Bilayer Thin Films That Combine Luminescent and Spin Crossover Properties for an Efficient and Reversible Fluorescence Switching
Abstract
1. Introduction
2. Experimental Section
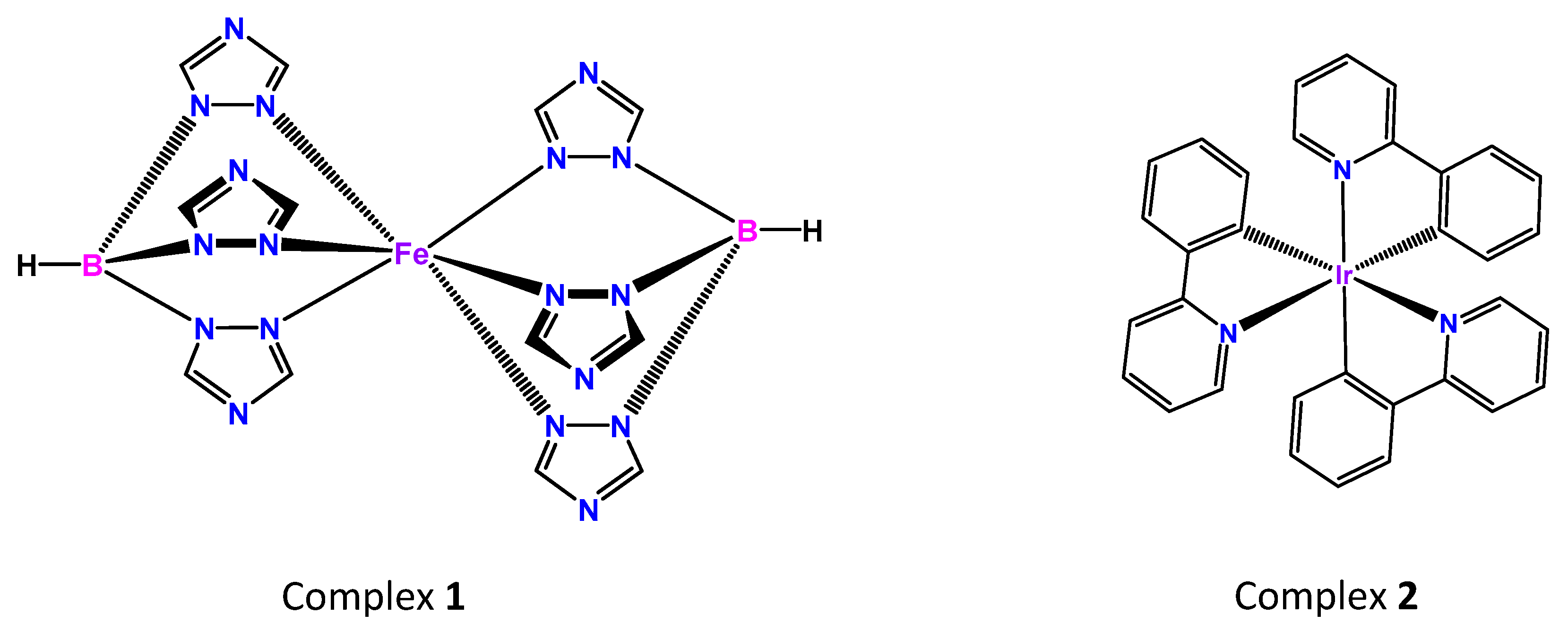
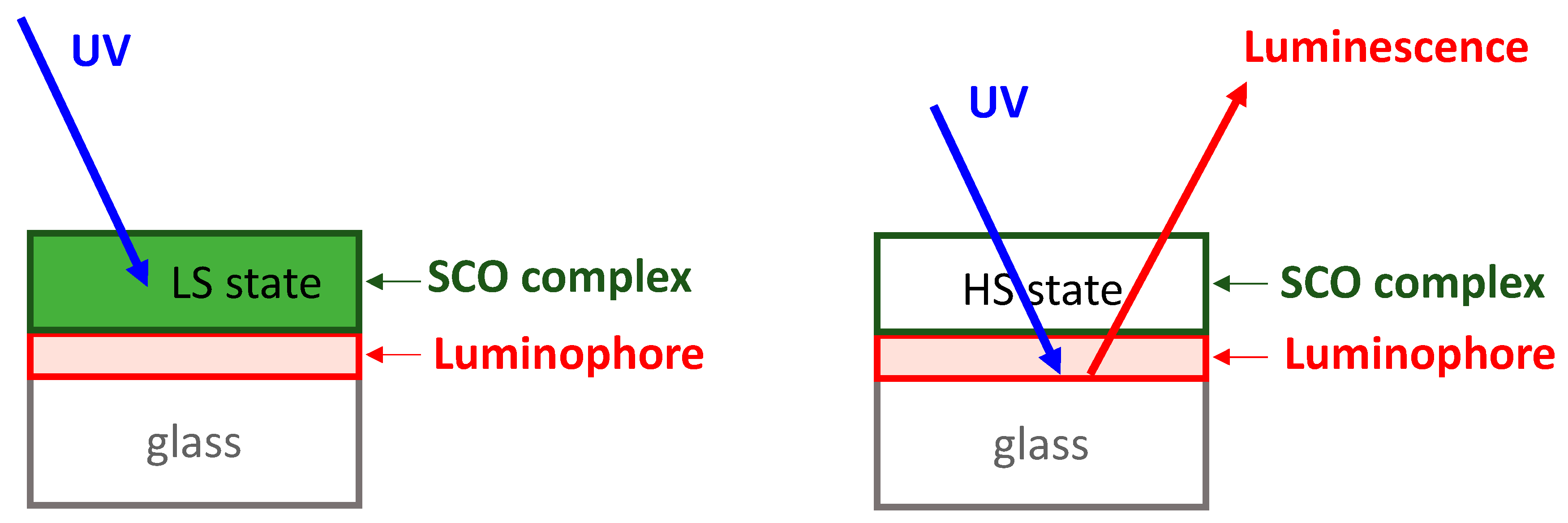
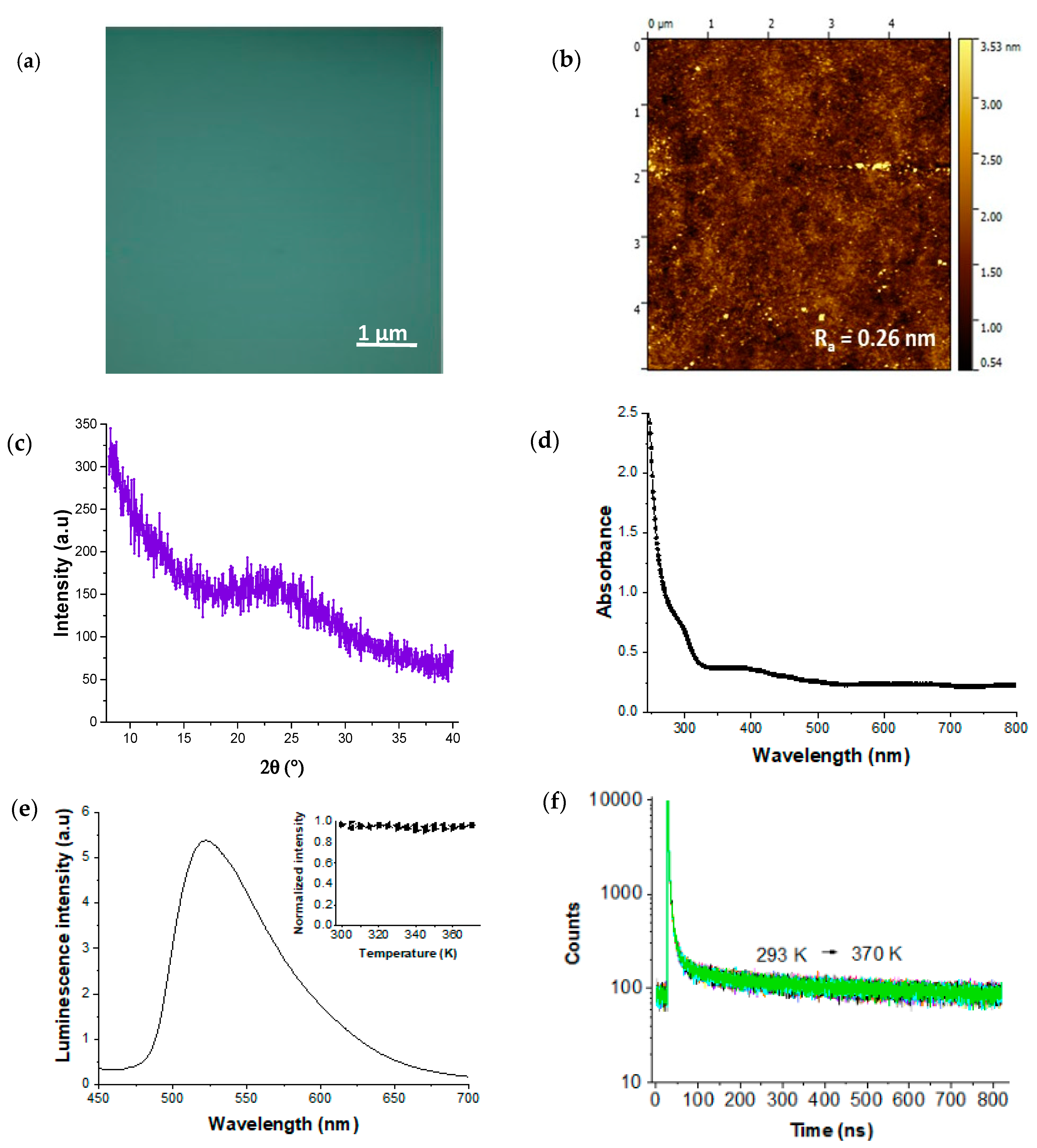
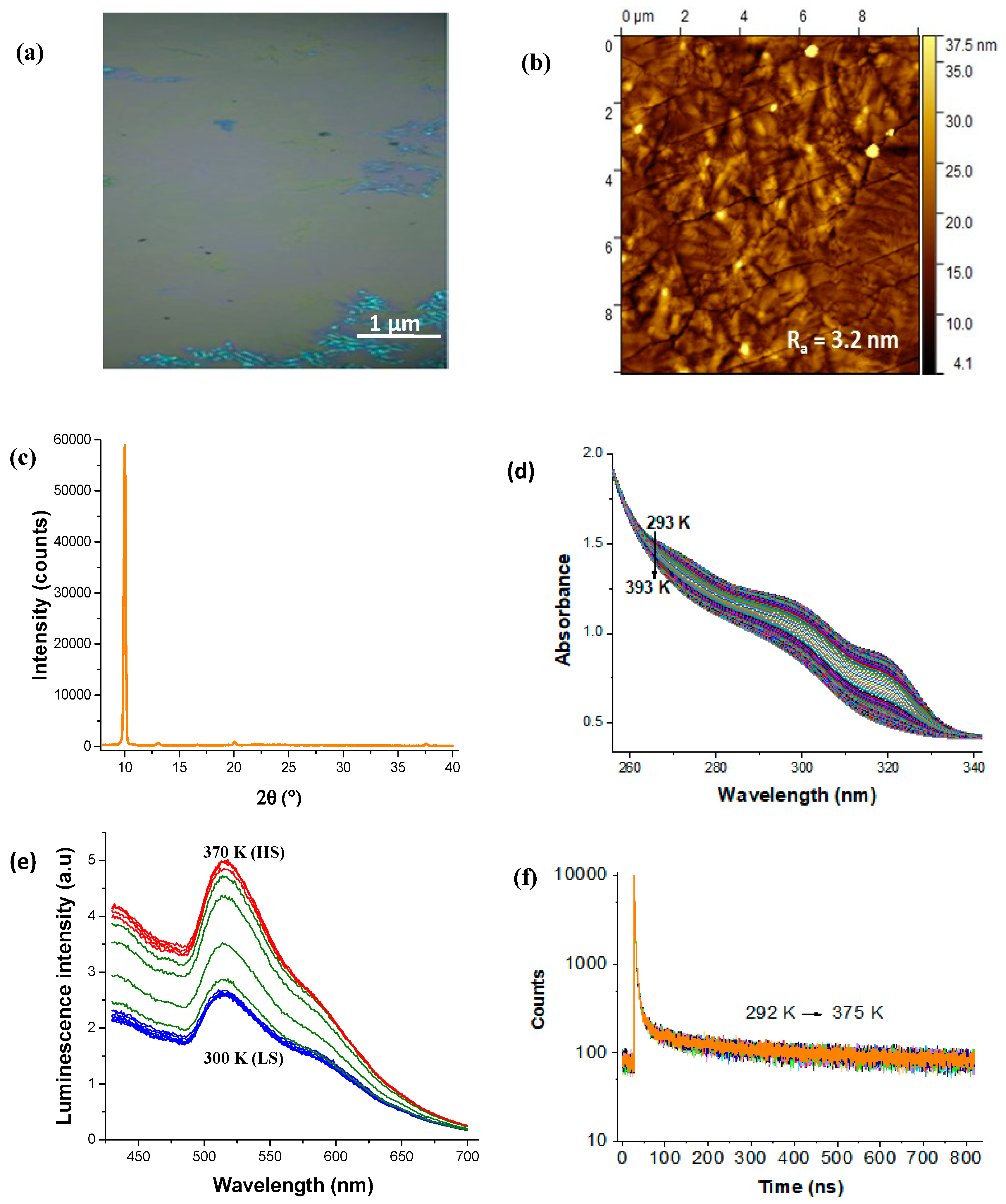
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Amendola, V.; Di Casa, M.; Fabbrizzi, L.; Licchelli, M.; Mangano, C.; Pallavicini, P.; Poggi, A. Mechanical switches of fluorescence. J. Incl. Phenom. Macrocycl. Chem. 2001, 41, 13–18. [Google Scholar] [CrossRef]
- Pischel, U. Molecular switches as platforms for information processing. Chimia 2014, 68, 505–511. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheng, Y.; Jie, W.; Chao, G.; Xin-Ge, L.; Bin, D.; Yan, L. A facile water-stable MOF-based “off-on” fluorescent switch for label-free detection of dopamine in biological fluid. J. Mater. Chem. B 2017, 5, 2524–2535. [Google Scholar] [CrossRef]
- Yang, L.; Jianping, W.; Liang, Y.; Cheng, Z.; Ruilong, Z.; Zhongping, Z.; Bianhua, L.; Changlong, J. Fluorescent paper sensor fabricated by carbazole-based probes for dual visual detection of Cu2+ and gaseous H2S. RSC Adv. 2016, 6, 5638456391. [Google Scholar] [CrossRef]
- Xin, L.; Tao, R.R.; Hong, L.-J.; Cheng, J.; Jiang, Q.; Lu, Y.-M.; Liao, M.-H.; Ye, W.-F.; Lu, N.-N.; Han, F.; et al. Visualizing peroxynitrite fluxes in endothelial cells reveals the dynamic progression of brain vascular injury. J. Am. Chem. Soc. 2015, 137, 12296–12303. [Google Scholar]
- Marc, V.; Benet, M.; Mena, S.; Rabih, O.; Al-Kaysi, J.H.; Guirado, G. Multistimuli-responsive fluorescent switches based on spirocyclic meisenheimer compounds: Smart molecules for the design of optical probes and electrochromic materials. J. Org. Chem. 2018, 83, 9166–9167. [Google Scholar]
- Bao-Hua, D.; Chen, Y.-L. Recent progress in organic mechanoluminescent materials. Chin. Chem. Lett. 2018, 29, 245–251. [Google Scholar]
- Jaume, G.-A.; Swaminathan, S.; Sortino, S.; Raymo, F.M. Plasmonic activation of a fluorescent carbazole-oxazine switch. Chem. Eur. J. 2014, 20, 10276–10284. [Google Scholar]
