First Molecular Superconductor with the Tris(Oxalato)Aluminate Anion, β″-(BEDT-TTF)4(H3O)Al(C2O4)3·C6H5Br, and Isostructural Tris(Oxalato)Cobaltate and Tris(Oxalato)Ruthenate Radical Cation Salts
Abstract
:1. Introduction
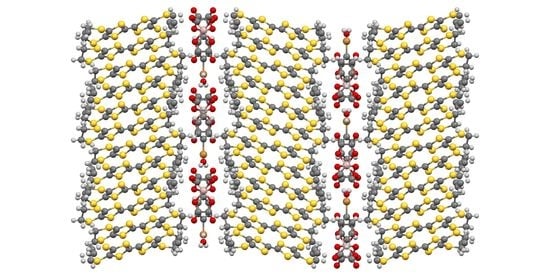
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis
3.2. Single-Crystal X-ray Crystallography
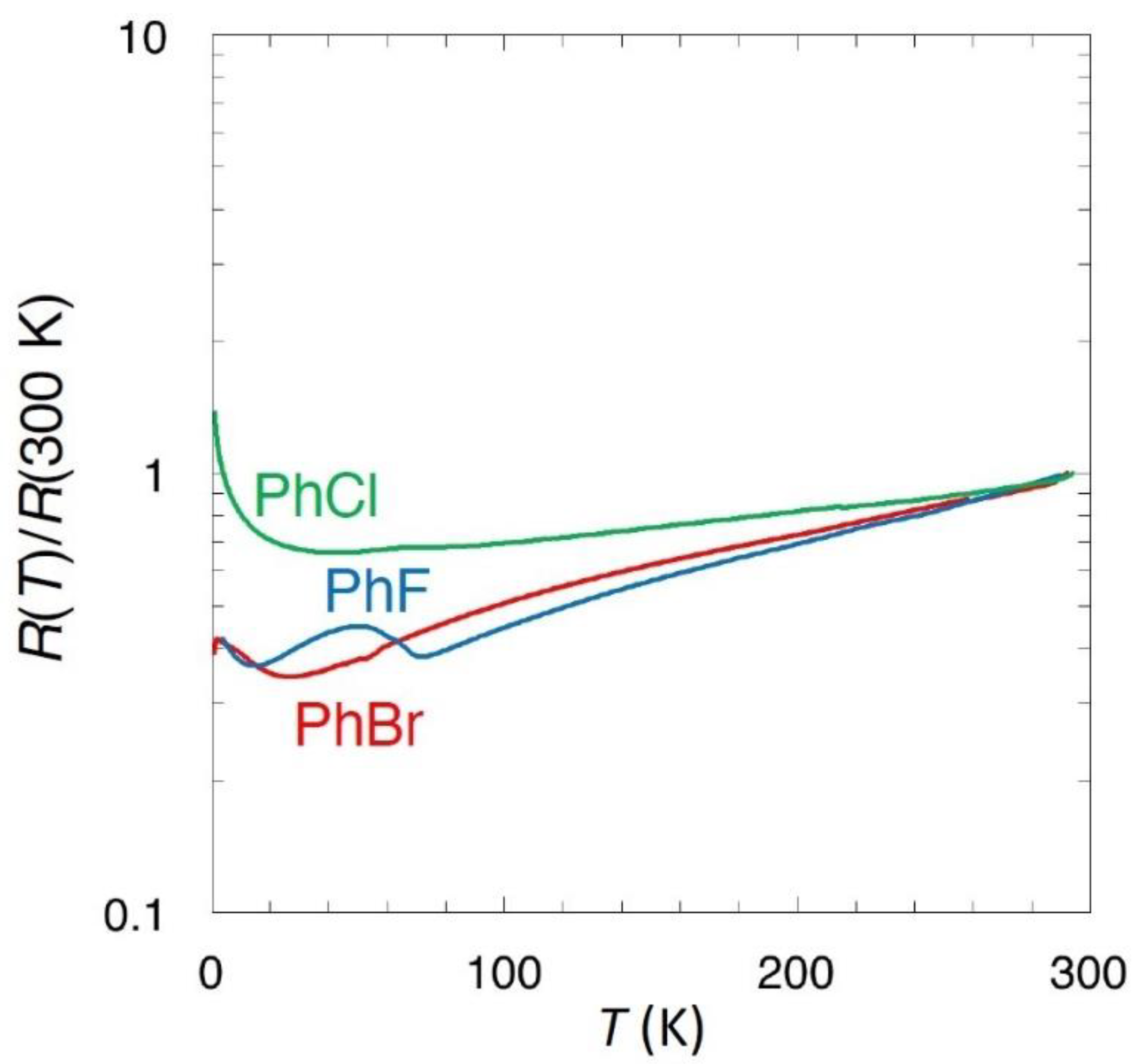
3.3. Conducting Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurmoo, M.; Graham, A.W.; Day, P.; Coles, S.J.; Hursthouse, M.B.; Caulfield, J.L.; Singleton, J.; Pratt, F.L.; Hayes, W.; Ducasse, L.; et al. Superconducting and Semiconducting Magnetic Charge Transfer Salts: (BEDT-TTF)4AFe(C2O4)3·C6H5CN (A = H2O, K, NH4). J. Am. Chem. Soc. 1995, 117, 12209–12217. [Google Scholar] [CrossRef]
- Martin, L. Molecular conductors of BEDT-TTF with tris(oxalato)metallate anions. Coord. Chem. Rev. 2018, 376, 277–291. [Google Scholar] [CrossRef] [Green Version]
- Coronado, E.; Galán-Mascarós, J.R.; Gómez-García, C.J.; Laukhin, V. Coexistence of ferromagnetism and metallic conductivity in a molecule-based layered compound. Nat. Cell Biol. 2000, 408, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.; Zhu, D. (BEDT-TTF)3Cu2(C2O4)3(CH3OH)2: An organic–inorganic hybrid antiferromagnetic semiconductor. Chem. Commun. 2011, 48, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Turner, S.S.; Day, P.; Light, M.E.; Hursthouse, M.B.; Firth, S.; Clark, R.J.H. The first molecular charge transfer salt containing proton channels. Chem. Commun. 2001, 16, 1462–1463. [Google Scholar] [CrossRef]
- Akutsu-Sato, A.; Akutsu, H.; Turner, S.S.; Day, P.; Probert, M.R.; Howard, J.A.K.; Akutagawa, T.; Takeda, S.; Nakamura, T.; Mori, T. The First Proton-Conducting Metallic Ion-Radical Salts. Angew. Chem. Int. Ed. 2004, 44, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wu, P.; Zhang, Q.; Zhu, D. The New Semiconducting Magnetic Charge Transfer Salt (BEDT-TTF)4 • H2O • Fe(C2O4)3 • C6H5NO2: Crystal Structure and Physical Properties. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 1998, 319, 259–269. [Google Scholar] [CrossRef]
- Sun, S.; Wu, P.; Zhang, Q.; Zhu, D. The new semiconducting magnetic charge transfer salt (BEDT-TTF)4·H2O·Fe(C2O4)3·C6H5NO2: Crystal structure and physical properties. Synth. Met. 1998, 94, 161–166. [Google Scholar] [CrossRef]
- Turner, S.S.; Day, P.; Malik, K.M.A.; Hursthouse, M.B.; Teat, S.J.; MacLean, E.J.; Martin, L.; French, S.A. Effect of Included Solvent Molecules on the Physical Properties of the Paramagnetic Charge Transfer Salts β″-(bedt-ttf)4[(H3O)Fe(C2O4)3]·Solvent (bedtttf = Bis(ethylenedithio)tetrathiafulvalene). Inorg. Chem. 1999, 38, 3543–3549. [Google Scholar] [CrossRef]
