Design, Characterization and Molecular Modeling of New Fluorinated Paramagnetic Contrast Agents for Dual 1H/19F MRI
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis
- Synthesis of the lanthanide complexes bearing an azide function:
- Synthesis of the diamagnetic fluorine compound:
- Synthesis of the fluorinated lanthanide complexes:
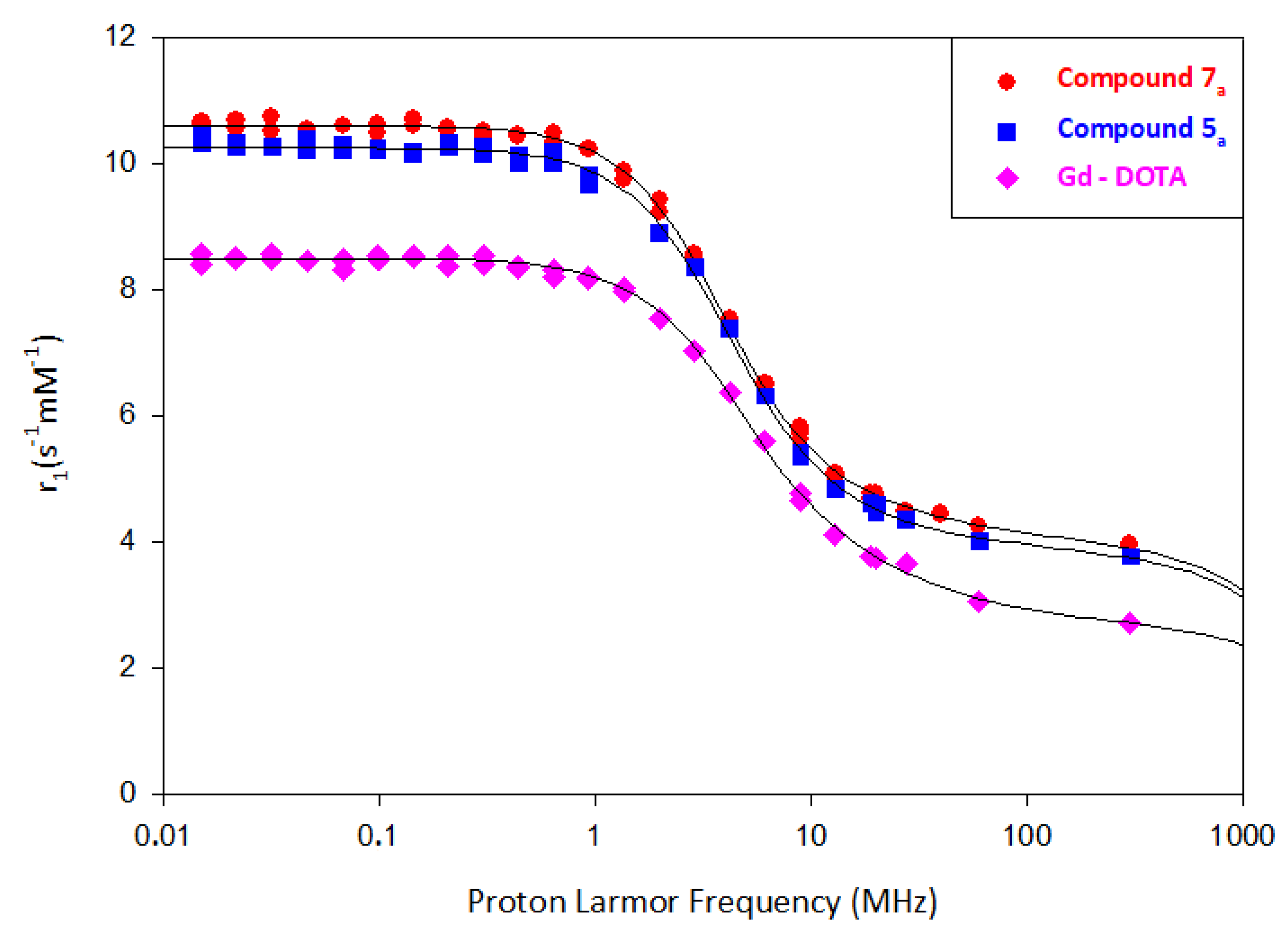
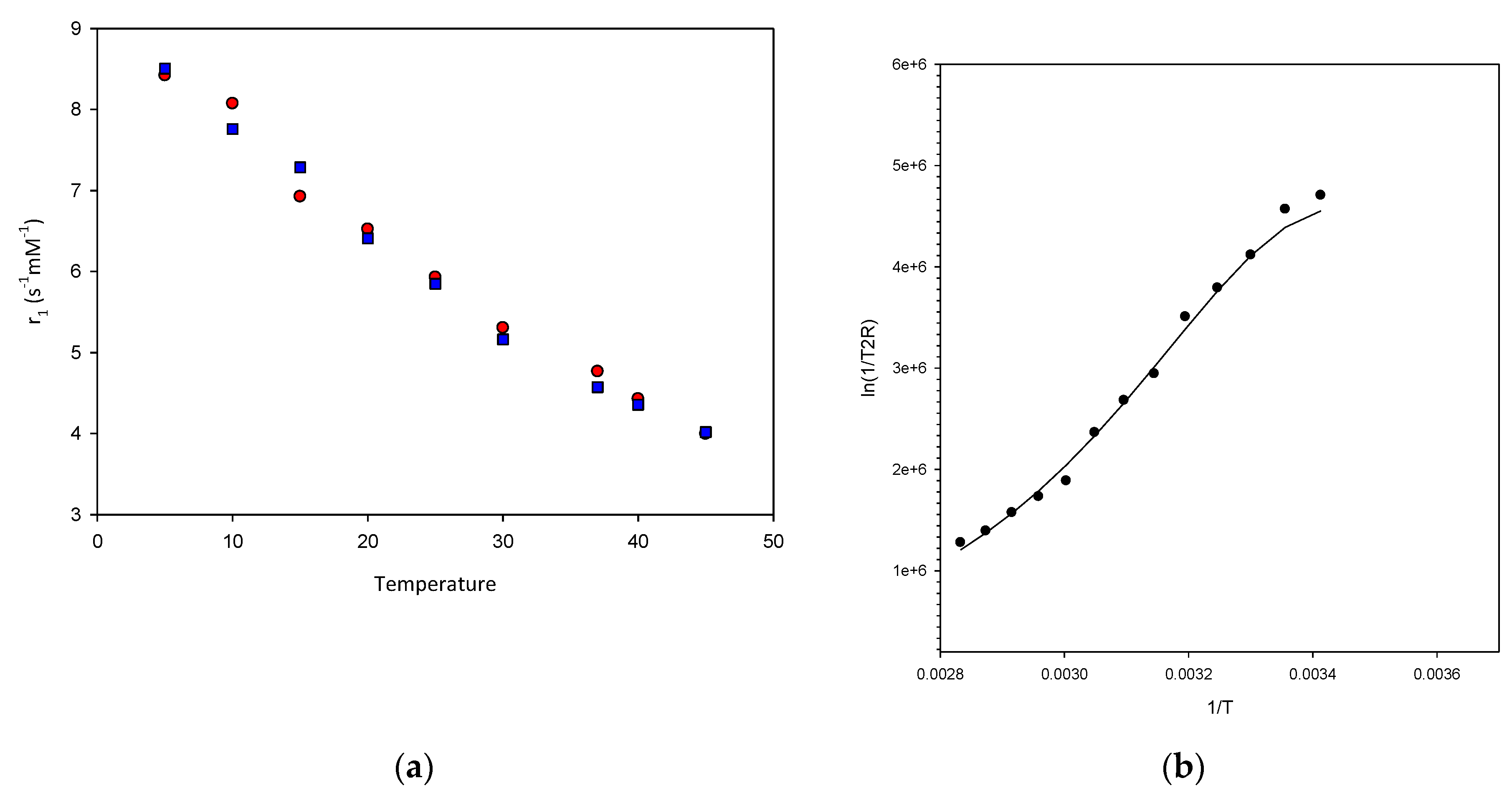
2.2. Relaxivity Measurements
2.3. Phantom Images
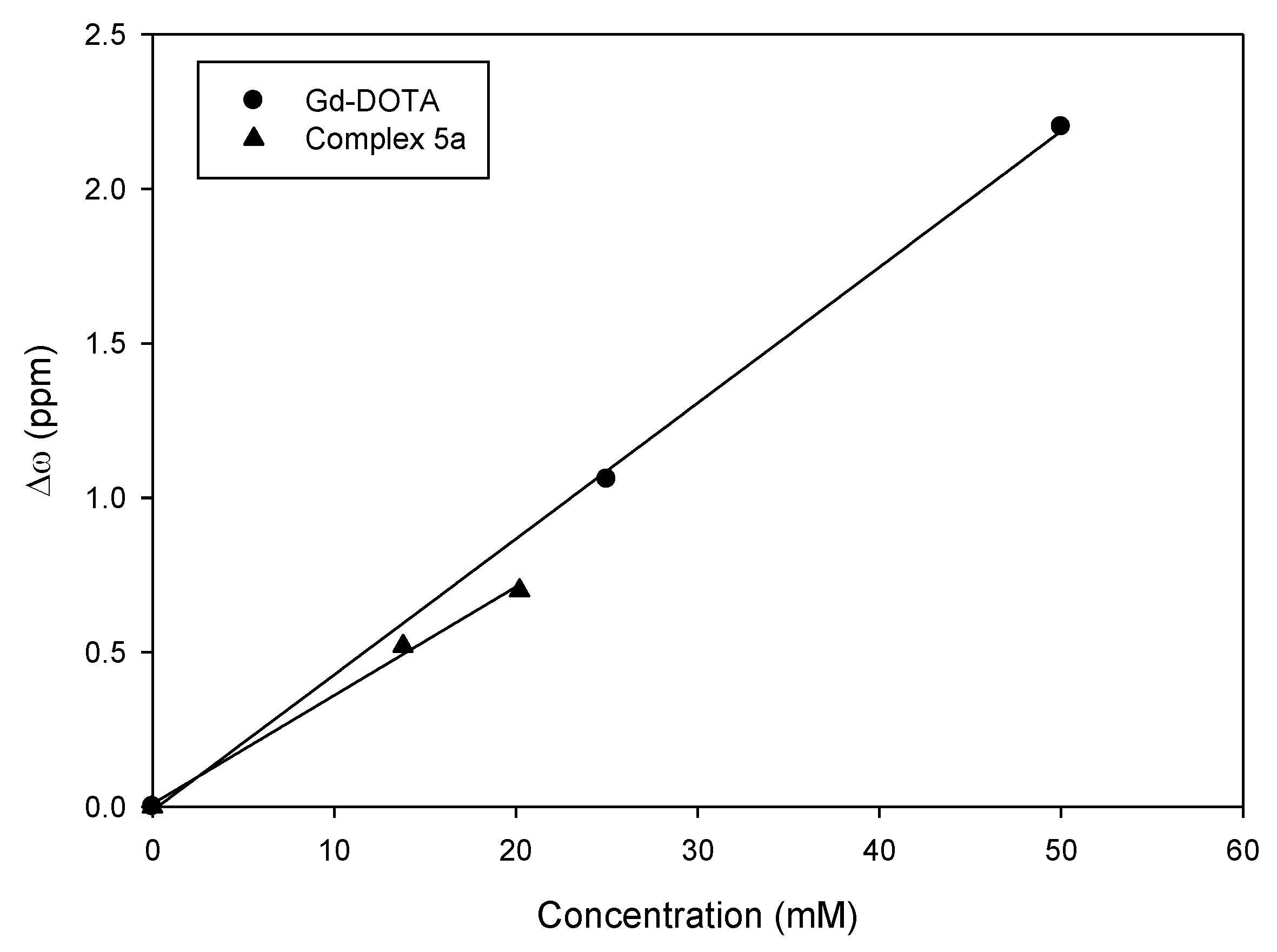
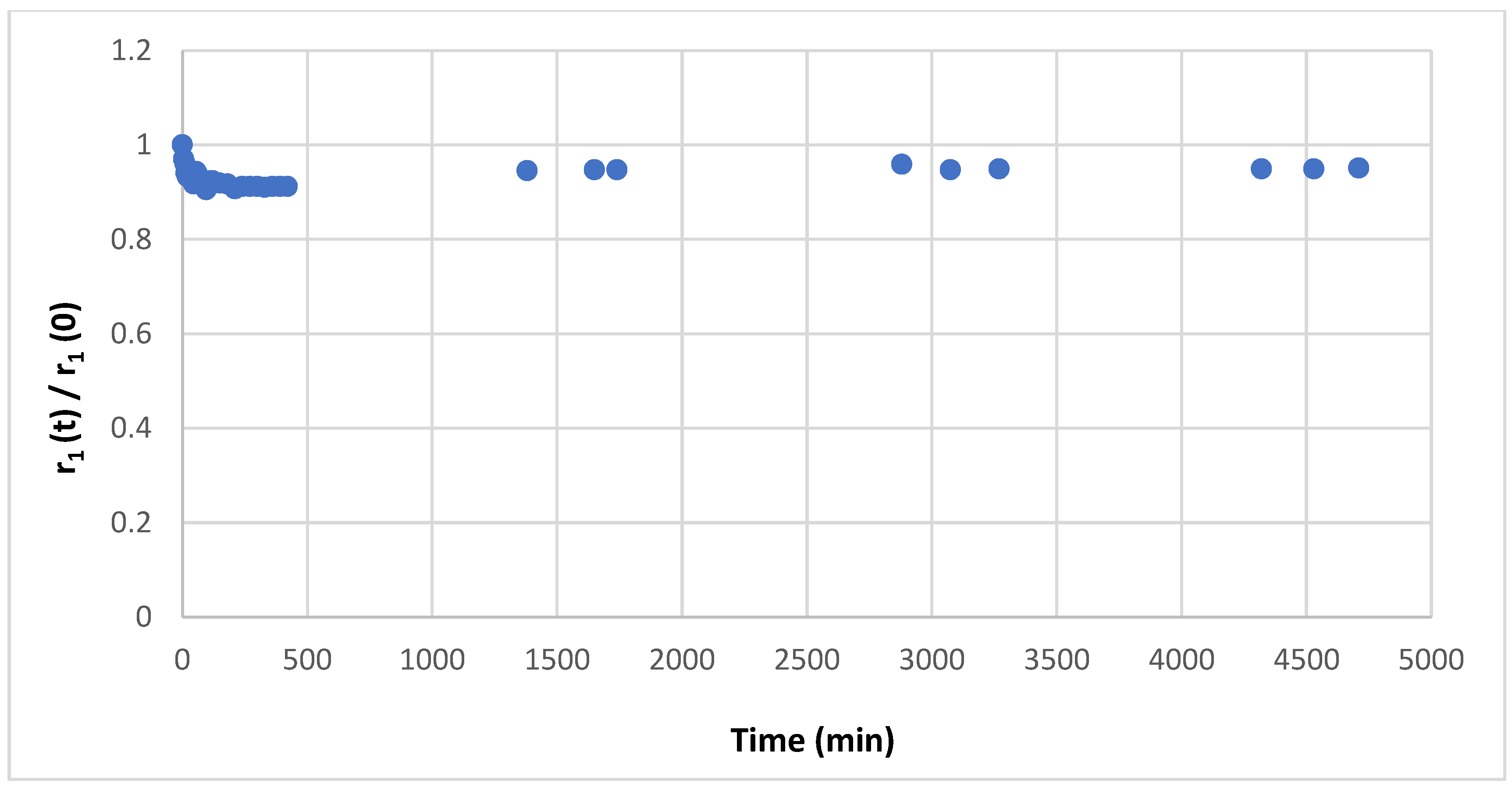
