Novel Structures and Magnetic Properties of Two [Mn2] Complexes with 2,4-di-2-pyridyl-2,4-pentanediol as the Ligand
Abstract
1. Introduction
2. Results and Discussion
2.1. Description of Structures
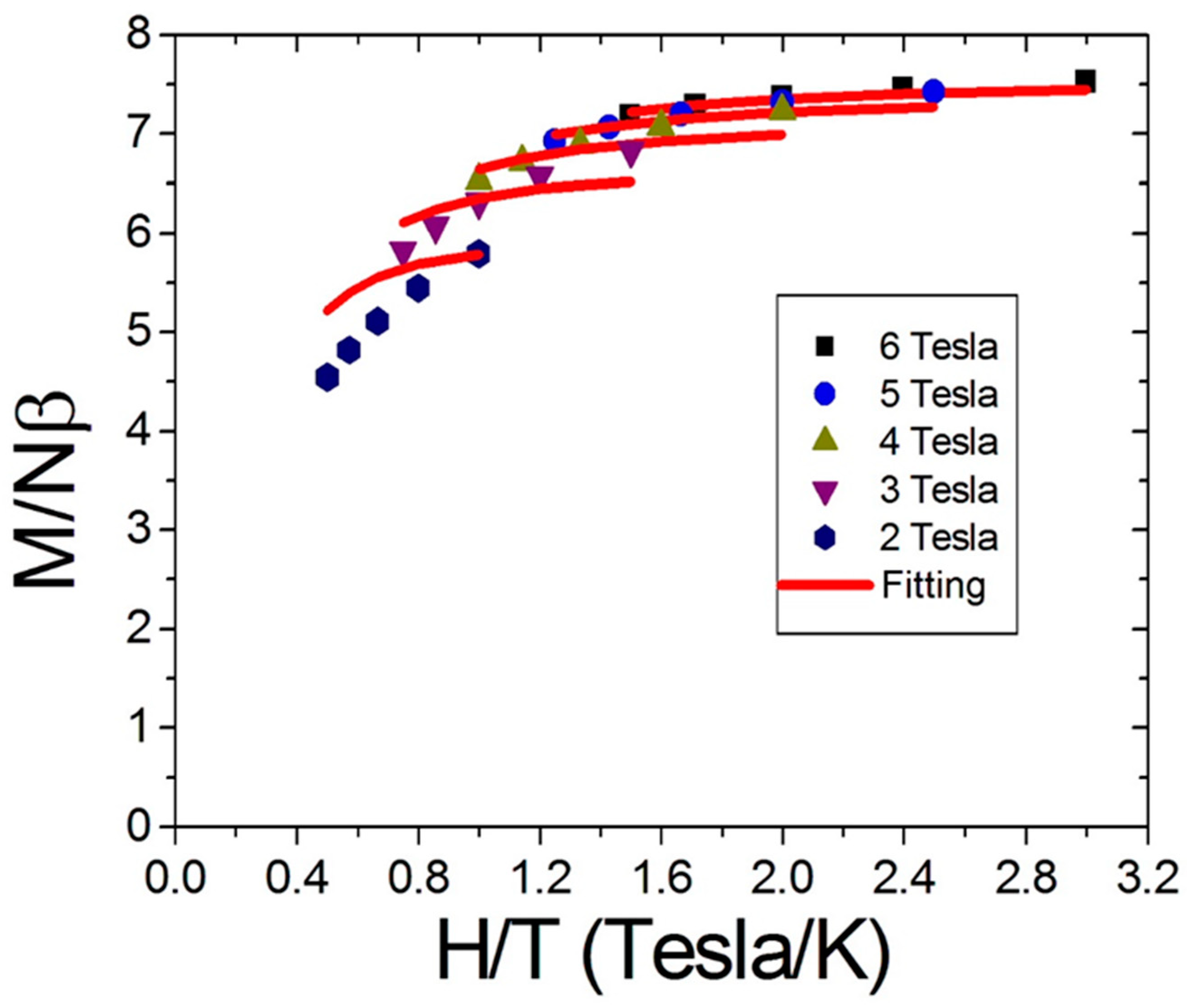
2.2. Magnetic Properties
3. Experimental Methods
3.1. Ligand Synthesis
3.2. Materials Synthesis
3.3. Physical Property Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Christou, G.; Gatteschi, D.; Hendrickson, D.N.; Sessoli, R. Single-Molecule Magnets. MRS Bull. 2000, 25, 66–71. [Google Scholar] [CrossRef]
- Aromi, G.; Aubin, S.M.J.; Bolcar, M.A.; Christou, G.; Eppley, H.J.; Folting, K.; Hendrickson, D.N.; Huffman, J.C.; Squire, R.C.; Tsai, H.L. Manganese Carboxylate Cluster: From Structural Aesthetics to Single-Molecule Magnets. Polyhedron 1998, 17, 3005–3020. [Google Scholar] [CrossRef]
- Christou, G. Single-Molecule Magnets: A Molecular Approach to Nanoscale Magnetic Materials. Polyhedron 2005, 24, 2065–2075. [Google Scholar] [CrossRef]
- Guo, M.; Corona, T.; Ray, K.; Nam, W. Heme and Nonheme High-Valent Iron and Manganese Oxo Cores in Biological and Abiological Oxidation Reactions. ACS Cent. Sci. 2019, 5, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Dismukes, G.C.; Brimblecombe, R.; Felton, G.A.N.; Pryadun, R.S.; Sheats, J.E.; Spiccia, L.; Swiegers, G.F. Development of bioinspired Mn4O4-cubane water oxidation catalysts: Lessons from photosynthesis. Acc. Chem. Res. 2009, 42, 1935–1943. [Google Scholar] [CrossRef] [PubMed]
- Conradie, J.; Tangen, E.; Ghosh, A. Trigonal bipyramidal iron(III) and manganese(III) oxo, sulfido, and selenido complexes. An electronic-structural overview. J. Inorg. Biochem. 2006, 100, 707–715. [Google Scholar] [CrossRef]
