Synthesis, Crystal Structures, and Magnetic Properties of Mixed-Valent Tetranuclear Complexes with Y-Shaped MnII2MnIII2 Core
Abstract
:1. Introduction
2. Results and Discussion
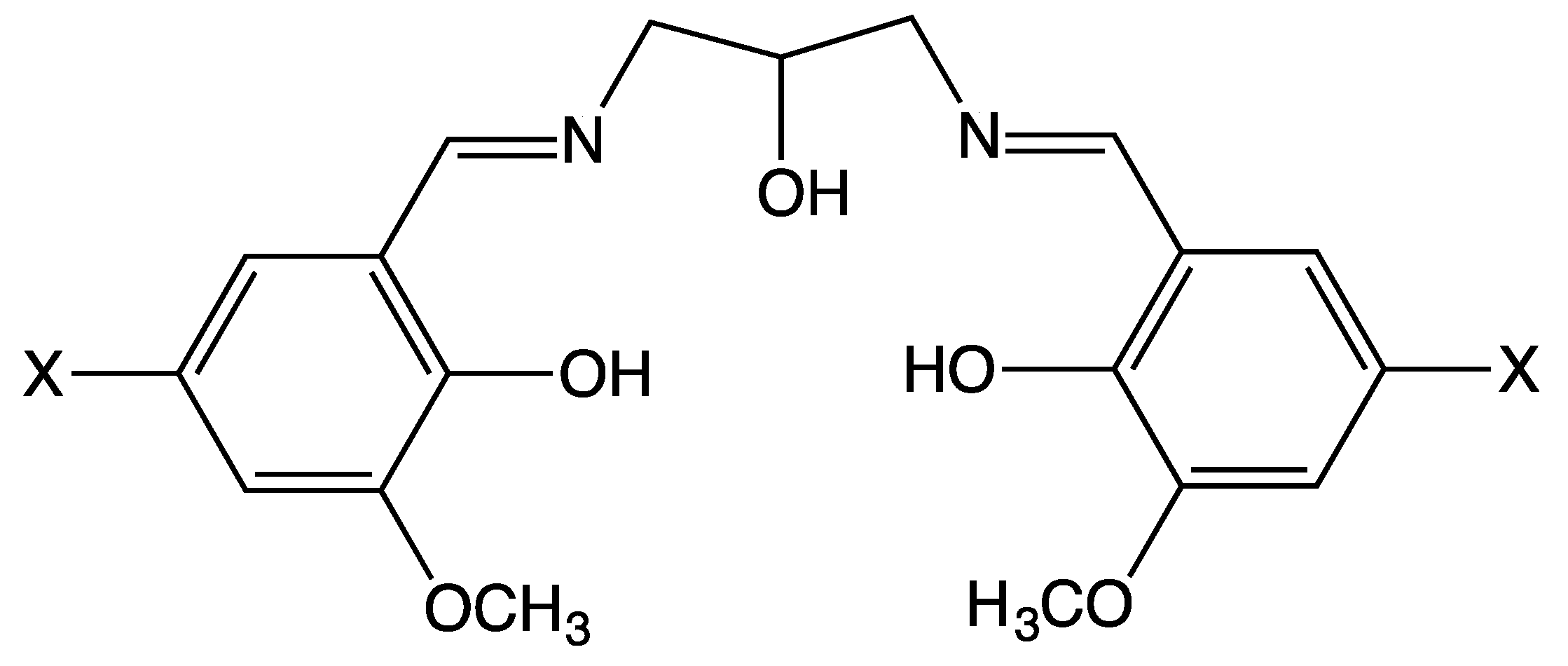
2.1. Synthesis of Tetranuclear Manganese Complexes
2.2. Infrared Spectra of Tetranuclear Manganese Complexes
2.3. Electronic Spectra of Tetranuclear Manganese Complexes
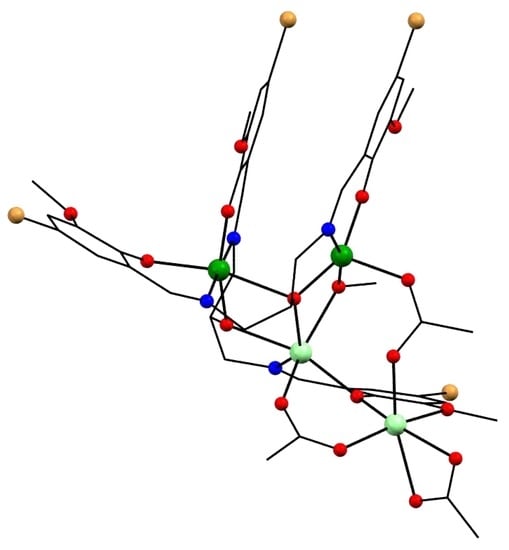
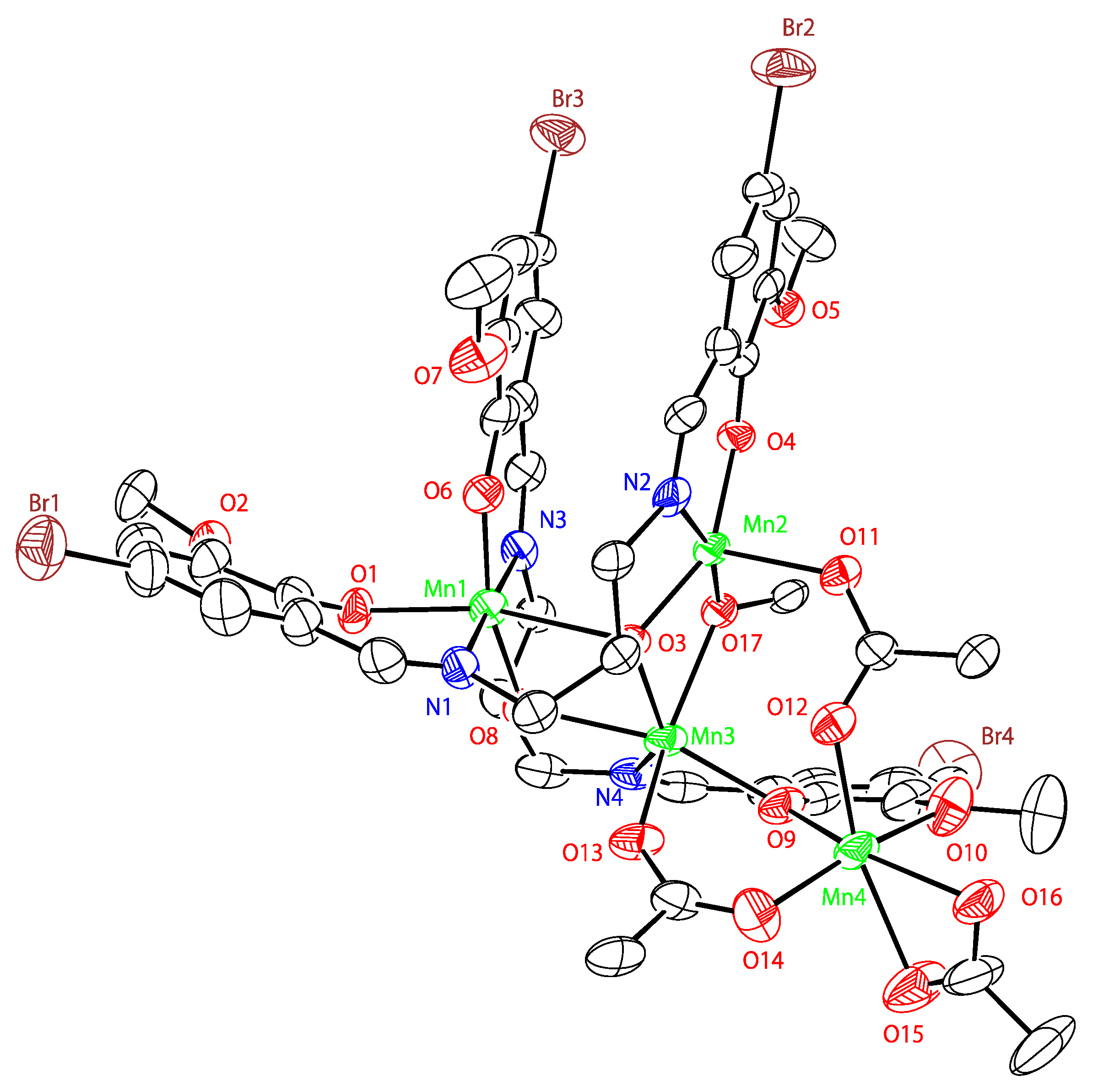
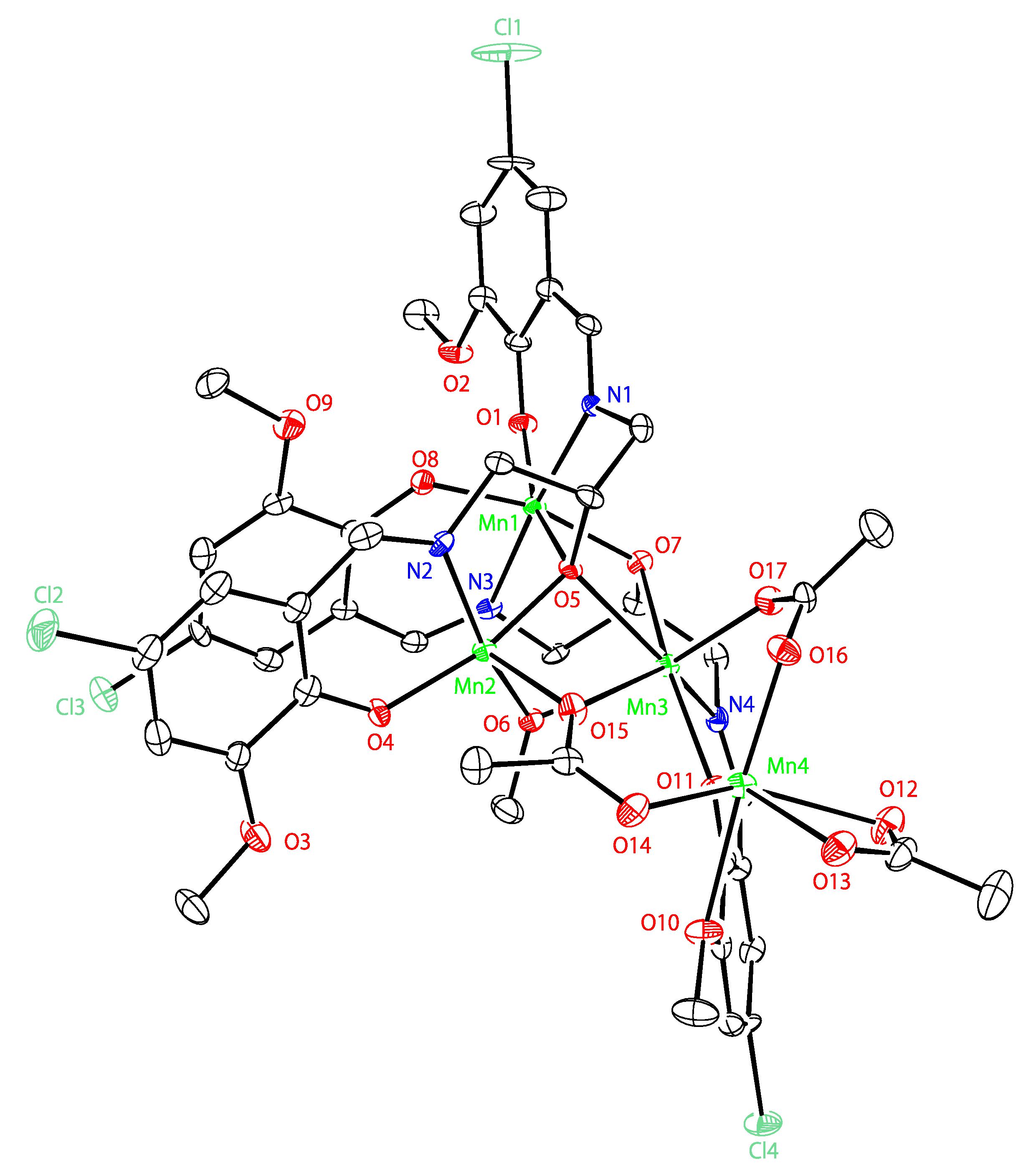
2.4. Crystal Structures of Tetranuclear Manganese Complexes
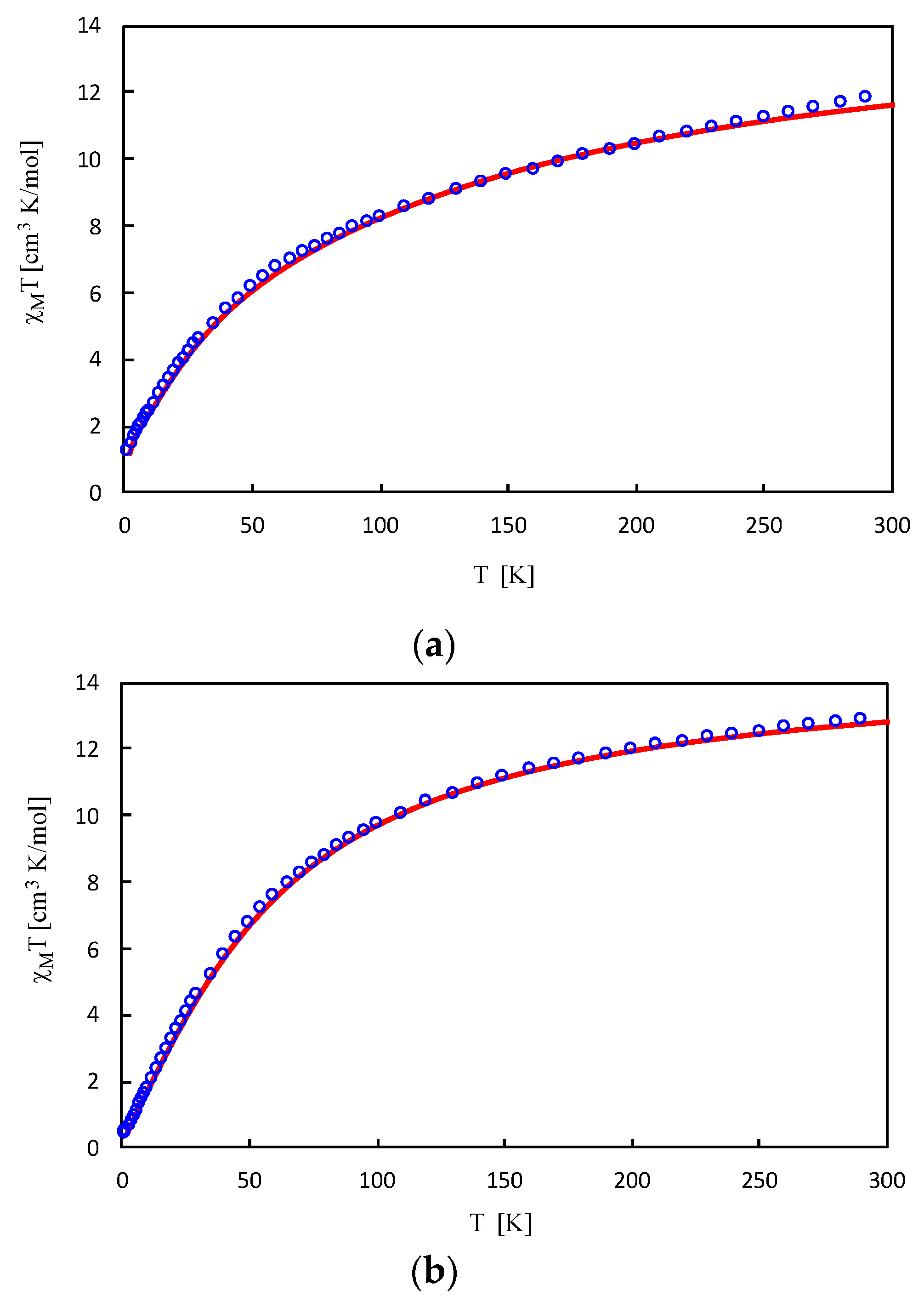
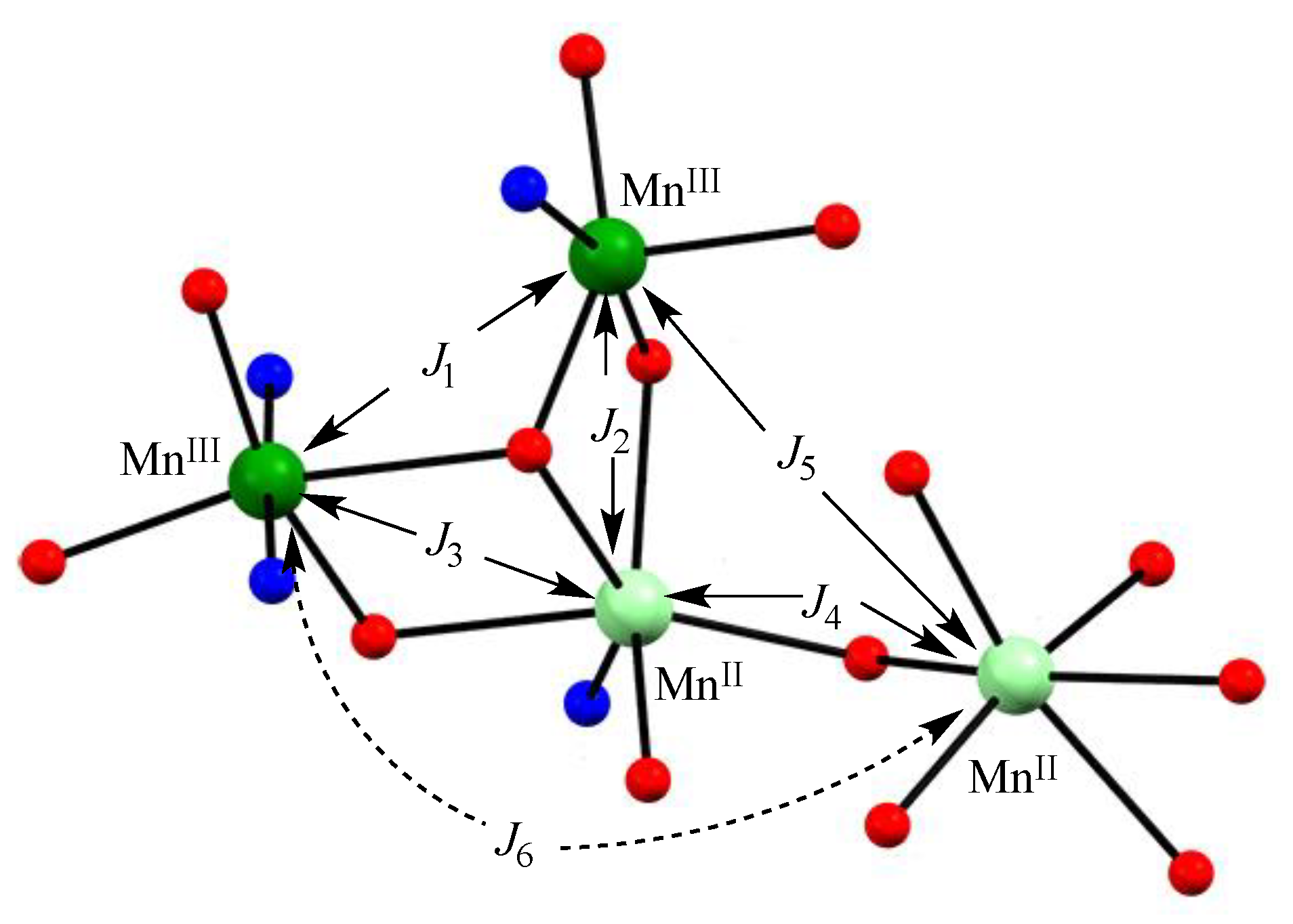
2.5. Magnetic Data of Tetranuclear Manganese Complexes
3. Materials and Methods
3.1. Synthesis of 1,3-Bis(5-bromo-3-methoxysalicylideneamino)-2-propanol (H3bmsap)
3.2. Synthesis of 1,3-Bis(5-chloro-3-methoxysalicylideneamino)-2-propanol (H3cmsap)
3.3. Synthesis of [Mn4(bmsap)2(CH3CO2)3(CH3O)] (3)
3.4. Synthesis of [Mn4(cmsap)2(CH3CO2)3(CH3O)] (4)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Brudvig, G.W.; Crabtree, R.H. Bioinorganic chemistry of manganese related to photosynthetic oxygen evolution. Prog. Inorg. Chem. 1989, 37, 99–142. [Google Scholar]
- Christou, G. Manganese carboxylate chemistry and its biological relevance. Acc. Chem. Res. 1989, 22, 328–335. [Google Scholar] [CrossRef]
- Dismukes, G.C. Manganese enzymes with binuclear active sites. Chem. Rev. 1996, 96, 2909–2926. [Google Scholar] [CrossRef]
- Yachandra, V.N.; Sauer, K.; Klein, M.P. Manganese cluster in photosynthesis: Where plants oxidize water to dioxygen. Chem. Rev. 1996, 96, 2927–2950. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Mandal, S.K.; Bhaduri, S.; Armstrong, W.H. Manganese cluster with relevance to photosystem II. Chem. Rev. 2004, 104, 3981–4026. [Google Scholar] [CrossRef] [PubMed]
