Two-Dimensional Doped Materials
Abstract
1. Introduction
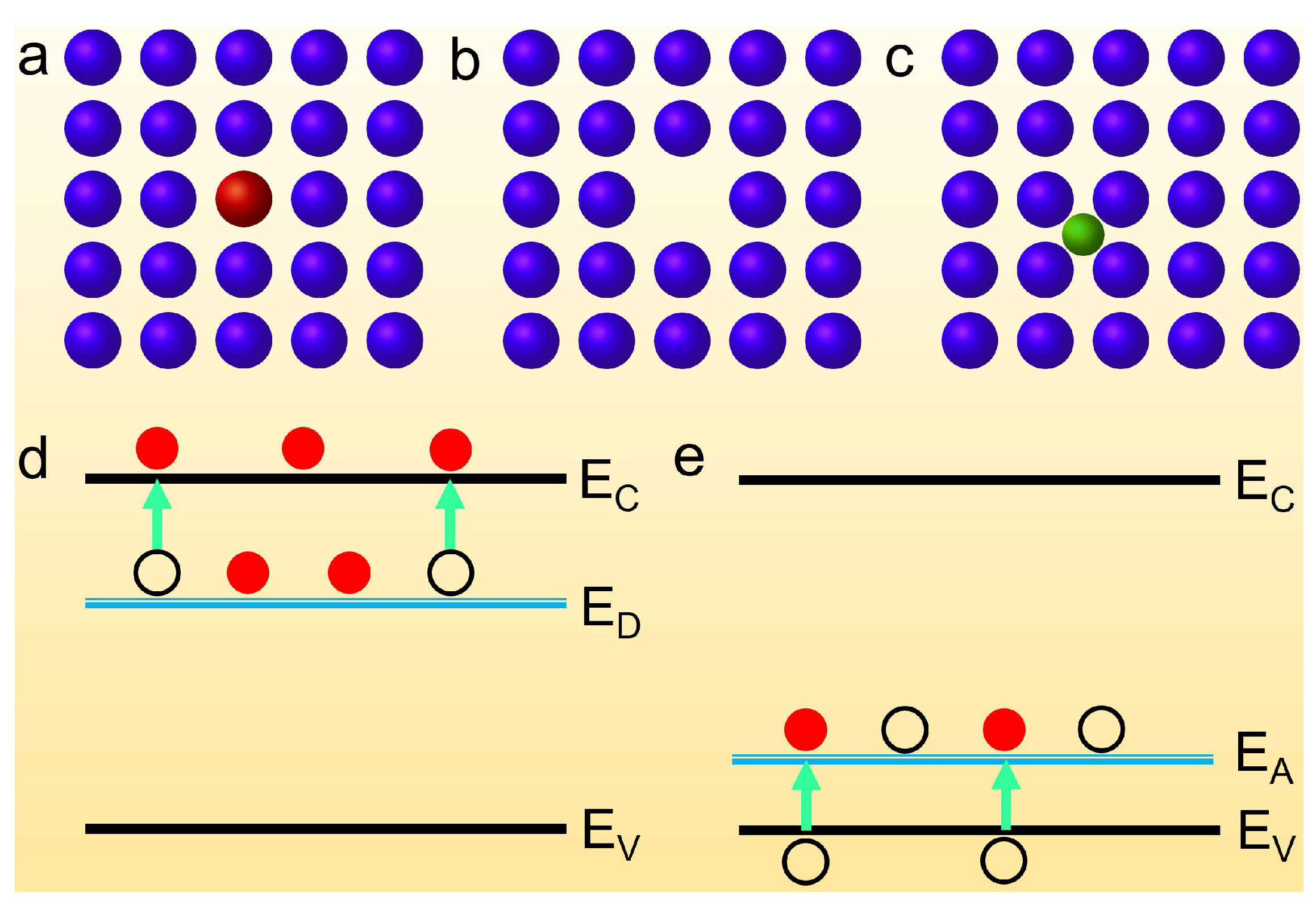
2. Classic Semiconductor Doping Type
2.1. Substitutional Doping
2.2. Vacancy
2.3. Interstitial Doping
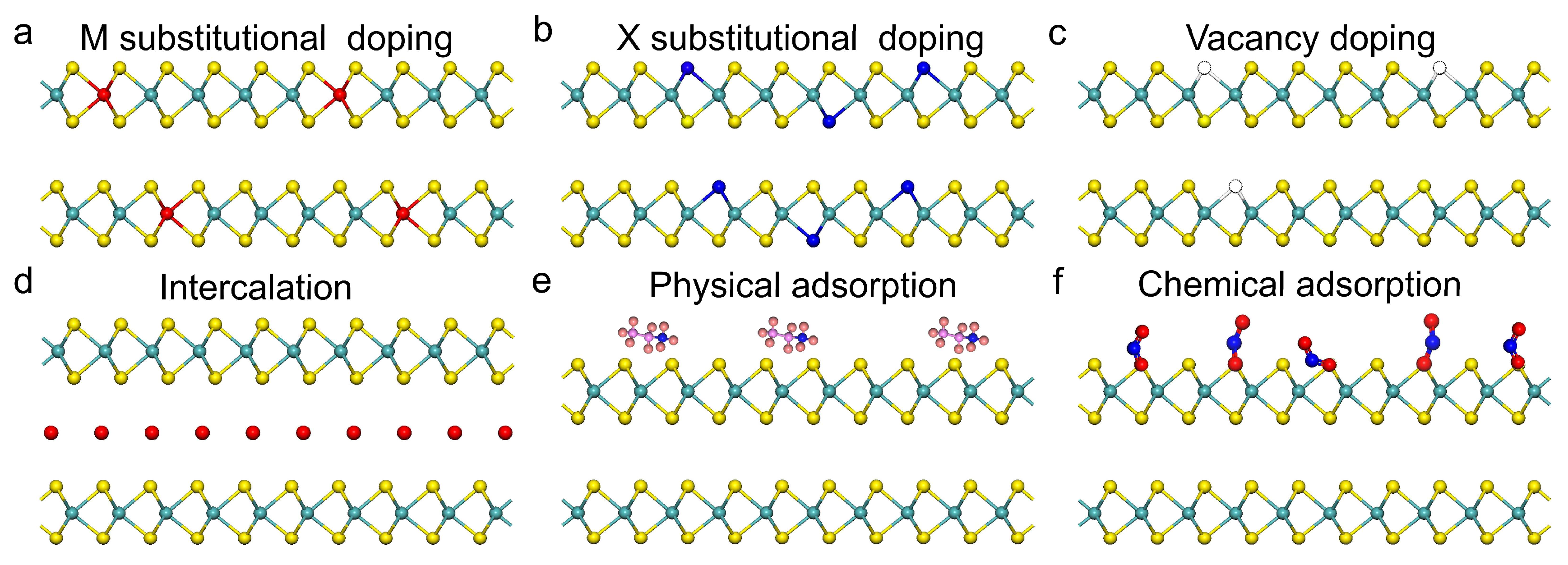
3. Doping Types in 2D Doped Materials
3.1. Substitutional Doping
3.2. Vacancy
3.3. Intercalation
3.4. Adsorption
4. Theoretical Investigation of 2D Doped Materials
5. Synthesis of 2D Doped Materials
5.1. Mechanical Exfoliation Method
5.2. Surface Functionalization
5.3. Vapor Phase Deposition Method
5.4. Plasma Treatment
5.5. Molecular Absorption Method
5.6. Electrochemical Intercalation Method
5.7. Thermal Evaporation Method
6. Characterization of 2D Doped Materials
6.1. Optical Microscopy
6.2. Scanning Transmission Electron Microscopy
6.3. Raman Spectroscopy
6.4. X-ray Photoelectron Spectroscopy
6.5. Aberration-Corrected Transmission Electron Microscopy
6.6. X-ray Diffraction Pattern
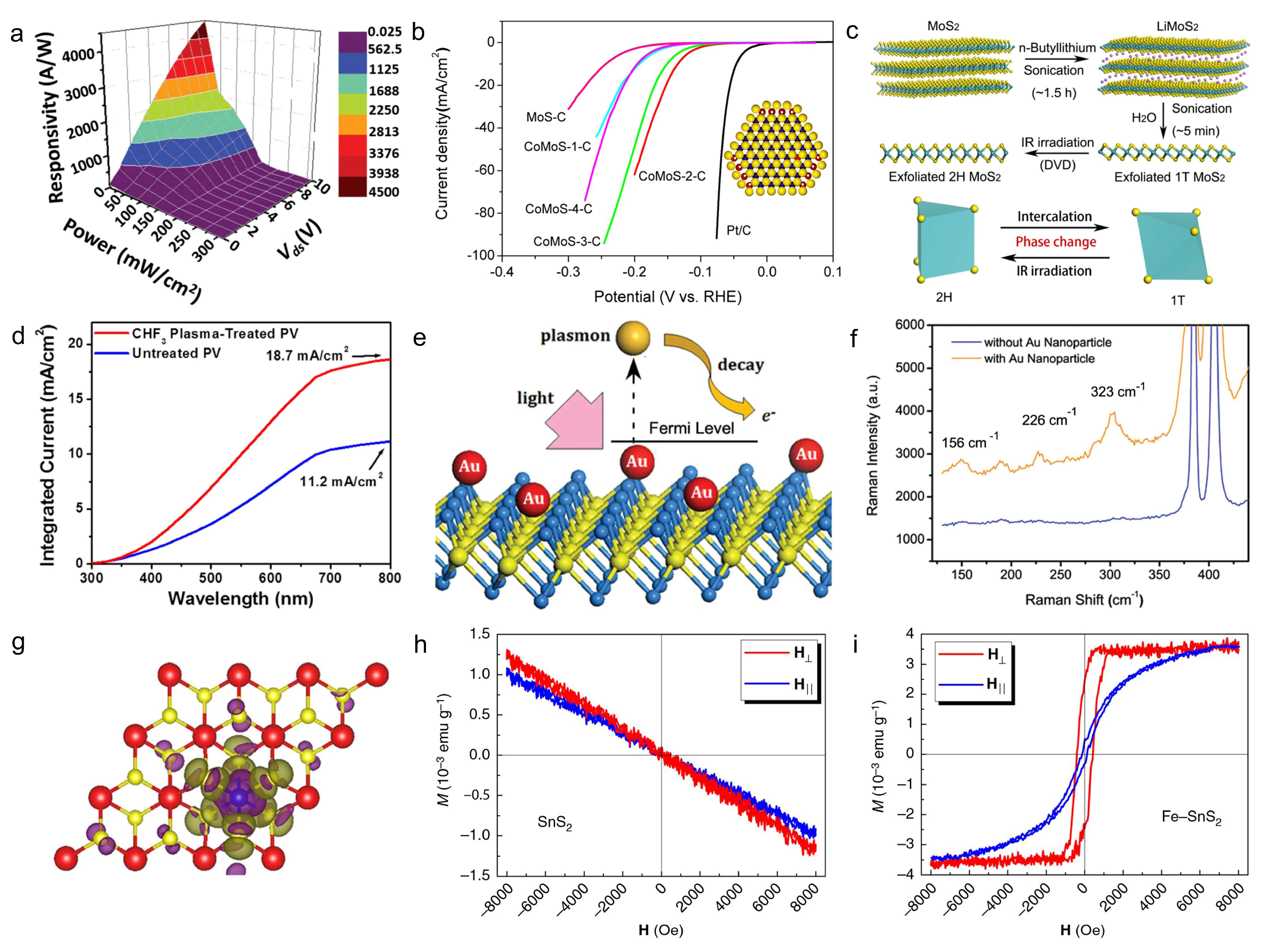
7. Properties and Applications of 2D Doped Materials
7.1. Electrical Transport and Devices
7.2. Optoelectronics, Catalysis and Magnetism
8. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wan, Z.; Liu, Y.; Xu, J.; Yang, X.; Shen, D.; Zhang, Z.; Guo, C.; Qian, Q.; Li, J.; et al. High-order superlattices by rolling up van der Waals heterostructures. Nature 2021, 591, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Z.; Li, B.; Duan, X. Synthesis of two-dimensional/one-dimensional heterostructures with tunable width. J. Semicond. 2021, 42, 092001. [Google Scholar] [CrossRef]
- Li, J.; Liang, J.; Yang, X.; Li, X.; Zhao, B.; Li, B.; Duan, X. Controllable Preparation of 2D Vertical van der Waals Heterostructures and Superlattices for Functional Applications. Small 2022, 18, 2107059. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Feng, J.; Ming, S.; Qu, F.; Xia, Y.; He, M.; Hu, Z.; Wang, J. Synthesis and electromagnetic transport of large-area 2D WTe2 thin film. J. Semicond. 2022, 43, 102002. [Google Scholar] [CrossRef]
- Qin, B.; Ma, H.; Hossain, M.; Zhong, M.; Xia, Q.; Li, B.; Duan, X. Substrates in the Synthesis of Two-Dimensional Materials via Chemical Vapor Deposition. Chem. Mater 2020, 32, 10321–10347. [Google Scholar] [CrossRef]
- Yuan, P.; Zhang, T.; Sun, J.; Liu, L.; Yao, Y.; Wang, Y. Recent progress in 2D group-V elemental monolayers: Fabrications and properties. J. Semicond. 2020, 41, 081003. [Google Scholar] [CrossRef]
- Li, B.; Wan, Z.; Wang, C.; Chen, P.; Huang, B.; Cheng, X.; Qian, Q.; Li, J.; Zhang, Z.; Sun, G.; et al. Van der Waals epitaxial growth of air-stable CrSe2 nanosheets with thickness-tunable magnetic order. Nat. Mater. 2021, 20, 818–825. [Google Scholar] [CrossRef]
- Li, Z.; Shu, W.; Li, Q.; Xu, W.; Zhang, Z.; Li, J.; Wang, Y.; Liu, Y.; Yang, J.; Chen, K.; et al. Nondegenerate P-Type In-Doped SnS2 Monolayer Transistor. Adv. Electron. Mater. 2021, 7, 2001168. [Google Scholar] [CrossRef]
- Li, B.; Deng, X.; Shu, W.; Cheng, X.; Qian, Q.; Wan, Z.; Zhao, B.; Shen, X.; Wu, R.; Shi, S.; et al. Air-stable ultrathin Cr3Te4 nanosheets with thickness-dependent magnetic biskyrmions. Mater. Today 2022, 57, 66–74. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, C.; Zhang, C.; Ji, W.; Li, S.; Wang, P. Magnetic tuning in a novel half-metallic Ir2TeI2 monolayer. J. Semicond. 2022, 43, 052001. [Google Scholar] [CrossRef]
- Mounet, N.; Gibertini, M.; Schwaller, P.; Campi, D.; Merkys, A.; Marrazzo, A.; Sohier, T.; Castelli, I.E.; Cepellotti, A.; Pizzi, G.; et al. Two-dimensional materials from high-throughput computational exfoliation of experimentally known compounds. Nat. Nanotechnol. 2018, 13, 246–252. [Google Scholar] [CrossRef]