- Vinslava, A.; Tasiopoulos, A.J.; Wernsdorfer, W.; Abboud, K.A.; Christou, G. Molecules at the Quantum-Classical Nanoparticle Interface: Giant Mn70 Single-Molecule Magnets of approximately 4 nm Diameter. Inorg. Chem. 2016, 55, 3419–3430. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Shiddiq, M.; Ghosh, T.; Abboud, K.A.; Hill, S.; Christou, G. Covalently Linked Dimer of Mn3 Single-Molecule Magnets and Retention of Its Structure and Quantum Properties in Solution. J. Am. Chem. Soc. 2015, 137, 7160–7168. [Google Scholar] [CrossRef]
- Constantina, P.; Eleni, E.M.; George, C.; Anastasios, J.T. Filling the Gap between the Quantum and Classical Worlds of Nanoscale Magnetism: Giant Molecular Aggregates Based on Paramagnetic 3d Metal Ions. Chem. Soc. Rev. 2016, 45, 1597–1628. [Google Scholar]
- Caneschi, A.; Ferraro, F.; Gatteschi, D.; Melandri, M.C.; Rey, P.; Sessoli, R. Structure and Magnetic Properties of a Dinuclear Manganese(II) Complex with One μ-Aqua and Two μ-Carboxylato Bridges. Angew. Chem. Int. Ed. Engl. 1989, 28, 1365–1367. [Google Scholar] [CrossRef]
- Penner-Hahn, J.E.; Fronko, R.M.; Pecoraro, V.L.; Yocum, C.F.; Betts, S.D.; Bowlby, N.R. Structural characterization of the manganese sites in the photosynthetic oxygen-evolving complex using x-ray absorption spectroscopy. J. Am. Chem. Soc. 1990, 112, 2549–2557. [Google Scholar] [CrossRef]
- Yu, S.B.; Wang, C.P.; Day, E.P.; Holm, R.H. A Binuclear Manganese System with Three Isolated Oxidation States: Synthesis, Structures, and Properties of Molecules with a Mn2(OR)2 Bridge Unit Containing MnIIIMnII, and MnIIMnII. Inorg. Chem. 1991, 30, 4067–4074. [Google Scholar] [CrossRef]
- Yu, S.B.; Lippard, S.J.; Shweky, I.; Bino, A. Dinuclear manganese(II) complexes with water and carboxylate bridges. Inorg. Chem. 1992, 31, 3502–3504. [Google Scholar] [CrossRef]
- Gultneh, Y.; Farooq, A.; Liu, S.; Karlin, K.D.; Zubieta, J. Synthesis, structure, and characterization of a phenolate-bridged dimanganese(II) complex of a dinucleating phenol ligand. Inorg. Chem. 1992, 31, 3607–3611. [Google Scholar] [CrossRef]
