High-temperature Spin Crossover of a Solvent-Free Iron(II) Complex with the Linear Hexadentate Ligand [Fe(L2-3-2Ph)](AsF6)2 (L2-3-2Ph = bis[N-(1-Phenyl-1H-1,2,3-triazol-4-yl)methylidene-2-aminoethyl]-1,3-propanediamine)
Abstract
:1. Introduction
2. Results and Discussion
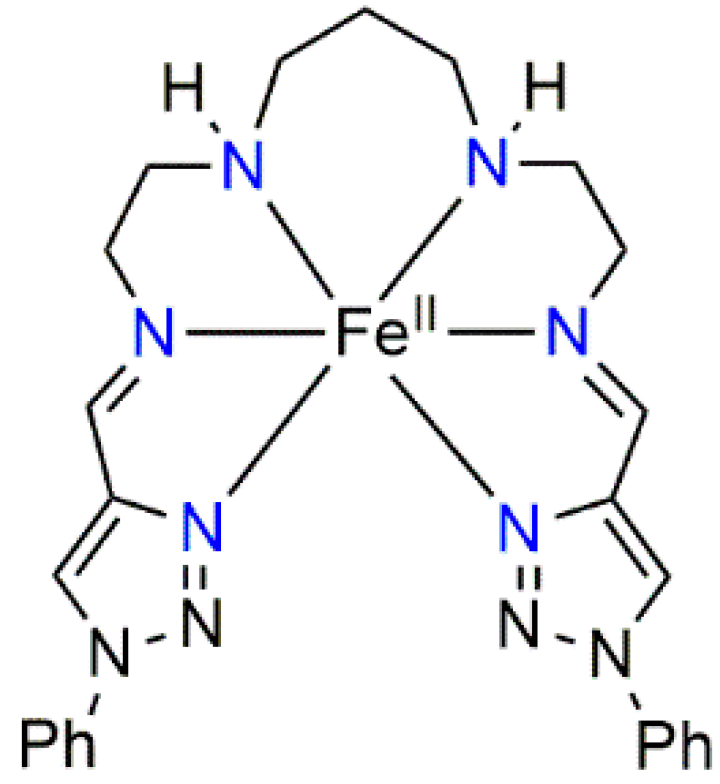
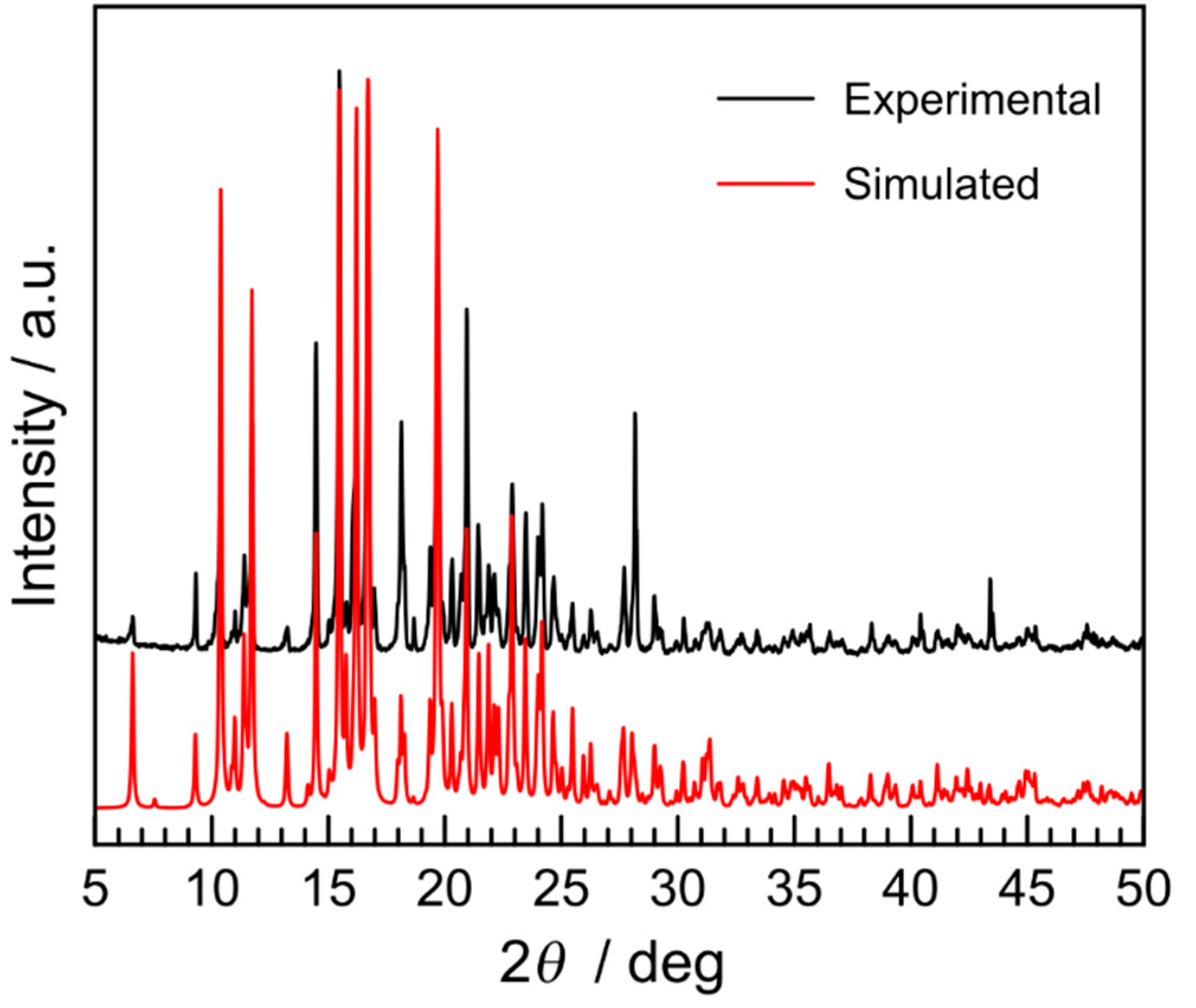
2.1. Synthesis and Characterization of [Fe(L2-3-2Ph)](AsF6)2 (1)
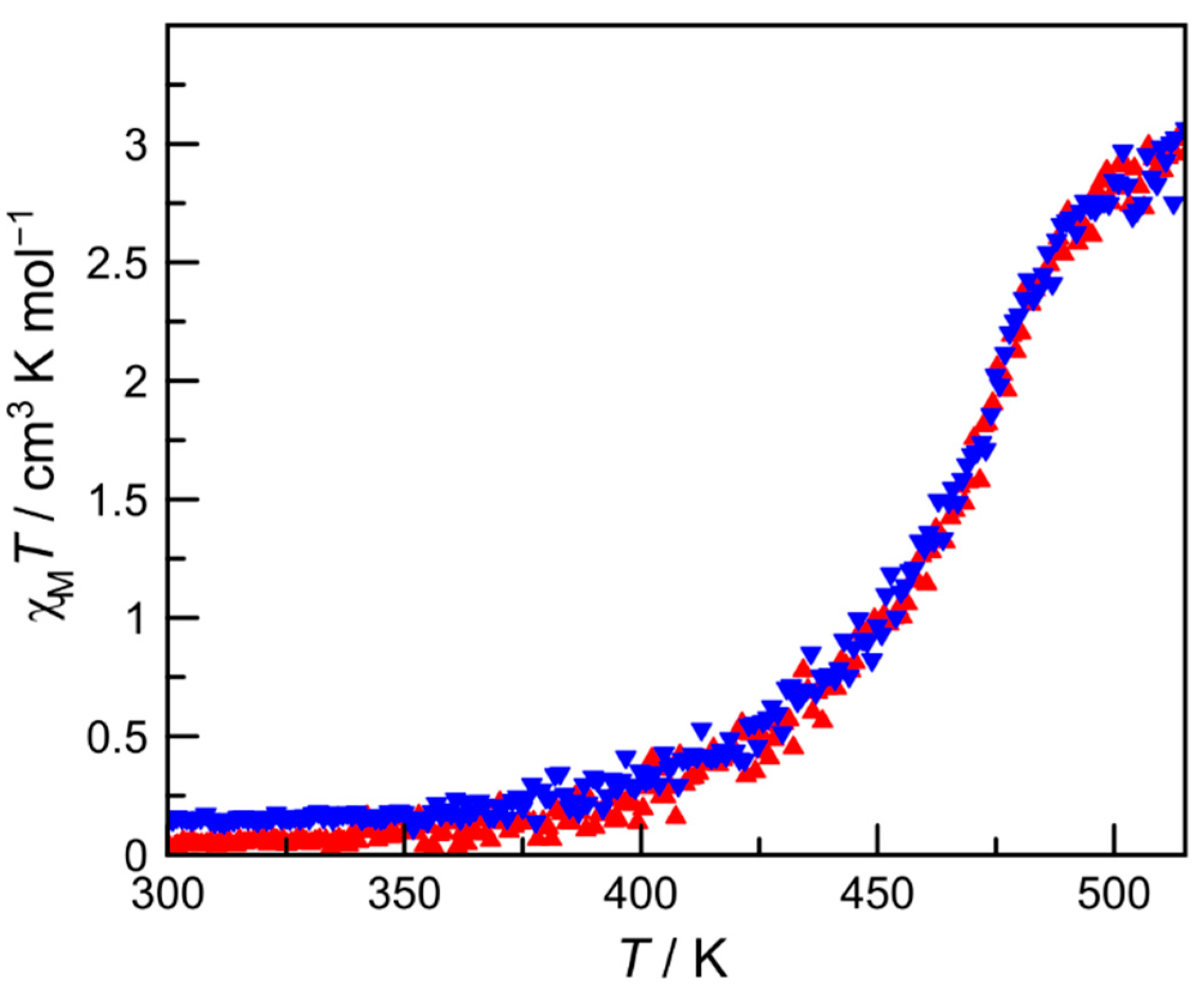
2.2. Magnetic Properties of Complex 1
2.3. Differential Scanning Calorimetry (DSC) of Complex 1
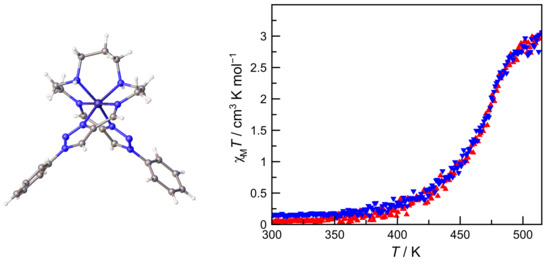
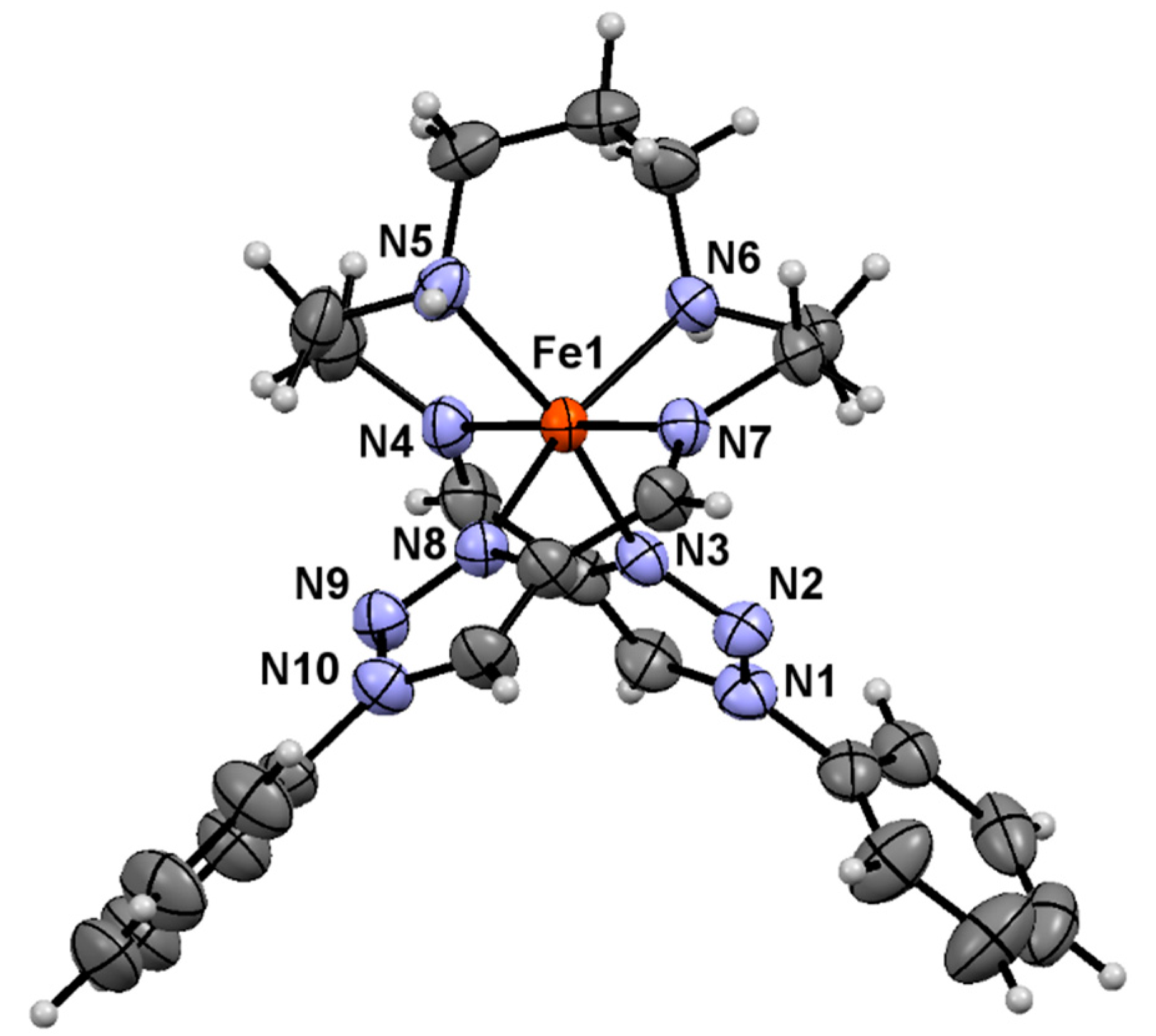
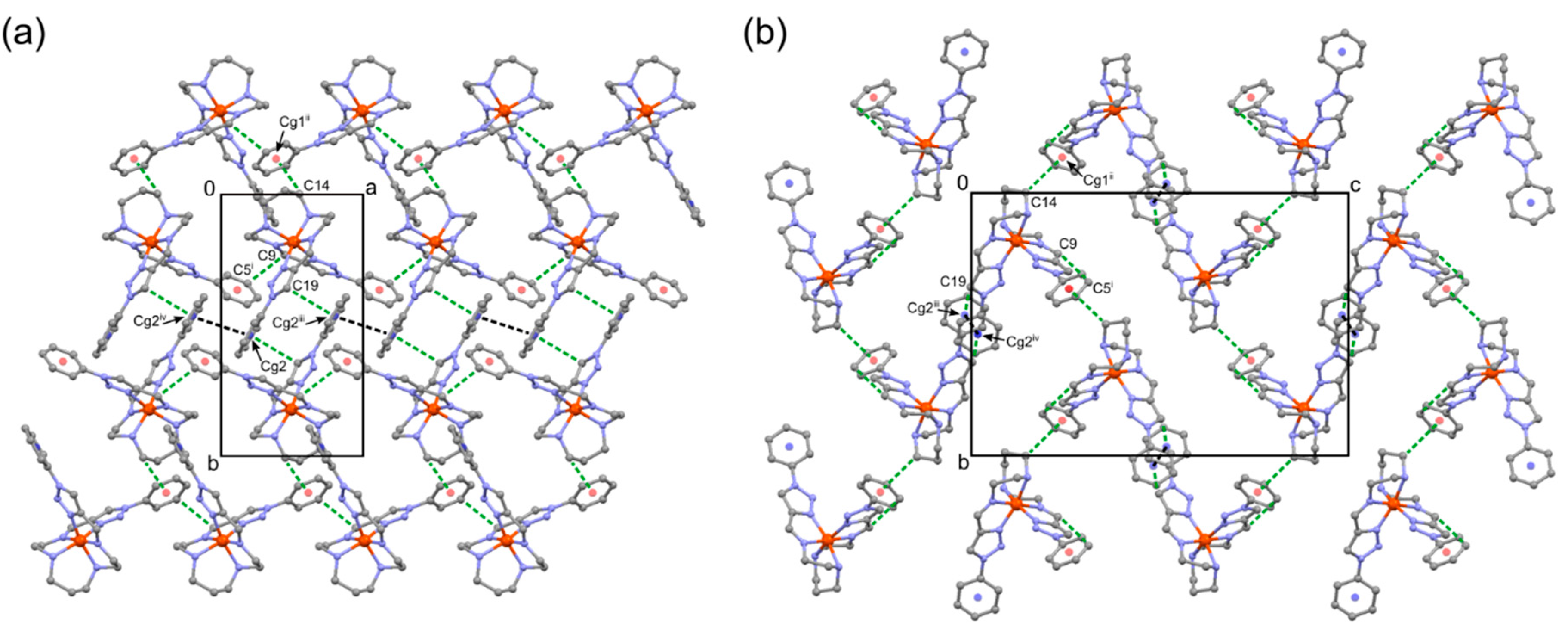
2.4. Crystal Structure of Complex 1
3. Materials and Methods
3.1. Synthesis of the FeII Complex
3.1.1. General
3.1.2. Synthesis of the Linear Hexadentate N6 Ligand L2-3-2Ph = bis[N-(1-Phenyl-1H-1,2,3-triazol-4-yl)methylidene-2-aminoethyl]-1,3-propanediamine)
3.1.3. Preparation of [Fe(L2-3-2Ph)](AsF6)2 (1)
3.2. Physical Measurements
3.3. Crystallographic Data Collection and Structure Analyses
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Gütlich, P.; Goodwin, H.A. (Eds.) Spin Crossover in Transition Metal Compounds I–III; Topics in Current Chemistry; Springer: Berlin, Germany, 2004; Volume 233–235. [Google Scholar]
- Halcrow, M.A. (Ed.) Spin-Crossover Materials–Properties and Applications; John Wiley & Sons: Chichester, UK, 2013. [Google Scholar]
- Gütlich, P.; Gaspar, A.B.; Garcia, Y. Spin state switching in iron coordination compounds. Beilstein J. Org. Chem. 2013, 9, 342–391. [Google Scholar] [CrossRef] [PubMed]
- Linares, J.; Codjovi, E.; Garcia, Y. Pressure and Temperature Spin Crossover Sensors with Optical Detection. Sensors 2012, 12, 4479–4492. [Google Scholar] [CrossRef] [PubMed]
- Halcrow, M.A. The spin-states and spin-transitions of mononuclear iron(II) complexes of nitrogen-donor ligands. Polyhedron 2007, 26, 3523–3576. [Google Scholar] [CrossRef]
- Nihei, M.; Shiga, T.; Maeda, Y.; Oshio, H. Spin crossover iron(III) complexes. Coord. Chem. Rev. 2007, 251, 2606–2621. [Google Scholar] [CrossRef]
- Bousseksou, A.; Molnár, G.; Real, J.A.; Tanaka, K. Spin crossover and photomagnetism in dinuclear iron(II) compounds. Coord. Chem. Rev. 2007, 251, 1822–1833. [Google Scholar] [CrossRef]
