Structures and Properties of New Organic Molecule-Based Metals, (D)2BrC2H4SO3 [D = BEDT-TTF and BETS]
Abstract
1. Introduction
2. Results and Discussion
2.1. Crystal Structures
2.1.1. Crystal Structure of β’’-β’’-(BEDT-TTF)2(BrC2H4SO3) (1)
2.1.2. Crystal Structure of β’’-β’’-(BETS)2(BrC2H4SO3) (2)
2.1.3. Crystal Structure of θ-(BETS)2(BrC2H4SO3) (3)
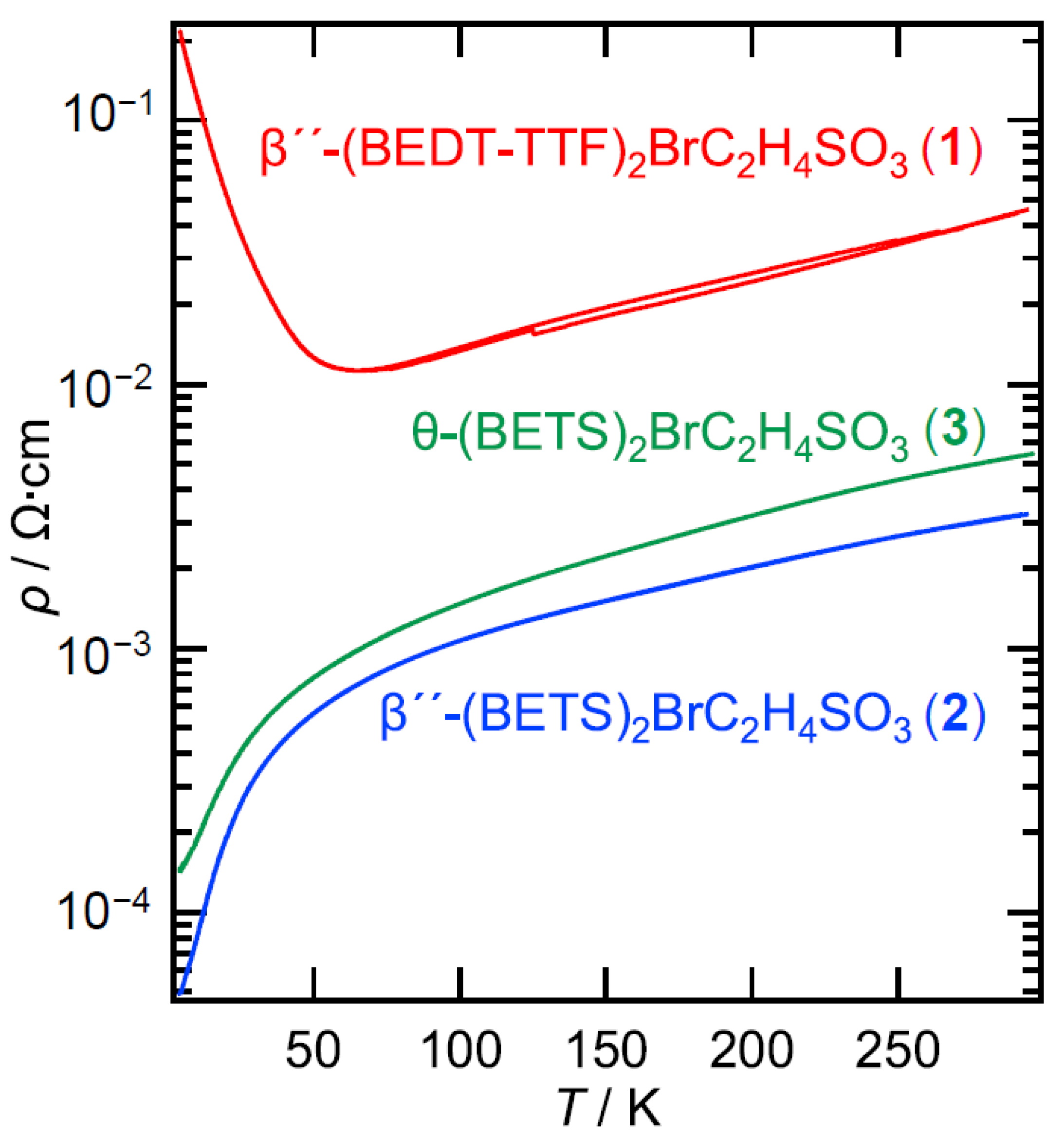
2.2. Electrical Resistivity
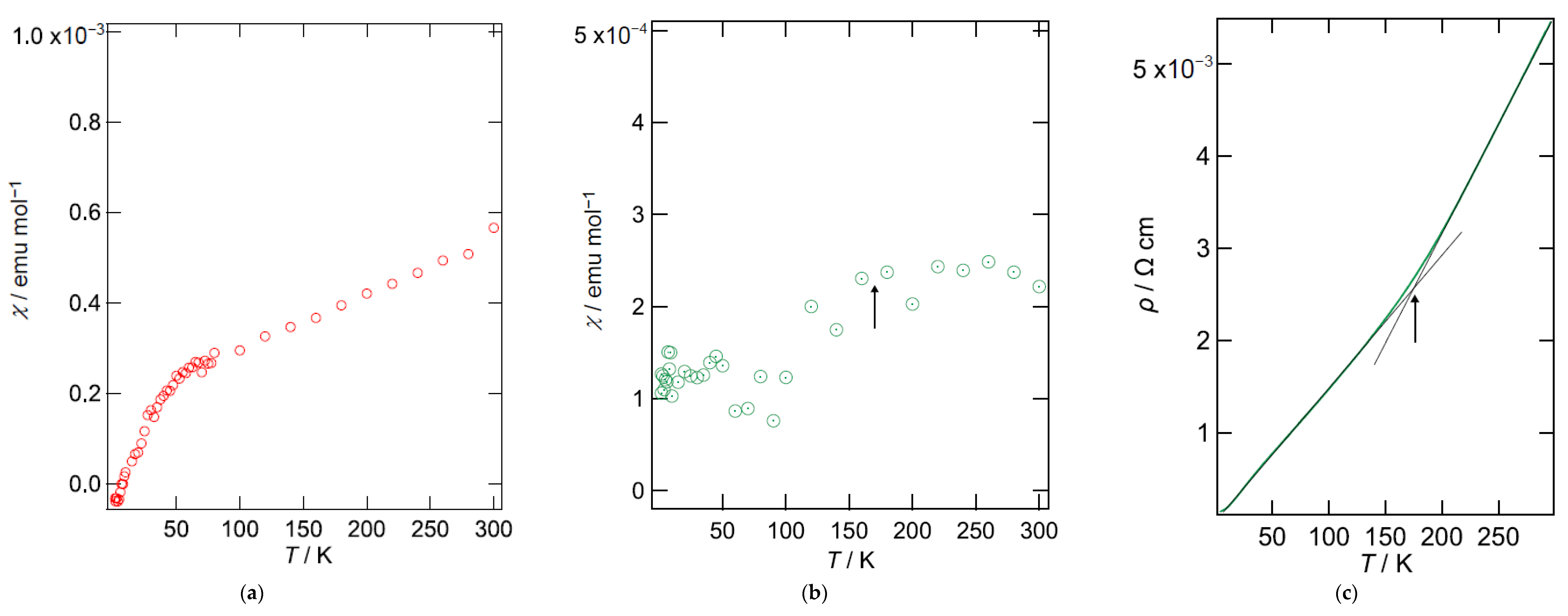
2.3. Magnetic Susceptibility
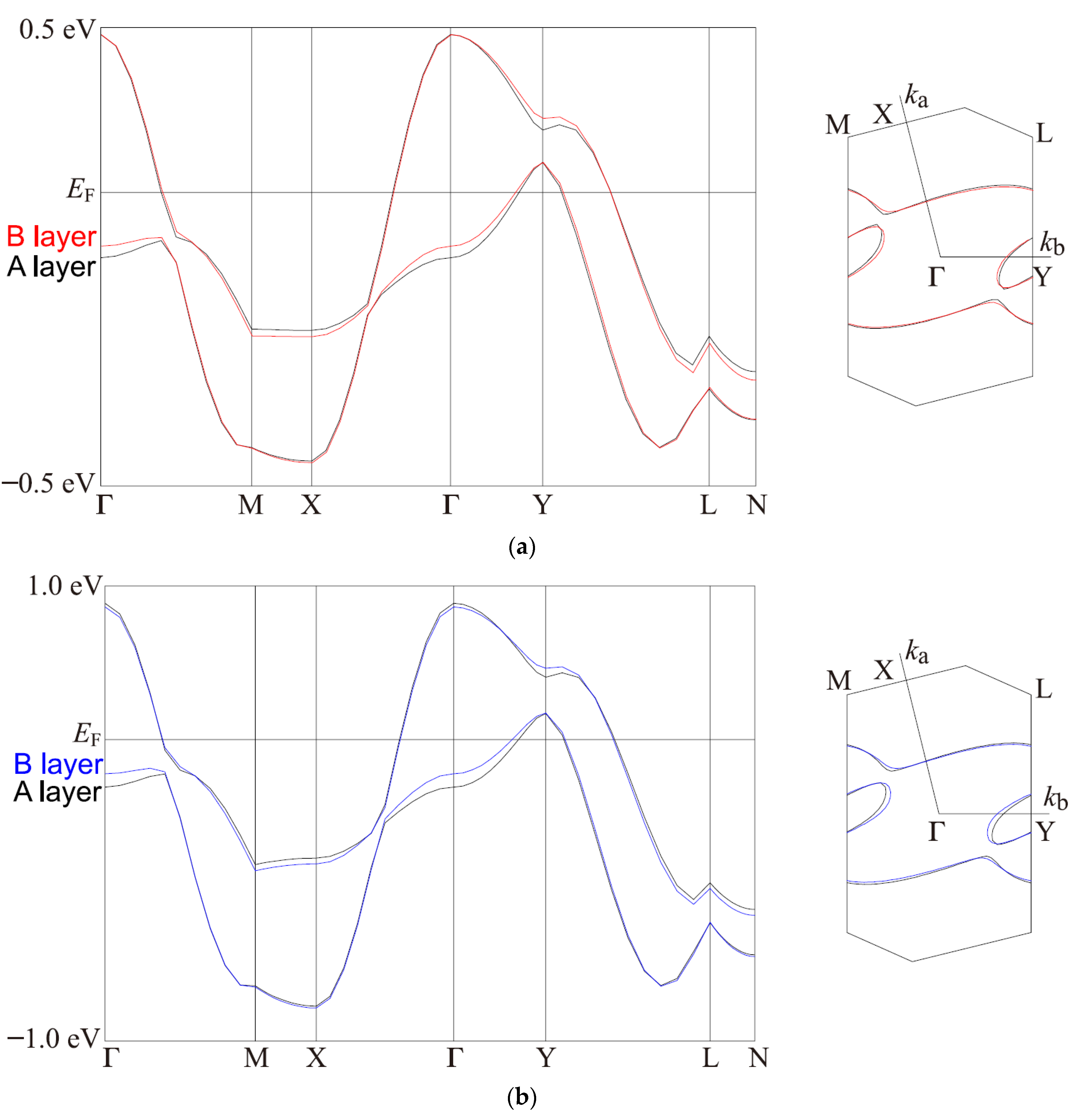
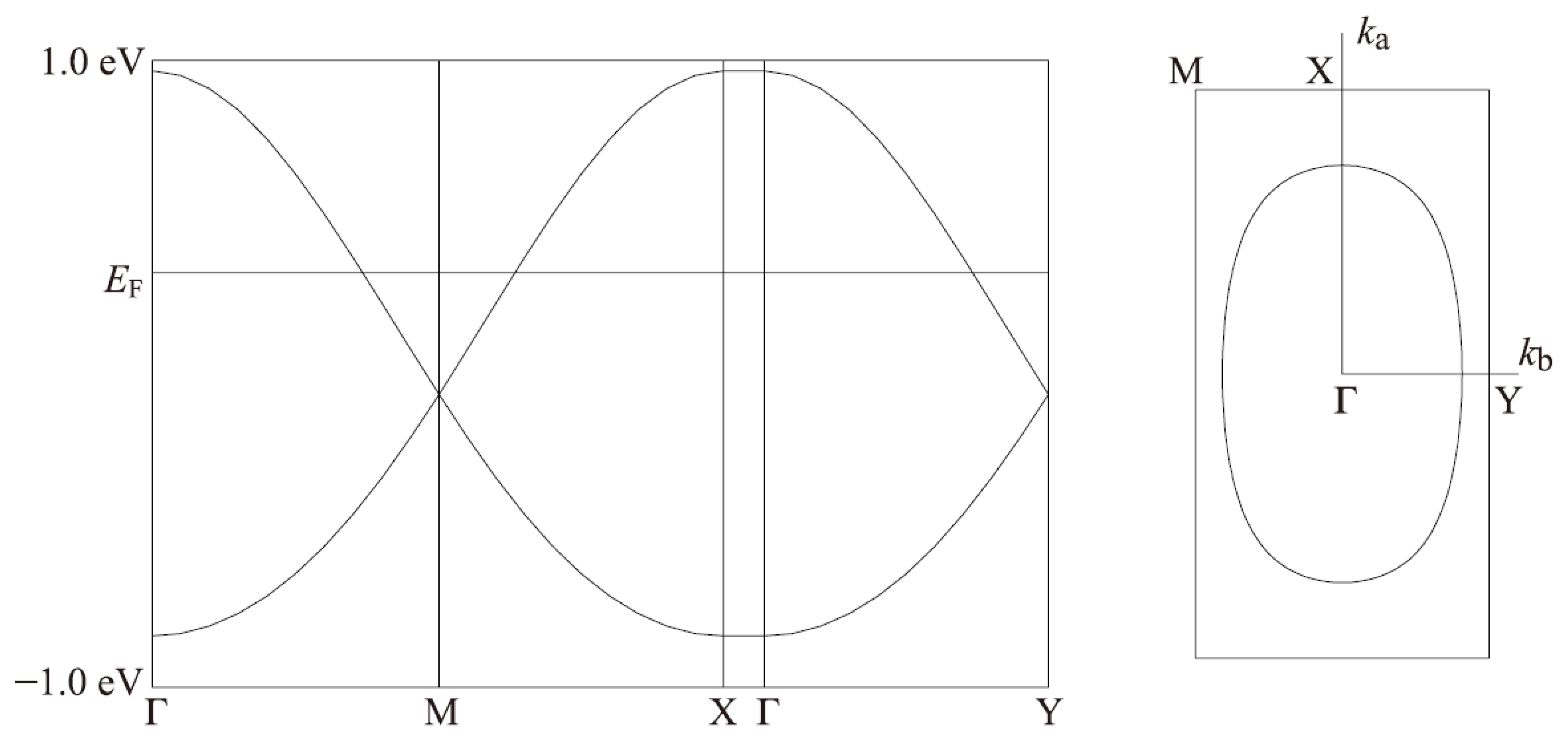
2.4. Band Structure Calculations
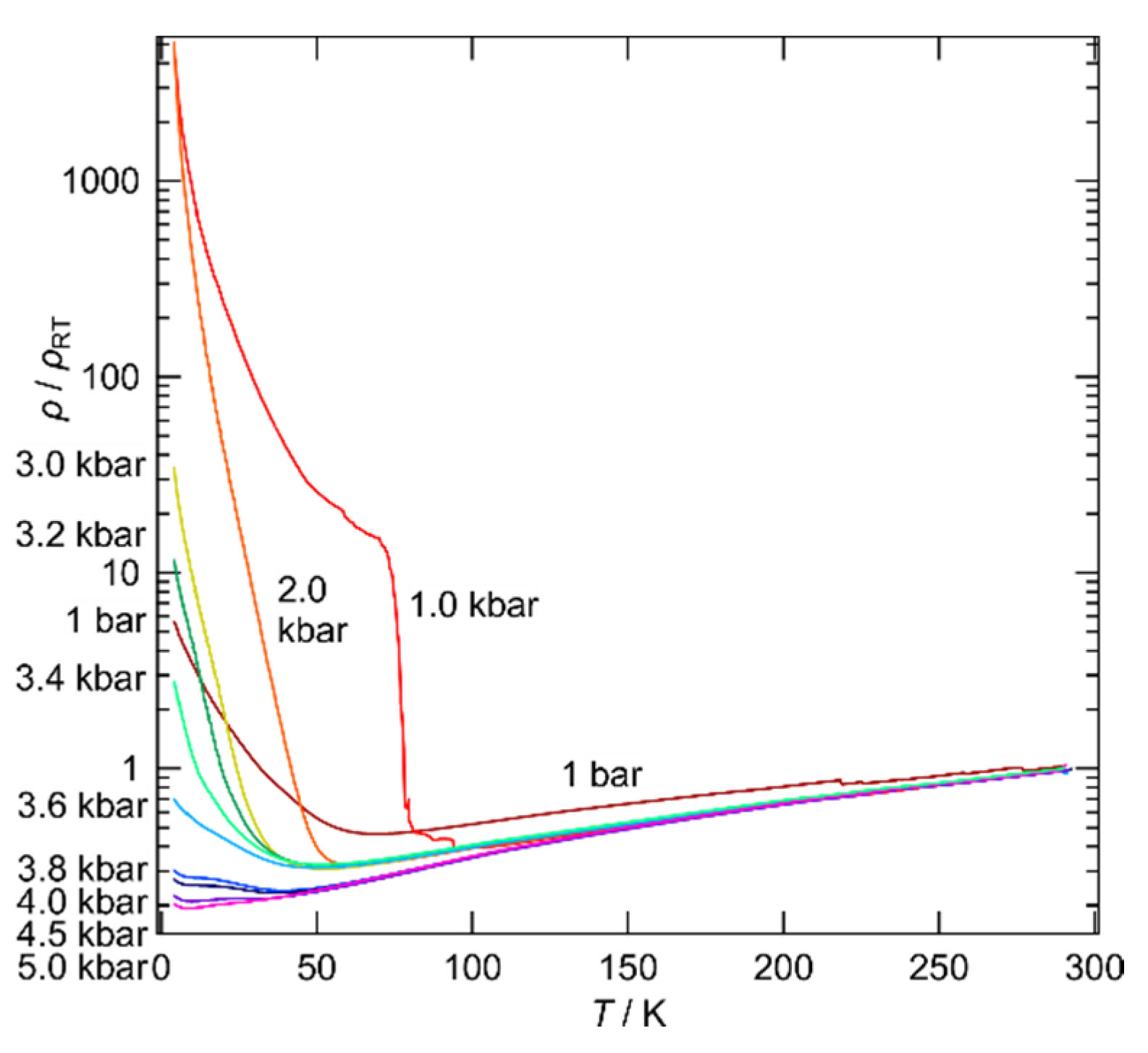
2.5. Electrical Resistivity under Pressure
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mori, T. Electronic Properties of Organic Conductors; Springer: Tokyo, Japan, 2016. [Google Scholar]
- Kaythirgamanan, P.; Mucklejohn, S.A.; Rosseinsky, D.R. Electrocrystallisation of Conductive Nonstoicheiornetric Adducts of Tetrathiafulvalene with Inorganic or Organic Anions, and of Similar Adducts of Tetracyanoquinodirnethane. J. Chem. Soc. Chem. Commun. 1979, 2, 86–87. [Google Scholar] [CrossRef]
- Geiser, U.; Schlueter, J.A. Conducting Organic Radical Cation Salts with Organic and Organometallic Anions. Chem. Rev. 2004, 104, 5203–5241. [Google Scholar] [CrossRef] [PubMed]
- Soling, H.; Rindorf, G.; Thorup, N. Di(4,4′,5,5′-tetramethyl-∆2,2′-bi-1,3-diselenolyliden)ium trifluoromethanesulfonate, C21H24F3O3SSe8, (TMTSF)2CF3SO3. Acta Cryst. 1983, C39, 490–491. [Google Scholar]
- Katayama, C.; Honda, M.; Kumagai, H.; Tanaka, J.; Saito, G.; Inokuchi, H. Crystal Structures of Complexes between Hexacyanobutadiene and Tetramethyltetrathiafulvalene and Tetramethylthiotetrathiafulvalene. Bull. Chem. Soc. Jpn. 1985, 58, 2272–2278. [Google Scholar] [CrossRef]
- Chasseau, D.; Watkin, D.; Rosseinsky, M.J.; Kurmoo, M.; Talham, D.R.; Day, P. Syntheses, crystal structures and physical properties of conducting salts (BEDT-TTF)2X (X = CF3SO3 or p-CH3C6H4SO3). Synth. Met. 1988, 24, 117–125. [Google Scholar] [CrossRef]