- Glowiak, T.; Kozlowski, H.; Erre, L.S.; Micera, G. Molecular structure and spectral properties of mono- and dinuclear complexes formed by manganese(II), 2,6-dimethoxybenzoate and 2,2’-bipyridine or 2-methylpyrazine. Inorg. Chim. Acta 1995, 236, 149–154. [Google Scholar] [CrossRef]
- Baldwin, M.J.; Pecoraro, V.L. Energetics of Proton-Coupled Electron Transfer in High-Valent Mn2(μ-O)2Systems: Models for Water Oxidation by the Oxygen-Evolving Complex of Photosystem II. J. Am. Chem. Soc. 1996, 118, 11325–11326. [Google Scholar] [CrossRef]
- Beuken, E.K.V.D.; Feringa, B.L. Bimetallic catalysis by late transition metal complexes. Tetrahedron 1998, 54, 12985–13011. [Google Scholar] [CrossRef]
- Barone, V.; Bencini, A.; Gatteschi, D.; Totti, F. DFT Description of the Magnetic Properties and Electron Localization in Dinuclear Di-μ-oxo-Bridged Manganese Complexes. Chem. Eur. J. 2002, 8, 5019–5027. [Google Scholar] [CrossRef]
- Thompson, L. Polynuclear coordination complexes—From dinuclear to nonanuclear and beyond. Co-ord. Chem. Rev. 2002, 193–206. [Google Scholar] [CrossRef]
- Okazawa, A.; Ishida, T.; Nogami, T. Dinuclear Manganese(II) Complex Containing Tetrakis(2-pyridyl)methane as a Spiro-fused Bridge. Chem. Lett. 2004, 33, 1478–1479. [Google Scholar] [CrossRef]
- Ren, Y.; Li, J.; Zhang, F.; Zhang, J.; Guo, H. Crystal Structure and Characterization of a New Mixed-valence Manganese(III/IV) Complex: [Mn2(cyclen)2(μ-O)2](ClO4)3•4H2O. Chin. J. Chem. 2005, 23, 418–420. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, L.; Gao, G.; Li, F.; Liu, X.; Guo, W. An Unexpected Ferromagnetic Coupling in a Dinuclear Manganese(II) Linked Trivacant Heteropolymolybdate Derivative. Eur. J. Inorg. Chem. 2009, 2009, 1460–1463. [Google Scholar] [CrossRef]