- Murray, K.S. Advances in Polynuclear Iron (II), Iron (III) and Cobalt (II) Spin-Crossover Compounds. Eur. J. Inorg. Chem. 2008, 3101–3121. [Google Scholar] [CrossRef]
- Weber, B. Spin crossover complexes with N4O2 coordination sphere—The influence of covalent linkers on cooperative interactions. Coord. Chem. Rev. 2009, 253, 2432–2449. [Google Scholar] [CrossRef]
- Halcrow, M.A. Iron(II) complexes of 2,6-di(pyrazol-1-yl)pyridines—A versatile system for spin-crossover research. Coord. Chem. Rev. 2009, 253, 2493–2514. [Google Scholar] [CrossRef]
- Aromí, G.; Barrios, L.A.; Roubeau, O.; Gamez, P. Triazoles and tetrazoles: Prime ligands to generate remarkable coordination materials. Coord. Chem. Rev. 2011, 255, 485–546. [Google Scholar] [CrossRef]
- Hayami, S.; Komatsu, Y.; Shimizu, T.; Kamihata, H.; Lee, Y.H. Spin-crossover in cobalt(II) compounds containing terpyridine and its derivatives. Coord. Chem. Rev. 2011, 255, 1981–1990. [Google Scholar] [CrossRef]
- Muñoz, M.C.; Real, J.A. Thermo-, piezo-, photo- and chemo-switchable spin crossover iron(II)-metallocyanate based coordination polymers. Coord. Chem. Rev. 2011, 255, 2068–2093. [Google Scholar] [CrossRef]
- Roubeau, O. Triazole-Based One-Dimensional Spin-Crossover Coordination Polymers. Chem. A Eur. J. 2012, 18, 15230–15244. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Wei, R.-J.; Huang, R.-B.; Zheng, L.-S. Polymorphism in spin-crossover systems. Chem. Soc. Rev. 2012, 41, 703–737. [Google Scholar] [CrossRef] [PubMed]
- Craig, G.A.; Roubeau, O.; Aromí, G. Spin state switching in 2,6-bis(pyrazol-3-yl)pyridine (3-bpp) based Fe(II) complexes. Coord. Chem. Rev. 2014, 269, 13–31. [Google Scholar] [CrossRef]
- Harding, D.J.; Harding, P.; Phonsri, W. Spin crossover in iron (III) complexes. Coord. Chem. Rev. 2016, 313, 38–61. [Google Scholar] [CrossRef]
- Attwood, M.; Turner, S.S. Back to back 2,6-bis(pyrazol-1-yl)pyridine and 2,2′:6′,2″-terpyridine ligands: Untapped potential for spin crossover research and beyond. Coord. Chem. Rev. 2017, 353, 247–277. [Google Scholar] [CrossRef]
- Scott, H.S.; Staniland, R.W.; Kruger, P.E. Spin crossover in homoleptic Fe (II) imidazolylimine complexes. Coord. Chem. Rev. 2018, 362, 24–43. [Google Scholar] [CrossRef]