- Gareau, D.; Desrosiers, A.; Vallee-Belisle, A. Programmable quantitative DNA nanothermometers. Nano Lett. 2016, 16, 3976–3981. [Google Scholar] [CrossRef] [PubMed]
- Bousseksou, A.; Molnar, G.; Salmon, L.; Nicolazzi, N. Molecular spin crossover phenomenon: Recent achievements and prospects. Chem. Soc. Rev. 2011, 40, 3313–3335. [Google Scholar] [CrossRef] [PubMed]
- Garcia, Y.; Gutlich, P. Thermal spin crossover in Mn(II), Mn(III), Cr(II), and Co(III) coordination compounds. Top. Curr. Chem. 2004, 234, 49–62. [Google Scholar]
- Halcrow, M.A. Spin crossover materials. In Properties and Applications; Halcrow, M.A., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Bousseksou, A. (Ed.) Spin crossover phenomenon. C. R. Chim. 2018, 12, 1055–1300. [Google Scholar]
- Decurtins, S.; Gütlich, P.; Köhler, C.P.; Spiering, H.; Hauser, A. Light-induced excited spin state trapping in a transition-metal complex: The hexa-1-propyltetrazole-iron (II) tetrafluoroborate spin-crossover system. Chem. Phys. Lett. 1984, 105, 1–4. [Google Scholar] [CrossRef]
- Bousseksou, A.; Boukheddaden, K.; Goiran, M.; Consejo, C.; Boillot, M.L.; Tuchagues, J.P. Dynamic response of the spin-crossover solid Co(H2(fsa)2en)(py)2 to a pulsed magnetic field. Phys. Rev. B Cover. Condens. Matter. Mater. Phys. 2002, 65. [Google Scholar] [CrossRef]
- Kuppusamy, S.K.; Ruben, M. Emerging trends in spin crossover (SCO) based functional materials and devices. Coord. Chem. Rev. 2017, 346, 176–205. [Google Scholar]
- Molnar, G.; Sylvain, R.; Salmon, L.; Nicolazzi, W.; Bousseksou, A. Spin crossover nanomaterials: From fundamental concepts to devices. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef]
- Shepherd, H.; Carlos, J.; Quintero, M.; Molnar, G.; Salmon, L.; Bousseksou, A. Luminescent Spin-Crossover Materials; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2013; pp. 347–373. [Google Scholar]
- Marfunin, A.S. Spectroscopy, Luminescence, and Radiation Centers in Minerals; Springer: Berlin, Germany, 1979; 352p. [Google Scholar]
- Suleimanov, I.; Molnar, G.; Salmon, L.; Bousseksou, A. Near-infrared luminescence switching in a spin-crossover polymer nanocomposite. Eur. J. Inorg. Chem. 2017, 3446–3451. [Google Scholar] [CrossRef]
