Two-Step Calcination-Method-Derived Al-Substituted W-Type SrYb Hexaferrites: Their Microstructural, Spectral, and Magnetic Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Techniques of Characterization
3. Results and Discussion
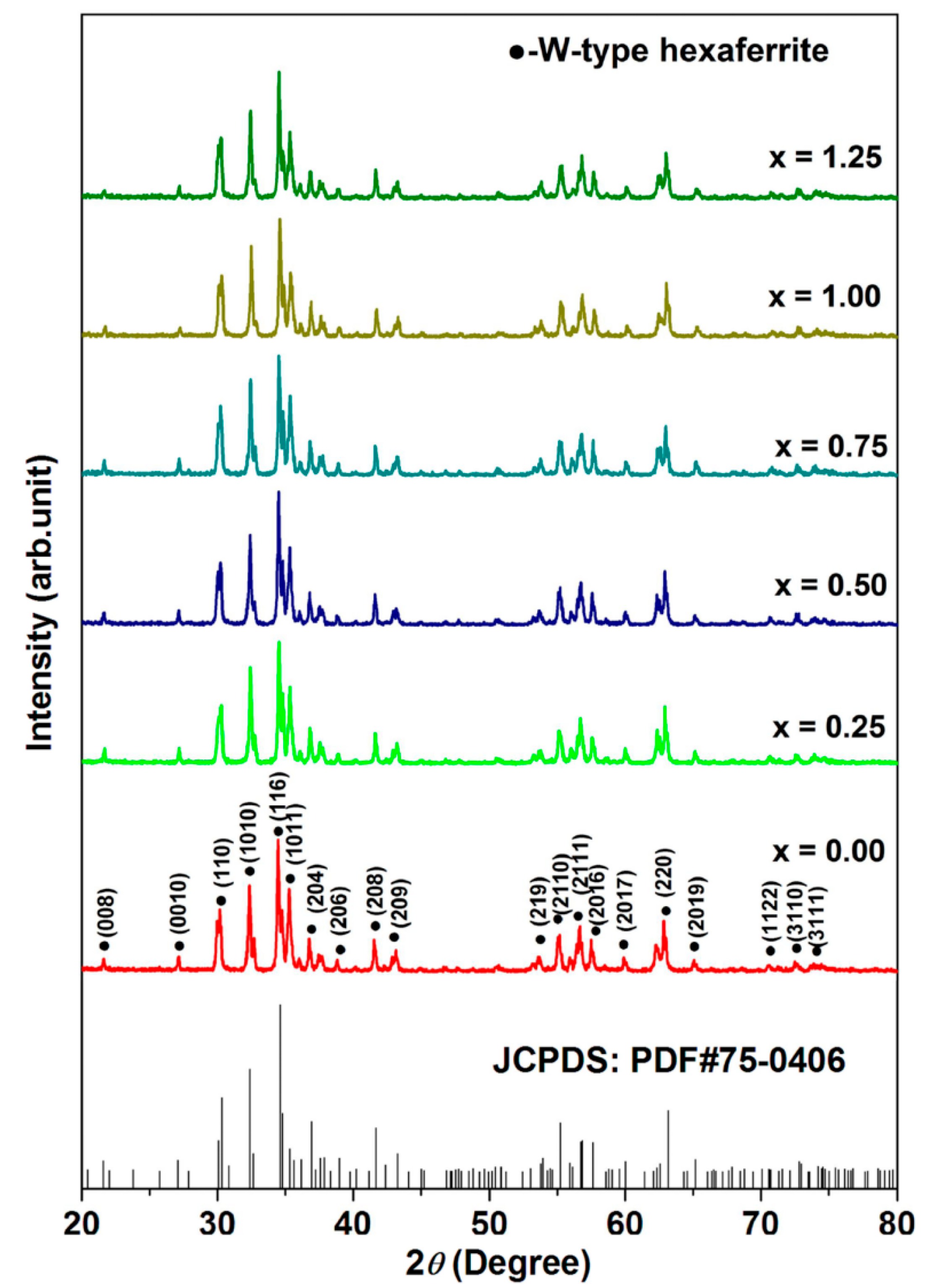
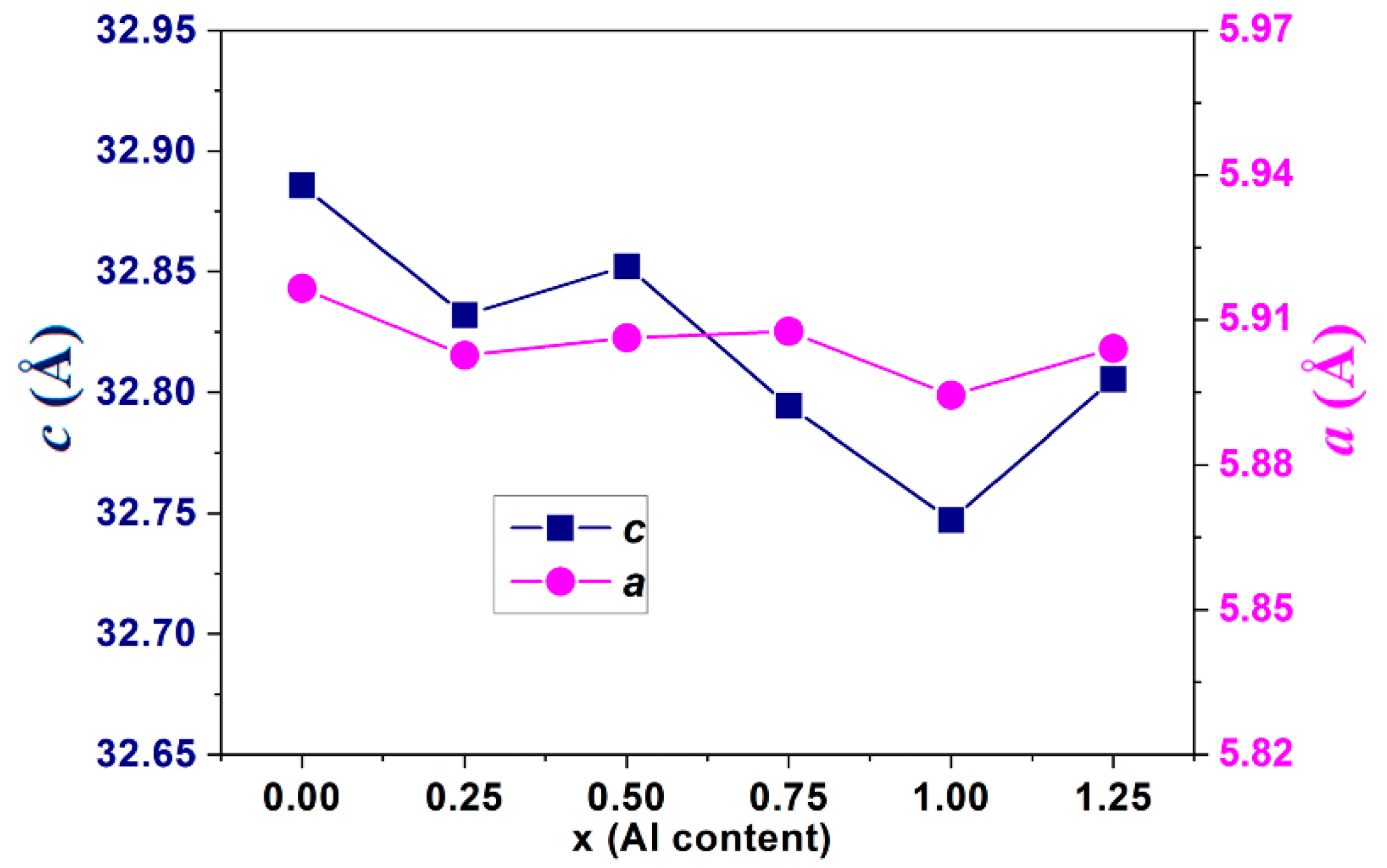
3.1. XRD Analysis
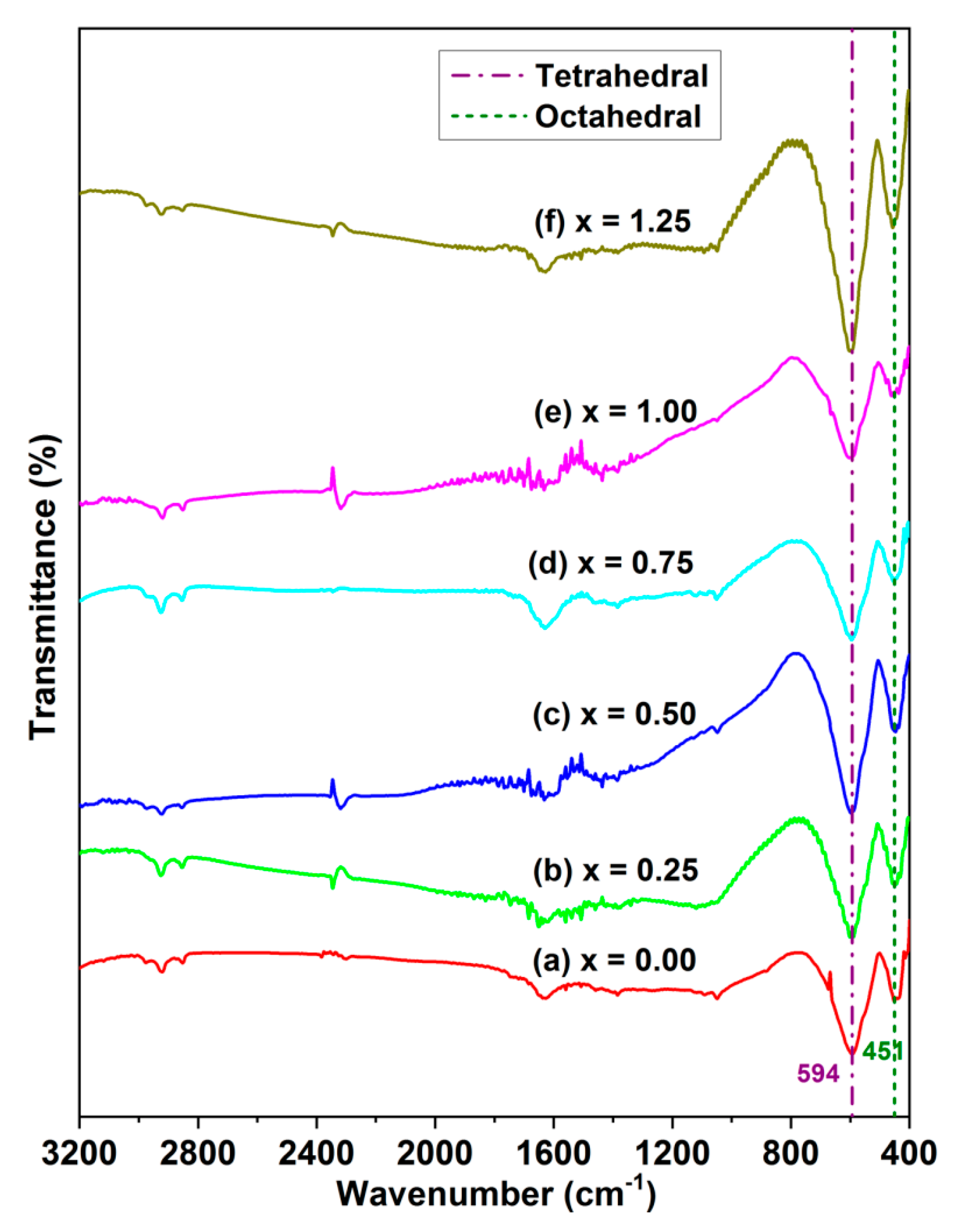
3.2. FT-IR
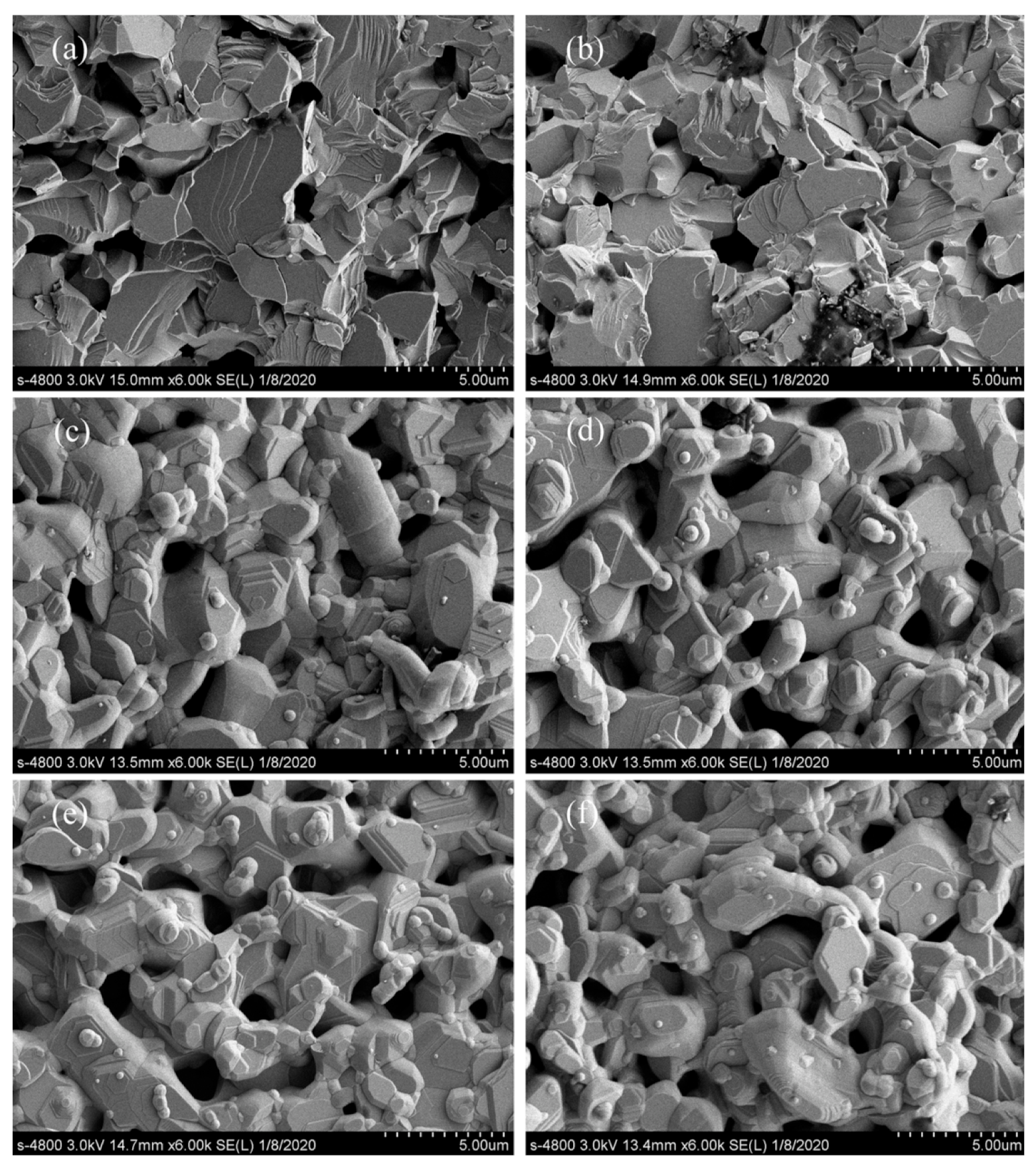
3.3. FE-SEM Images
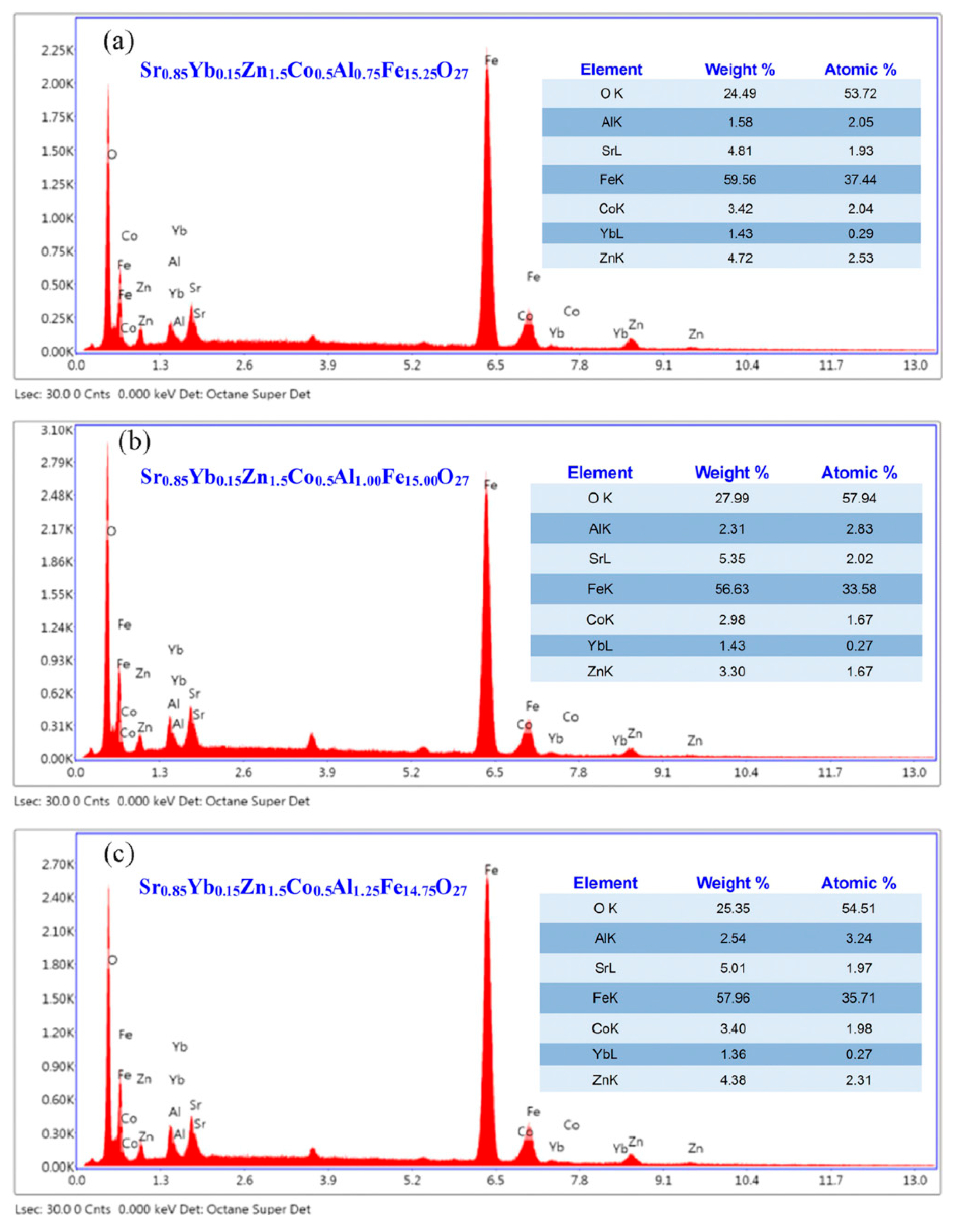
3.4. EDX Analysis
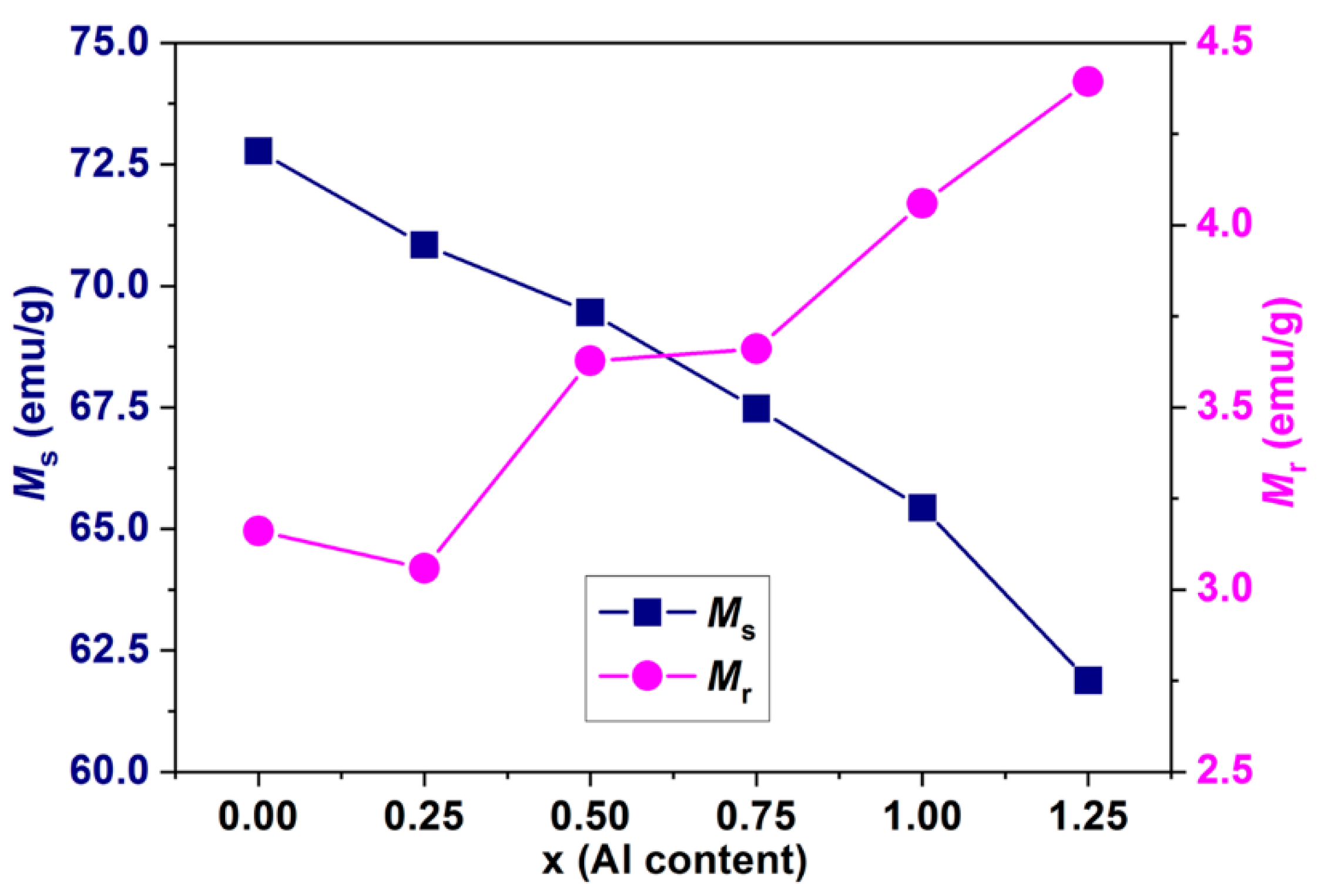
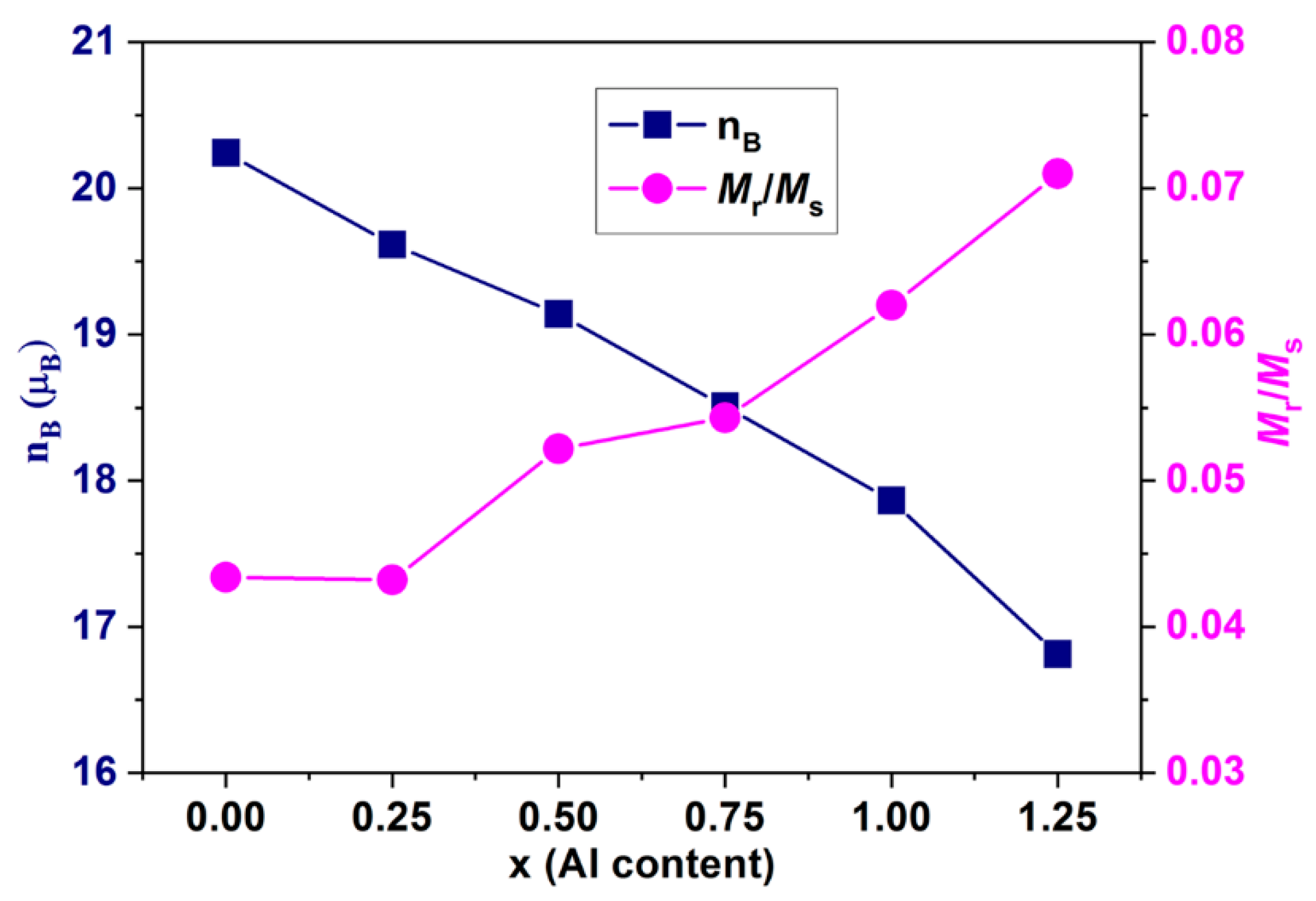
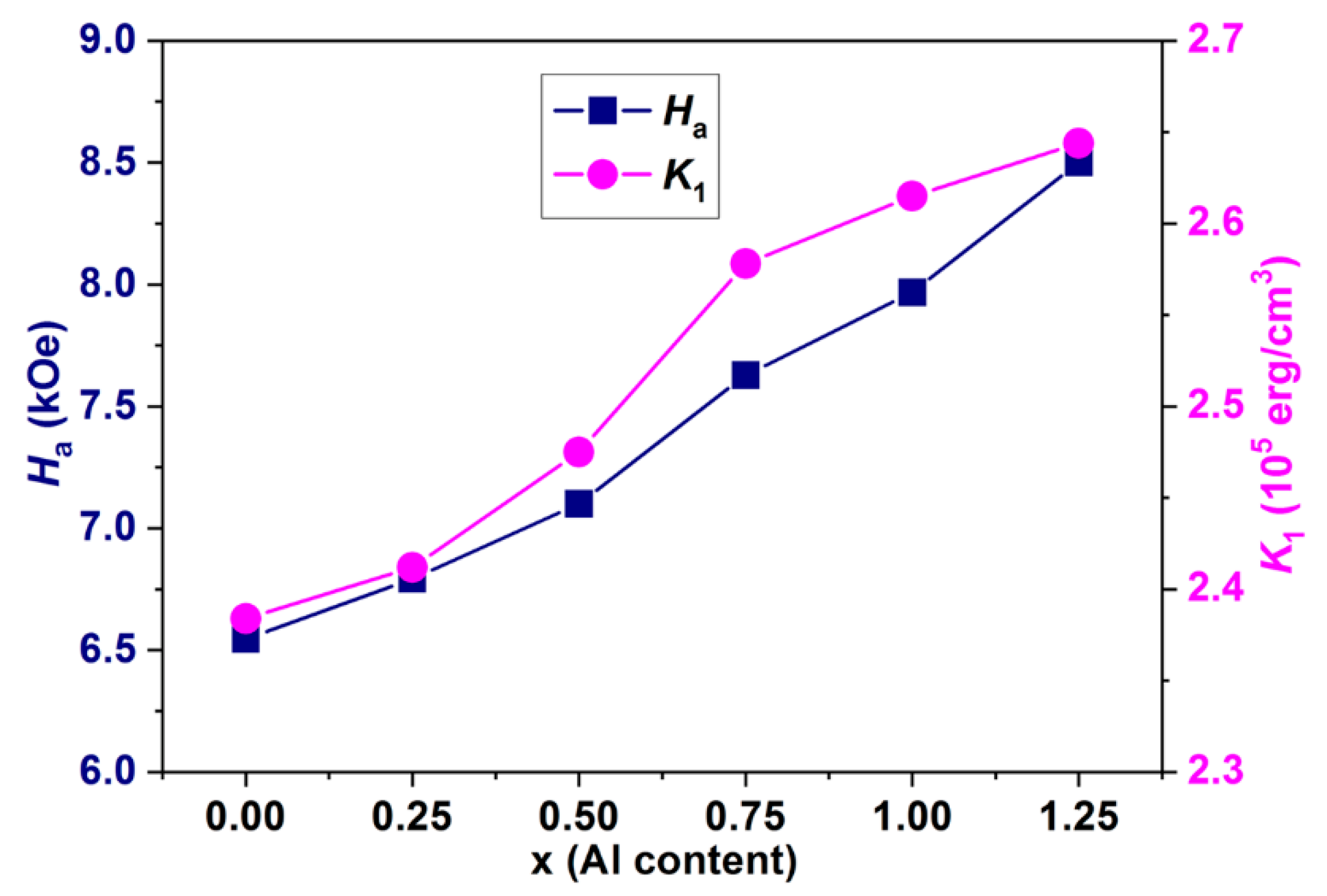
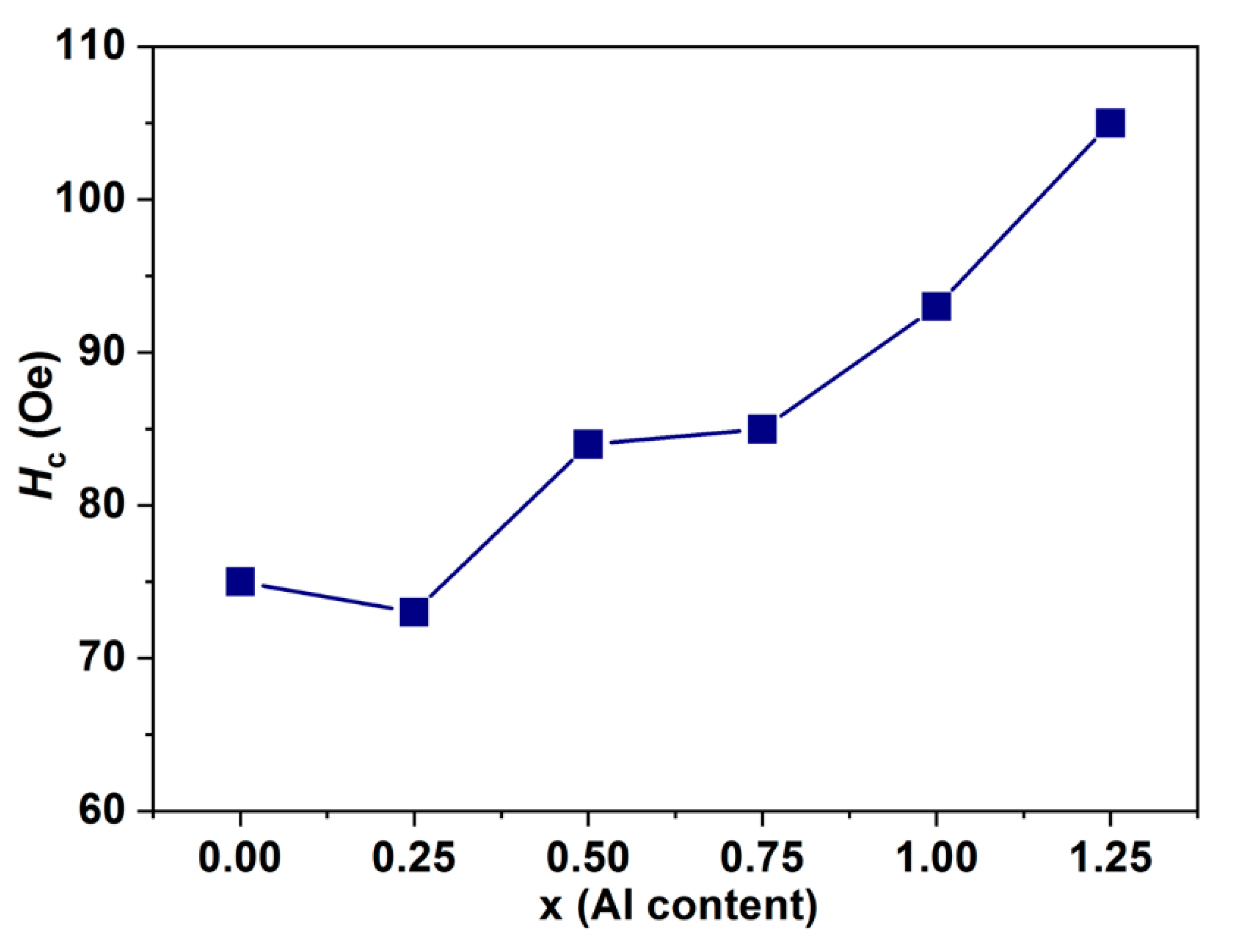
3.5. Magnetic Properties
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hemeda, D.M.; Al-Sharif, A.; Hemed, O.M. Effect of Co substitution on the structural and magnetic properties of Zn-W hexaferrite. J. Magn. Magn. Mater. 2017, 315, L1–L7. [Google Scholar] [CrossRef]
- Akhtar, M.N.; Khan, M.A. Structural, physical and magnetic evaluations of Ce-Zn substituted SrCo2 W-type hexaferrites prepared via sol gel auto combustion route. Ceram. Int. 2018, 44, 12921–12928. [Google Scholar] [CrossRef]
- Müller, R. Preparation of BaZn2−xCoxFe16O27 W-type hexaferrite powders by the glass crystallization method. J. Magn. Magn. Mater. 1993, 120, 61–63. [Google Scholar] [CrossRef]