- Cannizzo, A.; Milne, C.J.; Consani, C.; Gawelda, W.; Bressler, C.; van Mourik, F.; Chergui, M. Light-induced spin crossover in Fe(II)-based complexes: The full photocycle unraveled by ultrafast optical and X-ray spectroscopies. Coord. Chem. Rev. 2010, 254, 2677–2686. [Google Scholar] [CrossRef]
- Wolny, J.A.; Diller, R.; Schünemann, V. Vibrational spectroscopy of mono- and polynuclear spin-crossover systems. Eur. J. Inorg. Chem. 2012, 2635–2648. [Google Scholar] [CrossRef]
- Wolny, J.A.; Paulsen, H.; Trautwein, A.X.; Schünemann, V. Density functional theory calculations and vibrational spectroscopy on iron spin-crossover compounds. Coord. Chem. Rev. 2009, 253, 2423–2431. [Google Scholar] [CrossRef]
- Kitazawa, T.; Kishida, T.; Kawasaki, T.; Takahashi, M. Spin crossover behaviour in Hofmann-like coordination polymer Fe(py)2[Pd(CN)4] with 57Fe Mössbauer spectra. Hyperfine Interact. 2017, 238, 65. [Google Scholar] [CrossRef]
- Benaissa, H.; Rotaru, A.; Garcia, Y. Spin crossover in two 1D Fe(II) polymers with 1,2,4-triazole thiourea building blocks. Hyperfine Interact. 2018, 239, 37. [Google Scholar] [CrossRef]
- Nakashima, S.; Kaneko, M.; Yoshinami, K.; Iwai, S.; Dote, H. On/off spin-crossover phenomenon and control of the transition temperature in assembled Iron(II) complexes. Hyperfine Interact. 2018, 239, 39. [Google Scholar] [CrossRef]
- Paulsen, H.; Schünemann, V.; Wolny, J.A. Progress in Electronic Structure Calculations on Spin-Crossover Complexes. Eur. J. Inorg. Chem. 2013, 2013, 628–641. [Google Scholar] [CrossRef]
- Kepp, K.P. Consistent descriptions of metal–ligand bonds and spin-crossover in inorganic chemistry. Coord. Chem. Rev. 2013, 257, 196–209. [Google Scholar] [CrossRef]
- Ashley, D.C.; Jakubikova, E. Ironing out the photochemical and spin-crossover behavior of Fe(II) coordination compounds with computational chemistry. Coord. Chem. Rev. 2017, 337, 97–111. [Google Scholar] [CrossRef]
- Brooker, S. Spin crossover with thermal hysteresis: Practicalities and lessons learnt. Chem. Soc. Rev. 2015, 44, 2880–2892. [Google Scholar] [CrossRef]
- Bao, X.; Guo, P.-H.; Liu, W.; Tucek, J.; Zhang, W.-X.; Leng, J.-D.; Chen, X.-M.; Gural’skiy, I.; Salmon, L.; Bousseksou, A.; Tong, M.-L. Remarkably high-temperature spin transition exhibited by new 2D metal–organic frameworks. Chem. Sci. 2012, 3, 1629. [Google Scholar] [CrossRef]