- Pantazis, D.A.; Krewald, V.; Orio, M.; Neese, F. Theoretical magnetochemistry of dinuclear manganese complexes: broken symmetry density functional theory investigation on the influence of bridging motifs on structure and magnetism. Dalton Trans. 2010, 39, 4959. [Google Scholar] [CrossRef] [PubMed]
- Yahsi, Y.; Kara, H. Synthesis, structural analysis and magnetic properties of two novel doubly oxygen bridged binuclear manganese(III) and copper(II) complexes with ONO tridentate ligands. Inorg. Chim. Acta 2013, 397, 110–116. [Google Scholar] [CrossRef]
- Brimm, E.O.; Lynch, M.A.; Sesny, W.J. Preparation and Properties of Manganese Carbonyl. J. Am. Chem. Soc. 1954, 76, 3831–3835. [Google Scholar] [CrossRef]
- Mayer, P.; Hosten, E.; Gerber, T.I.A.; Abrahams, A. Synthesis and Structural Characterization of a Novel Ligand-bridged Dimer of Oxorhenium(V). Bull. Korean Chem. Soc. 2009, 30, 1204–1206. [Google Scholar]
- Shopov, D.Y.; Sharninghausen, L.S.; Sinha, S.B.; Borowski, J.E.; Mercado, B.Q.; Brudvig, G.W.; Crabtree, R.H. Synthesis of pyridine-alkoxide ligands for formation of polynuclear complexes. New J. Chem. 2017, 41, 6709–6719. [Google Scholar] [CrossRef]
- Sharninghausen, L.S.; Sinha, S.B.; Shopov, D.Y.; Mercado, B.Q.; Balcells, D.; Brudvig, G.W.; Crabtree, R.H. Synthesis and Characterization of Iridium(V) Coordination Complexes with an N,O-Donor Organic Ligand. Angew. Chem. Int. Ed. 2017, 56, 13047–13051. [Google Scholar] [CrossRef]
- Sunatsuki, Y.; Kishima, Y.; Kobayashi, T.; Yamaguchi, T.; Suzuki, T.; Kojima, M.; Krzystek, J.; Sundberg, M.R. A single tripodal ligand stabilizing three different oxidation states (II, III, and IV) of manganese. Chem. Commun. 2011, 47, 9149. [Google Scholar] [CrossRef]