- Salmon, L.; Molnar, G.; Zitouni, D.; Quintero, C.; Bergaud, C.; Micheau, J.-C.; Bousseksou, A. A novel approach for fluorescent thermometry and thermal imaging purposes using spin crossover nanoparticles. J. Mater. Chem. 2010, 20, 5499–5503. [Google Scholar] [CrossRef]
- Piguet, C.; Rivara-Minten, E.; Hopfgartner, G.; Buenzli, J.-C.G. Molecular magnetism and iron(II) spin-state equilibrium as structural probes in heterodinuclear d–f complexes. Helv. Chim. Acta 1995, 78, 1651–1672. [Google Scholar] [CrossRef]
- Wang, C.F.; Li, R.F.; Chen, X.Y.; Wei, R.J.; Zheng, L.S.; Tao, J. Synergetic spin crossover and fluorescence in one-dimensional hybrid complexes. Angew. Chem. Int. Ed. 2015, 54, 1574–1577. [Google Scholar] [CrossRef]
- Lochenie, C.; Wagner, K.G.; Karg, M.; Weber, B. Modulation of the ligand-based fluorescence of 3d metal complexes upon spin state change. J. Mater. Chem. C 2015, 3, 7925–7935. [Google Scholar] [CrossRef]
- Wang, C.F.; Sun, M.J.; Guo, Q.J.; Cao, Z.X.; Zheng, L.S.; Tao, J. Multiple correlations between spin crossover and fluorescence in a dinuclear compound. Chem. Commun. 2016, 52, 14322–14325. [Google Scholar] [CrossRef] [PubMed]
- Estrader, M.; Salinas Uber, J.; Barrios, L.A.; Garcia, J.; Lloyd-Williams, P.; Roubeau, O.; Teat, S.J.; Aromi, G. A magneto-optical molecular device: Interplay of spin crossover, luminescence, photomagnetism, and photochromism. Angew. Chem. Int. Ed. 2017, 56, 15622–15627. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy Senthil, K.; Salitros, I.; Moreno-Pineda, E.; Ruben, M. Spacer type mediated tunable spin crossover (SCO) characteristics of pyrene decorated 2,6-bis(pyrazol-1-yl)pyridine (bpp) based Fe(II) molecular spintronic modules. Dalton Trans. 2017, 46, 9765–9768. [Google Scholar]
- Schaefer, B.; Bauer, T.; Faus, I.; Wolny, J.A.; Dahms, F.; Fuhr, O.; Lebedkin, S.; Wille, H.-C.; Schlage, K.; Chevalier, K.; et al. A luminescent Pt2Fe spin crossover complex. Dalton Trans. 2017, 46, 2289–2302. [Google Scholar] [CrossRef]
- Wang, J.-L.; Qiang, L.; Yin-Shan, M.; Xin Liu, H.; Zheng, Q.S.; Chun-Ying, D.; Tao, L. Fluorescence modulation via photoinduced spin crossover switched energy transfer from fluorophores to FeII ions. Chem. Sci. 2018, 9, 2892–2897. [Google Scholar] [CrossRef]