- Liu, W.; Bao, X.; Li, J.-Y.; Qin, Y.-L.; Chen, Y.-C.; Ni, Z.-P.; Tong, M.-L. High-Temperature Spin Crossover in Two Solvent-Free Coordination Polymers with Unusual High Thermal Stability. Inorg. Chem. 2015, 54, 3006–3011. [Google Scholar] [CrossRef]
- Zheng, S.; Reintjens, N.R.M.; Siegler, M.A.; Roubeau, O.; Bouwman, E.; Rudavskyi, A.; Havenith, R.W.A.; Bonnet, S. Stabilization of the Low-Spin State in a Mononuclear Iron(II) Complex and High-Temperature Cooperative Spin Crossover Mediated by Hydrogen Bonding. Chem. A Eur. J. 2016, 22, 331–339. [Google Scholar] [CrossRef]
- Hagiwara, H.; Tanaka, T.; Hora, S. Synthesis, structure, and spin crossover above room temperature of a mononuclear and related dinuclear double helicate iron(II) complexes. Dalton Trans. 2016, 45, 17132–17140. [Google Scholar] [CrossRef] [PubMed]
- Hora, S.; Hagiwara, H. High-temperature Wide Thermal Hysteresis of an Iron(II) Dinuclear Double Helicate. Inorganics 2017, 5, 49. [Google Scholar] [CrossRef]
- Hagiwara, H.; Masuda, T.; Ohno, T.; Suzuki, M.; Udagawa, T.; Murai, K. Neutral Molecular Iron(II) Complexes Showing Tunable Bistability at Above, Below, and Just Room Temperature by a Crystal Engineering Approach: Ligand Mobility into a Three-Dimensional Flexible Supramolecular Network. Cryst. Growth Des. 2017, 17, 6006–6019. [Google Scholar] [CrossRef]
- Hagiwara, H.; Okada, S. A polymorphism-dependent T1/2 shift of 100 K in a hysteretic spin-crossover complex related to differences in intermolecular weak CH⋯X hydrogen bonds (X = S vs. S and N). Chem. Commun. 2016, 52, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, H.; Minoura, R.; Okada, S.; Sunatsuki, Y. Synthesis, Structure, and Magnetic Property of a New Mononuclear Iron(II) Spin Crossover Complex with a Tripodal Ligand Containing Three 1,2,3-Triazole Groups. Chem. Lett. 2014, 43, 950–952. [Google Scholar] [CrossRef]
- Bréfuel, N.; Shova, S.; Tuchagues, J.-P.; Matsumoto, N. An Abrupt Spin Crossover FeII Complex Based on Homochiral Chain. Chem. Lett. 2005, 34, 1092–1093. [Google Scholar] [CrossRef]
- Bréfuel, N.; Imatomi, S.; Torigoe, H.; Hagiwara, H.; Shova, S.; Meunier, J.-F.; Bonhommeau, S.; Tuchagues, J.-P.; Matsumoto, N. Structural−Electronic Correlation in the First-Order Phase Transition of [FeH2L2-Me](ClO4)2 (H2L2-Me = Bis[((2-methylimidazol-4-yl)methylidene)-3-aminopropyl]ethylenediamine). Inorg. Chem. 2006, 45, 8126–8135. [Google Scholar] [CrossRef]