- Dau, H.; Haumann, M. The manganese complex of photosystem II in its reaction cycle-basic framework and possible realization at the atomic level. Coord. Chem. Rev. 2008, 252, 273–295. [Google Scholar] [CrossRef]
- Kostakis, G.E.; Ako, A.M.; Powell, A.K. Structural motifs and topological representation of Mn coordination clusters. Chem. Soc. Rev. 2010, 39, 2238–2271. [Google Scholar] [CrossRef]
- Grundmeier, A.; Dau, H. Structural models of the manganese complex of photosystem II and mechanistic implications. Biochim. Biophys. Acta 2012, 1817, 88–105. [Google Scholar] [CrossRef]
- Umena, Y.; Kawakami, K.; Shen, J.-R.; Kamiya, N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 2011, 473, 55–60. [Google Scholar] [CrossRef]
- Suga, M.; Akita, F.; Hirata, K.; Ueno, G.; Murakami, H.; Nakajima, Y.; Shimizu, T.; Yamashita, K.; Yamamoto, M.; Ago, H.; et al. Native structure of photosystem II at 1.95 Å resolution viewed by femtosecond X-ray pulses. Nature 2015, 517, 99–103. [Google Scholar] [CrossRef]
- Alaimo, A.A.; Takahashi, D.; Cunha-Siva, L.; Christou, G.; Stamatatos, T.C. Emissive {MnIII4Ca} clusters with square pyramidal topologies: Synthesis and structural, spectroscopic, and physicochemical characterization. Inorg. Chem. 2015, 54, 2137–2151. [Google Scholar] [CrossRef]
- Chang, W.; Chen, C.; Dong, H.; Zhang, C. Artificail Mn4-oxido complexes mimic the oxygen-evolving center in photosynthesis. Sci. Bull. 2017, 62, 665–668. [Google Scholar] [CrossRef]