- Lochenie, C.; Schoetz, K.; Panzer, F.; Kurz, H.; Maier, B.; Puchtler, F.; Agarwal, S.; Koehler, A.; Weber, B. Spin-crossover iron(II) coordination polymer with fluorescent properties: Correlation between emission properties and spin state. J. Am. Chem. Soc. 2018, 140, 700–709. [Google Scholar] [CrossRef]
- Hasegawa, M.; Franz, R.; Hara, T.; Kikuchi, Y.; Fukuda, Y.; Okubo, J.; Hoshi, T.; Linert, W. Fluorescence spectra of Fe(II) spin crossover complexes with 2,6-bis(benzimidazole-2’-yl)pyridine. Chem. Phys. 2002, 277, 21–30. [Google Scholar] [CrossRef]
- Garcia, Y.; Robert, F.; Naik, A.D.; Zhou, G.; Tinant, B.; Robeyns, K.; Michotte, S.; Piraux, L. Spin transition charted in a fluorophore-tagged thermochromic dinuclear iron(II) complex. J. Am. Chem. Soc. 2011, 133, 15850–15853. [Google Scholar] [CrossRef]
- Gonzalez-Prieto, R.; Fleury, B.; Schramm, F.; Zoppellaro, G.; Chandrasekar, R.; Fuhr, O.; Lebedkin, S.; Kappes, M.; Ruben, M. Tuning the spin-transition properties of pyrene-decorated 2,6-bispyrazolylpyridine based Fe(II) complexes. Dalton Trans. 2011, 40, 7564–7570. [Google Scholar] [CrossRef]
- Santoro, A.; Kershaw Cook, L.J.; Kulmaczewski, R.; Barrett, S.A.; Cespedes, O.; Halcrow, M.A. Iron(II) complexes of tridentate indazolylpyridine ligands: Enhanced spin-crossover hysteresis and ligand-based fluorescence. Inorg. Chem. 2015, 54, 682–693. [Google Scholar] [CrossRef]
- Carine, E.; Piguet, C.; Bunzli, J.-C.G.; Hopfgartner, G. High-spin iron(II) as a semitransparent partner for tuning europium(III) luminescence in heterodimetallic d-f complexes. Chem. Eur. J. 2001, 7, 3014–3024. [Google Scholar]
- Piguet, C.; Rivara-Minten, E.; Bernardinelli, G.; Buenzli, J.-C.G.; Hopfgartner, G. Noncovalent lanthanide podates with predetermined physicochemical properties: Iron(II) spin-state equilibria in self-assembled heterodinuclear d-f supramolecular complexes. J. Chem. Soc. Dalton Trans. 1997, 421–433. [Google Scholar] [CrossRef]
- Quintero, C.M.; Gural’skiy, I.A.; Salmon, L.; Molnar, G.; Bergaud, C.; Bousseksou, A. Soft lithographic patterning of spin crossover complexes. Part 1: Fluorescent detection of the spin transition in single nanoobjects. J. Mater. Chem. 2012, 22, 3745–3751. [Google Scholar] [CrossRef]