- Brezgunova, M.; Shin, K.-S.; Auban-Senzier, P.; Jeannin, O.; Fourmigué, M. Combining halogen bonding and chirality in a two-dimensional organic metal (EDT-TTF-I2)2(D-camphorsulfonate)·H2O. Chem. Commun. 2010, 46, 3926–3928. [Google Scholar] [CrossRef]
- Lakhdar, Y.; El-Ghayoury, A.; Zorina, L.; Mercier, N.; Allain, M.; Mézière, C.; Auban-Senzier, P.; Batail, P.; Giffard, M. Acentric Polymeric Chains in Radical Cation Salts of Tetrathiafulvalene Derivatives with the p-Carboxybenzenesulfonate Anion. Eur. J. Inorg. Chem. 2010, 2010, 3338–3342. [Google Scholar] [CrossRef]
- Shin, K.-S.; Brezgunova, M.; Jeannin, O.; Roisnel, T.; Camerel, F.; Auban-Senzier, P.; Fourmigué, M. Strong Iodine···Oxygen Interactions in Molecular Conductors Incorporating Sulfonate Anions. Cryst. Growth Des. 2011, 11, 5337–5345. [Google Scholar] [CrossRef]
- Camerel, F.; Helloco, G.L.; Guizouarn, T.; Jeannin, O.; Fourmigué, M.; Frąckowiak, A.; Olejniczak, I.; Świetlik, R.; Marino, A.; Collet, E.; et al. Correlation between Metal–Insulator Transition and Hydrogen-Bonding Network in the Organic Metal δ-(BEDT-TTF)4[2,6-Anthracene-bis(sulfonate)]·(H2O)4. Cryst. Growth Des. 2013, 13, 5135–5145. [Google Scholar] [CrossRef]
- Akutsu, H.; Yamada, J.; Nakatsuji, S. A New Organic Anion Consisting of the TEMPO Radical for Organic Charge-Transfer Salts: 2,2,6,6-Tetramethylpiperidinyloxy-4-sulfamate (TEMPO-NHSO3−). Chem. Lett. 2001, 30, 208–209. [Google Scholar] [CrossRef]
- Akutsu, H.; Yamada, J.; Nakatsuji, S. Preparation and characterization of novel organic radical anions for organic conductors: TEMPO–NHSO3− and TEMPO-OSO3−. Synth. Met. 2001, 120, 871–872. [Google Scholar] [CrossRef]
- Akutsu, H.; Yamada, J.; Nakatsuji, S. New BEDT-TTF-based Organic Conductor Including an Organic Anion Derived from the TEMPO Radical, α-(BEDT-TTF)3(TEMPO–NHCOCH2SO3)2·6H2O. Chem. Lett. 2003, 32, 1118–1119. [Google Scholar] [CrossRef]
- Furuta, K.; Akutsu, H.; Yamada, J.; Nakatsuji, S. A Novel BEDT-TTF-based Organic Conducting Salt with a Ferrocene-containing Dianion, α-(BEDT-TTF)4(Fe(Cp–CONHCH2SO3)2)·4H2O. Chem. Lett. 2004, 33, 1214–1215. [Google Scholar] [CrossRef]
