Abstract
This paper reports the syntheses, crystal structures and magnetic properties of Mn(III) hexadentate Schiff base complexes [Mn(4-OH-sal-N-1,5,8,12)]NO3 (1) and [Mn(4-OH-sal-N-1,5,8,12)]ClO4 (2), where (4-OH-sal-N-1,5,8,12)2− (4,4′-((1E,13E)-2,6,9,13-tetraazatetradeca-1,13-diene-1,14-diyl)bis(3-methoxyphenol) is a new hydroxyl-substituted hexadentate Schiff base ligand. The introduction of the (4-OH-sal-N-1,5,8,12)2− ligand induces more hydrogen bonding interactions, in addition to promoting the formation of intermolecular interactions among the cations. However, the close-packing structures of both complexes lead to their stabilization in the high-spin state in the temperature range of 2−300 K.
1. Introduction
Spin crossover (SCO), which is a hot topic in modern materials science [1,2,3,4,5,6], occurs in octahedral transition metal complexes with electron configurations of 3d4−3d7. The phenomenon between a low-spin (LS) and a high-spin (HS) state may be triggered by the use of external stimuli, such as light, heat, magnetic field, and pressure [7,8,9,10,11].
Schiff bases are used widely because they are readily derivatized and generally easy to prepare. As versatile ligands, they can stabilize a wide range of geometries and oxidation states in transition metal complexes. This great diversity may permit the design of Schiff bases with a suitable ligand field strength to obtain Mn(III) SCO complexes. Notably, the first SCO d4 system [Mn(pyrol)3tren] with a hexadentate N6 Schiff base ligand was designed in 1981 by Sinn and Sim [12], breaking the conventional wisdom that it was too difficult to generate an amenable ligand-field strength to stabilize an LS state in Mn(III) complexes. Later, the prototypic gradual SCO phenomenon of a Mn(III) hexadentate N4O2 Schiff base complex was reported in 2006 by Morgan et al. [13] Subsequently, another ligand system, a tridentate Schiff base N2O ligand provided the third example of Mn(III) SCO compounds [14].
Mn(III) hexadentate Schiff base compounds with N4O2 donor sets are extensively studied [13,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30] from various aspects such as ligand substitution [31], the nature of counter anions [32,33,34], and the cocrystallized solvent molecules [35,36,37,38]. The chemical influences affect intermolecular interactions like crystal packing and hydrogen bonding, which offer more possibilities for cooperative SCO behaviors. In previous reports, we reported a series of Mn(III) hexadentate Schiff base compounds [Mn(sal-N-1,5,8,12)]Y (Y = Cl−, PF6−, AsF6−, SbF6−, and NO3−) [25] to confirm that the counter anion effects are clearly related to the SCO behavior. Moreover, cocrystallized solvent molecules affect the SCO profoundly. For instance, the complexes [Mn(3,5-diBr-sal-N-1,5,8,12)]ClO4·C2H5OH and [Mn(3,5-diBr-sal-N-1,5,8,12)] ClO4·0.5CH3CN show different SCO behaviors [19]. The ethanol solvate has a more complete SCO while the latter persists in the LS state over a considerable temperature range. Besides, the analysis of Mn(III) complexes with various hexadentate Schiff base ligands, such as (3-OMe-sal-N-1,5,8,12)2− [22], (5-OMe-sal-N-1,5,8,12)2− [24,30], (5-Br-sal-N-1,5,8,12)2− [18] and (naphth-sal-N-1,5,8,12)2− [29], has emphasized the impact of the substituent effects.
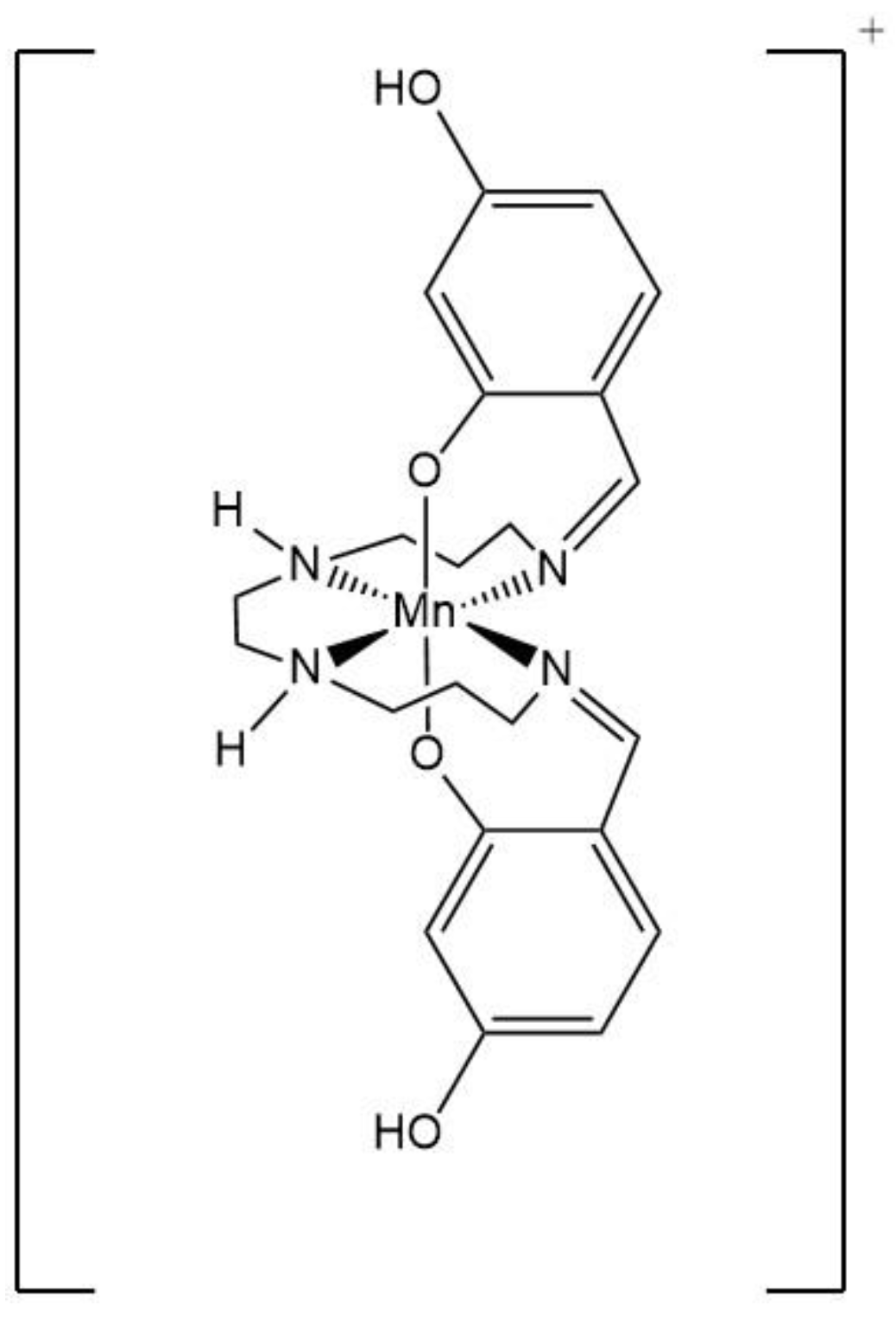
In addition, the report on the 4-position of salicylaldehyde of hexadentate Schiff base ligands is rare; however, a series of Mn(III) SCO compounds [Mn(4-R-sal-N-1,5,8,12)]Y (R = OC6H13, OC12H25, OC18H37) with gradual and uncompleted SCO behavior were reported by Albrecht and his co-workers [16]. Under this circumstance, we study to obtain complexes that can provide strong cooperation interactions and more complete SCO behaviors. Therefore, the complexes [Mn(4-OH-sal-N-1,5,8,12)]Y (Y = NO3− and ClO4−) (Scheme 1) were selected with the following main goals: increase the number of hydrogen bonds through the hydroxyl group, in order to strengthen the connection between anions and cations and improve the cooperativity of the system.
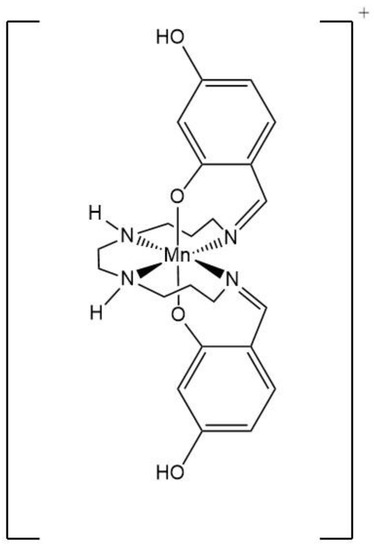
Scheme 1.
Structural unit of the cation [Mn(4-OH-sal-N-1,5,8,12)]+.
Herein, we report the synthesis, X-ray crystal structures, and magnetic properties of two isostructural compounds with the general formula [Mn(4-OH-sal-N-1,5,8,12)]Y (Y = NO3− and ClO4−). Correlations between properties and structures are discussed with respect to the alteration of the geometry and hydrogen-bonding acceptor of the anions.
