Molecular Probes for Evaluation of Oxidative Stress by In Vivo EPR Spectroscopy and Imaging: State-of-the-Art and Limitations
Abstract
1. Introduction
2. The Ideal Molecular Probe for Evaluation of Oxidative Stress by EPR
- The probe is transformed specifically by RONS produced by cells and is not sensitive to reductive processes. The variation of intensity of the EPR signal thus reflects the level of oxidative stress;
- Metabolization and excretion of the probe are slow compared to the typical time-scale of EPR imaging;
- The probe is highly water-soluble or can be easily formulated for in vivo administration;
- The probe distributes to the tissue of interest and accumulates to above the level of detection of the EPR spectrometer. Its compartmentalization corresponds to the site of RONS production;
- The spectral properties of the probe, especially its EPR linewidth, are compatible with the acquisition of EPR images with a resolution of a few millimeters;
- The probe is not toxic at the dose required for imaging.
3. Problems to Solve
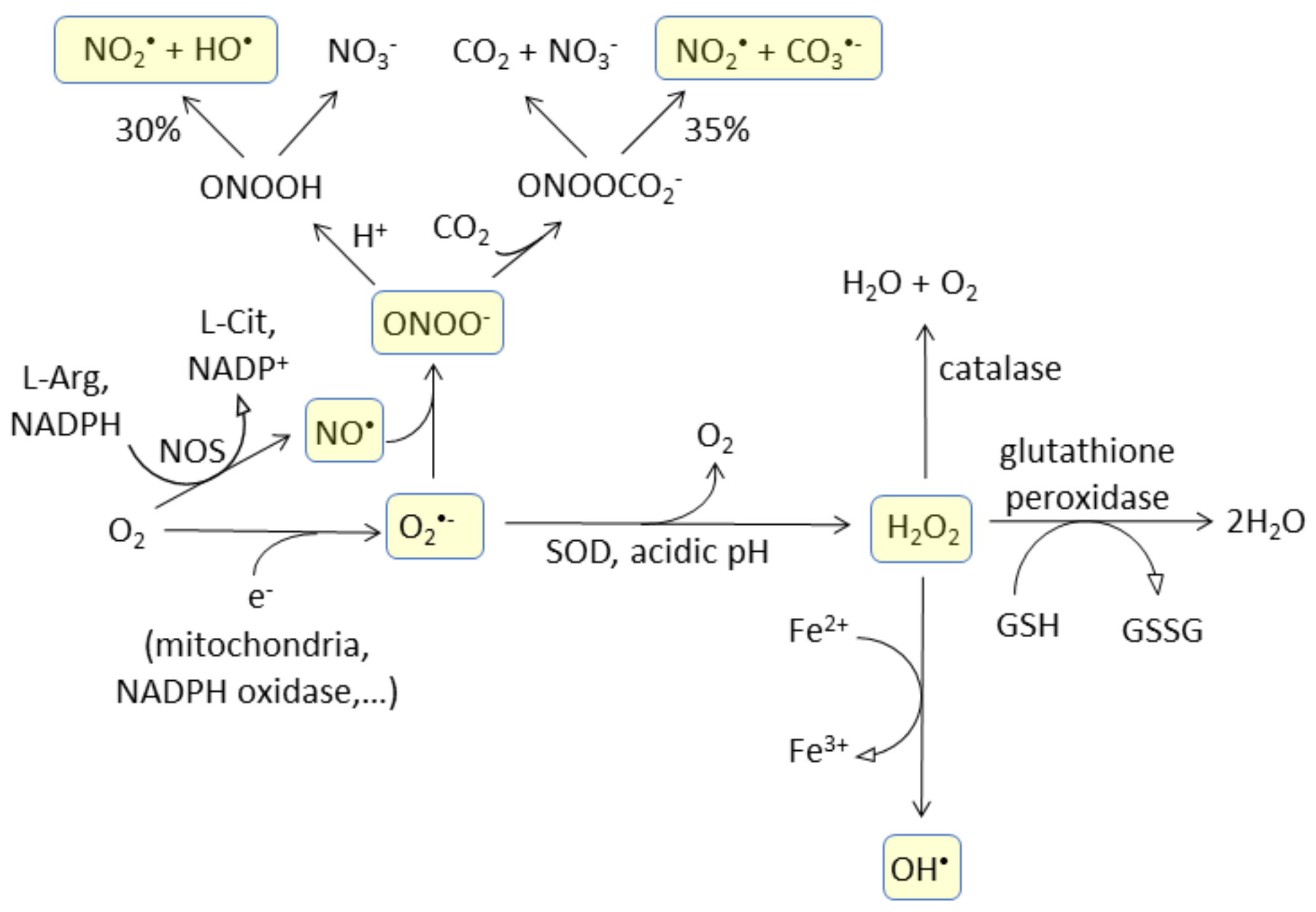
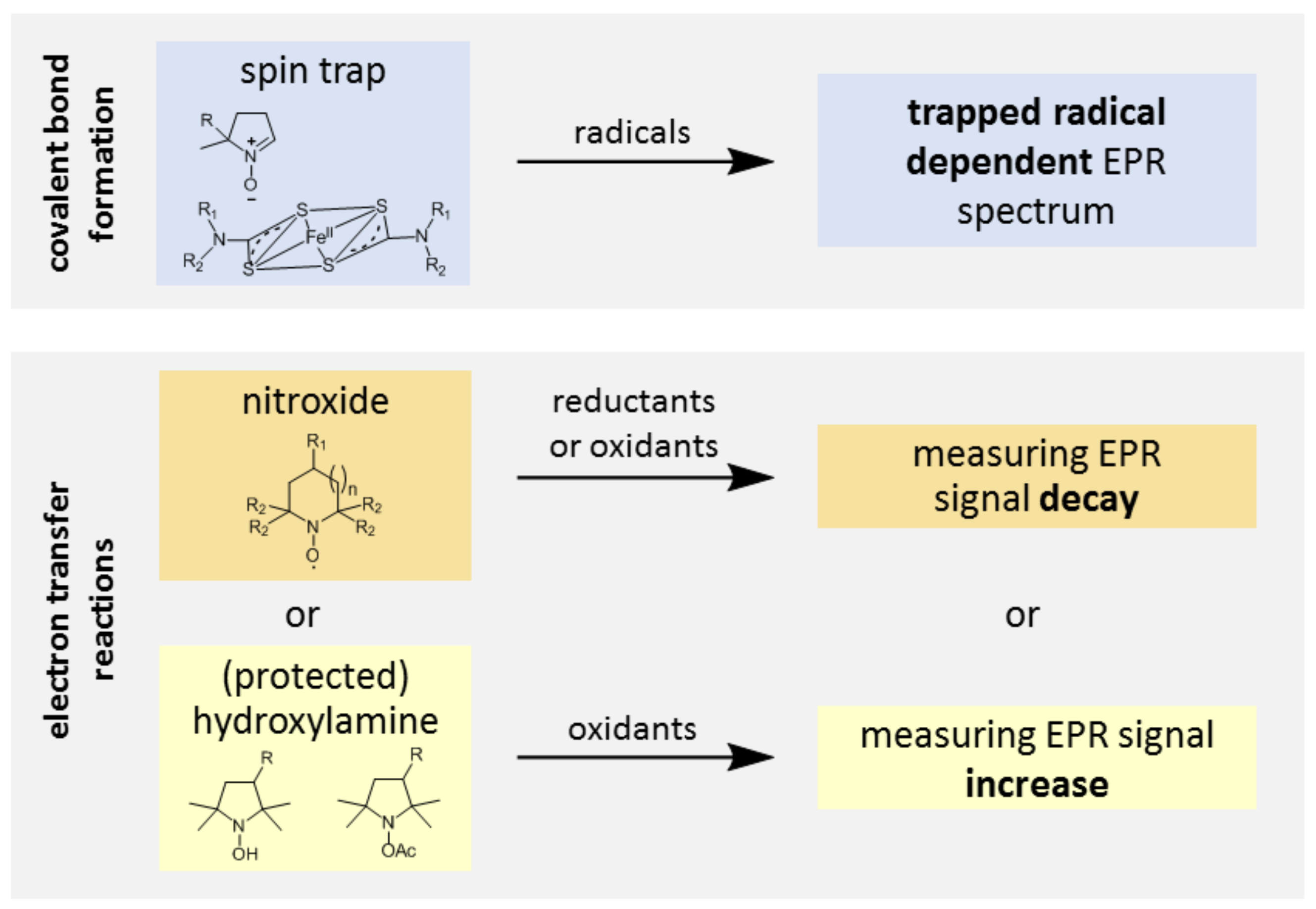
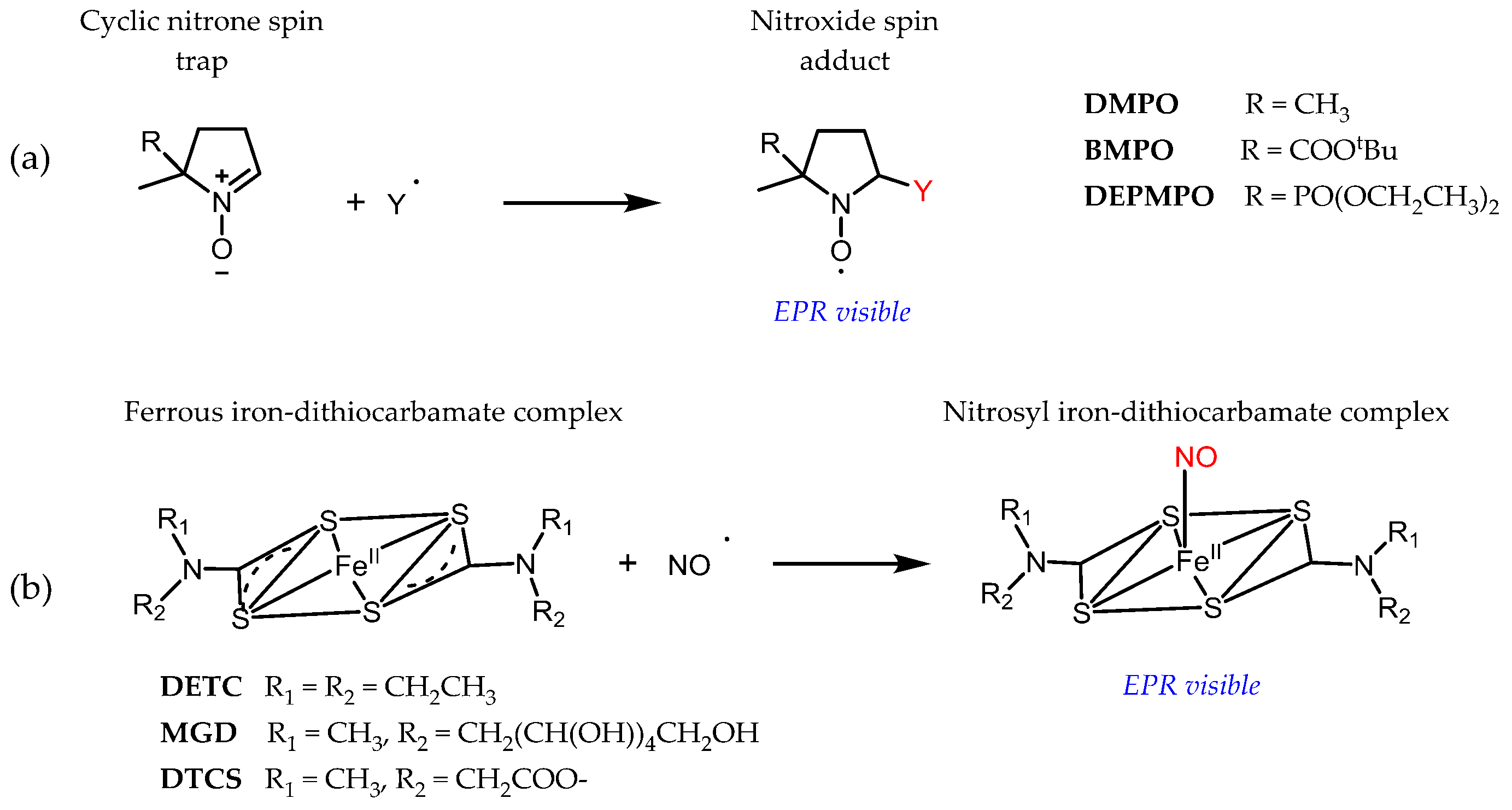
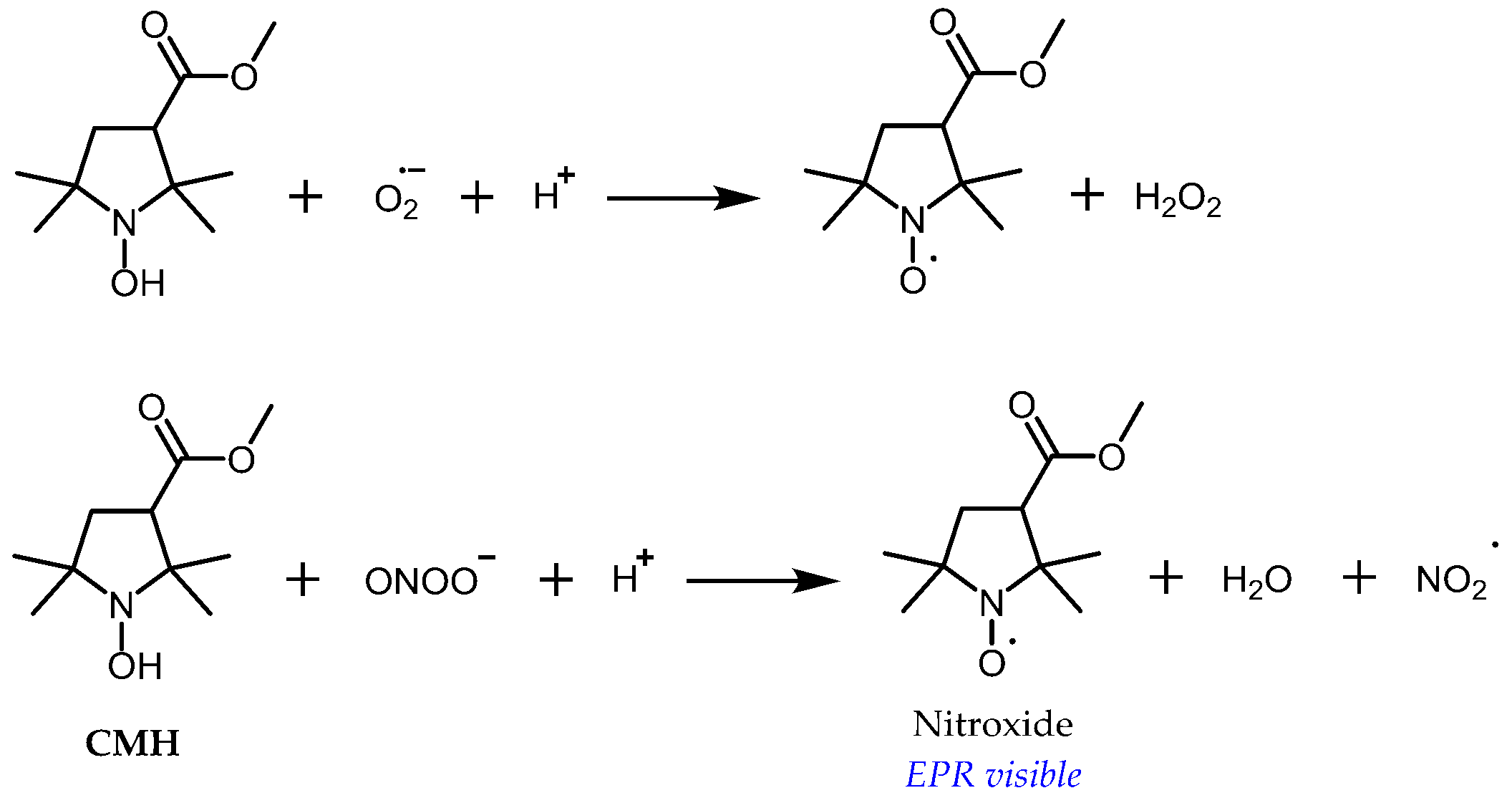
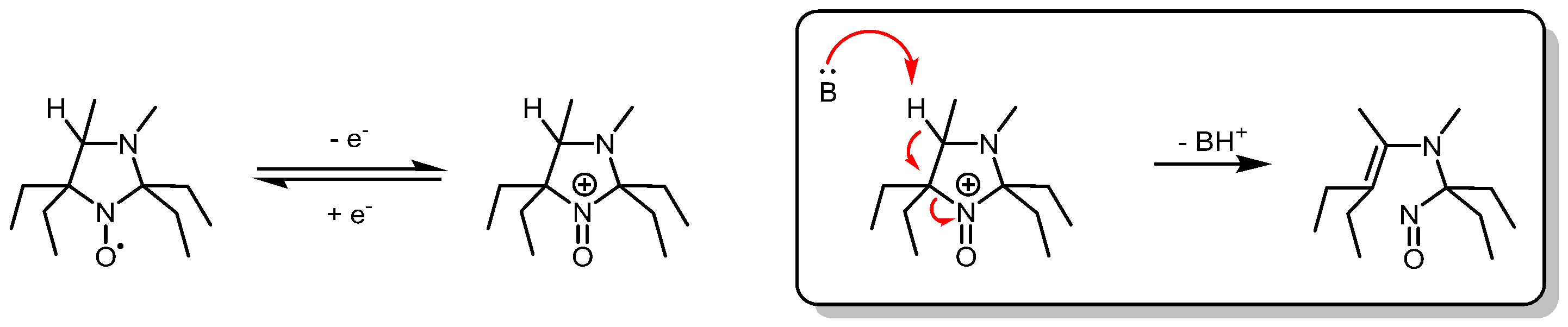
3.1. Probe Reactivity
3.2. Metabolic Biostability and Pharmacokinetics
3.3. Probe Formulation
3.4. Biodistribution
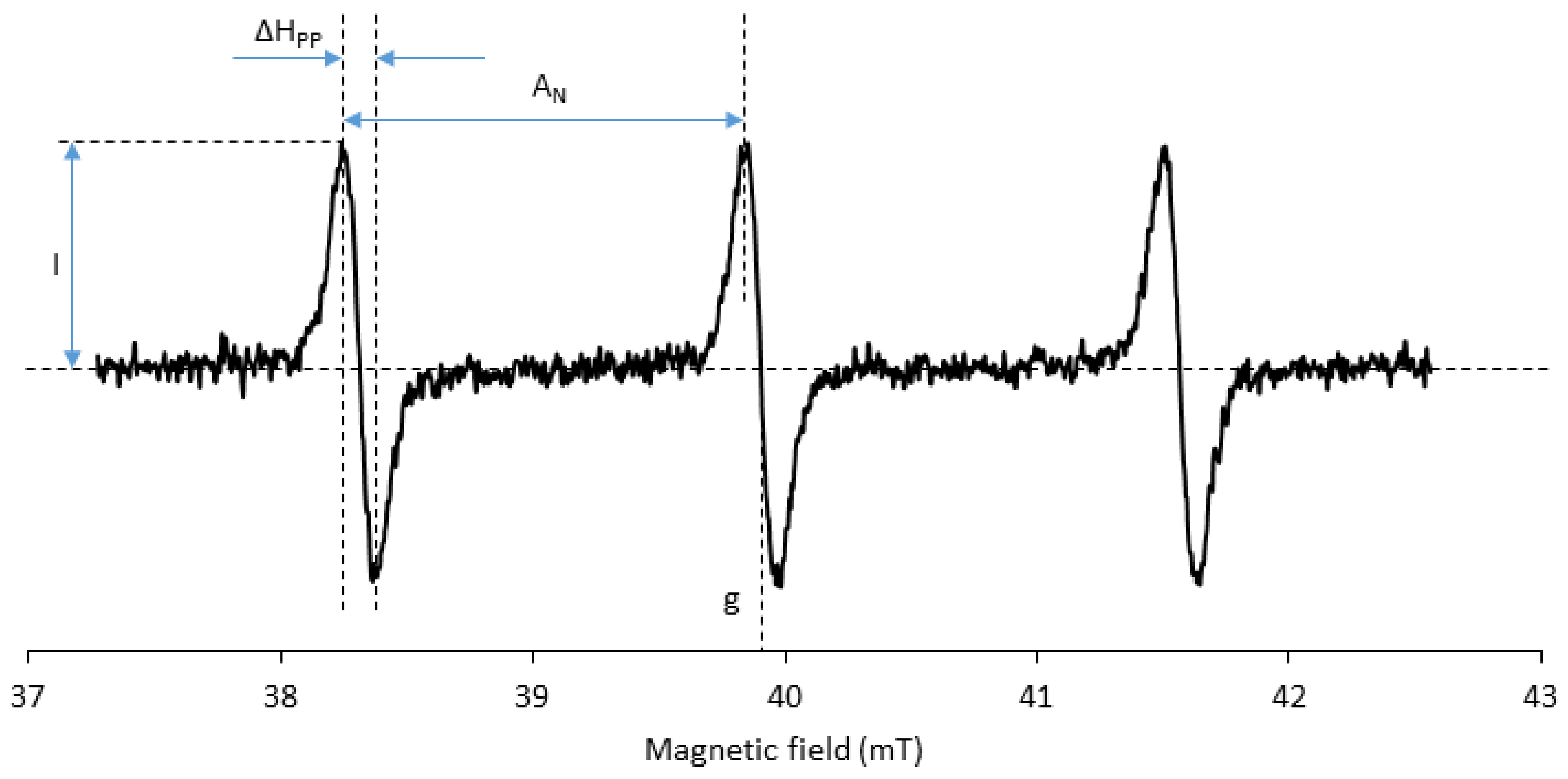
3.5. Spectral Properties
3.6. Toxicity
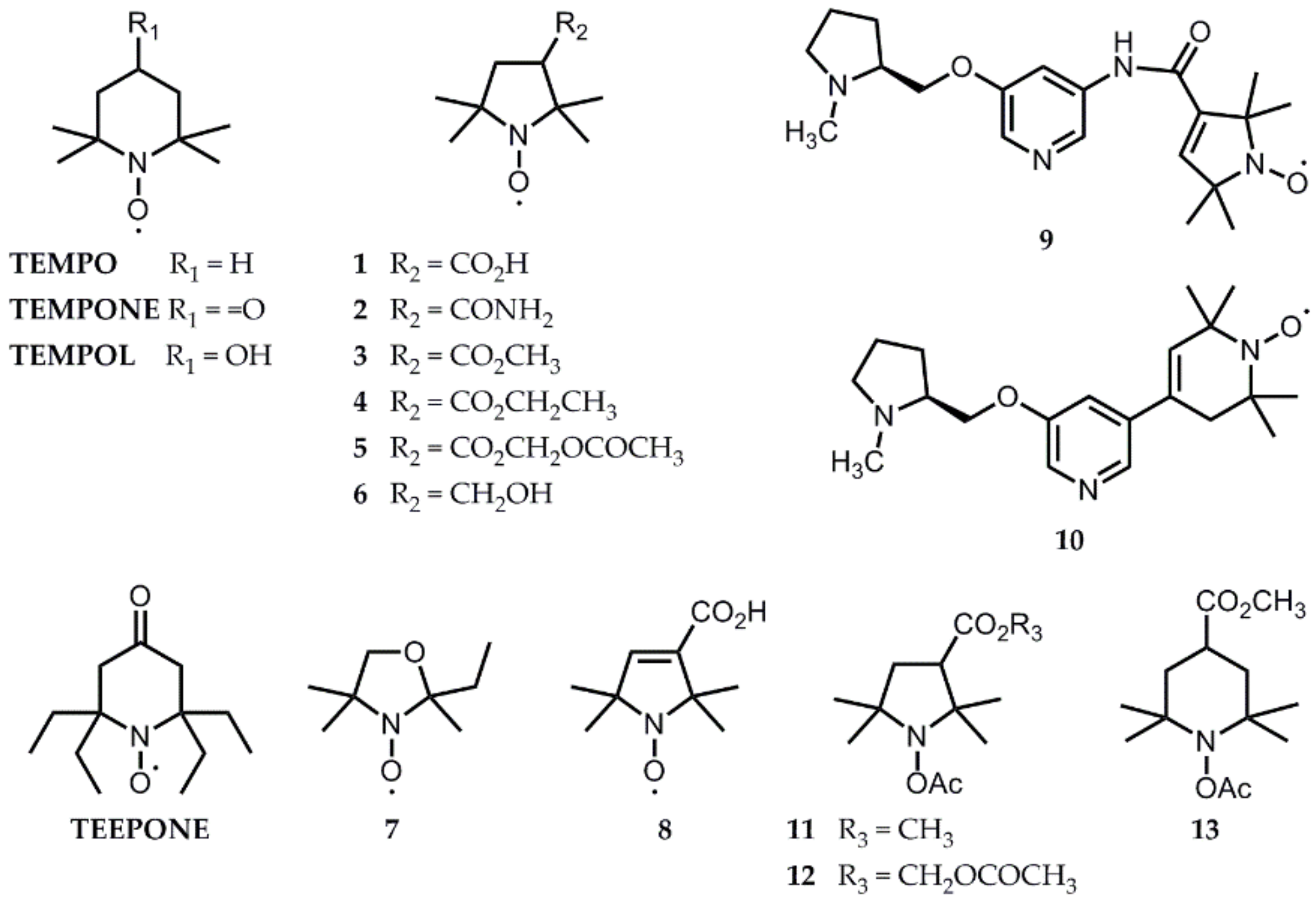
4. Solutions from the Literature That Have Already Been Tested In Vivo
4.1. Liver, Kidney, and Stomach
4.2. Brain Studies
4.3. Hind Limb-Implanted Tumor Studies
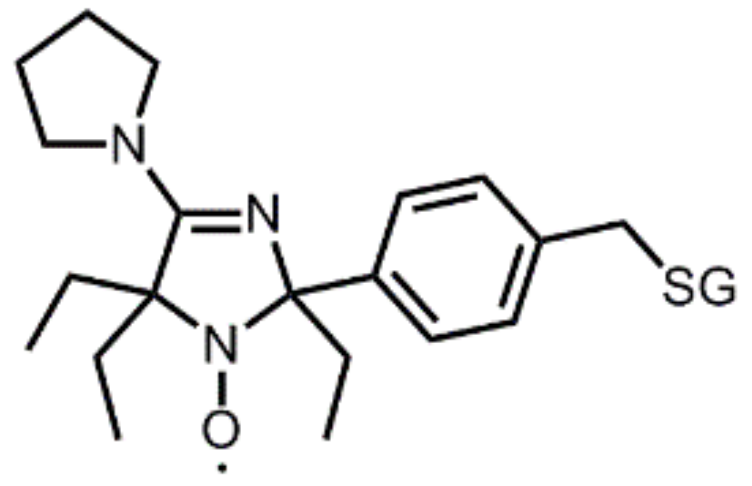
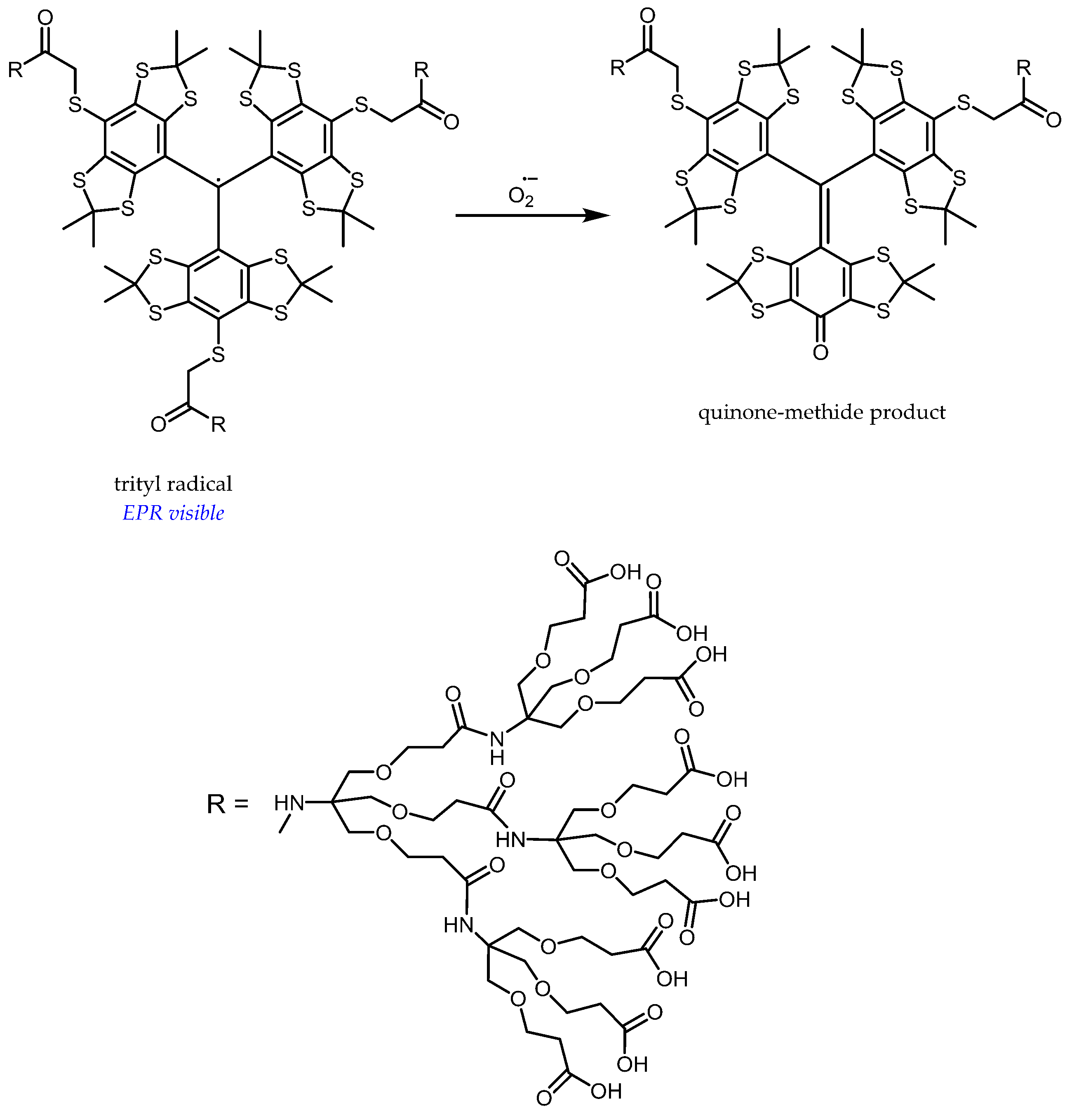
5. Perspectives from In Vitro Results
6. Conclusions
Abbreviations
References
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Swartz, H.M.; Williams, B.B.; Zaki, B.I.; Hartford, A.C.; Jarvis, L.A.; Chen, E.Y.; Comi, R.J.; Ernstoff, M.S.; Hou, H.; Khan, N.; et al. Clinical EPR: Unique opportunities and some challenges. Acad. Radiol. 2014, 21, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Epel, B.; Redler, G.; Tormyshev, V.; Halpern, H.J. Towards human oxygen images with electron paramagnetic resonance imaging. Adv. Exp. Med. Biol. 2016, 876, 363–369. [Google Scholar] [PubMed]
- Elas, M.; Ichikawa, K.; Halpern, H.J. Oxidative stress imaging in live animals with techniques based on electron paramagnetic resonance. Radiat. Res. 2012, 177, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, P.; Afeworki, M.; Shankar, R.A.; Coffin, D.; Krishna, M.C.; Hahn, S.M.; Mitchell, J.B.; Zweier, J.L. In vivo electron paramagnetic resonance imaging of tumor heterogeneity and oxygenation in a murine model. Cancer Res. 1998, 58, 1562–1568. [Google Scholar] [PubMed]
- Kuppusamy, P.; Li, H.; Ilangovan, G.; Cardounel, A.J.; Zweier, J.L.; Yamada, K.; Krishna, M.C.; Mitchell, J.B. Noninvasive imaging of tumor redox status and its modification by tissue glutathione levels. Cancer Res. 2002, 62, 307–312. [Google Scholar] [PubMed]
- Mikuni, T.; He, G.; Petryakov, S.; Fallouh, M.M.; Deng, Y.; Ishihara, R.; Kuppusamy, P.; Tatsuta, M.; Zweier, J.L. In vivo detection of gastric cancer in rats by electron paramagnetic resonance imaging. Cancer Res. 2004, 64, 6495–6502. [Google Scholar] [CrossRef]
- Hyodo, F.; Matsumoto, K.-I.; Matsumoto, A.; Mitchell, J.B.; Krishna, M.C. Probing the intracellular redox status of tumors with magnetic resonance imaging and redox-sensitive contrast agents. Cancer Res. 2006, 66, 9921–9928. [Google Scholar] [CrossRef]
- Takeshita, K.; Kawaguchi, K.; Fujii-Aikawa, K.; Ueno, M.; Okazaki, S.; Ono, M.; Krishna, M.C.; Kuppusamy, P.; Ozawa, T.; Ikota, N. Heterogeneity of regional redox status and relation of the redox status to oxygenation in a tumor model, evaluated using electron paramagnetic resonance imaging. Cancer Res. 2010, 70, 4133–4140. [Google Scholar] [CrossRef]