- Wittick, L.M.; Murray, K.S.; Moubaraki, B.; Batten, S.R.; Spiccia, L.; Berry, K.J. Synthesis, structure and magnetism of new single molecule magnets composed of MnII2MnIII2 alkoxo-carboxylate bridged clusters capped by triethanolamine ligands. Dalton Trans. 2004, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Sanudo, E.C.; Grillo, V.A.; Knapp, M.J.; Bollinger, J.C.; Huffman, J.C.; Hendrickson, D.N.; Christou, G. Tetranuclear manganese complexes with dimer-of-dimer and ladder structures from the use of a bis-bipyridyl ligand. Inorg. Chem. 2002, 41, 2441–2450. [Google Scholar] [CrossRef] [PubMed]
- Lecren, L.; Wernsdorfer, W.; Li, Y.-G.; Roubeau, O.; Miyasaka, H.; Clérac, R. Quantum tunneling and quantum phase interference in a [MnII2MnIII2] single-molecule magnet. J. Am. Chem. Soc. 2005, 127, 11311–11317. [Google Scholar] [CrossRef]
- Mikuriya, M.; Yamato, Y.; Tokii, T. 1,3-Bis(salicylideneamino)-2-propanol as the ligand for manganese(III) ions. Bull. Chem. Soc. Jpn. 1992, 65, 1466–1468. [Google Scholar] [CrossRef]
- Mikuriya, M.; Yamato, Y.; Tokii, T. Synthesis, properties, and crystal structures of a series of dinuclear manganese(III) complexes with 1,5-bis(salicylideneamino)-3-pentanol and various anions: Formation of a tetranuclear manganese(III) complex. Bull. Chem. Soc. Jpn. 1992, 65, 2624–2637. [Google Scholar] [CrossRef]
- Mikuriya, M.; Dai, J.; Kakuta, Y.; Tokii, T. Synthesis and characterization of a series of mononuclear manganese(IV) complexes with o-(salicylideneaminomethyl)phenol and its substituted derivatives. Bull. Chem. Soc. Jpn. 1993, 66, 1132–1139. [Google Scholar] [CrossRef]
- Mikuriya, M.; Fujii, T.; Tokii, T.; Kawamori, A. Synthesis and characterization of mononuclear (Mn(II) and Mn(III)) and dinuclear (Mn(II)Mn(II) and Mn(II)Mn(III)) complexes with 2,6-bis[N-(2-pyridylethyl)iminomethyl]-4-methylphenol. Bull. Chem. Soc. Jpn. 1993, 66, 1675–1686. [Google Scholar] [CrossRef]
- Mikuriya, M.; Hashimoto, Y.; Kawamori, A. Synthesis of a tetranuclear manganese complex with a cubane core at the MnIIMnIIIMnIIMnIII oxidation level. Chem. Lett. 1995, 1095–1096. [Google Scholar] [CrossRef]