- Rashid, S.; Turner, S.S.; Day, P.; Howard, J.A.K.; Guionneau, P.; McInnes, E.J.L.; Mabbs, F.E.; Clark, R.J.H.; Firth, S.; Biggs, T. New superconducting charge-transfer salts (BEDT-TTF)4[AM(C2O4)3]·C6H5NO2 (A = H3O or NH4, M = Cr or Fe, BEDT-TTF = bis(ethylenedithio)tetrathiafulvalene). J. Mater. Chem. 2001, 11, 2095–2101. [Google Scholar] [CrossRef]
- Prokhorova, T.; Khasanov, S.; Zorina, L.; Buravov, L.; Tkacheva, V.; Baskakov, A.; Morgunov, R.; Gener-Moret, M.; Canadell, E.; Shibaeva, R.; et al. Molecular Metals Based on BEDT-TTF Radical Cation Salts with Magnetic Metal Oxalates as Counterions: β″-(BEDT-TTF)4A[M(C2O4)3]·DMF (A = NH4+, K+; M = CrIII, FeIII). Adv. Funct. Mater. 2003, 13, 403–411. [Google Scholar] [CrossRef]
- Akutsu-Sato, A.; Kobayashi, A.; Mori, T.; Akutsu, H.; Yamada, J.; Nakatsuji, S.; Turner, S.; Day, P.; Tocher, D.; Light, M.; et al. Structures and Physical Properties of New β′-BEDT-TTF Tris-Oxalatometallate (III) Salts Containing Chlorobenzene and Halomethane Guest Molecules. Synth. Met. 2005, 152, 373–376. [Google Scholar] [CrossRef]
- Coronado, E.; Curreli, S.; Giménez-Saiz, C.; Gómez-García, C.J. A novel paramagnetic molecular superconductor formed by bis(ethylenedithio)tetrathiafulvalene, tris(oxalato)ferrate(iii) anions and bromobenzene as guest molecule: ET4[(H3O)Fe(C2O4)3]·C6H5Br. J. Mater. Chem. 2005, 15, 1429–1436. [Google Scholar] [CrossRef]
- Akutsu-Sato, A.; Turner, S.S.; Akutsu, H.; Yamada, J.; Nakatsuji, S.; Day, P. Suppression of superconductivity in a molecular charge transfer salt by changing guest molecule: -(BEDT-TTF)4[(H3O)Fe(C2O4)3](C6H5CN)x(C5H5N)1x. J. Mater. Chem. 2007, 17, 2497–2499. [Google Scholar] [CrossRef]
- Zorina, L.V.; Prokhorova, T.G.; Simonov, S.V.; Khasanov, S.S.; Shibaeva, R.P.; Manakov, A.I.; Zverev, V.N.; Buravov, L.I.; Yagubskiĭ, É.B. Structure and magnetotransport properties of the new quasi-two-dimensional molecular metal β″-(BEDT-TTF)4H3O[Fe(C2O4)3]·C6H4Cl2. J. Exp. Theor. Phys. 2008, 106, 347–354. [Google Scholar] [CrossRef]
- Prokhorova, T.G.; Korobenko, A.V.; Buravov, L.I.; Yagubskii, E.B.; Zorina, L.V.; Khasanov, S.S.; Simonov, S.; Shibaeva, R.P.; Zverev, V.N. Effect of electrocrystallization medium on quality, structural features, and conducting properties of single crystals of the (BEDT-TTF)4AI[FeIII(C2O4)3]G family. CrystEngComm 2011, 13, 537–545. [Google Scholar] [CrossRef]
- Coronado, E.; Curreli, S.; Giménez-Saiz, C.; Gómez-García, C.J. The Series of Molecular Conductors and Superconductors ET4[AFe(C2O4)3]PhX (ET = bis(ethylenedithio)tetrathiafulvalene; (C2O4)2–= oxalate; A+= H3O+, K+; X = F, Cl, Br, and I): Influence of the Halobenzene Guest Molecules on the Crystal Structure and Superconducting Properties. Inorg. Chem. 2011, 51, 1111–1126. [Google Scholar] [CrossRef] [PubMed]