- Bobko, A.A.; Eubank, T.D.; Voorhees, J.L.; Efimova, O.V.; Kirilyuk, I.A.; Petryakov, S.; Trofimiov, D.G.; Marsh, C.B.; Zweier, J.L.; Grigor’ev, I.A.; et al. In vivo monitoring of pH, redox status, and glutathione using L-band EPR for assessment of therapeutic effectiveness in solid tumors. Magn. Reson. Med. 2012, 67, 1827–1836. [Google Scholar] [CrossRef]
- Yamato, M.; Egashira, T.; Utsumi, H. Application of in vivo ESR spectroscopy to measurement of cerebrovascular ROS generation in stroke. Free Radic. Biol. Med. 2003, 35, 1619–1631. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Ueda, Y.; Itoh, O.; Ikeda, T.; Noor, J.I.; Ikenoue, T. EPR imaging to estimate the in vivo intracerebral reducing ability of mature rats after neonatal hypoxic-ischemic brain injury. Magn. Reson. Imaging 2004, 22, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, F.; Chuang, K.-H.; Goloshevsky, A.G.; Sulima, A.; Griffiths, G.L.; Mitchell, J.B.; Koretsky, A.P.; Krishna, M.C. Brain redox imaging using blood-brain barrier-permeable nitroxide MRI contrast agent. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2008, 28, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.G.; Sato-Akaba, H.; Emoto, M.C.; Itoh, K.; Ishihara, Y.; Hirata, H. Noninvasive mapping of the redox status in septic mouse by in vivo electron paramagnetic resonance imaging. Magn. Reson. Imaging 2013, 31, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-C.-I. Assessment of oxidative stress and antioxidant property using electron spin resonance (ESR) spectroscopy. J. Clin. Biochem. Nutr. 2013, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, S.; Tachibana, Y.; Koga-Ogawa, Y.; Takeshita, K. Redox evaluation in sepsis model mice by the in vivo ESR technique using acyl-protected hydroxylamine. Free Radic. Biol. Med. 2014, 68, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Emoto, M.C.; Yamato, M.; Sato-Akaba, H.; Yamada, K.; Fujii, H.G. Brain redox imaging in the pentylenetetrazole (PTZ)-induced kindling model of epilepsy by using in vivo electron paramagnetic resonance and a nitroxide imaging probe. Neurosci. Lett. 2015, 608, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, F.; Matsumoto, S.; Devasahayam, N.; Dharmaraj, C.; Subramanian, S.; Mitchell, J.B.; Krishna, M.C. Pulsed EPR imaging of nitroxides in mice. J. Magn. Reson. 2009, 197, 181–185. [Google Scholar] [CrossRef]
- Eaton, S.S.; Shi, Y.; Woodcock, L.; Buchanan, L.A.; McPeak, J.; Quine, R.W.; Rinard, G.A.; Epel, B.; Halpern, H.J.; Eaton, G.R. Rapid-scan EPR imaging. J. Magn. Reson. 2017, 280, 140–148. [Google Scholar] [CrossRef]
- Fujii, H.; Sato-Akaba, H.; Kawanishi, K.; Hirata, H. Mapping of redox status in a brain-disease mouse model by three-dimensional EPR imaging. Magn. Reson. Med. 2011, 65, 295–303. [Google Scholar] [CrossRef]
- Ahmad, R.; Samouilov, A.; Zweier, J.L. Accelerated dynamic EPR imaging using fast acquisition and compressive recovery. J. Magn. Reson. 2016, 273, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Komarov, D.A.; Hirata, H. Fast backprojection-based reconstruction of spectral-spatial EPR images from projections with the constant sweep of a magnetic field. J. Magn. Reson. 2017, 281, 44–50. [Google Scholar] [CrossRef]
- Halpern, H.J.; Yu, C.; Barth, E.; Peric, M.; Rosen, G.M. In situ detection, by spin trapping, of hydroxyl radical markers produced from ionizing radiation in the tumor of a living mouse. Proc. Natl. Acad. Sci. USA 1995, 92, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Pignitter, M.; Gorren, A.C.F.; Nedeianu, S.; Schmidt, K.; Mayer, B. Inefficient spin trapping of superoxide in the presence of nitric-oxide: Implications for studies on nitric-oxide synthase uncoupling. Free Radic. Biol. Med. 2006, 41, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Lauricella, R.P.; Bouteiller, J.-C.H.; Tuccio, B.N. Evidence of overestimation of rate constants for the superoxide trapping by nitrones in aqueous media. Phys. Chem. Chem. Phys. 2005, 7, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Bézière, N.; Frapart, Y.; Rockenbauer, A.; Boucher, J.-L.; Mansuy, D.; Peyrot, F. Metabolic stability of superoxide and hydroxyl radical adducts of a cyclic nitrone toward rat liver microsomes and cytosol: A stopped-flow ESR spectroscopy study. Free Radic. Biol. Med. 2010, 49, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Bézière, N.; Hardy, M.; Poulhès, F.; Karoui, H.; Tordo, P.; Ouari, O.; Frapart, Y.-M.; Rockenbauer, A.; Boucher, J.-L.; Mansuy, D.; et al. Metabolic stability of superoxide adducts derived from newly developed cyclic nitrone spin traps. Free Radic. Biol. Med. 2014, 67, 150–158. [Google Scholar] [CrossRef]
