A New FeIII Substituted Arsenotungstate [FeIII2(AsIIIW6O23)2(AsIIIO3H)2]12−: Synthesis, Structure, Characterization and Magnetic Properties
Abstract
1. Introduction
2. Experimental Section
Synthesis
3. Results and Discussion
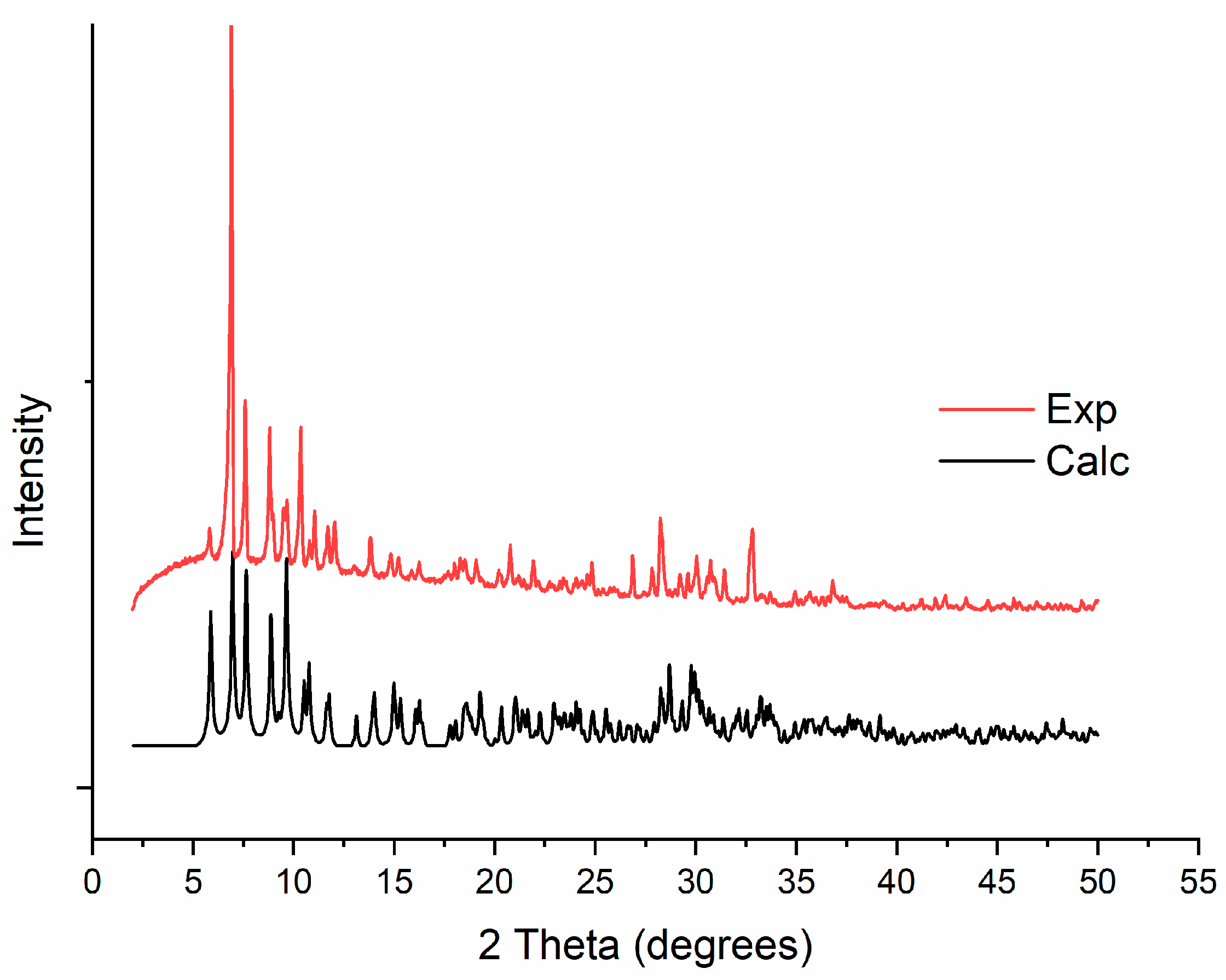
3.1. Synthesis
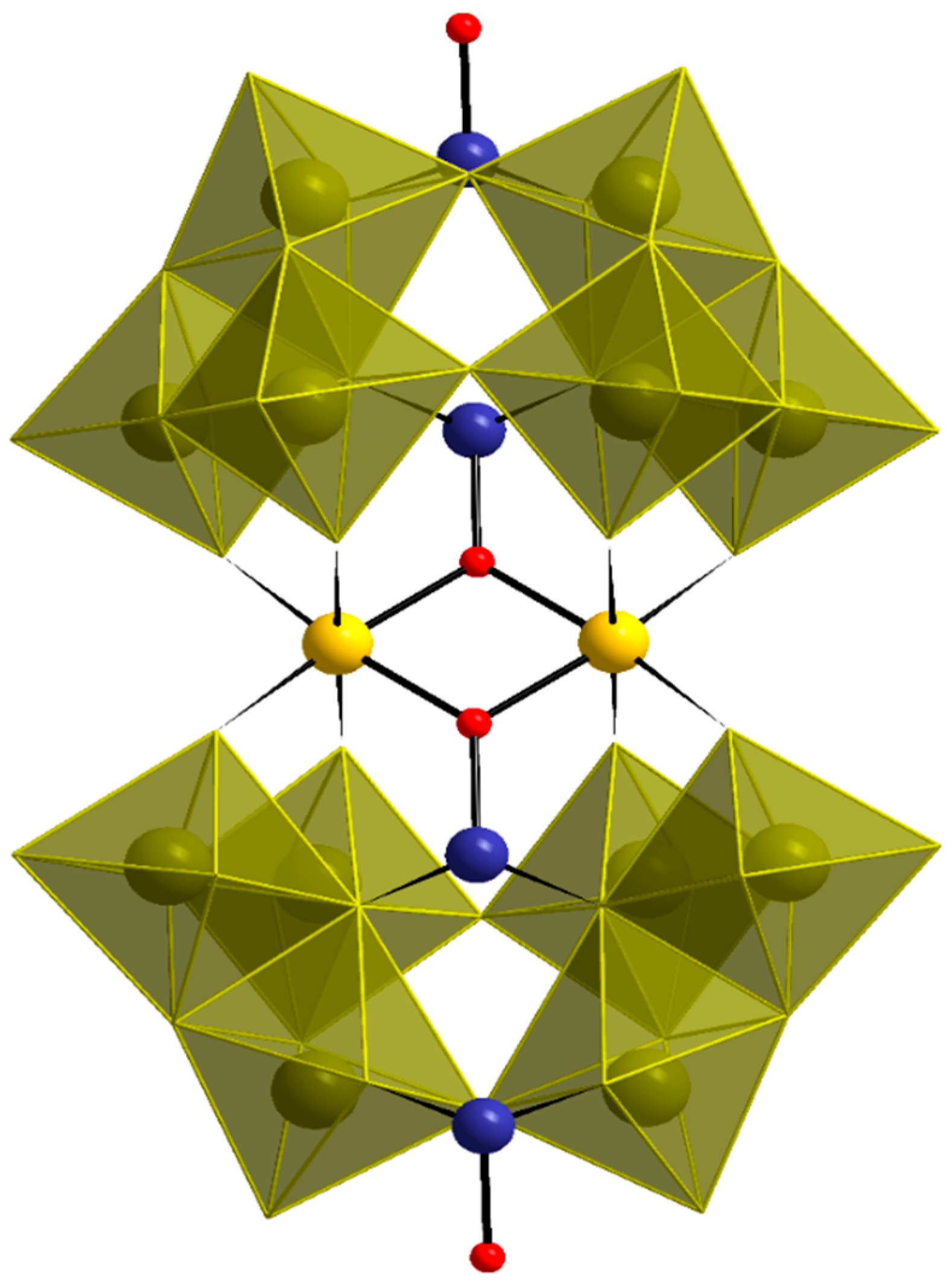
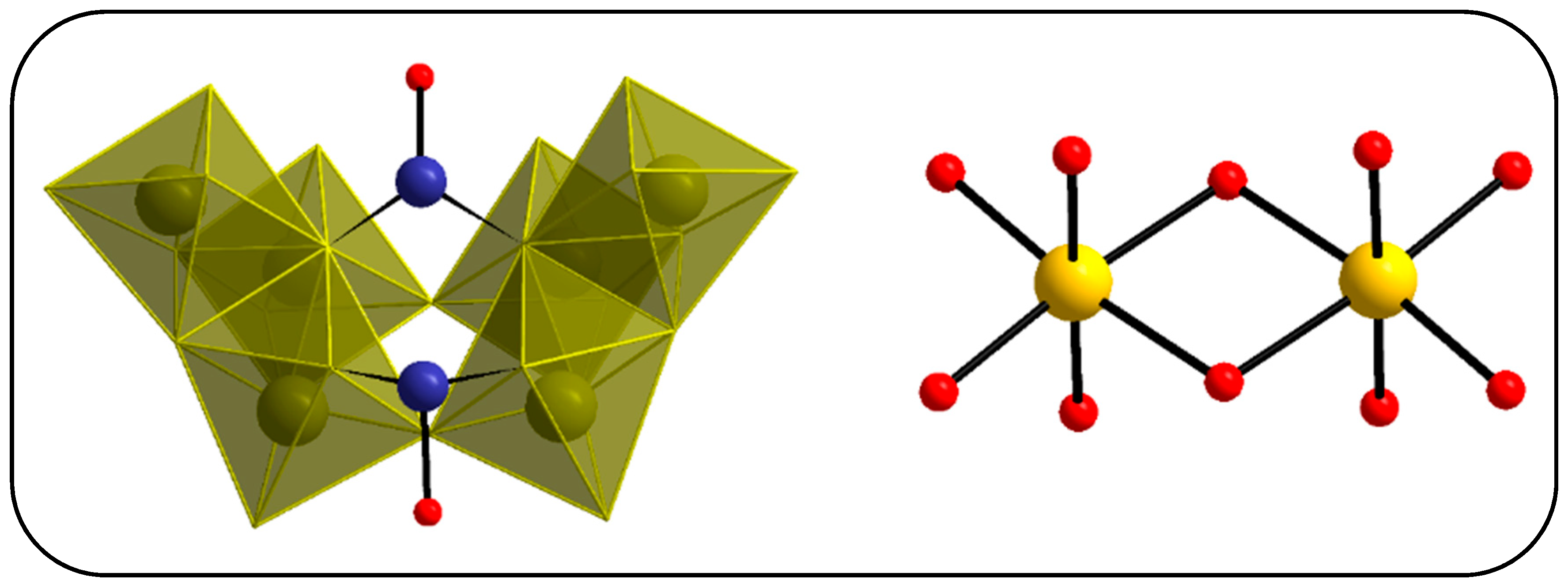
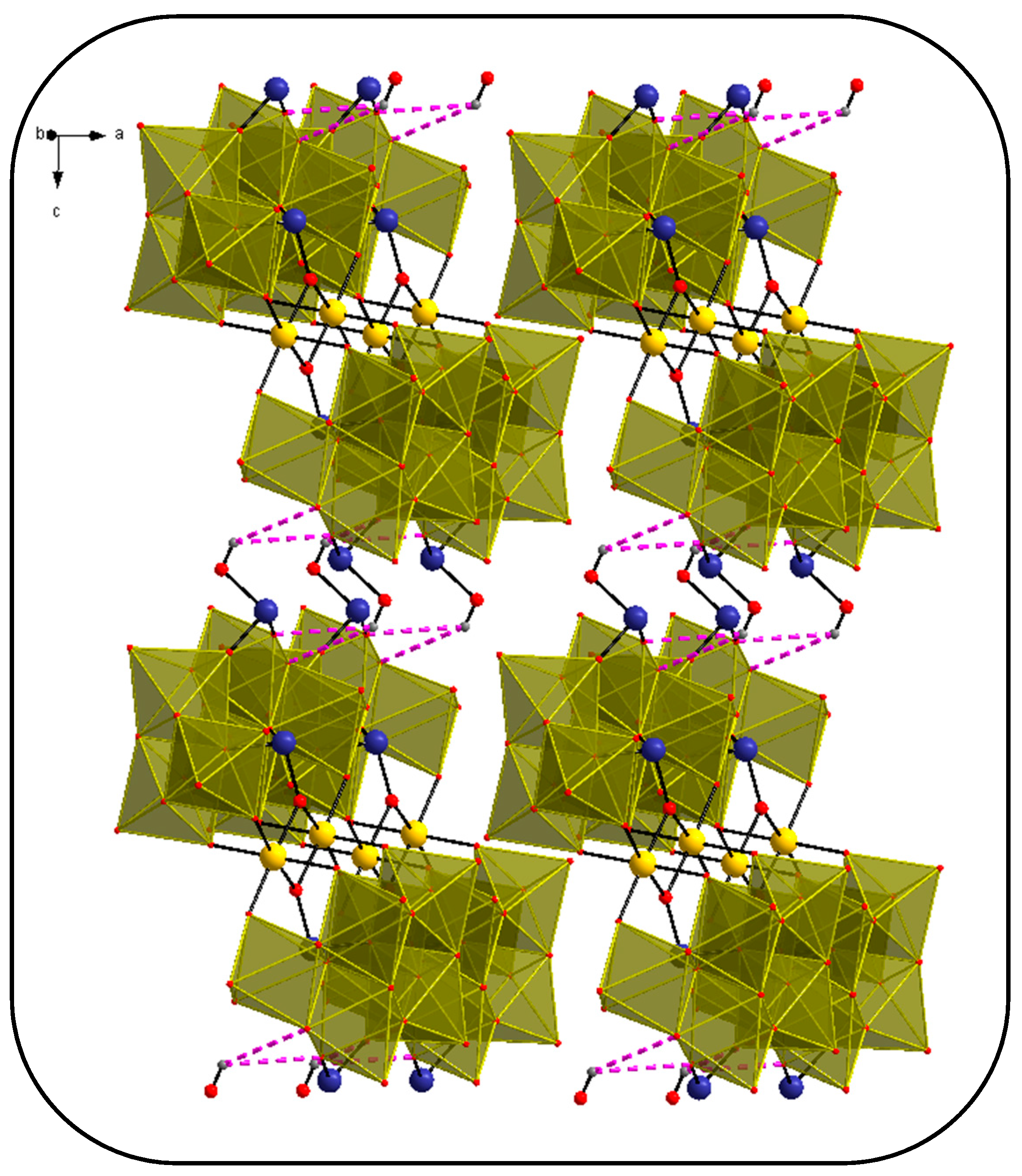
3.2. Single-Crystal X-ray Structure Determination
3.3. Characterizations
3.3.1. Vibrational Spectroscopy
3.3.2. Thermogravimetric Analysis
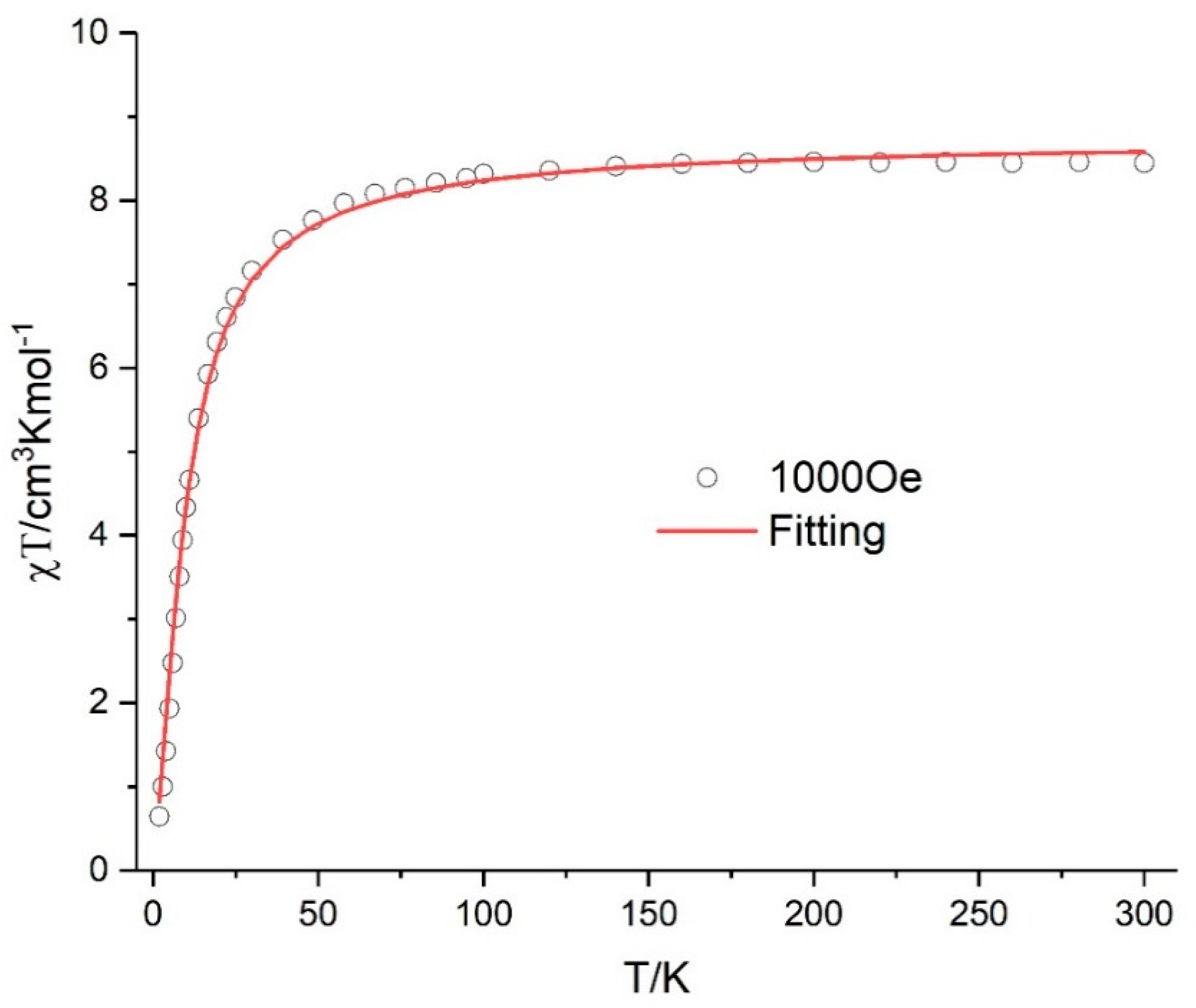
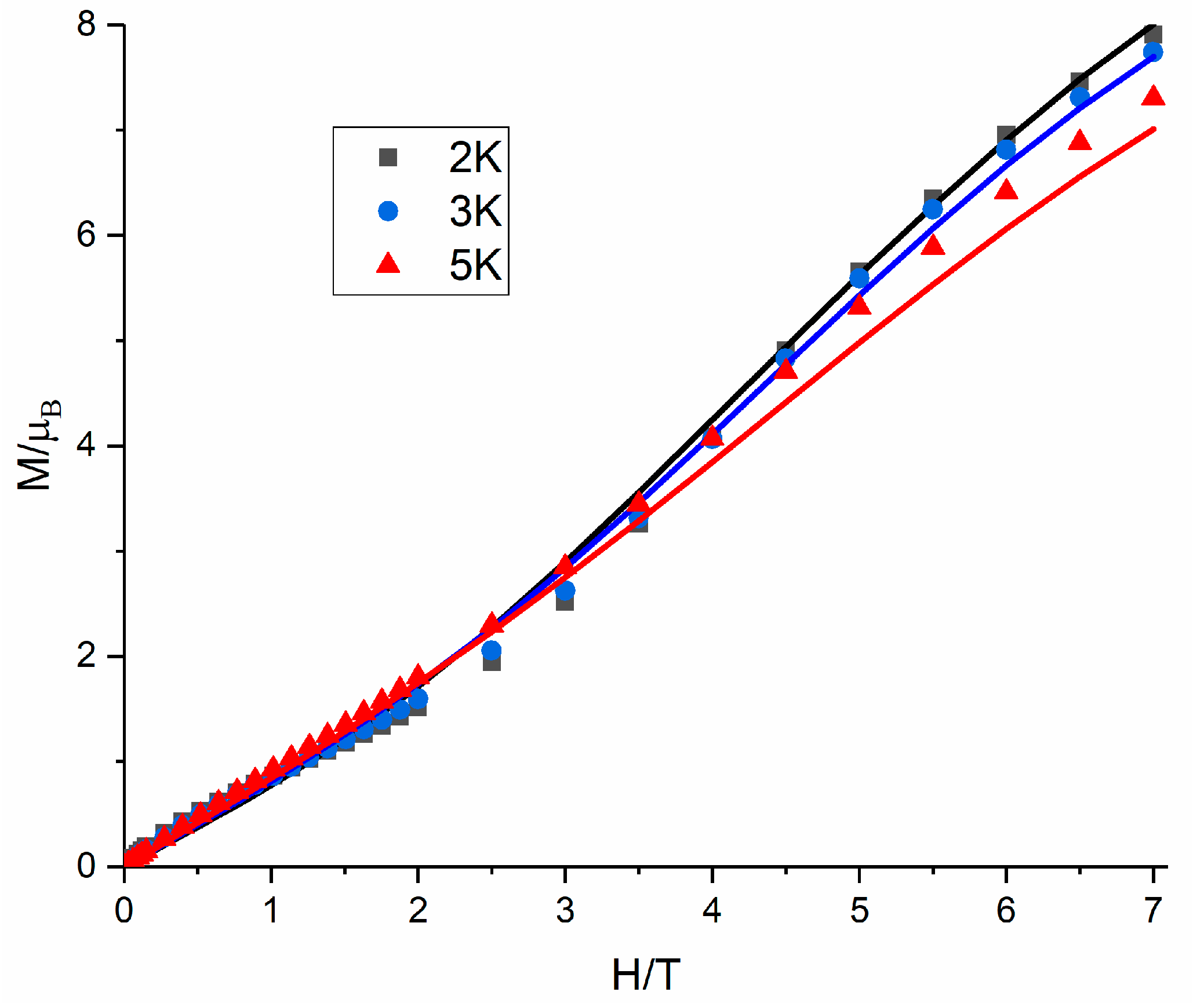
3.4. Magnetic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anyushin, A.V.; Kondinski, A.; Parac-Vogt, T.N. Hybrid polyoxometalates as post-functionalization platforms: From fundamentals to emerging applications. Chem. Soc. Rev. 2020, 49, 382–432. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.P.; Yin, Q.; Collins-Wildman, D.L.; Tao, M.; Geletii, Y.V.; Musaev, D.G.; Lian, T.; Hill, C.L. Multi-tasking POM systems. Front. Chem. 2018, 6, 365. [Google Scholar] [CrossRef] [PubMed]
- Long, D.L.; Tsunashima, R.; Cronin, L. Polyoxometalates: Building blocks for functional nanoscale systems. Angew. Chem. Int. Ed. 2010, 49, 1736–1758. [Google Scholar] [CrossRef] [PubMed]
