Microstructure, Martensitic Transformation, and Inverse Magnetocaloric Effect in Ni48Mn39.5Sn12.5−xAlx Metamagnetic Shape Memory Alloys
Abstract
1. Introduction
2. Experimental
3. Results and Discussion
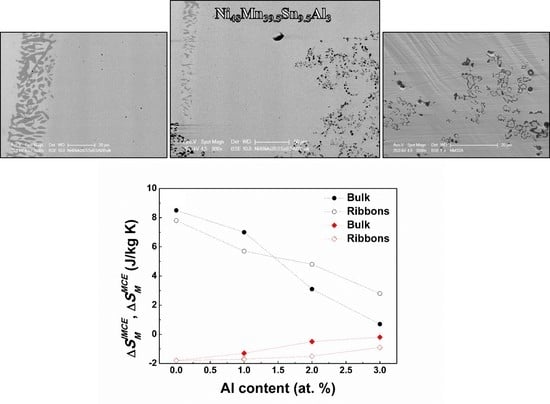
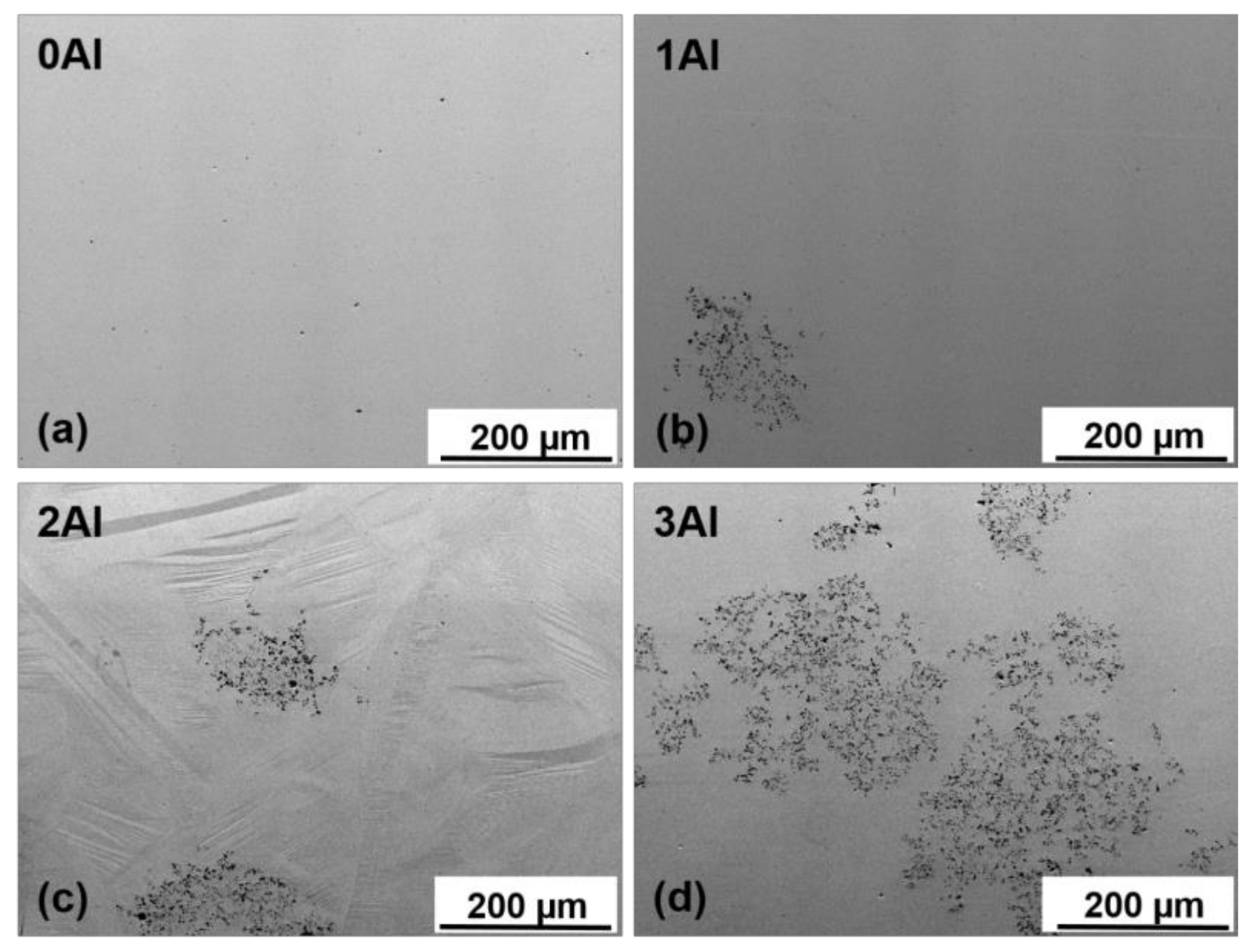
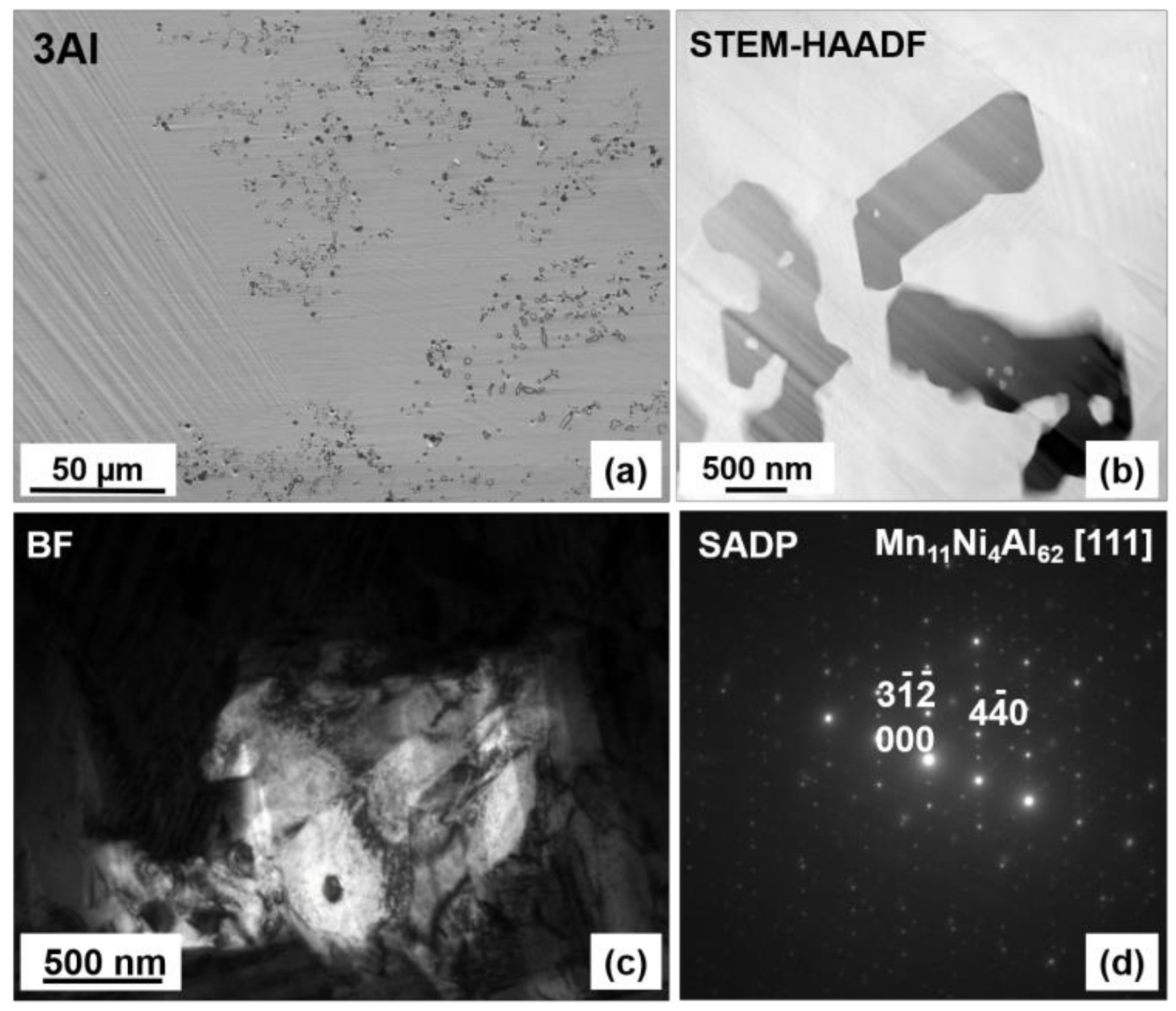
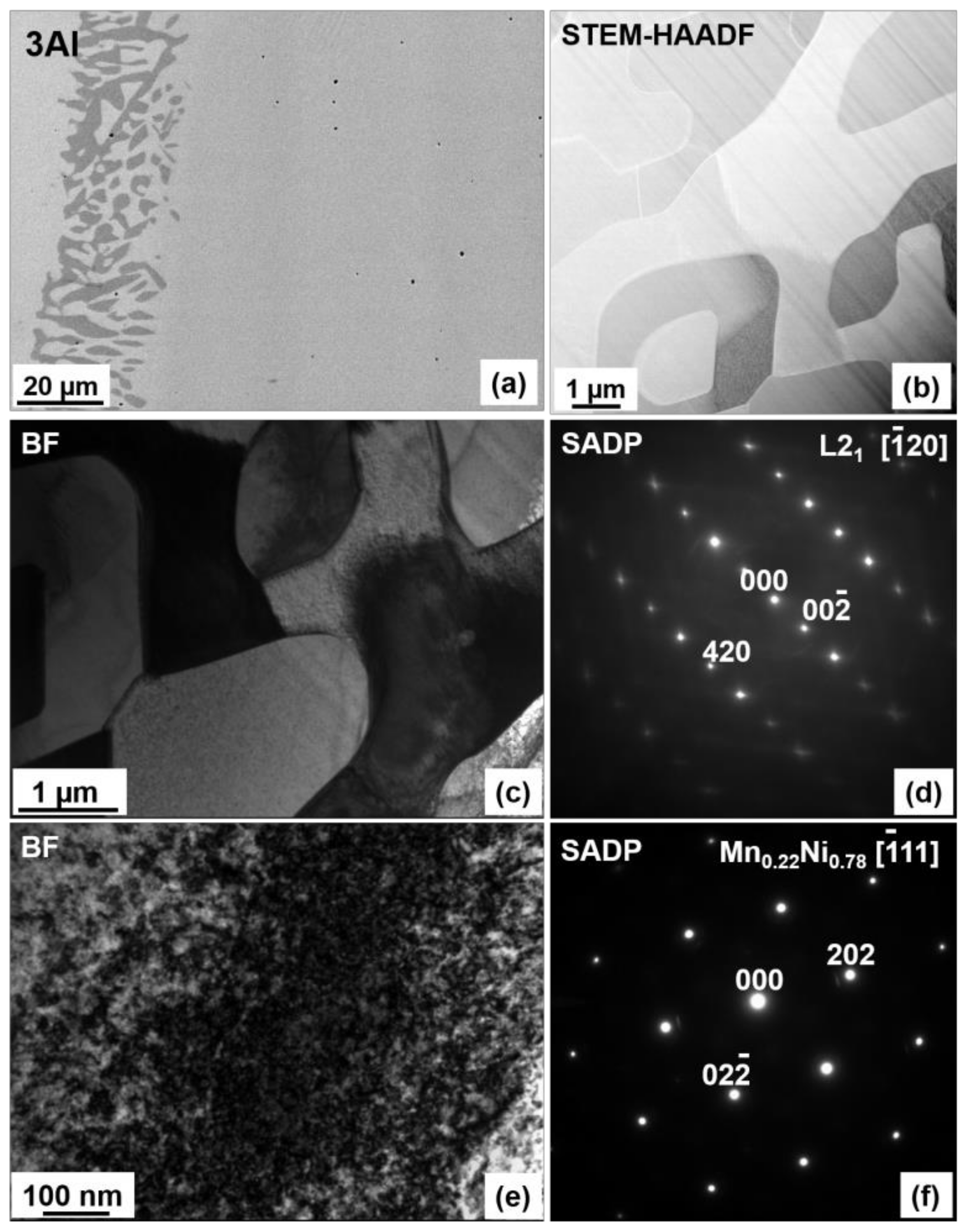
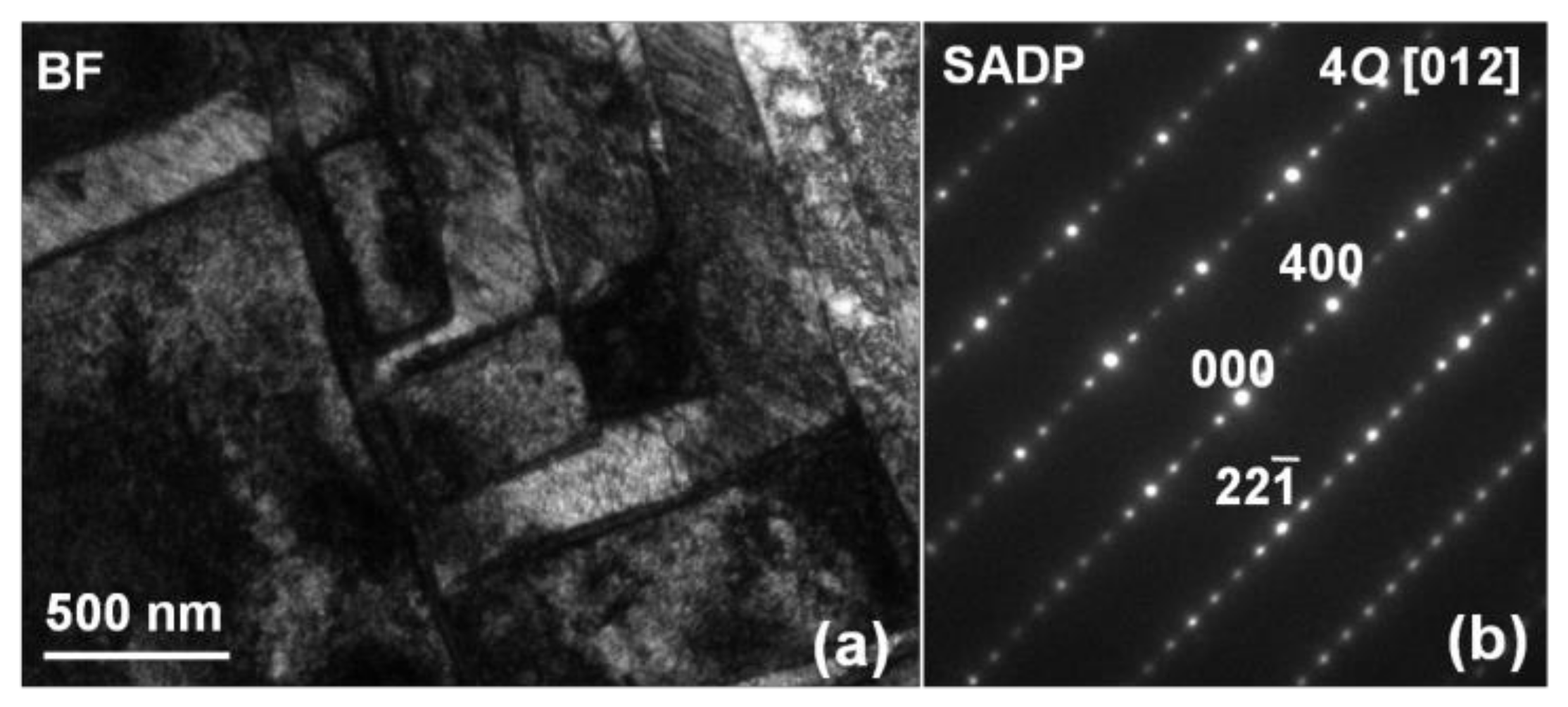
3.1. Microstructure
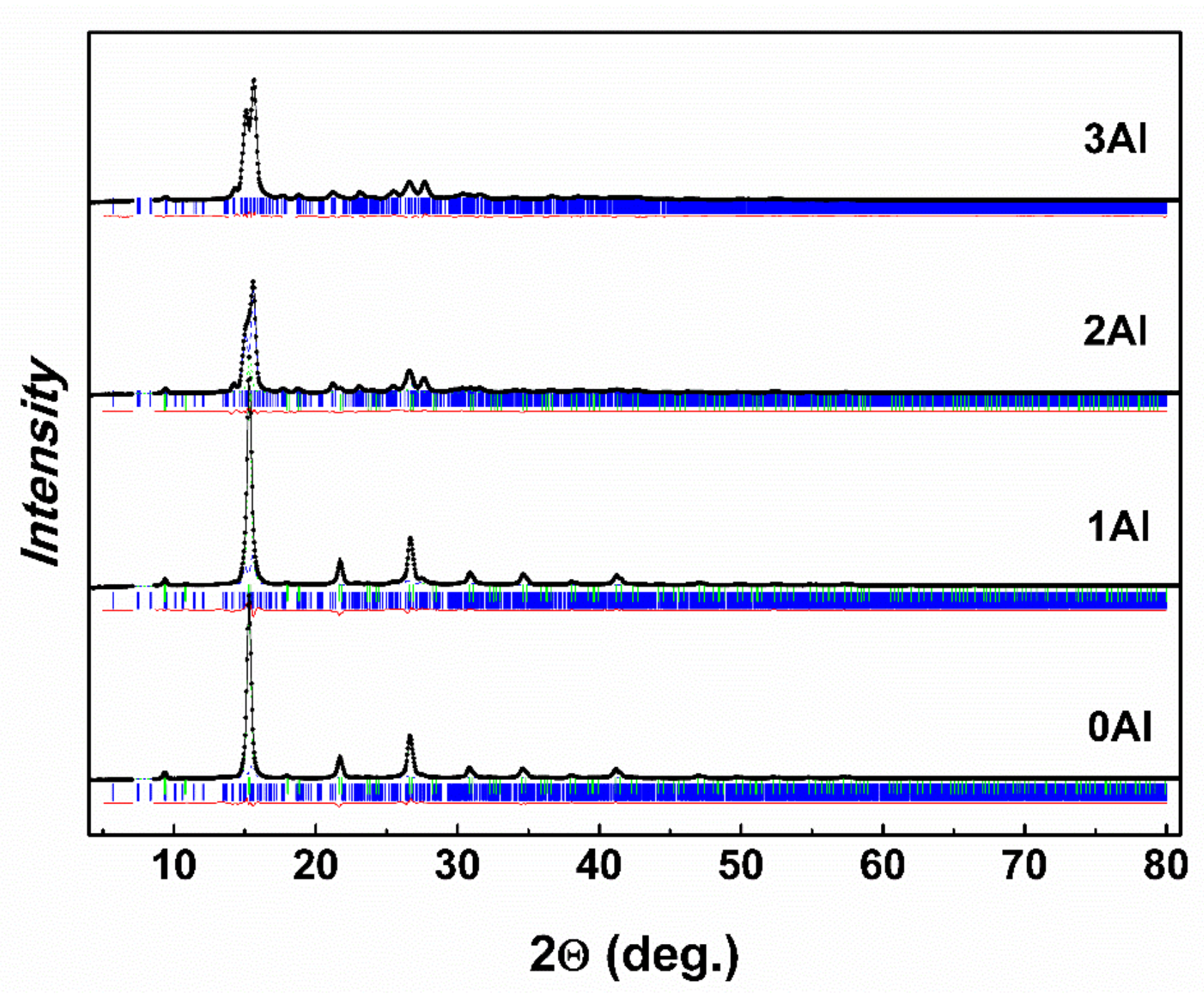
3.2. Crystal Structure
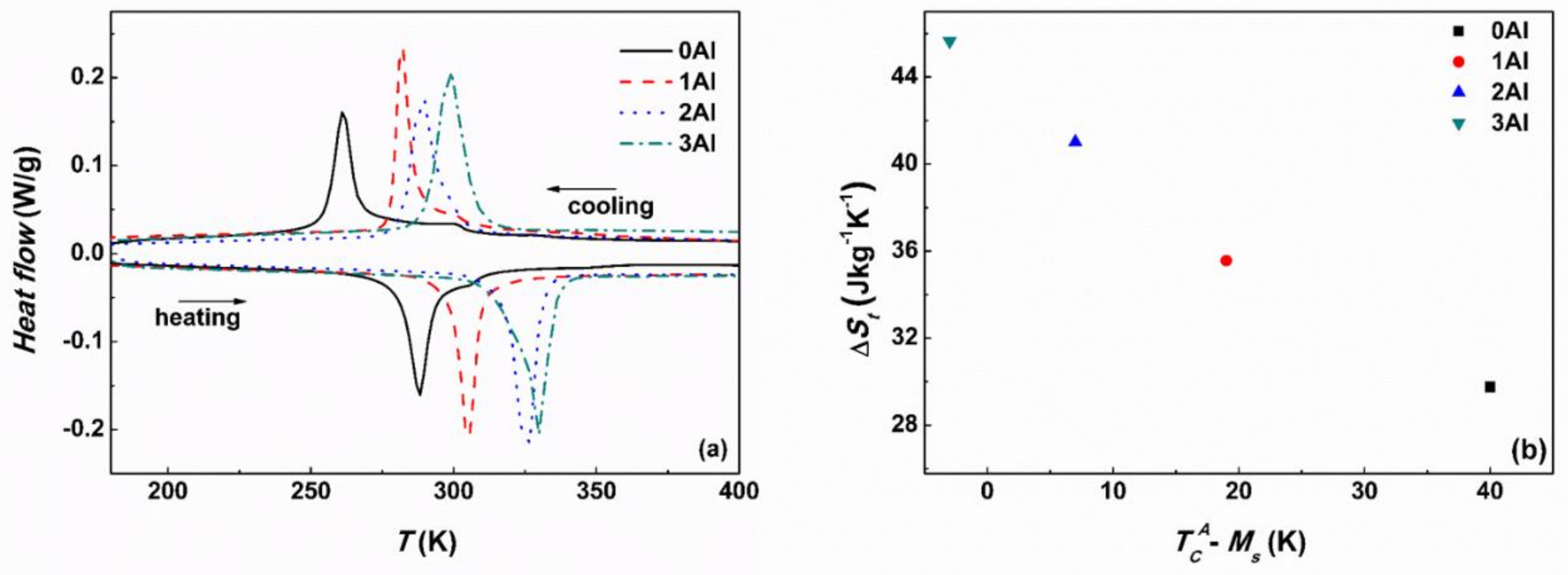
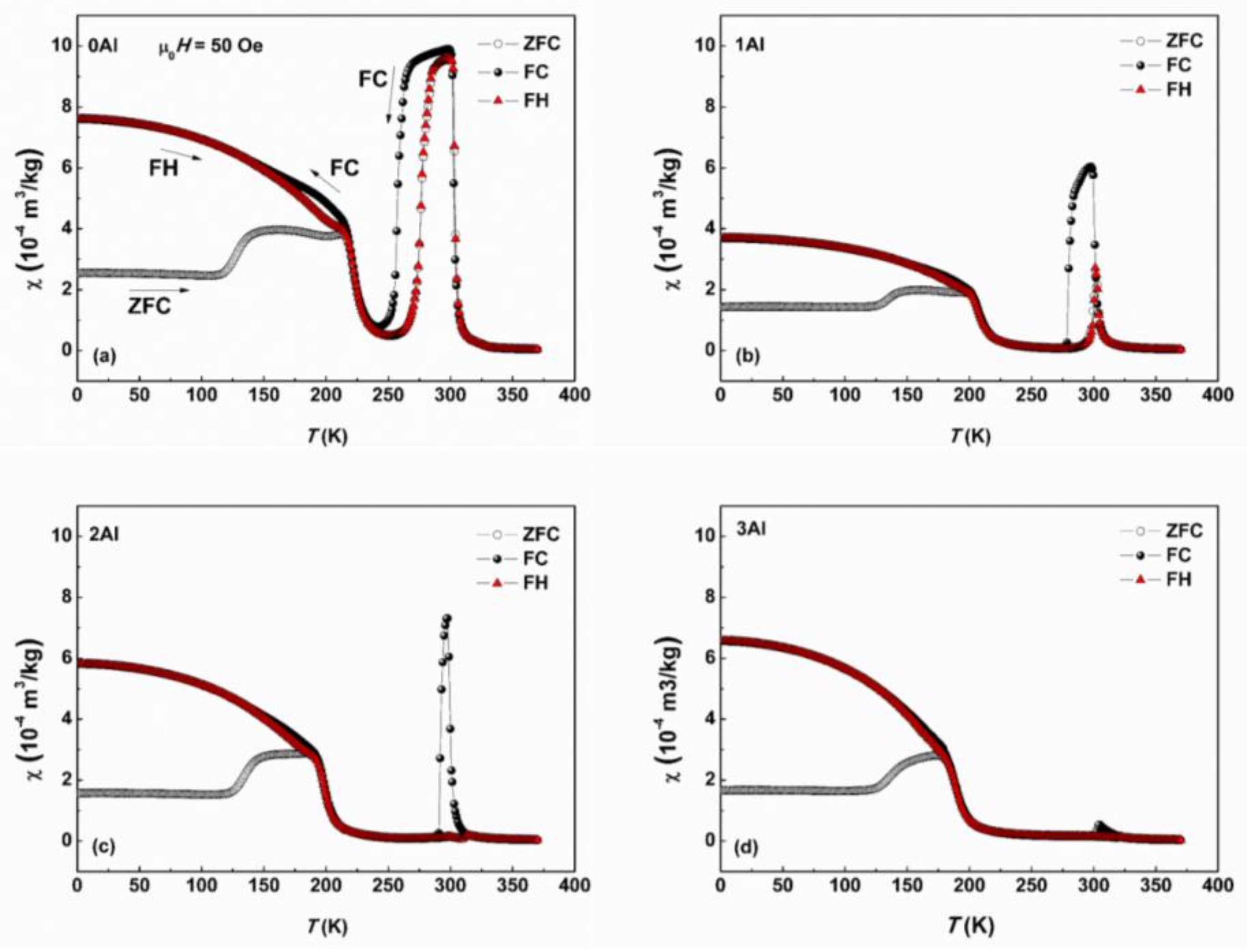
3.3. Martensitic Transformation and Magnetic Properties
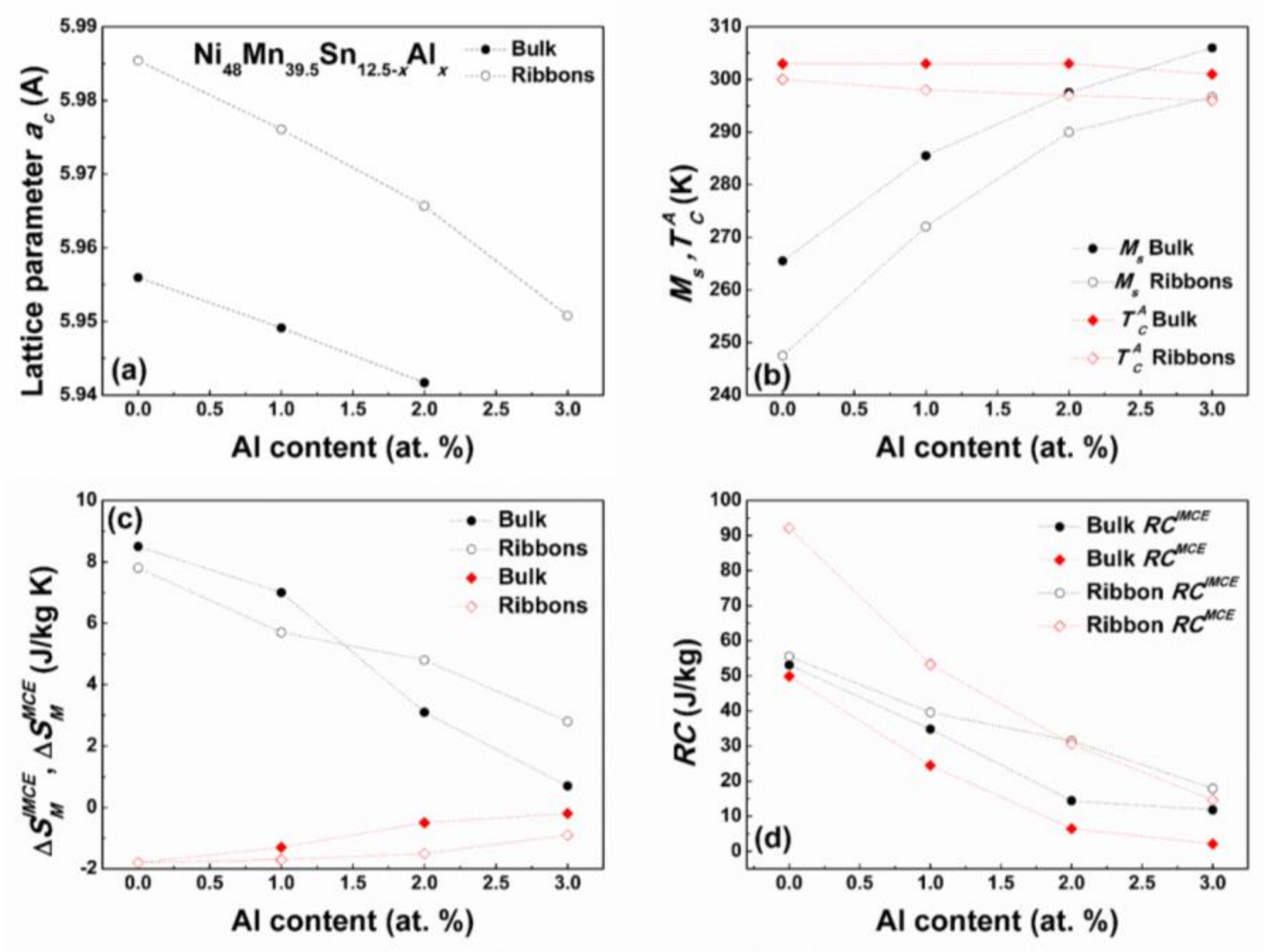
3.4. Bulk vs. Melt Spun Ribbon Alloys
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Soderberg, O.; Aaltio, I.; Ge, Y.; Heczko, O.; Hannula, S.P. Ni-Mn-Ga multifunctional compounds. Mater. Sci. Eng. A 2008, 481–482, 80–85. [Google Scholar] [CrossRef]
- Khan, M.; Pathak, A.K.; Paudel, M.R.; Dubenko, I.; Stadler, S.; Ali, N. Magnetoresistance and field-induced structural transitions in Ni50Mn50−xSnx Heusler alloys. J. Magn. Magn. Mater. 2008, 320, L21–L25. [Google Scholar] [CrossRef]
- Planes, A.; Manosa, L.; Moya, X.; Krenke, T.; Acet, M.; Wassermann, E.F. Magnetocaloric effect in Heusler shape-memory alloys. J. Magn. Magn. Mater. 2007, 310, 2767–2769. [Google Scholar] [CrossRef]
- Smith, A.; Bahl, C.R.; Bjork, R.; Engelbrecht, K.; Nielsen, K.K.; Pryds, N. Materials challenges for high performance magnetocaloric refrigeration devices. Adv. Eng. Mater. 2012, 2, 1288–1318. [Google Scholar] [CrossRef]
- Manosa, L.; Moya, X.; Planes, A.; Krenke, T.; Acet, M.; Wassermann, E.F. Ni-Mn-based magnetic shape memory alloys: Magnetic properties and martensitic transition. Mater. Sci. Eng. A 2008, 481–482, 49–56. [Google Scholar] [CrossRef]
- Pons, J.; Cesari, E.; Segui, C.; Masdeu, F.; Santamarta, R. Ferromagnetic shape memory alloys: Alternatives to Ni-Mn-Ga. Mater. Sci. Eng. A 2008, 481–482, 57–65. [Google Scholar] [CrossRef]
