The Rise of Single-Ion Magnets as Spin Qubits
Abstract
:1. Introduction
2. Single-Ion Magnetic Molecules with Transition Metal Centers
2.1. V(IV) Based Spin Qubits
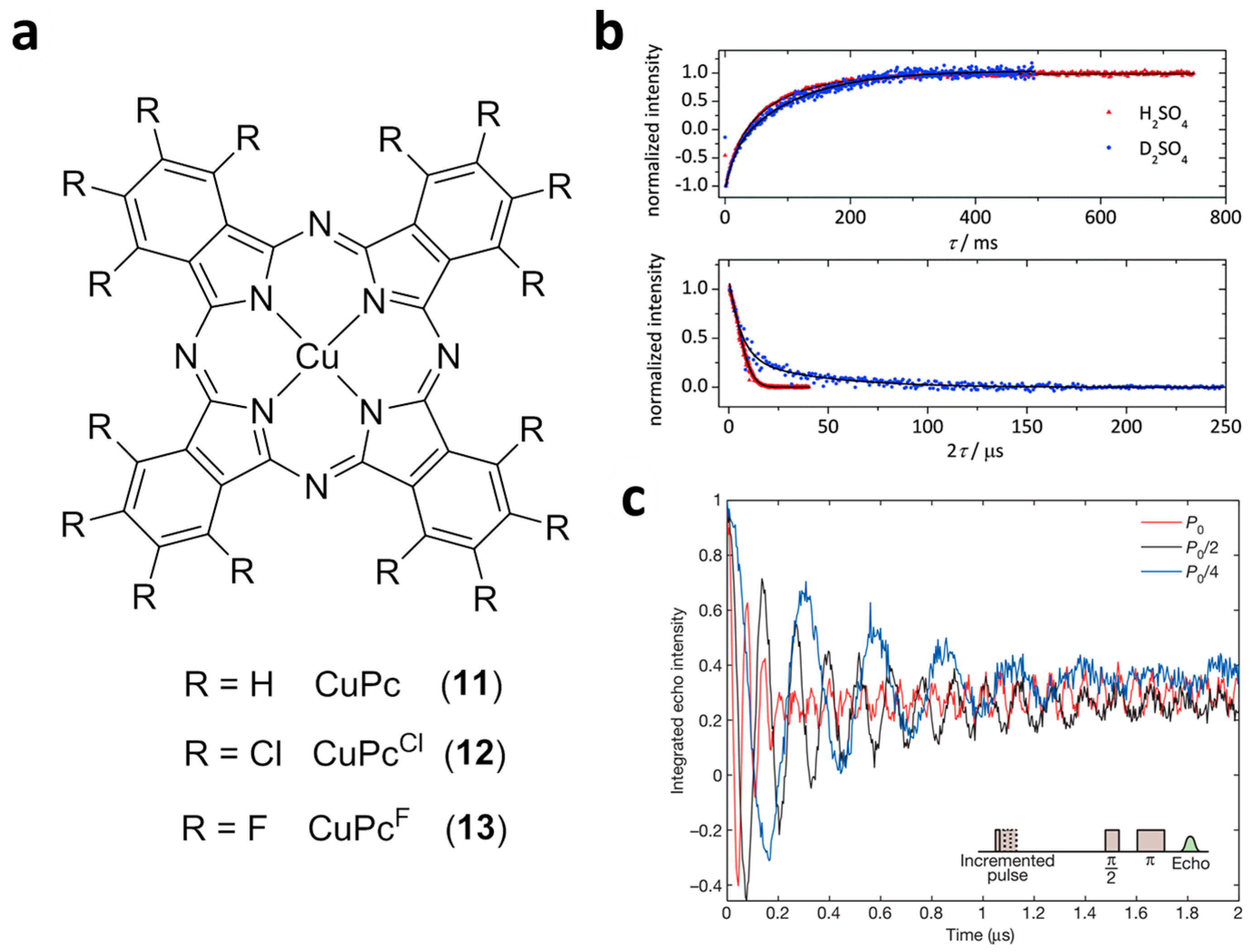
2.2. Cu(II) Based Single-Ion Spin Qubits
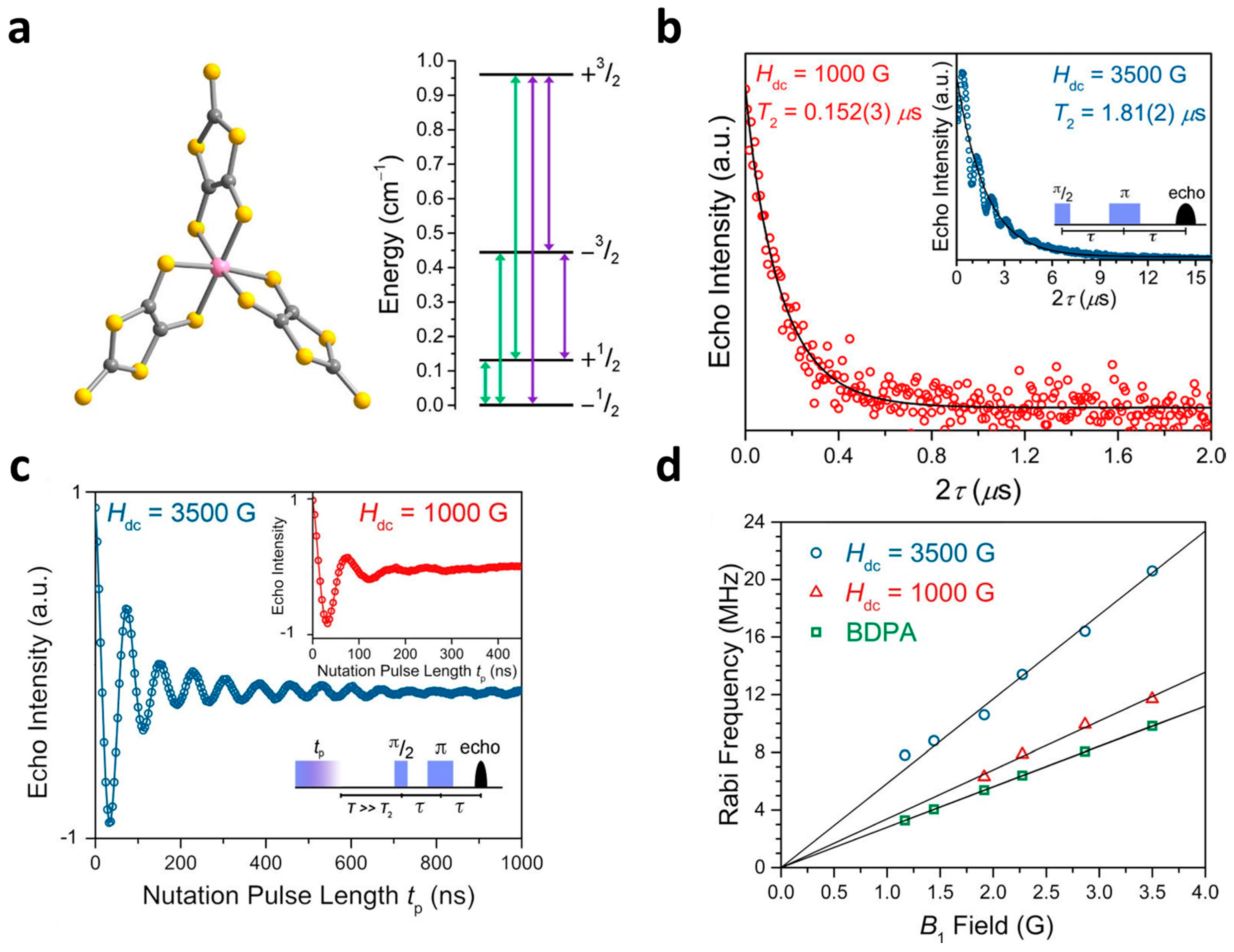
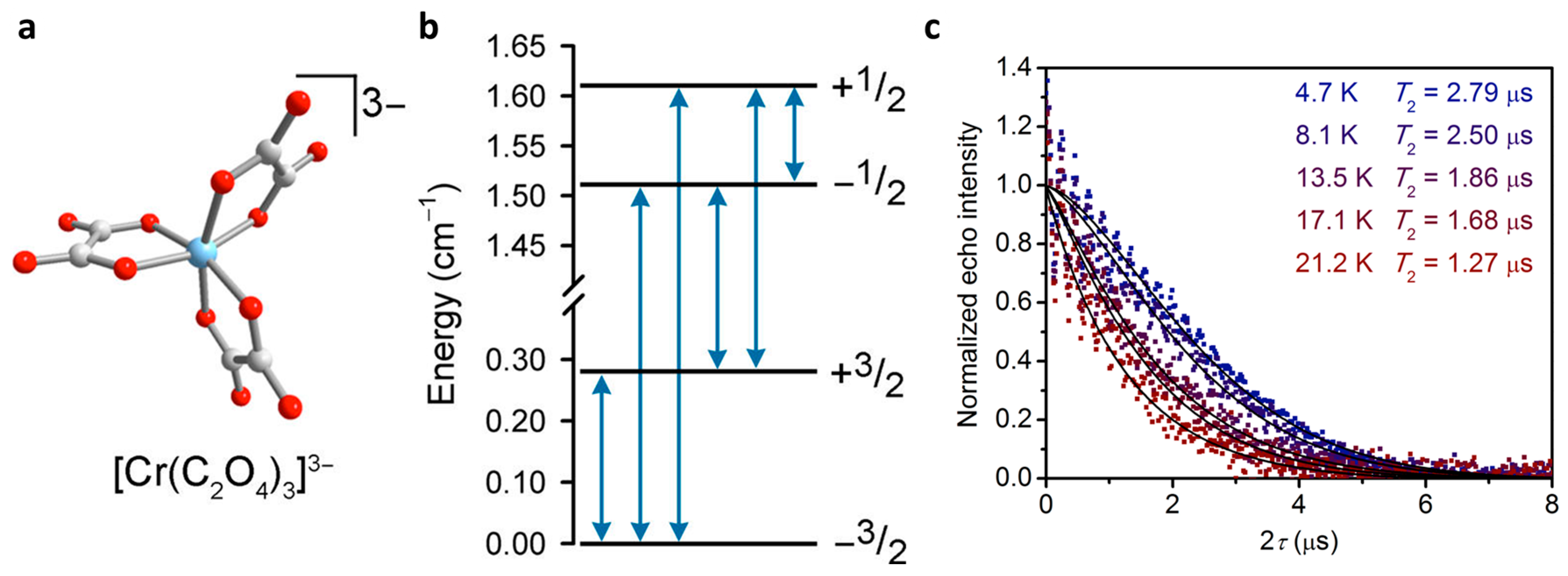
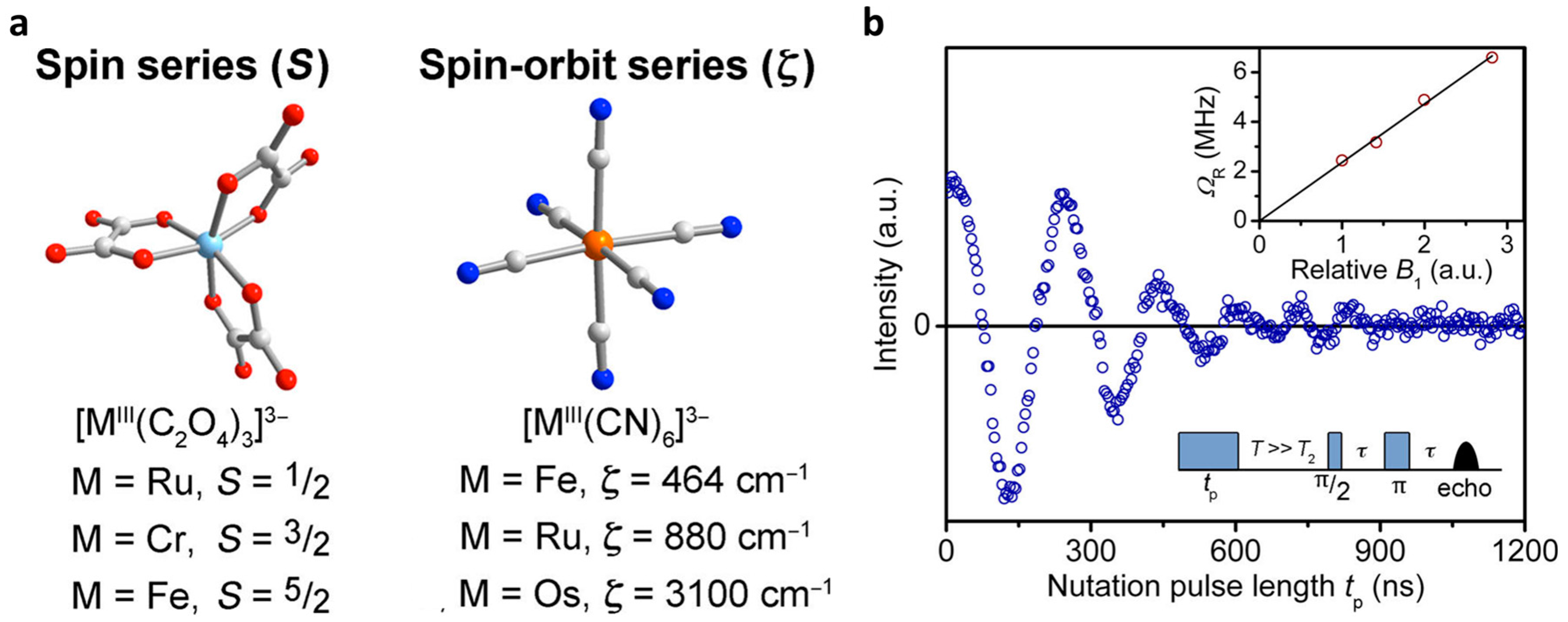
2.3. Other Transition Metals
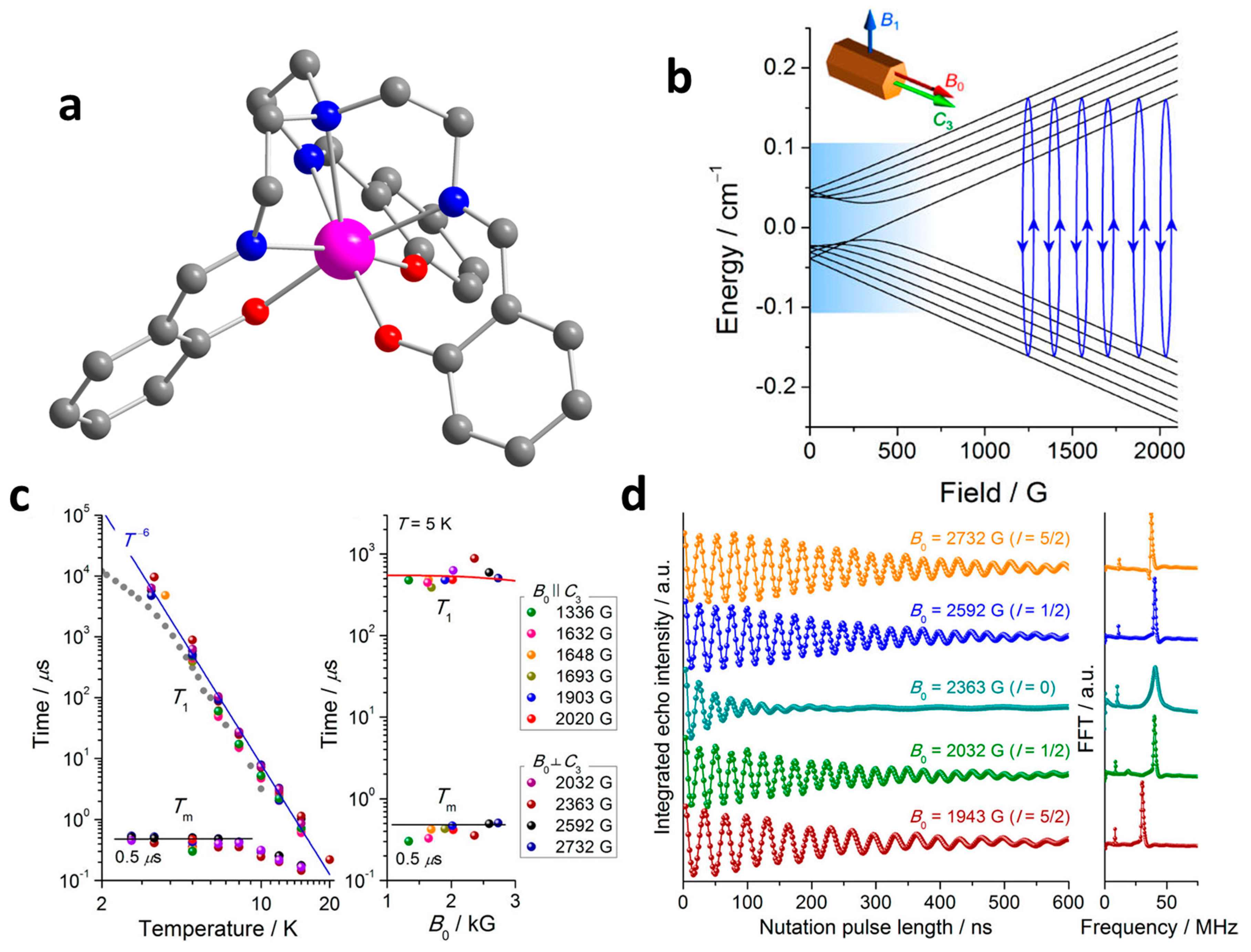
3. Single-Ion Magnetic Molecules with Single 4f Spins
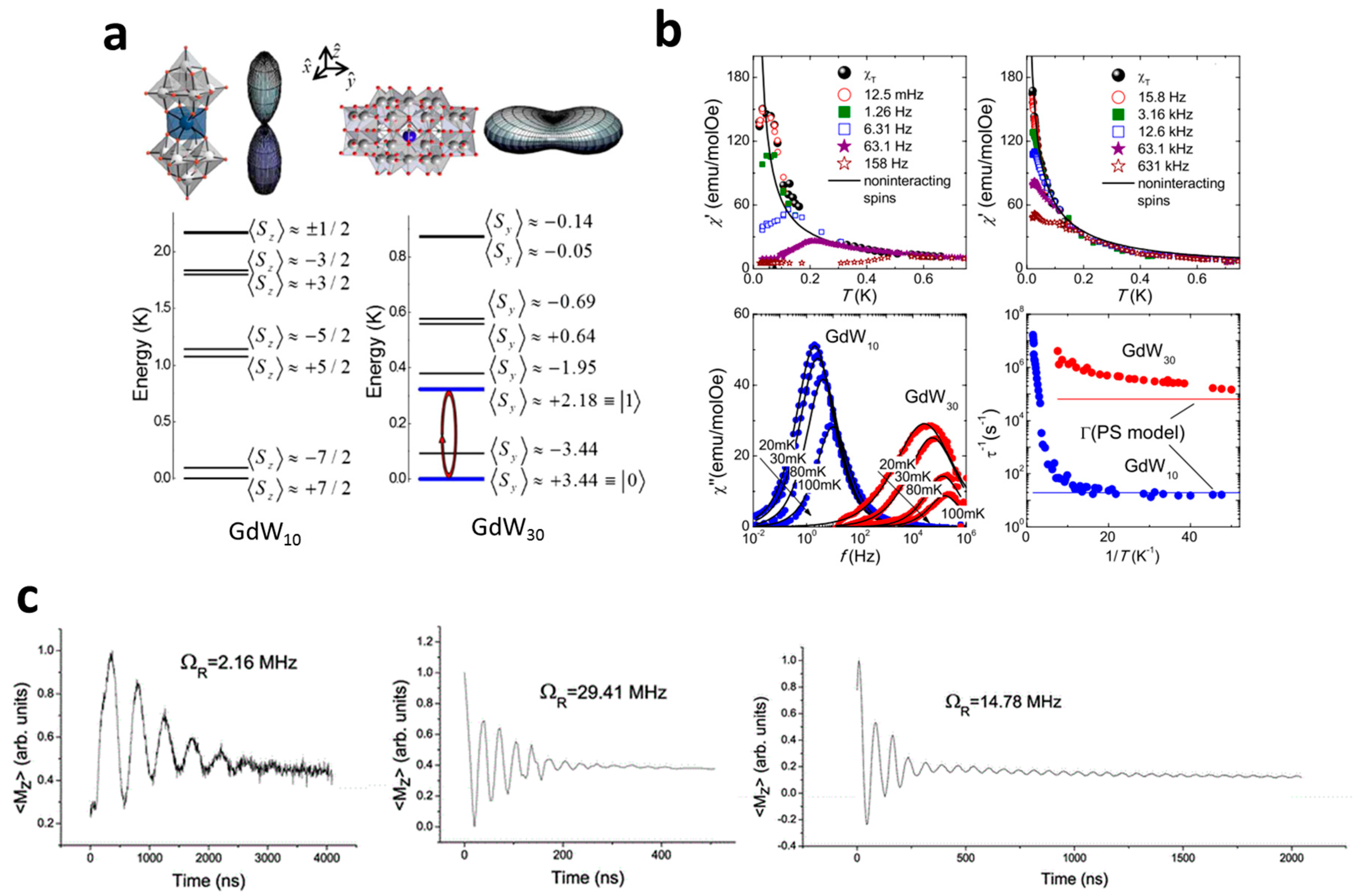
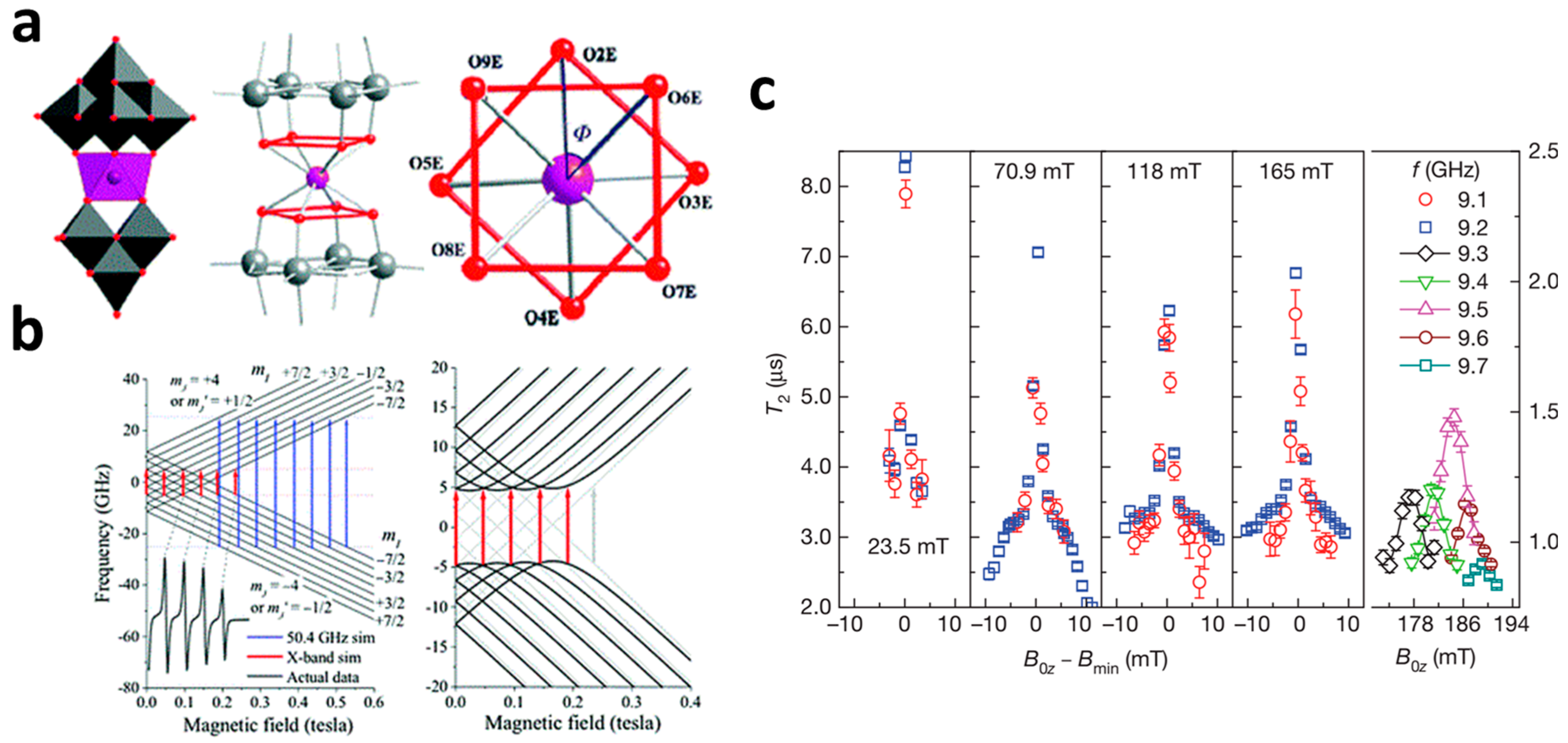
3.1. Single-Lanthanide Polyoxometalates as Spin Qubits
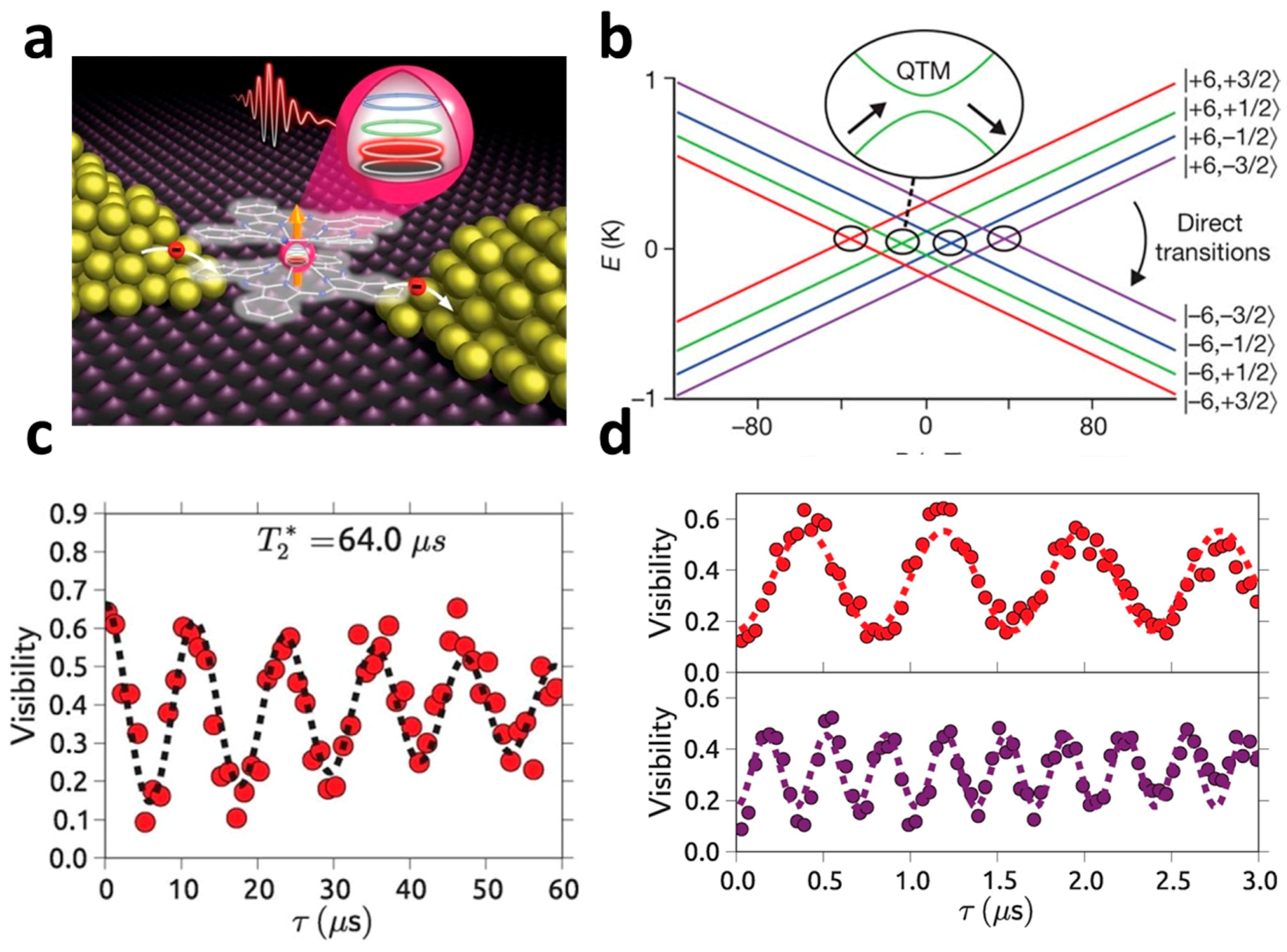
3.2. Tb(III) Based Nuclear-Spin Qubits
3.3. Yb(III) Based Spin Qubits
3.4. Lanthanide-Doped Spin Qubits
4. Conclusions and Perspectives
Acknowledgments
Conflicts of Interest
References
- Ishikawa, N.; Sugita, M.; Ishikawa, T.; Koshihara, S.Y.; Kaizu, Y. Lanthanide double-decker complexes functioning as magnets at the single-molecular level. J. Am. Chem. Soc. 2003, 125, 8694–8695. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, J.D.; Long, J.R. Exploiting single-ion anisotropy in the design of f-element single-molecule magnets. Chem. Sci. 2011, 2, 2078–2085. [Google Scholar] [CrossRef]
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide Single-Molecule Magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef] [PubMed]
- Feltham, H.L.C.; Brooker, S. Review of purely 4f and mixed-metal nd-4f single-molecule magnets containing only one lanthanide ion. Coord. Chem. Rev. 2014, 276, 1–33. [Google Scholar] [CrossRef]
- Craig, G.A.; Murrie, M. 3d single-ion magnets. Chem. Soc. Rev. 2015, 44, 2135–2147. [Google Scholar] [CrossRef] [PubMed]
- Gatteschi, D.; Sessoli, R.; Villain, J. Molecular Nanomagnets; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Benelli, C.; Gatteschi, D. Introduction to Molecular Magnetism: From Transition Metals to Lanthanides; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2015. [Google Scholar]
- Gao, S. Molecular Nanomagnets and Related Phenomena; Springer: New York, NY, USA, 2015; Volume 164. [Google Scholar]