- Stuckart, M.; Monakhov, K.Y. Polyoxometalates as components of supramolecular assemblies. Chem. Sci. 2019, 10, 4364–4376. [Google Scholar] [CrossRef]
- Oms, O.; Dolbecq, A.; Mialane, P. Diversity in structures and properties of 3d-incorporating polyoxotungstates. Chem. Soc. Rev. 2012, 41, 7497–7536. [Google Scholar] [CrossRef]
- Reinoso, S. Heterometallic 3d-4f polyoxometalates: Still an incipient field. Dalton Trans. 2011, 40, 6610–6615. [Google Scholar] [CrossRef]
- Boskovic, C. Rare earth polyoxometalates. Acc. Chem. Res. 2017, 50, 2205–2214. [Google Scholar] [CrossRef]
- Zhao, J.W.; Li, Y.Z.; Chen, L.J.; Yang, G.Y. Research progress on polyoxometalate-based transition-metal-rare-earth heterometallic derived materials: Synthetic strategies, structural overview and functional applications. Chem. Commun. 2016, 52, 4418–4445. [Google Scholar] [CrossRef]
- Ibrahim, M.; Mereacre, V.; Leblanc, N.; Wernsdorfer, W.; Anson, C.E.; Powell, A.K. Self-assembly of a giant tetrahedral 3 d-4 f single-molecule magnet within a polyoxometalate system. Angew. Chem. Int. Ed. 2015, 54, 15574–15578. [Google Scholar] [CrossRef]
- Ibrahim, M.; Lan, Y.; Bassil, B.S.; Xiang, Y.; Suchopar, A.; Powell, A.K.; Kortz, U. Hexadecacobalt(II)-containing polyoxometalate-based single-molecule magnet. Angew. Chem. Int. Ed. 2011, 50, 4708–4711. [Google Scholar] [CrossRef]