- Sutou, Y.; Imano, Y.; Koeda, N.; Omori, T.; Kainuma, R.; Ishida, K.; Oikawa, K. Magnetic and martensitic transformations of NiMnX(X = In, Sn, Sb) ferromagnetic shape memory alloys. Appl. Phys. Lett. 2004, 85, 4358–4360. [Google Scholar] [CrossRef]
- Koyama, K.; Watanabe, K.; Kanomata, T.; Kainuma, R.; Oikawa, K.; Ishida, K. Observation of field-induced reverse transformation in ferromagnetic shape memory alloy Ni50Mn36Sn14. Appl. Phys. Lett. 2006, 88, 132505. [Google Scholar] [CrossRef]
- Moya, X.; Manosa, L.; Planes, A.; Krenke, T.; Acet, M.; Wassermann, E.F. Martensitic transition and magnetic properties in Ni-Mn-X alloys. Mater. Sci. Eng. A 2006, 438–440, 911–915. [Google Scholar] [CrossRef]
- Kainuma, R.; Imano, Y.; Ito, W.; Sutou, Y.; Morito, H.; Okamoto, S.; Kitakami, O.; Oikawa, K.; Fujita, A.; Kanomata, T.; et al. Magnetic-field-induced shape recovery by reverse phase transformation. Nat. Lett. 2006, 439, 957–960. [Google Scholar] [CrossRef] [PubMed]
- Chernenko, V.A.; Barandiaran, J.M.; Fernandez, J.R.; Rojas, D.P.; Gutierrez, J.; Lazpita, P.; Orue, I. Magnetic and magnetocaloric properties of martensitic Ni2Mn1.4Sn0.6 Heusler alloy. J. Magn. Magn. Mater. 2012, 324, 3519–3523. [Google Scholar] [CrossRef]
- Koyama, K.; Kanomata, T.; Kainuma, R.; Oikawa, K.; Ishida, K.; Watanabe, K. High-field X-ray diffraction measurements of novel ferromagnetic shape-memory alloy Ni50Mn36Sn14. Physica B 2006, 383, 24–25. [Google Scholar] [CrossRef]
- Mukadam, M.D.; Yusuf, S.M.; Bhadtt, P. Tuning the magnetocaloric properties of the Ni2+xMn1−xSn Heusler alloys. J. Appl. Phys. 2013, 113, 173911. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, W.; Xue, S.; Zhai, Q.; Frenzel, J.; Luo, Z. Composition-dependent crystal structure and martensitic transformation in Heusler Ni-Mn-Sn alloys. Acta Mater. 2013, 61, 4648–4656. [Google Scholar] [CrossRef]
- McAlister, S.P.; Shiozaki, I.; Hurd, C.M.; Stager, C.V. Galvanomagnetic effects in the Ni2MnSn Heusler alloy. J. Phys. F Metal Phys. 1981, 11, 2129. [Google Scholar] [CrossRef]
