Tuning the Magnetocaloric Properties of the La(Fe,Si)13 Compounds by Chemical Substitution and Light Element Insertion
Abstract
1. Introduction
2. Sample Synthesis
Condition of Synthesis of La(FeSi) Type Alloys
3. Properties of the La(FeSi) Compounds
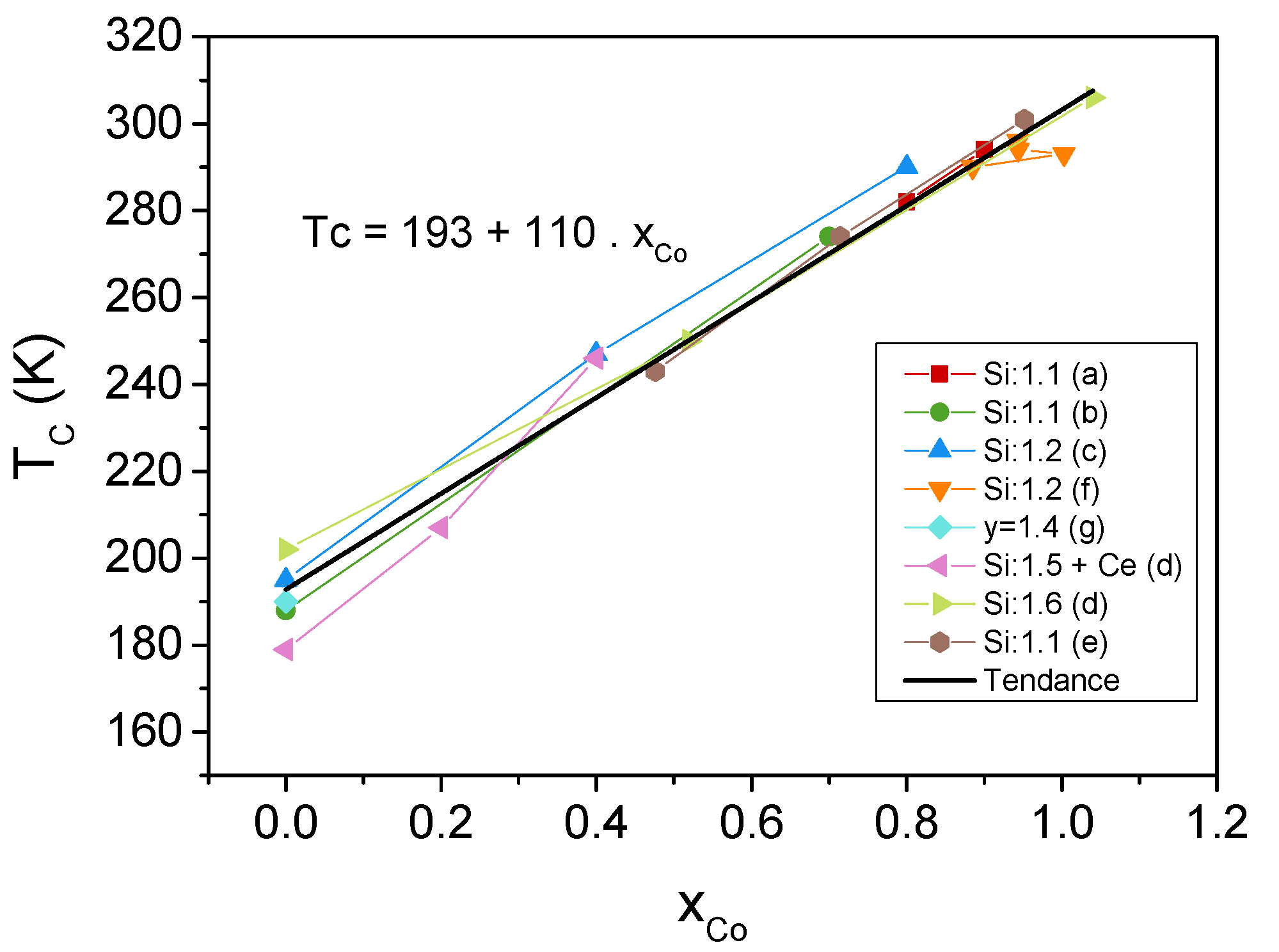
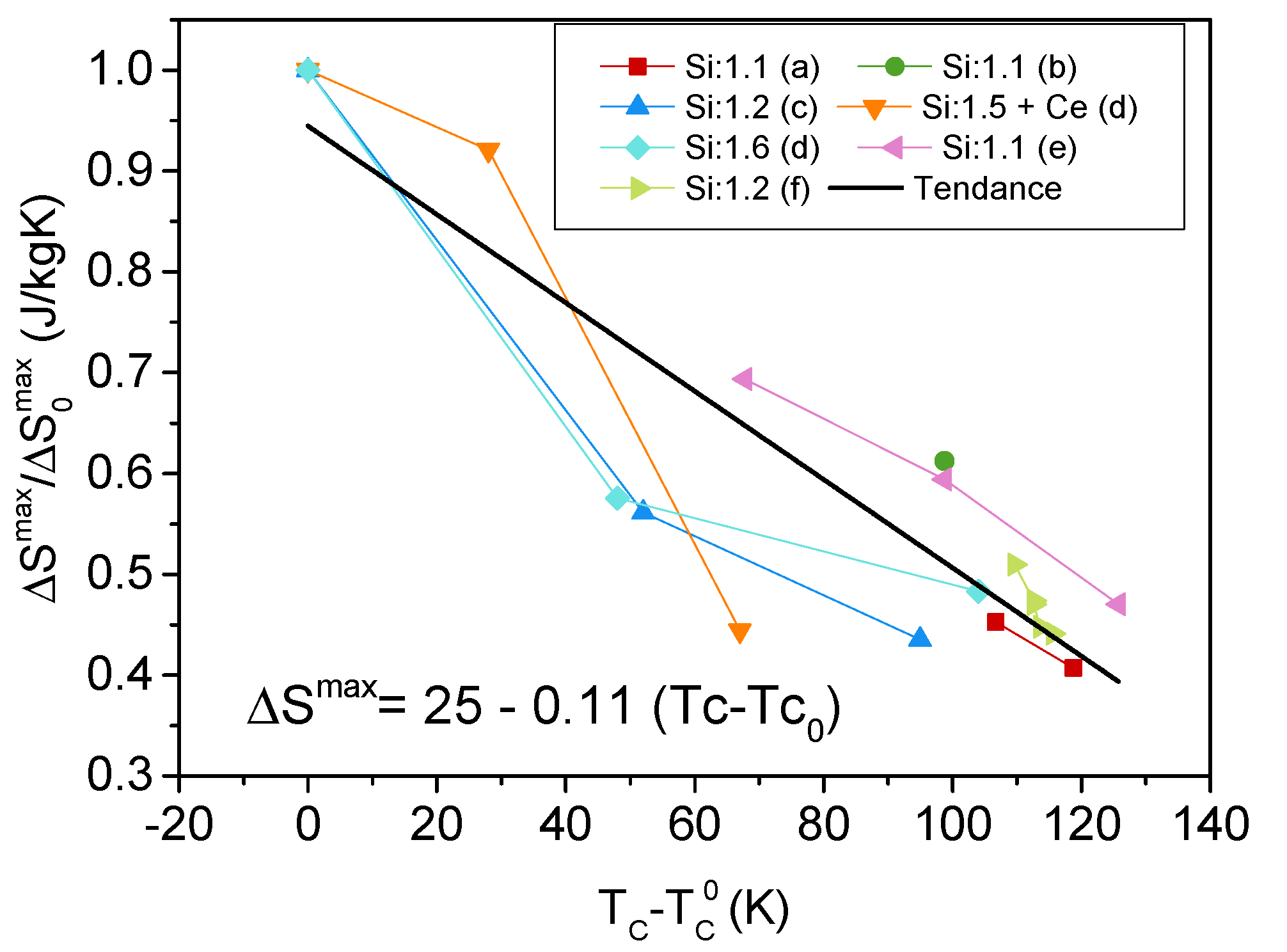
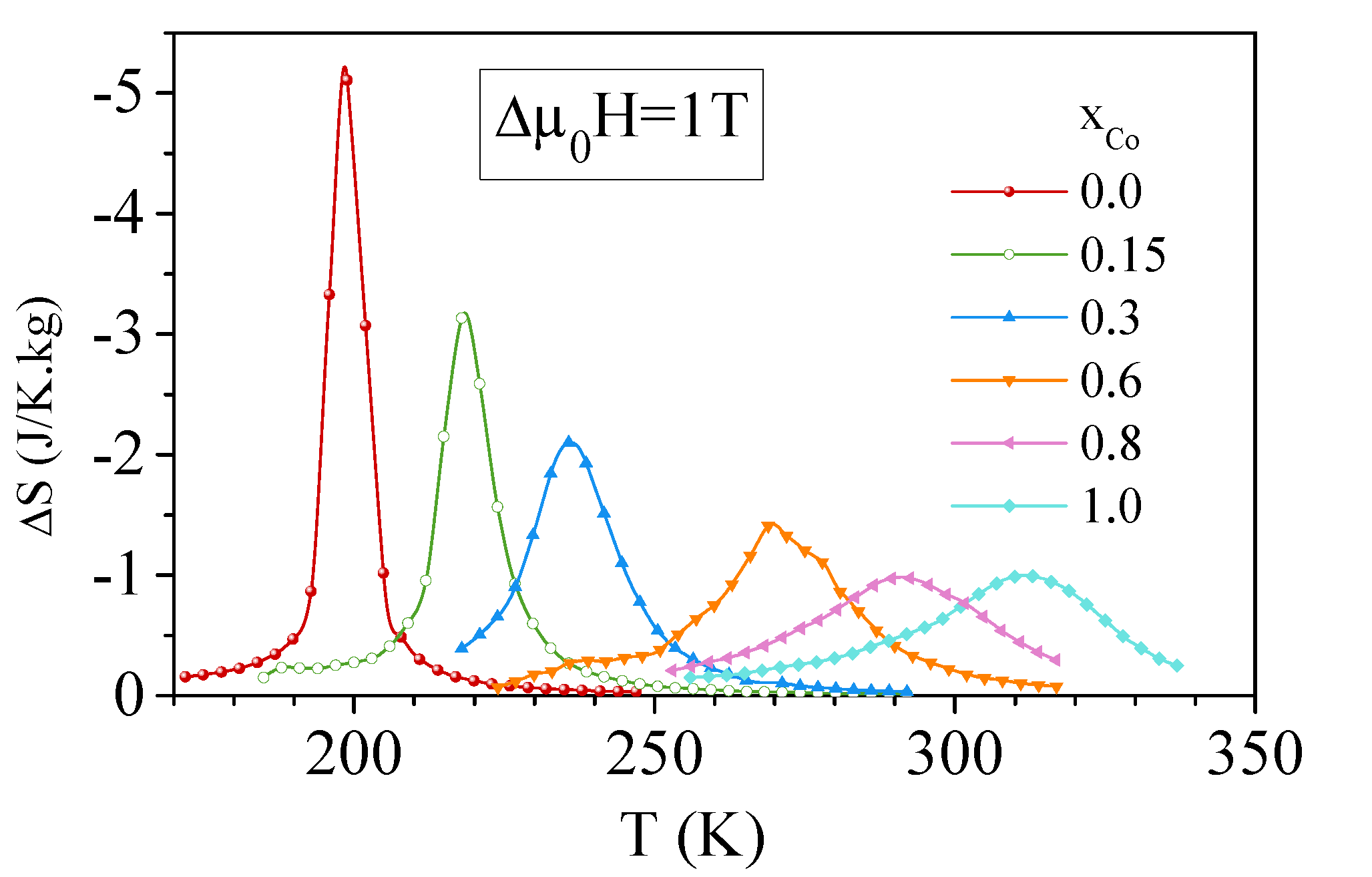
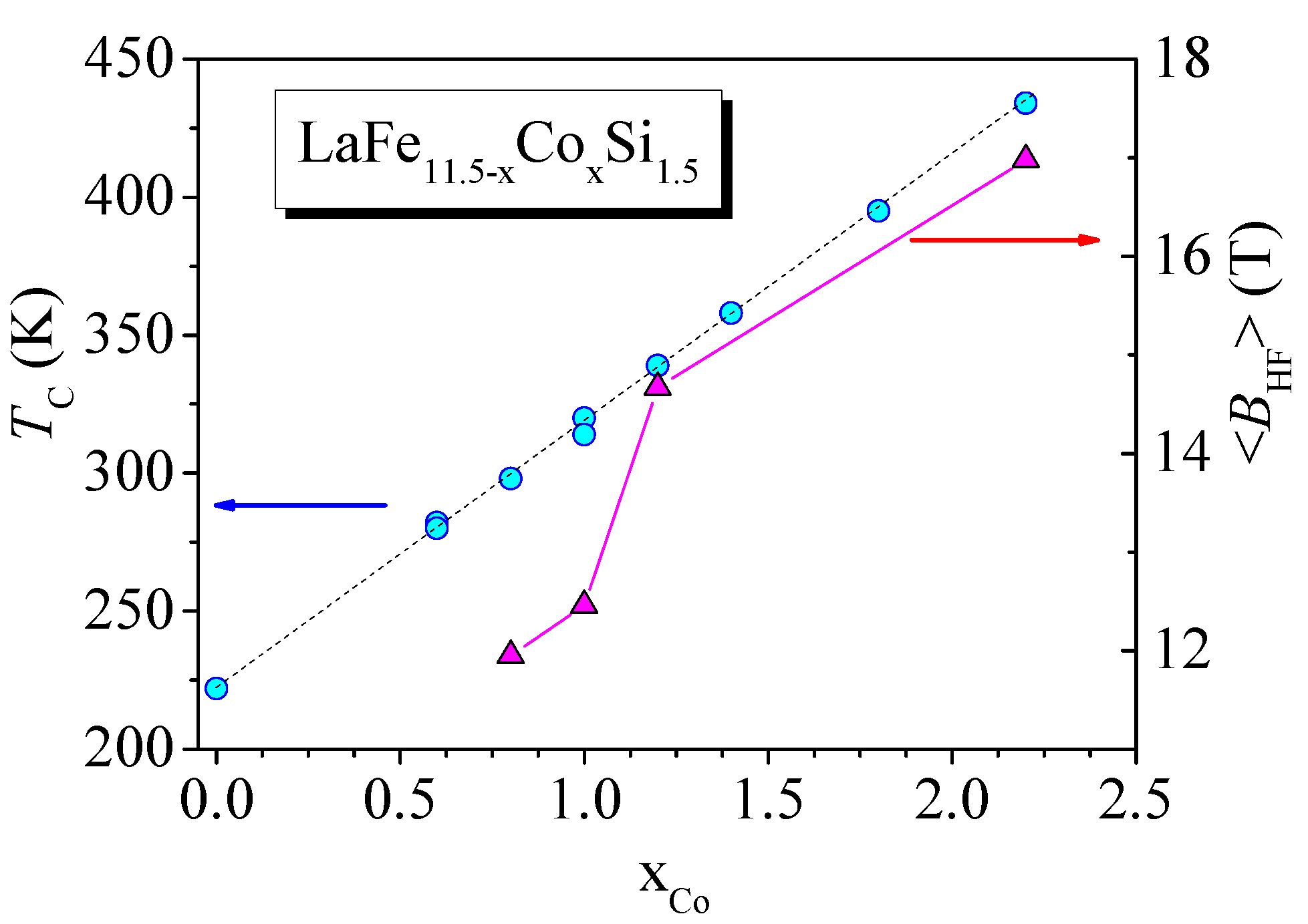
4. Effect of Iron Substitution in La(FeSiM) Compounds
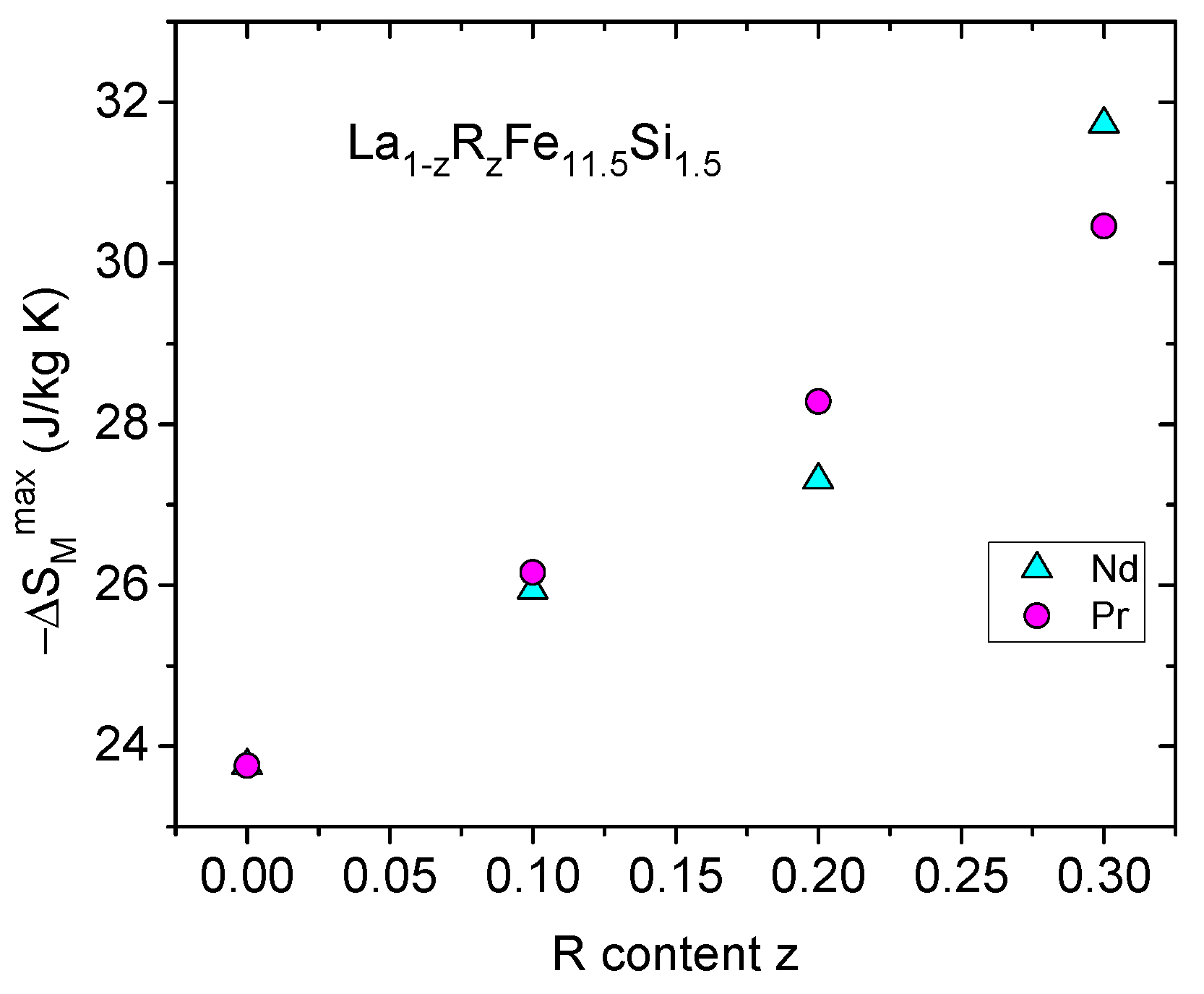
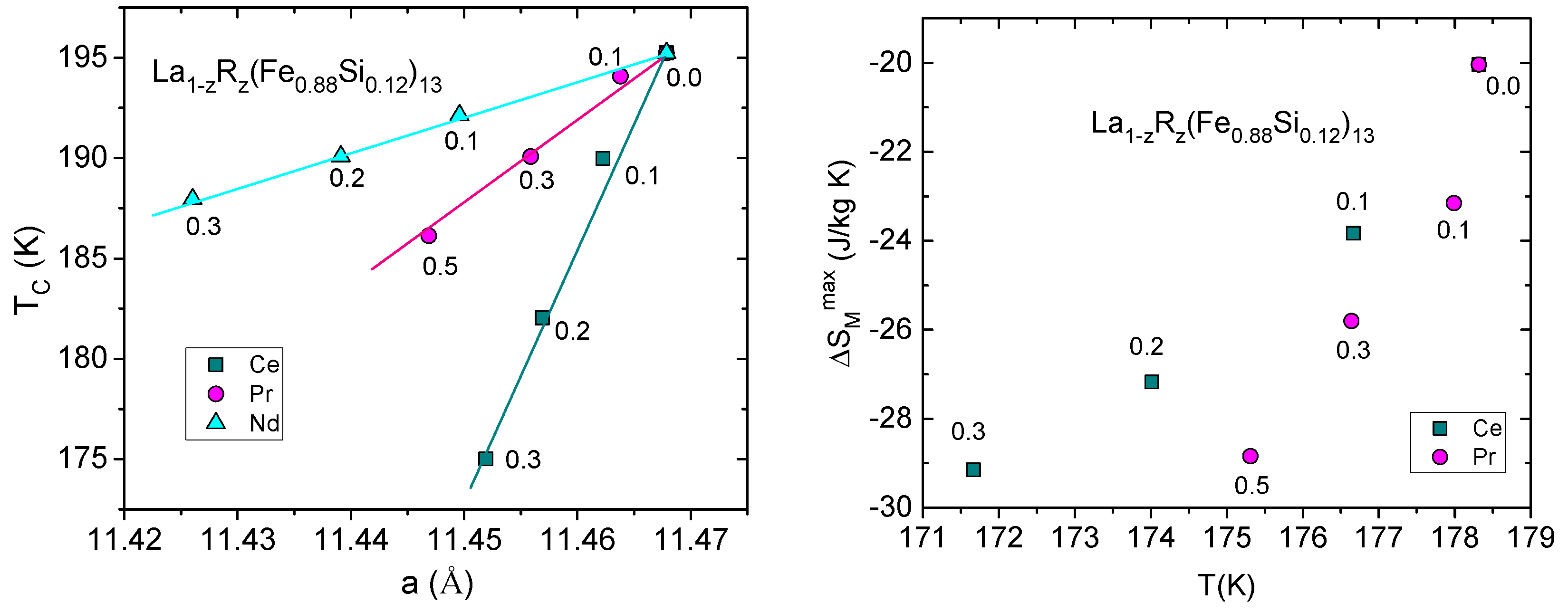
5. Effect of La Substitution in LaR(Fe,Si)
6. Effect of Light Element Insertion in La(FeSi)X
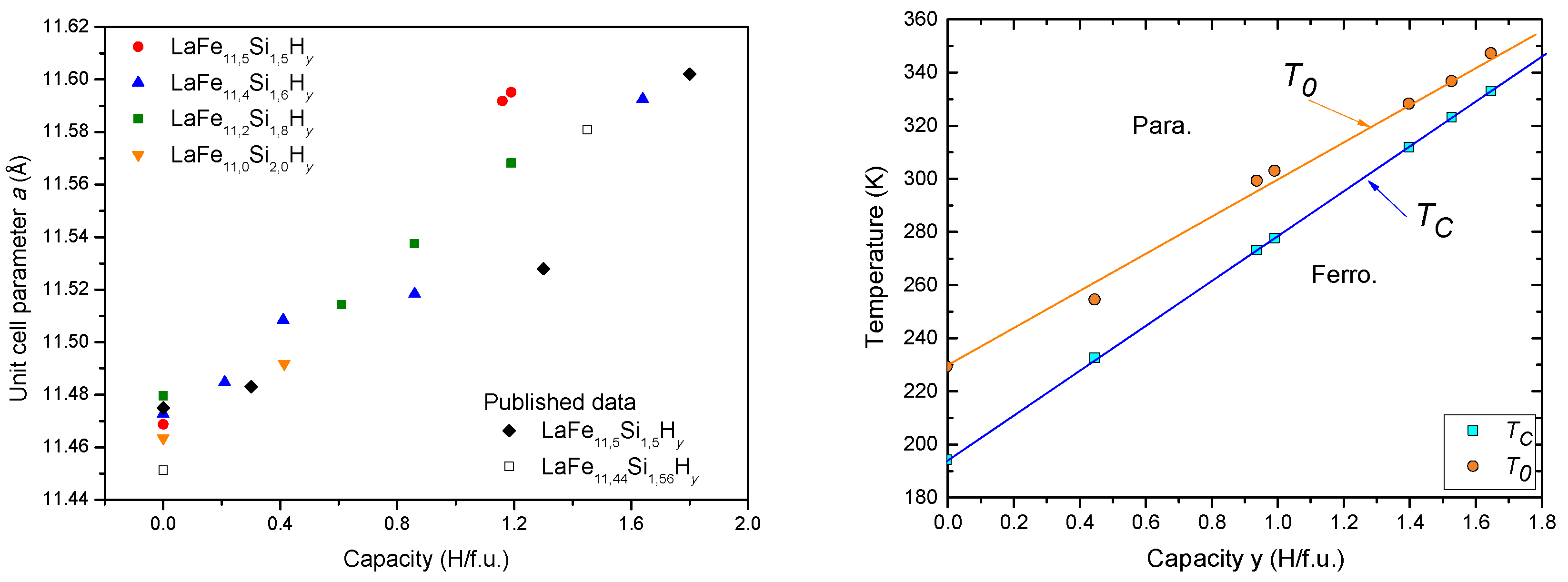
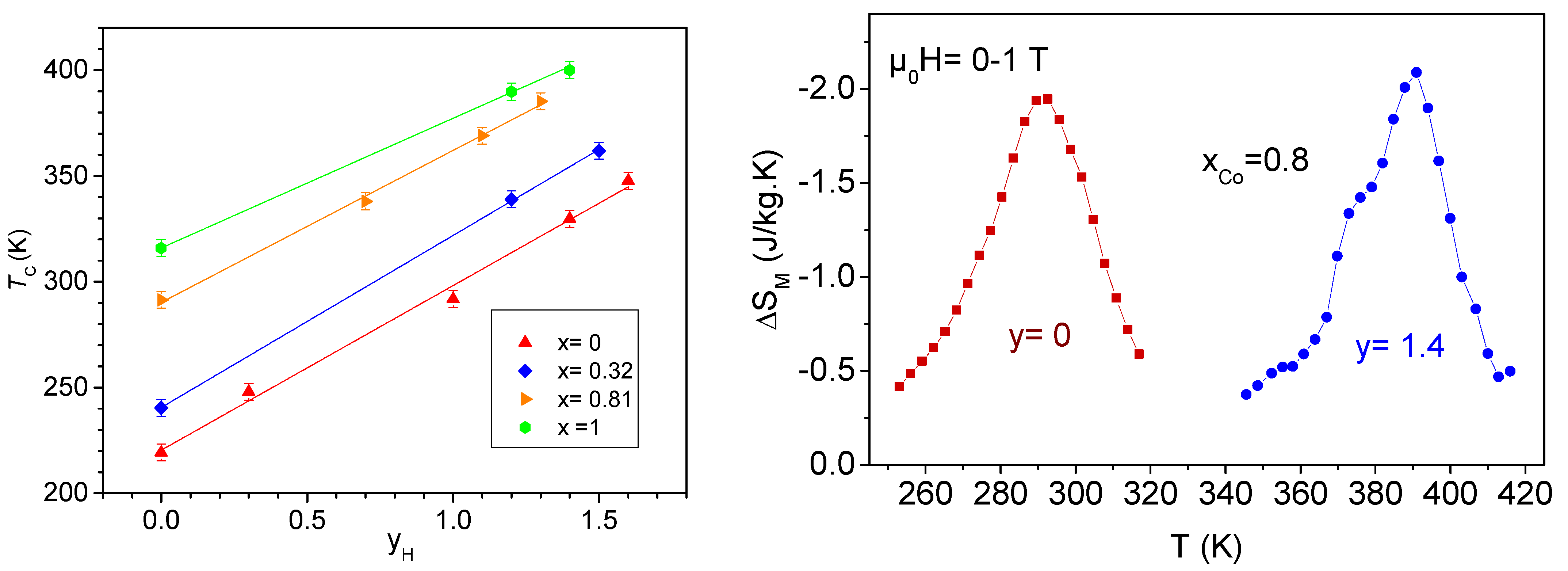
6.1. Hydrogen Insertion
6.2. Carbon Insertion
7. Combination of Substitution and Light Element Insertion
8. Implementation in Active Magnetic Regenerators
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Active Magnetic Regenerator |
| COP | Coefficient of Performance |
| DSC | Differential Scanning Calorimetry |
| FM | Ferromagnetic |
| IEM | Itinerant Electron Metamagnetic |
| MCE | Magnetocaloric effect |
| NPD | Neutron Powder Diffraction |
| PM | Paramagnetic |
| RT | Room Temperature |
| SPS | Spark Plasma Sintering |
| Curie Temperature | |
| TDR | Thermal Decomposition and Recombination |
| RCP | Relative Cooling Power |
| ZFC-FC | Zero Field Cooled - Field Cooled |
References
- Kitanovski, A.; Tusek, J.; Tomc, U.; Plaznik, U.; Ozbolt, M.; Poredos, A. Magnetocaloric Energy Conversion, From Theory to Applications; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Aprea, C.; Greco, A.; Maiorino, A.; Masselli, C. The employment of caloric-effect materials for solid-state heat pumping. Int. J. Refrig. 2020, 109, 1–11. [Google Scholar] [CrossRef]
- Nikitin, S.A.; Myalikgulyev, G.; Tishin, A.M.; Annaorazov, M.P.; Asatryan, K.A.; Tyurin, A.L. The magnetocaloric effect in Fe49Rh51 compound. Phys. Lett. A 1990, 148, 363–366. [Google Scholar] [CrossRef]
- Pecharsky, V.K.; Gschneidner, K.A. Giant Magnetocaloric Effect in Gd5(Si2Ge2). Phys. Rev. Lett. 1997, 78, 4494. [Google Scholar] [CrossRef]
- Wada, H.; Tomekawa, S.; Shiga, M. Magnetocaloric properties of a first-order magnetic transition system ErCo2. Cryogenics 1999, 39, 915–919. [Google Scholar] [CrossRef]
- Wada, H.; Tanabe, Y. Giant magnetocaloric effect of MnAs1-xSbx. Appl. Phys. Lett. 2001, 79, 3302–3304. [Google Scholar] [CrossRef]
- Valiev, E.; Gimaev, R.; Zverev, V.; Kamilov, K.; Pyatakov, A.; Kovalev, B.; Tishin, A. Application of the exchange-striction model for the calculation of the FeRh alloys magnetic properties. Intermetallics 2019, 108, 81–86. [Google Scholar] [CrossRef]
- Gimaev, R.R.; Vaulin, A.A.; Gubkin, A.F.; Zverev, V.I. Peculiarities of Magnetic and Magnetocaloric Properties of Fe-Rh Alloys in the Range of Antiferromagnet-Ferromagnet Transition. Phys. Metals Metallogr. 2020, 121, 823–850. [Google Scholar] [CrossRef]