- AlDamen, M.A.; Clemente-Juan, J.M.; Coronado, E.; Martí-Gastaldo, C.; Gaita-Arino, A. Mononuclear lanthanide single-molecule magnets based on polyoxometalates. J. Am. Chem. Soc. 2008, 130, 8874–8875. [Google Scholar] [CrossRef]
- Ritchie, C.; Ferguson, A.; Nojiri, H.; Miras, H.N.; Song, Y.F.; Long, D.L.; Burkholder, E.; Murrie, M.; Kögerler, P.; Brechin, E.K.; et al. Polyoxometalate-mediated self-assembly of single-molecule magnets: {[XW9O34]2[MnIII4MnII2O4(H2O)4]}12−. Angew. Chem Int. Ed. 2008, 47, 5609–5612. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhou, W.; Li, Y.; Ke, H.; Tang, J.; Clérac, R.; Wang, Y.; Su, Z.; Wang, E. Polyoxometalate-supported 3d-4f heterometallic single-molecule magnets. Inorg. Chem. 2012, 51, 2722–2724. [Google Scholar] [CrossRef]
- Izarova, N.V.; Kögerler, P. Polyoxometalate-based single-molecule magnets. In Trends in Polyoxometalates Research; Ruhlmann, L., Schaming, D., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2015; pp. 121–149. [Google Scholar]
- El Moll, H.; Dolbecq, A.; Marrot, J.; Rousseau, G.; Haouas, M.; Taulelle, F.; Rogez, G.; Wernsdorfer, W.; Keita, B.; Mialane, P. A stable hybrid bisphosphonate polyoxometalate single-molecule magnet. Chem. Eur. J. 2012, 18, 3845–3849. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.X.; Yang, T.; Cai, Z.W.; Zheng, S.T. Four-shell polyoxometalates featuring high-nuclearity Ln26Clusters: Structural transformations of nanoclusters into frameworks triggered by transition-metal ions. Angew. Chem. Int. Ed. 2017, 56, 2664–2669. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, C.; Moore, E.G.; Speldrich, M.; Kögerler, P.; Boskovic, C. Terbium polyoxometalate organic complexes: Correlation of structure with luminescence properties. Angew. Chem. Int. Ed. 2010, 49, 7702–7705. [Google Scholar] [CrossRef]
- Katsoulis, D.E. A survey of applications of polyoxometalates. Chem. Rev. 1998, 98, 359–388. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Juan, J.M.; Coronado, E.; Gaita-Ariño, A. Magnetic polyoxometalates: From molecular magnetism to molecular spintronics and quantum computing. Chem. Soc. Rev. 2012, 41, 7464–7478. [Google Scholar] [CrossRef]
- Coronado, E.; Gómez-García, C.J. Polyoxometalate-based molecular materials. Chem. Rev. 1998, 98, 273–296. [Google Scholar] [CrossRef]
- Vonci, M.; Boskovic, C. Polyoxometalate-supported lanthanoid single-molecule magnets. Aust. J. Chem. 2014, 67, 1542–1552. [Google Scholar] [CrossRef]