2.4. Transmetallation
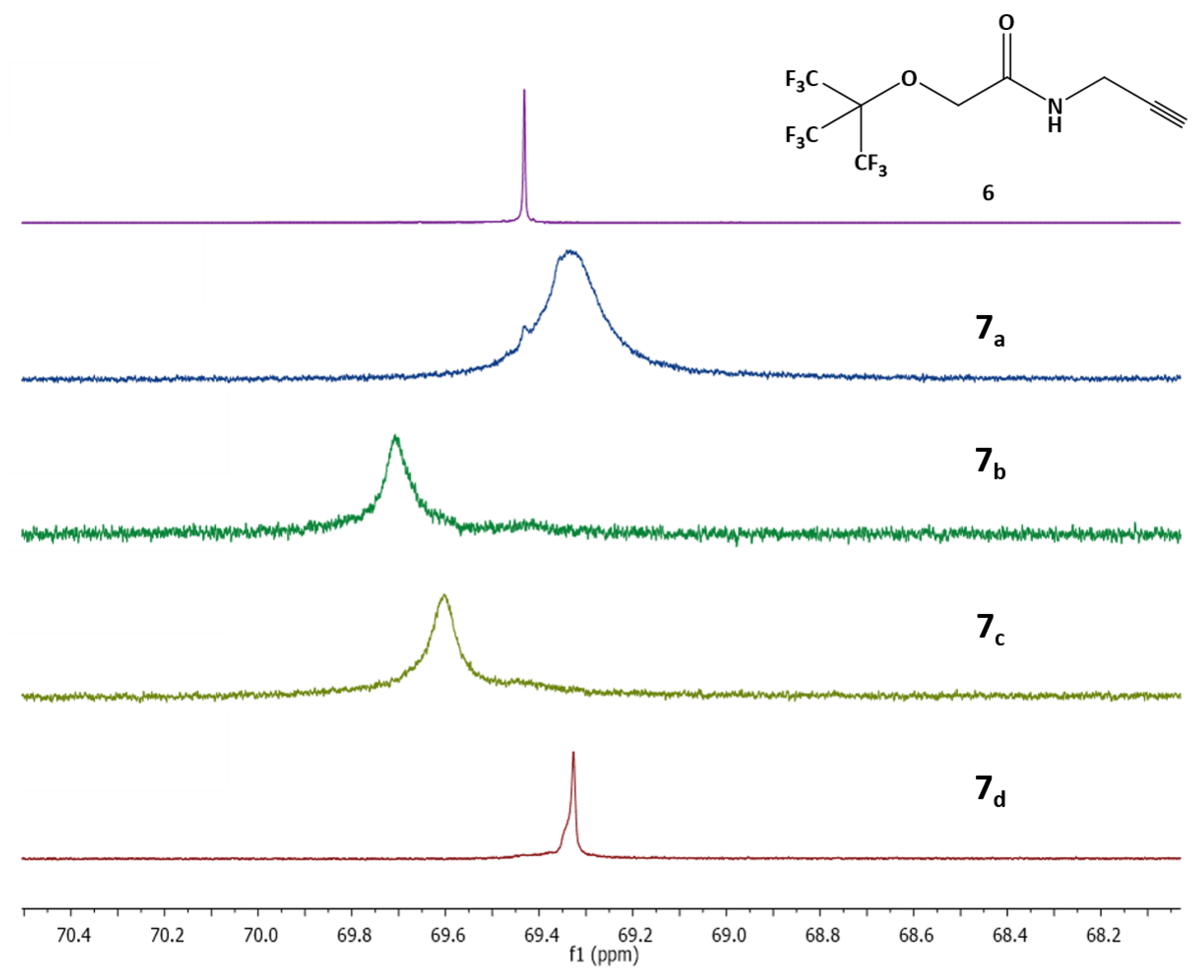
2.5. 19F Measurements
3. Materials and Methods
3.1. 1H Relaxation Measurements and 1H NMRD Profiles
3.2. 17O NMR
3.3. T1-Weighted Images
3.4. Transmetallation
3.5. 1H–13C–19F Measurements
- 1.