- Sanchez-Valdas, C.F.; Gimaev, R.R.; Lopez-Cruz, M.; Llamazares, J.L.S.; Zvereve, V.I.; Tishin, A.M.; Carvalho, A.M.G.; Aguiar, D.J.M.; Mudryk, Y.; Pecharsky, V.K. The effect of cooling rate on magnetothermal properties of Fe49Rh51. J. Magn. Magn. Mater. 2020, 498, 166130. [Google Scholar] [CrossRef]
- Zarkevich, N.A.; Zverev, V.I. Viable Materials with a Giant Magnetocaloric Effect. Crystals 2020, 10, 815. [Google Scholar] [CrossRef]
- Zverev, V.I.; Gimaev, R.R.; Miyanaga, T.; Vaulin, A.A.; Gubkin, A.F.; Kovalev, B.B.; dos Santos, A.M.; Lovell, E.; Cohen, L.F.; Zarkevich, N.A. Peculiarities of the phase transformation dynamics in bulk FeRh based alloys from magnetic and structural measurements. J. Magn. Magn. Mater. 2021, 522, 167560. [Google Scholar] [CrossRef]
- Raghavan, V. Fe-La-Si (Iron-Lanthanum-Silicon). J. Phase Equilib. 2001, 22, 158. [Google Scholar] [CrossRef]
- Niitsu, K.; Kainuma, R. Phase equilibria in the Fe-La-Si ternary system. Intermetallics 2012, 20, 160–169. [Google Scholar] [CrossRef]
- Liu, X.D.; Liu, X.B.; Altounian, Z.; Tu, G.H. Microstructures of (Fe0.88Co0.12)82La7Si11 prepared by arc-melting/melt spinning and subsequent annealing. Appl. Phys. A Mater. 2006, 82, 339–343. [Google Scholar] [CrossRef]
- Fujita, A.; Koiwai, S.; Fujieda, S.; Fukamichi, K.; Kobayashi, T.; Tsuji, H.; Kaji, S.; Saito, A.T. Magnetocaloric effect in spherical La(FexSi1-x)13 and their hydrides for active magnetic regenerator-type refrigerator. J. Appl. Phys. 2009, 105, 07A936. [Google Scholar] [CrossRef]
- Fujita, A.; Yako, H. Stability of metallic, magnetic and electronic states in NaZn13-type La(FexSi1-x)13 magnetocaloric compounds. Scripta Mater. 2012, 67, 578–583. [Google Scholar] [CrossRef]
- Gebara, P.; Pawlik, P.; Skorvanek, I.; Bednarcik, J.; Marcin, J.; Michalik, S.; Donges, J.; Wyslocki, J.J.; Michalski, B. Effect of Al content on the order of phase transition and magnetic entropy change in LaFe11Co0.8(Si1-xAlx)1.2 alloys. J. Magn. Magn. Mater. 2014, 372, 201–207. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Y.G.; Tang, Y.B.; Xiao, S.F.; Zhang, E.Y.; Wang, J.W. Structure and magnetic properties of shortly high temperature annealing LaFe11.6Si1.4 compound. J. Alloys Compd. 2009, 475, 672–675. [Google Scholar] [CrossRef]
- Gutfleisch, O.; Yan, A.; Muller, K.H. Large magnetocaloric effect in melt-spun LaFe13-xSix. J. Appl. Phys. 2005, 97, 10M305. [Google Scholar] [CrossRef]
- Liu, X.B.; Liu, X.D.; Altounian, Z.; Tu, G.H. Phase formation and structure in rapidly quenched La(Fe0.88Co0.12)13-xSix alloys. J. Alloys Compd. 2005, 397, 120–125. [Google Scholar] [CrossRef]
- Liu, X.B.; Liu, X.D.; Altounian, Z. Phase formation and magnetocaloric effect in rapidly quenched La(Fe1-xCox)11.4Si1.6. J. Appl. Phys. 2005, 98, 113904. [Google Scholar] [CrossRef]
- Yan, A. Structure and magnetocaloric effect in melt-spun La (Fe,Si)13 and MnFePGe compounds. Rare Met. 2006, 25, 544–549. [Google Scholar] [CrossRef]