- Huang, K.; Liu, X.; Feng, S.; Zhang, Z.; Yu, J.; Niu, X.; Lv, F.; Huang, X. Structural and magnetic properties of Ca-substituted barium W-type hexagonal hexaferrites. J. Magn. Magn. Mater. 2015, 379, 16–21. [Google Scholar] [CrossRef]
- Mandizadeh, S.; Salehabadi, A.; Amiri, O.; Salavati-Niasari, M. Amino acids assisted hydrothermal synthesis of W-type SrFe18O27 nanostructures; a potential hydrodesulfurization catalyst. Int. J. Hydrogen Energy 2019, 44, 15017–15025. [Google Scholar] [CrossRef]
- Yang, Z.H.; Li, Z.W.; Yang, Y.H. Structural and magnetic properties of plate-like W-type barium ferrites synthesized with a combination method of molten salt and sol-gel. Mater. Chem. Phys. 2014, 144, 568–574. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; You, X.; Liu, S.; Wu, C.; Liu, Q.; Zhang, H.; Harris, V.G. Crystallographically textured Zn2W-type barium hexaferrite for microwave and millimeter wave applications. J. Alloys Compd. 2019, 772, 1100–1104. [Google Scholar] [CrossRef]
- Ahmad, M.; Grössinger, R.; Kriegisch, M.; Kubel, F.; Rana, M.U. Characterization of Sr-substituted W-type hexagonal ferrites synthesized by sol-gel autocombustion method. J. Magn. Magn. Mater. 2013, 332, 137–145. [Google Scholar] [CrossRef]