- Synthesis of 6-azidohexanoic acid (1)
- 2.
- Synthesis of tert-butyl (2-(6-azidohexanamido)ethyl) carbamate (2)
- 3.
- Synthesis of N-(2-aminoethyl)-6-azidohexanamide (3)
- 4.
- Synthesis of 2,2′,2″-(10-(4-((2-(6-azidohexanamido)ethyl)amino)-1-carboxy-4-oxobutyl) -1,4,7,10-tetraazacyclododecane-1,4,7-triyl)triacetic acid (4)
- 5.
- Synthesis of {(2,2′,2″-(10-(4-((2-(6-azidohexanamido)ethyl)amino)-1-carboxylato-4-oxobutyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl)triacetate)}lanthanide (5)
- 6.
- Synthesis of 2-(nonafluorotert-but-oxy)-N-(prop-2-yn-1-yl)acetamide (6)
3.6. Synthesis of Complexes 7a–d
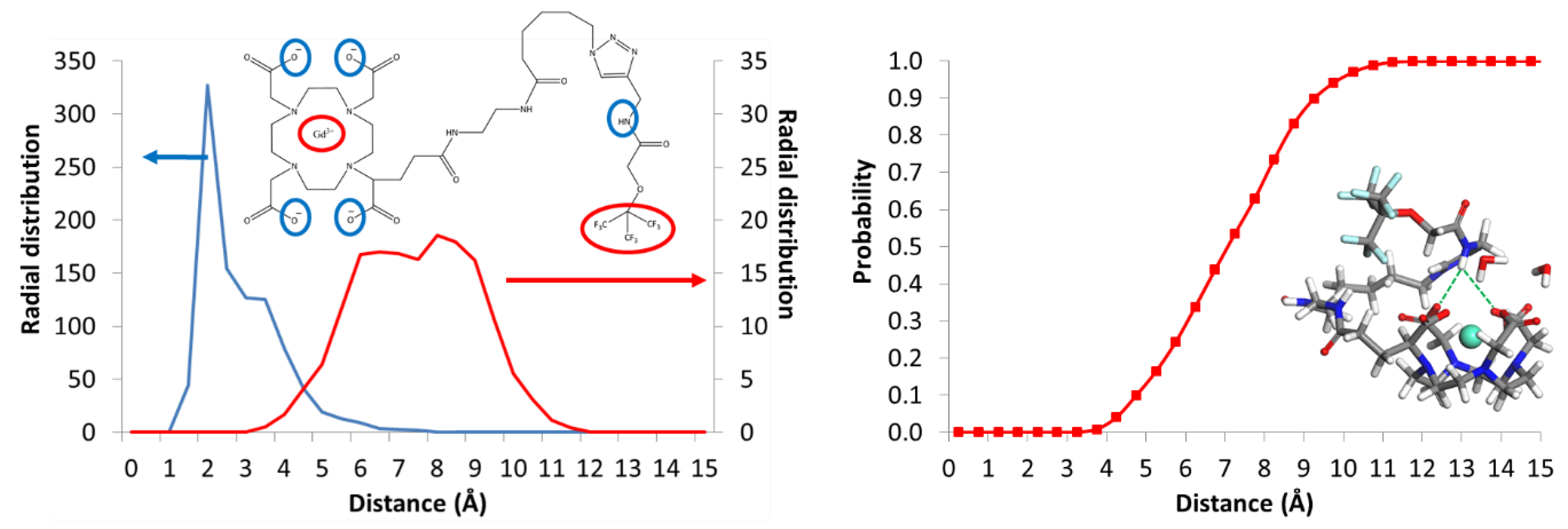
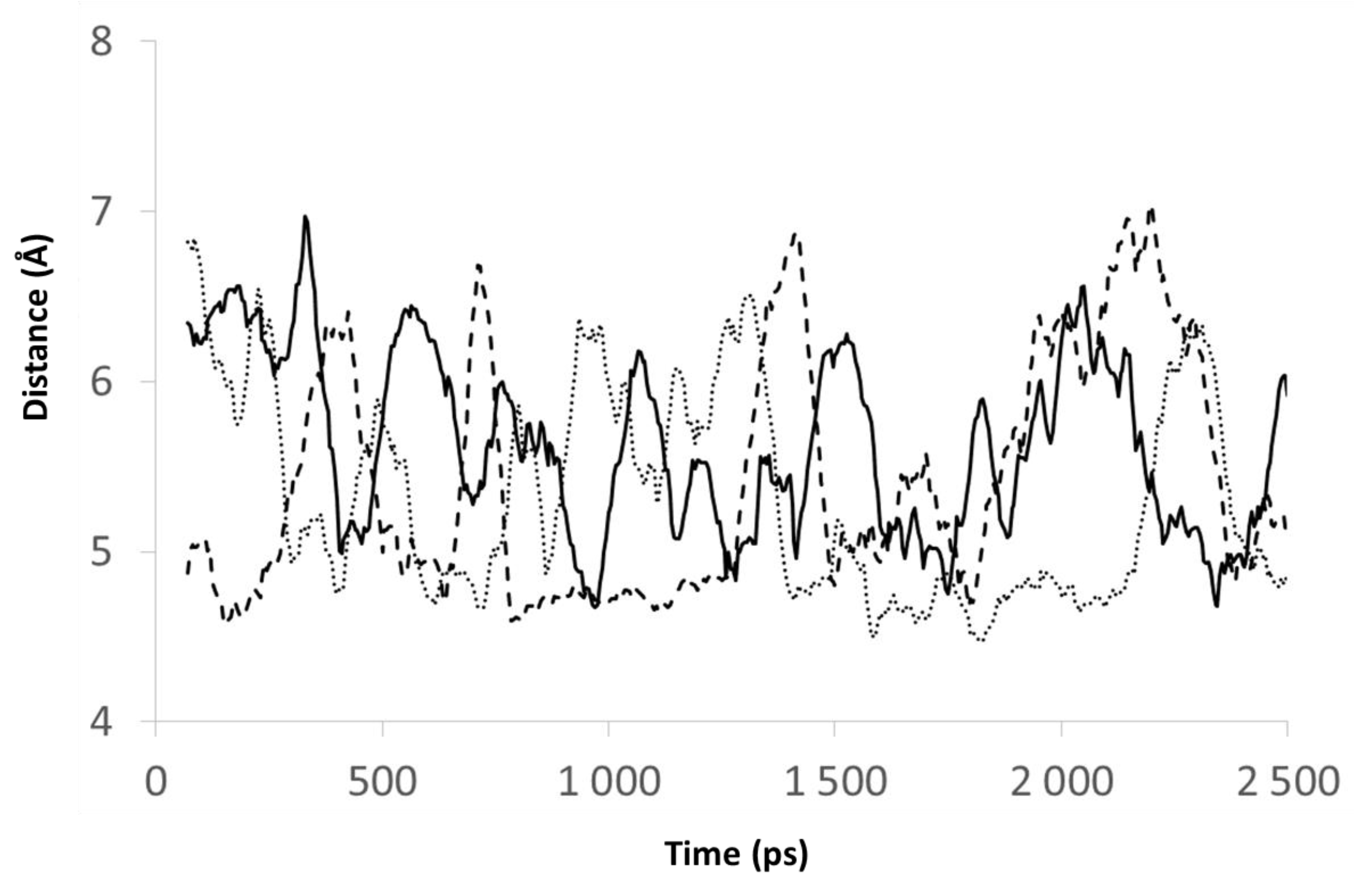
3.7. Modeling Strategy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wahsner, J.; Gale, E.M.; Rodriguez-Rodriguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef]
- De Leon Rodriguez, L.; Martins, A.; Pinho, M.; Rofsky, N.; Sherry, A.D. Basic MR Relaxation Mechanisms & Contrast Agent Design. J. Magn. Reason. Imaging 2015, 42, 545–565. [Google Scholar]
- Gianolio, E.; Di Gregorio, E.; Aime, S. Chemical Insights into the tissues of Gd-retention in brain and other tissues upon the admnistration of Gd-containing MRI contrast agents. Eur. J. Inorg. Chem. 2019, 2019, 137–151. [Google Scholar] [CrossRef]
- Runge, V.M. Critical Questions Regarding Gadolinium Deposition in the Brain and Body After Injections of the Gadolinium-Based Contrast Agents, Safety. Inve. Radiol. 2017, 52, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, I.; Roos, R.; van der Molen, A. Gadolinium retention after administration of contrast agents based on linear chelators and the recommendations of the European Medicines Agency. Eur. Radiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.; Toth, E. Next-Generation Magnetic Resonance Imaging Contrast Agents. Inorg. Chem. 2017, 56, 6029–6034. [Google Scholar] [CrossRef] [PubMed]
- Caschera, L.; Lazzara, A.; Piergallini, L.; Ricci, D.; Tuscano, B.; Vanzulli, A. Contrast agents in diagnostic imaging: Present and future. Pharmacol. Res. 2016, 110, 65–75. [Google Scholar] [CrossRef]
- Ruiz-Cabello, J.; Barnett, B.P.; Bottomley, P.A. Bulte JWM, Fluorine (19F) MRS and MRI in biomedicine. NMR Biomed. 2011, 24, 114–129. [Google Scholar] [CrossRef]
- Keliris, A.; Schefler, K.; Engelmann, J. 5. Responsve Probes for 19F MRS/MRI. In Fluorine Magnetic Resonance Imaging; Flögel, U., Ahrens, E., Eds.; Pan Stanford Publishing: Singapore (Singapore), 2017; Chapter 5; pp. 141–190. [Google Scholar]
- Kenwright, A.M.; Kuprov, I.; De Luca, E.; Parker, D.; Pandya, S.U.; Senanayake, P.K.; Smith, D.G. 19F NMR based pH probes: Lanthanide (III) complexes with pH-sensitive chemical shifts. Chem. Commun. 2008, 22, 2514–2516. [Google Scholar] [CrossRef]
- Peterson, K.L.; Srivastava, K.; Pierre, V.C. Fluorinated Paramagnetic Complexes: Sensitive and Responsive Probes for Magnetic Resonance Spectroscopy and Imaging. Front. Chem. 2018, 6, 120. [Google Scholar] [CrossRef]