- Brown, P.J.; Gandy, A.P.; Ishida, K.; Kainuma, R.; Kanomata, T.; Neumann, K.U.; Oikawa, K.; Ouladdiaf, B.; Ziebeck, K.R.A. The magnetic and structural properties of the magnetic shape memory compound Ni2Mn1.44Sn0.56. J. Phys. Condens. Matter. 2006, 18, 2249–2259. [Google Scholar] [CrossRef]
- Krenke, T.; Acet, M.; Wassermann, E.F. Martensitic transitions and the nature of ferromagnetism in the austenitic and martensitic states of Ni-Mn-Sn alloys. Phys. Rev. B 2005, 72, 014412. [Google Scholar] [CrossRef]
- Wang, R.L.; Yan, J.B.; Xiao, H.B.; Xu, L.S.; Marchenkov, V.V.; Xu, L.F.; Yang, C.P. Effect of electron density on the martensitic transition in Ni-Mn-Sn alloys. J. Alloys Compd. 2011, 509, 6834–6837. [Google Scholar] [CrossRef]
- Chen, J.; Han, Z.; Qian, B.; Zhang, P.; Wang, D.; Du, Y. The influence of Al substitution on the phase transitions and magnetocaloric effect in Ni43Mn46Sn11−xAlx alloys. J. Magn. Magn. Mater. 2011, 323, 248–251. [Google Scholar] [CrossRef]
- Wang, R.L.; Yan, J.B.; Xu, L.S.; Marchenkov, V.V.; Chen, S.S.; Tang, S.L.; Yang, C.P. Effect of Al doping on the martensitic transition and magnetic entropy change in Ni-Mn-Sn alloys. Solid State Commun. 2011, 151, 1196–1199. [Google Scholar] [CrossRef]
- Xiao, H.B.; Yang, C.P.; Wang, R.L.; Marchenkov, V.V.; Barner, K. Effect of alloying element Al substitution on Ni-Mn-Sn shape memory alloy by first-principle calculations. J. Appl. Phys. 2012, 112, 123723. [Google Scholar] [CrossRef]
- Agarwal, S.; Mukhopadhyay, P.K. The effect of Al replacement and heat treatment on magnetocaloric properties of Ni2Mn-Sn ferromagnetic shape memory alloys. J. Alloys Compd. 2014, 608, 329–333. [Google Scholar] [CrossRef]
- Agarwal, S.; Stern-Taulats, E.; Manosa, L.; Mukhopadhyay, P.K. Effect of low temperature annealing on magneto-caloric effect of Ni-Mn-Sn-Al ferromagnetic shape memory alloy. J. Alloys Compd. 2015, 641, 244–248. [Google Scholar] [CrossRef]