- Layfield, R.A.; Murugesu, M. Lanthanides and Actinides in Molecular Magnetism; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Bennett, C.H.; DiVincenzo, D.P. Quantum information and computation. Nature 2000, 404, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.A.; Chuang, I.L. Quantum Computation and Quantum Information; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Aromi, G.; Aguila, D.; Gamez, P.; Luis, F.; Roubeau, O. Design of magnetic coordination complexes for quantum computing. Chem. Soc. Rev. 2012, 41, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Bertaina, S.; Gambarelli, S.; Tkachuk, A.; Kurkin, I.N.; Malkin, B.; Stepanov, A.; Barbara, B. Rare-earth solid-state qubits. Nat. Nanotechnol. 2007, 2, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Ladd, T.D.; Jelezko, F.; Laflamme, R.; Nakamura, Y.; Monroe, C.; O Brien, J.L. Quantum computers. Nature 2010, 464, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Cirac, J.I.; Zoller, P. Quantum computations with cold trapped ions. Phys. Rev. Lett. 1995, 74, 4091–4094. [Google Scholar] [CrossRef] [PubMed]
- Monroe, C.; Kim, J. Scaling the ion trap quantum processor. Science 2013, 339, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Knill, E.; Laflamme, R.; Milburn, G.J. A scheme for efficient quantum computation with linear optics. Nature 2001, 409, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Gershenfeld, N.A.; Chuang, I.L. Bulk spin-resonance quantum computation. Science 1997, 275, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.; Wilhelm, F.K. Superconducting quantum bits. Nature 2008, 453, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Devoret, M.H.; Schoelkopf, R.J. Superconducting circuits for quantum information: An outlook. Science 2013, 339, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Loss, D.; DiVincenzo, D.P. Quantum computation with quantum dots. Phys. Rev. A 1998, 57, 120. [Google Scholar] [CrossRef]
- Awschalom, D.D.; Bassett, L.C.; Dzurak, A.S.; Hu, E.L.; Petta, J.R. Quantum spintronics: Engineering and manipulating atom-like spins in semiconductors. Science 2013, 339, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Hanson, R.; Awschalom, D.D. Coherent manipulation of single spins in semiconductors. Nature 2008, 453, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Nakazawa, S.; Rahimi, R.; Ise, T.; Nishida, S.; Yoshino, T.; Mori, N.; Toyota, K.; Shiomi, D.; Yakiyama, Y.; et al. Molecular electron-spin quantum computers and quantum information processing: Pulse-based electron magnetic resonance spin technology applied to matter spin-qubits. J. Mater. Chem. 2009, 19, 3739–3754. [Google Scholar] [CrossRef]
