Pressure Effects with Incorporated Particle Size Dependency in Graphene Oxide Layers through Observing Spin Crossover Temperature
Abstract
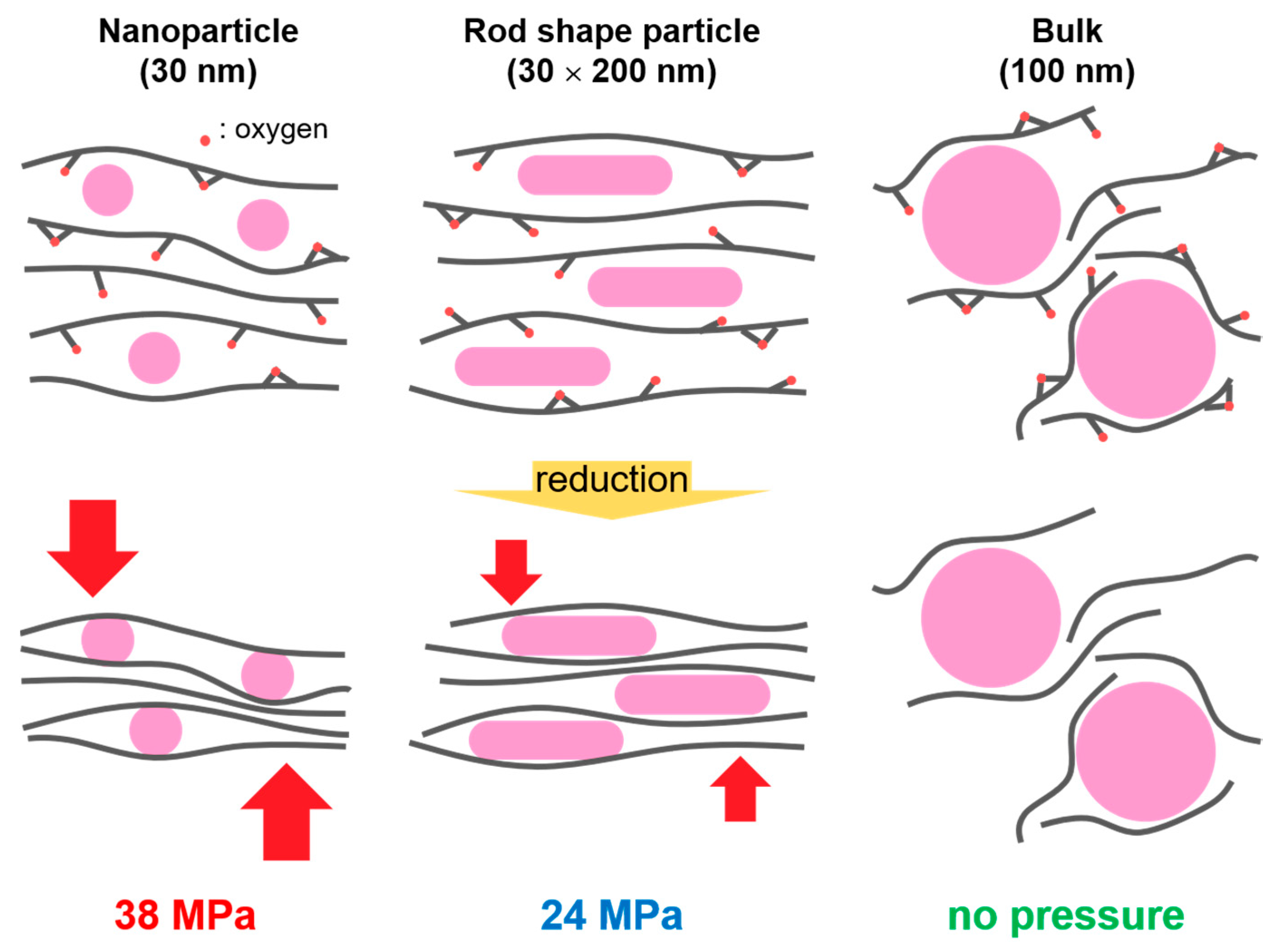
:1. Introduction
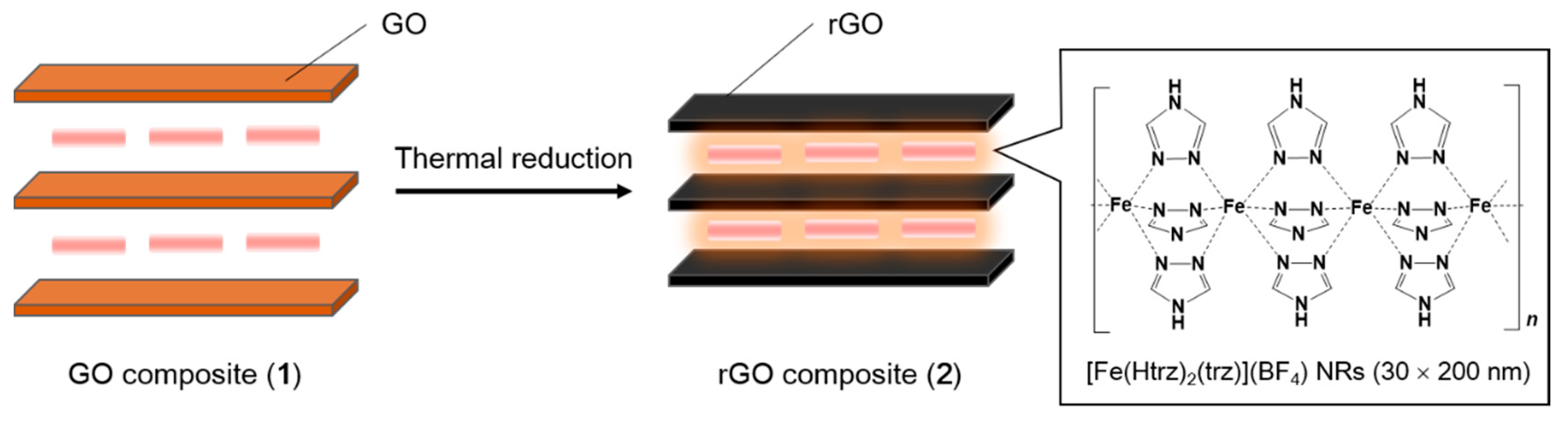
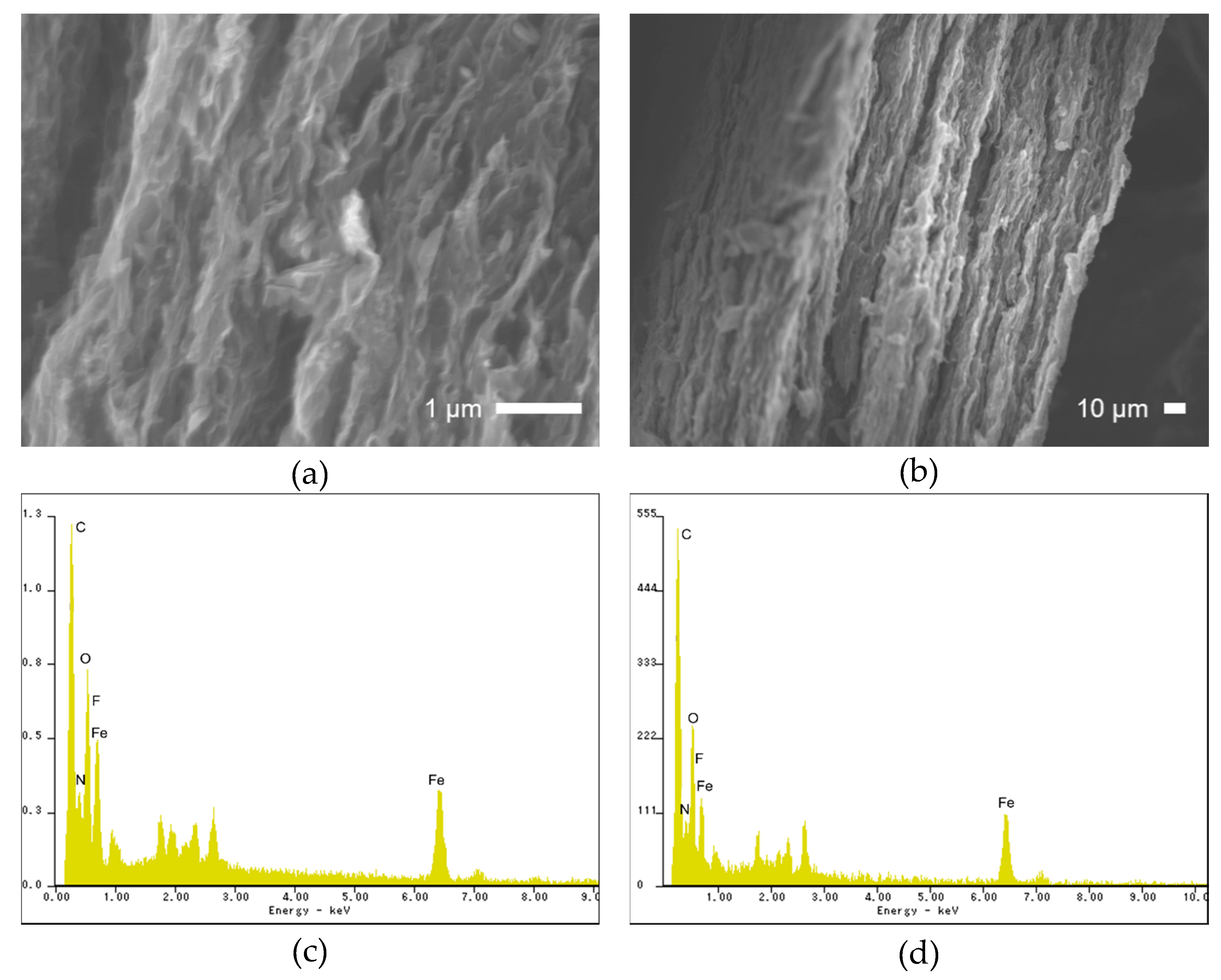
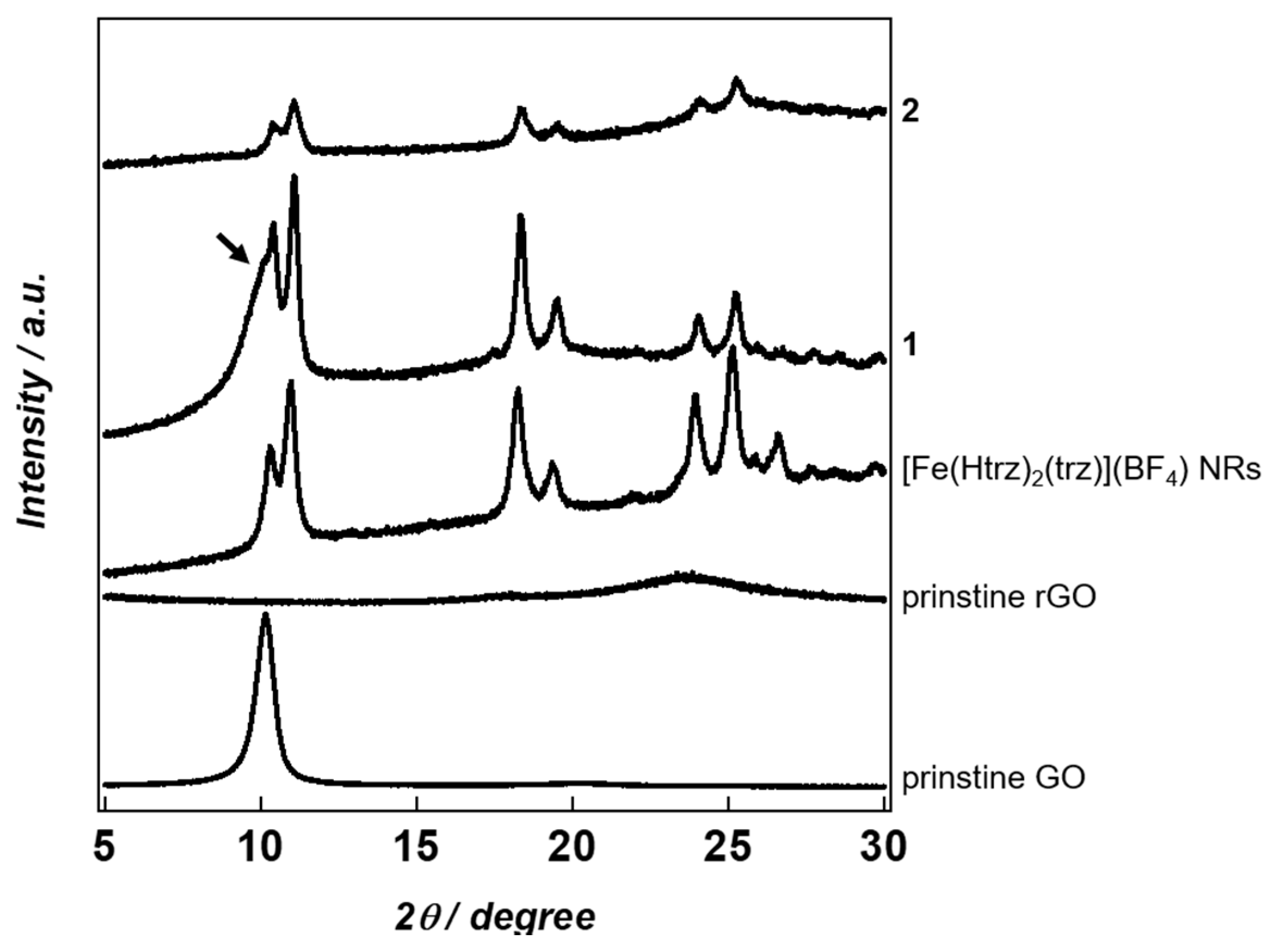
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis
3.1.1. [Fe(Htrz)2(trz)](BF4) NRs (30 × 200 nm)
3.1.2. Graphene Oxide (GO)
3.1.3. GO–[Fe(Htrz)2(trz)](BF4) NRs Composite (1)
3.1.4. rGO–[Fe(Htrz)2(trz)](BF4) NRs Composite (2)
3.2. Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Granick, S. Motions and Relaxations of Confined Liquids. Science 1991, 253, 1374–1379. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Fujimori, T.; Tanaka, H.; Urita, K.; Ohba, T.; Kanoh, H.; Itoh, T.; Asai, M.; Sakamoto, H.; Niimura, S.; et al. Anomaly of CH4 Molecular Assembly Confined in Single-Wall Carbon Nanohorn Spaces. J. Am. Chem. Soc. 2011, 133, 2022–2024. [Google Scholar] [CrossRef] [PubMed]
- Iiyama, T.; Nishikawa, K.; Otowa, T.; Kaneko, K. An Ordered Water Molecular Assembly Structure in a Slit-Shaped Carbon Nanospace. J. Phys. Chem. 1995, 99, 10075–10076. [Google Scholar] [CrossRef]