- Nagano, T.; Yoshimura, T. Bioimaging of Nitric Oxide. Chem. Rev. 2002, 102, 1235–1270. [Google Scholar] [CrossRef]
- Hong, H.; Sun, J.; Cai, W. Multimodality imaging of nitric oxide and nitric oxide synthases. Free Radic. Biol. Med. 2009, 47, 684–698. [Google Scholar] [CrossRef]
- Kocherginsky, N.; Swartz, H.M. Nitroxide Spin Labels: Reactions in Biology and Chemistry; CRC Press: Boca Raton, FL, USA, 1995; ISBN 978-0-8493-4204-2. [Google Scholar]
- Bačić, G.; Pavićević, A.; Peyrot, F. In vivo evaluation of different alterations of redox status by studying pharmacokinetics of nitroxides using magnetic resonance techniques. Redox Biol. 2016, 8, 226–242. [Google Scholar] [CrossRef]
- Giotta, G.J.; Wang, H.H. Reduction of nitroxide free radicals by biological materials. Biochem. Biophys. Res. Commun. 1972, 46, 1576–1580. [Google Scholar] [CrossRef]
- Chen, K.; Swartz, H.M. The products of the reduction of doxyl stearates in cells are hydroxylamines as shown by oxidation by 15N-perdeuterated Tempone. Biochim. Biophys. Acta 1989, 992, 131–133. [Google Scholar] [CrossRef]
- Swartz, H.M. Principles of the metabolism of nitroxides and their implications for spin trapping. Free Radic. Res. Commun. 1990, 9, 399–405. [Google Scholar] [CrossRef]
- Bobko, A.A.; Kirilyuk, I.A.; Grigor’ev, I.A.; Zweier, J.L.; Khramtsov, V.V. Reversible reduction of nitroxides to hydroxylamines: Roles for ascorbate and glutathione. Free Radic. Biol. Med. 2007, 42, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Itoh, O.; Ohya-Nishiguchi, H.; Kamada, H. Reducing ability of the striatum and cerebral cortex in rats following acute administration of risperidone or haloperidol: An estimation by in vivo electron paramagnetic resonance imaging. Neurochem. Res. 2002, 27, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.; Grahame, D.; Samuni, A.; Mitchell, J.; Russo, A. Oxoammonium cation intermediate in the nitroxide-catalyzed dismutation. Proc. Natl. Acad. Sci. USA 1992, 89, 5537–5541. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.; Samuni, A.; Hideg, K.; Merenyi, G. Structure-activity relationship of cyclic nitroxides as SOD mimics and scavengers of nitrogen dioxide and carbonate radicals. J. Phys. Chem. A 2006, 110, 3679–3685. [Google Scholar] [CrossRef]
- Goldstein, S.; Samuni, A.; Russo, A. Reaction of cyclic nitroxides with nitrogen dioxide: The intermediacy of the oxoammonium cations. J. Am. Chem. Soc. 2003, 125, 8364–8370. [Google Scholar] [CrossRef]
- Goldstein, S.; Samuni, A.; Merenyi, G. Reactions of nitric oxide, peroxynitrite, and carbonate radicals with nitroxides and their corresponding oxoammonium cations. Chem. Res. Toxicol. 2004, 17, 250–257. [Google Scholar] [CrossRef]
- Goldstein, S.; Merenyi, G.; Russo, A.; Samuni, A. The role of oxoammonium cation in the SOD-mimic activity of cyclic nitroxides. J. Am. Chem. Soc. 2003, 125, 789–795. [Google Scholar] [CrossRef]
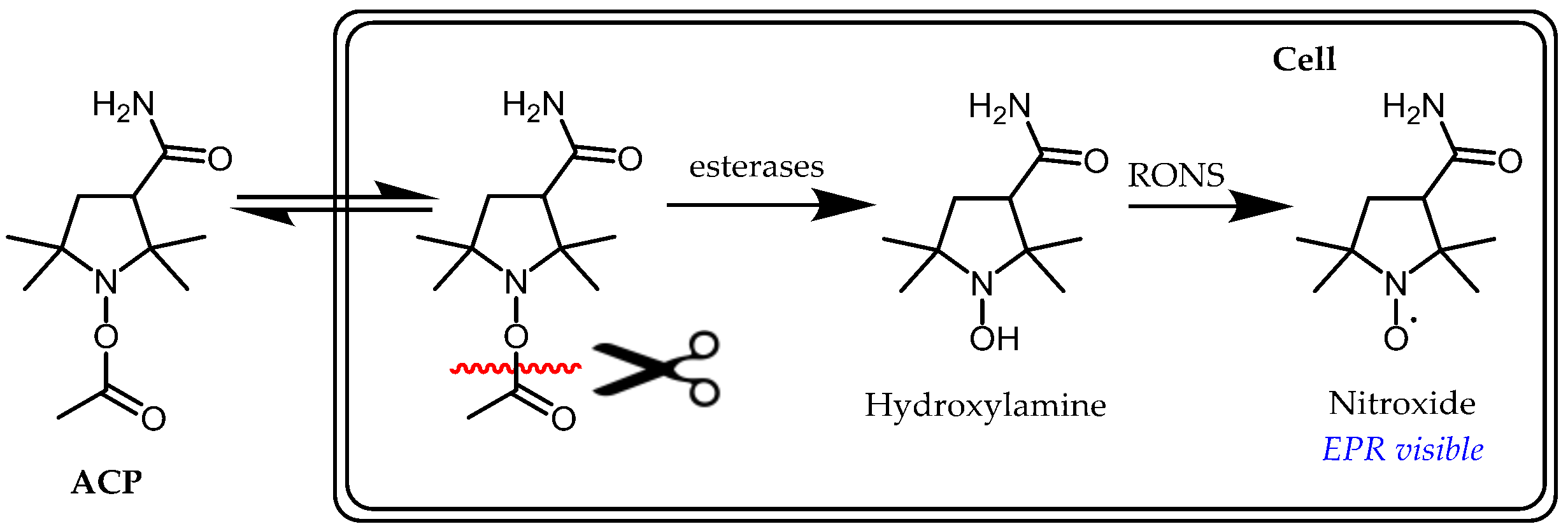
- Itoh, O.; Aoyama, M.; Yokoyama, H.; Obara, H.; Ohya, H.; Kamada, H. Sensitive ESR determination of intracellular oxidative stress by using acyl-protected hydroxylamines as new spin reagents. Chem. Lett. 2000, 4, 304–305. [Google Scholar] [CrossRef]
- Yokoyama, H.; Itoh, O.; Aoyama, M.; Obara, H.; Ohya, H.; Kamada, H. In vivo EPR imaging by using an acyl-protected hydroxylamine to analyze intracerebral oxidative stress in rats after epileptic seizures. Magn. Reson. Imaging 2000, 18, 875–879. [Google Scholar] [CrossRef]