- Luis, F.; Repollés, A.; Martínez-Pérez, M.J.; Aguilà, D.; Roubeau, O.; Zueco, D.; Alonso, P.J.; Evangelisti, M.; Camón, A.; Sesé, J. Molecular prototypes for spin-based CNOT and SWAP quantum gates. Phys. Rev. Lett. 2011, 107, 117203. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Gaita-Ari Nmacr, A.; Coronado, E.; Loss, D. Spin qubits with electrically gated polyoxometalate molecules. Nat. Nanotechnol. 2007, 2, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Leuenberger, M.N.; Loss, D. Quantum computing in molecular magnets. Nature 2001, 410, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Ardavan, A.; Rival, O.; Morton, J.J.; Blundell, S.J.; Tyryshkin, A.M.; Timco, G.A.; Winpenny, R.E. Will spin-relaxation times in molecular magnets permit quantum information processing? Phys. Rev. Lett. 2007, 98, 057201. [Google Scholar] [CrossRef] [PubMed]
- Wedge, C.J.; Timco, G.A.; Spielberg, E.T.; George, R.E.; Tuna, F.; Rigby, S.; McInnes, E.; Winpenny, R.; Blundell, S.J.; Ardavan, A. Chemical engineering of molecular qubits. Phys. Rev. Lett. 2012, 108, 107204. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, D.; Webber, A.L.; Wedge, C.J.; Liu, J.; Timco, G.A.; Vitorica-Yrezabal, I.J.; McInnes, E.J.; Winpenny, R.E.; Ardavan, A. Quantum spin coherence in halogen-modified Cr7Ni molecular nanomagnets. Phys. Rev. B 2014, 90, 184419. [Google Scholar] [CrossRef]
- Bader, K.; Winkler, M.; van Slageren, J. Tuning of molecular qubits: Very long coherence and spin-lattice relaxation times. Chem. Commun. 2016, 52, 3623–3626. [Google Scholar] [CrossRef] [PubMed]
- Fielding, A.J.; Fox, S.; Millhauser, G.L.; Chattopadhyay, M.; Kroneck, P.M.; Fritz, G.; Eaton, G.R.; Eaton, S.S. Electron spin relaxation of copper (II) complexes in glassy solution between 10 and 120 K. J. Magn. Reson. 2006, 179, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.J.; Zadrozny, J.M.; Shiddiq, M.; Anderson, J.S.; Fataftah, M.S.; Hill, S.; Freedman, D.E. Influence of Electronic Spin and Spin–Orbit Coupling on Decoherence in Mononuclear Transition Metal Complexes. J. Am. Chem. Soc. 2014, 136, 7623–7626. [Google Scholar] [CrossRef] [PubMed]