- Compain, J.D.; Mialane, P.; Dolbecq, A.; Mbomekallé, I.M.; Marrot, J.; Sécheresse, F.; Riviére, E.; Rogez, G.; Wernsdorfer, W. Iron polyoxometalate single-molecule magnets. Angew. Chem. Int. Ed. 2009, 48, 3077–3081. [Google Scholar] [CrossRef] [PubMed]
- Pichon, C.; Dolbecq, A.; Mialane, P.; Marrot, J.; Rivière, E.; Sécheresse, F. Square versus tetrahedral iron clusters with polyoxometalate ligands. J. Chem. Soc. Dalton Trans. 2007, 707, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Haider, A.; Xiang, Y.; Bassil, B.S.; Carey, A.M.; Rullik, L.; Jameson, G.B.; Doungmene, F.; Mbomekallé, I.M.; de Oliveira, P.; et al. Tetradecanuclear iron(III)-oxo nanoclusters stabilized by trilacunary heteropolyanions. Inorg. Chem. 2015, 54, 6136–6146. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Chen, Z.; Ma, P.; Zhang, D.; Drew, M.G.B.; Niu, J.; Wang, J. Unprecedented {Fe14}/{Fe10} Polyoxotungstate-based nanoclusters with efficient photocatalytic H2 evolution activity: Synthesis, structure, magnetism, and electrochemistry. Chem. Eur. J. 2016, 22, 10983–10989. [Google Scholar] [CrossRef]
- Botar, B.; Geletii, Y.V.; Kögerler, P.; Musaev, D.G.; Morokuma, K.; Weinstock, I.A.; Hill, C.L. The true nature of the di-iron(III) γ-Keggin structure in water: Catalytic aerobic oxidation and chemistry of an unsymmetrical trimer. J. Am. Chem. Soc. 2006, 128, 11268–11277. [Google Scholar] [CrossRef]