- Zorina, L.V.; Khasanov, S.S.; Simonov, S.V.; Shibaeva, R.P.; Bulanchuk, P.O.; Zverev, V.N.; Canadell, E.; Prokhorova, T.G.; Yagubskii, E.B. Structural phase transition in the β″-(BEDT-TTF)4H3O[Fe(C2O4)3]G crystals (where G is a guest solvent molecule). CrystEngComm 2011, 14, 460–465. [Google Scholar] [CrossRef]
- Prokhorova, T.G.; Buravov, L.I.; Yagubskii, E.B.; Zorina, L.V.; Simonov, S.; Zverev, V.N.; Shibaeva, R.P.; Canadell, E. Effect of Halopyridine Guest Molecules on the Structure and Superconducting Properties of β″-[Bis(ethylenedithio)tetra thiafulvalene]4(H3O)[Fe(C2O4)3]·Guest Crystals. Eur. J. Inorg. Chem. 2015, 2015, 5611–5620. [Google Scholar] [CrossRef]
- Martin, L.; Turner, S.S.; Day, P.; Mabbs, F.E.; McInnes, E.J.L. New molecular superconductor containing paramagnetic chromium(iii) ions. Chem. Commun. 1997, 1367–1368. [Google Scholar] [CrossRef]
- Martin, L.; Turner, S.S.; Day, P.; Malik, K.M.A.; Coles, S.J.; Hursthouse, M.B. Polymorphism based on molecular stereoisomerism in tris(oxalato) Cr(III) salts of bedt-ttf [bis(ethylenedithio)tetrathiafulvalene]. Chem. Commun. 1999, 513–514. [Google Scholar] [CrossRef]
- Rashid, S.; Turner, S.S.; Le Pevelen, D.; Day, P.; Light, M.E.; Hursthouse, M.B.; Firth, S.; Clark, R.J.H. β″-(BEDT-TTF)4[(H3O)Cr(C2O4)3]CH2Cl2: Effect of Included Solvent on the Structure and Properties of a Conducting Molecular Charge-Transfer Salt. Inorg. Chem. 2001, 40, 5304–5306. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Curreli, S.; Giménez-Saiz, C.; Gómez-García, C. New magnetic conductors and superconductors based on BEDT-TTF and BEDS-TTF. Synth. Met. 2005, 154, 245–248. [Google Scholar] [CrossRef]
- Prokhorova, T.G.; Yagubskii, E.B.; Zorina, L.V.; Simonov, S.V.; Zverev, V.N.; Shibaeva, R.P.; Buravov, L.I. Specific Structural Disorder in an Anion Layer and Its Influence on Conducting Properties of New Crystals of the (BEDT-TTF)4A+[M3+(ox)3]G Family, Where G Is 2-Halopyridine; M Is Cr, Ga; A+ Is [K0.8(H3O)0.2]+. Crystals 2018, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Martin, L.; Turner, S.S.; Day, P.; Guionneau, P.; Howard, J.A.K.; Hibbs, D.E.; Light, M.E.; Hursthouse, M.B.; Uruichi, M.; Yakushi, K. Crystal Chemistry and Physical Properties of Superconducting and Semiconducting Charge Transfer Salts of the Type (BEDT-TTF)4[AIMIII(C2O4)3]PhCN (AI = H3O, NH4, K.; MIII = Cr, Fe, Co, Al; BEDT-TTF = Bis(ethylenedithio)tetrathiafulvalene). Inorg. Chem. 2001, 40, 1363–1371. [Google Scholar] [CrossRef]
- Benmansour, S.; Sánchez-Máez, Y.; Gómez-García, C.J.; Sánchez-Máñez, Y. Mn-Containing Paramagnetic Conductors with Bis(ethylenedithio)tetrathiafulvalene (BEDT-TTF). Magnetochemistry 2017, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Akutsu, H.; Akutsu-Sato, A.; Turner, S.S.; Le Pevelen, D.; Day, P.; Laukhin, V.; Klehe, A.