- Harvey, P.; Kuprov, I.; Parker, D. Lanthanide Complexes as Paramagnetic Probes for 19F Magnetic Resonance. Eur. J. Inorg. Chem. 2012, 2012, 2015–2022. [Google Scholar] [CrossRef]
- Knight, J.C.; Edwards, G.; Paisey, S.J. Fluorinated contrast agents for magnetic resonance imaging; a review of recent developments. RSC Adv. 2011, 1, 1415–1425. [Google Scholar] [CrossRef]
- Tirotta, I.; Dichiarante, V.; Pigliacelli, C.; Cavallo, G.; Terraneo, G.; Bombelli, F.B.; Metrangolo, P.; Resnati, G. 19F Magnetic Resonance Imaging (MRI): From Design of Materials to Clinical Applications. Chem. Rev. 2015, 115, 1106–1129. [Google Scholar] [CrossRef] [PubMed]
- Hequet, E.; Henoumont, C.; Muller, R.N.; Laurent, S. Fluorinated MRI contrast agents and their versatile applications in the biomedical field. Future Med. Chem. 2019, 11, 1157–1175. [Google Scholar] [CrossRef] [PubMed]
- Boccalon, M.; Franchi, P.; Lucarini, M.; Delgado, J.J.; Sousa, F.; Stellacci, F.; Zucca, I.; Scotti, A.; Spreafico, R.; Pengo, P.; et al. Gold nanoparticles protected by fluorinated ligands for 19F MRI. Chem. Commun. 2013, 49, 8794–8796. [Google Scholar] [CrossRef]
- Pengo, P.; Pasquato, L. Gold nanoparticles protected by fluorinated ligands: Syntheses, properties and applications. J. Fluorine Chem. 2015, 177, 2–10. [Google Scholar] [CrossRef]
- Sologan, M.; Padelli, F.; Giachetti, I.; Aquino, D.; Boccalon, M.; Adami, G.; Pengo, P.; Pasquato, L. Functionalized Gold Nanoparticles as Contrast Agents for Proton and Dual Proton/Fluorine MRI. Nanomaterials 2019, 9, 879. [Google Scholar] [CrossRef]
- Nakamura, T.; Sugihara, F.; Matsushita, H.; Yoshioka, Y.; Mizukami, S.; Kikuchi, K. Mesoporous silica nanoparticles for 19F resonance imaging, fluorescence imaging, and drug delivery. Chem. Sci. 2015, 6, 1986–1990. [Google Scholar] [CrossRef]
- Matsushita, H.; Mizukami, S.; Sugihara, F.; Nakanishi, Y.; Yoshioka, Y.; Kikuchi, Y. Multifunctional core-shell silica nanoparticles for highly sensitive 19F magnetic resonance imaging. Angew. Chem. Int. Ed. 2014, 53, 1008–1011. [Google Scholar] [CrossRef]
- Bouchoucha, M.; van Heeswijk, R.B.; Gossuin, Y.; Kleitz, F.; Fortin, M.-A. Fluorinated Mesoporous Silica Nanoparticles for Binuclear Probes in 1H and 19F Magnetic Resonance Imaging. Langmuir 2017, 33, 10531–10542. [Google Scholar] [CrossRef]
- Yu, Y.B. Fluorinated dendrimers as imaging agents for 19F MRI. WIRES Nanomed Nanobiotechnol. 2013, 5, 646–661. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ainsa, S.; Barberá, J. Fluorinated liquid crystalline dendrimers. J. Fluorine Chem. 2015, 177, 37–45. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, Y.; Bo, S.; Li, Y.; Yang, Z.; Zhou, X.; Chen, S.; Jiang, Z.-X. Monitoring Fluorinated Dendrimer-Based Self-Assembled Drug-Delivery Systems with 19F Magnetic Resonance. Eur. J. Org. Chem. 2017, 2017, 4461–4468. [Google Scholar] [CrossRef]
- Biaggi, C.; Benaglia, M.; Ortenzi, M.; Micotti, E.; Perego, C.; De Simoni, M.-G. Easily available, low cost 19F MRI agents: Poly(ethylene-glycol)-functionalized fluorinated ethers. J. Fluorine Chem. 2013, 153, 172–177. [Google Scholar] [CrossRef]