- Furuta, K.; Akutsu, H.; Yamada, J.; Nakatsuji, S. New organic functional anions: Ferrocenyl-(CONHCH2SO3−)n (n = 1–2) and their TTF salts. Synth. Met. 2005, 152, 381–384. [Google Scholar] [CrossRef]
- Akutsu, H.; Yamada, J.; Nakatsuji, S. Novel organic magnetic conductors based on organochalcogen donors and an organic magnetic anion, TEMPO-NHCOCH2SO3−. Synth. Met. 2005, 152, 377–380. [Google Scholar] [CrossRef]
- Akutsu, H.; Masaki, K.; Mori, K.; Yamada, J.; Nakatsuji, S. New organic free radical anions TEMPO-A-CO-(o-, m-, p-)C6H4SO3− (A = NH, NCH3, O) and their TTF and/or BEDT-TTF salts. Polyhedron 2005, 24, 2126–2132. [Google Scholar] [CrossRef]
- Yamashita, A.; Akutsu, H.; Yamada, J.; Nakatsuji, S. New organic magnetic anions TEMPO–CONA(CH2)nSO3− (n = 0–3 for A = H, n = 2 for A = CH3) and their TTF, TMTSF and/or BEDT-TTF salts. Polyhedron 2005, 16, 2796–2802. [Google Scholar] [CrossRef]
- Furuta, K.; Akutsu, H.; Yamada, J.; Nakatsuji, S.; Turner, S.S. The first organic molecule-based metal containing ferrocene. J. Mater. Chem. 2006, 16, 1504–1506. [Google Scholar] [CrossRef]
- Akutsu, H.; Yamada, J.; Nakatsuji, S.; Turner, S.S. A novel BEDT-TTF-based purely organic magnetic conductor, α-(BEDT-TTF)2 (TEMPO-N(CH3)COCH2SO3)·3H2O. Solid State Commun. 2006, 140, 256–260. [Google Scholar] [CrossRef]
- Furuta, K.; Akutsu, H.; Yamada, J.; Nakatsuji, S.; Turner, S.S. The first metallic salt containing ferrocene, β′′-(BEDT-TTF)4(Fe(Cp-CONHCH2SO3)2)·2H2O. In Multifunctional Conducting Molecular Materials; Saito, G., Wudl, F., Haddon, R.C., Tanigaki, K., Enoki, T., Katz, H.E., Maesato, M., Eds.; RSC Publishing: Dorchester, UK, 2007; pp. 147–150. [Google Scholar]
- Akutsu, H.; Yamada, J.; Nakatsuji, S.; Turner, S.S. An anionic weak acceptor 2-aminomethylsulfo-3,5,6-trichloro-1, 4-benzoquinone and its BEDT-TTF-based charge-transfer salts. Solid State Commun. 2007, 144, 144–147. [Google Scholar] [CrossRef]
