Hydrogen Peroxide-Assisted Hydrothermal Synthesis of BiFeO3 Microspheres and Their Dielectric Behavior
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Method
2.3. Characterization
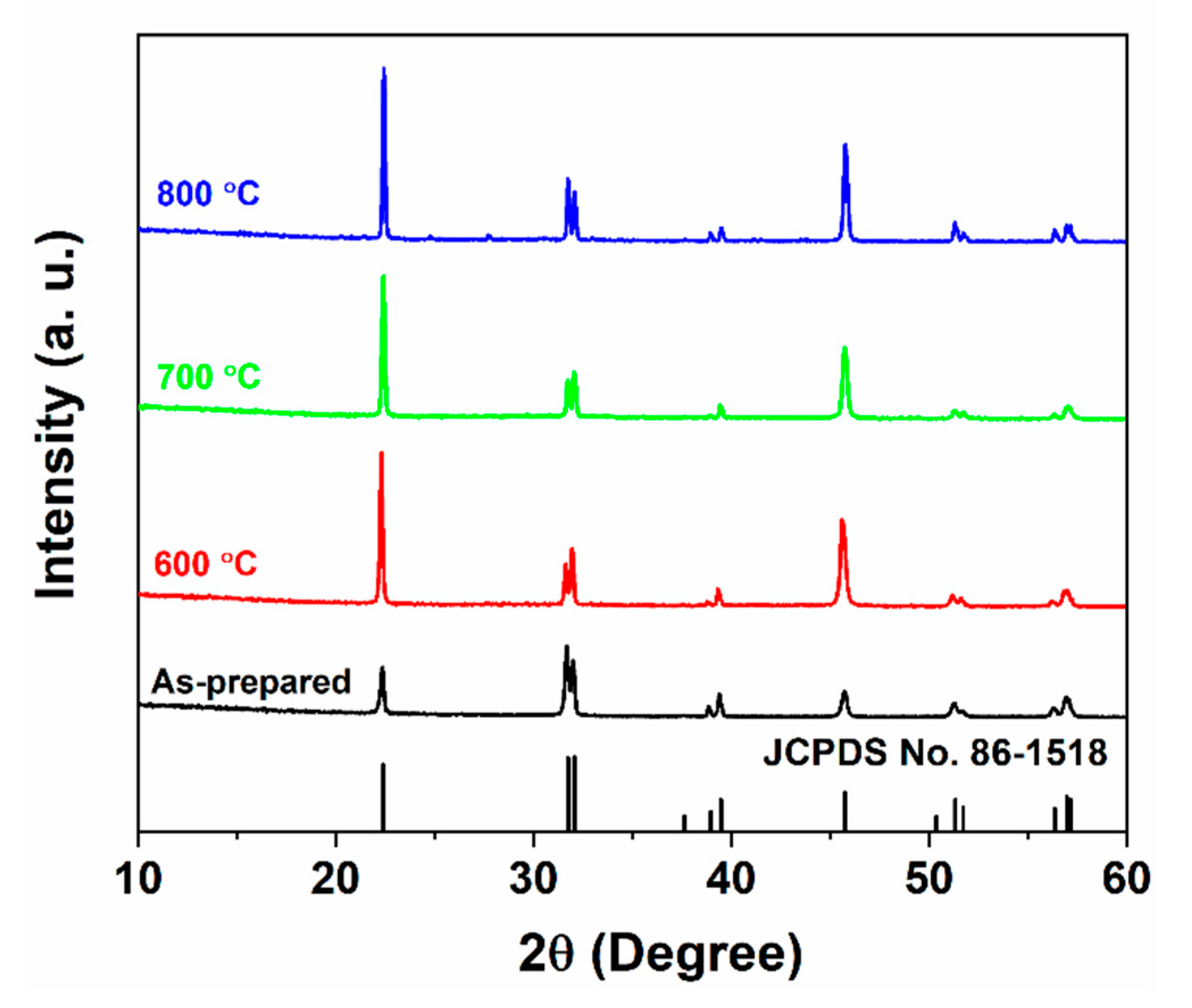
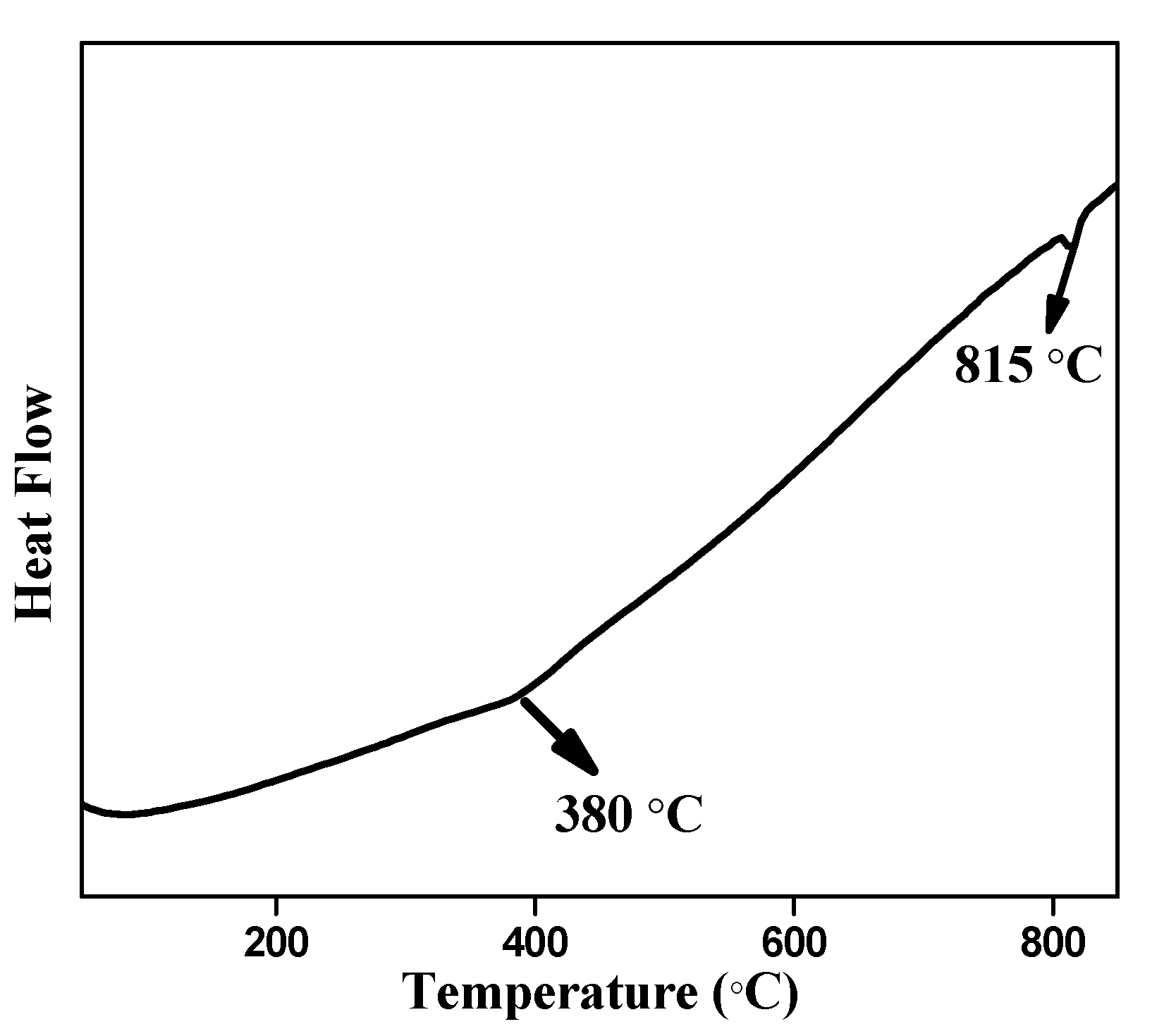
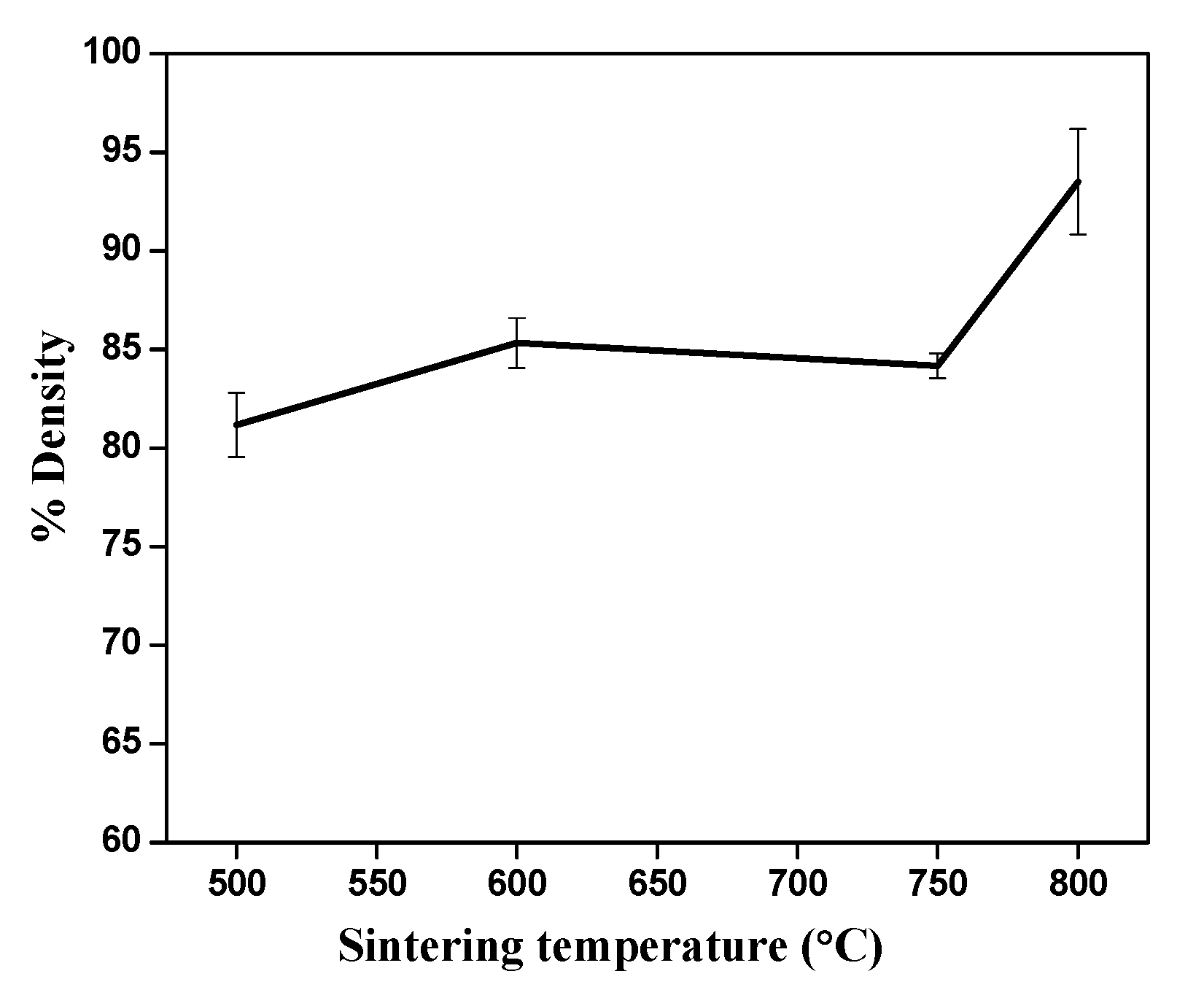
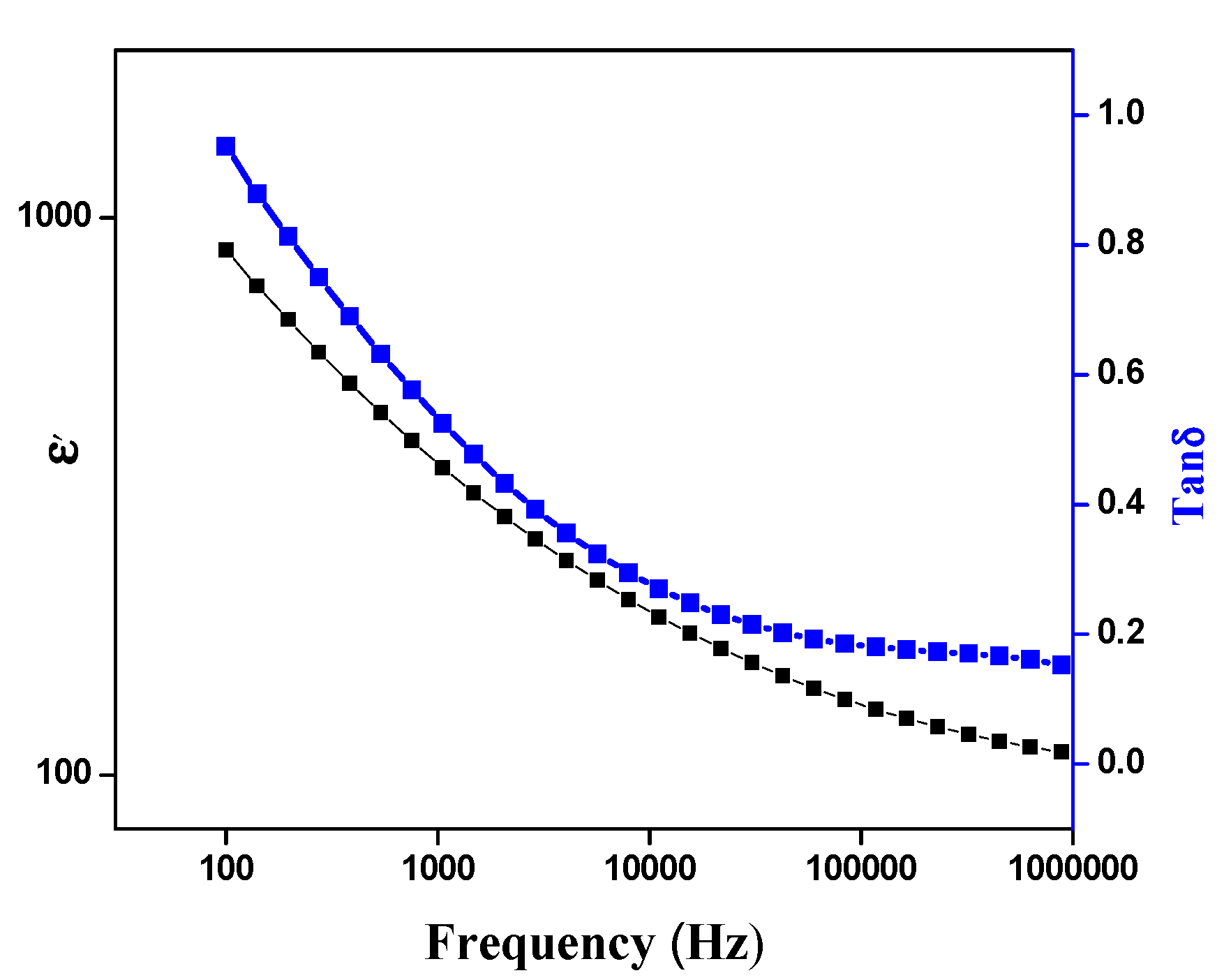
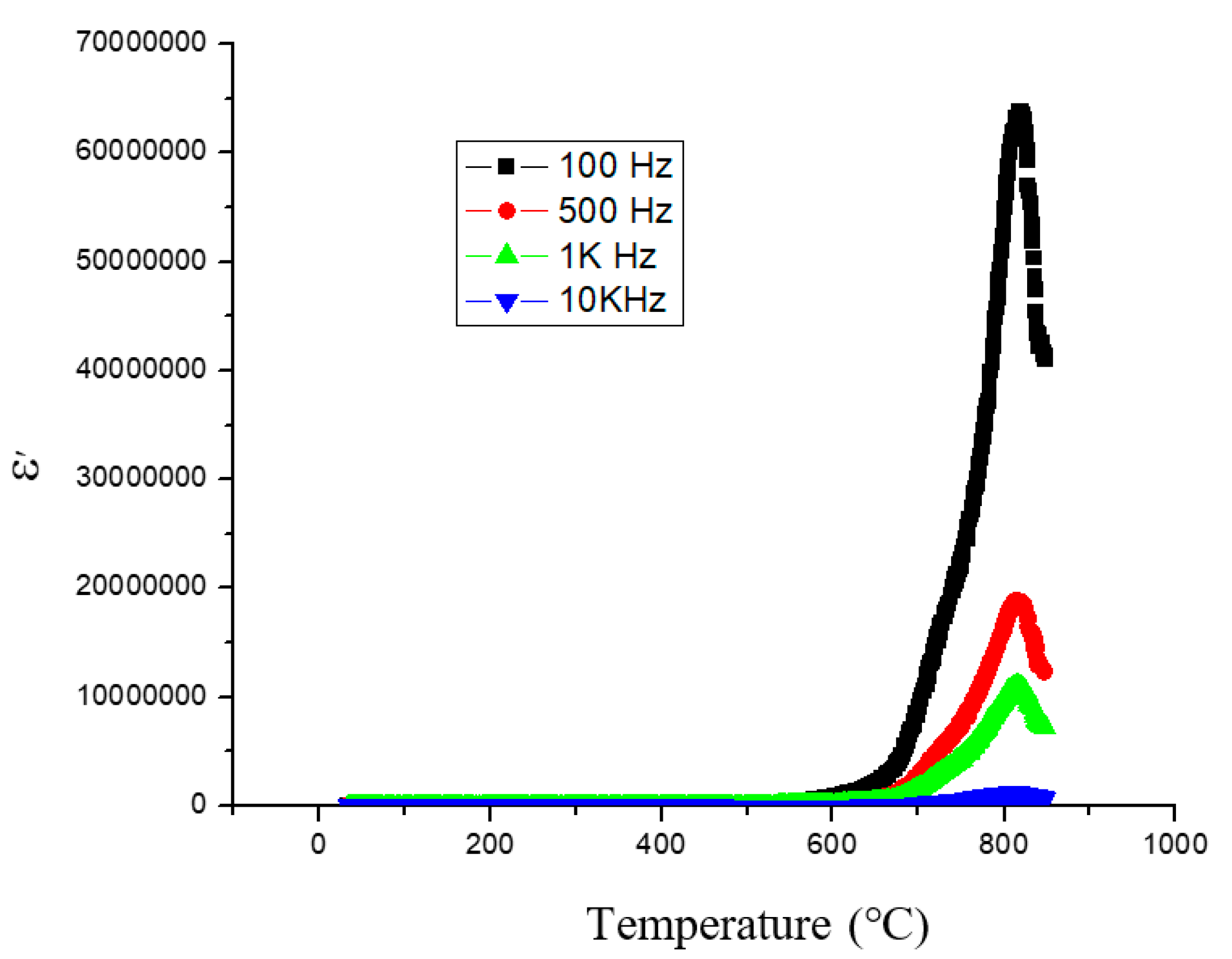
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ma, Y.; Lv, P.; Duan, F.; Sheng, J.; Lu, S.; Zhu, H.; Du, M.; Chen, M. Direct Z-scheme Bi2S3/BiFeO3 heterojunction nanofibers with enhanced photocatalytic activity. J. Alloys Compd. 2020, 834, 155158. [Google Scholar] [CrossRef]
- Carvalho, T.T.; Manjunath, B.; Pérez de la Cruz, J.; Amaral, V.S.; Fernandes, J.R.A.; Almeida, A.; Moreira, J.A.; Vilarinho, R.; Tavares, P.B. Enhancement of resistivity and magnetization of Bi1-xLaxFe1-yMnyO3 ceramics by composition optimization. J. Alloys Compd. 2020, 835, 155404. [Google Scholar] [CrossRef]