- Sedlacek, O.; Jirak, D.; Galisova, A.; Jager, E.; Laaser, J.E.; Lodge, T.P.; Stepanek, P.; Hruby, M. 19F Magnetic Resonance Imaging of Injectable Polymeric Implants with Multiresponsive Behavior. Chem. Mater. 2018, 30, 4892–4896. [Google Scholar] [CrossRef]
- Jirak, D.; Galisova, A.; Kolouchova, K.; Babuka, D.; Hruby, M. Fluorine polymer probes for magnetic resonance imaging: Quo vadis? Magn. Reson. Mater. Phys. Biol. Med. 2019, 32, 173–185. [Google Scholar] [CrossRef]
- Schmieder, A.H.; Caruthers, S.D.; Keupp, J.; Wickline, S.A.; Lanza, G.M. Recent Advances in 19Fluorine Magnetic Resonance Imaging with Perfluorocarbon Emulsions. Engineering 2015, 1, 475–489. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Q.; Tian, J.-H.; Xing, J.-F.; Guo, W.; Liang, X.-J. Perfluorocarbon-based nanomedicine: Emerging strategy for diagnosis and treatment of diseases. Mater. Res. Soc. 2018, 8, 303–313. [Google Scholar] [CrossRef]
- Chapelin, F.; Capitini, C.M.; Ahrens, E.T. Fluorine-19 MRI for detection and quantification of immune cell therapy for cancer. J. Immunother. Cancer 2018, 6, 105. [Google Scholar] [CrossRef]
- Akazawa, K.; Sugihara, F.; Nakamura, T.; Matsushita, H.; Mukai, H.; Akimoto, R.; Minoshima, M.; Mizukami, S.; Kakuchi, K. Perfluorocarbon-based 19F MRI Nanoprobes for In Vivo Multicolor Imaging. Angew. Chem. Int. Ed. 2018, 57, 16742–16747. [Google Scholar] [CrossRef]
- Senanayake, P.K.; Kenwright, A.M.; Parker, D.; Van Der Hoorn, S.K. Responsive fluorinated lanthanide probes for 19F magnetic resonance spectroscopy. Chem. Commun. 2007, 28, 2923–2925. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, K.H.; De Luca, E.; Hogg, N.H.M.; Kenwright, A.M.; Kuprov, I.; Parker, D.; Botta, M.; Wilson, J.I.; Blamire, D.A.M. Design principles and theory of paramagnetic fluorine-labelled lanthanide complexes as probes for 19F magnetic resonance: A proof-of-concept study. Chem. Eur. J. 2010, 16, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Wolters, M.; Mohades, S.G.; Hackeng, T.M.; Post, M.J.; Kooi, M.E.; Backes, W.H. Clinical Perspectives of Hybrid Proton-Fluorine Magnetic Resonance Imaging and Spectroscopy. Inve. Radiol. 2012, 48, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Schmid, F.; Höltke, C.; Parker, D.; Faber, C. Boosting 19F MRI-SNR efficient detection of paramagnetic contrast agents using ultrafast sequences. Magn. Reson. Med. 2013, 69, 1056–1062. [Google Scholar] [CrossRef]
- Bartusik, D.; Aebisher, D. 19F applications in drug development and imaging—A review. Biomed. Pharmacother. 2014, 68, 813–817. [Google Scholar] [CrossRef]
- Peng, Q.; Li, Y.; Bo, S.; Yuan, Y.; Yang, Z.; Chen, S.; Zhou, X.; Jiang, Z.-X. Paramagnetic nanoemulsions with unified signals for sensitive 19F MRI cell tracking. Chem. Commun. 2018, 54, 6000–6003. [Google Scholar] [CrossRef]
- Cakić, N.; Savić, T.; Stricker-Shaver, J.; Truffault, V.; Platas-Iglesias, C.; Mirkes, C.; Pohmann, R.; Scheffler, K.; Angelovski, G. Paramagnetic lanthanide chelates for multicontrast MRI. Chem. Commun. 2016, 52, 9224–9227. [Google Scholar] [CrossRef]