2. Experimental Section
All of the reagents and chemicals were analytically pure, purchased from commercial sources, and were used without further purification. Although we experienced no problems with the compounds reported in this work, the manganese perchlorate salt with the organic ligand is potentially explosive and should be handled with great care and used in small amounts. Elemental analyses of C, H, and N were performed on a Vario EL III elemental analyzer. IR spectra of the solid samples (KBr tablets) in the range 400−4000 cm−1 were recorded by an FT-IR Perkin Elmer spectrometer. The properties of the Hirshfeld surface for complexes 1 and 2 were generated using a CrystalExplorer 17.5. The Hirshfeld surface was generated using a high resolution and mapped with the dnorm and shape-indexed functions. 2D fingerprint plots were prepared using the same software. Magnetic susceptibility was measured in a sweep mode upon cooling from 300 to 2 K under a 0.1 T applied magnetic field by the use of a Quantum Design MPMS SQUID VSM magnetometer. A freshly prepared crystalline sample was placed in a gelatin capsule holder. Magnetic data were calibrated with the sample holder and diamagnetic corrections were estimated from Pascal’s constants. Magnetic behavior was primarily analyzed by using variable-temperature magnetic susceptibility measurements.
[Mn(4-OH-sal-N-1,5,8,12)]NO3 (1) 2,4-dihydroxybenzaldehyde (31.4 mg, 0.228 mmol) and N,N-bis(3-aminopropyl)ethylenediamine (21.4 mg, 0.114 mmol) dissolved in methanol (6 mL), and then solid manganese(II) nitrate tetrahydrate (33.4 mg, 0.114 mmol) was added. The resulting dark brown solution was stirred for half an hour and then filtered. Black crystals formed upon evaporation of the solvent (56.9%). Anal. calcd for C22H28MnN5O7: C, 49.91; H, 5.33; N, 13.23. Found: C, 49.86; H, 5.35; N, 13.16. IR (cm−1): 3550 (m, sh), 1613 (m, sh), 1338 (m, sh).
[Mn(4-OH-sal-N-1,5,8,12)]ClO4 (2) 2,4-dihydroxybenzaldehyde (45.8 mg, 0.332 mmol) and N,N-bis(3-aminopropyl)ethylenediamine (28.9 mg,0.166 mmol) dissolved in methanol (6 mL), and then solid manganese(II) perchlorate hexahydrate (41.9 mg, 0.166 mmol) was added. The resulting dark brown solution was stirred for half an hour and then filtered. Black crystals formed upon evaporation of the solvent (63.8%). Anal. calcd for C22H28ClMnN4O8: C, 46.61; H, 4.98; N, 9.88. Found: C, 46.66; H, 4.94; N, 9.83. IR (cm−1): 3552 (m, sh), 1618 (m, sh), 1076 (st,sh), 619 (m, b).
3. Results and Discussion
3.1. Crystallographic Studies
Single-crystal X-ray diffraction data for the compounds [Mn(4-OH-sal-N-1,5,8,12)]NO3 (1) and [Mn(4-OH-sal-N-1,5,8,12)]ClO4 (2) were collected at 100 and 298 K, respectively. Table 1 presents the most relevant parameters for single-crystal determination.

Table 1.
Selected crystallographic data for complexes 1 and 2.
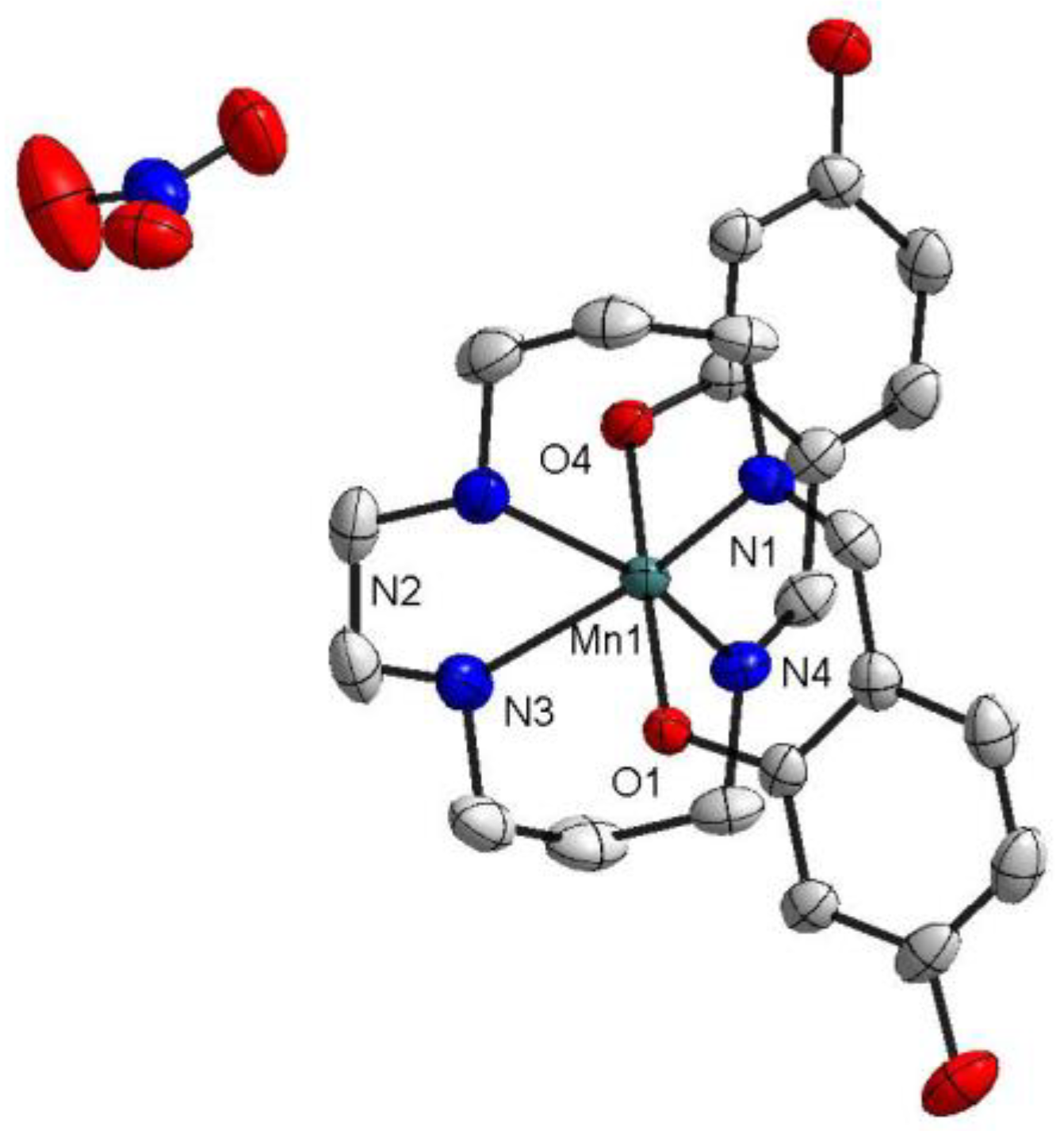
At both temperatures (100 and 298 K), the two isomorphic complexes crystallized in the triclinic space group. The asymmetric unit of 1 contained one [Mn(4-OH-sal-N-1,5,8,12)]+ cation and one NO3− counter anion (Figure 1), while one [Mn(4-OH-sal-N-1,5,8,12)]+ cation and one ClO4− anion were observed in the structure of 2 (Figure 2). In these compounds, the Mn3+ ion was coordinated pseudo-octahedrally by trans-O (O1 and O2) donors in the axial position and pairs of cis-imine (N1 and N4) and cis-amine (N2 and N3) in the equatorial plane. The Mn-Nimine (N1 and N4) (2.075–2.135 Å), Mn-Namine (N2 and N3) (2.233–2.291 Å), and Mn-O (1.864–1.880 Å) bond lengths at 100 K were in greatagreement with those observed in other HS Mn(III) hexadentate Schiff base complexes. Moreover, the bond lengths of the complexes at 298 K had no significant change compared with those at 100 K (Table 2).
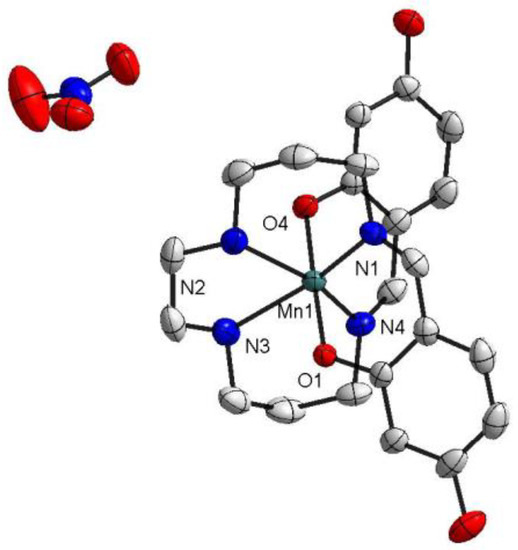
Figure 1.
The structure of [Mn(4-OH-sal-N-1,5,8,12)]NO3 at T = 100 K. Ellipsoids are drawn at the 50% probability level. Hydrogen atoms were omitted for clarity.