- Khan, I.; Sadiq, I.; Ashiq, M.N.; Rana, M.-U.-D. Role of Ce-Mn substitution on structural, electrical and magnetic properties of W-type strontium hexaferrites. J. Alloys Compd. 2011, 509, 8042–8046. [Google Scholar] [CrossRef]
- Soroka, M.; Buršík, J.; Kužel, R.; Prokleška, J.; Aguirr, M.H. Characterization of W-type hexaferrite thin films prepared by chemical solution deposition. Thin Solid Film. 2021, 726, 138670. [Google Scholar] [CrossRef]
- Albanese, G.; Carbucicchio, M.; Pareti, L.; Rinaldi, S. Cation distribution and magnetic properties of BaZn2Fe16−xInxO27 and BaZn2Fe16−xScxO27 hexagonal ferrites. Phys. Stat. Sol. A 1982, 73, K193–K197. [Google Scholar] [CrossRef]
- Qi, G.; Liu, Y.; Chen, Y.; Liu, Q.; Chen, J.; Yin, Q.; Zhang, H. Crystallographically oriented W-type barium hexaferrite BaZn2Fe16−xScxO27 with high squareness ratio. J. Mater. Sci. Mater. Electron. 2021, 32, 25769–25781. [Google Scholar] [CrossRef]
- Ahmad, M.; Aen, F.; Islam, M.U.; Niazi, S.B.; Rana, M.U. Structural, physical, magnetic and electrical properties of La-substituted W-type hexagonal ferrites. Ceram. Int. 2011, 37, 3691–3696. [Google Scholar] [CrossRef]
- Sharbati, A.-; Khani, J.-M.V.; Amiri, G.R.; Mousarezaei, R. Influence of dysprosium addition on the structural, morphological, electrical and magnetic properties of nano-crystalline W-type hexaferrites. Bull. Mater. Sci. 2015, 38, 1–5. [Google Scholar] [CrossRef]