- Matsuda, M.; Hikaru, I.; Hiroyuki, T. Electroluminescence quenching caused by a spin-crossover transition. Chem. Lett. 2008, 37, 374–375. [Google Scholar] [CrossRef]
- Matsuda, M.; Hikaru, I.; Hiroyuki, T. Reproducible on-off switching of the light emission from the electroluminescent device containing a spin crossover complex. Thin Solid Films 2008, 517, 1465–1467. [Google Scholar] [CrossRef]
- Matsuda, M.; Keita, K.; Ryoma, U.; Nobuaki, K.; Hiroyuki, T. Characteristics of organic light-emitting devices consisting of dye-doped spin crossover complex films. Thin Solid Films 2013, 531, 451–453. [Google Scholar] [CrossRef]
- Gural’skiy, I.A.; Quintero, C.M.; Abdul-Kader, K.; Lopes, M.; Bartual-Murgui, C.; Salmon, L.; Zhao, P.; Molnar, G.; Astruc, D.; Bousseksou, A. Detection of molecular spin-state changes in ultrathin films by photonic methods. J. Nanophotonics 2012, 6, 63517. [Google Scholar] [CrossRef]
- Tovee, C.A.; Kilner, C.A.; Thomas, J.A.; Halcrow, M.A. Co-crystallizing two functional complex molecules in a terpyridine embrace lattice. Cryst. Eng. Comm. 2009, 11, 2069–2077. [Google Scholar] [CrossRef]
- Kershaw Cook, L.J.; Halcrow, M.A. Doping ruthenium complexes into a molecular spin-crossover material. Polyhedron 2015, 87, 91–97. [Google Scholar] [CrossRef]
- Matsukizono, H.; Keita, K.; Nobuo, K. Self-assembly-directed spin conversion of iron(II) 1,2,4-triazole complexes in solution and their effect on photorelaxation processes of fluorescent counter ions. Chem. Lett. 2008, 37, 446–447. [Google Scholar] [CrossRef]
- Titos-Padilla, S.; Herrera, J.M.; Chen, X.-W.; Delgado, J.J.; Colacio, E. Bifunctional Hybrid SiO2 Nanoparticles Showing Synergy between Core Spin Crossover and Shell Luminescence Properties. Angew. Chem. Int. Ed. 2011, 50, 3290–3293. [Google Scholar] [CrossRef]
- Suleimanov, I.; Kraieva, O.; Molnar, G.; Salmon, L.; Bousseksou, A. Enhanced luminescence stability with a Tb-spin crossover nanocomposite for spin state monitoring. Chem. Commun. 2015, 51, 15098–15101. [Google Scholar] [CrossRef] [PubMed]