- Yordanov, A.T.; Yamada, K.; Krishna, M.C.; Russo, A.; Yoo, J.; English, S.; Mitchell, J.B.; Brechbiel, M.W. Acyl-protected hydroxylamines as spin label generators for EPR brain imaging. J. Med. Chem. 2002, 45, 2283–2288. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S.I.; Polienko, Y.F.; Kirilyuk, I. Electron paramagnetic resonance measurements of reactive oxygen species by cyclic hydroxylamine spin probes. Antioxid. Redox Signal. 2018, 28, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Goldstein, S.; Samuni, A. Kinetics of superoxide-induced exchange among nitroxide antioxidants and their oxidized and reduced forms. Free Radic. Biol. Med. 1999, 26, 1245–1252. [Google Scholar] [CrossRef]
- Griesser, M.; Shah, R.; Van Kessel, A.T.; Zilka, O.; Haidasz, E.A.; Pratt, D.A. The catalytic reaction of nitroxides with peroxyl radicals and its relevance to their cytoprotective properties. J. Am. Chem. Soc. 2018, 140, 3798–3808. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S.; Skatchkov, M.; Bassenge, E. Quantification of peroxynitrite, superoxide, and peroxyl radicals by a new spin trap hydroxylamine 1-hydroxy-2,2,6,6-tetramethyl-4-oxo-piperidine. Biochem. Biophys. Res. Commun. 1997, 230, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Swartz, H.M. Oxidation of hydroxylamines to nitroxide spin labels in living cells. Biochim. Biophys. Acta 1988, 970, 270–277. [Google Scholar] [CrossRef]
- Giustarini, D.; Colombo, G.; Garavaglia, M.L.; Astori, E.; Portinaro, N.M.; Reggiani, F.; Badalamenti, S.; Aloisi, A.M.; Santucci, A.; Rossi, R.; et al. Assessment of glutathione/glutathione disulphide ratio and S-glutathionylated proteins in human blood, solid tissues, and cultured cells. Free Radic. Biol. Med. 2017, 112, 360–375. [Google Scholar] [CrossRef]
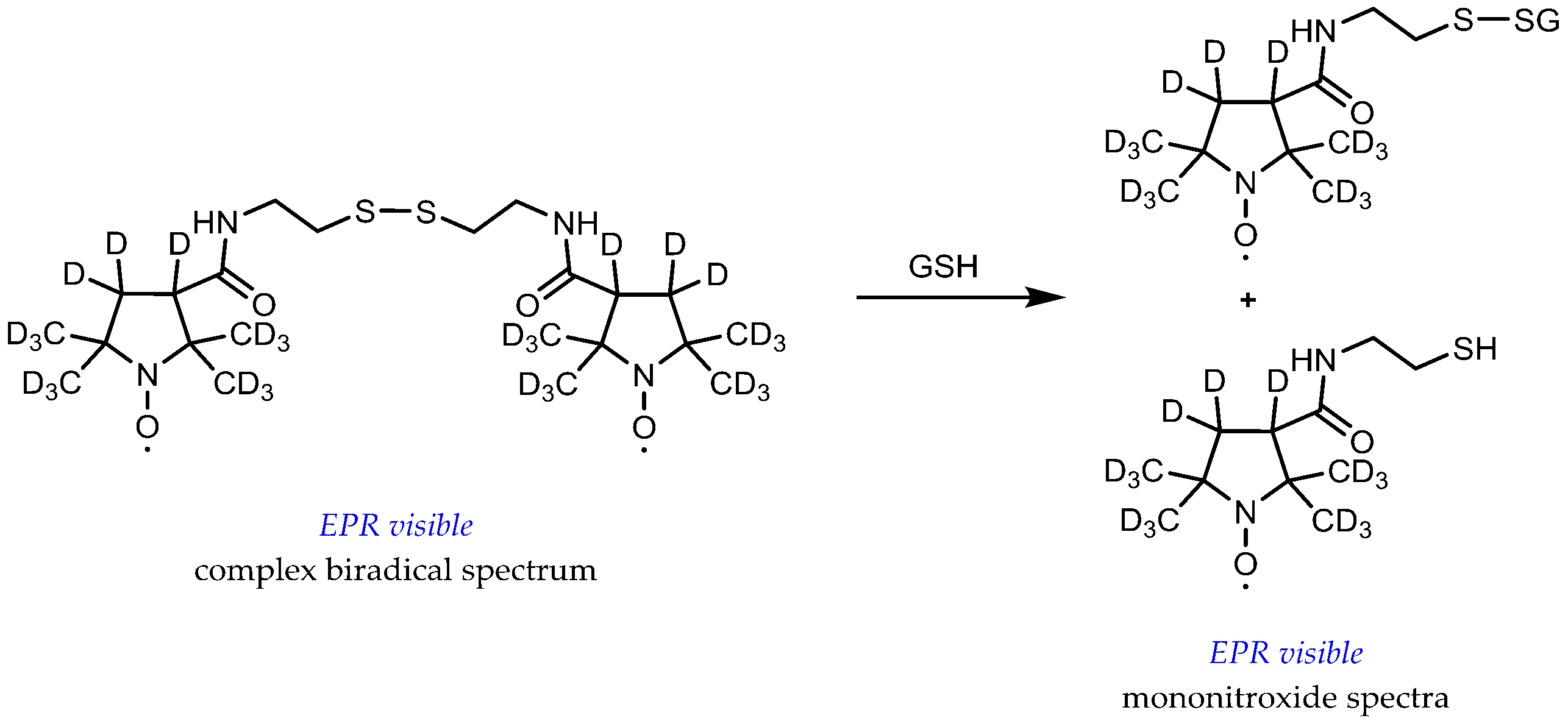
- Roshchupkina, G.I.; Bobko, A.A.; Bratasz, A.; Reznikov, V.A.; Kuppusamy, P.; Khramtsov, V.V. In vivo EPR measurement of glutathione in tumor-bearing mice using improved disulfide biradical probe. Free Radic. Biol. Med. 2008, 45, 312–320. [Google Scholar] [CrossRef]
- Epel, B.; Sundramoorthy, S.V.; Krzykawska-Serda, M.; Maggio, M.C.; Tseytlin, M.; Eaton, G.R.; Eaton, S.S.; Rosen, G.M.; Kao, J.P.Y.; Halpern, H.J. Imaging thiol redox status in murine tumors in vivo with rapid-scan electron paramagnetic resonance. J. Magn. Reson. 2017, 276, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Khramtsov, V.V.; Yelinova, V.I.; Glazachev, Y.; Reznikov, V.A.; Zimmer, G. Quantitative determination and reversible modification of thiols using imidazolidine biradical disulfide label. J. Biochem. Biophys. Methods 1997, 35, 115–128. [Google Scholar] [CrossRef]
- Chen, K.Y.; McLaughlin, M.G. Differences in the reduction kinetics of incorporated spin labels in undifferentiated and differentiated mouse neuroblastoma cells. Biochim. Biophys. Acta 1985, 845, 189–195. [Google Scholar] [CrossRef]
- Couet, W.R.; Eriksson, U.G.; Tozer, T.N.; Tuck, L.D.; Wesbey, G.E.; Nitecki, D.; Brasch, R.C. Pharmacokinetics and metabolic fate of two nitroxides potentially useful as contrast agents for magnetic resonance imaging. Pharm. Res. 1984, 1, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Bacic, G.; Nilges, M.J.; Magin, R.L.; Walczak, T.; Swartz, H.M. In vivo localized ESR spectroscopy reflecting metabolism. Magn. Reson. Med. 1989, 10, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, H.; Muto, E.; Masuda, S.; Hamada, A. In vivo ESR measurement of free radicals in whole mice. Biochem. Biophys. Res. Commun. 1990, 172, 1342–1348. [Google Scholar] [CrossRef]
- Miura, Y.; Utsumi, H.; Hamada, A. Effects of inspired oxygen concentration on in vivo redox reaction of nitroxide radicals in whole mice. Biochem. Biophys. Res. Commun. 1992, 182, 1108–1114. [Google Scholar] [CrossRef]
- Vianello, F.; Momo, F.; Scarpa, M.; Rigo, A. Kinetics of nitroxide spin label removal in biological systems: An in vitro and in vivo ESR study. Magn. Reson. Imaging 1995, 13, 219–226. [Google Scholar] [CrossRef]