- Aen, F.; Ahmad, M.; Rana, M.U. The role of Ga substitution on magnetic and electromagnetic properties of nano-sized W-type hexagonal ferrites. Curr. Appl. Phys. 2013, 13, 41–46. [Google Scholar] [CrossRef]
- Ahmad, M.; Grössinger, R.; Ali, I.; Ahmad, I.; Rana, M.U. Synthesis and characterization of Al-substituted W-type hexagonal ferrites for high frequency applications. J. Alloys Compd. 2013, 577, 382–388. [Google Scholar] [CrossRef]
- Rehman, A.U.; Shaukat, S.F.; Haidyrah, A.S.; Akhtar, M.N.; Ahmad, M. Synthesis and investigations of structural, magnetic and dielectric properties of Cr-substituted W-type Hexaferrites for high frequency applications. J. Electroceram. 2021, 46, 93–106. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Khan, R.A. Enhancement of electrical and dielectric properties of Cr doped BaZn2 W-type hexaferrite for potential applications in high frequency devices. J. Alloys Compd. 2009, 478, 847–852. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, F.; Shao, J.; Huang, D.; He, H.; Trukhanov, A.V.; Trukhanov, S.V. Influence of Nd-NbZn co-substitution on structural, spectral and magnetic properties of M-type calcium-strontium hexaferrites Ca0.4Sr0.6−xNdxFe12.0−x(Nb0.5Zn0.5)xO19. J. Alloys Compd. 2018, 765, 616–623. [Google Scholar] [CrossRef]