- Chang, C.; Chen, W.; Chen, Y.; Chen, Y.; Chen, Y.; Ding, F.; Fan, C.; Jin Fan, H.; Fan, Z.; Gong, C.; et al. Recent Progress on Two-Dimensional Materials. Acta Phys-Chim. Sin. 2021, 37, 2108017. [Google Scholar] [CrossRef]
- Liu, W.; Bao, H.; Li, Y.; Ma, F. Highly tunable electronic structure and linear dichroism in 90 degrees twisted alpha-phosphorus carbide bilayer: A first-principles calculation. Phys. Chem. Chem. Phys. 2021, 23, 7080–7087. [Google Scholar] [CrossRef]
- Xiang, Y.; Xia, Q.-l.; Luo, J.-h.; Liu, Y.-p.; Peng, Y.-d.; Wang, D.-w.; Nie, Y.-z.; Guo, G.-h. Observation of ferromagnetism in black phosphorus nanosheets with high magnetization by liquid exfoliation. Solid State Commun. 2018, 281, 1–5. [Google Scholar] [CrossRef]
- Shi, S.; Feng, Y.; Li, B.; Zhang, H.; Li, Q.; Mo, Z.; Zhou, X.; Lu, Z.; Dang, W.; Lin, X.; et al. Broadband and high-performance SnS2/FePS3/graphene van der Waals heterojunction photodetector. Appl. Phys. Lett. 2022, 120, 081101. [Google Scholar] [CrossRef]
- Liu, Y.; Weiss, N.O.; Duan, X.; Cheng, H.-C.; Huang, Y.; Duan, X. Van der Waals heterostructures and devices. Nat. Rev. Mater. 2016, 1, 16042. [Google Scholar] [CrossRef]
- Wang, X.; Pan, L.; Yang, J.; Li, B.; Liu, Y.Y.; Wei, Z. Direct Synthesis and Enhanced Rectification of Alloy-to-Alloy 2D Type-II MoS2(1-x)Se2x/SnS2(1-y)Se2y Heterostructures. Adv. Mater. 2021, 33, e2006908. [Google Scholar] [CrossRef]
- Wang, P.; Han, S.; Quhe, R. Quantum transport simulation of the two-dimensional GaSb transistors. J. Semicond. 2021, 42, 122001. [Google Scholar] [CrossRef]
- Huang, M.; Zheng, Z.; Dai, Z.; Guo, X.; Wang, S.; Jiang, L.; Wei, J.; Chen, S. DASP: Defect and Dopant ab-initio Simulation Package. J. Semicond. 2022, 43, 042101. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, C.; Song, L.; Zhang, H.; Huang, Z.; He, C.; Li, B.; Lin, X.; Zhang, Z.; Shi, S.; et al. Promoting the optoelectronic and ferromagnetic properties of Cr2S3 nanosheets via Se doping. Sci. China Phys. Mech. 2022, 65, 276811. [Google Scholar] [CrossRef]
- Zhu, H.; Gan, X.; McCreary, A.; Lv, R.; Lin, Z.; Terrones, M. Heteroatom doping of two-dimensional materials: From graphene to chalcogenides. Nano Today 2020, 30, 100829. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Han, C.; Chen, W. Surface charge transfer doping for two-dimensional semiconductor-based electronic and optoelectronic devices. Nano Res. 2020, 14, 1682–1697. [Google Scholar] [CrossRef]
- Loh, L.; Zhang, Z.; Bosman, M.; Eda, G. Substitutional doping in 2D transition metal dichalcogenides. Nano Res. 2021, 14, 1668–1681. [Google Scholar] [CrossRef]
- Rana, F.; Koksal, O.; Jung, M.; Shvets, G.; Vamivakas, A.N.; Manolatou, C. Exciton-Trion Polaritons in Doped Two-Dimensional Semiconductors. Phys. Rev. Lett. 2021, 126, 127402. [Google Scholar] [CrossRef]
- Shen, D.; Zhao, B.; Zhang, Z.; Zhang, H.; Yang, X.; Huang, Z.; Li, B.; Song, R.; Jin, Y.; Wu, R.; et al. Synthesis of Group VIII Magnetic Transition-Metal-Doped Monolayer MoSe2. ACS Nano 2022, 16, 10623–10631. [Google Scholar] [CrossRef]
- Luo, J.-H.; Li, B.; Zhang, J.-M.; Zhong, M.-Z.; Xia, Q.-L.; Nie, Y.-Z.; Guo, G.-H. Bi doping-induced ferromagnetism of layered material SnSe2 with extremely large coercivity. J. Magn. Magn. Mater. 2019, 486, 165269. [Google Scholar] [CrossRef]
- Xin, K.; Wang, X.; Grove-Rasmussen, K.; Wei, Z. Twist-angle two-dimensional superlattices and their application in (opto)electronics. J. Semicond. 2022, 43, 011001. [Google Scholar] [CrossRef]