- Mikuriya, M.; Nakadera, K.; Kotera, T.; Tokii, T.; Mori, W. Sythesis and characterization of tetranuclear manganese(III) complexes with 2,6-bis(salicylideneaminomethyl)-4-methylphenol. Bull. Chem. Soc. Jpn. 1995, 68, 3077–3083. [Google Scholar] [CrossRef]
- Mikuriya, M.; Nukada, R.; Tokami, W.; Hashimoto, Y.; Fujii, T. Syntheis and characterization of manganese(III) complexes with 2-{N-[2-(4-imidazolyl)ethyl]iminomethyl}phenol or 2-{N-[2-(2-pyridyl)ethyl]iminomethyl}phenol. Bull. Chem. Soc. Jpn. 1996, 69, 1573–1578. [Google Scholar] [CrossRef]
- Mikuriya, M.; Hatano, Y.; Asato, E. Synthesis and structural characterization of manganese(II) complexes with N,N′-bis(2-pyridylmethylene)-1,3-diaminopropan-2-ol or N,N′-bis(2-pyridylmethylene)-1,3-propanediamine. Bull. Chem. Soc. Jpn. 1997, 70, 2495–2507. [Google Scholar] [CrossRef]
- Wada, S.; Mikuriya, M. Synthesis and structural characterization of dinuclear manganese(III) complexes with cyclam-based macrocyclic ligands having Schiff-base pendant arms as chelating agents. Bull. Chem. Soc. Jpn. 2008, 81, 348–357. [Google Scholar] [CrossRef]
- Mikuriya, M. Mononuclear and oligonuclear manganese complexes with organic multidentate ligands. J. Cryst. Soc. Jpn. 2011, 53, 193–200. [Google Scholar] [CrossRef]
- Mikuriya, M.; Nagao, N.; Kurahashi, S.; Tabuchi, A.; Tomohara, S.; Tsuboi, M.; Yoshioka, D.; Sakiyama, H.; Fuyuhiro, A. Mixed-valent tetranuclear MnIIMnIII3 complex with 1,3-diamino-2-hydroxypropane-N,N′,N″,N′′′-tetraacetic acid. Chem. J. Mold. 2014, 9, 100–105. [Google Scholar] [CrossRef]
- Mikuriya, M.; Matsushima, I.; Yoshioka, D. Trinuclear mixed-valent manganese complex with non-schiff-base tetradentate ligand showing a ferromagnetic coupling. Chem. J. Mold. 2017, 12, 34–40. [Google Scholar] [CrossRef]