- Du, X.; Ding, Y.; Song, F.; Ma, B.; Zhao, J.; Song, J. Efficient photocatalytic water oxidation catalyzed by polyoxometalate [Fe11(H2O)14(OH)2(W3O10)2(α-SbW9O33)6]27− based on abundant metals. Chem. Commun. 2015, 51, 13925–13928. [Google Scholar] [CrossRef]
- Ritchie, C.; Li, F.; Pradeep, C.P.; Long, D.L.; Xu, L.; Cronin, L. A functional hybrid polyoxometalate framework based on a ‘trilacunary’ heteropolyanion [(P4W6O34)2Co2Na2(H2O)2]18−. J. Chem. Soc. Dalton Trans. 2009, 2, 6483–6486. [Google Scholar] [CrossRef]
- Wenjing, L. Synthesis of Chromium-Containing and Some other Heteropolytungstates and Study of Their Magnetic, Electrochemical, and Biological Properties. Ph.D. Thesis, Jacobs University Bremen, Bremen, Germany, 2015. [Google Scholar]
- Detusheva, L.G.; Kuznetsova, L.I.; Dovlitova, L.S.; Likholobov, V.A. Study of the equilibrium of formation of arsenic(III) lacunar heteropolytungstates by Raman spectroscopy. Russ. Chem. Bull. 2003, 52, 370–374. [Google Scholar] [CrossRef]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef]
- Xiang, H.; Mereacre, V.; Lan, Y.; Lu, T.-B.; Anson, C.E.; Powell, A.K. Direct observation of the role of lanthanides in stabilizing a ferromagnetic spin orientation in a weak FeIII-FeIII antiferromagnet. Chem. Commun. 2013, 49, 7385–7387. [Google Scholar] [CrossRef]