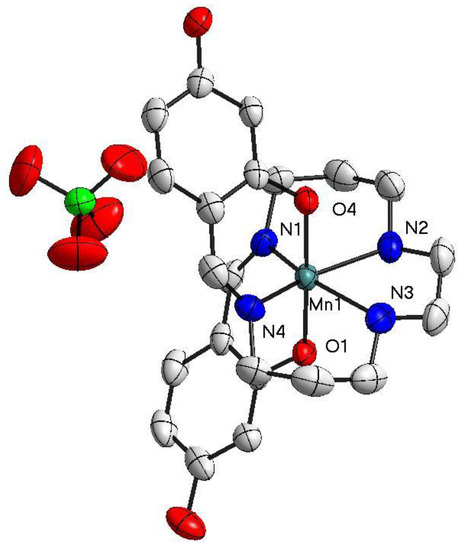
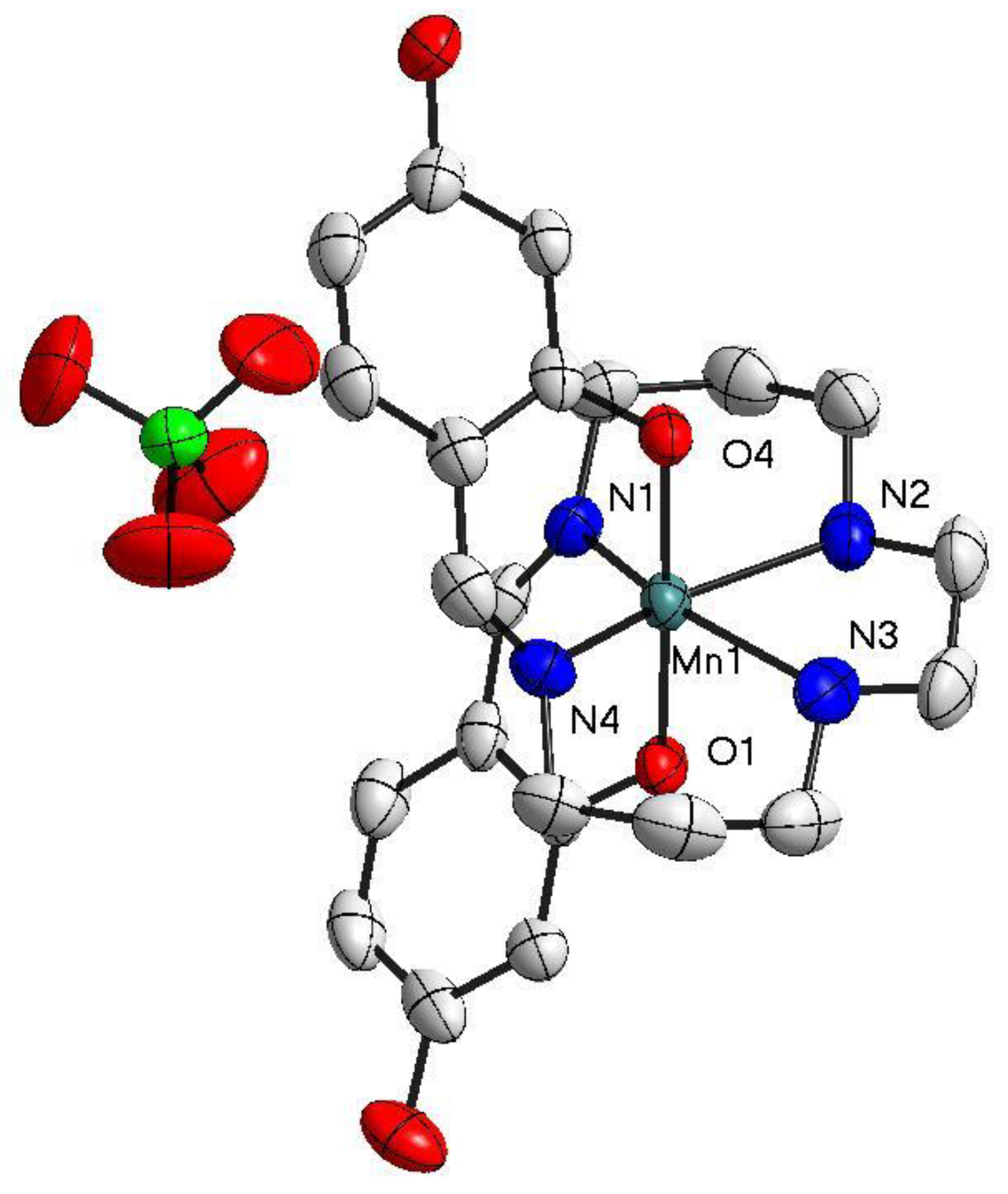
Figure 2.
The structure of [Mn(4-OH-sal-N-1,5,8,12)]ClO4 at T = 100 K. Ellipsoids are drawn at the 50% probability level. Hydrogen atoms were omitted for clarity.

Table 2.
Selected bond distances (Å), angles (°), and octahedral distortion parameters (°) for complexes 1 and 2.
The octahedral distortion parameters Σ and θ can intuitively reflect the changes of the Mn(III) coordination sphere. For complex 1, θ was from 292.38° at 100 K to 291.98° at 298 K, while the change in Σ was small, with values of 79.59° and 79.41°, respectively. Both parameters, with a little change, further demonstrate that there was no SCO behavior in complex 1. The central Mn3+ ion of compound 2, which is similar to complex 1, was furnishing a distorted octahedral geometry (θ = 289.93° and Σ = 77.66° at 100 K; θ = 294.06° and Σ = 78.90° at 298 K).
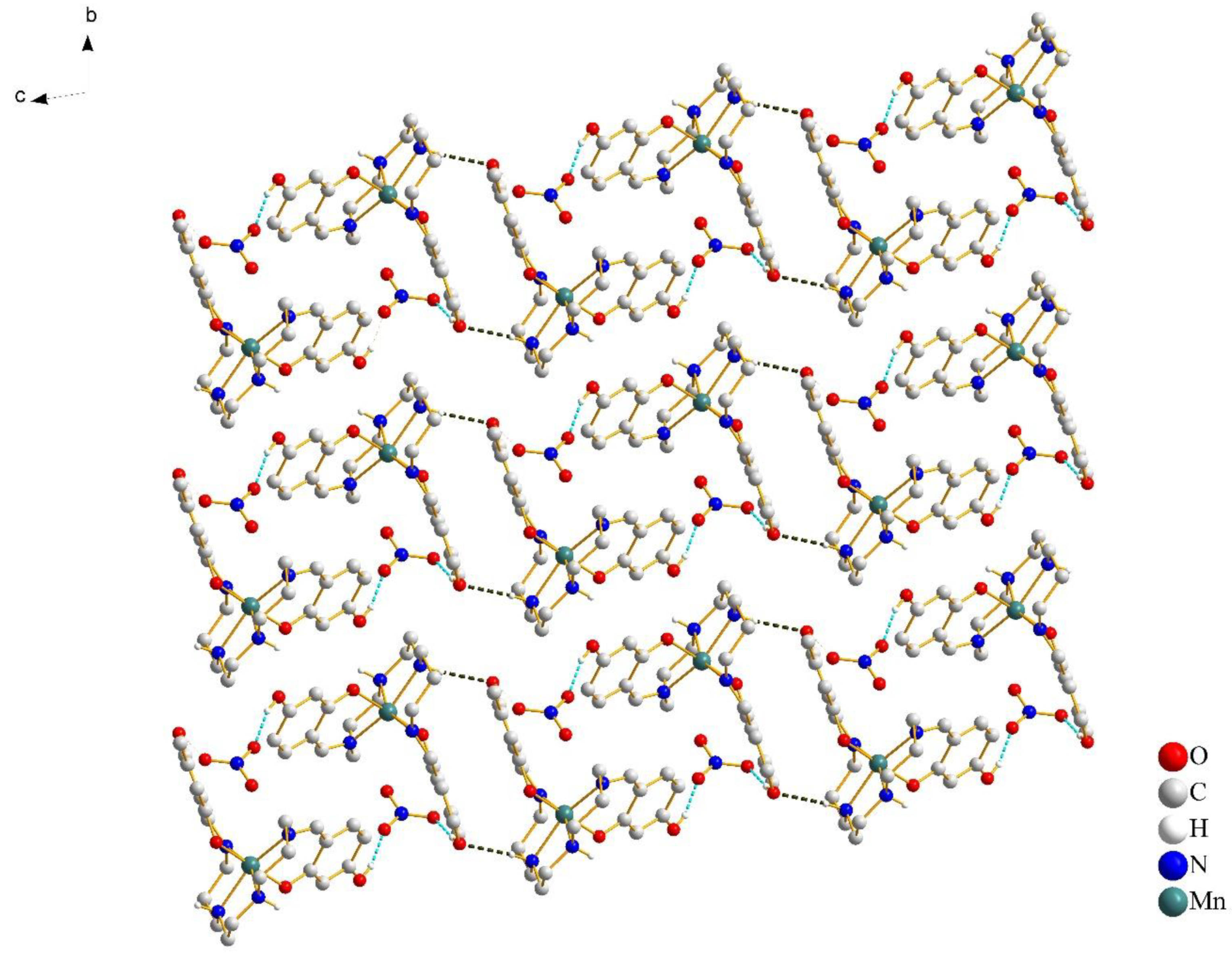
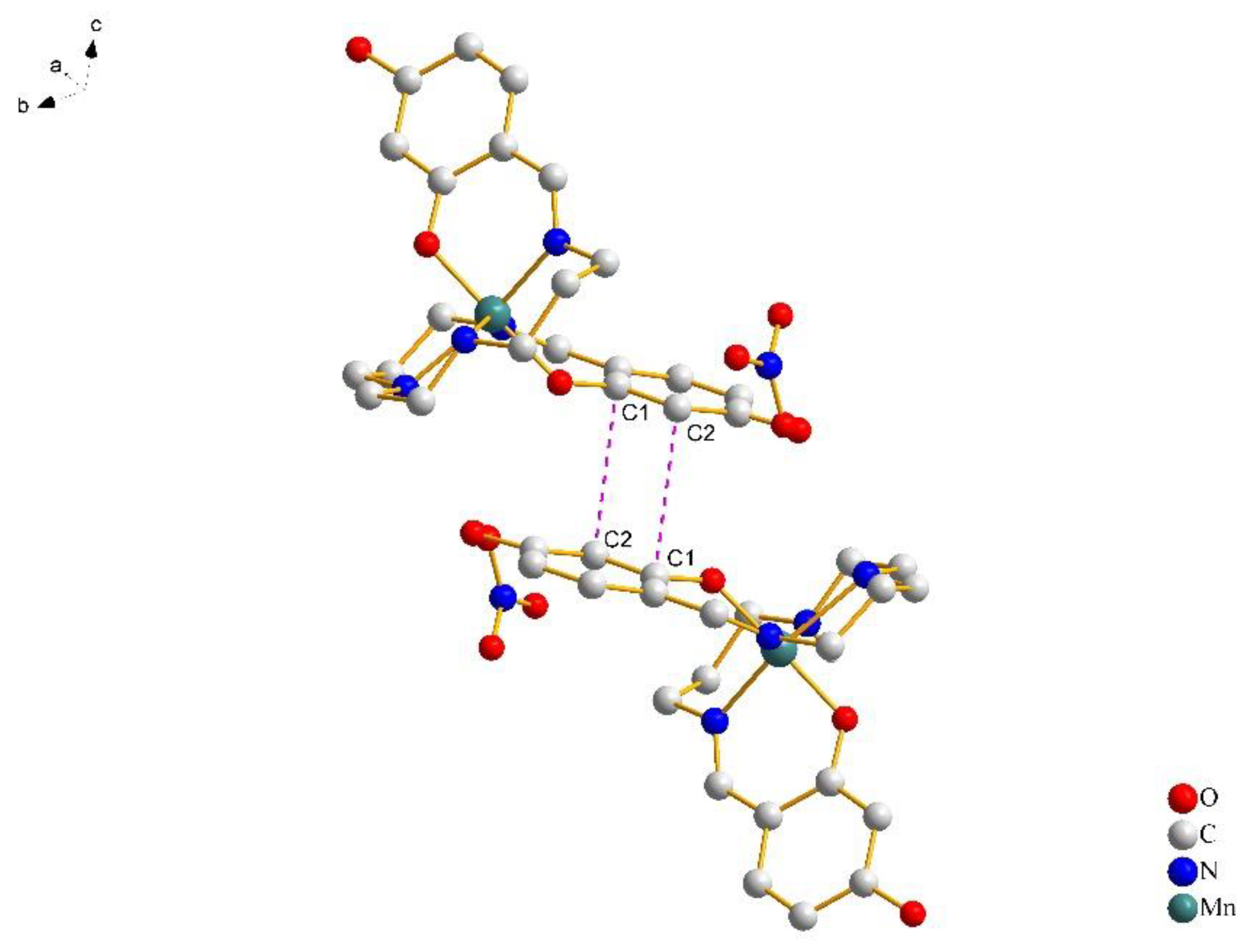
In Figure 3, a pair of [Mn(4-OH-sal-N-1,5,8,12)]+ cations form a centrosymmetric dimer through the N–H···O hydrogen bonds between the amino nitrogen atoms and peripheral hydroxy oxygen atoms. Moreover, weak edge-to-edge π···π contacts exist between the two sets of C(1)–C(2) atoms (Figure 4), and stabilize the dimer structure.