- Casco, M.E.; Silvestre-Albero, J.; Ramírez-Cuesta, A.J.; Rey, F.; Jordá, J.L.; Bansode, A.; Urakawa, A.; Peral, I.; Martínez-Escandell, M.; Kaneko, K.; et al. Methane hydrate formation in confined nanospace can surpass nature. Nat. Commun. 2015, 6, 6432–6439. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Banhart, F.; Krasheninnikov, A.V.; Rodríguez-Manzo, J.A.; Terrones, M.; Ajayan, P.M. Carbon Nanotubes as High-Pressure Cylinders and Nanoextruders. Science 2006, 312, 1199–1202. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Yanai, N.; Watanabe, S.; Tanaka, H.; Numaguchi, R.; Miyahara, M.T.; Ohta, Y.; Nagaoka, M.; Kitagawa, S. Unveiling thermal transitions of polymers in subnanometre pores. Nat. Commun. 2010, 1, 83–90. [Google Scholar] [CrossRef]
- Urita, K.; Shiga, Y.; Fujimori, T.; Iiyama, T.; Hattori, Y.; Kanoh, H.; Ohba, T.; Tanaka, H.; Yudasaka, M.; Iijima, S.; et al. Confinement in Carbon Nanospace-Induced Production of KI Nanocrystals of High-Pressure Phase. J. Am. Chem. Soc. 2011, 133, 10344–10347. [Google Scholar] [CrossRef]
- Kitamura, R.; Kitagawa, S.; Kubota, Y.; Kobayashi, T.C.; Kindo, K.; Mita, Y.; Matsuo, A.; Kobayashi, M.; Chang, H.-C.; Ozawa, C.T.; et al. Formation of a One-Dimensional Array of Oxygen in a Microporous Metal-Organic Solid. Science 2002, 298, 2358–2361. [Google Scholar] [CrossRef] [PubMed]