- Repollés Rabinad, A.M. Quantum Computing with Molecular Magnets; Prensas de la Universidad de Zaragoza: Zaragoza, Spain, 2016; Volume 131. [Google Scholar]
- Timco, G.A.; Carretta, S.; Troiani, F.; Tuna, F.; Pritchard, R.J.; Muryn, C.A.; McInnes, E.J.; Ghirri, A.; Candini, A.; Santini, P. Engineering the coupling between molecular spin qubits by coordination chemistry. Nat. Nanotechnol. 2009, 4, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Klyatskaya, S.; Ruben, M.; Fuhr, O.; Wernsdorfer, W.; Candini, A.; Corradini, V.; Rizzini, A.L.; Del Pennino, U.; Troiani, F. Magnetic interplay between two different lanthanides in a tris-phthalocyaninato complex: A viable synthetic route and detailed investigation in the bulk and on the surface. J. Mater. Chem. C 2015, 3, 9794–9801. [Google Scholar] [CrossRef]
- Aguilà, D.; Barrios, L.A.; Velasco, V.; Roubeau, O.; Repollés, A.; Alonso, P.J.; Sesé, J.; Teat, S.J.; Luis, F.; Aromí, G. Heterodimetallic [LnLn′] lanthanide complexes: Toward a chemical design of two-qubit molecular spin quantum gates. J. Am. Chem. Soc. 2014, 136, 14215–14222. [Google Scholar] [CrossRef] [PubMed]
- Ferrando-Soria, J.; Pineda, E.M.; Chiesa, A.; Fernandez, A.; Magee, S.A.; Carretta, S.; Santini, P.; Vitorica-Yrezabal, I.J.; Tuna, F.; Timco, G.A. A modular design of molecular qubits to implement universal quantum gates. Nat. Commun. 2016, 7, 11377. [Google Scholar] [CrossRef] [PubMed]
- Vincent, R.; Klyatskaya, S.; Ruben, M.; Wernsdorfer, W.; Balestro, F. Electronic read-out of a single nuclear spin using a molecular spin transistor. Nature 2012, 488, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Thiele, S.; Balestro, F.; Ballou, R.; Klyatskaya, S.; Ruben, M.; Wernsdorfer, W. Electrically driven nuclear spin resonance in single-molecule magnets. Science 2014, 344, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Zadrozny, J.M.; Niklas, J.; Poluektov, O.G.; Freedman, D.E. Multiple Quantum Coherences from Hyperfine Transitions in a Vanadium(IV) Complex. J. Am. Chem. Soc. 2014, 136, 15841–15844. [Google Scholar] [CrossRef] [PubMed]
- Zadrozny, J.M.; Niklas, J.; Poluektov, O.G.; Freedman, D.E. Millisecond Coherence Time in a Tunable Molecular Electronic Spin Qubit. ACS Cent. Sci. 2015, 1, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Stanwix, P.L.; Pham, L.M.; Maze, J.R.; Le Sage, D.; Yeung, T.K.; Cappellaro, P.; Hemmer, P.R.; Yacoby, A.; Lukin, M.D.; Walsworth, R.L. Coherence of nitrogen-vacancy electronic spin ensembles in diamond. Phys. Rev. B 2010, 82, 201201. [Google Scholar] [CrossRef]