- Lyubina, J.; Gutfleisch, O.; Kuzmin, M.D.; Richter, M. La(Fe,Si)13-based magnetic refrigerants obtained by novel processing routes. J. Magn. Magn. Mater. 2008, 320, 2252–2258. [Google Scholar] [CrossRef]
- Dong, J.D.; Yan, A.R.; Liu, J. Microstructure and magnetocaloric properties of melt-extracted La-Fe-Si microwires. J. Magn. Magn. Mater. 2014, 357, 73–76. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, P.N.; Dai, F.P.; Yan, A.R. A new approach to prepare spherical La-Fe-Si-Co magnetocaloric refrigerant particles. Scripta Mater. 2013, 69, 485–488. [Google Scholar] [CrossRef]
- Fujita, A.; Nakayama, Y.; Kano, M.; Matsunami, D. Improvement of low-field magnetic entropy change by increasing Fe concentration in solid-state reactive sintered La(FexSi1-x)13. J. Alloys Compd. 2014, 601, 158–161. [Google Scholar] [CrossRef]
- Mayer, C.; Dubrez, A.; Pierronnet, M.; Vikner, P. Towards the large scale production of (La1-zCez)(Fe1-x-yMnySix)13H-n products for room temperature refrigeration. Phys. Status Solidi C. 2014, 11, 1059–1063. [Google Scholar] [CrossRef]
- Katter, M.; Zellmann, V.; Reppel, G.W.; Uestuener, K. Magnetocaloric Properties of La(Fe,Co,Si)13 Bulk Material Prepared by Powder Metallurgy. IEEE Trans. Magn. 2008, 44, 3044–3047. [Google Scholar] [CrossRef]
- Katter, M.; Zellmann, V.; Barcza, A. Sintering behavior and thermally induced decomposition and recomposition processe of LaFe13-x-yCoxSiy. In Proceedings of the Fourth IIF-IIR International Conference on Magnetic Refrigeration at Room Temperature, Thermag IV, IIF-IIR, Baotou, China, 23–27 August 2010. [Google Scholar]
- Phejar, M.; Paul-Boncour, V.; Bessais, L. Structural and magnetic properties of magnetocaloric LaFe13-xSix compounds synthesized by high energy ball-milling. Intermetallics 2010, 18, 2301–2307. [Google Scholar] [CrossRef]
- Patissier, A.; Paul-Boncour, V. Fast synthesis of LaFe13-xSix magnetocaloric compounds by reactive Spark Plasma Sintering. J. Alloys Compd. 2015, 645, 143–150. [Google Scholar] [CrossRef]
- Moore, J.D.; Klemm, D.; Lindackers, D.; Grasemann, S.; Trager, R.; Eckert, J.; Lober, L.; Scudino, S.; Katter, M.; Barcza, A.; et al. Selective laser melting of La(Fe,Co,Si)13 geometries for magnetic refrigeration. J. Appl. Phys. 2013, 114, 043907. [Google Scholar] [CrossRef]
- Paul-Boncour, V.; Pattisier, A.; Nakouri, K.; Bessais, L. Magnetocaloric compounds prepared by reactive spark plasma sintering. In Proceedings of the 7th International Conference on Caloric Cooling (Thermag VII), Turin, Italy, 11–14 September 2016. [Google Scholar]
- Niitsu, K.; Fujieda, S.; Fujita, A.; Kainuma, R. Microstructure and magnetic properties of as-quenched cubic and tetragonal La(Fe1-xSix)13 compounds. J. Alloys Compd. 2013, 578, 220–227. [Google Scholar] [CrossRef]
- Fujita, A.; Fujieda, S.; Hasegawa, Y.; Fukamichi, K. Itinerant-electron metamagnetic transition and large magnetocaloric effects in La(FexSi1-x)13 compounds and their hydrides. Phys. Rev. B 2003, 67, 104416. [Google Scholar] [CrossRef]