- Vasu, K.S.; Prestat, E.; Abraham, J.; Dix, J.; Kashtiban, R.J.; Beheshtian, J.; Sloan, J.; Carbone, P.; Neek-Amal, M.; Haigh, S.J.; et al. Van der Waals pressure and its effect on trapped interlayer molecules. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef]
- Chialvo, A.A.; Vlcek, L.; Cummings, P.T. Surface Strain Effects on the Water-Graphene Interfacial and Confinement Behavior. J. Phys. Chem. C 2014, 118, 19701–19711. [Google Scholar] [CrossRef]
- Khestanova, E.; Guinea, F.; Fumagalli, L.; Geim, A.K.; Grigorieva, I.V. Universal shape and pressure inside bubbles appearing in van der Waals heterostuctures. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Algara-Siller, G.; Lehtinen, O.; Wang, F.C.; Nair, R.R.; Kaiser, U.; Wu, H.A.; Geim, A.K.; Grigorieva, I.V. Square ice in graphene nanocapollaries. Nature 2015, 519, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.R.; Takehira, H.; Matsui, T.; Murashima, Y.; Ohtani, R.; Nakamura, M.; Hayami, S. Graphene and Graphene Oxide as Super Materials. Curr. Inorg. Chem. 2014, 4, 191–219. [Google Scholar] [CrossRef]