-K.; Singleton, J.; Tocher, D.; Probert, M.R.; et al. Effect of Included Guest Molecules on the Normal State Conductivity and Superconductivity of β″-(ET)4[(H3O)Ga(C2O4)3]G (G = Pyridine, Nitrobenzene). J. Am. Chem. Soc. 2002, 124, 12430–12431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prokhorova, T.G.; Buravov, L.I.; Yagubskii, E.B.; Zorina, L.V.; Simonov, S.; Shibaeva, R.P.; Zverev, V.N. Metallic Bi- and Monolayered Radical Cation Salts Based on Bis(ethylenedithio) tetrathiafulvalene (BEDT-TTF) with the Tris(oxalato)gallate Anion. Eur. J. Inorg. Chem. 2014, 2014, 3933–3940. [Google Scholar] [CrossRef]
- Prokhorova, T.G.; Zorina, L.V.; Simonov, S.V.; Zverev, V.N.; Canadell, E.; Shibaeva, R.P.; Yagubskii, E.B. The first molecular superconductor based on BEDT-TTF radical cation salt with paramagnetic tris(oxalato)ruthenate anion. CrystEngComm 2013, 15, 7048. [Google Scholar] [CrossRef]
- Martin, L.; Morritt, A.L.; Lopez, J.R.; Nakazawa, Y.; Akutsu, H.; Imajo, S.; Ihara, Y.; Zhang, B.; Zhang, Y.; Guo, Y. Molecular conductors from bis(ethylenedithio)tetrathiafulvalene with tris(oxalato)rhodate. Dalton Trans. 2017, 46, 9542–9548. [Google Scholar] [CrossRef] [Green Version]
- Imajo, S.; Akutsu, H.; Akutsu-Sato, A.; Morritt, A.L.; Martin, L.; Nakazawa, Y. Effects of Electron Correlations and Chemical Pressures on Superconductivity of β″-Type Organic Compounds. Phys. Rev. Res. 2019, 1, 33184. [Google Scholar] [CrossRef] [Green Version]
- Akutsu, H.; Akutsu-Sato, A.; Turner, S.S.; Day, P.; Canadell, E.; Firth, S.; Clark, R.J.H.; Yamada, J.-I.; Nakatsuji, S. Superstructures of donor packing arrangements in a series of molecular charge transfer salts. Chem. Commun. 2004, 18–19. [Google Scholar] [CrossRef] [PubMed]
- Zorina, L.V.; Khasanov, S.S.; Simonov, S.V.; Shibaeva, R.P.; Zverev, V.N.; Canadell, E.; Prokhorova, T.G.; Yagubskii, E.B. Coexistence of two donor packing motifs in the stable molecular metal α-‘pseudo-κ’-(BEDT-TTF)4(H3O)[Fe(C2O4)3]·C6H4Br2. CrystEngComm 2011, 13, 2430–2438. [Google Scholar] [CrossRef]
- Martin, L.; Day, P.; Akutsu, H.; Yamada, J.-I.; Nakatsuji, S.; Clegg, W.; Harrington, R.W.; Horton, P.N.; Hursthouse, M.B.; McMillan, P.; et al. Metallic molecular crystals containing chiral or racemic guest molecules. CrystEngComm 2007, 9, 865–867. [Google Scholar] [CrossRef]
- Martin, L.; Engelkamp, H.; Akutsu, H.; Nakatsuji, S.; Yamada, J.; Horton, P.; Hursthouse, M.B. Radical-cation salts of BEDT-TTF with lithium tris(oxalato)metallate(iii). Dalton Trans. 2015, 44, 6219–6223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, L.; Day, P.; Horton, P.; Nakatsuji, S.; Yamada, J.; Akutsu, H. Chiral conducting salts of BEDT-TTF containing a single enantiomer of tris(oxalato)chromate(III) crystallised from a chiral solvent. J. Mater. Chem. 2010, 20, 2738–2742. [Google Scholar] [CrossRef]