- Akutsu, H.; Ohnishi, R.; Yamada, J.; Nakatsuji, S.; Turner, S.S. Novel Bis(ethylenedithio)tetrathiafulvalene-Based Organic Conductor with 1,1-Ferrocenedisulfonate. Inorg. Chem. 2007, 46, 8472–8474. [Google Scholar] [CrossRef]
- Akutsu, H.; Sato, K.; Yamashita, S.; Yamada, J.; Nakatsuji, S.; Turner, S.S. The first organic paramagnetic metal containing the aminoxyl radical. J. Mater. Chem. 2008, 18, 3313–3315. [Google Scholar] [CrossRef]
- Akutsu, H.; Yamashita, S.; Yamada, J.; Nakatsuji, S.; Turner, S.S. Novel Purely Organic Conductor with an Aminoxyl Radical, α-(BEDT-TTF)2(PO–CONHCH2SO3)·2H2O (PO = 2,2,5,5-Tetramethyl-3-pyrrolin-1-oxyl Free Radical). Chem. Lett. 2008, 37, 882–883. [Google Scholar] [CrossRef]
- Akutsu, H.; Yamada, J.; Nakatsuji, S.; Turner, S.S. A new anionic acceptor, 2-sulfo-3,5,6-trichloro-1,4-benzoquinone and its charge-transfer salts. CrystEngComm 2009, 11, 2588–2592. [Google Scholar] [CrossRef]
- Akutsu, H.; Sasai, T.; Yamada, J.; Nakatsuji, S.; Turner, S.S. New anionic acceptors Br2XQNHCH2SO3− [X = Br, BryCl1−y (y ≈ 0.5), and Cl; Q=1,4-benzoquinone] and their charge-transfer salts. Physica B 2010, 405, S2–S5. [Google Scholar] [CrossRef]
- Akutsu, H.; Yamashita, S.; Yamada, J.; Nakatsuji, S.; Hosokoshi, Y.; Turner, S.S. A Purely Organic Paramagnetic Metal, κ-β″-(BEDT-TTF)2(PO–CONHC2H4SO3), Where PO = 2,2,5,5-Tetramethyl-3-pyrrolin-1-oxyl Free Radical. Chem. Mater. 2011, 23, 762–764. [Google Scholar] [CrossRef]
- Akutsu, H.; Kawamura, A.; Yamada, J.; Nakatsuji, S.; Turner, S.S. Anion polarity-induced dual oxidation states in a dual-layered purely organic paramagnetic charge-transfer salt, (TTF)3(PO–CON(CH3)C2H4SO3)2, where PO = 2,2,5,5-tetramethyl-3-pyrrolin-1-oxyl free radical. CrystEngComm 2011, 13, 5281–5284. [Google Scholar] [CrossRef]
- Akutsu, H.; Maruyama, Y.; Yamada, J.; Nakatsuji, S.; Turner, S.S. A new BEDT-TTF-based organic metal with an anionic weak acceptor 2-sulfo-1,4-benzoquinone. Synth. Met. 2011, 161, 2339–2343. [Google Scholar] [CrossRef]
- Akutsu, H.; Yamada, J.; Nakatsuji, S.; Turner, S.S. A New BEDT-TTF-Based Organic Charge Transfer Salt with a New Anionic Strong Acceptor, N,N′-Disulfo-1,4-benzoquinonediimine. Crystals 2012, 2, 182–192. [Google Scholar] [CrossRef]