- Karim, M.R.; Hatakeyama, K.; Matsui, T.; Takehira, H.; Taniguchi, T.; Koinuma, M.; Matsumoto, Y.; Akutagawa, T.; Nakamura, T.; Noro, S.; et al. Graphene Oxide Nanosheet with High Proton Conductivity. J. Am. Chem. Soc. 2013, 135, 8079–8100. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, N.; Suzuki, H.; Takeuchi, Y.; Kawaguchi, S.; Kunisu, M.; Bielawski, C.W.; Nishina, Y. Real-Time, in Situ Monitoring of the Oxidation of Graphite: Lessons Learned. Chem. Mater. 2017, 29, 2150–2156. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Eda, G.; Chhowalla, M. Chemically Derived Graphene Oxide: Towards Large-Area Thin-Film Electronics and Optoelectronics. Adv. Mater. 2010, 22, 2392–2415. [Google Scholar] [CrossRef]
- Hatakeyama, K.; Tateishi, H.; Taniguchi, T.; Koinuma, M.; Kida, T.; Hayami, S.; Yokoi, H.; Matsumoto, Y. Tunable Graphene Oxide Proton/Electron Mixed Conductor that Functions at Room Temperature. Chem. Mater. 2014, 26, 5598–5604. [Google Scholar] [CrossRef]
- Pan, Q.; Chung, C.-C.; He, N.; Jones, J.L.; Gao, W. Accelerated Thermal Decomposition of Graphene Oxide Films in Air via in Situ X-ray Diffraction Analysis. J. Phys. Chem. C 2016, 120, 14984–14990. [Google Scholar] [CrossRef]