- Koehl, W.F.; Buckley, B.B.; Heremans, F.J.; Calusine, G.; Awschalom, D.D. Room temperature coherent control of defect spin qubits in silicon carbide. Nature 2011, 479, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Tesi, L.; Lucaccini, E.; Cimatti, I.; Perfetti, M.; Mannini, M.; Atzori, M.; Morra, E.; Chiesa, M.; Caneschi, A.; Sorace, L. Quantum coherence in a processable vanadyl complex: New tools for the search of molecular spin qubits. Chem. Sci. 2016, 7, 2074–2083. [Google Scholar] [CrossRef]
- Tesi, L.; Lunghi, A.; Atzori, M.; Lucaccini, E.; Sorace, L.; Totti, F.; Sessoli, R. Giant spin-phonon bottleneck effects in evaporable vanadyl-based molecules with long spin coherence. Dalton. Trans. 2016, 45, 16635–16643. [Google Scholar] [CrossRef] [PubMed]
- Atzori, M.; Morra, E.; Tesi, L.; Albino, A.; Chiesa, M.; Sorace, L.; Sessoli, R. Quantum Coherence Times Enhancement in Vanadium (IV)-based Potential Molecular Qubits: The Key Role of the Vanadyl Moiety. J. Am. Chem. Soc. 2016, 138, 11234–11244. [Google Scholar] [CrossRef] [PubMed]
- Atzori, M.; Tesi, L.; Morra, E.; Chiesa, M.; Sorace, L.; Sessoli, R. Room-Temperature Quantum Coherence and Rabi Oscillations in Vanadyl Phthalocyanine: Toward Multifunctional Molecular Spin Qubits. J. Am. Chem. Soc. 2016, 138, 2154–2157. [Google Scholar] [CrossRef] [PubMed]
- Warner, M.; Din, S.; Tupitsyn, I.S.; Morley, G.W.; Stoneham, A.M.; Gardener, J.A.; Wu, Z.; Fisher, A.J.; Heutz, S.; Kay, C.W. Potential for spin-based information processing in a thin-film molecular semiconductor. Nature 2013, 503, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Bader, K.; Dengler, D.; Lenz, S.; Endeward, B.; Jiang, S.; Neugebauer, P.; van Slageren, J. Room temperature quantum coherence in a potential molecular qubit. Nat. Commun. 2014, 5, 6304. [Google Scholar] [CrossRef] [PubMed]
- Fataftah, M.S.; Zadrozny, J.M.; Coste, S.C.; Graham, M.J.; Rogers, D.M.; Freedman, D.E. Employing Forbidden Transitions as Qubits in a Nuclear Spin-Free Chromium Complex. J. Am. Chem. Soc. 2016, 138, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Mor, H.H.; Weihe, H.; Bendix, J. Fitting of EPR spectra: The importance of a flexible bandwidth. J. Magn. Reson. 2010, 207, 283–286. [Google Scholar]