- Moore, D.; Morrison, K.; Sandeman, K.G.; Katter, M.; Cohen, L.F. Reducing extrinsic hysteresis in first-order La(Fe,Co,Si)13 magnetocaloric systems. Appl. Phys. Lett. 2009, 95, 252504. [Google Scholar] [CrossRef]
- Yako, H.; Fujieda, S.; Fujita, A.; Fukamichi, K. Influence of Demagnetization Effect on the Kinetics of the Itinerant Electron Metamagnetic Transition in Magnetic Refrigerant La(Fe0.88Si0.12)13. IEEE Trans. Magn. 2011, 47, 2482–2485. [Google Scholar] [CrossRef]
- Lovell, E.; Pereira, A.M.; Caplin, A.D.; Lyubina, J.; Cohen, L.F. Dynamics of the First-Order Metamagnetic Transition in Magnetocaloric La(Fe,Si)13: Reducing Hysteresis. Adv. Energy Mater. 2015, 5, 1401639. [Google Scholar] [CrossRef]
- Cohen, L.F. Contributions to Hysteresis in Magnetocaloric Materials. Phys. Status Solidi B 2018, 255, 1700317. [Google Scholar] [CrossRef]
- Lovell, E.; Morrison, K.; Pereira, A.M.; Caplin, A.D.; Gutfleisch, O.; Cohen, L.F. Scanning Hall Probe Imaging of LaFe13-xSix. Adv. Sci. Tech. 2014, 93, 219–224. [Google Scholar] [CrossRef]
- Ido, H.; Sohn, J.C.; Pourarian, F.; Cheng, S.F.; Wallace, W.E. Magnetic properties of LaCo13-based systems. J. Appl. Phys. 1990, 67, 4978. [Google Scholar] [CrossRef]
- Hu, F.X.; Shen, B.G.; Sun, J.R.; Wang, G.J.; Cheng, Z.H. Very large magnetic entropy change near room temperature in LaFe11.2Co0.7Si1.1. Appl. Phys. Lett. 2002, 80, 826–828. [Google Scholar] [CrossRef]
- Bjork, R.; Bahl, C.R.H.; Katter, M. Magnetocaloric properties of LaFe13-x-yCoxSiy and commercial grade Gd. J. Magn. Magn. Mater. 2010, 322, 3882–3888. [Google Scholar] [CrossRef]
- Hansen, B.R.; Kuhn, L.T.; Bahl, C.R.H.; Lundberg, M.; Ancona-Torres, C.; Katter, M. Properties of magnetocaloric La(Fe,Co,Si)13 produced by powder metallurgy. J. Magn. Magn. Mater. 2010, 322, 3447–3454. [Google Scholar] [CrossRef]
- Hu, F.X.; Qian, X.L.; Sun, J.R.; Wang, G.J.; Zhang, X.X.; Cheng, Z.H.; Shen, B.G. Magnetic entropy change and its temperature variation in compounds La(Fe1-xCox)11.2Si1.8. J. Appl. Phys. 2002, 92, 3620–3623. [Google Scholar] [CrossRef]
- Balli, M.; Fruchart, D.; Sari, O.; Gignoux, D.; Huang, J.H.; Hu, J.; Egolf, P.W. Direct measurement of the magnetocaloric effect on La(Fe13-x-yCoy)Six. J. Appl. Phys. 2009, 106, 023902. [Google Scholar] [CrossRef]
- Ilyn, M.; Tishin, A.; Hu, F.; Gao, J.; Sun, J.; Shen, B. Magnetocaloric properties of the LaFe11.7Si1.3 and LaFe11.2Co0.7Si1.1 systems. J. Magn. Magn. Mater. 2005, 290, 712. [Google Scholar] [CrossRef]
- Yan, A.; Muller, K.H.; Gutfleish, O. Magnetocaloric properties of the LaFe11.7Si1.3 and LaFe11.2Co0.7Si1.1 systems. J. Alloys Compd. 2008, 450, 18. [Google Scholar] [CrossRef]