- Krzystek, J.; Yeagle, G.J.; Park, J.H.; Britt, R.D.; Meisel, M.W.; Brunel, L.C.; Telser, J. High-Frequency and -Field EPR Spectroscopy of Tris(2,4-pentanedionato)manganese(III): Investigation of Solid-State versus Solution Jahn−Teller Effects. Inorg. Chem. 2003, 42, 4610–4618. [Google Scholar] [CrossRef]
- Cotton, F.A.; Extine, M.; Rice, G.W. Sensitivity of the chromium-chromium quadruple bond in dichromium tetracarboxylates to axial coordination and changes in inductive effects. Inorg. Chem. 1978, 17, 176–186. [Google Scholar] [CrossRef]
- Cotton, F.A.; Millar, M. An Exceedingly Short Metal-Metal Bond in a Bis(o-alkoxyphenyl) Dicarboxylatodichromium Compound. Inorg. Chem. 1978, 17, 2014–2017. [Google Scholar] [CrossRef]
- Brown, I.D. Recent Developments in the Methods and Applications of the Bond Valence Model. Chem. Rev. 2009, 109, 6858–6919. [Google Scholar] [CrossRef]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database. Acta Cryst. Sect. B Struct. Sci. 1985, 41, 244–247. [Google Scholar] [CrossRef]
- Thorp, H.H. Bond valence sum analysis of metal-ligand bond lengths in metalloenzymes and model complexes. Inorg. Chem. 1992, 31, 1585–1588. [Google Scholar] [CrossRef]
- Liu, W.; Thorp, H.H. Bond valence sum analysis of metal-ligand bond lengths in metalloenzymes and model complexes. 2. Refined distances and other enzymes. Inorg. Chem. 1993, 32, 4102–4105. [Google Scholar] [CrossRef]
- Manohar, A.; Ramalingam, K.; Karpagavel, K.; Bocelli, G. Crystallographic Distances Based Bond Valence Sum (BVS) Analysis on Nickel(II) Complexes Containing Ni-S and Ni-P Bonds. Adv. Mater. Res. 2012, 584, 84–87. [Google Scholar] [CrossRef]
- Kambe, K. On the Paramagnetic Susceptibilities of Some Polynuclear Complex Salts. J. Phys. Soc. Jpn. 1950, 5, 48–51. [Google Scholar] [CrossRef]
- Cotton, F.A.; Murillo, C.A.; Walton, R.A. Multiple Bonds between Metal Atoms, 3rd ed.; Springer: Berlin, Germany, 2005. [Google Scholar]