- Shen, G.; Zhao, Y.; Bai, Y.; Liu, J.; Xie, H.; Dong, Z.; Yang, J.; Yu, D. Photoluminescene study acceptor defects in lightly doped n type GaSb single crystals. J. Semicond. 2019, 40, 042101. [Google Scholar] [CrossRef]
- Sze, S.M.; Lee, M.-K. Semiconductor Devices: Physics and Technology, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012; p. 592. [Google Scholar]
- Chen, Y.; Xi, J.; Dumcenco, D.O.; Liu, Z.; Suenaga, K.; Wang, D.; Shuai, Z.; Huang, Y.-S.; Xie, L. Tunable band gap photoluminescence from atomically thin transition-metal dichalcogenide alloys. ACS Nano 2013, 7, 4610–4616. [Google Scholar] [CrossRef]
- Chen, Y.; Dumcenco, D.O.; Zhu, Y.; Zhang, X.; Mao, N.; Feng, Q.; Zhang, M.; Zhang, J.; Tan, P.-H.; Huang, Y.-S.; et al. Composition-dependent Raman modes of Mo1-xWxS2 monolayer alloys. Nanoscale 2014, 6, 2833–2839. [Google Scholar] [CrossRef] [PubMed]
- Qingliang, F.; Yiming, Z.; Jinhua, H.; Mei, Z.; Wenjie, D.; Nannan, M.; Juanxia, W.; Hua, X.; Fengliang, D.; Fang, L.; et al. Growth of large-area 2D MoS2(1-x)Se2x semiconductor alloys. Adv. Mater. 2014, 26, 2648–2653. [Google Scholar]
- Feng, Q.; Mao, N.; Wu, J.; Xu, H.; Wang, C.; Zhang, J.; Xie, L. Growth of MoS2(1-x)Se2x (x = 0.41–1.00) monolayer alloys with controlled morphology by physical vapor deposition. ACS Nano 2015, 9, 7450–7455. [Google Scholar] [CrossRef]
- Li, H.; Duan, X.; Wu, X.; Zhuang, X.; Zhou, H.; Zhang, Q.; Zhu, X.; Hu, W.; Ren, P.; Guo, P.; et al. Growth of alloy MoS2xSe2(1–x) nanosheets with fully tunable chemical compositions and optical properties. J. Am. Chem. Soc. 2014, 136, 3756–3759. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Q.; Duan, X.; Wu, X.; Fan, X.; Zhu, X.; Zhuang, X.; Hu, W.; Zhou, H.; Pan, A.; et al. Lateral growth of composition graded atomic layer MoS2(1-x)Se2x nanosheets. J. Am. Chem. Soc. 2015, 137, 5284–5287. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Wang, C.; Fan, Z.; Hao, G.; Kou, L.; Halim, U.; Li, H.; Wu, X.; Wang, Y.; Jiang, J.; et al. Synthesis of WS2xSe2–2x Alloy Nanosheets with Composition-Tunable Electronic Properties. Nano Lett. 2016, 16, 264–269. [Google Scholar] [CrossRef]
- Mann, J.; Ma, Q.; Odenthal, P.M.; Isarraraz, M.; Le, D.; Preciado, E.; Barroso, D.; Yamaguchi, K.; von Son Palacio, G.; Nguyen, A.; et al. 2-dimensional transition metal dichalcogenides with tunable direct band gaps: MoS2(1–x)Se2x monolayers. Adv. Mater. 2014, 26, 1399–1404. [Google Scholar] [CrossRef]
- Dou, C.X.; Wen, W.; Wang, J.L.; Ma, M.Y.; Xie, L.M.; Ho, C.H.; Wei, Z.Y. Ternary ReS2(1-x)Se2x alloy saturable absorber for passively Q-switched and mode-locked erbium-doped all-fiber lasers. Photonics Res. 2019, 7, 283–288. [Google Scholar] [CrossRef]
- Yu, P.; Lin, J.; Sun, L.; Le, Q.L.; Yu, X.; Gao, G.; Hsu, C.-H.; Wu, D.; Chang, T.-R.; Zeng, Q.; et al. Metal–semiconductor phase-transition in WSe2(1-x)Te2x monolayer. Adv. Mater. 2017, 29, 1603991. [Google Scholar] [CrossRef]
- Song, J.-G.; Ryu, G.H.; Lee, S.J.; Sim, S.; Lee, C.W.; Choi, T.; Jung, H.; Kim, Y.; Lee, Z.; Myoung, J.-M.; et al. Controllable synthesis of molybdenum tungsten disulfide alloy for vertically composition-controlled multilayer. Nat. Commun. 2015, 6, 7817. [Google Scholar] [CrossRef]
- Kudrynskyi, Z.R.; Wang, X.; Sutcliffe, J.; Bhuiyan, M.A.; Fu, Y.; Yang, Z.; Makarovsky, O.; Eaves, L.; Solomon, A.; Maslyuk, V.T.; et al. Van der Waals SnSe2(1−x)S2x alloys: Composition-dependent bowing coefficient and electron–phonon interaction. Adv. Funct. Mater. 2020, 30, 1908092. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, L.; Li, B.; Shang, J.; Xia, C.; Fan, C.; Deng, H.-X.; Wei, Z.; Li, J. Composition-tunable 2D SnSe2(1-x)S2x alloys towards efficient bandgap engineering and high performance (opto) electronics. J. Mater. Chem. C 2017, 5, 84–90. [Google Scholar] [CrossRef]