- Dong, Q.Y.; Zhang, H.W.; Zhao, T.Y.; Sun, J.R.; Shen, B.G. Realization of a small hysteresis loss and a large magnetic entropy change in NaZn13-type La-Fe-Si compound. Solid State Commun. 2008, 147, 266–270. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, J.; Xu, Z.Y.; Zheng, X.Q.; Hu, F.X.; Sun, J.R.; Shen, B.G. Simultaneous enhancements of Curie temperature and magnetocaloric effects in the La1-xCexFe11.5Si1.5Cy compounds. J. Magn. Magn. Mater. 2013, 324, 484–487. [Google Scholar] [CrossRef]
- Hu, F.X.; Gao, J.; Qian, X.L.; Ilyn, M.; Tishin, A.M.; Sun, J.R.; Shen, B.G. Magnetocaloric effect in itinerant electron metamagnetic systems La(Fe1-xCox)11.9Si1.1. J. Appl. Phys. 2005, 97, 10M303. [Google Scholar] [CrossRef]
- Paul-Boncour, V.; Nakouri, K.; Pattisier, A.; Bessais, L. Fast synthesis and magnetocaloric properties of La(Fe, Co, Si)13 compounds and their hydrides. In Proceedings of the 8th International Conference on Caloric Cooling (Thermag VIII), Darmstadt, Germany, 16–20 September 2018; pp. 593–598. [Google Scholar]
- Gebara, P.; Pawlik, P. Broadening of temperature working range inmagnetocaloric La(Fe,Co,Si)13- based multicomposite. J. Magn. Magn. Mater. 2017, 442, 145–151. [Google Scholar] [CrossRef]
- Pathak, A.K.; Basnyat, P.; Dubenko, I.; Stadler, S.; Ali, N. Influence of the small substitution of Z=Ni, Cu, Cr, V for Fe on the magnetic, magnetocaloric, and magnetoelastic properties of LaFe11.4Si1.6. J. Magn. Magn. Mater. 2010, 322, 692–697. [Google Scholar] [CrossRef]
- Barcza, A.; Katter, M.; Zellmann, V.; Russek, S.; Jacobs, S.; Zimm, C. Stability and Magnetocaloric Properties of Sintered La(Fe,Mn,Si)13Hz Alloys. IEEE Trans. Magn. 2011, 47, 3391–3394. [Google Scholar] [CrossRef]
- Anh, D.T.K.; Thuy, N.P.; Duc, N.H.; Nhien, T.T.; Nong, N.V. Magnetism and magnetocaloric effect in La1-yNdy(Fe0.88Si0.12)13 compounds. J. Magn. Magn. Mater. 2003, 262, 427–431. [Google Scholar]
- Fujita, A.; Fujieda, S.; Fukamichi, K. Control of Magnetocaloric Effects by Partial Substitution in Itinerant-Electron Metamagnetic La(FexSi1-x)13 for Application to Magnetic Refrigeration. IEEE Trans. Magn. 2009, 45, 2620–2625. [Google Scholar] [CrossRef]
- Fujita, A.; Fujieda, S.; Fukamichi, K. Changes in electronic states and magnetic free energy in La1-zCez(FexSi1-x )13 magnetic refrigerants. J. Phys. D Appl. Phys. 2011, 44, 064013. [Google Scholar] [CrossRef]
- Fujieda, S.; Fujita, A.; Fukamichi, K. Enhancements of magnetocaloric effects in La(Fe0.90Si0.10)13 and its hydride by partial substitution of Ce for La. Mater. Trans. 2004, 45, 3228–3231. [Google Scholar] [CrossRef]
- Fujieda, S.; Fujita, A.; Fukamichi, K.; Hirano, N.; Nagaya, S. Large magnetocaloric effects enhanced by partial substitution of Ce for La in La(Fe0.88Si0.12)13 compound. J. Alloys Compd. 2006, 408, 1165–1168. [Google Scholar] [CrossRef]
- Shen, B.G.; Sun, J.R.; Hu, F.X.; Zhang, H.W.; Cheng, Z.H. Recent Progress in Exploring Magnetocaloric Materials. Adv. Mater. 2009, 21, 4545–4564. [Google Scholar] [CrossRef]