- Seimenis, I.; Foster, M.A.; Lurie, D.J.; Hutchison, J.M.S.; Whiting, P.H.; Payne, S. The excretion mechanism of the spin label proxyl carboxylic acid (PCA) from the rat monitored by X-band ESR and PEDRI. Magn. Reson. Med. 1997, 37, 552–558. [Google Scholar] [CrossRef]
- Kroll, C.; Borchert, H.H. Metabolism of the stable nitroxyl radical 4-oxo-2,2,6, 6-tetramethylpiperidine-N-oxyl (TEMPONE). Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 1999, 8, 5–9. [Google Scholar]
- Ueda, A.; Nagase, S.; Yokoyama, H.; Tada, M.; Noda, H.; Ohya, H.; Kamada, H.; Hirayama, A.; Koyama, A. Importance of renal mitochondria in the reduction of TEMPOL, a nitroxide radical. Mol. Cell. Biochem. 2003, 244, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.-I.; Krishna, M.C.; Mitchell, J.B. Novel pharmacokinetic measurement using electron paramagnetic resonance spectroscopy and simulation of in vivo decay of various nitroxyl spin probes in mouse blood. J. Pharmacol. Exp. Ther. 2004, 310, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Okajo, A.; Matsumoto, K.; Mitchell, J.B.; Krishna, M.C.; Endo, K. Competition of nitroxyl contrast agents as an in vivo tissue redox probe: Comparison of pharmacokinetics by the bile flow monitoring (BFM) and blood circulating monitoring (BCM) methods using X-band EPR and simulation of decay profiles. Magn. Reson. Med. 2006, 56, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Zhelev, Z.; Matsumoto, K.-I.; Gadjeva, V.; Bakalova, R.; Aoki, I.; Zheleva, A.; Anzai, K. EPR signal reduction kinetic of several nitroxyl derivatives in blood in vitro and in vivo. Gen. Physiol. Biophys. 2009, 28, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Mitra, K.; Stearns, R.A.; Baillie, T.A.; Kumar, S. Conversion of the 2,2,6,6-tetramethylpiperidine moiety to a 2,2-dimethylpyrrolidine by cytochrome P450: Evidence for a mechanism involving nitroxide radicals and heme iron. Biochemistry 2004, 43, 5455–5466. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Takeshita, K.; Anzai, K.; Ozawa, T. Pharmacokinetic study of acyl-protected hydroxylamine probe, 1-acetoxy-3-carbamoyl-2,2,5,5-tetramethylpyrrolidine, for in vivo measurements of reactive oxygen species. Free Radic. Biol. Med. 2004, 36, 517–525. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L. Modern methods for delivery of drugs across the blood-brain barrier. Adv. Drug Deliv. Rev. 2012, 64, 640–665. [Google Scholar] [CrossRef]
- Testa, B.; Crivori, P.; Reist, M.; Carrupt, P.-A. The influence of lipophilicity on the pharmacokinetic behavior of drugs: Concepts and examples. Perspect. Drug Discov. Des. 2000, 19, 179–211. [Google Scholar] [CrossRef]
- Sosnovsky, G.; Rao, N.; Li, S.; Swartz, H. Synthesis of nitroxyl (aminoxyl) labeled probes for studies of intracellular environment by EPR and MRI. J. Org. Chem. 1989, 54, 3667–3674. [Google Scholar] [CrossRef]
- Hu, H.P.; Sosnovsky, G.; Li, S.W.; Rao, N.U.; Morse, P.D.; Swartz, H.M. Development of nitroxides for selective localization inside cells. Biochim. Biophys. Acta 1989, 1014, 211–218. [Google Scholar] [CrossRef]
- Dikalov, S.I.; Dikalova, A.E.; Morozov, D.A.; Kirilyuk, I.A. Cellular accumulation and antioxidant activity of acetoxymethoxycarbonyl pyrrolidine nitroxides. Free Radic. Res. 2018, 52, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Binet, L.; Gourier, D.; Derenne, S. Potential of EPR imaging to detect traces of primitive life in sedimentary rocks. Earth Planet. Sci. Lett. 2008, 273, 359–366. [Google Scholar] [CrossRef]
- Burks, S.R.; Makowsky, M.A.; Yaffe, Z.A.; Hoggle, C.; Tsai, P.; Muralidharan, S.; Bowman, M.K.; Kao, J.P.Y.; Rosen, G.M. The effect of structure on nitroxide EPR spectral linewidth. J. Org. Chem. 2010, 75, 4737–4741. [Google Scholar] [CrossRef] [PubMed]
- Biller, J.R.; Meyer, V.; Elajaili, H.; Rosen, G.M.; Kao, J.P.Y.; Eaton, S.S.; Eaton, G.R. Relaxation times and line widths of isotopically-substituted nitroxides in aqueous solution at X-band. J. Magn. Reson. 2011, 212, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Afzal, V.; Brasch, R.C.; Nitecki, D.E.; Wolff, S. Nitroxyl spin label contrast enhancers for magnetic resonance imaging. Studies of acute toxicity and mutagenesis. Investig. Radiol. 1984, 19, 549–552. [Google Scholar] [CrossRef]