- Perumal, P.; Ulaganathan, R.K.; Sankar, R.; Liao, Y.-M.; Sun, T.-M.; Chu, M.-W.; Chou, F.C.; Chen, Y.-T.; Shih, M.-H.; Chen, Y.-F. Ultra-thin layered ternary single crystals Sn(SxSe1-x)2 with bandgap engineering for high performance phototransistors on versatile substrates. Adv. Funct. Mater. 2016, 26, 3630–3638. [Google Scholar] [CrossRef]
- Liu, H.; Antwi, K.K.A.; Chua, S.; Chi, D. Vapor-phase growth and characterization of Mo1-xWxS2 (0 < x < 1) atomic layers on 2-inch sapphire substrates. Nanoscale 2014, 6, 624–629. [Google Scholar] [PubMed]
- Tongay, S.; Narang, D.S.; Kang, J.; Fan, W.; Ko, C.; Luce, A.V.; Wang, K.X.; Suh, J.; Patel, K.D.; Pathak, V.M.; et al. Two-dimensional semiconductor alloys: Monolayer Mo1-xWxSe2. Appl. Phys. Lett. 2014, 104, 012101. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, Z.; Lupini, A.R.; Shi, G.; Lin, J.; Najmaei, S.; Lin, Z.; Elias, A.L.; Berkdemir, A.; You, G.; et al. Band gap engineering and layer-by-layer mapping of selenium-doped molybdenum disulfide. Nano Lett. 2014, 14, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, X.J.; Zhu, X.Y.; Dumcenco, X.D.O.; Hong, J.; Mao, N.; Deng, S.; Chen, Y.; Yang, Y.; Jin, C.; et al. Two-Dimensional Molybdenum Tungsten Diselenide Alloys: Photoluminescence, Raman Scattering, and Electrical Transport. ACS Nano 2014, 8, 7130–7137. [Google Scholar] [CrossRef] [PubMed]
- Xie, L. Two-dimensional transition metal dichalcogenide alloys: Preparation, characterization and applications. Nanoscale 2015, 7, 18392–18401. [Google Scholar] [CrossRef]
- Liang, F.; Xu, H.; Dong, Z.; Xie, Y.; Luo, C.; Xia, Y.; Zhang, J.; Wang, J.; Wu, X. Substrates and interlayer coupling effects on Mo1−xWxSe2 alloys. J. Semicond. 2019, 40, 062005. [Google Scholar] [CrossRef]
- Feng, L.P.; Su, J.; Liu, Z.T. Effect of vacancies on structural, electronic and optical properties of monolayer MoS2: A first-principles study. J. Alloy Compd. 2014, 613, 122–127. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, J.; Zhong, M.; Xia, Q.; Li, B.; Duan, X.; Wei, Z. Intercalation of Two-dimensional Layered Materials. Chem. Res. Chin. Univ. 2020, 36, 584–596. [Google Scholar] [CrossRef]
- Gong, Y.; Yuan, H.; Wu, C.L.; Tang, P.; Yang, S.Z.; Yang, A.; Li, G.; Liu, B.; van de Groep, J.; Brongersma, M.L.; et al. Spatially controlled doping of two-dimensional SnS2 through intercalation for electronics. Nat. Nanotechnol. 2018, 13, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.-Q.; Guo, J.-J.; Luo, Z.-Y.; Zhong, M.-Z.; Li, B.; Wang, X.-G.; Nie, Y.-Z.; Xia, Q.-L.; Guo, G.-H. Anisotropic Magnetoresistance Effect of Intercalated Ferromagnet FeTa3S6. Front. Phys. 2022, 10, 847402. [Google Scholar] [CrossRef]
- Qi, B.-T.; Guo, J.-J.; Miao, Y.-Q.; Zhong, M.-Z.; Li, B.; Luo, Z.-Y.; Wang, X.-G.; Nie, Y.-Z.; Xia, Q.-L.; Guo, G.-H. Abnormal Magnetoresistance Transport Properties of van der Waals Antiferromagnetic FeNbTe2. Front. Phys. 2022, 10, 851838. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, D.; Ouyang, B.; Raja, A.; Song, J.; Heinz, T.F.; Brus, L.E. Probing the dynamics of the metallic-to-semiconducting structural phase transformation in MoS2 crystals. Nano Lett. 2015, 15, 5081–5088. [Google Scholar] [CrossRef]