- Suleimanov, I.; Kraieva, O.; Sanchez Costa, J.; Fritsky, I.O.; Molnar, G.; Salmon, L.; Bousseksou, A. Electronic communication between fluorescent pyrene excimers and spin crossover complexes in nanocomposite particles. J. Mater. Chem. C 2015, 3, 5026–5032. [Google Scholar] [CrossRef]
- Kraieva, O.; Suleimanov, I.; Molnar, G.; Salmon, L.; Bousseksou, A. CdTe quantum dot fluorescence modulation by spin crossover. Magnetochemistry 2016, 2, 11. [Google Scholar] [CrossRef]
- Luo, Y.-H.; Jing-Wen, W.; Wen, W.; Xiao-Tong, H.; Dan-Li, H.; Chen Chen, T.X.; Qiyue, S.; Bai-Wang, S. Bidirectional photoswitching via alternating NIR and UV irradiation on a core-shell UCNP-SCO nanosphere. ACS Appl. Mater. Interfaces 2018, 10, 16666–16673. [Google Scholar] [CrossRef]
- Herrera, J.M.; Titos-Padilla, S.; Pope, S.J.A.; Berlanga, I.; Zamora, F.; Delgado, J.J.; Kamenev, K.V.; Wang, X.; Prescimone, A.; Brechin, E.K.; et al. Studies on bifunctional Fe(II)-triazole spin crossover nanoparticles: Time-dependent luminescence, surface grafting and the effect of a silica shell and hydrostatic pressure on the magnetic properties. J. Mater. Chem. C 2015, 3, 7819–7829. [Google Scholar] [CrossRef]
- Rat, S.; Ridier, K.; Vendier, L.; Molnar, G.; Salmon, L.; Bousseksou, A. Solvatomorphism and structural-spin crossover property relationship in bis[hydrotris(1,2,4-triazol-1-yl)borate]iron(II). Cryst. Eng. Comm. 2017, 19, 3271–3280. [Google Scholar] [CrossRef]
- Shalabaeva, V.; Mikolasek, M.; Manrique-Juarez, M.D.; Bas, A.-C.; Rat, S.; Salmon, L.; Nicolazzi, W.; Molnar, G.; Bousseksou, A. Unprecedented size effect on the phase stability of molecular thin films displaying a spin transition. J. Phys. Chem. C 2017, 121, 25617–25621. [Google Scholar] [CrossRef]
- Shalabaeva, V.; Rat, S.; Manrique-Juarez, M.D.; Bas, A.-C.; Vendier, L.; Salmon, L.; Molnar, G.; Bousseksou, A. Vacuum deposition of high-quality thin films displaying spin transition near room temperature. J. Mater. Chem. C 2017, 5, 4419–4425. [Google Scholar] [CrossRef]
- Manrique-Juarez, M.D.; Mathieu, F.; Shalabaeva, V.; Cacheux, J.; Rat, S.; Nicu, L.; Leichle, T.; Salmon, L.; Molnar, G.; Bousseksou, A. A bistable microelectromechanical system actuated by spin-crossover molecules. Angew. Chem. Int. Ed. 2017, 56, 8074–8078. [Google Scholar] [CrossRef]