- Mikuriya, M.; Kudo, S.; Matsumoto, C.; Kurahashi, S.; Tomohara, S.; Koyama, Y.; Yoshioka, D.; Mitsuhashi, R. Mixed-valent tetranuclear manganese complexes with pentadentate Schiff-base ligand having a Y-shaped core. Chem. Pap. 2018, 72, 853–862. [Google Scholar] [CrossRef]
- Bonadies, J.A.; Kirk, M.L.; Lah, M.S.; Kessissoglou, D.P.; Hatfield, W.E.; Pecoraro, V.L. Structurally diverse manganese(III) schiff-base complexes: Chains, dimers, and cages. Inorg. Chem. 1989, 28, 2037–2044. [Google Scholar] [CrossRef]
- Bertoncello, K.; Fallon, G.D.; Murray, K.S.; Tiekink, E.R.T. Manganese(III) complexes of a binucleating Schiff-base ligand based on the 1,3-diaminopropan-2-ol backbone. Inorg. Chem. 1991, 30, 3562–3568. [Google Scholar] [CrossRef]
- Gelasco, A.; Pecoraro, V.L. [Mn(III)(2-OHsalpn)]2 is an efficient functional model for the manganese catalases. J. Am. Chem. Soc. 1993, 115, 7928–7929. [Google Scholar] [CrossRef]
- Bagai, R.; Abboud, K.A.; Christou, G. Ligand-induced distortion of a tetranuclear manganese butterfly complex. Dalton Trans. 2006, 3306–3312. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 64–67. ISBN 978-0-471-74493-1. [Google Scholar]
- Wada, S.; Saka, K.; Yoshioka, D.; Mikuriya, M. Synthesis, crystal structures, and magnetic properties of dinuclear and hexanuclear copper(II) complexes with cyclam-based macrocyclic ligands having four schiff-base pendant arms. Bull. Chem. Soc. Jpn. 2010, 83, 364–374. [Google Scholar] [CrossRef]
