plate with 1.5 mL cell medium (DMEM + 10% FBS) and cultured at 37 °C, 5% CO2. After 24 h, the culture medium was withdrawn and replaced with the conditioned DMEM + 10% FBS culture media of condition 1 or 2, while untreated DMEM + 10% FBS was used for the positive and negative controls. After 72 h, the supernatant was removed, and the cells were treated with alamarBlue® cell viability stain. The relative cell metabolic activity, total cell number and cell morphology were compared between the trial conditions and the control groups.
4.5. Cell Separation
For all cell separations, the adherent growing cell were trypsinated and used as single cell suspension either as single cell types or mixed as heterogenous populations. Prior to negative selection of receptor positive HER2 expressing SKBR3 cells, the magnetic beads MAGicBeads were conjugated with anti-HER2 antibody as detailed in the text and stored in sterile phosphate buffered saline (PBS) pH 7.4 until use. For the cell separation, 1 × 105 cells, SKBR3 cells or a mixture of hMSCs and SKBR3 cells, according to the text, were added to 20 µL beads, which had been washed three times with PBS.
The beads and cells were incubated for 45 min on an end-to-end shaker (PTR-35, Grant Instruments Ltd., Shepreth, UK) at 7 or 50 rpm at room temperature (RT) to ensure complete interaction with the HER2+ cells. After incubation, the MAGicBeads beads were separated using a magnetic field created by a magnet, and the desired cell type was retained in the supernatant. While the supernatant was subject to analyses, the beads were subsequently washed three times with PBS and sanitized with 0.1 M NaOH. After the cleaning procedure, the beads could be re-used following a new conjugation with the selective antibody.
4.6. Flow Cytometer (FC) Analysis
The cell distribution was quantified by flow cytometer (FC) analysis with the flow through cell fraction (1000 µL). For FC analysis, cells were washed with PBS and concentrated by centrifugation, followed by incubation with 5 and 20 µL specific fluorescence Ab (Anti-HU-CD90-APC and Anti-HER-2/NEU-FITC, respectively) for 30 min at 4 °C. The unbound antibodies were washed away by centrifugation and resuspension of the cells with PBS. Lastly, the cell sorting efficiency was analyzed by residue cell content using Guava® easyCyteTM (Luminex, Austin, TX, USA) and InCyte software.
4.7. Cell Count
The cell counts for the biocompatibility assay, all the cell sorting experiments and for the mixtures of heterogenous cell populations were performed with Trypan blue exclusion and enumeration using a Bürker hemocytometer. The percentages reported in the graphs for the cell separation are related to the number of cells prior to cell separation.
4.8. Metabolic Activity Assay
The cell metabolic activity for the biocompatibility assay was assessed with an alamarBlue® cell viability stain (Invitrogen, Waltham, MA, USA). For the staining protocol, the supernatant was removed from the culture, and the cells were incubated for 2 h with 1:10 alamarBlue®:cell medium. alamarBlue® supernatant was withdrawn, and the fluorescence intensity (ƛex: 544 nm and ƛem: 595 nm) measured by a 96-well plate reader (CLARIOstar, BMG LABTECH, Ortenberg, Germany) and relative metabolic activity were compared.
5. Conclusions
With the great advances in cell-based therapies, such as tissue renewal with progenitor cells, the demand for reliable isolation methods has also increased in order to safely remove pluripotent cells prior to administration. In comparison with FACS, magnetic bead-based isolation possesses interesting advantages, such as processing of large cell amounts, parallel throughput, low cell loss, fast processing time and low equipment cost. However, it has low flexibility as dedicated beads are used for the isolation of specific cells.
On the contrary, the MAGicBeads Pr A beads offer a very flexible platform, where the antibody binding the cells can easily be exchanged. The present process based on these beads provided high cell isolation efficiency of 91%. In the present case, an in-house produced antibody was used to isolate HER2+ cells. The flexibility of the MAGicBeads Pr A beads and isolation efficiency together with the robustness of the beads for mechanical stress makes this system highly promising for autologous cell therapy or large-scale manufacturing aimed at allogenic cell therapy.