- Bréfuel, N.; Watanabe, H.; Toupet, L.; Come, J.; Matsumoto, N.; Collet, E.; Tanaka, K.; Tuchagues, J.-P. Concerted spin crossover and symmetry breaking yield three thermally and one light-induced cristallographic phases of a molecular material. Angew. Chem. Int. Ed. 2009, 48, 9304–9307. [Google Scholar] [CrossRef]
- Bréfuel, N.; Collet, E.; Watanabe, H.; Kojima, M.; Matsumoto, N.; Toupet, L.; Tanaka, K.; Tuchagues, J.-P. Nanoscale self-hosting of molecular spin-states in the intermediate phase of a spin-crossover material. Chem. A Eur. J. 2010, 16, 14060–14068. [Google Scholar] [CrossRef]
- Hagiwara, H.; Kawano, A.; Fujinami, T.; Matsumoto, N.; Sunatsuki, Y.; Kojima, M.; Miyamae, H. Conformational effect of a spin crossover iron(II) complex: Bis[N-(2-methylimidazol-4-yl)methylidene-2-aminoethyl]propanediamineiron(II) perchlorate. Inorg. Chim. Acta 2011, 367, 141–150. [Google Scholar] [CrossRef]
- Collet, E.; Watanabe, H.; Bréfuel, N.; Palatinus, L.; Roudaut, L.; Toupet, L.; Tanaka, K.; Tuchagues, J.-P.; Fertey, P.; Ravy, S.; Toudic, B.; Cailleau, H. Aperiodic Spin State Ordering of Bistable Molecules and Its Photoinduced Erasing. Phys. Rev. Lett. 2012, 109, 257206. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Bréfuel, N.; Collet, E.; Toupet, L.; Tanaka, K.; Tuchagues, J.-P. Competing symmetry breaking and spin crossover in [FeH2L2-Me](ClO4)2. Eur. J. Inorg. Chem. 2013, 1, 710–715. [Google Scholar] [CrossRef]
- Sinn, E.; Sim, G.; Dose, E.V.; Tweedle, M.F.; Wilson, L.J. Iron(III) chelates with hexadentate ligands from triethylenetetramine and.beta.-diketones or salicylaldehyde. Spin state dependent crystal and molecular structures of [Fe(acac)2trien]PF6 (S = 5/2), [Fe(acacCl)2trien]PF6 (S = 5/2), [Fe(sal)2trien]Cl∙2H2O (S = 1/2), and [Fe(sal)2trien]NO3∙H2O (S = 1/2). J. Am. Chem. Soc. 1978, 100, 3375–3390. [Google Scholar] [CrossRef]
- Maeda, Y.; Oshio, H.; Tanigawa, Y.; Oniki, T.; Takashima, Y. Physical Characteristic and Molecular Structure of Spin-Crossover Iron(III) Complexes of Monoclinic Form with Hexadentate Ligands Derived from Triethylenetetramine and Salicylaldehyde [Fe(sal2trien)]BPh4·acetone. Bull. Chem. Soc. Jpn. 1991, 64, 1522–1527. [Google Scholar] [CrossRef]