- Canada-Vilalta, C.; O’Brien, T.A.; Brechin, E.K.; Pink, M.; Davidson, E.R.; Christou, G. Large spin differences in structurally related Fe6 molecular clusters and their magnetostructural explanation. Inorg. Chem. 2004, 43, 5505–5521. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.J.; Abboud, K.A.; Christou, G. Magnetostructural correlation for high-nuclearity iron(III)/oxo complexes and application to Fe5, Fe6, and Fe8 clusters. Inorg. Chem. 2016, 55, 6597–6608. [Google Scholar] [CrossRef]
- Ako, A.M.; Lan, Y.; Mereacre, V.; Ruiz, E.; Aravena, D.; Anson, C.E.; Powell, A.K. Spins on a curved surface: A FeIII14 ferracalixarene. Dalton Trans. 2013, 42, 9606–9612. [Google Scholar] [CrossRef] [PubMed]
| Compound | Na-1 |
|---|---|
| Formula | As4 H90 Fe2 Na12 O96 W12 |
| Formula weight | 4520.17 |
| Crystal System | Triclinic |
| Space Group | P |
| a/Å | 11.9906 (9) |
| b/Å | 13.1591 (9) |
| c/Å | 15.0490 (9) |
| α/° | 88.242 (5) |
| β/° | 89.361 (6) |
| γ/° | 74.518 (5) |
| U/Å3 | 2287.3 (3) |
| Z | 1 |
| T/K | 150 (2) |
| F(000) | 2062 |
| Dc/Mg m−3 | 3.282 |
| μ(Ga-Kα)/mm−1 | 23.123 |
| Data Measured | 21,626 |
| Unique Data | 9508 |
| Rint | 0.0378 |
| Data with I ≥ 2σ(I) | 9017 |
| wR2 (all data) | 0.1663 |