- Kahn, O. Molecular Magnetism; VCH Publishers Inc.: New York, NY, USA, 1993. [Google Scholar]
- Carlin, R.L. Magnetochemistry; Springer: Berlin, Germany, 1986. [Google Scholar]
- Bruker. APEX3, SAINT and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2016. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]

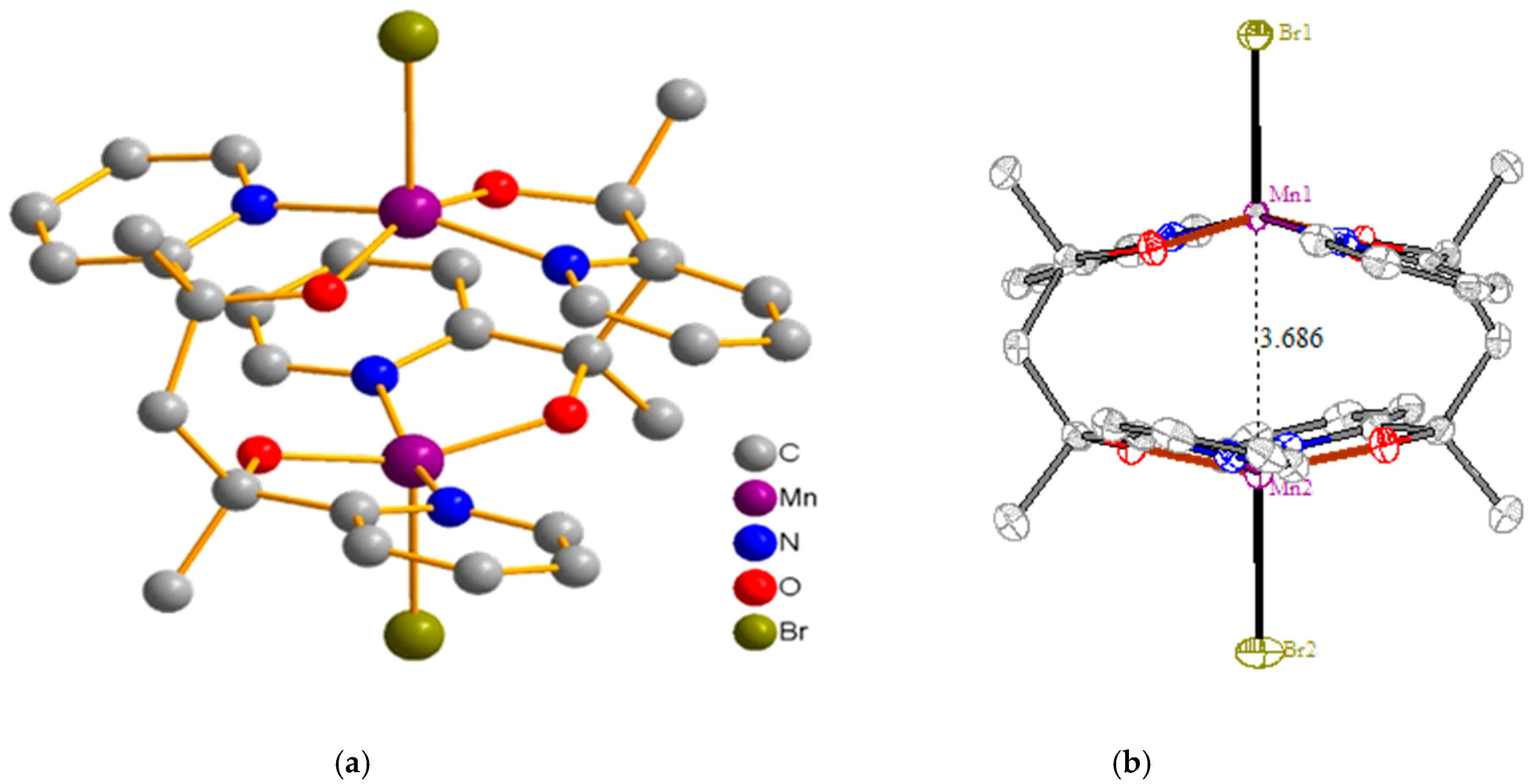
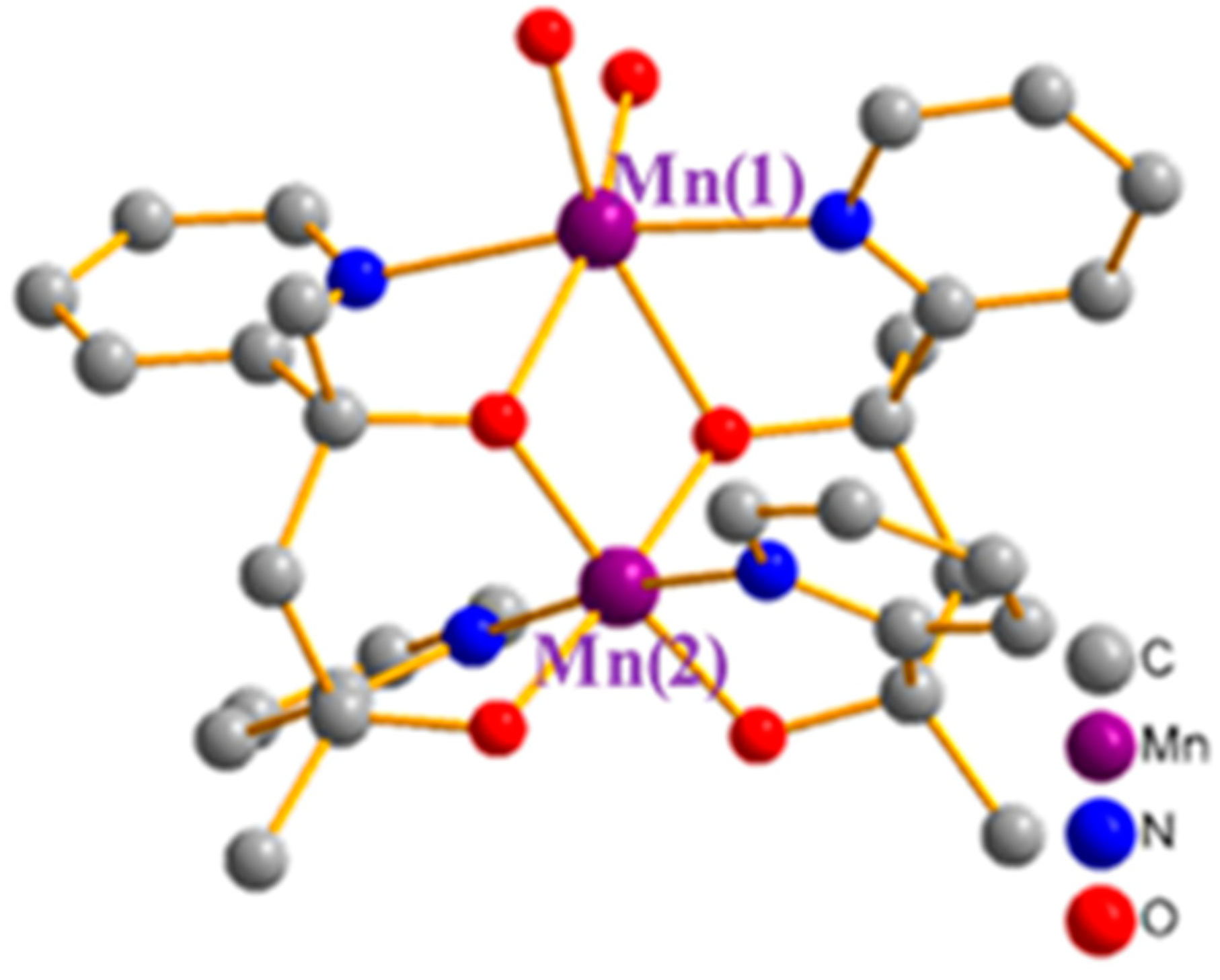



| Empirical Formula | C30H32N4O4Br2Mn2 | C30H44N4O10Br2Mn2 |
|---|---|---|
| Fw | 782.3 | 890.39 |
| Space Group | P41212 | C2/c |
| a, Å | 10.1069(5) | 27.9847(12) |
| b, Å | 10.1069(5) | 8.3508(3) |
| c, Å | 29.1085(16) | 19.8000(8) |
| α, deg | 90 | 90 |
| β, deg | 90 | 129.3794(10) |
| γ, deg | 90 | 90 |
| V, (Å3) | 2973.4(3) | 3576.6(2) |
| Z | 4 | 4 |
| ρ(calc), Mg/m3 | 1.748 | 1.654 |
| T, K | 150(2) | 150(2) |
| λ, Å | 0.71073 | 0.71073 |
| θ range (deg) | 2.133–27.499 | 2.614–30.000 |
| hkl ranges | −10<=h<=12, −13<=k<=13, −37<=l<=37 | −39<=h<=39, −11<=k<=11, −27<=l<=27 |
| Reflections collected | 18008 | 17265 |
| Independent reflections | 3411 [R(int) = 0.0354] | 5220 [R(int) = 0.0262] |
| μ, mm−1 | 3.580 | 2.999 |
| GOF | 1.139 | 1.052 |
| R1 [I > 2σ(I)] | 0.0224 | 0.0228 |
| wR2 | 0.0547 | 0.0571 |
| Flack parameter | 0.009(5) |
| Mn(1)-O(1) | 1.846(2) | Mn(2)-O(2) | 1.854(2) |
| Mn(1)-O(1)#1 | 1.846(2) | Mn(2)-O(2)#1 | 1.854(2) |
| Mn(1)-N(1) | 2.003(2) | Mn(2)-N(2) | 2.004(2) |
| Mn(1)-N(1)#1 | 2.003(2) | Mn(2)-N(2)#1 | 2.004(2) |
| Mn(1)-Br(1) | 2.5853(7) | Mn(2)-Br(2) | 2.5710(7) |
| O(1)-Mn(1)-O(1)#1 | 152.66(13) | O(2)-Mn(2)-O(2)#1 | 155.75(15) |
| O(1)-Mn(1)-N(1) | 82.98(10) | O(2)-Mn(2)-N(2) | 82.57(9) |
| O(1)#1-Mn(1)-N(1) | 92.34(10) | O(2)#1-Mn(2)-N(2) | 93.92(9) |
| O(1)-Mn(1)-N(1)#1 | 92.34(10) | O(2)-Mn(2)-N(2)#1 | 93.92(9) |
| O(1)#1-Mn(1)-N(1)#1 | 82.98(10) | O(2)#1-Mn(2)-N(2)#1 | 82.58(9) |
| N(1)-Mn(1)-N(1)#1 | 160.16(13) | N(2)-Mn(2)-N(2)#1 | 163.33(16) |