- Murakami, Y.; Sakata, K. Kireto Kagaku (In Japanese); Ueno, K., Ed.; Nankodo: Tokyo, Japan, 1976; Volume 1, pp. 91–396. [Google Scholar]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; Rjin, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen-sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Brown, I.D.; Wu, K.K. Empirical parameters for calculating cation-oxygen bond valences. Acta Cryst. Sect. B 1976, 32, 1957–1959. [Google Scholar] [CrossRef]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the inorganic crystal structure database. Acta Cryst. Sect. B 1985, 41, 244–247. [Google Scholar] [CrossRef]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. Sect A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]

| 3 | 4 | |
|---|---|---|
| Empirical formula | C45H46Br4Mn4N4O17 | C48.3H54.2Cl4Mn4N7O18.3 |
| Formula weight | 1454.26 | 1359.12 |
| Temperature/K | 90 | 90 |
| Crystal dimensions/mm | 0.23 × 0.12 × 0.08 | 0.08 × 0.15 × 0.35 |
| Crystal system | monoclinic | triclinic |
| Space group | C2/c | P |
| a/Å | 32.754(9) | 12.5259(14) |
| b/Å | 15.121(4) | 15.5053(17) |
| c/Å | 29.893(13) | 16.8716(18) |
| α/o | 90 | 102.278(2) |
| β/o | 117.132(3) | 104.869(2) |
| γ/o | 90 | 110.789(2) |
| V/Å3 | 13176(8) | 2788.6(5) |
| Z | 8 | 2 |
| dcalcd./g·cm−3 | 1.466 | 1.619 |
| μ/mm−1 | 3.234 | 1.153 |
| F(000) | 5760 | 1387 |
| Reflections collected | 41795 | 12414 |
| Independent reflections | 16231 | 12414 |
| θ range for data collection | 1.397 to 28.715° | 2.569 to 27.496° |
| Data/restraints/parameters | 16231/0/675 | 12414/0/744 |
| R1, wR2 [I > 2σ(I)] a | 0.0785, 0.1756 | 0.0725, 0.1432 |
| R1, wR2 (all data) a | 0.1798, 0.2135 | 0.1252, 0.1593 |
| Goodness-of-fit on F2 | 0.976 | 0.979 |
| CCDC (The Cambridge Crystallographic Data Centren) number | 1887620 | 1887482 |
| [Mn4(bmsap)2(CH3CO2)3(CH3O)] (3) | |||
|---|---|---|---|
| Bond | Bond Length/Å | Bond | Bond Angle/° |
| Mn1···Mn2 | 3.530(2) | Mn1-O3-Mn2 | 110.2(3) |
| Mn2···Mn3 | 3.237(1) | Mn2-O3-Mn3 | 99.9(2) |