| S (all data) | 1.056 |
| R1 [I ≥ 2σ(I)] | 0.0578 |
| Parameters/Restraints | 598/1 |
| Biggest diff. peak/hole/eÅ−3 | 2.46/−3.14 |
| CSD number | 2,022,893 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, M.; Peng, Y.; Anson, C.E. A New FeIII Substituted Arsenotungstate [FeIII2(AsIIIW6O23)2(AsIIIO3H)2]12−: Synthesis, Structure, Characterization and Magnetic Properties. Magnetochemistry 2020, 6, 54. https://doi.org/10.3390/magnetochemistry6040054
Ibrahim M, Peng Y, Anson CE. A New FeIII Substituted Arsenotungstate [FeIII2(AsIIIW6O23)2(AsIIIO3H)2]12−: Synthesis, Structure, Characterization and Magnetic Properties. Magnetochemistry. 2020; 6(4):54. https://doi.org/10.3390/magnetochemistry6040054
Chicago/Turabian StyleIbrahim, Masooma, Yan Peng, and Christopher E. Anson. 2020. "A New FeIII Substituted Arsenotungstate [FeIII2(AsIIIW6O23)2(AsIIIO3H)2]12−: Synthesis, Structure, Characterization and Magnetic Properties" Magnetochemistry 6, no. 4: 54. https://doi.org/10.3390/magnetochemistry6040054
APA StyleIbrahim, M., Peng, Y., & Anson, C. E. (2020). A New FeIII Substituted Arsenotungstate [FeIII2(AsIIIW6O23)2(AsIIIO3H)2]12−: Synthesis, Structure, Characterization and Magnetic Properties. Magnetochemistry, 6(4), 54. https://doi.org/10.3390/magnetochemistry6040054