| O(1)-Mn(1)-Br(1) | 103.67(7) | O(2)-Mn(2)-Br(2) | 102.12(8) |
| O(1)#1-Mn(1)-Br(1) | 103.67(7) | O(2)#1-Mn(2)-Br(2) | 102.12(8) |
| N(1)-Mn(1)-Br(1) | 99.92(6) | N(2)-Mn(2)-Br(2) | 98.33(8) |
| N(1)#1-Mn(1)-Br(1) | 99.92(6) | N(2)#1-Mn(2)-Br(2) | 98.33(8) |
| Mn(1)-O(3)#1 | 2.1223(10) | Mn(2)-O(2)#1 | 1.8435(9) |
| Mn(1)-O(3) | 2.1223(10) | Mn(2)-O(2) | 1.8436(9) |
| Mn(1)-O(1) | 2.1832(9) | Mn(2)-O(1) | 1.9049(8) |
| Mn(1)-O(1)#1 | 2.1832(9) | Mn(2)-O(1)#1 | 1.9049(8) |
| Mn(1)-N(1) | 2.3073(11) | Mn(2)-N(2)#1 | 1.9862(11) |
| Mn(1)-N(1)#1 | 2.3073(11) | Mn(2)-N(2) | 1.9862(11) |
| O(3)#1-Mn(1)-O(3) | 90.13(6) | O(1)-Mn(2)-O(1)#1 | 85.63(5) |
| O(3)#1-Mn(1)-O(1) | 102.84(4) | O(2)#1-Mn(2)-N(2)#1 | 80.90(4) |
| O(3)-Mn(1)-O(1) | 156.24(4) | O(2)-Mn(2)-N(2)#1 | 93.45(4) |
| O(3)#1-Mn(1)-O(1)#1 | 156.24(4) | O(1)-Mn(2)-N(2)#1 | 92.28(4) |
| O(3)-Mn(1)-O(1)#1 | 102.84(4) | O(1)#1-Mn(2)-N(2)#1 | 93.67(4) |
| O(1)-Mn(1)-O(1)#1 | 72.74(4) | O(2)#1-Mn(2)-N(2) | 93.45(4) |
| O(3)#1-Mn(1)-N(1) | 99.60(4) | O(2)-Mn(2)-N(2) | 80.90(4) |
| O(3)-Mn(1)-N(1) | 86.97(4) | O(1)-Mn(2)-N(2) | 93.67(4) |
| O(1)-Mn(1)-N(1) | 71.43(4) | O(1)#1-Mn(2)-N(2) | 92.28(4) |
| O(1)#1-Mn(1)-N(1) | 100.88(4) | N(2)#1-Mn(2)-N(2) | 171.90(6) |
| O(3)#1-Mn(1)-N(1)#1 | 86.96(4) | O(2)#1-Mn(2)-Mn(1) | 133.89(3) |
| O(3)-Mn(1)-N(1)#1 | 99.60(4) | O(2)-Mn(2)-Mn(1) | 133.89(3) |
| O(1)-Mn(1)-N(1)#1 | 100.87(4) | O(1)-Mn(2)-Mn(1) | 42.82(3) |
| O(1)#1-Mn(1)-N(1)#1 | 71.43(4) | O(1)#1-Mn(2)-Mn(1) | 42.82(3) |
| N(1)-Mn(1)-N(1)#1 | 170.75(6) | N(2)#1-Mn(2)-Mn(1) | 94.05(3) |
| O(3)#1-Mn(1)-Mn(2) | 134.94(3) | N(2)-Mn(2)-Mn(1) | 94.05(3) |
| O(3)-Mn(1)-Mn(2) | 134.94(3) | C(6)-O(1)-Mn(2) | 127.13(7) |
| O(1)-Mn(1)-Mn(2) | 36.37(2) | C(6)-O(1)-Mn(1) | 115.89(7) |
| O(1)#1-Mn(1)-Mn(2) | 36.37(2) | Mn(2)-O(1)-Mn(1) | 100.81(4) |