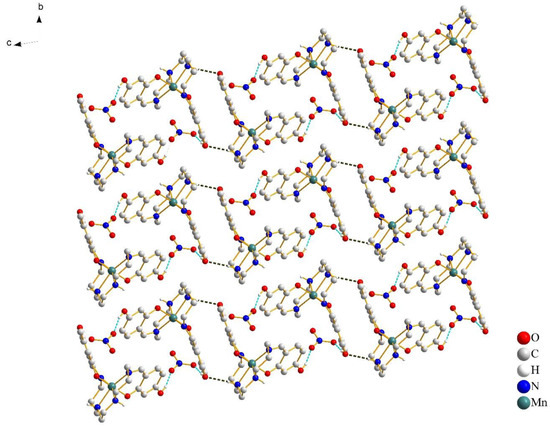
Figure 3.
Crystal packing of complex 1 at 100 K, viewed along the a-axis. The cation–anion O–H···O hydrogen bonds are shown with cyan blue dash lines and the cation–cation N–H···O hydrogen bonds are shown with black lines. Most of the hydrogen atoms were omitted for clarity, except for those involved in hydrogen bonds.
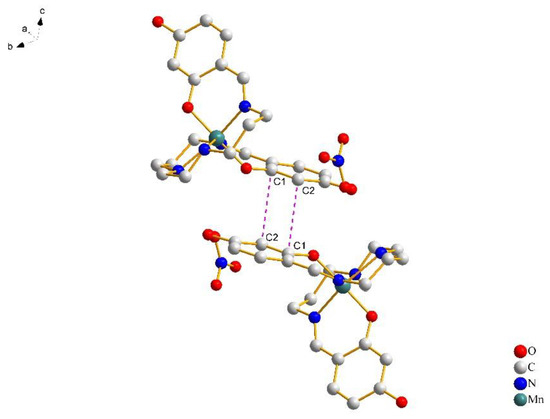
Figure 4.
The details of π···π stacking interactions between phenolate rings of complex 1 at 100 K. Hydrogen atoms were omitted for clarity.
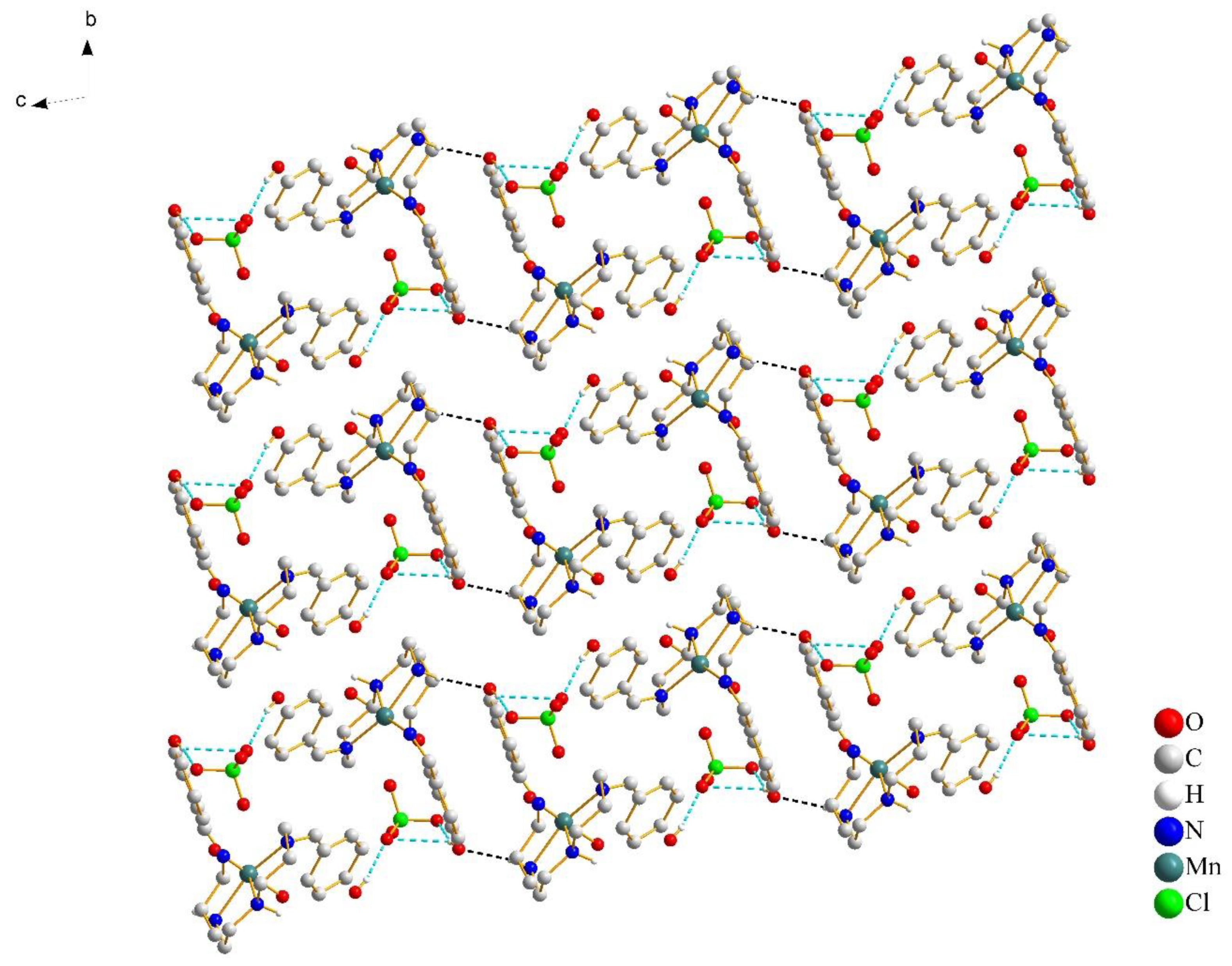
The anions connected cationic dimers located in chains via the O–H···O hydrogen bonds (Figure 3). The supramolecular chains were relatively independent and extended infinitely in the bc-plane. With the increase of the temperature, there was no significant change in the strength of the hydrogen bonds (Table 3). The intrachain Mn-Mn separation was 8.337 Å at 100 K and 8.349 Å at 298 K. However, the formation of dimers resulted in the short interchain Mn···Mn distance, which was 6.709 Å at 100 K and 6.749 Å at 298 K, respectively. The close Mn–Mn distance gave the [Mn(4-OH-sal-N-1,5,8,12)]+ cation less space to change the coordination geometry.