- Yuhasz, W.M.; Schlagel, D.L.; Xing, Q.; McCallum, R.W.; Lograsso, T.A. Metastability of ferromagnetic Ni-Mn-Sn Heusler alloys. J. Alloys Compd. 2010, 492, 681–684. [Google Scholar] [CrossRef]
- Yuhasz, W.M.; Schlagel, D.L.; Xing, Q.; Dennis, K.W.; McCallum, R.W.; Lograsso, T.A. Influence of annealing and phase decomposition on the magnetostructural transitions in Ni50Mn39Sn11. J. Appl. Phys. 2009, 105, 07A921. [Google Scholar] [CrossRef]
- Czaja, P.; Maziarz, W.; Przewoźnik, J.; Kapusta, C.; Hawelek, L.; Chrobak, A.; Drzymała, P.; Fitta, M.; Kolano-Burian, A. Magnetocaloric properties and exchange bias effect in Al for Sn substituted Ni48Mn39.5Sn12.5 Heusler alloy ribbons. J. Magn. Magn. Mater. 2014, 358–359, 142–148. [Google Scholar] [CrossRef]
- Maziarz, W.; Czaja, P.; Szczerba, M.J.; Przewoźnik, J.; Kapusta, C.; Żywczak, A.; Stobiecki, T.; Cesari, E.; Dutkiewicz, J. Room temperature magneto-structural transition in Al for Sn substituted Ni-Mn-Sn melt spun ribbons. J. Magn. Magn. Mater. 2013, 348, 8–16. [Google Scholar] [CrossRef]
- Czaja, P.; Maziarz, W.; Przewoźnik, J.; Żywczak, A.; Ozga, P.; Bramowicz, M.; Kulesza, S.; Dutkiewicz, J. Surface topography, microstructure and magnetic domains in Al for Sn substituted metamagnetic Ni-Mn-Sn Heusler alloy ribbons. Intermetallics 2014, 55, 1–8. [Google Scholar] [CrossRef]
- Guo, Z.; Pan, L.; Yasir Rafique, M.; Zheng, X.; Qiu, H.; Liu, Z. Metamagnetic phase transformation and magnetocaloric effect in quinary Ni45Co5Mn40InxSn10−x Heusler alloy. J. Alloys Compd. 2013, 577, 174–178. [Google Scholar] [CrossRef]
- Caron, L.; Ou, Z.Q.; Nguyen, T.T.; Cam Thanh, D.T.; Tegus, O.; Bruck, E. On the determination of the magnetic entropy change in materials with first-order transitions. J. Magn. Magn. Mater. 2009, 321, 3559–3566. [Google Scholar] [CrossRef]
- Tocado, L.; Palacios, E.; Burriel, R. Entropy determinations and magnetocaloric parameters in systems with first-order transitions: Study of MnAs. J. Appl. Phys. 2009, 105, 093918. [Google Scholar] [CrossRef]