| Mn3···Mn1 | 3.466(2) | Mn1-O3-Mn3 | 94.4(2) |
| Mn4···Mn3 | 3.494(2) | Mn2-O17-Mn3 | 105.1(2) |
| Mn1-O1 | 2.067(5) | Mn3-O9-Mn4 | 110.2(2) |
| Mn1-O3 | 2.399(4) | Mn1-O8-Mn3 | 113.0(2) |
| Mn1-O6 | 1.911(5) | - | - |
| Mn1-O8 | 1.889(5) | - | - |
| Mn1-N1 | 2.056(6) | - | - |
| Mn1-N3 | 1.993(5) | - | - |
| Mn2-O3 | 1.891(4) | - | - |
| Mn2-O4 | 1.881(4) | - | - |
| Mn2-O11 | 2.085(5) | - | - |
| Mn2-O17 | 1.872(5) | - | - |
| Mn2-N2 | 1.981(6) | - | - |
| Mn3-O3 | 2.324(4) | - | - |
| Mn3-O8 | 2.261(5) | - | - |
| Mn3-O9 | 2.162(5) | - | - |
| Mn3-O13 | 2.080(4) | - | - |
| Mn3-O17 | 2.196(4) | - | - |
| Mn3-N4 | 2.241(6) | - | - |
| Mn4-O9 | 2.098(5) | - | - |
| Mn4-O10 | 2.343(7) | - | - |
| Mn4-O12 | 2.123(5) | - | - |
| Mn4-O14 | 2.081(6) | - | - |
| Mn4-O15 | 2.310(6) | - | - |
| Mn4-O16 | 2.196(5) | - | - |
| [Mn4(cmsap)2(CH3CO2)3(CH3O)] (4) | |||
|---|---|---|---|
| Bond | Bond Length/Å | Bond | Bond Angle/° |
| Mn1···Mn2 | 3.590(1) | Mn1-O5-Mn2 | 113.8(2) |
| Mn2···Mn3 | 3.181(1) | Mn2-O5-Mn3 | 99.4(1) |
| Mn3···Mn1 | 3.391(1) | Mn1-O5-Mn3 | 93.3(1) |
| Mn4···Mn3 | 3.562(1) | Mn2-O6-Mn3 | 100.8(2) |
| Mn1-O1 | 2.041(3) | Mn3-O11-Mn4 | 113.0(2) |
| Mn1-O5 | 2.387(3) | Mn1-O7-Mn3 | 108.6(2) |
| Mn1-O7 | 1.892(3) | - | - |
| Mn1-O8 | 1.885(3) | - | - |
| Mn1-N1 | 2.064(4) | - | - |
| Mn1-N3 | 2.000(4) | - | - |
| Mn2-O4 | 1.860(3) | - | - |
| Mn2-O5 | 1.885(3) | - | - |
| Mn2-O6 | 1.877(3) | - | - |
| Mn2-O15 | 2.100(3) | - | - |
| Mn2-N2 | 1.975(4) | - | - |
| Mn3-O5 | 2.273(3) | - | - |
| Mn3-O6 | 2.240(3) | - | - |
| Mn3-O7 | 2.275(3) | - | - |
| Mn3-O11 | 2.125(3) | - | - |
| Mn3-O17 | 2.086(3) | - | - |
| Mn3-N4 | 2.210(4) | - | - |
| Mn4-O10 | 2.336(3) | - | - |
| Mn4-O11 | 2.148(3) | - | - |
| Mn4-O12 | 2.306(4) | - | - |
| Mn4-O13 | 2.262(4) | - | - |