- Stamp, P.C.; Gaita-Arino, A. Spin-based quantum computers made by chemistry: Hows and whys. J. Mater. Chem. 2009, 19, 1718–1730. [Google Scholar] [CrossRef]
- Morello, A.; Stamp, P.; Tupitsyn, I.S. Pairwise decoherence in coupled spin qubit networks. Phys. Rev. Lett. 2006, 97, 207206. [Google Scholar] [CrossRef] [PubMed]
- Bertaina, S.; Gambarelli, S.; Mitra, T.; Tsukerblat, B.; Müller, A.; Barbara, B. Quantum oscillations in a molecular magnet. Nature 2008, 453, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Bendix, J.; Brorson, M.; Schaffer, C.E. Accurate empirical spin-orbit coupling parameters. zeta. nd for gaseous ndq transition metal ions. The parametrical multiplet term model. Inorg. Chem. 1993, 32, 2838–2849. [Google Scholar] [CrossRef]
- Bendix, J.; Steenberg, P.; Søtofte, I. Isolation and molecular structure of hexacyanoruthenate (III). Inorg. Chem. 2003, 42, 4510–4512. [Google Scholar] [CrossRef] [PubMed]
- Albores, P.; Slep, L.D.; Baraldo, L.M.; Baggio, R.; Garland, M.T.; Rentschler, E. Crystal structure and electronic and magnetic properties of hexacyanoosmate (III). Inorg. Chem. 2006, 45, 2361–2363. [Google Scholar] [CrossRef] [PubMed]
- Zadrozny, J.M.; Freedman, D.E. Qubit Control Limited by Spin–Lattice Relaxation in a Nuclear Spin-Free Iron (III) Complex. Inorg. Chem. 2015, 54, 12027–12031. [Google Scholar] [CrossRef] [PubMed]
- Zadrozny, J.M.; Graham, M.J.; Krzyaniak, M.D.; Wasielewski, M.R.; Freedman, D.E. Unexpected suppression of spin-lattice relaxation via high magnetic field in a high-spin iron (III) complex. Chem. Commun. 2016, 52, 10175–10178. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Eaton, G.R.; Eaton, S.S. Electron spin relaxation in vanadyl, copper (II), and silver (II) porphyrins in glassy solvents and doped solids. J. Magn. Reson. Ser. A 1996, 119, 240–246. [Google Scholar] [CrossRef]
- Gupta, S.K.; Rajeshkumar, T.; Rajaraman, G.; Murugavel, R. An air-stable Dy (iii) single-ion magnet with high anisotropy barrier and blocking temperature. Chem. Sci. 2016, 7, 5181–5191. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Jia, J.; Liu, J.; Vieru, V.; Ungur, L.; Chibotaru, L.F.; Lan, Y.; Wernsdorfer, W.; Gao, S. A Stable Pentagonal-Bipyramidal Dy (III) Single-Ion Magnet with a Record Magnetization Reversal Barrier over 1000 K. J. Am. Chem. Soc. 2016, 138, 5441–5450. [Google Scholar] [CrossRef] [PubMed]