- Ridier, K.; Rat, S.; Salmon, L.; Nicolazzi, W.; Molnar, G.; Bousseksou, A. Scan-rate and vacuum pressure dependence of the nucleation and growth dynamics in a spin-crossover single crystal: The role of latent heat. Phys. Chem. Chem. Phys. 2018, 20, 913–945. [Google Scholar] [CrossRef]
- Shalabaeva, V.; Ridier, K.; Rat, S.; Manrique-Juarez, M.D.; Salmon, L.; Seguy, I.; Rotaru, A.; Molnar, G.; Bousseksou, A. Room temperature current modulation in large area electronic junctions of spin crossover thin films. Appl. Phys. Lett. 2018, 112. [Google Scholar] [CrossRef]
- Mikolasek, M.; Manrique-Juarez, M.D.; Shepherd, H.J.; Ridier, K.; Rat, S.; Shalabaeva, V.; Bas, A.-C.; Collings, I.E.; Mathieu, F.; Cacheux, J.; et al. Complete set of elastic moduli of a spin-crossover solid: Spin-state dependence and mechanical actuation. J. Am. Chem. Soc. 2018, 140, 8970–8979. [Google Scholar] [CrossRef]
- Ridier, K.; Rat, S.; Shepherd, H.J.; Salmon, L.; Nicolazzi, W.; Molnar, G.; Bousseksou, A. Spatiotemporal dynamics of the spin transition in [Fe(HB(tz)3)2] single crystals. Phys. Rev. B 2017, 96, 134106. [Google Scholar] [CrossRef]
- Adachi, C.; Baldo, M.A.; Forrest, S.R.; Thompson, M.E. High-efficiency organic electrophosphorescent devices with tris(2-phenylpyridine)iridium doped into electron-transporting materials. Appl. Phys. Lett. 2000, 77, 904–906. [Google Scholar] [CrossRef]
- Kawamura, Y.; Brooks, J.; Brown, J.J.; Sasabe, H.; Adachi, C. Intermolecular interaction and a concentration-quenching mechanism of phosphorescent Ir(III) complexes in a solid film. Phys. Rev. Lett. 2006, 96, 017404. [Google Scholar] [CrossRef]
- Taiju, T.; Aljaroudi, N. Energy transfer between Ir(ppy)3 molecules in neat film and concentration quenching of phosphorescence. Opt. Mater. 2008, 30, 1375–1381. [Google Scholar]
- Namdas, E.B.; Ruseckas, A.; Samuel, I.D.W.; Lo, S.-C.; Burn, P.L. Photophysics of Fac-Tris(2-Phenylpyridine) iridium(III) cored electroluminescent dendrimers in Solution and films. J. Phys. Chem. B 2004, 108, 1570–1577. [Google Scholar] [CrossRef]