- Hahn, S.M.; Sullivan, F.J.; DeLuca, A.M.; Bacher, J.D.; Liebmann, J.; Krishna, M.C.; Coffin, D.; Mitchell, J.B. Hemodynamic effect of the nitroxide superoxide dismutase mimics. Free Radic. Biol. Med. 1999, 27, 529–535. [Google Scholar] [CrossRef]
- Davis, R.M.; Matsumoto, S.; Bernardo, M.; Sowers, A.; Matsumoto, K.-I.; Krishna, M.C.; Mitchell, J.B. Magnetic resonance imaging of organic contrast agents in mice: Capturing the whole-body redox landscape. Free Radic. Biol. Med. 2011, 50, 459–468. [Google Scholar] [CrossRef]
- Ueda, A.; Nagase, S.; Yokoyama, H.; Tada, M.; Ohya, H.; Kamada, H.; Hirayama, A.; Koyama, A. Identification by an EPR technique of decreased mitochondrial reducing activity in puromycin aminonucleoside-induced nephrosis. Free Radic. Biol. Med. 2002, 33, 1082–1088. [Google Scholar] [CrossRef]
- Ueda, A.; Hirayama, A.; Nagase, S.; Inoue, M.; Oteki, T.; Aoyama, M.; Yokoyama, H. In vivo detection of intrinsic reactive oxygen species using acyl-protected hydroxylamine in puromycin nephrosis. Free Radic. Res. 2007, 41, 823–828. [Google Scholar] [CrossRef]
- Ueda, A.; Yokoyama, H.; Nagase, S.; Hirayama, A.; Koyama, A.; Ohya, H.; Kamada, H. In vivo temporal EPR imaging for estimating the kinetics of a nitroxide radical in the renal parenchyma and pelvis in rats. Magn. Reson. Imaging 2002, 20, 77–82. [Google Scholar] [CrossRef]
- Hirayama, A.; Yoh, K.; Nagase, S.; Ueda, A.; Itoh, K.; Morito, N.; Hirayama, K.; Takahashi, S.; Yamamoto, M.; Koyama, A. EPR imaging of reducing activity in Nrf2 transcriptional factor-deficient mice. Free Radic. Biol. Med. 2003, 34, 1236–1242. [Google Scholar] [CrossRef]
- Sonta, T.; Inoguchi, T.; Matsumoto, S.; Yasukawa, K.; Inuo, M.; Tsubouchi, H.; Sonoda, N.; Kobayashi, K.; Utsumi, H.; Nawata, H. In vivo imaging of oxidative stress in the kidney of diabetic mice and its normalization by angiotensin II type 1 receptor blocker. Biochem. Biophys. Res. Commun. 2005, 330, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Reyes, L.A.; Johnson, D.H.; Velayutham, M.; Yang, C.; Samouilov, A.; Zweier, J.L. Fast gated EPR imaging of the beating heart: Spatiotemporally resolved 3D imaging of free-radical distribution during the cardiac cycle. Magn. Reson. Med. 2013, 69, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.H.; Ahmad, R.; Liu, Y.; Chen, Z.; Samouilov, A.; Zweier, J.L. Uniform spinning sampling gradient electron paramagnetic resonance imaging. Magn. Reson. Med. 2014, 71, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Caia, G.L.; Efimova, O.V.; Velayutham, M.; El-Mahdy, M.A.; Abdelghany, T.M.; Kesselring, E.; Petryakov, S.; Sun, Z.; Samouilov, A.; Zweier, J.L. Organ specific mapping of in vivo redox state in control and cigarette smoke-exposed mice using EPR/NMR co-imaging. J. Magn. Reson. 2012, 216, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Yokoyama, H.; Ohya-Nishiguchi, H.; Kamada, H. ESR spectroscopy for analysis of hippocampal elimination of a nitroxide radical during kainic acid-induced seizure in rats. Magn. Reson. Med. 1998, 40, 491–493. [Google Scholar] [CrossRef]
- Miura, Y.; Anzai, K.; Takahashi, S.; Ozawa, T. A novel lipophilic spin probe for the measurement of radiation damage in mouse brain using in vivo electron spin resonance (ESR). FEBS Lett. 1997, 419, 99–102. [Google Scholar] [CrossRef]
- Utsumi, H.; Sano, H.; Naruse, M.; Matsumoto, K.; Ichikawa, K.; Oi, T. Nitroxyl probes for brain research and their application to brain imaging. Methods Enzymol. 2002, 352, 494–506. [Google Scholar]
- Kinoshita, Y.; Yamada, K.; Yamasaki, T.; Mito, F.; Yamato, M.; Kosem, N.; Deguchi, H.; Shirahama, C.; Ito, Y.; Kitagawa, K.; et al. In vivo evaluation of novel nitroxyl radicals with reduction stability. Free Radic. Biol. Med. 2010, 49, 1703–1709. [Google Scholar] [CrossRef]
- Sano, H.; Naruse, M.; Matsumoto, K.; Oi, T.; Utsumi, H. A new nitroxyl-probe with high retention in the brain and its application for brain imaging. Free Radic. Biol. Med. 2000, 28, 959–969. [Google Scholar] [CrossRef]