- Liu, J.; Niu, M.; Wang, L.; Peng, C.; Xu, D. Effect of tuning A/B substitutions on multiferroic characteristics of BiFeO3-based ternary system ceramics. J. Mag. Mag. Mater. 2020, 510, 166928–166937. [Google Scholar] [CrossRef]
- Wang, J.; Neaton, J.B.; Zheng, H.; Nagarajan, V.; Ogale, S.B.; Liu, B.; Viehland, D.; Vaithyanathan, V.; Schlom, D.G.; Waghmare, U.V.; et al. Epitaxial BiFeO3 Multiferroic Thin Film Heterostructures. Science 2003, 299, 1719–1722. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Maggard, P.A. Hydrothermal Synthesis and Photocatalytic Activities of SrTiO3-Coated Fe2O3 and BiFeO3. Adv. Mater. 2006, 18, 514–517. [Google Scholar]
- Li, S.; Lin, Y.H.; Zhang, B.P.; Li, J.F.; Nan, C.W.J. BiFeO3/TiO2 core-shell structured nanocomposites as visible-active photocatalysts and their optical response mechanism. Appl. Phys. 2009, 105, 054310. [Google Scholar] [CrossRef]
- Gao, F.; Yuan, Y.; Wang, K.F.; Chen, X.Y.; Chen, F.; Liu, J.M.; Ren, Z.F. Preparation and photoabsorption characterization of nanowires. Appl. Phys. Lett. 2006, 89, 102506. [Google Scholar] [CrossRef]