- Hayami, S.; Matoba, T.; Nomiyama, S.; Kojima, T.; Osaki, S.; Maeda, Y. Structures and Magnetic Properties of Some Fe(III) Complexes with Hexadentate Ligands: In Connection with Spin-Crossover Behavior. Bull. Chem. Soc. Jpn. 1997, 70, 3001–3009. [Google Scholar] [CrossRef]
- Martinho, P.N.; Harding, C.J.; Müller-Bunz, H.; Albrecht, M.; Morgan, G.G. Inducing Spin Crossover in Amphiphilic Iron(III) Complexes. Eur. J. Inorg. Chem. 2010, 2010, 675–679. [Google Scholar] [CrossRef]
- Griffin, M.; Shakespeare, S.; Shepherd, H.J.; Harding, C.J.; Létard, J.-F.; Desplanches, C.; Goeta, A.E.; Howard, J.A.K.; Powell, A.K.; Mereacre, V.; et al. A Symmetry-Breaking Spin-State Transition in Iron(III). Angew. Chem. Int. Ed. 2011, 50, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Vieira, B.J.C.; Coutinho, J.T.; Santos, I.C.; Pereira, L.C.J.; Waerenborgh, J.C.; da Gama, V. [Fe(nsal2trien)]SCN, a New Two-Step Iron(III) Spin Crossover Compound, with Symmetry Breaking Spin-State Transition and an Intermediate Ordered State. Inorg. Chem. 2013, 52, 3845–3850. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, A.J.; Martinho, P.N.; Gildea, B.J.; Holbrey, J.D.; Morgan, G.G. Robust Room Temperature Hysteresis in an FeIII Spin Crossover Metallomesogen. Eur. J. Inorg. Chem. 2016, 2016, 2025–2029. [Google Scholar] [CrossRef]
- Martinho, P.N.; Kühne, I.A.; Gildea, B.; McKerr, G.; O’Hagan, B.; Keyes, T.E.; Lemma, T.; Gandolfi, C.; Albrecht, M.; Morgan, G.G. Self-Assembly Properties of Amphiphilic Iron(III) Spin Crossover Complexes in Water and at the Air–Water Interface. Magnetochemistry 2018, 4, 49. [Google Scholar] [CrossRef]
- Morgan, G.G.; Murnaghan, K.D.; Müller-Bunz, H.; McKee, V.; Harding, C.J. A Manganese(III) Complex That Exhibits Spin Crossover Triggered by Geometric Tuning. Angew. Chem. Int. Ed. 2006, 45, 7192–7195. [Google Scholar] [CrossRef] [PubMed]
- Pandurangan, K.; Gildea, B.; Murray, C.; Harding, C.J.; Müller-Bunz, H.; Morgan, G.G. Lattice effects on the spin-crossover profile of a mononuclear manganese(III) cation. Chem. A Eur. J. 2012, 18, 2021–2029. [Google Scholar] [CrossRef] [PubMed]
- Martinho, P.N.; Gildea, B.; Harris, M.M.; Lemma, T.; Naik, A.D.; Müller-Bunz, H.; Keyes, T.E.; Garcia, Y.; Morgan, G.G. Cooperative Spin Transition in a Mononuclear Manganese(III) Complex. Angew. Chem. 2012, 124, 12765–12769. [Google Scholar] [CrossRef]