- Srivastava, K.; Ferrauto, G.; Young, V.G.; Aime, S.; Pierre, V.C. Eight-Coordinate, Stable Fe(II) Complex as a Dual 19F and CEST Contrast Agent for Ratiometric pH Imaging. Inorg. Chem. 2017, 56, 12206–12213. [Google Scholar] [CrossRef]
- Pujales-Paradela, R.; Savić, T.; Brandariz, I.; Perez-Lourido, P.; Angelovski, G.; Esteban-Gómez, D.; Platas-Iglesias, C. Reinforced Ni(II)-cyclam derivatives as dual 1H/19F MRI Probes. Chem. Commun. 2019, 55, 4115–4118. [Google Scholar] [CrossRef]
- Pujales-Paradela, R.; Savić, T.; Perez-Lourido, P.; Esteban-Gómez, D.; Angelovski, G.; Botta, M.; Platas-Iglesias, C. Lanthanide Complexes with 1H paraCEST and 19F Response for Magnetic Resonance Imaging Applications. Inorg. Chem. 2019, 58, 7571–7583. [Google Scholar] [CrossRef]
- Yu, M.; Bouley, B.S.; Xie, D.; Que, E.L. Highly fluorinated metal complexes as dual 19F and PARACEST imaging agents. Dalton Trans. 2019, 48, 9337–9341. [Google Scholar] [CrossRef] [PubMed]
- De Luca, E.; Harvey, P.; Chalmers, K.H.; Mishra, A.; Senanayake, P.K.; Wilson, J.I.; Botta, M.; Fekete, M.; Blamire, A.M.; Parker, D. Characterisation and evaluation of paramagnetic fluorine labelled glycol chitosan conjugates for 19F and 1H magnetic resonance imaging. J. Biol. Inorg. Chem. 2014, 19, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Pujales-Paradela, R.; Savić, T.; Esteban-Gómez, D.; Angelovski, G.; Carniato, F.; Botta, M.; Platas-Iglesias, C. Gadolinium (III)-Based Dual 1H/19F Magnetic Resonance Imaging Probes. Chem. Eur. J. 2019, 25, 4782–4792. [Google Scholar] [CrossRef] [PubMed]
- Berg, R.; Straub, B.F. Advancements in the mechanistic understanding of the copper-catalyzed azide—alkyne cycloaddition. Beilstein J. Org. Chem. 2013, 9, 2715–2750. [Google Scholar] [CrossRef]
- Solomon, I. Relaxation Processes in a System of Two Spins. Phys. Rev. 1955, 99, 559–565. [Google Scholar] [CrossRef]
- Bloembergen, N. Proton Relaxation Times in Paramagnetic Solutions. J. Chem. Phys. 1957, 27, 572–573. [Google Scholar] [CrossRef]
- Bloembergen, N.; Morgan, L.O. Proton Relaxation Times in Paramagnetic Solutions. Effects of Electron Spin Relaxation. J. Chem. Phys. 1961, 34, 842–850. [Google Scholar] [CrossRef]
- Henoumont, C.; Vander Elst, L.; Laurent, S.; Muller, R.N. Synthesis and Physicochemical Characterization of Gd-C4-Thyroxin-DTPA, a Potential MRI Contrast Agent. J. Phys. Chem. B 2010, 114, 3689–3697. [Google Scholar] [CrossRef]
- Laurent, S.; Henoumont, C.; Stanicki, D.; Boutry, S.; Lipani, E.; Belaid, S.; Muller, R.N.; Vander Elst, L. Contrast Agents From Molecules to Particles; Springer: Singapore (Singapore), 2016. [Google Scholar]
- Laurent, S.; Vander Elst, L.; Henoumont, C.; Muller, R.N. How to measure the transmetallation of a gadolinium complex. Contrast Media Mol. Imaging 2010, 5, 305–308. [Google Scholar] [CrossRef]
- Mayo, S.L.; Olafson, B.D.; Goddard, W.A. DREIDING: A Generic Force Field for Molecular Simulations. J. Phys. Chem. 1990, 94, 8897–8909. [Google Scholar] [CrossRef]