- Tongay, S.; Zhou, J.; Ataca, C.; Liu, J.; Kang, J.S.; Matthews, T.S.; You, L.; Li, J.; Grossman, J.C.; Wu, J. Broad-range modulation of light emission in two-dimensional semiconductors by molecular physisorption gating. Nano Lett. 2013, 13, 2831–2836. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Xie, X.; Kang, J.; Zhang, H.; Liu, W.; Navarrete, J.; Moskovits, M.; Banerjee, K. Functionalization of transition metal dichalcogenides with metallic nanoparticles: Implications for doping and gas-sensing. Nano Lett. 2015, 15, 2852–2862. [Google Scholar] [CrossRef]
- Kang, D.-H.; Shim, J.; Jang, S.K.; Jeon, J.; Jeon, M.H.; Yeom, G.Y.; Jung, W.-S.; Jang, Y.H.; Lee, S.; Park, J.-H. Controllable nondegenerate p-type doping of tungsten diselenide by octadecyltrichlorosilane. ACS Nano 2015, 9, 1099–1107. [Google Scholar] [CrossRef]
- Zhao, P.; Kiriya, D.; Azcatl, A.; Zhang, C.; Tosun, M.; Liu, Y.-S.; Hettick, M.; Kang, J.S.; McDonnell, S.; KC, S.; et al. Air Stable p-Doping of WSe2 by Covalent Functionalization. ACS Nano 2014, 8, 10808–10814. [Google Scholar] [CrossRef]
- Yang, L.; Majumdar, K.; Liu, H.; Du, Y.; Wu, H.; Hatzistergos, M.; Hung, P.Y.; Tieckelmann, R.; Tsai, W.; Hobbs, C.; et al. Chloride molecular doping technique on 2D materials: WS2 and MoS2. Nano Lett. 2014, 14, 6275–6280. [Google Scholar] [CrossRef]
- Kang, J.; Tongay, S.; Li, J.; Wu, J. Monolayer semiconducting transition metal dichalcogenide alloys: Stability and band bowing. J. Appl. Phys. 2013, 113, 143703. [Google Scholar] [CrossRef]
- Mishra, R.; Zhou, W.; Pennycook, S.J.; Pantelides, S.T.; Idrobo, J.-C. Long-range ferromagnetic ordering in manganese-doped two-dimensional dichalcogenides. Phys. Rev. B 2013, 88, 144409. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Zhu, Z.Y.; Mi, W.B.; Guo, Z.B.; Schwingenschlögl, U. Prediction of two-dimensional diluted magnetic semiconductors: Doped monolayer MoS2 systems. Phys. Rev. B 2013, 87, 100401. [Google Scholar] [CrossRef]
- Kan, M.; Wang, J.Y.; Li, X.W.; Zhang, S.H.; Li, Y.W.; Kawazoe, Y.; Sun, Q.; Jena, P. Structures and Phase Transition of a MoS2 Monolayer. J. Phys. Chem. C 2014, 118, 1515–1522. [Google Scholar] [CrossRef]
- Zhao, S.; Xue, J.; Kang, W. Gas adsorption on MoS2 monolayer from first-principles calculations. Chem. Phys. Lett. 2014, 595–596, 35–42. [Google Scholar] [CrossRef]
- Ramasubramaniam, A.; Naveh, D. Mn-doped monolayer MoS2: An atomically thin dilute magnetic semiconductor. Phys. Rev. B 2013, 87, 195201. [Google Scholar] [CrossRef]
- Singh, N.; Schwingenschlogl, U. A Route to Permanent Valley Polarization in Monolayer MoS2. Adv. Mater. 2017, 29, 1600970. [Google Scholar] [CrossRef]
- Li, Q.-Q.; Li, S.; Wu, D.; Ding, Z.-K.; Cao, X.-H.; Huang, L.; Pan, H.; Li, B.; Chen, K.-Q.; Duan, X.-D. Magnetic properties manipulation of CrTe2 bilayer through strain and self-intercalation. Appl. Phys. Lett. 2021, 119, 162402. [Google Scholar] [CrossRef]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef]
- Benavente, E.; Santa Ana, M.A.; Mendizábal, F.; González, G. Intercalation chemistry of molybdenum disulfide. Coord. Chem. Rev. 2002, 224, 87–109. [Google Scholar] [CrossRef]
- Eda, G.; Fujita, T.; Yamaguchi, H.; Voiry, D.; Chen, M.; Chhowalla, M. Coherent atomic and electronic heterostructures of single-layer MoS2. ACS Nano 2012, 6, 7311–7317. [Google Scholar] [CrossRef] [PubMed]
- Ataca, C.; Sahin, H.; Ciraci, S. Stable, single-layer MX2 transition-metal oxides and dichalcogenides in a honeycomb-like structure. J. Phys. Chem. C 2012, 116, 8983–8999. [Google Scholar] [CrossRef]
- Rocquefelte, X.; Boucher, F.; Gressier, P.; Ouvrard, G.; Blaha, P.; Schwarz, K. Mo cluster formation in the intercalation compound LiMoS2. Phys. Rev. B 2000, 62, 2397–2400. [Google Scholar] [CrossRef]