- Robinson, K. The Determination of the Crystal Structure of Ni4Mn11Al60. Acta Crystallogr. 1954, 7, 494–497. [Google Scholar] [CrossRef]
- Passamani, E.C.; Xavier, F.; Favre-Nicolin, E.; Larica, C.; Takeuchi, A.Y.; Castro, I.L.; Proveti, J.R. Magnetic properties of NiMn-based Heusler alloys influenced by Fe atoms replacing Mn. J. Appl. Phys. 2009, 105, 033919. [Google Scholar] [CrossRef]
- Mishima, Y.; Ochiai, S.; Suzuki, T. Lattice parameters of Ni(γ), Ni3Al(γ′) and Ni3Ga(γ′) solid solutions with additions of transition and B-subgroup elements. Acta Metall. 1985, 33, 1161–1169. [Google Scholar] [CrossRef]
- Sanchez-Alarcos, V.; Recarte, V.; Perez-Landazabal, J.I.; Chapelon, J.R.; Rodriguez-Velamazan, J.A. Structural and magnetic properties of Cr-doped Ni-Mn-In metamagnetic shape memory alloys. J. Phys. D Appl. Phys. 2011, 44, 395001. [Google Scholar] [CrossRef]
- Ingale, B.D.; Kuo, Y.K.; Ram, S. Phase Transformation, Microstructure and Magnetocaloric Properties in Polycrystalline Bulk Ni50Mn50−zSnzAlloys. IEEE Trans. Magn. 2011, 47, 3395–3398. [Google Scholar] [CrossRef]
- Cesari, E.; Salas, D.; Kustov, S. Entropy Changes in Ferromagnetic Shape Memory Alloys. Mater. Sci. Forum 2011, 684, 49–60. [Google Scholar] [CrossRef]
- Recarte, V.; Perez-Landazabal, J.I.; Sanchez-Alcaros, V.; Zablotskii, V.; Cesari, E.; Kustov, S. Entropy change linked to the martensitic transformation in metamagnetic shape memory alloys. Acta Mater. 2012, 60, 3168–3175. [Google Scholar] [CrossRef]
- Tao, Q.; Han, Z.D.; Wang, J.J.; Qian, B.; Zhang, P.; Jiang, X.F.; Wang, D.H.; Du, Y.W. Phase stability and magnetic-field-induced martensitic transformation in Mn-rich NiMnSn alloys. AIP Adv. 2012, 2, 042181. [Google Scholar] [CrossRef]
- Phan, T.L.; Zhang, P.; Dan, N.H.; Yen, N.H.; Thanh, P.T.; Thanh, T.D.; Phan, M.H.; Yu, S.C. Coexistence of conventional and inverse magnetocaloric effects and critical behaviors in Ni50Mn50−xSnx (x = 13 and 14) alloy ribbons. Appl. Phys. Lett. 2012, 101, 212403. [Google Scholar] [CrossRef]