Table 3.
Hydrogen bond distances and parameters for the complexes of 1 and 2 (Å, °).
As for complex 2, increasing the size of the anion from planar NO3− to tetrahedral ClO4− resulted in more intricate interconnections, but there was no change in crystal packing. Moreover, the formations of supramolecular dimers indicate the close contacts between the [Mn(4-OH-sal-N-1,5,8,12)]+ cations. The existence of O(2)–H(2)···O(7), O(7)–H(27)···O(3) and O(6)–H(27)···O(3) hydrogen bonds contributed to the close stacking between the anions and cations, which hindered the flexibility of the whole ligand and prevented the distortion of the Mn(III) coordination geometry (Figure 5). In addition, the interchain Mn-Mn separation changed from 6.841 at 298 K to 6.740 Å at 100 K because of its bigger anion size. However, this did not give the Mn(III) cation enough space to change its conformation to meet the structural requirements of SCO.
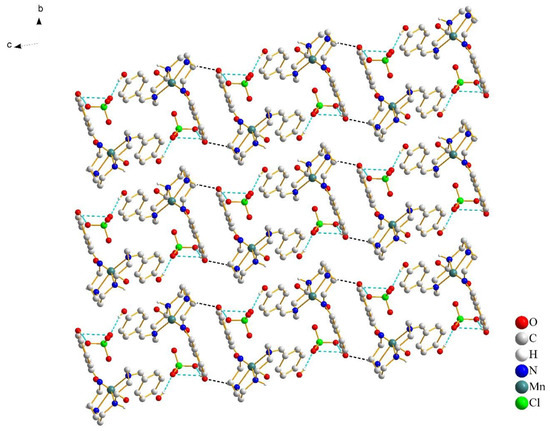
Figure 5.
Crystal packing of complex 2 at 100 K, viewed along the a-axis. The cation–anion O–H···O hydrogen bonds are shown with cyan blue dash lines and the cation–cation N–H···O hydrogen bonds are shown with black lines. Most of the hydrogen atoms were omitted for clarity, except for those involved in hydrogen bonds.
3.2. Hirshfeld Surface Analysis
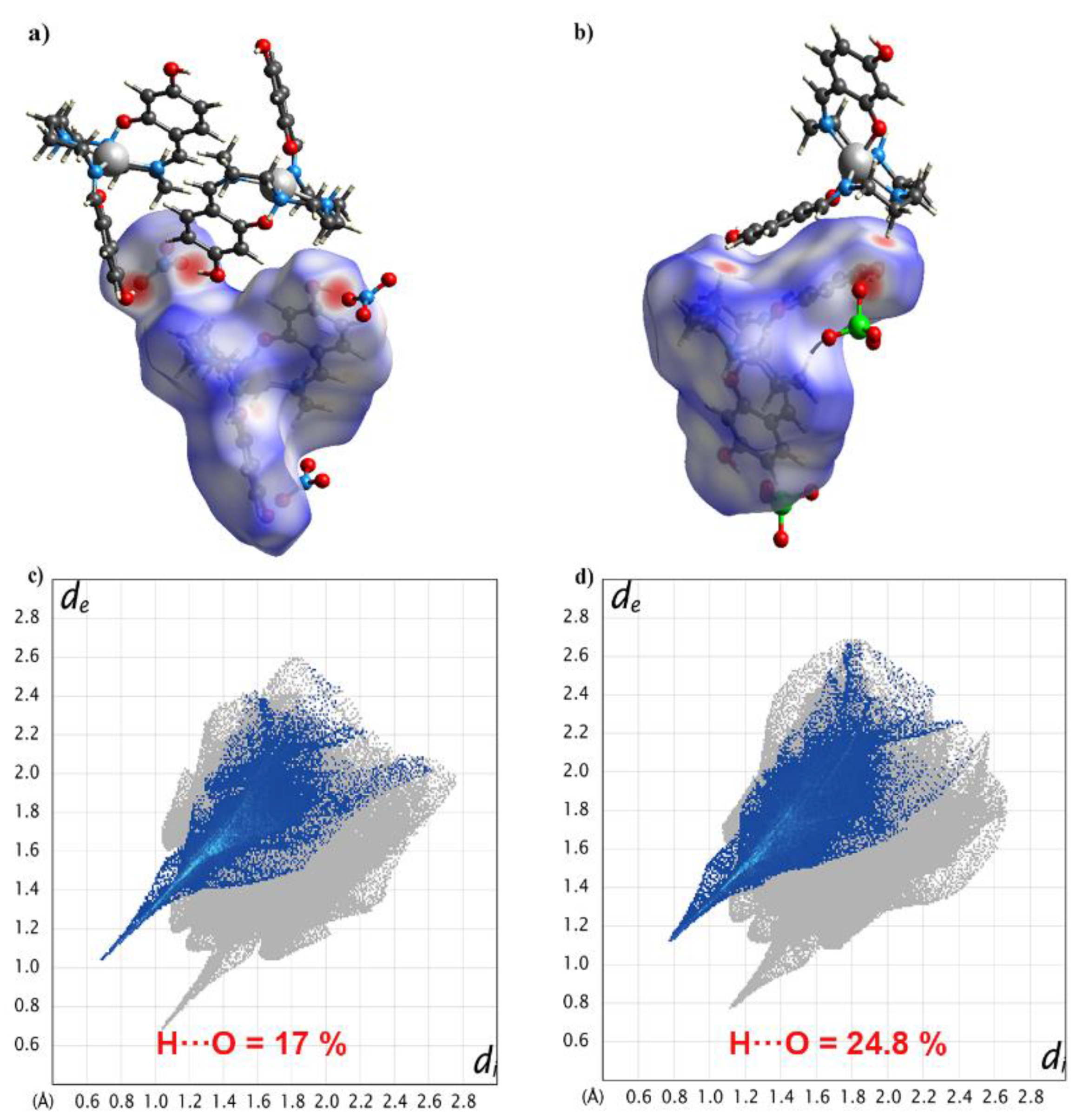
To gain deeper insight into the supramolecular contacts in 1 and 2, we undertook Hirshfeld surface analysis using CrystalExplorer 17.5. The Hirshfeld surfaces for the cations of complexes 1 and 2 were mapped with the dnorm function [39,40], which shows several red spots. For 1, the four strongest red spots were due to N–H···O and O–H···O hydrogen bonding interactions, and the weak red spots were due to C–H···O interactions (Figure 6a). The hydrogen bond was one of the major interactions here, contributing to 17% of all interactions in Figure 6c.
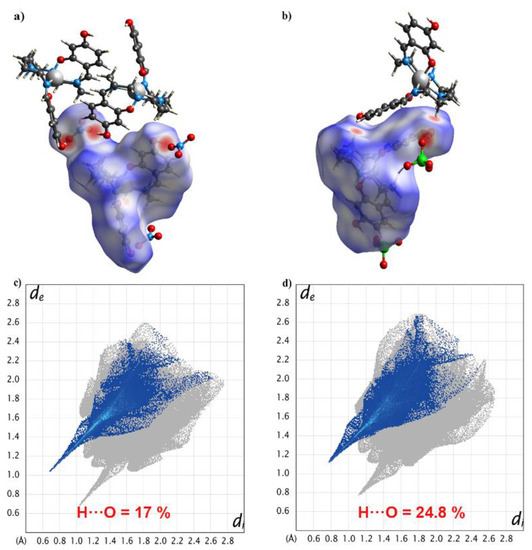
Figure 6.
The N–H···O and O–H···O hydrogen bonding interactions in 1 (a) and 2 (b) through Hirshfeld surface mapping by dnorm function. 2D fingerprint plots of all contacts: H···O for 1 (c) and 2 (d) at 298 K.
For complex 2, owing to the change in anions, the hydrogen bond contributed to 24.8% of all interactions (Figure 6d). This supports the discussion above and suggests that the OH group in the (4-OH-sal-N-1,5,8,12)2− ligand is critical in linking the [Mn(4-OH-sal-N-1,5,8,12)]+ cations together in these structures.