- Anzai, K.; Saito, K.; Takeshita, K.; Takahashi, S.; Miyazaki, H.; Shoji, H.; Lee, M.C.; Masumizu, T.; Ozawa, T. Assessment of ESR-CT imaging by comparison with autoradiography for the distribution of a blood-brain-barrier permeable spin probe, MC-PROXYL, to rodent brain. Magn. Reson. Imaging 2003, 21, 765–772. [Google Scholar] [CrossRef]
- Diehl, K.H.; Hull, R.; Morton, D.; Pfister, R.; Rabemampianina, Y.; Smith, D.; Vidal, J.M.; van de Vorstenbosch, C. European Federation of Pharmaceutical Industries Association and European Centre for the Validation of Alternative Methods A good practice guide to the administration of substances and removal of blood, including routes and volumes. J. Appl. Toxicol. JAT 2001, 21, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Itoh, O.; Aoyama, M.; Obara, H.; Ohya, H.; Kamada, H. In vivo temporal EPR imaging of the brain of rats by using two types of blood-brain barrier-permeable nitroxide radicals. Magn. Reson. Imaging 2002, 20, 277–284. [Google Scholar] [CrossRef]
- Yokoyama, H.; Ishida, S.-I.; Ogata, T. In vivo temporal EPR study using a region-selected intensity determination method to estimate cerebral reducing ability in rats treated with olanzapine. Magn. Reson. Imaging 2010, 28, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Emoto, M.C.; Sato-Akaba, H.; Hirata, H.; Fujii, H.G. Dynamic changes in the distribution and time course of blood–brain barrier-permeative nitroxides in the mouse head with EPR imaging: Visualization of blood flow in a mouse model of ischemia. Free Radic. Biol. Med. 2014, 74, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Emoto, M.C.; Yamato, M.; Sato-Akaba, H.; Yamada, K.; Matsuoka, Y.; Fujii, H.G. Brain imaging in methamphetamine-treated mice using a nitroxide contrast agent for EPR imaging of the redox status and a gadolinium contrast agent for MRI observation of blood-brain barrier function. Free Radic. Res. 2015, 49, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, A.; Emoto, M.C.; Suzuki, S.; Iwahara, N.; Hisahara, S.; Kawamata, J.; Suzuki, H.; Yamauchi, A.; Sato-Akaba, H.; Fujii, H.G.; et al. Evaluation of oxidative stress in the brain of a transgenic mouse model of Alzheimer disease by in vivo electron paramagnetic resonance imaging. Free Radic. Biol. Med. 2015, 85, 165–173. [Google Scholar] [CrossRef]
- Fang, D.; Zhang, Z.; Li, H.; Yu, Q.; Douglas, J.T.; Bratasz, A.; Kuppusamy, P.; Yan, S.S. Increased electron paramagnetic resonance signal correlates with mitochondrial dysfunction and oxidative stress in an Alzheimer’s disease mouse brain. J. Alzheimers Dis. 2016, 51, 571–580. [Google Scholar] [CrossRef]
- Emoto, M.C.; Matsuoka, Y.; Yamada, K.-I.; Sato-Akaba, H.; Fujii, H.G. Non-invasive imaging of the levels and effects of glutathione on the redox status of mouse brain using electron paramagnetic resonance imaging. Biochem. Biophys. Res. Commun. 2017, 485, 802–806. [Google Scholar] [CrossRef]
- Emoto, M.C.; Sato-Akaba, H.; Matsuoka, Y.; Yamada, K.-I.; Fujii, H.G. Non-invasive mapping of glutathione levels in mouse brains by in vivo electron paramagnetic resonance (EPR) imaging: Applied to a kindling mouse model. Neurosci. Lett. 2018, 690, 6–10. [Google Scholar] [CrossRef]
- Paletta, J.T.; Pink, M.; Foley, B.; Rajca, S.; Rajca, A. Synthesis and reduction kinetics of sterically shielded pyrrolidine nitroxides. Org. Lett. 2012, 14, 5322–5325. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, A.P.; Krstic, I.; Kunjir, N.C.; Hänsel, R.; Prisner, T.F.; Sigurdsson, S.T. Sterically shielded spin labels for in-cell EPR spectroscopy: Analysis of stability in reducing environment. Free Radic. Res. 2015, 49, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Emoto, M.; Mito, F.; Yamasaki, T.; Yamada, K.-I.; Sato-Akaba, H.; Hirata, H.; Fujii, H. A novel ascorbic acid-resistant nitroxide in fat emulsion is an efficient brain imaging probe for in vivo EPR imaging of mouse. Free Radic. Res. 2011, 45, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Emoto, M.C.; Yamada, K.-I.; Yamato, M.; Fujii, H.G. Novel ascorbic acid-resistive nitroxide in a lipid emulsion: An efficient brain imaging contrast agent for MRI of small rodents. Neurosci. Lett. 2013, 546, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Emoto, M.; Miyake, Y.; Itto, K.; Xu, S.; Fujii, H.; Hirata, H.; Arimoto, H. Novel blood-brain barrier-permeable spin probe for in vivo electron paramagnetic resonance imaging. Bioorg. Med. Chem. Lett. 2016, 26, 4947–4949. [Google Scholar] [CrossRef] [PubMed]