- Barrera, V.; Betancourt, I. Hard magnetic properties of nanosized Sr(Fe,Al)12O19 hexaferrites obtained by Pechini method. J. Phys. Chem. Solids 2016, 93, 1–6. [Google Scholar] [CrossRef]
- Liu, M.Q.; Shen, X.Q.; Song, F.Z.; Xiang, J.; Meng, X.F. Microstructure and magnetic properties of electrospun one-dimensional Al3+-substituted SrFe12O19 nanofibers. J. Solid State Chem. 2011, 184, 871–876. [Google Scholar] [CrossRef]
- Luo, H.; Rai, B.K.; Mishra, S.R.; Nguyen, V.V.; Liu, J.P. Physical and magnetic properties of highly aluminum doped strontium ferrite nanoparticles prepared by auto-combustion route. J. Magn. Magn. Mater. 2012, 324, 2602–2608. [Google Scholar] [CrossRef]
- Saafan, S.A.; Assar, S.T.; Moharram, B.M.; El Nimr, M.K. Comparison study of some structural and magnetic properties of nano-structured and bulk Li–Ni–Zn ferrite samples. J. Magn. Magn. Mater. 2010, 322, 628–632. [Google Scholar] [CrossRef]
- Rafiq, M.A.; Waqar, M.; Mirza, T.A.; Frooq, A.; Zulfiqar, A. Effect of Ni2+ substitution on the structural, magnetic, and dielectric properties of barium hexagonal ferrites (BaFe12O19). J. Electron. Mater. 2017, 46, 241–246. [Google Scholar] [CrossRef]
- Singhal, S.; Namgyal, T.; Singh, J.; Chandra, K.; Bansal, S. A comparative study on the magnetic properties of MFe12O19 and MAlFe11O19 (M = Sr, Ba and Pb) hexaferrites with different morphologies. Ceram. Int. 2011, 37, 1833–1837. [Google Scholar] [CrossRef]