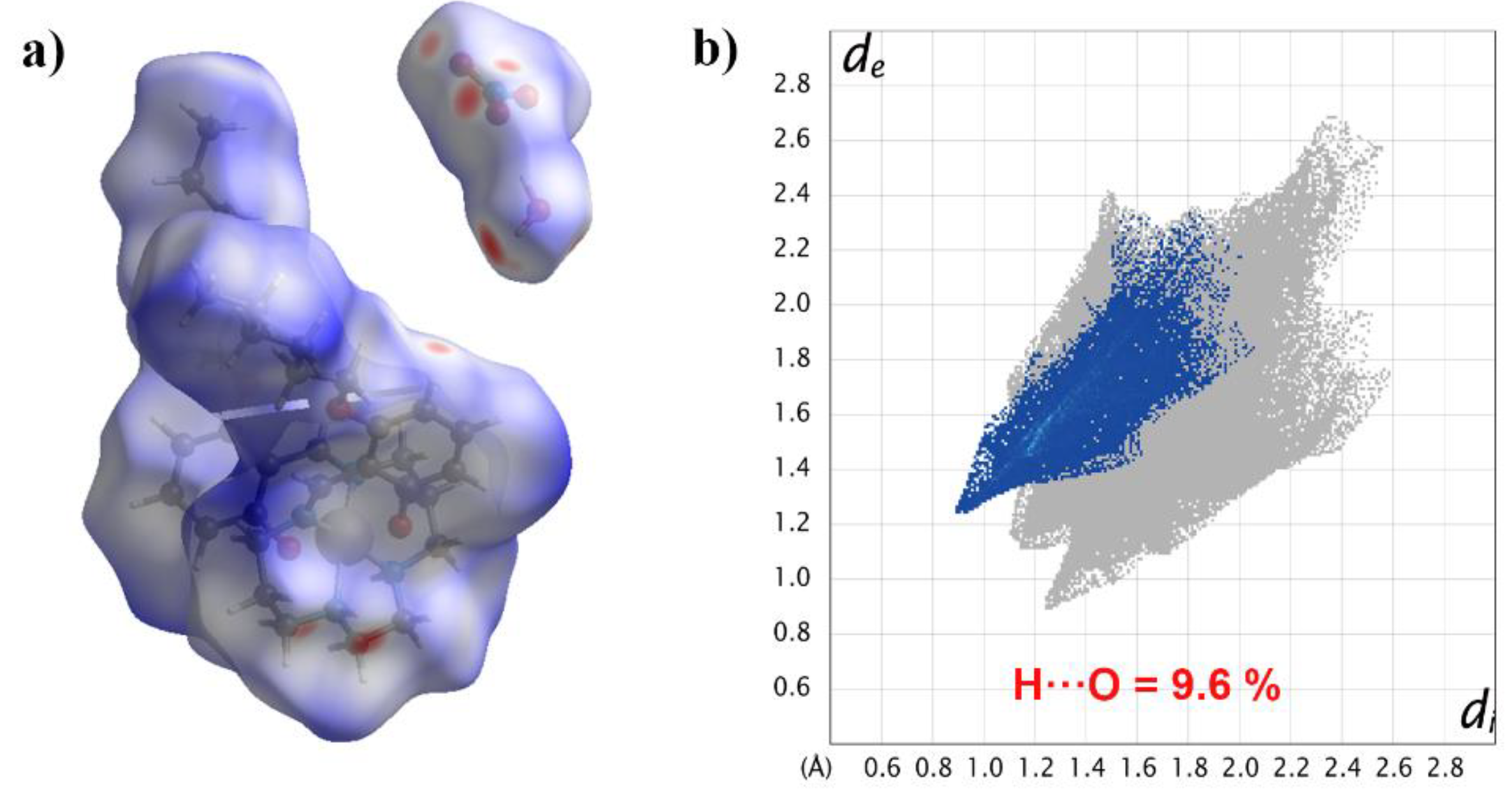
In order to more intuitively study the influence of hydroxyl on the hydrogen bond interaction, we introduced Hirshfeld surface analysis on the complex [Mn(4-OC6H13-sal-N-1,5,8,12)]NO3·H2O [16] (Figure 7). Though it crystallizes as an H2O solvate, the hydrogen bond contributed to only 9.6% of all interactions. All in all, the OH group played a significant role in non-covalent interactions.
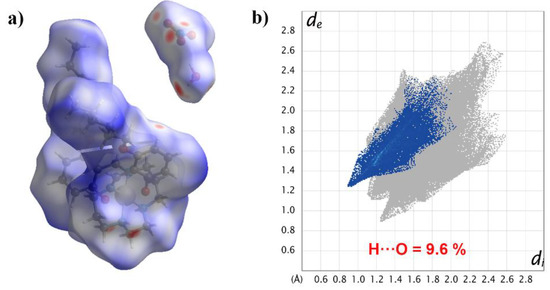
Figure 7.
Hirshfeld surface mapped with dnorm (a); 2D fingerprint plots of H···O for [Mn(4-OC6H13-sal-N-1,5,8,12)]NO3·H2O (b).
From the Hirshfeld analysis, it was very clear that the hydroxyl group effectively enhanced the hydrogen bonding interactions in both complexes. However, these non-covalent intermolecular forces were insufficient to result in a cooperative SCO. The cation structures were tightly packed, hindering the distortion required to undergo SCO.
3.3. Magnetic Characterization
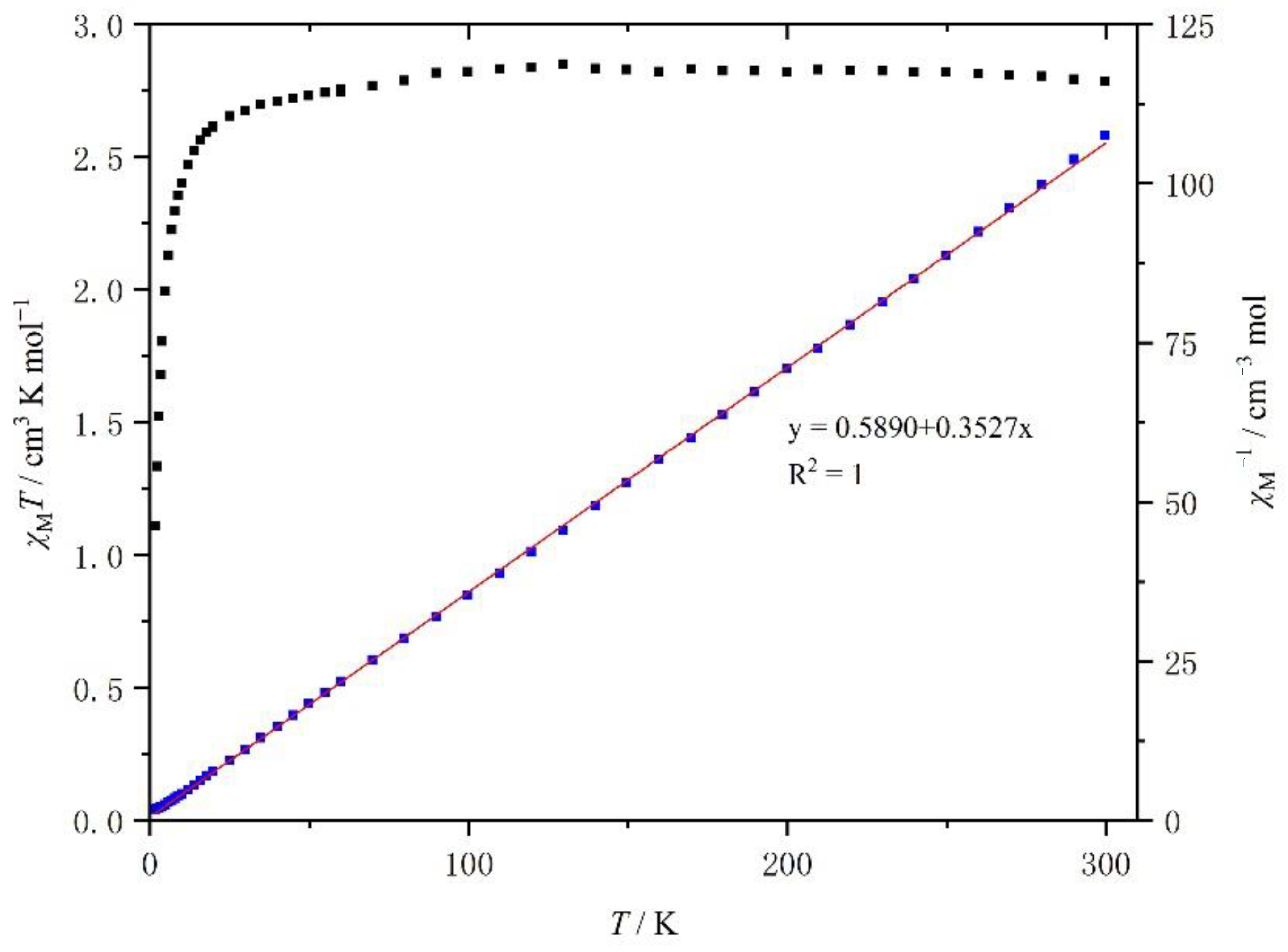
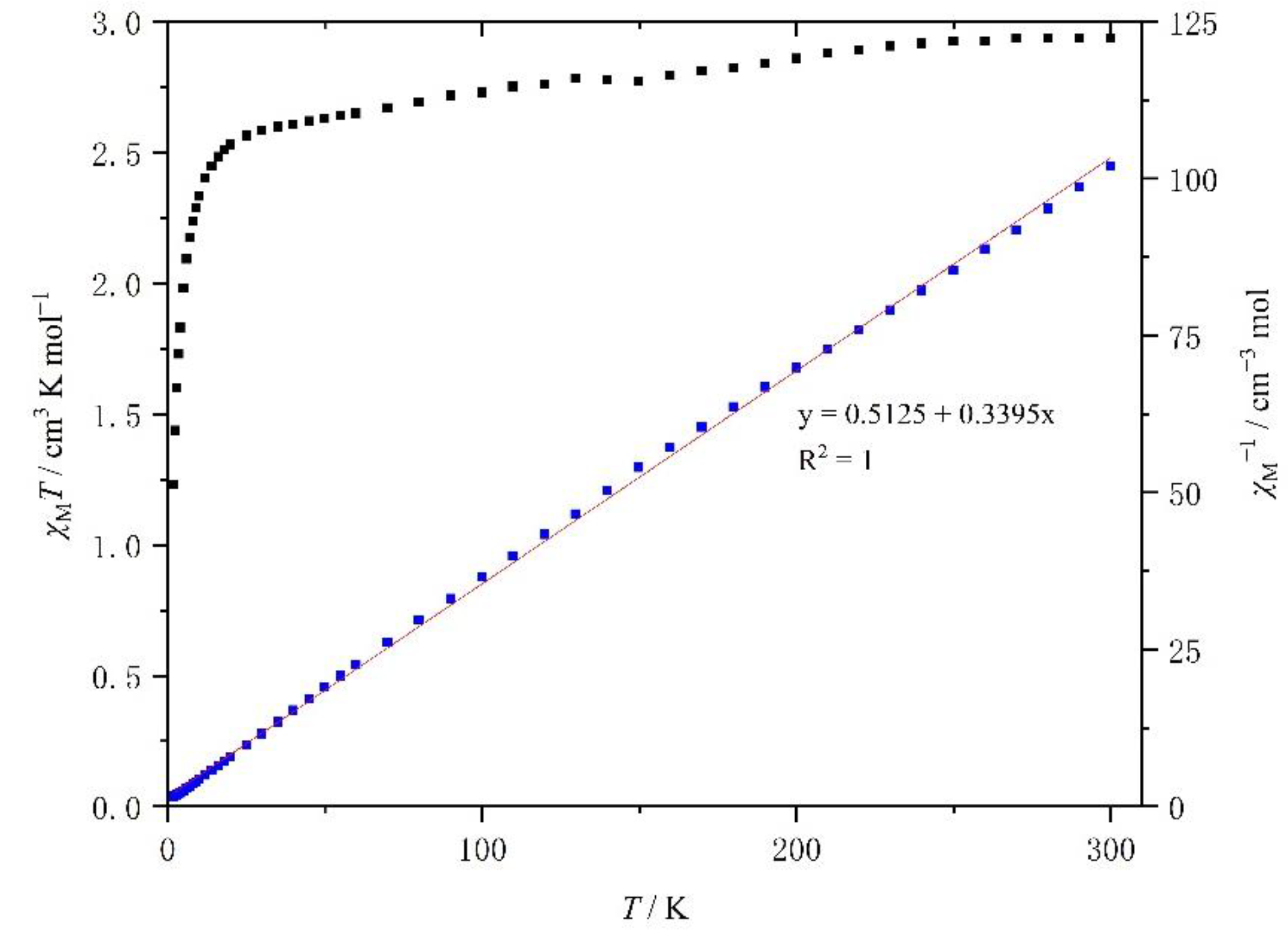
The temperature dependence of the product χMT (χM is the molar paramagnetic susceptibility) versus T plots for the crystalline samples of complexes 1 and 2 is shown in Figure 8 and Figure 9, respectively.