- Shen, J.; Li, Y.X.; Sun, J.R.; Shen, B.G. Effect of R substitution on magnetic properties and magnetocaloric effects of La1-xRxFe11.5Si1.5 compounds with R=Ce, Pr and Nd. Chin. Phys. B 2009, 18, 2058–2062. [Google Scholar]
- Gebara, P.; Kovac, J. Magnetocaloric effect of the LaFe11.2Co0.7Si1.1 modified by partial substitution of La by Pr or Ho. Mater. Des. 2017, 129, 111–115. [Google Scholar] [CrossRef]
- Gebara, P.; Kovac, J. The influence of partial substitution of La by Dy on structure and thermomagnetic properties of the LaFe11.0Co0.7Si1.3 alloy. J. Magn. Magn. Mater. 2018, 454, 298–303. [Google Scholar] [CrossRef]
- Fujieda, S.; Fujita, A.; Fukamichi, K.; Yamazaki, Y.; Iijima, Y. Giant isotropic magnetostriction of itinerant-electron metamagnetic La(Fe0.88Si0.12)13Hy. Appl. Phys. Lett. 2001, 79, 653–655. [Google Scholar] [CrossRef]
- Chen, Y.F.; Wang, F.; Shen, B.G.; Wang, G.J.; Sun, J.R. Magnetism and magnetic entropy change of LaFe11.6Si1.4Cx (x = 0–0.6) interstitial compounds. J. Appl. Phys. 2003, 93, 1323–1325. [Google Scholar] [CrossRef]
- Rosca, M.; Balli, M.; Fruchart, D.; Gignoux, D.; Hill, E.K.; Miraglia, S.; Ouladdiaf, B.; Wolfers, P. Neutron diffraction study of LaFe11.31Si1.69 and LaFe11.31Si1.69H1.45 compounds. J. Alloys Compd. 2010, 490, 50–55. [Google Scholar] [CrossRef]
- Phejar, M.; Paul-Boncour, V.; Bessais, L. Investigation on structural and magnetocaloric properties of LaFe13-xSix(H,C)y compounds. J. Solid State Chem. 2016, 233, 95–102. [Google Scholar] [CrossRef]
- Gebara, P.; Cesnek, M.; Bednarcik, J. Anomalous behavior of thermal expansion of α-Fe impurities in the La(Fe,Co,Si)13- based alloys modified by Mn or selected lanthanides (Ce, Pr, Ho). Curr. Appl. Phys. 2019, 19, 188–192. [Google Scholar] [CrossRef]
- Paul-Boncour, V.; Nakouri, K.; Bessais, L. Optimization of Magnetocaloric Properties of Ball-Milled La(Fe,Co,Si)13(H,C)y. In Proceedings of the TMS 2019 148th Annual Meeting and Exhibition, San Antonio, TX, USA, 10–14 March 2019. [Google Scholar]
- Mandal, K.; Pal, D.; Gutfleisch, O.; Kerschi, P.; Müller, K. Magnetocaloric effect in reactively-milled LaFe11.57Si1.43Hy intermetalic compounds. J. Appl. Phys. 2007, 102, 053806. [Google Scholar] [CrossRef]
- Zhao, J.L.; Shen, J.; Hu, F.X.; Li, Y.X.; Sun, J.R.; Shen, B.G. Reduction of magnetic hysteresis loss in La0.5Pr0.5Fe11.4Si1.6Hx hydrides with large magnetocaloric effects. J. Appl. Phys. 2011, 107, 113911. [Google Scholar] [CrossRef]
- Li, J.Q.; Liu, F.S.; Ao, W.Q.; Zhuang, Y.H.; Zhou, K.W. Influence of carbon on the giant magnetocaloric effect of LaFe11.7Si1.3. Rare Met. 2006, 25, 556–561. [Google Scholar] [CrossRef]
- Wang, Z.C.; He, L.H.; Cuevas, F.; Shen, M.L.J.; Wang, F.W. Hydrogenation, structure and magnetic properties of La(Fe0.91Si0.09)13 hydrides and deuterides. Chin. Phys. B 2011, 20, 067502. [Google Scholar] [CrossRef]
- Baumfeld, O.L.; Gercsi, Z.; Krautz, M.; Gutfleisch, O.; Sandeman, K.G. The dynamics of spontaneous hydrogen segregation in LaFe13-xSixHy. J. Appl. Phys. 2014, 115, 203905. [Google Scholar] [CrossRef]