- Han, Z.D.; Wang, D.H.; Zhang, C.L.; Xuan, H.C.; Gu, B.X.; Du, Y.W. Low-field inverse magnetocaloric effect in Ni50−xMn39+xSn11 Heusler alloys. Appl. Phys. Lett. 2007, 90, 042507. [Google Scholar] [CrossRef]
- Liu, F.S.; Wang, Q.B.; Li, S.P.; Ao, W.Q.; Li, J.Q. The martensitic transition and magnetocaloric properties of Ni51Mn49−xSnx. Phys. B 2013, 412, 74–78. [Google Scholar] [CrossRef]
- Xuan, H.C.; Wang, D.H.; Zhang, C.L.; Han, Z.D.; Gu, B.X.; Du, Y.W. Boron’s effect on martensitic transformation and magnetocaloric effect in Ni43Mn46Sn11Bx alloys. Appl. Phys. Lett. 2008, 92, 102503. [Google Scholar] [CrossRef]
- Chatterjee, S.; Giri, S.; De, S.K.; Majumdar, S. Giant magneto-caloric effect near room temperature in Ni-Mn-Sn-Ga alloys. J. Alloys Compd. 2010, 503, 273–276. [Google Scholar] [CrossRef]
- Fukushima, K.; Sano, K.; Kanomata, T.; Nishihara, H.; Furutani, Y.; Shishido, T.; Ito, W.; Umetsu, R.Y.; Kainuma, R.; Oikawa, K.; et al. Phase diagram of Fe-substituted Ni-Mn-Sn shape memory alloys. Scr. Mater. 2009, 61, 813–816. [Google Scholar] [CrossRef]
- Han, Z.D.; Wang, D.H.; Zhang, C.L.; Xuan, H.C.; Zhang, J.R.; Gu, B.X.; Du, Y.W. Effect of lattice contraction on martensitic transformation and magnetocaloric effect in Ge doped Ni-Mn-Sn alloys. Mater. Sci. Eng. B 2009, 157, 40–43. [Google Scholar] [CrossRef]
- Donner, P. Microstructure and phase-transformation in meltspun shape memory alloys. J. Phys. IV France 1991, 1, C4-355–C4-360. [Google Scholar] [CrossRef][Green Version]
- Bai, J.; Raulot, J.M.; Zhang, Y.D.; Esling, C.; Zhao, X.; Zuo, L. Defect formation energy and magnetic structure of shape memory alloys Ni-X-Ga (X = Mn, Fe, Co) by first principle calculation. J. Appl. Phys. 2010, 108, 064904. [Google Scholar] [CrossRef]
- Das, R.; Perumal, A.; Srinivasan, A. Effect of particle size on the magneto-caloric properties of Ni51Mn34In14Si1 alloy. J. Alloys Compd. 2013, 572, 192–198. [Google Scholar] [CrossRef]