- Fitzpatrick, A.J.; Trzop, E.; Müller-Bunz, H.; Dîrtu, M.M.; Garcia, Y.; Collet, E.; Morgan, G.G. Electronic vs. structural ordering in a manganese(III) spin crossover complex. Chem. Commun. 2015, 51, 17540–17543. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Yamada, M.; Hagiwara, H.; Torigoe, H.; Matsumoto, N.; Kojima, M.; Dahan, F.; Tuchagues, J.-P.; Re, N.; Iijima, S. A variety of spin-crossover behaviors depending on the counter anion: Two-dimensional complexes constructed by NH⋯Cl− hydrogen bonds, [FeIIH3LMe]Cl·X (X = PF6−, AsF6−, SbF6−, CF3SO3−; H3LMe = tris[2-{[(2methylimidazol-4-yl)methylidene]amino}ethyl]amine). Chem. A Eur. J. 2006, 12, 4536–4549. [Google Scholar] [CrossRef]
- Guionneau, P.; Marchivie, M.; Bravic, G.; Létard, J.-F.; Chasseau, D. Structural aspects of spin crossover. Examples of the [FeIILn(NCS)2] complexes. Top. Curr. Chem. 2004, 234, 97–128. [Google Scholar]
- Marchivie, M.; Guionneau, P.; Létard, J.-F.; Chasseau, D. Photo-induced spin-transition: The role of the iron(II) environment distortion. Acta Crystallogr. Sect. B 2005, 61, 25–28. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE2.1. Program for Calculating Continuous Shape Measures of Polyhedral Structures; Universitat de Barcelona: Barcelona, Spain, 2013. [Google Scholar]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Siddiki, A.A.; Takale, B.S.; Telvekar, V.N. One pot synthesis of aromatic azide using sodium nitrite and hydrazine hydrate. Tetrahedron Lett. 2013, 54, 1294–1297. [Google Scholar] [CrossRef]
- Pathigoolla, A.; Pola, R.P.; Sureshan, K.M. A versatile solvent-free azide-alkyne click reaction catalyzed by in situ generated copper nanoparticles. Appl. Catal. A Gen. 2013, 453, 151–158. [Google Scholar] [CrossRef]
- L’abbé, G.; Bruynseels, M.; Delbeke, P.; Toppet, S. Molecular rearrangements of 4-iminomethyl-1,2,3-triazoles. Replacement of 1-aryl substituents in 1 H -1,2,3-triazole-4-carbaldehydes. J. Heterocycl. Chem. 1990, 27, 2021–2027. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH: Weinheim, Germany, 1993. [Google Scholar]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- SCALE3 ABSPACK, version: 1.0.4; gui: 1.03; an Oxford Diffraction Program; Oxford Diffraction Ltd.: Abingdon, UK, 2005.