- Ali, I.; Islam, M.U.; Awan, M.S.; Ahmad, M. Effects of Ga–Cr substitution on structural and magnetic properties of hexaferrite (BaFe12O19) synthesized by sol–gel auto-combustion route. J. Alloys Compd. 2013, 547, 118–125. [Google Scholar] [CrossRef]
- Xie, T.; Xu, L.; Liu, C. Synthesis and properties of composite magnetic material SrCoxFe12−xO19 (x = 0–0.3). Powder Technol. 2012, 232, 87–92. [Google Scholar] [CrossRef]
- Wohlfarth, E.P. Handbook of Magnetic Materials; North-Holland Publishing Company: Amesterdam, The Netherlands, 1982; Volume 3, pp. 395–396. [Google Scholar]
- Iqbal, M.J.; Khan, R.A.; Takeda, S.; Mizukami, S.; Miyazaki, T. W-type hexaferrite nanoparticles: A consideration for microwave attenuation at wide frequency band of 0.5–10 GHz. J. Alloys Compd. 2011, 509, 7618–7624. [Google Scholar] [CrossRef]
- Albanese, G.; Carbucicchio, M.; Pareti, L.; Rinaldi, S.; Lucchini, E.; Slokar, G. Magnetic and Mössbauer study of Al, Ga, In and Sc substituted Zn2−W hexagonal ferrites. J. Magn. Magn. Mater. 1980, 15–18, 1453–1454. [Google Scholar] [CrossRef]
- Albanese, G. Mössbauer investigation of aluminium substituted barium hexaferrite in the paramagnetic state. J. Magn. Magn. Mater. 1995, 147, 421–426. [Google Scholar] [CrossRef]