- Quintana-Nedelcos, A.; Sanchez Llamazares, J.L.; Rios-Jara, D.; Lara-Rodriguez, A.G.; Garcia-Fernandez, T. Effect of quenching rate on the average grain size and martensitic transformation temperature in rapidly solidified polycrystalline Ni50Mn37Sn13 alloy ribbons. Phys. Status Solidi A 2013, 210, 2159–2165. [Google Scholar] [CrossRef]


| Alloy | Ni (at %) | Mn (at %) | Sn (at %) | Al (at %) | O (at %) | e/a | |
|---|---|---|---|---|---|---|---|
| 0Al | Nominal | 48 | 39.5 | 12.5 | - | - | 8.065 |
| SEM-EDS | Matrix | 49.3 | 38.4 | 12.3 | - | - | 8.109 |
| Al-phase | - | - | - | - | - | - | |
| 1Al | Nominal | 48 | 39.5 | 11.5 | 1 | - | 8.055 |
| SEM-EDS | Matrix | 48.5 | 39 | 11.5 | 1 | - | 8.071 |
| Al-phase | 33.8 | 27.2 | 8.1 | 30.9 | - | 6.533 | |
| Ni-phase | 63.7 | 30.6 | 4.7 | 1 | - | 8.731 | |
| 2Al | Nominal | 48 | 39.5 | 10.5 | 2 | - | 8.045 |
| SEM-EDS | Matrix | 48.8 | 39 | 10.4 | 1.8 | - | 8.081 |
| Al-phase | 34.6 | 27.8 | 7.4 | 30.2 | - | 6.61 | |
| 3Al | Nominal | 48 | 39.5 | 9.5 | 3 | - | 8.035 |
| SEM-EDS | Matrix | 48.3 | 39 | 9.7 | 3 | - | 8.036 |
| Al-phase | 37.5 | 31.5 | 7.8 | 23.2 | - | 6.967 | |
| Ni-phase | 66.2 | 28.2 | 3 | 2.6 | - | 8.794 | |
| SEM-WDS | Al-phase | 14.8 | 10.6 | 2.2 | 69.7 | 2.7 | - |
| TEM-EDX | Al-phase | 13.5 | 11.1 | 1.1 | 74.3 | - | 4.397 |
| Matrix | 51.9 | 39.6 | 6.8 | 1.7 | - | 8.288 | |
| Ni-phase | 66.8 | 29.1 | 2.5 | 1.5 | - | 8.87 | |
| Matrix | 55 | 28.1 | 14.8 | 2.1 | - | 8.124 |
| Alloy | Austenite | Martensite | |||||
|---|---|---|---|---|---|---|---|
| ac (Å) | Vc (Å3) | pc (%) | ao (Å) | bo (Å) | co (Å) | Vc (Å3) | |
| 0Al | 5.9560 (4) | 211.28 (2) | 87 (5) | 8.585 (15) | 5.656 (10) | 4.320 (10) | 209.7 (7) |
| 1Al | 5.9491 (5) | 210.55 (3) | 79 (5) | 8.562 (10) | 5.643 (8) | 4.321 (6) | 208.8 (5) |
| 2Al | 5.942 (2) | 209.77 (12) | 16 (3) | 8.546 (3) | 5.615 (2) | 4.334 (2) | 208.0 (1) |
| 3Al | - | - | - | 8.524 (2) | 5.6093 (2) | 4.3306 (2) | 207.1 (1) |