- Lei, S.; Wang, X.; Li, B.; Kang, J.; He, Y.; George, A.; Ge, L.; Gong, Y.; Dong, P.; Jin, Z.; et al. Surface functionalization of two-dimensional metal chalcogenides by Lewis acid-base chemistry. Nat. Nanotechnol. 2016, 11, 465–471. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Zhu, X.; Duan, X.; Pan, A. Composition modulation in one-dimensional and two-dimensional chalcogenide semiconductor nanostructures. Chem. Soc. Rev. 2018, 47, 7504–7521. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Yuan, S.; Zhao, Y.; Yang, Z.; Choi, S.Y.; Chai, Y.; Yu, S.F.; Lau, S.P.; Hao, J. 2D Layered Materials of Rare-Earth Er-Doped MoS2 with NIR-to-NIR Down- and Up-Conversion Photoluminescence. Adv. Mater. 2016, 28, 7472–7477. [Google Scholar] [CrossRef]
- Ye, G.; Gong, Y.; Lin, J.; Li, B.; He, Y.; Pantelides, S.T.; Zhou, W.; Vajtai, R.; Ajayan, P.M. Defects Engineered Monolayer MoS2 for Improved Hydrogen Evolution Reaction. Nano Lett. 2016, 16, 1097–1103. [Google Scholar] [CrossRef]
- Wang, C.; He, Q.; Halim, U.; Liu, Y.; Zhu, E.; Lin, Z.; Xiao, H.; Duan, X.; Feng, Z.; Cheng, R.; et al. Monolayer atomic crystal molecular superlattices. Nature 2018, 555, 231–236. [Google Scholar] [CrossRef]
- Chen, C.-H.; Wu, C.-L.; Pu, J.; Chiu, M.-H.; Kumar, P.; Takenobu, T.; Li, L.-J. Hole mobility enhancement and p -doping in monolayer WSe2 by gold decoration. 2D Mater. 2014, 1, 034001. [Google Scholar] [CrossRef]
- Li, B.; Huang, L.; Zhong, M.; Huo, N.; Li, Y.; Yang, S.; Fan, C.; Yang, J.; Hu, W.; Wei, Z.; et al. Synthesis and transport properties of large-scale alloy Co0.16Mo0.84S2 bilayer nanosheets. ACS Nano 2015, 9, 1257–1262. [Google Scholar] [CrossRef]
- George, A.S.; Mutlu, Z.; Ionescu, R.; Wu, R.J.; Jeong, J.S.; Bay, H.H.; Chai, Y.; Mkhoyan, K.A.; Ozkan, M.; Ozkan, C.S. Wafer scale synthesis and high resolution structural characterization of atomically thin MoS2 layers. Adv. Funct. Mater. 2014, 24, 7461–7466. [Google Scholar] [CrossRef]
- Fang, H.; Chuang, S.; Chang, T.C.; Takei, K.; Takahashi, T.; Javey, A. High-performance single layered WSe2 p-FETs with chemically doped contacts. Nano Lett. 2012, 12, 3788–3792. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.; Park, T.E.; Lin, D.Y.; Fu, D.; Park, J.; Jung, H.J.; Chen, Y.; Ko, C.; Jang, C.; Sun, Y.; et al. Doping against the native propensity of MoS2: Degenerate hole doping by cation substitution. Nano Lett. 2014, 14, 6976–6982. [Google Scholar] [CrossRef]
- Qiu, H.; Xu, T.; Wang, Z.; Ren, W.; Nan, H.; Ni, Z.; Chen, Q.; Yuan, S.; Miao, F.; Song, F.; et al. Hopping transport through defect-induced localized states in molybdenum disulphide. Nat. Commun. 2013, 4, 2642. [Google Scholar] [CrossRef]
- Krivanek, O.L.; Chisholm, M.F.; Nicolosi, V.; Pennycook, T.J.; Corbin, G.J.; Dellby, N.; Murfitt, M.F.; Own, C.S.; Szilagyi, Z.S.; Oxley, M.P.; et al. Atom-by-atom structural and chemical analysis by annular dark-field electron microscopy. Nature 2010, 464, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Hartel, P.; Rose, H.; Dinges, C. Conditions and reasons for incoherent imaging in STEM. Ultramicroscopy 1996, 63, 93–114. [Google Scholar] [CrossRef]
- Fu, Q.; Yang, L.; Wang, W.; Han, A.; Huang, J.; Du, P.; Fan, Z.; Zhang, J.; Xiang, B. Synthesis and enhanced electrochemical catalytic performance of monolayer WS2(1–x)Se2x with a tunable band gap. Adv. Mater. 2015, 27, 4732–4738. [Google Scholar] [CrossRef]
- Dumcenco, D.O.; Kobayashi, H.; Liu, Z.; Huang, Y.S.; Suenaga, K. Visualization and quantification of transition metal atomic mixing in Mo1-xWxS2 single layers. Nat. Commun. 2013, 4, 1351. [Google Scholar] [CrossRef]