- Krautz, M.; Skokov, K.; Gottschall, T.; Teixeira, C.S.; Waske, A.; Liu, J.; Schultz, J.L.; Gutfleisch, O. Systematic investigation of Mn substituted La(Fe,Si)13 alloys and their hydrides for room-temperature magnetocaloric application. J. Alloys Compd. 2014, 598, 27–32. [Google Scholar] [CrossRef]
- Piazzi, M.; Bennati, C.; Curcio, C.; Kuepferling, M.; Basso, V. Modeling specific heat and entropy change in La(Fe-Mn-Si)13-H compounds. J. Magn. Magn. Mater. 2016, 400, 349–355. [Google Scholar] [CrossRef]
- Morrison, K.; Sandeman, K.G.; Cohen, L.F.; Sasso, C.P.; Basso, V.; Barcza, A.; Katter, M.; Moore, J.D.; Skokov, K.P.; Gutfleisch, O. Evaluation of the reliability of the measurement of key magnetocaloric properties: A round robin study of La(Fe,Si,Mn)Hδ conducted by the SSEEC consortium of European laboratories. Int. J. Refrig. Rev. Int. Froid 2012, 35, 1528–1536. [Google Scholar] [CrossRef]
- Mu, L.; Huang, J.; Zhang, W.; Liu, C.; Wang, G.; Zhao, Z. Influence of partial substitution of cerium for lanthanum on magnetocaloric properties of La1-xCexFe11.44Si1.56 and their hydrides. J. Rare Earth 2014, 32, 1135–1139. [Google Scholar] [CrossRef]
- Hai, X.Y.; Mayer, C.; Colin, C.V.; Miraglia, S. In-situ neutron investigation of hydrogen absorption kinetics in La(Fe1-xSix)13 magnetocaloric alloys for room-temperature refrigeration application. J. Magn. Magn. Mater. 2016, 400, 344–348. [Google Scholar] [CrossRef]
- Hai, X.Y.; Porcher, F.; Mayer, C.; Miraglia, S. Structural effects in the interstitial solid solution system (La,Ce)(Fe,Si)13CxH: Correlation with hydrogenation kinetics. J. Appl. Phys. 2018, 123, 7. [Google Scholar] [CrossRef]
- Xia, W.; Huang, J.; Sun, N.; Lui, C.; Ou, Z.; Song, L. Influence of powder bonding on mechanical properties and magnetocaloric effects of La0.9Ce0.1(Fe,Mn)11.7Si1.3H1.8. J. Alloys Compd. 2015, 635, 124–128. [Google Scholar] [CrossRef]
- Sun, N.; Zhang, Y.; Zhao, X.; Guo, J.; Cheng, J.; Huang, J.; Zhang, Z. Microstructure, mechanical and magnetocaloric properties of bulk La0.9Ce0.1Fe11.7-xMnxSi1.3 hydrides prepared by high-hydrogen-pressure sintering. J. Magn. Magn. Mater. 2020, 495, 165889. [Google Scholar] [CrossRef]
- Brown, G.V. Magnetic heat pumping near room-temperature. J. Appl. Phys. 1976, 47, 3673. [Google Scholar] [CrossRef]
- Rowe, A.; Tura, A. Experimental investigation of a three-material layered active magnetic regenerator. Int. J. Refrig. 2006, 29, 1286–1293. [Google Scholar] [CrossRef]
- Balli, M.; Sari, O.; Zamni, L.; Mahmed, C.; Forchelet, J. Implementation of La(Fe, Co)13-xSix materials in magnetic refrigerators: Practical aspects. Mater. Sci. Eng. B Adv. 2012, 177, 629–634. [Google Scholar] [CrossRef]
- Tusek, J.; Kitanovski, A.; Poredos, A. Geometrical optimization of packed-bed and parallel-plate active magnetic regenerators. Int. J. Refrig. 2013, 36, 1456–1464. [Google Scholar] [CrossRef]