| Mn4-O14 | 2.166(3) | - | - |
| Mn4-O16 | 2.094(4) | - | - |
| Complex 1 | Complex 2 | Complex 3 | Complex 4 | |
|---|---|---|---|---|
| J1 | −4.91 cm−1 | −7.43 cm−1 | −10.78 cm−1 | −5.36 cm−1 |
| J2 | −1.77 cm−1 | −2.56 cm−1 | −7.01 cm−1 | −2.30 cm−1 |
| J3 | −0.75 cm−1 | −2.63 cm−1 | −5.19 cm−1 | −2.99 cm−1 |
| J4 | −2.20 cm−1 | −2.07 cm−1 | −2.14 cm−1 | −3.29 cm−1 |
| J5 | 7.84 cm−1 | 2.33 cm−1 | 0.61 cm−1 | 1.69 cm−1 |
| J6 | 0 cm−1 | 0 cm−1 | 0 cm−1 | 0 cm−1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikuriya, M.; Kurahashi, S.; Tomohara, S.; Koyama, Y.; Yoshioka, D.; Mitsuhashi, R.; Sakiyama, H. Synthesis, Crystal Structures, and Magnetic Properties of Mixed-Valent Tetranuclear Complexes with Y-Shaped MnII2MnIII2 Core. Magnetochemistry 2019, 5, 8. https://doi.org/10.3390/magnetochemistry5010008
Mikuriya M, Kurahashi S, Tomohara S, Koyama Y, Yoshioka D, Mitsuhashi R, Sakiyama H. Synthesis, Crystal Structures, and Magnetic Properties of Mixed-Valent Tetranuclear Complexes with Y-Shaped MnII2MnIII2 Core. Magnetochemistry. 2019; 5(1):8. https://doi.org/10.3390/magnetochemistry5010008
Chicago/Turabian StyleMikuriya, Masahiro, Satoshi Kurahashi, Seiki Tomohara, Yoshiki Koyama, Daisuke Yoshioka, Ryoji Mitsuhashi, and Hiroshi Sakiyama. 2019. "Synthesis, Crystal Structures, and Magnetic Properties of Mixed-Valent Tetranuclear Complexes with Y-Shaped MnII2MnIII2 Core" Magnetochemistry 5, no. 1: 8. https://doi.org/10.3390/magnetochemistry5010008
APA StyleMikuriya, M., Kurahashi, S., Tomohara, S., Koyama, Y., Yoshioka, D., Mitsuhashi, R., & Sakiyama, H. (2019). Synthesis, Crystal Structures, and Magnetic Properties of Mixed-Valent Tetranuclear Complexes with Y-Shaped MnII2MnIII2 Core. Magnetochemistry, 5(1), 8. https://doi.org/10.3390/magnetochemistry5010008