- Shin, H.-J.; Kim, K.K.; Benayad, A.; Yoon, S.-M.; Park, H.K.; Jung, I.-S.; Jin, M.H.; Jeong, H.-K.; Kim, J.M.; Choi, J.-Y.; et al. Efficient Reduction of Graphite Oxide by Sodium Borohydride and Its Effect on Electrical Conductance. Adv. Funct. Mater. 2009, 19, 1987–1992. [Google Scholar] [CrossRef]
- Tien, H.-W.; Huang, Y.-L.; Yang, S.-Y.; Wang, J.-Y.; Ma, C.-C.M. The production of graphene nanosheets decorated with silver nanoparticles for use in transparent, conductive films. Carbon 2011, 49, 1550–1560. [Google Scholar] [CrossRef]
- Sekimoto, Y.; Ohtani, R.; Nakamura, M.; Koinuma, M.; Lindoy, L.F.; Hayami, S. Tunable pressure effects in graphene oxide layers. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Kröber, J.; Audière, J.-P.; Claude, R.; Codjovi, E.; Kahn, O. Spin Transitions and Thermal Hystereses in the Molecular-Based Materials[Fe(Htrz)2(trz)](BF4)2 and [Fe(Htrz)3](BF4)2·H2O (Htrz = 1,2,4-4H-triazole; trz = 1,2,4-triazolato). Chem. Mater. 1994, 6, 1404–1412. [Google Scholar] [CrossRef]
- Coronado, E.; Galán-Mascarós, J.R.; Monrabal-Capilla, M.; García-Martínez, J.; Pardo-Ibáñez, P. Bistable Spin-Crossover Nanoparticles Showing Magnetic Thermal Hysteresis near Room Temperature. Adv. Mater. 2007, 19, 1359–1361. [Google Scholar] [CrossRef]