- Kanbayashi, N.; Akutsu, H.; Yamada, J.; Nakatsuji, S.; Turner, S.S. A new ferrocene-containing charge-transfer salt, (TTF)2[Fe(C5H4-CH(CH3)NHCOCH2SO3)2]. Inorg. Chem. Commun. 2012, 21, 122–124. [Google Scholar] [CrossRef][Green Version]
- Akutsu, H.; Yamada, J.; Nakatsuji, S.; Turner, S.S. Structures and properties of a BEDT-TTF-based organic charge transfer salt and the zwitterion of ferrocenesulfonate. Dalton Trans. 2013, 42, 16351–16354. [Google Scholar] [CrossRef] [PubMed]
- Akutsu, H.; Hashimoto, R.; Yamada, J.; Nakatsuji, S.; Nakazawa, Y.; Turner, S.S. Structures and properties of new ferrocene-based paramagnetic anion octamethylferrocenedisulfonate and its TTF salt. Inorg. Chem. Commun. 2015, 61, 41–47. [Google Scholar] [CrossRef]
- Akutsu, H.; Ishihara, K.; Yamada, J.; Nakatsuji, S.; Turner, S.S.; Nakazawa, Y. A strongly polarized organic conductor. CrystEngComm 2016, 18, 8151–8154. [Google Scholar] [CrossRef]
- Akutsu, H.; Ishihara, K.; Ito, S.; Nishiyama, F.; Yamada, J.; Nakatsuji, S.; Turner, S.S.; Nakazawa, Y. Anion Polarity-Induced Self-doping in Purely Organic Paramagnetic Conductor, α′-α′-(BEDT-TTF)2(PO-CONH-m-C6H4SO3)·H2O where BEDT-TTF is Bis(ethylenedithio)tetrathiafulvalene and PO is 2,2,5,5-Tetramethyl-3-pyrrolin-1-oxyl Free Radical. Polyhedron 2017, 136, 23–29. [Google Scholar] [CrossRef]
- Akutsu, H.; Hashimoto, R.; Yamada, J.; Nakatsuji, S.; Turner, S.S.; Nakazawa, Y. Structure and Properties of a BEDT-TTF-Based Organic Conductor with a Ferrocene-Based Magnetic Anion Octamethylferrocenedisulfonate. Eur. J. Inorg. Chem. 2018, 3249–3252. [Google Scholar] [CrossRef]
- Ito, H.; Edagawa, Y.; Pu, J.; Akutsu, H.; Suda, M.; Yamamoto, H.M.; Kawasugi, Y.; Haruki, R.; Kumai, R.; Takenobu, T. Electrolyte-Gating-Induced Metal-Like Conduction in Nonstoichiometric Organic Crystalline Semiconductors under Simultaneous Bandwidth Control. Phys. Status Solidi 2019, 13, 1900162–1900175. [Google Scholar] [CrossRef]