- Joshi, U.A.; Jang, J.S.; Borse, P.H.; Lee, J.S. Microwave synthesis of single-crystalline perovskite BiFeO3 nanocubes for photoelectrode and photocatalytic applications. Appl, Phys. Lett. 2008, 92, 242106. [Google Scholar] [CrossRef]
- Tahir, M.; Riaz, S.; Khan, U.; Hussain, S.S.; Nairan, A.; Akbar, A.; Saleem, M.; Atiq, S.; Naseem, S. Enhanced structural and magnetic ordering in as-synthesized Ca doped bismuth iron oxide nanoceramics. J. Alloys Compd. 2020, 832, 154725. [Google Scholar] [CrossRef]
- Auromun, K.; Choudhary, R.N.P. Structural, Dielectric and Electrical investigation of Zirconium and Tin modified 0.5BFO-0.5BST. Mater. Chem. Phys. 2020, 250, 123033. [Google Scholar] [CrossRef]
- Selbach, M.; Einarsrud, M.-A.; Grande, T. On the Thermodynamic Stability of BiFeO3. Chem. Mater. 2009, 21, 169–173. [Google Scholar] [CrossRef]
- Achenbach, G.D.; James, W.J.; Gerson, R. Preparation of Single-Phase Polycrystalline BiFeO3. J. Am. Ceram. Soc. 1967, 50, 437. [Google Scholar] [CrossRef]
- Valant, M.; Axelsson, A.K.; Alford, N. Peculiarities of a Solid-State Synthesis of Multiferroic Polycrystalline BiFeO3. Chem. Mater. 2007, 19, 5431–5436. [Google Scholar] [CrossRef]
- Shetty, S.; Palkar, V.R.; Pinto, R. Size effect study in magnetoelectric BiFeO3 system. J. Phys. 2002, 58, 1027–1030. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, S.S.; Kim, W.J. Sol–Gel synthesis and properties of multiferroic BiFeO3. Mater. Lett. 2005, 59, 4006–4009. [Google Scholar] [CrossRef]
- Chen, C.; Cheng, J.; Yu, S.; Che, L.; Meng, Z. Hydrothermal synthesis of perovskite bismuth ferrite crystallites. J. Cryst. Growth 2006, 291, 135–139. [Google Scholar] [CrossRef]
- Tabares-Munoz, C.; Rivera, J.P.; Monnier, A.; Schmid, H. Measurement of the quadratic magnetoelectric effect on single crystalline BiFeO3. J. Appl. Phys. Suppl. 1985, 24, 1051–1053. [Google Scholar] [CrossRef]