- Consentino, U.; Villa, A.; Pitea, D.; Moro, G.; Barone, V.; Maiocchi, A. Conformational Characterization of Lanthanide(III)–DOTA Complexes by ab Initio Investigation in Vacuo and in Aqueous Solution. J. Am. Chem. Soc. 2002, 124, 4901–4909. [Google Scholar] [CrossRef] [PubMed]
- Rappe, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A.; Skiff, W.M. UFF, a Full Periodic Table Force Field for Molecular Mechanics and Dynamics Simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Opina, A.C.L.; Strickland, M.; Lee, Y.-S.; Tjandra, N.; Byrd, R.R.; Swenson, R.E.; Vasalatiy, O. Analysis of the isomer ratios of polymethylated-DOTA complexes and the implications on protein structural studies. Dalton Trans. 2016, 45, 4673–4687. [Google Scholar] [CrossRef] [PubMed]
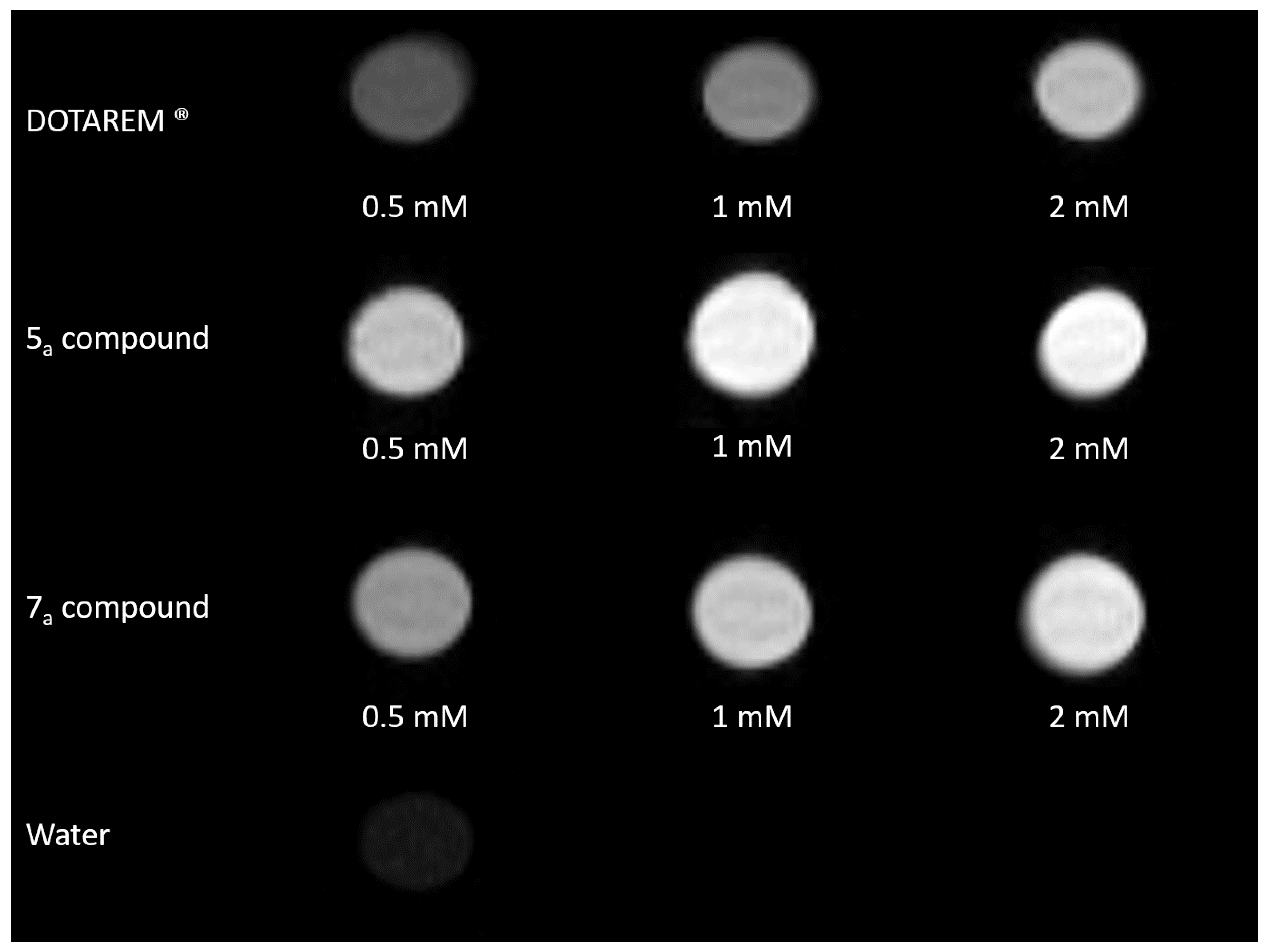
| DOTAREM® | Compound 5a | Compound 7a | |
|---|---|---|---|
| d (nm) * | 0.36 | 0.36 | 0.36 |
| D (m2·s−1) * | 3.30 10−9 | 3.30 10−9 | 3.30 × 10−9 |
| r (nm) * | 0.31 | 0.31 | 0.31 |
| τR (ps) | 49.5 ± 0.4 | 79.9 ± 2.4 | 83.0 ± 2.0 |
| τM (ns) * | 122 | 50 | 53 |
| τS0 (ps) | 450.0 ± 24.0 | 301.0 ± 28.3 | 296.0 ± 19.4 |
| τV (ps) | 4.4 ± 1.1 | 6.5 ± 1.7 | 9.5 ± 1.3 |
| q * | 1 | 1 | 1 |
| Compound 6 | Gadolinium Complex 7a (1.7 mM) | Dysprosium Complex 7b (1.3 mM) | Terbium Complex 7c (1.9 mM) | Europium Complex 7d (2.0 mM) | |||||
|---|---|---|---|---|---|---|---|---|---|
| T1 (ms) | T2 (ms) | T1 (ms) | T2 (ms) | T1 (ms) | T2 (ms) | T1 (ms) | T2 (ms) | T1 (ms) | T2 (ms) |
| 2000 | 1800 | 13.3 | 8.6 | 206.6 | 77.3 | 468.1 | 186.9 | 1380 | 1177 |
| 1H T1 (ms) | 19F T1 (ms) | |
|---|---|---|
| Aqueous solution of 7a | 155 | 13.3 |
| Seronorm-based solution of 7a | 84 | 14.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hequet, E.; Henoumont, C.; Djouana Kenfack, V.; Lemaur, V.; Lazzaroni, R.; Boutry, S.; Vander Elst, L.; Muller, R.N.; Laurent, S. Design, Characterization and Molecular Modeling of New Fluorinated Paramagnetic Contrast Agents for Dual 1H/19F MRI. Magnetochemistry 2020, 6, 8. https://doi.org/10.3390/magnetochemistry6010008
Hequet E, Henoumont C, Djouana Kenfack V, Lemaur V, Lazzaroni R, Boutry S, Vander Elst L, Muller RN, Laurent S. Design, Characterization and Molecular Modeling of New Fluorinated Paramagnetic Contrast Agents for Dual 1H/19F MRI. Magnetochemistry. 2020; 6(1):8. https://doi.org/10.3390/magnetochemistry6010008
Chicago/Turabian StyleHequet, Emilie, Céline Henoumont, Vera Djouana Kenfack, Vincent Lemaur, Roberto Lazzaroni, Sébastien Boutry, Luce Vander Elst, Robert N. Muller, and Sophie Laurent. 2020. "Design, Characterization and Molecular Modeling of New Fluorinated Paramagnetic Contrast Agents for Dual 1H/19F MRI" Magnetochemistry 6, no. 1: 8. https://doi.org/10.3390/magnetochemistry6010008
APA StyleHequet, E., Henoumont, C., Djouana Kenfack, V., Lemaur, V., Lazzaroni, R., Boutry, S., Vander Elst, L., Muller, R. N., & Laurent, S. (2020). Design, Characterization and Molecular Modeling of New Fluorinated Paramagnetic Contrast Agents for Dual 1H/19F MRI. Magnetochemistry, 6(1), 8. https://doi.org/10.3390/magnetochemistry6010008