- Akhtar, M.N.; Javed, S.; Ahmad, M.; Sulong, A.B.; Khan, M.A. Sol gel derived MnTi doped Co2 W-type hexagonal ferrites: Structural, physical, spectral and magnetic evaluations. Ceram. Int. 2020, 46, 7842–7849. [Google Scholar] [CrossRef]
- Grössinger, R. A Critical Examination of the Law of Approach to Saturation I. Fit Procedure. Phys. Stat. Sol. A 1981, 66, 665–674. [Google Scholar] [CrossRef]
- Ahmad, M.; Grössinger, R.; Kriegisch, M.; Kubel, F.; Rana, M.U. Magnetic and microwave attenuation behavior of Al-substituted Co2W hexaferrites synthesized by sol-gel autocombustion process. Curr. Appl. Phys. 2012, 12, 1413–1420. [Google Scholar] [CrossRef]
- Sadat, M.E.; Patel, R.; Sookoor, J.; Bud’ko, S.L.; Ewing, R.C.; Zhang, J.; Xu, H.; Wang, Y.; Pauletti, G.M.; Mast, D.B.; et al. Effect of spatial confinement on magnetic hyperthermia via dipolar interactions in Fe3O4 nanoparticles for biomedical applications. Mater. Sci. Eng. C 2014, 42, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Güner, S.; Auwal, I.A.; Baykal, A.; Sözeri, H. Synthesis, characterization and magneto optical properties of BaBixLaxYxFe12−3xO19 (0.0 ≤ x ≤ 0.33) hexaferrites. J. Magn. Magn. Mater. 2016, 416, 261–268. [Google Scholar] [CrossRef]