- Martin, L.; Day, P.; Nakatsuji, S.; Yamada, J.; Akutsu, H.; Horton, P. A molecular charge transfer salt of BEDT-TTF containing a single enantiomer of tris(oxalato)chromate(III) crystallised from a chiral solvent. CrystEngComm 2009, 12, 1369–1372. [Google Scholar] [CrossRef]
- Martin, L.; Akutsu, H.; Horton, P.N.; Hursthouse, M.B. Chirality in charge-transfer salts of BEDT-TTF of tris(oxalato)chromate(III). CrystEngComm 2015, 17, 2783–2790. [Google Scholar] [CrossRef] [Green Version]
- Martin, L.; Akutsu, H.; Horton, P.N.; Hursthouse, M.B.; Harrington, R.W.; Clegg, W. Chiral Radical-Cation Salts of BEDT-TTF Containing a Single Enantiomer of Tris(oxalato)aluminate(III) and -chromate(III). Eur. J. Inorg. Chem. 2015, 2015, 1865–1870. [Google Scholar] [CrossRef]
- Martin, L.; Morritt, A.L.; Lopez, J.R.; Akutsu, H.; Nakazawa, Y.; Imajo, S.; Ihara, Y. Ambient-pressure molecular superconductor with a superlattice containing layers of tris(oxalato)rhodate enantiomers and 18-crown-6. Inorg. Chem. 2017, 56, 717–720. [Google Scholar] [CrossRef] [Green Version]
- Martin, L.; Lopez, J.R.; Akutsu, H.; Nakazawa, Y.; Imajo, S. Bulk Kosterlitz–Thouless Type Molecular Superconductor β″-(BEDT-TTF)2[(H2O)(NH4)2Cr(C2O4)3]18-crown-6. Inorg. Chem. 2017, 56, 14045–14052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morritt, A.L.; Lopez, J.R.; Blundell, T.; Canadell, E.; Akutsu, H.; Nakazawa, Y.; Imajo, S.; Martin, L. 2D Molecular Superconductor to Insulator Transition in the β″-(BEDT-TTF)2[(H2O)(NH4)2M(C2O4)3]18-crown-6 Series (M = Rh, Cr, Ru, Ir). Inorg. Chem. 2019, 58, 10656–10664. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Liu, F.; Guo, Y. Synthesis, crystal structure, and characterization of charge-transfer salt: (BEDT-TTF)5[Fe(C2O4)3]·(H2O)2·CH2Cl2 (BEDT-TTF = bis(ethylenedithio)tetrathiafulvalene). CrystEngComm 2009, 11, 2523–2528. [Google Scholar] [CrossRef]
- Martin, L.; Day, P.; Horton, P.; Bingham, A.; Hursthouse, M.B. The first molecular charge transfer salt containing layers of an alkali metal. J. Low Temp. Phys. 2006, 142, 417–420. [Google Scholar] [CrossRef]
- Martin, L.; Day, P.; Clegg, W.; Harrington, R.W.; Horton, P.N.; Bingham, A.; Hursthouse, M.B.; McMillan, P.; Firth, S. Multi-layered molecular charge-transfer salts containing alkali metal ions. J. Mater. Chem. 2007, 17, 3324–3329. [Google Scholar] [CrossRef]
- Martin, L.; Day, P.; Barnett, S.A.; Tocher, D.A.; Horton, P.N.; Hursthouse, M.B. Magnetic molecular charge-transfer salts containing layers of water and tris(oxalato)ferrate(iii) anions. CrystEngComm 2008, 10, 192–196. [Google Scholar] [CrossRef]
- Martin, L.; Turner, S.S.; Day, P.; Guionneau, P.; Howard, J.A.K.; Uruichi, M.; Yakushi, K. Synthesis, crystal structure and properties of the semiconducting molecular charge-transfer salt (bedtttf)2Ge(C2O4)3 PhCN [bedt-ttfbis(ethylenedithio)tetrathiafulvalene]. J. Mater. Chem. 1999, 9, 2731–2736. [Google Scholar] [CrossRef]