- Kahn, O.; Codjovi, E. Iron(II)-1,2,4-triazole spin transition molecular materials. Philos. Trans. R. Soc. Lond. A 1996, 354, 359–379. [Google Scholar] [CrossRef]
- Holovchenko, A.; Dugay, J.; Giménez-Maequés, M.; Torres-Cavanillas, R.; Coronado, E.; van der Zant, H.S.J. Near Room-Temperature Memory Devices Based on Hybrid Spin-Crossover@SiO2 Nanoparticles Coupled to Single-Layer Graphene Nanoelectrodes. Adv. Mater. 2016, 28, 7228–7233. [Google Scholar] [CrossRef] [PubMed]
- Herrera, J.M.; Titos-Padilla, S.; Pope, S.J.A.; Berlanga, I.; Zamora, F.; Delgado, J.J.; Kamenev, K.V.; Wang, X.; Prescimone, A.; Brechin, E.K.; et al. Studies on bifunctional Fe(II)-triazole spin crossover nanoparticles: time-dependent luminescence, surface grafting and the effect of a silica shell and hydrostatic pressure on the magnetic properties. J. Mater. Chem. C 2015, 3, 7819–7829. [Google Scholar] [CrossRef]
- Rotaru, A.; Cural’skiy, I.A.; Molnár, G.; Salmon, L.; Demont, P.; Bousseksou, A. Spin state dependence of electrical conductivity of spin crossover materials. Chem. Commun. 2012, 48, 4163–4165. [Google Scholar] [CrossRef]
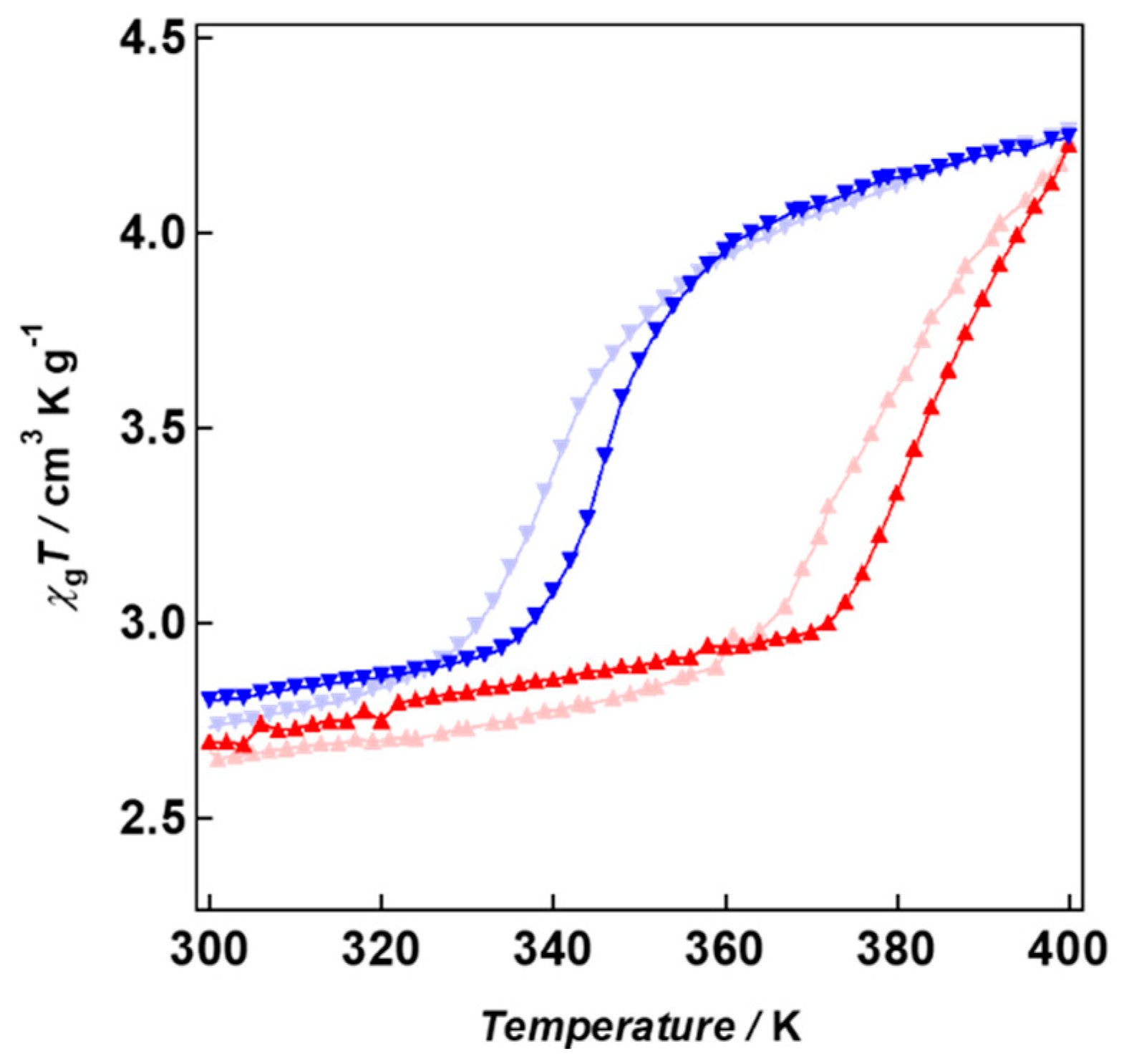
| T1/2 (K) | Pseudo-Pressure | |
|---|---|---|
| 1 (GO/NRs) | 357 | 24 MPa |
| 2 (rGO/NRs) | 364 | |
| GO/NPs | 351 | 38 MPa |