- Pullar, R.C.; Bdikin, I.K.; Bhattacharya, A.K. Magnetic properties of randomly oriented BaM, SrM, Co2Y, Co2Z and Co2W hexagonal ferrite fibres. J. Eur. Ceram. Soc. 2012, 32, 905–913. [Google Scholar] [CrossRef]
- Wei, F.L.; Lu, M.; Yang, Z. The temperature dependence of magnetic properties of of Zn-Ti substituted Ba-ferrite particles for magnetic recording. J. Magn. Magn. Mater. 1999, 191, 249–253. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y. Two-Step Calcination-Method-Derived Al-Substituted W-Type SrYb Hexaferrites: Their Microstructural, Spectral, and Magnetic Properties. Magnetochemistry 2022, 8, 118. https://doi.org/10.3390/magnetochemistry8100118
Yang Y. Two-Step Calcination-Method-Derived Al-Substituted W-Type SrYb Hexaferrites: Their Microstructural, Spectral, and Magnetic Properties. Magnetochemistry. 2022; 8(10):118. https://doi.org/10.3390/magnetochemistry8100118
Chicago/Turabian StyleYang, Yujie. 2022. "Two-Step Calcination-Method-Derived Al-Substituted W-Type SrYb Hexaferrites: Their Microstructural, Spectral, and Magnetic Properties" Magnetochemistry 8, no. 10: 118. https://doi.org/10.3390/magnetochemistry8100118
APA StyleYang, Y. (2022). Two-Step Calcination-Method-Derived Al-Substituted W-Type SrYb Hexaferrites: Their Microstructural, Spectral, and Magnetic Properties. Magnetochemistry, 8(10), 118. https://doi.org/10.3390/magnetochemistry8100118