- Akutsu, H.; Koyama, Y.; Turner, S.S.; Furuta, K.; Nakazawa, Y. Structures and Properties of New Organic Conductors: BEDT-TTF, BEST and BETS Salts of the HOC2H4SO3− Anion. Crystals 2020, 10, 775. [Google Scholar] [CrossRef]
- Akutsu, H.; Kohno, A.; Turner, S.S.; Nakazawa, Y. Structure and Properties of a New Purely Organic Magnetic Conductor, δ´-(BEDT-TTF)2(PO-CONHCH(cyclopropyl)SO3)·1.7H2O. Chem. Lett. 2020, 49, 1345–1348. [Google Scholar] [CrossRef]
- Akutsu, H.; Kohno, A.; Turner, S.S.; Yamashita, S.; Nakazawa, Y. Different electronic states in the isomorphous chiral vs. racemic organic conducting salts, β′′-(BEDT-TTF)2(S- and rac-PROXYL-CONHCH2SO3). Mat. Adv. 2020, 1, 3171–3175. [Google Scholar] [CrossRef]
- Minemawari, H.; Naito, T.; Inabe, T. (ET)3(Br3)5: A Metallic Conductor with an Unusually High Oxidation State of ET (ET = Bis(ethylenedithio)tetrathiafulvalene). Chem. Lett. 2007, 36, 74–75. [Google Scholar] [CrossRef]
- Guionneau, P.; Kepert, C.J.; Bravic, G.; Chasseau, D.; Truter, R.M.; Kurmoo, M.; Day, P. Determine the charge distribution in BEDT-TTF salts. Synth. Met. 1997, 86, 1973–1974. [Google Scholar] [CrossRef]
- Akutsu, H.; Turner, S.S.; Nakazawa, Y. New Dmit-Based Organic Magnetic Conductors (PO-CONH-C2H4N(CH3)3)[M(dmit)2]2 (M = Ni, Pd) Including an Organic Cation Derived from a 2,2,5,5-Tetramethyl-3-pyrrolin-1-oxyl (PO) Radical. Magnetochemisry 2017, 3, 11. [Google Scholar] [CrossRef]
- Akutsu, H.; Ito, S.; Kadoya, T.; Yamada, J.; Nakatsuji, S.; Turner, S.S.; Nakazawa, Y. A new Ni(dmit)2-based organic magnetic charge-transfer salt, (m-PO-CONH-N-methylpyridinium)[Ni(dmit)2]·CH3CN. Inorg. Chim. Acta 2018, 482, 654–658. [Google Scholar] [CrossRef]
- Stewart, J.J.P. MOPAC7; Stewart Computational Chemistry: Colorado Springs, CO, USA, 1993. [Google Scholar]
- Mori, H.; Tanaka, S.; Mori, T. Systematic study of the electronic state in θ-type BEDT-TTF organic conductors by changing the electronic correlation. Phys. Rev. B 1998, 57, 12023–12029. [Google Scholar] [CrossRef]