- Khramtsov, V.V. In vivo molecular electron paramagnetic resonance-based spectroscopy and imaging of tumor microenvironment and redox using functional paramagnetic Probes. Antioxid. Redox Signal. 2018, 28, 1365–1377. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, J.; Yachi, K.; Nagane, M.; Yasui, H.; Miyake, Y.; Inanami, O.; Bobko, A.A.; Khramtsov, V.V.; Hirata, H. In vivo tumour extracellular pH monitoring using electron paramagnetic resonance: The effect of X-ray irradiation. NMR Biomed. 2014, 27, 453–458. [Google Scholar] [CrossRef]
- Biller, J.R.; Tseitlin, M.; Mitchell, D.G.; Yu, Z.; Buchanan, L.A.; Elajaili, H.; Rosen, G.M.; Kao, J.P.Y.; Eaton, S.S.; Eaton, G.R. Improved sensitivity for imaging spin trapped hydroxyl radical at 250 MHz. ChemPhysChem 2015, 16, 528–531. [Google Scholar] [CrossRef]
- Tan, X.; Tao, S.; Liu, W.; Rockenbauer, A.; Villamena, F.A.; Zweier, J.L.; Song, Y.; Liu, Y. Synthesis and characterization of the perthiatriarylmethyl radical and its dendritic derivatives with high sensitivity and selectivity to superoxide radical. Chem. Eur. J. 2018, 24, 6958–6967. [Google Scholar] [CrossRef]
- Poncelet, M.; Driesschaert, B.; Bobko, A.A.; Khramtsov, V.V. Triarylmethyl-based biradical as a superoxide probe. Free Radic. Res. 2018, 52, 373–379. [Google Scholar] [CrossRef]
- Decroos, C.; Li, Y.; Bertho, G.; Frapart, Y.; Mansuy, D.; Boucher, J.-L. Oxidation of tris-(p-carboxyltetrathiaaryl)methyl radical EPR probes: Evidence for their oxidative decarboxylation and molecular origin of their specific ability to react with O2*-. Chem. Commun. 2009, 1416–1418. [Google Scholar] [CrossRef] [PubMed]
- Bobko, A.A.; Efimova, O.V.; Voinov, M.A.; Khramtsov, V.V. Unique oxidation of imidazolidine nitroxides by potassium ferricyanide: Strategy for designing paramagnetic probes with enhanced sensitivity to oxidative stress. Free Radic. Res. 2012, 46, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Glazachev, Y.I.; Grigor’ev, I.A.; Reijerse, E.J.; Khramtsov, V.V. EPR studies of 15N- and 2H-substituted pH-sensitive spin probes of imidazoline and imidazolidine types. Appl. Magn. Reson. 2001, 20, 489–505. [Google Scholar] [CrossRef]
- Bobko, A.A.; Kirilyuk, I.A.; Gritsan, N.P.; Polovyanenko, D.N.; Grigor’ev, I.A.; Khramtsov, V.V.; Bagryanskaya, E.G. EPR and quantum chemical studies of the pH-sensitive imidazoline and imidazolidine nitroxides with bulky substituents. Appl. Magn. Reson. 2010, 39, 437–451. [Google Scholar] [CrossRef] [PubMed]
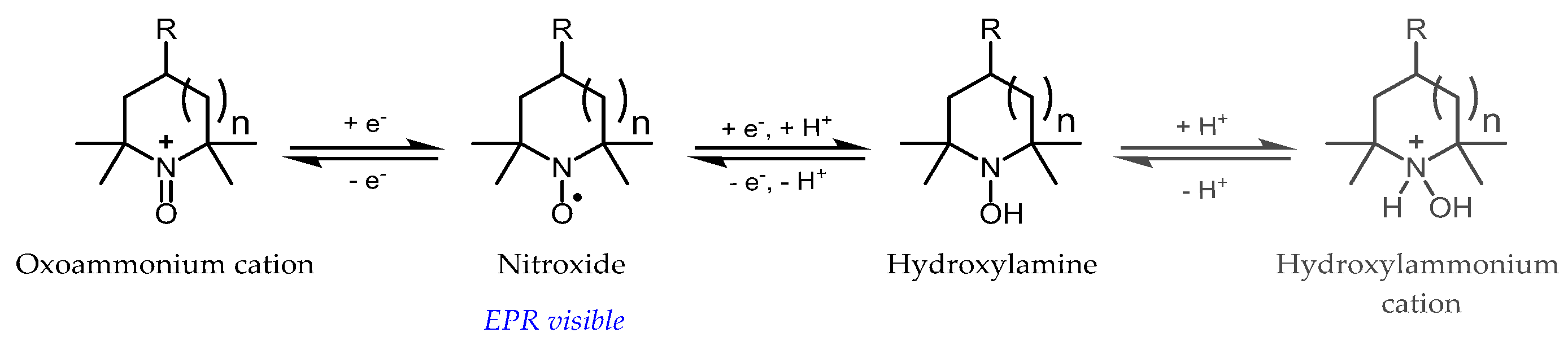
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babić, N.; Peyrot, F. Molecular Probes for Evaluation of Oxidative Stress by In Vivo EPR Spectroscopy and Imaging: State-of-the-Art and Limitations. Magnetochemistry 2019, 5, 13. https://doi.org/10.3390/magnetochemistry5010013
Babić N, Peyrot F. Molecular Probes for Evaluation of Oxidative Stress by In Vivo EPR Spectroscopy and Imaging: State-of-the-Art and Limitations. Magnetochemistry. 2019; 5(1):13. https://doi.org/10.3390/magnetochemistry5010013
Chicago/Turabian StyleBabić, Nikola, and Fabienne Peyrot. 2019. "Molecular Probes for Evaluation of Oxidative Stress by In Vivo EPR Spectroscopy and Imaging: State-of-the-Art and Limitations" Magnetochemistry 5, no. 1: 13. https://doi.org/10.3390/magnetochemistry5010013
APA StyleBabić, N., & Peyrot, F. (2019). Molecular Probes for Evaluation of Oxidative Stress by In Vivo EPR Spectroscopy and Imaging: State-of-the-Art and Limitations. Magnetochemistry, 5(1), 13. https://doi.org/10.3390/magnetochemistry5010013