- Low, P.; Kim, B.; Takama, N.; Bergaud, C. High-spatial-resolution surface-temperature mapping using fluorescent thermometry. Small 2008, 4, 908–914. [Google Scholar] [CrossRef]
- Vetrone, F.; Naccache, R.; Zamarron, A.; Juarranz de la Fuente, A.; Sanz-Rodriguez, F.; Martinez Maestro, L.; Martin Rodriguez, E.; Jaque, D.; Sole, H.G.; Capobianco, J.A. Temperature Sensing Using Fluorescent Nanothermometers. ACS Nano 2010, 4, 3254–3258. [Google Scholar] [CrossRef] [PubMed]
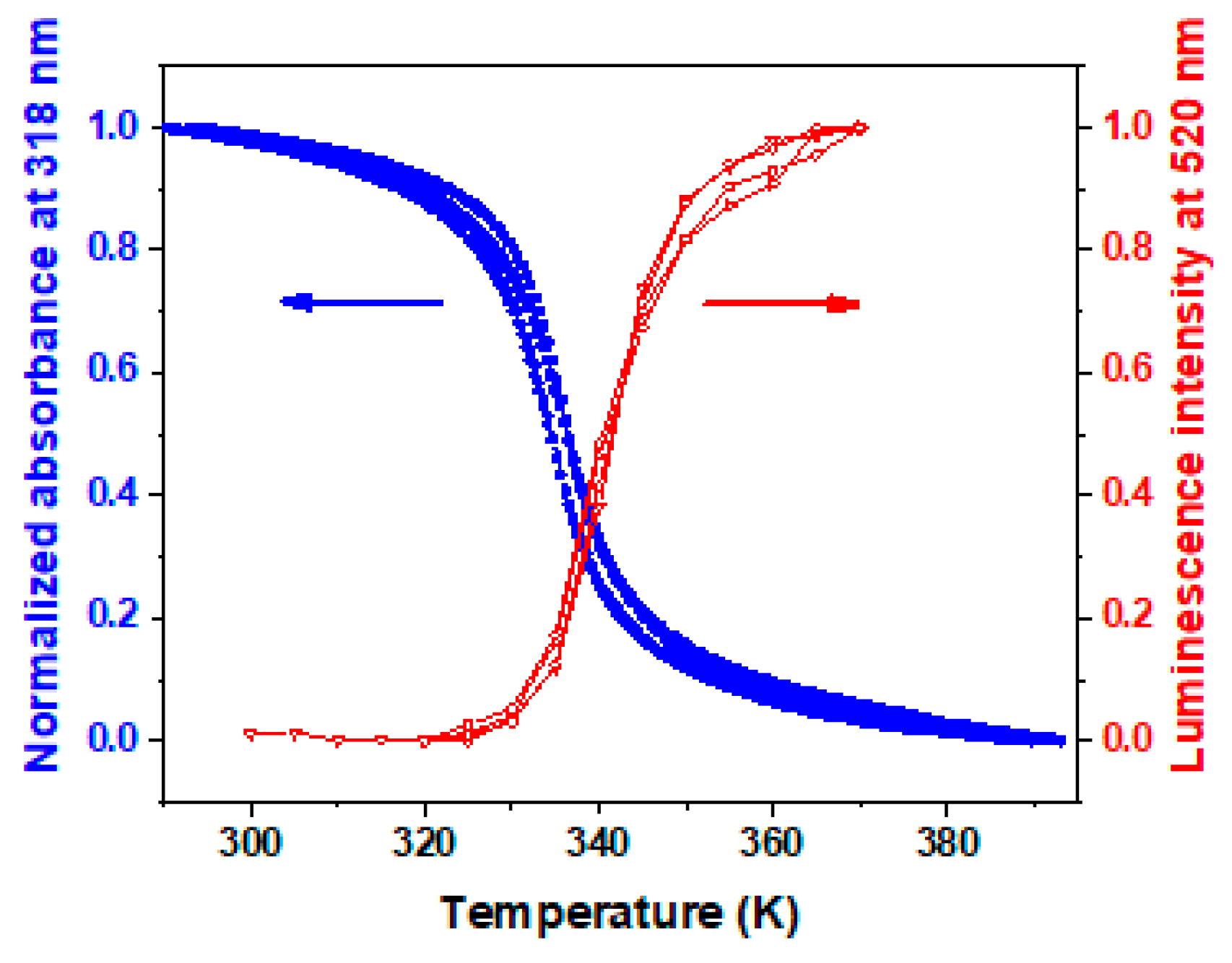
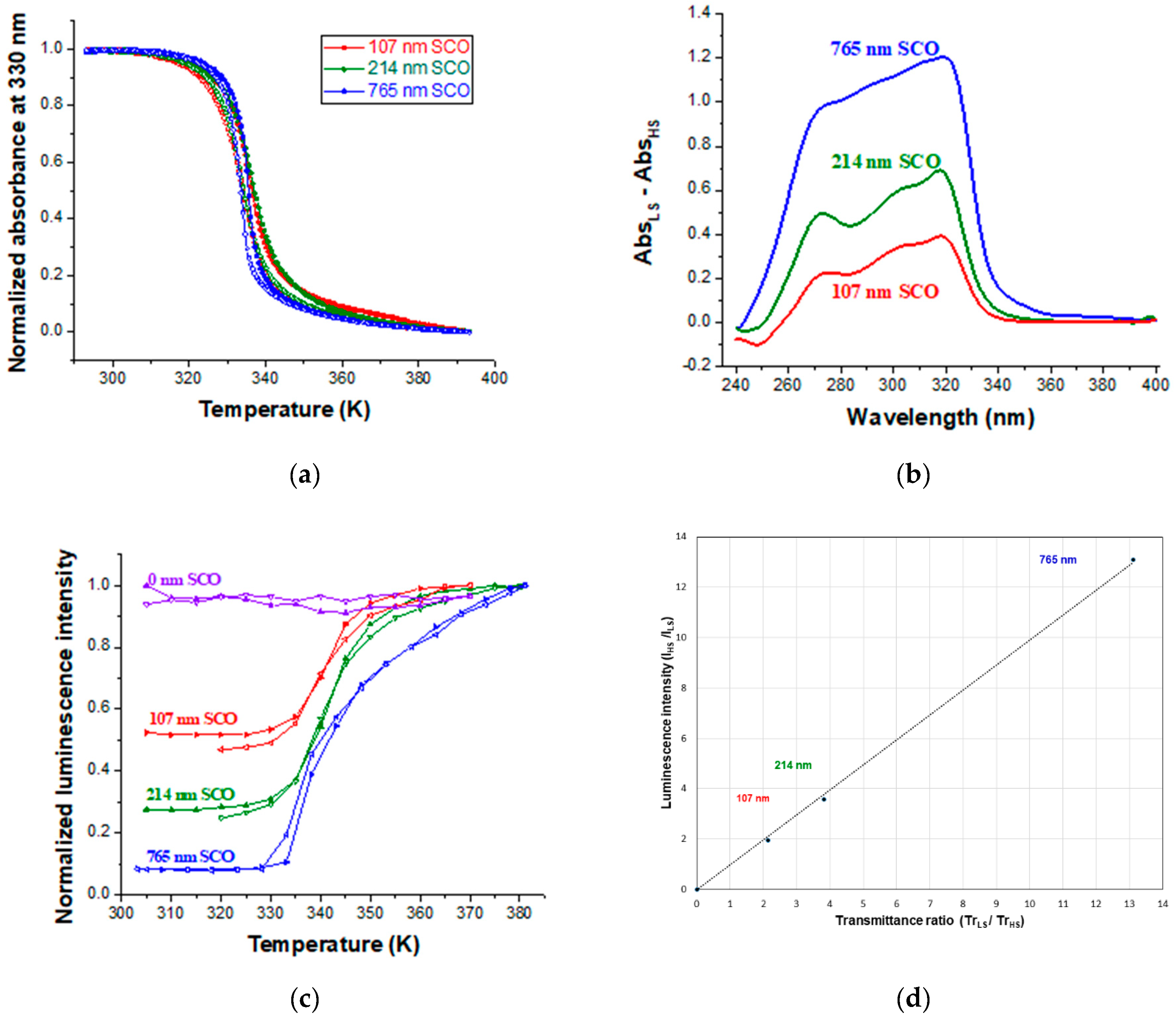
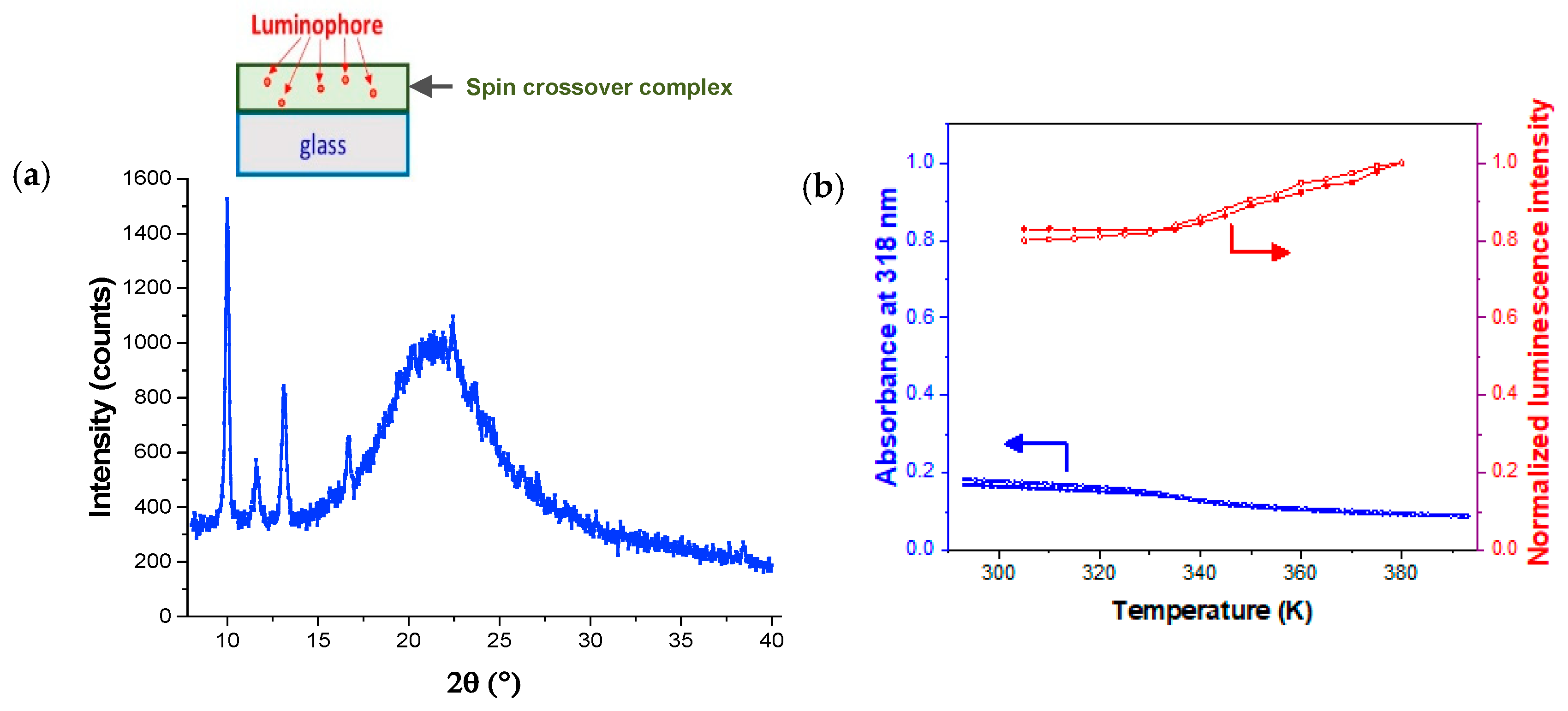
| Thickness of SCO Layer | ΔAbs = AbsLS − AbsHS | Trmax/Trmin at 300 nm | Imax/Imin | |
|---|---|---|---|---|
| At 300 nm | At 318 nm | |||
| 107 nm | 0.331 | 0.392 | 2.14 | 1.95 |
| 214 nm | 0.582 | 0.755 | 3.82 | 3.59 |
| 765 nm | 1.118 | 1.204 | 13.05 | 13.12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bas, A.-C.; Thompson, X.; Salmon, L.; Thibault, C.; Molnár, G.; Palamarciuc, O.; Routaboul, L.; Bousseksou, A. Bilayer Thin Films That Combine Luminescent and Spin Crossover Properties for an Efficient and Reversible Fluorescence Switching. Magnetochemistry 2019, 5, 28. https://doi.org/10.3390/magnetochemistry5020028
Bas A-C, Thompson X, Salmon L, Thibault C, Molnár G, Palamarciuc O, Routaboul L, Bousseksou A. Bilayer Thin Films That Combine Luminescent and Spin Crossover Properties for an Efficient and Reversible Fluorescence Switching. Magnetochemistry. 2019; 5(2):28. https://doi.org/10.3390/magnetochemistry5020028
Chicago/Turabian StyleBas, Alin-Ciprian, Xavier Thompson, Lionel Salmon, Christophe Thibault, Gábor Molnár, Oleg Palamarciuc, Lucie Routaboul, and Azzedine Bousseksou. 2019. "Bilayer Thin Films That Combine Luminescent and Spin Crossover Properties for an Efficient and Reversible Fluorescence Switching" Magnetochemistry 5, no. 2: 28. https://doi.org/10.3390/magnetochemistry5020028
APA StyleBas, A.-C., Thompson, X., Salmon, L., Thibault, C., Molnár, G., Palamarciuc, O., Routaboul, L., & Bousseksou, A. (2019). Bilayer Thin Films That Combine Luminescent and Spin Crossover Properties for an Efficient and Reversible Fluorescence Switching. Magnetochemistry, 5(2), 28. https://doi.org/10.3390/magnetochemistry5020028