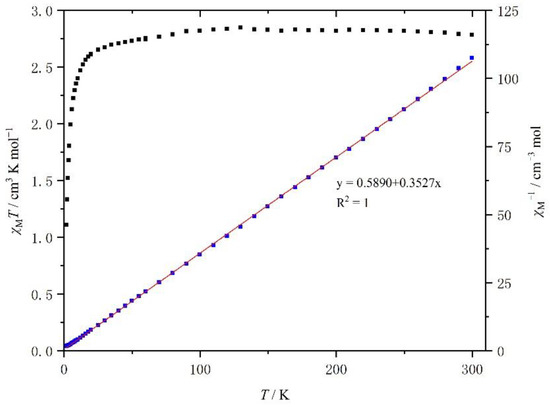
Figure 8.
Temperature dependence of the χMT of [Mn(4-OH-sal-N-1,5,8,12)]NO3 between 2 and 300 K.
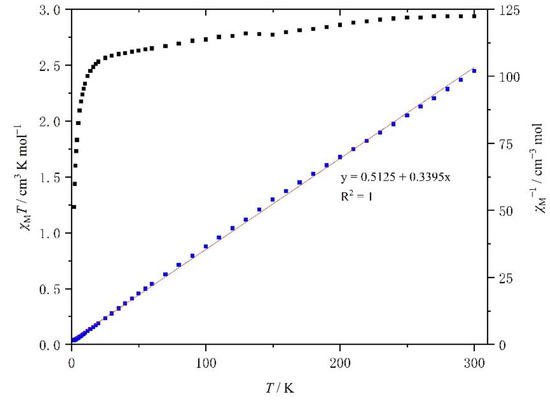
Figure 9.
Temperature dependence of the χMT of [Mn(4-OH-sal-N-1,5,8,12)]ClO4 between 2 and 300 K.
At room temperature, the χMT value of 1 was about 2.79 cm3 K mol−1, which is typical of an HS Mn3+ center (S = 2) with a g value of 2.0 (Figure 7). As the temperature went down, the χMT value was constant until 20 K, when it decreased rapidly because of the ZFS (zero field split) effects of HS Mn3+ ions. However, within the temperature range, the χMT value did not drop to 1.0 cm3 K mol−1. The temperature dependence of the χM−1 of complex 1 is shown in Figure 7. It is linear between 2 and 300 K and a linear least-squares fit yields a Curie constant of 2.84 emu K mol−1, a Weiss temperature θ of −1.67 K, while the Curie constant and θ value of compound 2 was 2.95 emu K mol−1 and −1.51 K (Figure 8).
The magnetic characterization of [Mn(4-OH-sal-N-1,5,8,12)]ClO4 was similar to that of complex 1, and increasing the size of the anion from NO3− from ClO4− seems to have had no effect on the magnetic behavior.
4. Conclusions
In an effort to synthesize new Mn(III) SCO complexes, we have described two new isomorphic [Mn(4-OH-sal-N-1,5,8,12)]Y (Y = NO3− and ClO4−) complexes. The crystal structure of these compounds is rich in non-covalent contacts (hydrogen bonding and π···π interactions) between the mononuclear cations and anions. Whereas, compared with other Mn(III) hexadentate Schiff base SCO complexes, such as [Mn(5-OMe-sal-N-1,5,8,12)]Cl and [Mn(sal-N-1,5,8,12)]NO3, the introduction of hydroxyl groups makes the Mn(III) cations dimerized and weakens the interactions between anions and cations. Besides, the Mn···Mn separations are short and the connections between Mn3+ centers are extremely close. They may limit the Mn3+ cations to change their coordination geometry.
Author Contributions
Methodology, Z.-M.Y.; formal analysis, Y.-T.W. and P.-Y.X.; investigation, Y.-T.W. and P.-Y.X.; resources, Y.-H.L.; project administration, S.W.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
We gratefully acknowledge financial support from the Natural Science Foundations of China (grant no. 21771110) and from the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions (grant no. YX03002).
Conflicts of Interest
There is no conflict to declare.
References
- Vogelsberg, C.S.; Garcia-Garibay, M.A. Crystalline molecular machines: Function, phase order, dimensionality, and composition. Chem. Soc. Rev. 2012, 41, 1892–1910. [Google Scholar] [CrossRef] [PubMed]
- Ohkoshi, S.; Tokoro, H. Photomagnetism in Cyano-Bridged Bimetal Assemblies. Acc. Chem. Res. 2012, 45, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Koumousi, E.S.; Jeon, I.-R.; Gao, Q.; Dechambenoit, P.; Woodruff, D.N.; Merzeau, P.; Buisson, L.; Jia, X.; Lionel, B.; Volatron, F.; et al. Metal-to-Metal Electron Transfer in Co/Fe Prussian Blue Molecular Analogues: The Ultimate Miniaturization. J. Am. Chem. Soc. 2014, 136, 15461–15464. [Google Scholar] [CrossRef] [PubMed]
- Irie, M.; Fukaminato, T.; Matsuda, K.; Kobatake, S. Photochromism of Diarylethene Molecules and Crystals: Memories, Switches, and Actuators. Chem. Rev. 2014, 114, 12174–12277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Zhong, J.-Q.; Lin, J.D.; Hu, W.P.; Wu, K.; Xu, G.Q.; Wee, A.T.S.; Chen, W. Towards single molecule switches. Chem. Soc. Rev. 2015, 44, 2998–3022. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, L.; Edelmann, K.; Homberg, J.; Valasek, M.; Bahoosh, S.G.; Lukas, M.; Pauly, F.; Mayor, M.; Wulfhekel, W. An electronically actuated molecular toggle switch. Nat. Commun. 2017, 8, 14672. [Google Scholar] [CrossRef]
- Kahn, O.; Martinez, C.J. Spin-transition Polymers: From Molecular Materials Toward Memory Devices. Science 1998, 279, 44–48. [Google Scholar] [CrossRef]
- Southon, P.D.; Liu, L.; Fellows, E.A.; Price, D.J.; Halder, G.J.; Chapman, K.W.; Moubaraki, B.; Murray, K.S.; Létard, J.-F.; Kepert, C.J. Dynamic Interplay between Spin-Crossover and Host−Guest Function in a Nanoporous Metal−Organic Framework Material. J. Am. Chem. Soc. 2009, 131, 10998–11009. [Google Scholar] [CrossRef]
- Bousseksou, A.; Molnár, G.; Salmon, L.; Nicolazzi, W. Molecular spin crossover phenomenon: Recent achievements and prospects. Chem. Soc. Rev. 2011, 40, 3313–3335. [Google Scholar] [CrossRef]
- Linares, J.; Codjovi, E.; Garcia, Y. Pressure and Temperature Spin Crossover Sensors with Optical Detection. Sensors 2012, 12, 4479–4492. [Google Scholar] [CrossRef]
- Gao, D.; Liu, Y.; Miao, B.; Wei, C.; Ma, J.-G.; Cheng, P.; Yang, G.-M. Pressure Sensor with a Color Change at Room Temperature Based on Spin-Crossover Behavior. Inorg. Chem. 2018, 57, 12475–12479. [Google Scholar] [CrossRef] [PubMed]
- Sim, P.G.; Sinn, E. First manganese(III) spin crossover and first d4 crossover: Comment on cytochrome-oxidase. J. Am. Chem. Soc. 1981, 103, 241–243. [Google Scholar] [CrossRef]
- Morgan, G.G.; Murnaghan, K.D.; Müller-Bunz, H.; McKee, V.; Harding, C.J. A Manganese(III) Complex That Exhibits Spin Crossover Triggered by Geometric Tuning. Angew. Chem. Int. Ed. 2006, 45, 7192–7195. [Google Scholar] [CrossRef] [PubMed]
- Ossinger, S.; Naggert, H.; Kipgen, L.; Jasper-Toennies, T.; Rai, A.; Rudnik, J.; Nickel, F.; Arruda, L.M.; Bernien, M.; Kuch, W.; et al. Vacuum-Evaporable Spin-Crossover Complexes in Direct Contact with a Solid Surface: Bismuth versus Gold. J. Phys. Chem. C 2017, 121, 1210–1219. [Google Scholar] [CrossRef]
- Wang, S.; Ferbinteanu, M.; Marinescu, C.; Dobrinescu, A.; Ling, Q.-D.; Huang, W. Case Study on a Rare Effect: The Experimental and Theoretical Analysis of a Manganese(III) Spin-Crossover System. Inorg. Chem. 2010, 49, 9839–9851. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, C.; Cotting, T.; Martinho, P.N.; Sereda, O.; Neels, A.; Morgan, G.G.; Albrecht, M. Synthesis and self-assembly of spin-labile and redox-active manganese(iii) complexes. Dalton Trans. 2010, 40, 1855–1865. [Google Scholar] [CrossRef] [PubMed]
- Martinho, P.N.; Gildea, B.; Harris, M.M.; Lemma, T.; Naik, A.D.; Müller-Bunz, H.; Keyes, T.E.; Garcia, Y.; Morgan, G.G. Cooperative Spin Transition in a Mononuclear Manganese(III) Complex. Angew. Chem. Int. Ed. 2012, 51, 12597–12601. [Google Scholar] [CrossRef]