| Alloy | Method | Ms | Mf | As | Af | ∆Th. | TM | TRM | ΔSt | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cool | Heat | |||||||||||
| (K) | (J kg−1 K−1) | |||||||||||
| 0Al | χ(T) | 263 | 253 | 270 | 283 | 20 | 257 | 276 | 303 | 222 | - | - |
| DSC | 268 | 254 | 280 | 294 | 26 | 261 | 288 | - | - | 29.8 | 37.4 | |
| 1Al | χ(T) | 284 | 277 | 297 | 302 | 18 | 280 | 300 | 303 | 207 | - | - |
| DSC | 287 | 278 | 298 | 311 | 24 | 282 | 305 | - | - | 35.6 | 31.9 | |
| 2Al | χ(T) | 296 | 290 | 309 | 313 | 17 | 292 | 311 | 303 | 197 | - | - |
| DSC | 299 | 282 | 318 | 331 | 32 | 290 | 327 | - | - | 41.0 | 36.7 | |
| 3Al | χ(T) | 304 | 301 | 318 | 321 | 17 | 302 | 320 | 301 | 189 | - | - |
| DSC | 308 | 290 | 322 | 337 | 29 | 299 | 330 | - | - | 45.6 | 39.9 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czaja, P.; Wróblewski, R.; Grzonka, J.; Przewoźnik, J.; Maziarz, W. Microstructure, Martensitic Transformation, and Inverse Magnetocaloric Effect in Ni48Mn39.5Sn12.5−xAlx Metamagnetic Shape Memory Alloys. Magnetochemistry 2018, 4, 19. https://doi.org/10.3390/magnetochemistry4020019
Czaja P, Wróblewski R, Grzonka J, Przewoźnik J, Maziarz W. Microstructure, Martensitic Transformation, and Inverse Magnetocaloric Effect in Ni48Mn39.5Sn12.5−xAlx Metamagnetic Shape Memory Alloys. Magnetochemistry. 2018; 4(2):19. https://doi.org/10.3390/magnetochemistry4020019
Chicago/Turabian StyleCzaja, P., R. Wróblewski, J. Grzonka, J. Przewoźnik, and W. Maziarz. 2018. "Microstructure, Martensitic Transformation, and Inverse Magnetocaloric Effect in Ni48Mn39.5Sn12.5−xAlx Metamagnetic Shape Memory Alloys" Magnetochemistry 4, no. 2: 19. https://doi.org/10.3390/magnetochemistry4020019
APA StyleCzaja, P., Wróblewski, R., Grzonka, J., Przewoźnik, J., & Maziarz, W. (2018). Microstructure, Martensitic Transformation, and Inverse Magnetocaloric Effect in Ni48Mn39.5Sn12.5−xAlx Metamagnetic Shape Memory Alloys. Magnetochemistry, 4(2), 19. https://doi.org/10.3390/magnetochemistry4020019