| rGO/NPs | 362 | |
| GO/BPs | 357 | No pressure |
| rGO/BPs | 352 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitayama, H.; Akiyoshi, R.; Nakamura, M.; Hayami, S. Pressure Effects with Incorporated Particle Size Dependency in Graphene Oxide Layers through Observing Spin Crossover Temperature. Magnetochemistry 2019, 5, 26. https://doi.org/10.3390/magnetochemistry5020026
Kitayama H, Akiyoshi R, Nakamura M, Hayami S. Pressure Effects with Incorporated Particle Size Dependency in Graphene Oxide Layers through Observing Spin Crossover Temperature. Magnetochemistry. 2019; 5(2):26. https://doi.org/10.3390/magnetochemistry5020026
Chicago/Turabian StyleKitayama, Hikaru, Ryohei Akiyoshi, Masaaki Nakamura, and Shinya Hayami. 2019. "Pressure Effects with Incorporated Particle Size Dependency in Graphene Oxide Layers through Observing Spin Crossover Temperature" Magnetochemistry 5, no. 2: 26. https://doi.org/10.3390/magnetochemistry5020026
APA StyleKitayama, H., Akiyoshi, R., Nakamura, M., & Hayami, S. (2019). Pressure Effects with Incorporated Particle Size Dependency in Graphene Oxide Layers through Observing Spin Crossover Temperature. Magnetochemistry, 5(2), 26. https://doi.org/10.3390/magnetochemistry5020026