| N(1)-Mn(1)-Mn(2) | 85.38(3) | C(8)-O(2)-Mn(2) | 111.86(7) |
| N(1)#1-Mn(1)-Mn(2) | 85.38(3) | C(5)-N(1)-C(1) | 118.48(11) |
| O(2)#1-Mn(2)-O(2) | 92.22(5) | C(5)-N(1)-Mn(1) | 114.76(8) |
| O(2)#1-Mn(2)-O(1) | 172.42(4) | C(1)-N(1)-Mn(1) | 125.45(9) |
| O(2)-Mn(2)-O(1) | 91.48(4) | C(13)-N(2)-C(9) | 121.39(11) |
| O(2)#1-Mn(2)-O(1)#1 | 91.48(4) | C(13)-N(2)-Mn(2) | 126.02(9) |
| O(2)-Mn(2)-O(1)#1 | 172.42(4) | C(9)-N(2)-Mn(2) | 112.58(8) |
| MnII-O | MnII-N | MnII-Br |
| 1.765 | 1.849 | 2.34 |
| MnIII-O | MnIII-N | MnIII-Br |
| 1.732 | 1.837 | 2.315 |
| MnIV-O | MnIV-N | MnIV-Br |
| 1.75 | 1.822 | 2.41 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, E.-C.; Chang, Y.-Y.; Huang, S.-Y.; Hong, L.-X.; Lee, G.-H.; Sheu, H.-S.; Chang, C.-K. Novel Structures and Magnetic Properties of Two [Mn2] Complexes with 2,4-di-2-pyridyl-2,4-pentanediol as the Ligand. Magnetochemistry 2019, 5, 43. https://doi.org/10.3390/magnetochemistry5030043
Yang E-C, Chang Y-Y, Huang S-Y, Hong L-X, Lee G-H, Sheu H-S, Chang C-K. Novel Structures and Magnetic Properties of Two [Mn2] Complexes with 2,4-di-2-pyridyl-2,4-pentanediol as the Ligand. Magnetochemistry. 2019; 5(3):43. https://doi.org/10.3390/magnetochemistry5030043
Chicago/Turabian StyleYang, En-Che, Yu-Ying Chang, Shi-Yi Huang, Ling-Xuan Hong, Gene-Hsiang Lee, Hwo-Shuenn Sheu, and Chung-Kai Chang. 2019. "Novel Structures and Magnetic Properties of Two [Mn2] Complexes with 2,4-di-2-pyridyl-2,4-pentanediol as the Ligand" Magnetochemistry 5, no. 3: 43. https://doi.org/10.3390/magnetochemistry5030043
APA StyleYang, E.-C., Chang, Y.-Y., Huang, S.-Y., Hong, L.-X., Lee, G.-H., Sheu, H.-S., & Chang, C.-K. (2019). Novel Structures and Magnetic Properties of Two [Mn2] Complexes with 2,4-di-2-pyridyl-2,4-pentanediol as the Ligand. Magnetochemistry, 5(3), 43. https://doi.org/10.3390/magnetochemistry5030043