- Gildea, B.; Gavin, L.C.; Murray, C.A.; Müller-Bunz, H.; Harding, C.J.; Morgan, G.G. Supramolecular modulation of spin crossover profile in manganese(III). Supramol. Chem. 2012, 24, 641–653. [Google Scholar] [CrossRef]
- Pandurangan, K.; Gildea, B.; Murray, C.; Harding, C.J.; Müller-Bunz, H.; Morgan, G.G. Lattice Effects on the Spin-Crossover Profile of a Mononuclear Manganese(III) Cation. Chem. A Eur. J. 2012, 18, 2021–2029. [Google Scholar] [CrossRef]
- Wang, S.; He, W.-R.; Ferbinteanu, M.; Li, Y.-H.; Huang, W. Tetragonally compressed high-spin Mn(III) Schiff base complex: Synthesis, crystal structure, magnetic properties and theoretical calculations. Polyhedron 2013, 52, 1199–1205. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, F.; Wei, R.-M.; Zhang, Y.; Zhang, Y.-Q.; Song, Y. Spin-crossover phenomena of the mononuclear MnIII complex tuned by metal dithiolene counteranions. Dalton Trans. 2014, 43, 3783–3791. [Google Scholar] [CrossRef] [PubMed]
- Gildea, B.; Harris, M.M.; Gavin, L.C.; Murray, C.A.; Ortin, Y.; Müller-Bunz, H.; Harding, C.J.; Lan, Y.; Powell, A.K.; Morgan, G.G. Substituent Effects on Spin State in a Series of Mononuclear Manganese(III) Complexes with Hexadentate Schiff-Base Ligands. Inorg. Chem. 2014, 53, 6022–6033. [Google Scholar] [CrossRef] [PubMed]
- Morgan, G.G.; Fitzpatrick, A.J.; Trzop, E.; Müller-Bunz, H.; Dîrtu, M.M.; Garcia, Y.; Collet, E. Electronic vs. structural ordering in a manganese(III) spin crossover complex. Chem. Commun. 2015, 51, 17540–17543. [Google Scholar]
- Wang, S.; Li, Y.-H.; Huang, W. Effects of Big Planar Anions on the Spin Transition of a Mononuclear Manganese(III) Complex with a Hexadentate Schiff-Base Ligand. Eur. J. Inorg. Chem. 2015, 2015, 2237–2244. [Google Scholar] [CrossRef]
- Wang, S.; Xu, W.-T.; He, W.-R.; Takaishi, S.; Li, Y.-H.; Yamashita, M.; Huang, W. Structural insights into the counterion effects on the manganese(iii) spin crossover system with hexadentate Schiff-base ligands. Dalton Trans. 2016, 45, 5676–5688. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.-J.; Ju, F.-F.; Xu, W.-T.; Kagesawa, K.; Li, Y.-H.; Yamashita, M.; Huang, W. The molecular and supramolecular aspects in mononuclear manganese(iii) Schiff-base spin crossover complexes. Dalton Trans. 2017, 46, 11063–11077. [Google Scholar] [CrossRef]
- Barker, A.; Kelly, C.T.; Kühne, I.A.; Hill, S.; Krzystek, J.; Wix, P.; Esien, K.; Felton, S.; Müller-Bunza, H.; Morgan, G.G. Spin state solvomorphism in a series of rare S = 1 manganese(III) complexes. Dalton Trans. 2019, 48, 15560–15566. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, A.V.; Tiunova, A.V.; Korchagin, D.V.; Shilov, G.V.; Yagubskii, E.B.; Zverev, V.N.; Yang, S.C.; Lin, J.; Lee, J.; Maximova, O.V.; et al. The First Conducting Spin-Crossover Compound Combining a Mn III Cation Complex with Electroactive TCNQ Demonstrating an Abrupt Spin Transition with a Hysteresis of 50 K. Chem. A Eur. J. 2019, 25, 10204–10213. [Google Scholar] [CrossRef]
- Zhao, S.-Z.; Qin, C.-Y.; Wang, S.; Yamashita, M.; Li, Y.-H.; Huang, W. Structure function correlations in mononuclear manganese(III) spin crossover systems with a big conjugated hexadentate Schiff-base ligand. Dalton Trans. 2020, 49, 4293–4305. [Google Scholar] [CrossRef]
- Villaman, D.; McMonagle, C.J.; Probert, M.R.; Peña, O.; Moreno, Y.; Fuentealba, M. Structural studies of a manganese(iii) complex with spin-crossover and thermochromic properties. CrystEngComm 2020, 22, 3221–3233. [Google Scholar] [CrossRef]
- Krüger, C.; Augustín, P.; Dlháň, L.; Pavlik, J.; Moncol’, J.; Nemec, I.; Boča, R.; Renz, F. Iron(III) complexes with pentadentate Schiff-base ligands: Influence of crystal packing change and pseudohalido coligand variations on spin crossover. Polyhedron 2015, 87, 194–201. [Google Scholar] [CrossRef]
- Yamada, M.; Hagiwara, H.; Torigoe, H.; Matsumoto, N.; Kojima, M.; Dahan, F.; Tuchagues, J.P.; Re, N.; Iijima, S. A variety of spin-crossover behaviors depending on the counter anion: Two-dimensional complexes constructed by NH Cl hydrogen bonds, [FeIIH3LMe]Cl·X (X=PF6, AsF6, SbF6, CF3SO3; H3LMe=Tris[2-{[(2-methylimidazol-4-yl) methylidene]amino}ethyl]amine). Chem. Eur. J. 2006, 12, 4536–4549. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; McDaniel, A.M.; Shores, M.P. Ambient temperature anion-dependent spin state switching observed in “mostly low spin” heteroleptic iron(ii) diimine complexes. Chem. Sci. 2010, 1, 615–621. [Google Scholar] [CrossRef]
- Nemec, I.; Herchel, R.; Šalitroš, I.; Trávníček, Z.; Moncoľ, J.; Fuess, H.; Ruben, M.; Linert, W. Anion driven modulation of magnetic intermolecular interactions and spin crossover properties in an isomorphous series of mononuclear iron(iii) complexes with a hexadentate Schiff base ligand. CrystEngComm 2012, 14, 7015. [Google Scholar] [CrossRef]
- Li, B.; Wei, R.-J.; Tao, J.; Huang, R.-B.; Zheng, L.-S.; Zheng, Z. Solvent-Induced Transformation of Single Crystals of a Spin-Crossover (SCO) Compound to Single Crystals with Two Distinct SCO Centers. J. Am. Chem. Soc. 2010, 132, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.-J.; Tao, J.; Huang, R.-B.; Zheng, L.-S. Reversible and Irreversible Vapor-Induced Guest Molecule Exchange in Spin-Crossover Compounds. Inorg. Chem. 2011, 50, 8553–8564. [Google Scholar] [CrossRef]
- Costa, J.S.; Rodríguez-Jiménez, S.; Craig, G.A.; Barth, B.; Beavers, C.M.; Teat, S.J.; Aromí, G. Three-way crystal-tocrystal reversible transformation and controlled spin switching by a nonporous molecular material. J. Am. Chem. Soc. 2014, 136, 3869–3874. [Google Scholar] [CrossRef]
- Wannarit, N.; Nassirinia, N.; Amani, S.; Masciocchi, N.; Youngme, S.; Roubeau, O.; Teat, S.J.; Gamez, P. Drastic Effect of Lattice Propionitrile Molecules on the Spin-Transition Temperature of a 2,2′-Dipyridylamino/s-triazine-Based Iron(II) Complex. Inorg. Chem. 2014, 53, 9827–9836. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm. 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Hassan, N.H.H.; Abdullah, A.A.; Arshad, S.; Khalib, N.C.; Razak, I.A. Crystal structure and Hirshfeld surface analysis of (E)-3-(2-chloro-6-fluorophenyl)-1-(3-fluoro-4-methoxyphenyl)prop-2-en-1-one. Acta Cryst. Sec. E 2016, 72, 716–719. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).