- Mori, H.; Sakurai, N.; Tanaka, S.; Moriyama, H.; Mori, T.; Kobayashi, H.; Kobayashi, A. Control of electronic state by dihedral angle in θ-type bis(ethylenedithio)tetraselenafulvalene salts. Chem. Matter. 2000, 12, 2984–2987. [Google Scholar] [CrossRef]
- Mori, T. Structural Genealogy of BEDT-TTF-Based Organic Conductors I. Parallel Molecules: β and β″ Phases. Bull. Chem. Soc. Jpn. 1998, 71, 2509–2526. [Google Scholar] [CrossRef]
- Williams, J.M.; Ferraro, J.R.; Thorn, R.J.; Carlson, K.D.; Geiser, U.; Wang, H.H.; Kini, A.M.; Whangbo, M.H. Organic Superconductors: Synthesis, Structure, Properties, and Theory; Prentice Hall: Englewood Cliffs, NJ, USA, 1992. [Google Scholar]
- Kobayashi, A.; Sato, A.; Arai, E.; Kobayashi, H.; Faulmann, C.; Kushch, N.; Cassoux, P. A stable molecular metal with a binuclear magnetic anion, θ-(BETS)2Cu2Cl6. Solid State Commun. 1997, 103, 371–374. [Google Scholar] [CrossRef]
- Mori, T.; Kobayashi, A.; Sasaki, Y.; Kobayashi, H.; Saito, G.; Inokuchi, H. The Intermolecular Interaction of Tetrathiafulvalene and Bis(ethylenedithio)tetrathiafulvalene in Organic Metals. Calculation of Orbital Overlaps and Models of Energy-band Structures. Bull. Chem. Soc. Jpn. 1984, 57, 627–633. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
| 1 | 2,083,397 |
| 2 | 2,088,144 |



| Compound | 1 | 2 | 3 | 3 | 3 |
|---|---|---|---|---|---|
| Formula | C22H20O3S17Br | C22H20O3S9Se8Br | C22H20O3S9Se8Br | C22H20O3S9Se8Br | C22H20O3S9Se8Br |
| Fw *1 | 957.32 | 1332.52 | 1267.52 | 1267.52 | 1267.52 |
| Space Group | P | P | C2/c | C2/c | C2/c |
| a (Å) | 5.8671(2) | 5.93509(18) | 35.850(2) | 35.686(2) | 35.633(2) |
| b (Å) | 8.7790(2) | 8.8364(2) | 5.1587(3) | 5.1193(3) | 5.0986(4) |
| c (Å) | 33.3201(7) | 34.0428(9) | 9.9900(6) | 9.9154(7) | 9.9154(7) |
| α (°) | 89.076(6) | 88.939(6) | 90.0 | 90.0 | 90.0 |
| β (°) | 85.469(6) | 85.697(6) | 93.346(7) | 92.695(7) | 92.673(7) |
| γ (°) | 75.793(5) | 76.167(5) | 90.0 | 90.0 | 90.0 |
| V (Å3) | 1658.53(9) | 1728.70(9) | 1844.38(19) | 1809.43(19) | 1798.1(2) |
| Z | 2 | 2 | 2 | 2 | 2 |
| T (K) | 150 | 150 | 290 | 150 | 110 |
| dcalc (g·cm−1) | 1.917 | 2.560 | 2.399 | 2.446 | 2.461 |