- Rigaku Oxford Diffraction. CrysAlisPro Software System, version 1.171.39.46; Rigaku Corporation: Oxford, UK, 2018. [Google Scholar]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Wakita, K. Yadokari-XG, Software for Crystal Structure Analyses, 2001; Release of Software (Yadokari-XG 2009) for Crystal Structure Analyses: Kabuto, C.; Akine, S.; Nemoto, T.; Kwon, E.J. Cryst. Soc. Jpn. 2009, 51, 218–224. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]


| Temperature/K | 296 | 448 |
| Formula | C25H30N10As2F12Fe | |
| Formula weight | 904.28 | |
| Crystal system | monoclinic | |
| Space group | P21/n (No.14) | |
| a/Å | 8.8724(2) | 8.9968(2) |
| b/Å | 16.2976(4) | 16.5743(6) |
| c/Å | 23.4941(6) | 23.6197(7) |
| β/deg | 94.865(2) | 95.333(2) |
| V/Å3 | 3384.98(14) | 3506.82(18) |
| Z | 4 | 4 |
| dcalcd./g cm−3 | 1.774 | 1.713 |
| μ (Mo Kα)/mm−1 | 2.487 | 2.401 |
| R1a (I > 2sigma(I)) | 0.0471 | 0.0648 |
| wR2b (I > 2sigma(I)) | 0.1300 | 0.1931 |
| R1a (all data) | 0.0570 | 0.0879 |
| wR2b (all data) | 0.1374 | 0.2163 |
| S | 1.036 | 1.044 |
| CCDC number | 1,887,351 | 1,887,352 |
| Temperature | 296 | 448 |
| Fe–N3 | 1.980(2) | 2.034(3) |
| Fe–N4 | 1.930(3) | 1.964(4) |
| Fe–N5 | 2.062(3) | 2.078(4) |
| Fe–N6 | 2.041(2) | 2.086(3) |
| Fe–N7 | 1.930(2) | 1.973(3) |
| Fe–N8 | 1.986(2) | 2.024(4) |
| Average Fe–N | 1.988 | 2.027 |
| N3–Fe1–N4 | 80.12(11) | 78.67(15) |
| N3–Fe1–N5 | 161.93(11) | 160.11(17) |
| N3–Fe1–N6 | 86.96(10) | 87.76(14) |
| N3–Fe1–N7 | 98.13(10) | 98.47(14) |
| N3–Fe1–N8 | 92.78(10) | 93.32(14) |
| N4–Fe1–N5 | 81.88(12) | 81.45(19) |
| N4–Fe1–N6 | 97.13(11) | 98.80(16) |
| N4–Fe1–N7 | 178.24(11) | 177.14(16) |
| N4–Fe1–N8 | 99.98(11) | 100.86(15) |
| N5–Fe1–N6 | 96.76(10) | 95.93(15) |
| N5–Fe1–N7 | 99.87(11) | 101.41(18) |
| N5–Fe1–N8 | 88.88(10) | 89.78(15) |
| N6–Fe1–N7 | 82.91(10) | 81.14(15) |
| N6–Fe1–N8 | 162.58(10) | 160.14(14) |
| N7–Fe1–N8 | 79.88(10) | 79.08(15) |
| Σ | 84.0 | 90.9 |
| Θ | 250.7 | 282.5 |
| S(Oh) | 1.748 | 2.131 |
| Octahedral volume (Å3) | 10.200 | 10.734 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagiwara, H. High-temperature Spin Crossover of a Solvent-Free Iron(II) Complex with the Linear Hexadentate Ligand [Fe(L2-3-2Ph)](AsF6)2 (L2-3-2Ph = bis[N-(1-Phenyl-1H-1,2,3-triazol-4-yl)methylidene-2-aminoethyl]-1,3-propanediamine). Magnetochemistry 2019, 5, 10. https://doi.org/10.3390/magnetochemistry5010010
Hagiwara H. High-temperature Spin Crossover of a Solvent-Free Iron(II) Complex with the Linear Hexadentate Ligand [Fe(L2-3-2Ph)](AsF6)2 (L2-3-2Ph = bis[N-(1-Phenyl-1H-1,2,3-triazol-4-yl)methylidene-2-aminoethyl]-1,3-propanediamine). Magnetochemistry. 2019; 5(1):10. https://doi.org/10.3390/magnetochemistry5010010
Chicago/Turabian StyleHagiwara, Hiroaki. 2019. "High-temperature Spin Crossover of a Solvent-Free Iron(II) Complex with the Linear Hexadentate Ligand [Fe(L2-3-2Ph)](AsF6)2 (L2-3-2Ph = bis[N-(1-Phenyl-1H-1,2,3-triazol-4-yl)methylidene-2-aminoethyl]-1,3-propanediamine)" Magnetochemistry 5, no. 1: 10. https://doi.org/10.3390/magnetochemistry5010010
APA StyleHagiwara, H. (2019). High-temperature Spin Crossover of a Solvent-Free Iron(II) Complex with the Linear Hexadentate Ligand [Fe(L2-3-2Ph)](AsF6)2 (L2-3-2Ph = bis[N-(1-Phenyl-1H-1,2,3-triazol-4-yl)methylidene-2-aminoethyl]-1,3-propanediamine). Magnetochemistry, 5(1), 10. https://doi.org/10.3390/magnetochemistry5010010