- Martin, L.; Day, P.; Nakatsuji, S.; Yamada, J.-I.; Akutsu, H.; Horton, P.N. BEDT-TTF Tris(oxalato)germanate(IV) Salts with Novel Donor Packing Motifs. Bull. Chem. Soc. Jpn. 2010, 83, 419–423. [Google Scholar] [CrossRef]
- Lopez, J.R.; Akutsu, H.; Martin, L. Radical-cation salt with novel BEDT-TTF packing motif containing tris(oxalato)germanate(IV). Synth. Met. 2015, 209, 188–191. [Google Scholar] [CrossRef] [Green Version]
- Guionneau, P.; Kepert, C.; Bravic, G.; Chasseau, D.; Truter, M.; Kurmoo, M.; Day, P. Determining the charge distribution in BEDT-TTF salts. Synth. Met. 1997, 86, 1973–1974. [Google Scholar] [CrossRef]
- Audouard, A.; Laukhin, V.N.; Brossard, L.; Prokhorova, T.G.; Yagubskii, E.B.; Canadell, E. Combination frequencies of magnetic oscillations in β″−(BEDT−TTF)4(NH4)[Fe(C2O4)3]⋅DMF. Phys. Rev. B 2004, 69. [Google Scholar] [CrossRef]
- Uji, S.; Iida, Y.; Sugiura, S.; Isono, T.; Sugii, K.; Kikugawa, N.; Terashima, T.; Yasuzuka, S.; Akutsu, H.; Nakazawa, Y.; et al. Fulde-Ferrell-Larkin-Ovchinnikov superconductivity in the layered organic superconductor β″−(BEDT−TTF)4[(H3O)Ga(C2O4)3]C6H5NO2. Phys. Rev. B 2018, 97, 144505. [Google Scholar] [CrossRef] [Green Version]
- Bangura, A.F.; Coldea, A.I.; Singleton, J.; Ardavan, A.; Akutsu-Sato, A.; Akutsu, H.; Turner, S.S.; Day, P.; Yamamoto, T.; Yakushi, K. Robust superconducting state in the low-quasiparticle-density organic metals β″−(BEDT−TTF)4[(H3O)M(C2O4)3]Y: Superconductivity due to proximity to a charge-ordered state. Phys. Rev. B 2005, 72, 014543. [Google Scholar] [CrossRef] [Green Version]
- Coldea, A.I.; Bangura, A.F.; Singleton, J.; Ardavan, A.; Akutsu-Sato, A.; Akutsu, H.; Turner, S.S.; Day, P. Fermi-surface topology and the effects of intrinsic disorder in a class of charge-transfer salts containing magnetic ions: β″−(BEDT−TTF)4[(H3O)M(C2O4)3]Y(M = Ga, Cr, Fe;Y = C5H5N). Phys. Rev. B 2004, 69, 085112. [Google Scholar] [CrossRef] [Green Version]
- Ihara, Y.; Seki, H.; Kawamoto, A. 13C NMR Study of Superconductivity near Charge Instability Realized in β″-(BEDT-TTF)4 [(H3O)Ga(C2O4)3]C6H5NO2. J. Phys. Soc. Jpn. 2013, 82, 83701. [Google Scholar] [CrossRef] [Green Version]
- Ihara, Y.; Jeong, M.; Mayaffre, H.; Berthier, C.; Horvatić, M.; Seki, H.; Kawamoto, A. C 13NMR study of the charge-ordered state near the superconducting transition in the organic superconductor β″−(BEDT-TTF)4[(H3O)Ga(C2O4)3]·C6H5NO2. Phys. Rev. B 2014, 90, 121106. [Google Scholar] [CrossRef] [Green Version]
- Ihara, Y.; Moribe, K.; Fukuoka, S.; Kawamoto, A. Microscopic coexistence of superconductivity and charge order in the organic superconductor β″−(BEDT-TTF)4[(H3O)Ga(C2O4)3]·C6H5NO2. Phys. Rev. B 2019, 100, 060505. [Google Scholar] [CrossRef] [Green Version]