- Zhang, K.; Feng, S.; Wang, J.; Azcatl, A.; Lu, N.; Addou, R.; Wang, N.; Zhou, C.; Lerach, J.; Bojan, V.; et al. Manganese doping of monolayer MoS2: The substrate is critical. Nano Lett. 2015, 15, 6586–6591. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, X.; Deng, L.; Shi, Z.; Liu, S.; Wei, Q.; Zhang, L.; Cheng, Y.; Zhang, L.; Lu, H.; et al. Enhanced Valley Zeeman Splitting in Fe-Doped Monolayer MoS2. ACS Nano 2020, 14, 4636–4645. [Google Scholar] [CrossRef]
- Li, B.; Xing, T.; Zhong, M.; Huang, L.; Lei, N.; Zhang, J.; Li, J.; Wei, Z. A two-dimensional Fe-doped SnS2 magnetic semiconductor. Nat. Commun. 2017, 8, 1958. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, S.; Addou, R.; Buie, C.; Wallace, R.M.; Hinkle, C.L. Defect-Dominated Doping and Contact Resistance in MoS2. ACS Nano 2014, 8, 2880–2888. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhong, M.; Liu, X.; Sun, G.; Chen, P.; Zhang, Z.; Li, J.; Ma, H.; Zhao, B.; Wu, R.; et al. Two-dimensional plumbum-doped tin diselenide monolayer transistor with high on/off ratio. Nanotechnology 2018, 29, 474002. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Chen, Z.; Miao, L.; Liu, X.; Li, B.; Tang, L.; Chen, K.; Liu, Y.; Li, J.; et al. Tunable Schottky barrier width and enormously enhanced photoresponsivity in Sb doped SnS2 monolayer. Nano Res. 2019, 12, 463–468. [Google Scholar] [CrossRef]
- Wang, N.; Tang, H.; Shi, M.; Zhang, H.; Zhuo, W.; Liu, D.; Meng, F.; Ma, L.; Ying, J.; Zou, L.; et al. Transition from ferromagnetic semiconductor to ferromagnetic metal with enhanced Curie temperature in Cr2Ge2Te6 via organic ion intercalation. J. Am. Soc. Chem. 2019, 141, 17166–17173. [Google Scholar] [CrossRef] [PubMed]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, I.V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Guo, S. Janus MSiGeN4 (M = Zr and Hf) monolayers derived from centrosymmetric β-MA2Z4: A first-principles study. J. Semicond. 2021, 42, 122002. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, H.; Peng, S.a.; Xiong, G.; Zhu, C.; Huang, X.; Cao, S.; Zhang, J.; Yan, Y.; Yao, Y.; et al. Radiation-hardened property of single-walled carbon nanotube film-based field-effect transistors under low-energy proton irradiation. J. Semicond. 2021, 42, 112002. [Google Scholar] [CrossRef]
- Yin, Z.; Li, H.; Li, H.; Jiang, L.; Shi, Y.; Sun, Y.; Lu, G.; Zhang, Q.; Chen, X.; Zhang, H. Single-layer MoS2 phototransistors. ACS Nano 2012, 6, 74–80. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Chen, Z.; Reinecke, B.N.; Jaramillo, T.F. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nat. Mater. 2012, 11, 963. [Google Scholar] [CrossRef]
- Kang Kim, K. Two-dimensional air-stable CrSe2 nanosheets with thickness-tunable magnetism. J. Semicond. 2021, 42, 100401. [Google Scholar] [CrossRef]
- Taur, Y.; Ning, T. Fundamentals of Modern VLSI Devices; Cambridge University Press: Cambrige, UK, 1998. [Google Scholar]
- Liu, Y.; Duan, X.D.; Huang, Y.; Duan, X.F. Two-dimensional transistors beyond graphene and TMDCs. Chem. Soc. Rev. 2018, 47, 6388–6409. [Google Scholar] [CrossRef] [PubMed]
- Kiriya, D.; Tosun, M.; Zhao, P.; Kang, J.S.; Javey, A. Air-stable surface charge transfer doping of MoS2 by benzyl viologen. J. Am. Chem. Soc. 2014, 136, 7853–7856. [Google Scholar] [CrossRef] [PubMed]
- Dolui, K.; Rungger, I.; Das Pemmaraju, C.; Sanvito, S. Possible doping strategies for MoS2 monolayers: An ab initio study. Phys. Rev. B 2013, 88, 075420. [Google Scholar] [CrossRef]
- Qian, Q.; Ren, H.; Zhou, J.; Wan, Z.; Zhou, J.; Yan, X.; Cai, J.; Wang, P.; Li, B.; Sofer, Z.; et al. Chiral molecular intercalation superlattices. Nature 2022, 606, 902–908. [Google Scholar] [CrossRef]