- Palkar, V.R.; John, J.; Pinto, R. Observation of saturated polarization and dielectric anomaly in magnetoelectric thin films. Appl. Phys. Lett. 2002, 80, 1628–1631. [Google Scholar] [CrossRef]
- Iakovlev, S.; Solterbeck, C.H.; Kuhnke, M.; Es-Souni, M. Multiferroic thin films processed via chemical solution deposition: Structural and electrical characterization. J. Appl. Phys. 2005, 97, 094901. [Google Scholar] [CrossRef]
- Fabienne, R.; Michael, T.; Thorsten, B.; Hans, H.; Peter, D.J.; Josef, R.; Juan, C.; Miguel, F. Particle density determination of pellets and briquettes. Biomass Bioenergy 2006, 30, 954–963. [Google Scholar]
- Mazumder, R.; Chakravarty, D.; Bhattacharya, D.; Sen, A. Spark plasma sintering of BiFeO3. Mater. Res. Bull. 2009, 44, 555–559. [Google Scholar] [CrossRef]
- Fang, T.T.; Ting, C.C.; Miao, J.H. A Template-Free Synthesis of the One-Dimensional Nanostructure of Multiferroic BiFeO3. J. Am. Ceram. Soc. 2009, 92, 3065–3069. [Google Scholar] [CrossRef]