- Imajo, S.; Akutsu, H.; Kurihara, R.; Yajima, T.; Kohama, Y.; Tokunaga, M.; Kindo, K.; Nakazawa, Y. Anisotropic Fully Gapped Superconductivity Possibly Mediated by Charge Fluctuations in a Nondimeric Organic Complex. Phys. Rev. Lett. 2020, 125, 177002. [Google Scholar] [CrossRef]
- Bailar, J.C., Jr.; Jones, E.M.; Booth, H.S.; Grennert, M. Trioxalato Salts (Trioxalatoaluminiate, -Ferriate, -Chromiate, and -Cobaltiate). Inorg. Synth. 1939, 1, 35–38. [Google Scholar]
- Kaziro, R.; Hambley, T.W.; Binstead, R.A.; Beattie, J.K. Potassium tris(oxalato)ruthenate (III). Inorg. Chim. Acta 1989, 164, 85–91. [Google Scholar] [CrossRef]





| Salt | Al-PhBr 290 K | Al-PhBr 110 K | Co-PhBr 150 K | Ru-PhF 293 K | Ru-PhCl 298 K |
|---|---|---|---|---|---|
| Formula | C52H40AlBrO13S32 | C52H40AlBrO13S32 | C52H40BrCoO13S32 | C52H40FO13RuS32 | C52H40O13S32ClRu |
| Fw (g mol−1) | 2005.65 | 2005.65 | 2037.60 | 2018.83 | 2035.28 |
| Crystal System | monoclinic | monoclinic | monoclinic | monoclinic | monoclinic |
| Space group | C2/c | C2/c | C2/c | C2/c | C2/c |
| Z | 4 | 4 | 4 | 4 | 4 |
| T (K) | 290 (2) | 110 (2) | 150 (2) | 293 (2) | 293 (2) |
| a | 10.2851 (2) | 10.2520 (3) | 10.2306 (3) | 10.32786 (19) | 10.32017 (19) |
| b | 19.9472 (4) | 19.7919 (7) | 19.7508 (5) | 19.9521 (4) | 20.0264 (4) |
| c | 35.597 (3) | 35.4275 (11) | 35.2520 (9) | 34.9966 (6) | 35.161 (3) |
| 90 | 90 | 90 | 90 | 90 | |
| 93.399 (7) | 93.843 (7) | 93.938 (7) | 93.010 (7) | 93.586 (7) | |
| 90 | 90 | 90 | 90 | 90 | |
| Volume | 7290.1 (6) | 7172.3 (4) | 7106.3 (3) | 7201.5 (2) | 7252.6 (6) |
| Density (g cm−3) | 1.827 | 1.857 | 1.905 | 1.862 | 1.864 |
| (mm−1) | 1.553 | 1.578 | 1.806 | 1.209 | 1.235 |
| 0.0547 | 0.0688 | 0.0431 | 0.0460 | 0.0442 | |
| 0.1401 | 0.1526 | 0.0914 | 0.318 | 0.1089 |
| Contact (Å) | Al-PhBr 290 K | Al-PhBr 110 K | Co-PhBr 150 K | Ru-PhF 293 K | Ru-PhCl 298 K |
|---|---|---|---|---|---|
| S1…S7 | 3.4069 (13) | 3.3795 (19) | 3.3683 (11) | 3.4015 (14) | 3.4283 (11) |
| S3…S7 | 3.5046 (13) | 3.458 (2) | 3.4544 (11) | 3.5369 (15) | 3.5290 (11) |
| S2…S9 | 3.3138 (14) | 3.2838 (19) | 3.2864 (11) | 3.3744 (15) | 3.3528 (11) |
| S2…S11 | 3.3720 (13) | 3.340 (2) | 3.3413 (11) | 3.3954 (15) | 3.3842 (11) |
| S6…S15 | 3.5231 (14) | 3.463 (2) | 3.4475 (12) | 3.5236 (17) | 3.5190 (13) |
| S8…S15 | 3.5704 (15) | 3.502 (2) | 3.4904 (13) | 3.6182 (17) | 3.5869 (12) |
| S8…S10 | 3.5889 (14) | 3.550 (2) | 3.5551 (13) | 3.6169 (16) | 3.6031 (13) |

| Salt | Donor | a | b | c | d | δ | Q |
|---|---|---|---|---|---|---|---|
| Al-PhBr 290 K | A | 1.376 | 1.73925 | 1.7515 | 1.352 | 0.763 | 0.65 |
| B | 1.373 | 1.74025 | 1.753 | 1.347 | 0.773 | 0.58 | |
| Al-PhBr 110 K | A | 1.364 | 1.74475 | 1.75675 | 1.346 | 0.792 | 0.44 |
| B | 1.376 | 1.7435 | 1.75725 | 1.3455 | 0.779 | 0.53 | |
| Co-PhBr 150 K | A | 1.367 | 1.73775 | 1.75275 | 1.345 | 0.779 | 0.54 |