- Stepanow, S.; Honolka, J.; Gambardella, P.; Vitali, L.; Abdurakhmanova, N.; Tseng, T.; Rauschenbach, S.; Tait, S.L.; Sessi, V.; Klyatskaya, S. Spin and orbital magnetic moment anisotropies of monodispersed bis (phthalocyaninato) terbium on a copper surface. J. Am. Chem. Soc. 2010, 132, 11900–11901. [Google Scholar] [CrossRef] [PubMed]
- Margheriti, L.; Chiappe, D.; Mannini, M.; Car, P.E.; Sainctavit, P.; Arrio, M.A.; de Mongeot, F.B.; Cezar, J.C.; Piras, F.M.; Magnani, A. X-Ray Detected Magnetic Hysteresis of Thermally Evaporated Terbium Double-Decker Oriented Films. Adv. Mater. 2010, 22, 5488–5493. [Google Scholar] [CrossRef] [PubMed]
- Gonidec, M.; Biagi, R.; Corradini, V.; Moro, F.; De Renzi, V.; Del Pennino, U.; Summa, D.; Muccioli, L.; Zannoni, C.; Amabilino, D.B. Surface supramolecular organization of a terbium (III) double-decker complex on graphite and its single molecule magnet behavior. J. Am. Chem. Soc. 2011, 133, 6603–6612. [Google Scholar] [CrossRef] [PubMed]
- Mannini, M.; Bertani, F.; Tudisco, C.; Malavolti, L.; Poggini, L.; Misztal, K.; Menozzi, D.; Motta, A.; Otero, E.; Ohresser, P. Magnetic behaviour of TbPc2 single-molecule magnets chemically grafted on silicon surface. Nat. Commun. 2014, 5, 5582. [Google Scholar] [CrossRef] [PubMed]
- Gaita-Ariño, A.; Prima-Garcia, H.; Cardona-Serra, S.; Escalera-Moreno, L.; Rosaleny, L.E.; Baldovi, J.J. Coherence and organisation in lanthanoid complexes: From single ion magnets to spin qubits. Inorg. Chem. Front. 2016, 3, 568–577. [Google Scholar] [CrossRef]
- Clemente-Juan, J.M.; Coronado, E.; Gaita-Ariño, A. Magnetic polyoxometalates: From molecular magnetism to molecular spintronics and quantum computing. Chem. Soc. Rev. 2012, 41, 7464–7478. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pérez, M.J.; Cardona-Serra, S.; Schlegel, C.; Moro, F.; Alonso, P.J.; Prima-García, H.; Clemente-Juan, J.M.; Evangelisti, M.; Gaita-Ariño, A.; Sesé, J. Gd-based single-ion magnets with tunable magnetic anisotropy: Molecular design of spin qubits. Phys. Rev. Lett. 2012, 108, 247213. [Google Scholar] [CrossRef] [PubMed]