- Legait, U.; Guillou, F.; Kedous-Lebouc, A.; Hardy, V.; Almanza, M. An experimental comparison of four magnetocaloric regenerators using three different materials. Int. J. Refrig. 2014, 37, 147–155. [Google Scholar] [CrossRef]
- Pulko, B.; Tusek, J.; Moore, J.D.; Weise, B.; Skokov, K.; Mityashkin, O.; Kitanovski, A.; Favero, C.; Fajfar, P.; Gutfleisch, O.; et al. Epoxy-bonded La-Fe-Co-Si magnetocaloric plates. J. Magn. Magn. Mater. 2015, 375, 65–73. [Google Scholar] [CrossRef]
- Vasile, C.; Muller, C. Innovative design of a magnetocaloric system. Int. J. Refrig. 2006, 29, 1318–1326. [Google Scholar] [CrossRef]
- Forchelet, J.; Zamni, L.; El Alami, S.E.; Hu, J.; Balli, M.; Sari, O. Corrosion behavior of gadolinium and La-Fe-Co-Si compounds in various heat conducting fluids. Int. J. Refrig. 2014, 37, 307–313. [Google Scholar] [CrossRef]
- Aprea, C.; Greco, A.; Maiorino, A.; Masselli, C. Analyzing the energetic performances of AMR regenerator working with different magnetocaloric materials: Investigations and viewpoints. Int. J. Heat Technol. 2017, 35, S383–S390. [Google Scholar] [CrossRef]
- Aprea, C.; Greco, A.; Maiorino, A.; Masselli, C. The environmental impact of solid-state materials working in an active caloric refrigerator compared to a vapor compression cooler. Int. J. Heat Technol. 2018, 36, 1155–1162. [Google Scholar] [CrossRef]
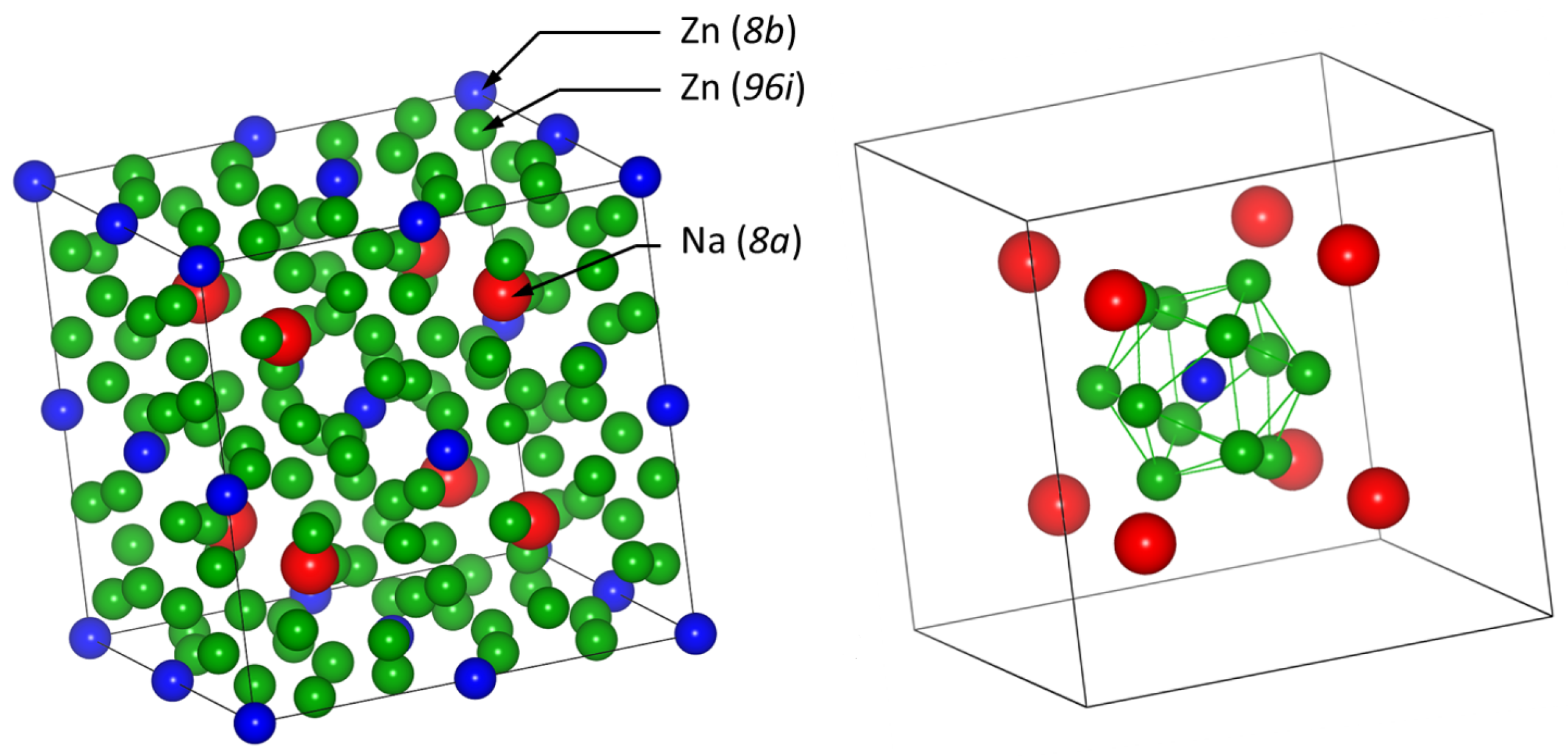

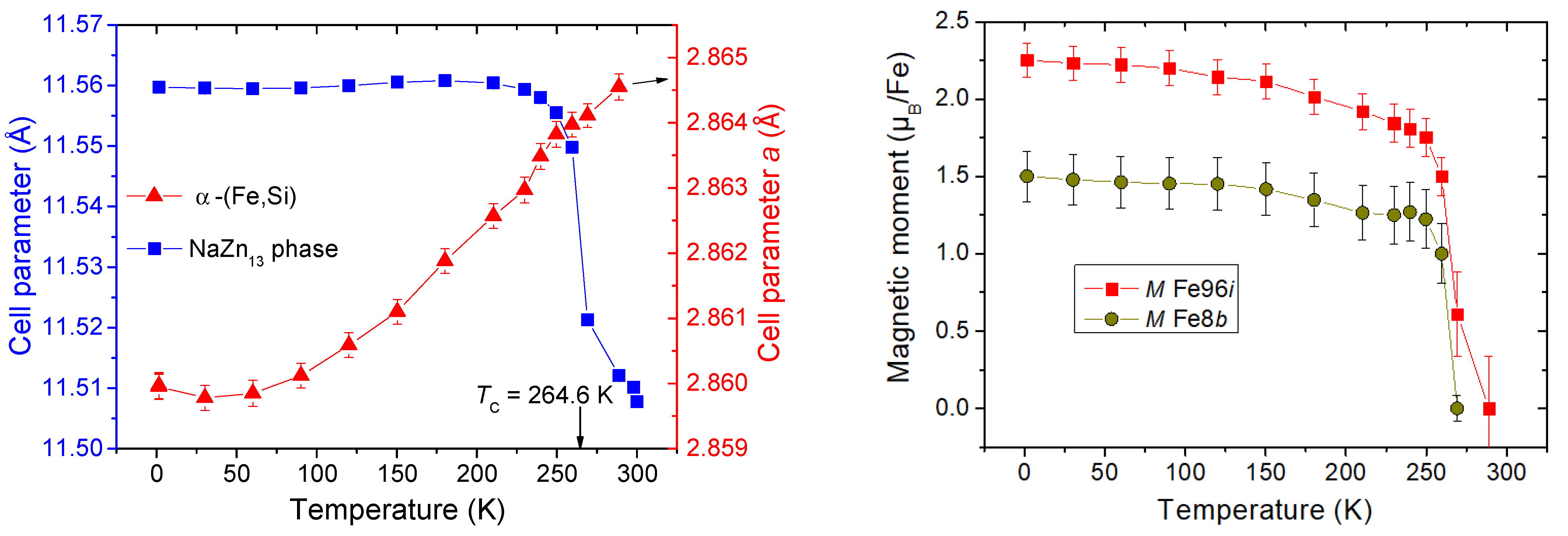
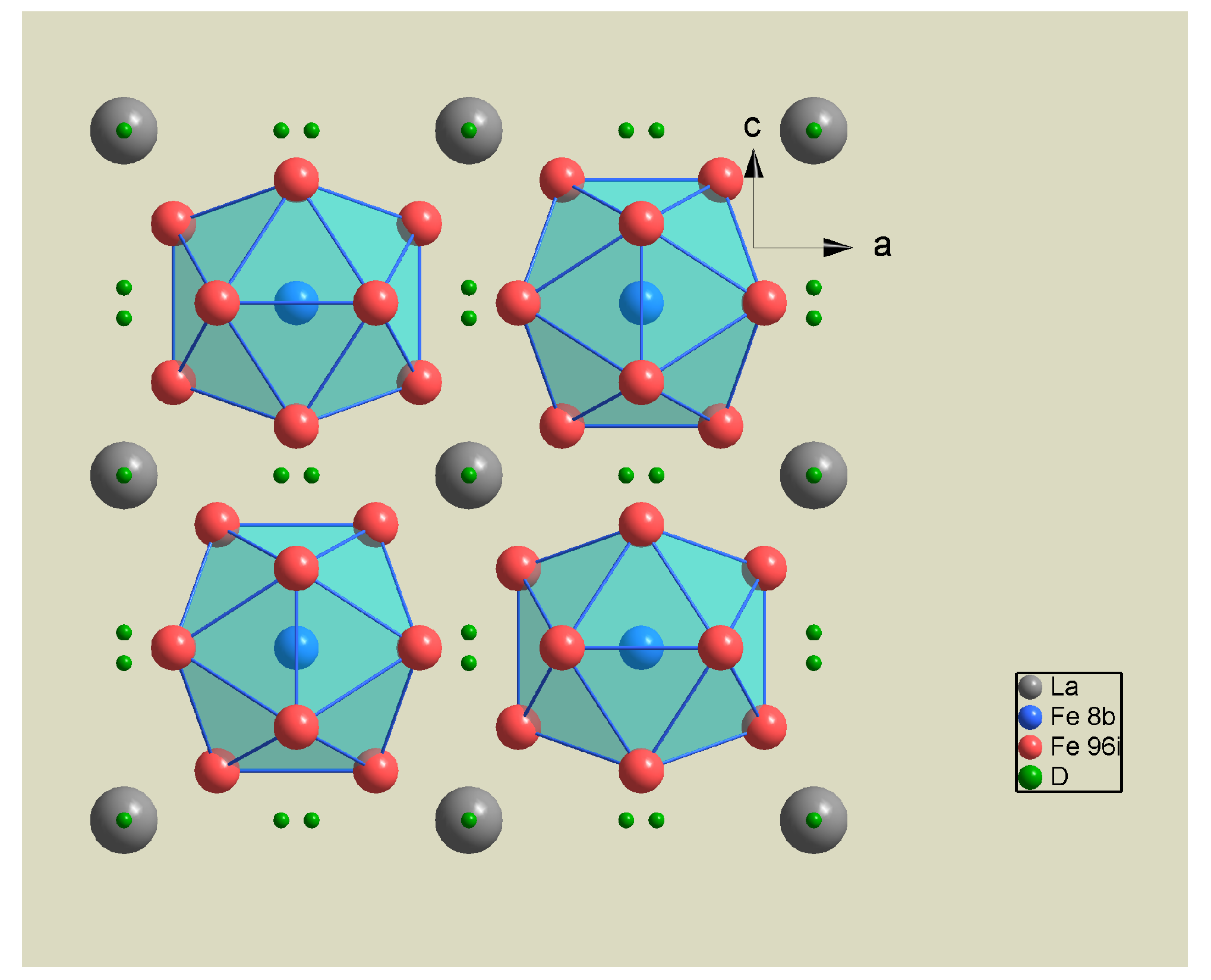
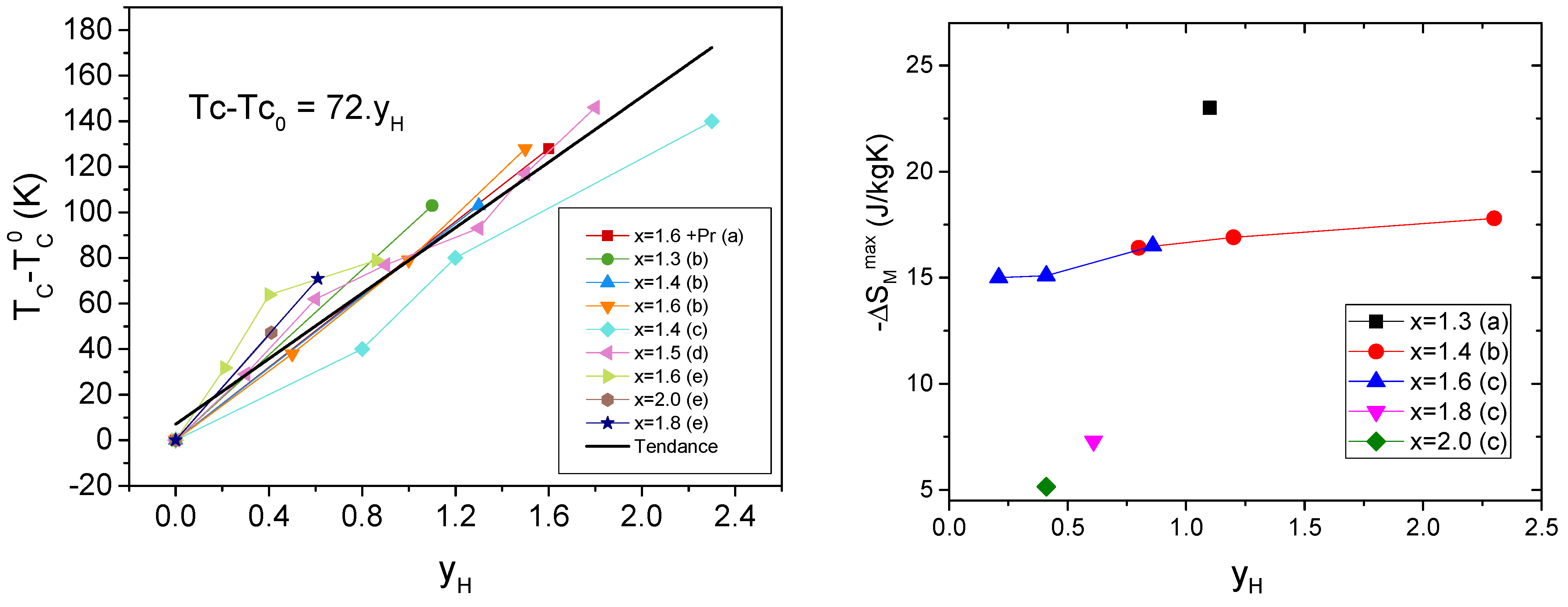
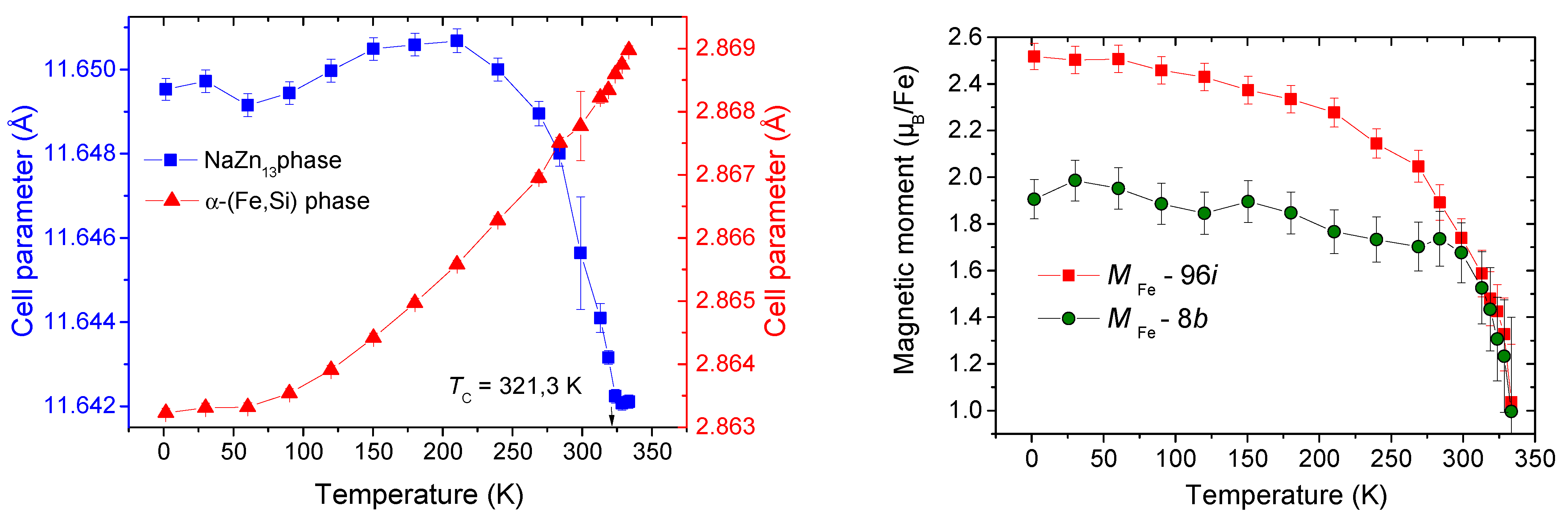
| Phase | Pearson | Space Group | Atm. | Wyck. | Sym. | Position |
|---|---|---|---|---|---|---|
| NaZn | cF112 | La | 432 | 1/4, 1/4, 1/4 | ||
| Fe | 0, 0, 0 | |||||
| Fe, Si | 0, 0.1806, 0.1192 | |||||
| CeNiSi | tI56 | La | 422 | 0, 0, 1/4 | ||
| Fe1 | 0, 1/2, 0 | |||||
| Fe2 | 0.2024, 0.0691, 0 | |||||
| Fe3 | 0.1294, 0.6294, 0.1832 | |||||
| Si | 0.33, 0.83, 0.118 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul-Boncour, V.; Bessais, L. Tuning the Magnetocaloric Properties of the La(Fe,Si)13 Compounds by Chemical Substitution and Light Element Insertion. Magnetochemistry 2021, 7, 13. https://doi.org/10.3390/magnetochemistry7010013
Paul-Boncour V, Bessais L. Tuning the Magnetocaloric Properties of the La(Fe,Si)13 Compounds by Chemical Substitution and Light Element Insertion. Magnetochemistry. 2021; 7(1):13. https://doi.org/10.3390/magnetochemistry7010013
Chicago/Turabian StylePaul-Boncour, Valérie, and Lotfi Bessais. 2021. "Tuning the Magnetocaloric Properties of the La(Fe,Si)13 Compounds by Chemical Substitution and Light Element Insertion" Magnetochemistry 7, no. 1: 13. https://doi.org/10.3390/magnetochemistry7010013
APA StylePaul-Boncour, V., & Bessais, L. (2021). Tuning the Magnetocaloric Properties of the La(Fe,Si)13 Compounds by Chemical Substitution and Light Element Insertion. Magnetochemistry, 7(1), 13. https://doi.org/10.3390/magnetochemistry7010013