Author Contributions
Conceptualization, methodology, N.A.B. and V.C.; formal analysis, investigation, writing—original draft preparation, visualization, N.A.B.; writing—review and editing, N.A.B., M.J., A.H., R.H., J.H., K.E. and V.C.; supervision, project administration, funding acquisition V.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by AdBIOPRO, Competence Centre for Advanced BioProduction by Continuous Processing (Grant/Award number: 2016-05181) and CAMP, Competence Centre for Advanced Medical Products (Grant/Award number: 2017-02130), both funded by the Swedish Agency for Innovation Systems VINNOVA. The APC was funded by KTH library.
Institutional Review Board Statement
Not applicable as the studies did not involve humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that supports the findings of this study are available from the corresponding author on reasonable request.
Conflicts of Interest
N.A.B., M.J., A.H., R.H., J.H. and V.C. declare no conflict of interest. K.E. is an employee of MAGic Bioprocessing, the company that owns the intellectual property regarding the magnetic beads.
References
- Hanna, E.; Rémuzat, C.; Auquier, P.; Toumi, M. Advanced therapy medicinal products: Current and future perspectives. J. Mark. Access Health Policy 2016, 4, 31036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roex, M.C.; Hageman, L.; Heemskerk, M.T.; Veld, S.A.; van Liempt, E.; Kester, M.G.; Germeroth, L.; Stemberger, C.; Falkenburg, J.F.; Jedema, I. The simultaneous isolation of multiple high and low frequent T-cell populations from donor peripheral blood mononuclear cells using the major histocompatibility complex I-Streptamer isolation technology. Cytotherapy 2018, 20, 543–555. [Google Scholar] [CrossRef]
- Körbling, M.; Drach, J.; Champlin, R.E.; Engel, H.; Huynh, L.; Kleine, H.D.; Berenson, R.; Deisseroth, A.B.; Andreeff, M. Large-scale preparation of highly purified, frozen/thawed CD34+, HLA-DR- hematopoietic progenitor cells by sequential immunoadsorption (CEPRATE SC) and fluorescence-activated cell sorting: Implications for gene transduction and/or transplantation. Bone Marrow Transplant. 1994, 13, 649–654. [Google Scholar] [PubMed]
- Zhu, B.; Murthy, S.K. Stem cell separation technologies. Curr. Opin. Chem. Eng. 2013, 2, 3–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makkar, R.R.; Smith, R.R.; Cheng, K.; Malliaras, K.; Thomson, L.E.; Berman, D.; Czer, L.S.; Marbán, L.; Mendizabal, A.; Johnston, P.V.; et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): A prospective, randomised phase 1 trial. Lancet 2012, 379, 895–904. [Google Scholar] [CrossRef] [Green Version]
- Janssens, S.; Dubois, C.; Bogaert, J.; Theunissen, K.; Deroose, C.; Desmet, W.; Kalantzi, M.; Herbots, L.; Sinnaeve, P.; Dens, J.; et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: Double-blind, randomised controlled trial. Lancet 2006, 367, 113–121. [Google Scholar] [CrossRef]
- Lunde, K.; Solheim, S.; Aakhus, S.; Arnesen, H.; Abdelnoor, M.; Egeland, T.; Endresen, K.; Ilebekk, A.; Mangschau, A.; Fjeld, J.G.; et al. Intracoronary Injection of Mononuclear Bone Marrow Cells in Acute Myocardial Infarction. N. Engl. J. Med. 2006, 355, 1199–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, G.P.; Wollert, K.C.; Lotz, J.; Steffens, J.; Lippolt, P.; Fichtner, S.; Hecker, H.; Schaefer, A.; Arseniev, L.; Hertenstein, B.; et al. Intracoronary Bone Marrow Cell Transfer After Myocardial Infarction. Circulation 2006, 113, 1287–1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, R.A.; Parmar, M.; Studer, L.; Takahashi, J. Human Trials of Stem Cell-Derived Dopamine Neurons for Parkinson’s Disease: Dawn of a New Era. Cell Stem Cell 2017, 21, 569–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hentze, H.; Soong, P.L.; Wang, S.T.; Phillips, B.W.; Putti, T.C.; Dunn, N.R. Teratoma