- Deng, N.; Tian, H.; Zhang, J.; Jian, J.; Wu, F.; Shen, Y.; Yang, Y.; Ren, T.-L. Black phosphorus junctions and their electrical and optoelectronic applications. J. Semicond. 2021, 42, 081001. [Google Scholar] [CrossRef]
- Zhou, Z.; Cui, Y.; Tan, P.-H.; Liu, X.; Wei, Z. Optical and electrical properties of two-dimensional anisotropic materials. J. Semicond. 2019, 40, 061001. [Google Scholar] [CrossRef]
- Zhang, F.; Yu, Y.; Mo, Z.; Huang, L.; Xia, Q.; Li, B.; Zhong, M.; He, J. Alloying-engineered high-performance broadband polarized Bi1.3In0.7Se3 photodetector with ultrafast response. Nano Res. 2022, 15, 8451–8457. [Google Scholar] [CrossRef]
- Mo, Z.; Zhang, F.; Wang, D.; Cui, B.; Xia, Q.; Li, B.; He, J.; Zhong, M. Ultrafast-response and broad-spectrum polarization sensitive photodetector based on Bi1.85In0.15S3 nanowire. Appl. Phys. Lett. 2022, 120, 201105. [Google Scholar] [CrossRef]
- Zhong, M.; Meng, H.; Ren, Z.; Huang, L.; Yang, J.; Li, B.; Xia, Q.; Wang, X.; Wei, Z.; He, J. Gate-controlled ambipolar transport in b-AsP crystals and their VIS-NIF photodetection. Nanoscale 2021, 13, 10579–10586. [Google Scholar] [CrossRef]
- Dai, X.; Du, K.; Li, Z.; Liu, M.; Ma, Y.; Sun, H.; Zhang, X.; Yang, Y. Co-Doped MoS2 Nanosheets with the Dominant CoMoS Phase Coated on Carbon as an Excellent Electrocatalyst for Hydrogen Evolution. ACS Appl. Mater. Interfaces 2015, 7, 27242–27253. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Xu, P.; Zhou, D.; Sun, Y.; Li, Y.C.; Nguyen, M.A.T.; Terrones, M.; Mallouk, A.T.E. Fast and Efficient Preparation of Exfoliated 2H MoS2 Nanosheets by Sonication-Assisted Lithium Intercalation and Infrared Laser-Induced 1T to 2H Phase Reversion. Nano Lett. 2015, 15, 5956–5960. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.; Chang, S.; Qin, S.; Li, J. Functionalization of monolayer MoS2 by substitutional doping: A first-principles study. Phys. Lett. A 2013, 377, 1362–1367. [Google Scholar] [CrossRef]
- Chen, M.; Nam, H.; Wi, S.; Ji, L.; Ren, X.; Bian, L.; Lu, S.; Liang, X. Stable few-layer MoS2 rectifying diodes formed by plasma-assisted doping. Appl. Phys. Lett. 2013, 103, 142110. [Google Scholar] [CrossRef]
- Wi, S.; Kim, H.; Chen, M.; Nam, H.; Guo, L.J.; Meyhofer, E.; Liang, X. Enhancement ofPhotovoltaic Response in Multilayer MoS2 Induced by Plasma Doping. ACS Nano 2014, 8, 5270–5281. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Najmaei, S.; Liu, Z.; Bao, Y.; Wang, Y.; Zhu, X.; Halas, N.J.; Nordlander, P.; Ajayan, P.M.; Lou, J.; et al. Plasmonic hot electron induced structural phase transition in a MoS2 monolayer. Adv. Mater. 2014, 26, 6467–6471. [Google Scholar] [CrossRef]
- Fang, J.; Song, H.; Li, B.; Zhou, Z.; Yang, J.; Lin, B.; Liao, Z.; Wei, Z. Large unsaturated magnetoresistance of 2D magnetic semiconductor Fe-SnS2 homojunction. J. Semicond. 2022, 43, 092501. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Li, B.; Li, Q. Two-Dimensional Doped Materials. Magnetochemistry 2022, 8, 172. https://doi.org/10.3390/magnetochemistry8120172
Liu J, Li B, Li Q. Two-Dimensional Doped Materials. Magnetochemistry. 2022; 8(12):172. https://doi.org/10.3390/magnetochemistry8120172
Chicago/Turabian StyleLiu, Junchi, Bo Li, and Qiuqiu Li. 2022. "Two-Dimensional Doped Materials" Magnetochemistry 8, no. 12: 172. https://doi.org/10.3390/magnetochemistry8120172
APA StyleLiu, J., Li, B., & Li, Q. (2022). Two-Dimensional Doped Materials. Magnetochemistry, 8(12), 172. https://doi.org/10.3390/magnetochemistry8120172