- Xiaobo, H.; Lian, G. Synthesis of pure phase BiFeO3 powders in molten alkali metal nitrates. Ceram. Int. 2009, 35, 975–978. [Google Scholar]
- Bondioli, F.; Bonamartini, C.A.; Leonelli, C.; Manfredini, T. Nanosized CeO2 powders obtained by flux method. Mater. Res. Bull. 1999, 34, 2159–2166. [Google Scholar] [CrossRef]
- Du, Y.; Cheng, Z.X.; Dou, S.X.; Shahbazi, M.; Wang, X.L. Enhancement of magnetization and dielectric properties of chromium-doped BiFeO3 with tunable morphologies. Thin Solid Film. 2010, 518, e5–e8. [Google Scholar] [CrossRef]
- Chang, F.; Zhang, N.; Yang, F.; Wang, S.; Song, G. Effect of Cr substitution on the structure and electrical properties of BiFeO3 ceramics. J. Phys. D 2007, 40, 7799–7803. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Z.; Liu, Q.; Yao, K. Ferroelectric properties of BiFeO3 films grown by sol–gel process. Thin Solid Films 2006, 500, 105–109. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syed, A.; Siddaramanna, A.; Elgorban, A.M.; Hakeem, D.A.; Nagaraju, G. Hydrogen Peroxide-Assisted Hydrothermal Synthesis of BiFeO3 Microspheres and Their Dielectric Behavior. Magnetochemistry 2020, 6, 42. https://doi.org/10.3390/magnetochemistry6030042
Syed A, Siddaramanna A, Elgorban AM, Hakeem DA, Nagaraju G. Hydrogen Peroxide-Assisted Hydrothermal Synthesis of BiFeO3 Microspheres and Their Dielectric Behavior. Magnetochemistry. 2020; 6(3):42. https://doi.org/10.3390/magnetochemistry6030042
Chicago/Turabian StyleSyed, Asad, Ashoka Siddaramanna, Abdallah M. Elgorban, D. A. Hakeem, and G. Nagaraju. 2020. "Hydrogen Peroxide-Assisted Hydrothermal Synthesis of BiFeO3 Microspheres and Their Dielectric Behavior" Magnetochemistry 6, no. 3: 42. https://doi.org/10.3390/magnetochemistry6030042
APA StyleSyed, A., Siddaramanna, A., Elgorban, A. M., Hakeem, D. A., & Nagaraju, G. (2020). Hydrogen Peroxide-Assisted Hydrothermal Synthesis of BiFeO3 Microspheres and Their Dielectric Behavior. Magnetochemistry, 6(3), 42. https://doi.org/10.3390/magnetochemistry6030042