formation by human embryonic stem cells: Evaluation of essential parameters for future safety studies. Stem Cell Res. 2009, 2, 198–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Yamazaki, S.; Yamaguchi, T.; Okabe, M.; Masaki, H.; Takaki, S.; Otsu, M.; Nakauchi, H. Generation of Engraftable Hematopoietic Stem Cells From Induced Pluripotent Stem Cells by Way of Teratoma Formation. Mol. Ther. 2013, 21, 1424–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahlke, J.; Schott, J.W.; Vollmer Barbosa, P.; Klatt, D.; Selich, A.; Lachmann, N.; Morgan, M.; Moritz, T.; Schambach, A. Efficient Genetic Safety Switches for Future Application of iPSC-Derived Cell Transplants. J. Pers. Med. 2021, 11, 565. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; Tang, C.; Rao, M.S.; Weissman, I.L.; Wu, J.C. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat. Med. 2013, 19, 998–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orfao, A.; Ruiz-Argüelles, A. General concepts about cell sorting techniques. Clin. Biochem. 1996, 29, 5–9. [Google Scholar] [CrossRef]
- Tang, W.; Jiang, D.; Li, Z.; Zhu, L.; Shi, J.; Yang, J.; Xiang, N. Recent advances in microfluidic cell sorting techniques based on both physical and biochemical principles. Electrophoresis 2019, 40, 930–954. [Google Scholar] [CrossRef] [PubMed]
- Sutermaster, B.A.; Darling, E.M. Considerations for high-yield, high-throughput cell enrichment: Fluorescence versus magnetic sorting. Sci. Rep. 2019, 9, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, J.; Wan, J. Methodological comparison of FACS and MACS isolation of enriched microglia and astrocytes from mouse brain. J. Immunol. Methods 2020, 486, 112834. [Google Scholar] [CrossRef]
- Miltenyi, S.; Muller, W.; Weichel, W.; Radbruch, A. High gradient magnetic cell separation with MACS. Cytometry 1990, 11, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Simaria, A.S.; Varadaraju, H.; Gupta, S.; Warren, K.; Farid, S.S. Allogeneic cell therapy bioprocess economics and optimization: Downstream processing decisions. Regen. Med. 2015, 10, 591–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brechmann, N.A.; Eriksson, P.-O.; Eriksson, K.; Oscarsson, S.; Buijs, J.; Shokri, A.; Hjälm, G.; Chotteau, V. Pilot-scale process for magnetic bead purification of antibodies directly from non-clarified CHO cell culture. Biotechnol. Prog. 2019, 35, e2775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brechmann, N.A.; Schwarz, H.; Eriksson, P.; Eriksson, K.; Shokri, A.; Chotteau, V. Antibody capture process based on magnetic beads from very high cell density suspension. Biotechnol. Bioeng. 2021, 118, 3499–3510. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells—Current trends and future prospective. Biosci. Rep. 2015, 35, e00191. [Google Scholar] [CrossRef] [PubMed]
- Grützkau, A.; Radbruch, A. Small but mighty: How the MACS®-technology based on nanosized superparamagnetic particles has helped to analyze the immune system within the last 20 years. Cytom. Part A 2010, 77A, 643–647. [Google Scholar] [CrossRef]
- Markides, H.; Rotherham, M.; El Haj, A.J. Biocompatibility and Toxicity of Magnetic Nanoparticles in Regenerative Medicine. J. Nanomater. 2012, 2012, 614094. [Google Scholar] [CrossRef] [Green Version]
- Laghmouchi, A.; Hoogstraten, C.; Falkenburg, J.H.F.; Jedema, I. Long-term in vitro persistence of magnetic properties after magnetic bead-based cell separation of T cells. Scand. J. Immunol. 2020, 92, e12924. [Google Scholar] [CrossRef] [PubMed]
- Zborowski, M.; Chalmers, J.J. Magnetic cell sorting. In Immunochemical Protocols; Springer: Berlin, Germany, 2005; pp. 291–300. [Google Scholar]
- Bong, K.W.; Chapin, S.C.; Doyle, P.S. Magnetic Barcoded Hydrogel Microparticles for Multiplexed Detection. Langmuir 2010, 26, 8008–8014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).