| μ (cm−1) *2 | 23.434 | 101.896 | 95.505 | 97.349 | 97.963 |
| F(000) *3 | 966 | 1254 | 1254 | 1254 | 1254 |
| 2θ range (°) | 4–55 | 4–55 | 4–55 | 4–55 | 4–55 |
| Total ref. | 16,127 | 16,694 | 8164 | 7936 | 8018 |
| Unique ref. | 7559 | 7871 | 2110 | 2081 | 2063 |
| Rint | 0.0325 | 0.0672 | 0.0733 | 0.0391 | 0.0431 |
| Parameters | 407 | 395 | 118 | 118 | 118 |
| R1 (I > 2σ(I)) | 0.032 | 0.049 | 0.063 | 0.051 | 0.059 |
| wR2 (all data) | 0.089 | 0.158 | 0.205 | 0.138 | 0.166 |
| S *4 | 1.009 | 1.042 | 1.060 | 1.119 | 1.088 |
| Δρmax (e Å−3) | 1.49 | 1.94 | 1.18 | 2.18 | 2.20 |
| Δρmin (e Å−3) | −0.72 | −1.73 | −0.62 | −1.92 | −2.06 |
| CCDC number | 2,083,397 | 2,083,398 | 2,088,144 | 2,083,399 | 2,088,145 |
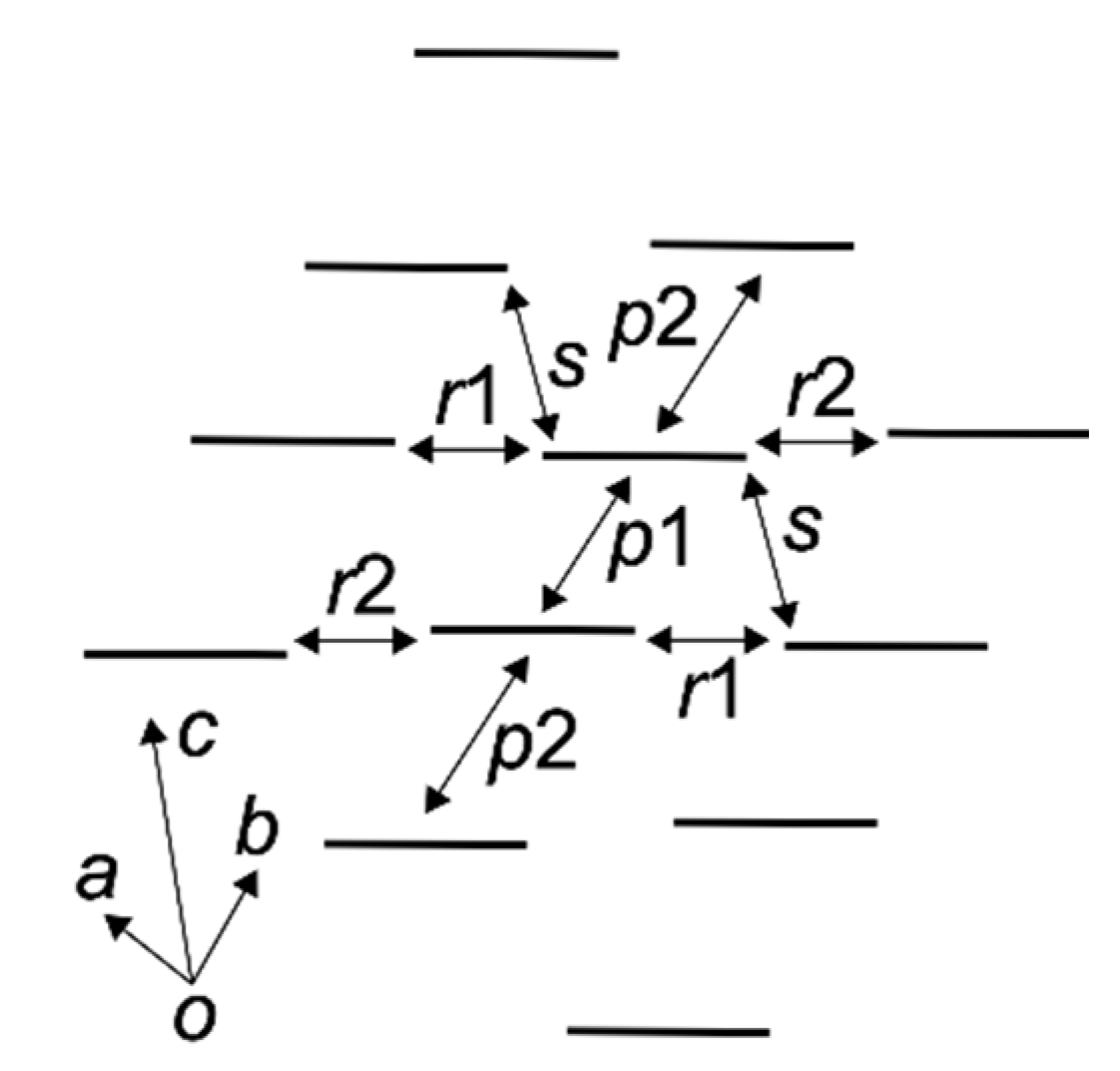
| Salts | 1 | 2 | ||
|---|---|---|---|---|
| Layers | A | B | A | B |
| p1 | −5.03 | −4.88 | −6.43 | −5.97 |
| p2 | −0.36 | −0.06 | +2.53 | +3.50 |
| r1 | −9.80 | −9.89 | −17.73 | −17.27 |
| r2 | −10.74 | −9.60 | −18.70 | −16.87 |
| s | −14.50 | −14.98 | −26.02 | −26.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akutsu, H.; Koyama, Y.; Turner, S.S.; Nakazawa, Y. Structures and Properties of New Organic Molecule-Based Metals, (D)2BrC2H4SO3 [D = BEDT-TTF and BETS]. Magnetochemistry 2021, 7, 91. https://doi.org/10.3390/magnetochemistry7070091
Akutsu H, Koyama Y, Turner SS, Nakazawa Y. Structures and Properties of New Organic Molecule-Based Metals, (D)2BrC2H4SO3 [D = BEDT-TTF and BETS]. Magnetochemistry. 2021; 7(7):91. https://doi.org/10.3390/magnetochemistry7070091
Chicago/Turabian StyleAkutsu, Hiroki, Yuta Koyama, Scott S. Turner, and Yasuhiro Nakazawa. 2021. "Structures and Properties of New Organic Molecule-Based Metals, (D)2BrC2H4SO3 [D = BEDT-TTF and BETS]" Magnetochemistry 7, no. 7: 91. https://doi.org/10.3390/magnetochemistry7070091
APA StyleAkutsu, H., Koyama, Y., Turner, S. S., & Nakazawa, Y. (2021). Structures and Properties of New Organic Molecule-Based Metals, (D)2BrC2H4SO3 [D = BEDT-TTF and BETS]. Magnetochemistry, 7(7), 91. https://doi.org/10.3390/magnetochemistry7070091