| B | 1.369 | 1.738 | 1.7515 | 1.346 | 0.775 | 0.57 | |
| Ru-PhF 293 K | A | 1.36 | 1.7385 | 1.74475 | 1.349 | 0.774 | 0.57 |
| B | 1.367 | 1.73325 | 1.74525 | 1.354 | 0.758 | 0.69 | |
| Ru-PhCl 298 K | A | 1.366 | 1.737 | 1.7475 | 1.349 | 0.770 | 0.60 |
| B | 1.366 | 1.73575 | 1.74625 | 1.349 | 0.767 | 0.62 |
| Salt Temp. | Al-PhBr 290 K | Al-PhBr 110 K | Co-PhBr 150 K | Ru-PhF 293 K | Ru-PhCl 298 K |
|---|---|---|---|---|---|
| Distances (Å) | |||||
| a | 6.269 (3) | 6.255 (6) | 6.249 (3) | 6.292 (3) | 6.312 (2) |
| b | 6.387 (5) | 6.111 (10) | 6.286 (6) | 6.380 (5) | 6.377 (4) |
| c | 4.5360 (16) | 4.487 (3) | 4.5212 (10) | 4.804 (13) | 4.331 (16) |
| d | 1.894 (6) | 1.905 (10) | 1.902 (6) | 1.377 (14) | 1.737 (6) |
| e | 4.401 (9) | 4.338 (19) | 4.296 (10) | 4.786 (13) | 4.543 (9) |
| h | 13.560 (5) | 13.481 (10) | 13.464 (6) | 13.572 (5) | 13.649 (4) |
| w | 10.2851 (2) | 10.2520 (3) | 10.2306 (3) | 10.32786 (19) | 10.32017 (19) |
| O4-cation | 3.066 (5) | 3.004 (11) | 2.985 (6) | 2.956 (6) | 2.962 (5) |
| O6-cation | 2.857 (3) | 2.851 (6) | 2.842 (3) | 2.846 (6) | 2.831 (3) |
| O1-cation | 3.083 (5) | 3.073 (9) | 3.112 (5) | 2.941 (5) | 2.996 (4) |
| Angles (°) | |||||
| 33.522 (3) | 33.378 (3) | 33.60 (13) | 33.74 (19) | 32.677 (3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blundell, T.J.; Brannan, M.; Mburu-Newman, J.; Akutsu, H.; Nakazawa, Y.; Imajo, S.; Martin, L. First Molecular Superconductor with the Tris(Oxalato)Aluminate Anion, β″-(BEDT-TTF)4(H3O)Al(C2O4)3·C6H5Br, and Isostructural Tris(Oxalato)Cobaltate and Tris(Oxalato)Ruthenate Radical Cation Salts. Magnetochemistry 2021, 7, 90. https://doi.org/10.3390/magnetochemistry7070090
Blundell TJ, Brannan M, Mburu-Newman J, Akutsu H, Nakazawa Y, Imajo S, Martin L. First Molecular Superconductor with the Tris(Oxalato)Aluminate Anion, β″-(BEDT-TTF)4(H3O)Al(C2O4)3·C6H5Br, and Isostructural Tris(Oxalato)Cobaltate and Tris(Oxalato)Ruthenate Radical Cation Salts. Magnetochemistry. 2021; 7(7):90. https://doi.org/10.3390/magnetochemistry7070090
Chicago/Turabian StyleBlundell, Toby James, Michael Brannan, Joey Mburu-Newman, Hiroki Akutsu, Yasuhiro Nakazawa, Shusaku Imajo, and Lee Martin. 2021. "First Molecular Superconductor with the Tris(Oxalato)Aluminate Anion, β″-(BEDT-TTF)4(H3O)Al(C2O4)3·C6H5Br, and Isostructural Tris(Oxalato)Cobaltate and Tris(Oxalato)Ruthenate Radical Cation Salts" Magnetochemistry 7, no. 7: 90. https://doi.org/10.3390/magnetochemistry7070090
APA StyleBlundell, T. J., Brannan, M., Mburu-Newman, J., Akutsu, H., Nakazawa, Y., Imajo, S., & Martin, L. (2021). First Molecular Superconductor with the Tris(Oxalato)Aluminate Anion, β″-(BEDT-TTF)4(H3O)Al(C2O4)3·C6H5Br, and Isostructural Tris(Oxalato)Cobaltate and Tris(Oxalato)Ruthenate Radical Cation Salts. Magnetochemistry, 7(7), 90. https://doi.org/10.3390/magnetochemistry7070090