- Baldovi, J.J.; Cardona-Serra, S.; Clemente-Juan, J.M.; Coronado, E.; Gaita-Ariño, A.; Prima-Garcia, H. Coherent manipulation of spin qubits based on polyoxometalates: The case of the single ion magnet [GdW30P5O110]14−. Chem. Commun. 2013, 49, 8922–8924. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Datta, S.; Friend, L.; Cardona-Serra, S.; Gaita-Ariño, A.; Coronado, E.; Hill, S. Multi-frequency EPR studies of a mononuclear holmium single-molecule magnet based on the polyoxometalate [HoIII(W5O18)2]9−. Dalton. Trans. 2012, 41, 13697–13704. [Google Scholar] [CrossRef] [PubMed]
- Shiddiq, M.; Komijani, D.; Duan, Y.; Gaita-Ariño, A.; Coronado, E.; Hill, S. Enhancing coherence in molecular spin qubits via atomic clock transitions. Nature 2016, 531, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Urdampilleta, M.; Klayatskaya, S.; Ruben, M.; Wernsdorfer, W. Magnetic Interaction between a Radical Spin and a Single-Molecule Magnet in a Molecular Spin-Valve. ACS Nano 2015, 9, 4458–4464. [Google Scholar] [CrossRef] [PubMed]
- Urdampilleta, M.; Klyatskaya, S.; Cleuziou, J.; Ruben, M.; Wernsdorfer, W. Supramolecular spin valves. Nat. Mater. 2011, 10, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.S.; Ariciu, A.; McAdams, S.; Weihe, H.; Bendix, J.; Tuna, F.; Piligkos, S. Toward Molecular 4f Single-Ion Magnet Qubits. J. Am. Chem. Soc. 2016, 138, 5801–5804. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, R.J.; Ungur, L.; Korobkov, I.; Chibotaru, L.F.; Murugesu, M. Observation of unusual slow-relaxation of the magnetisation in a Gd-EDTA chelate. Dalton. Trans. 2015, 44, 20321–20325. [Google Scholar] [CrossRef] [PubMed]
- Ganivet, C.R.; Ballesteros, B.; de la Torre, G.; Clemente Juan, J.M.; Coronado, E.; Torres, T. Influence of Peripheral Substitution on the Magnetic Behavior of Single-Ion Magnets Based on Homo-and Heteroleptic TbIII Bis (phthalocyaninate). Chem. Eur. J. 2013, 19, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, N.; Sugita, M.; Wernsdorfer, W. Quantum Tunneling of Magnetization in Lanthanide Single-Molecule Magnets: Bis (phthalocyaninato) terbium and Bis (phthalocyaninato) dysprosium Anions. Angew. Chem. Int. Ed. 2005, 44, 2931–2935. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.S.; Dreiser, J.; Weihe, H.; Sibille, R.; Johannesen, H.V.; Sørensen, M.A.; Nielsen, B.E.; Sigrist, M.; Mutka, H.; Rols, S. Design of single-molecule magnets: Insufficiency of the anisotropy barrier as the sole criterion. Inorg. Chem. 2015, 54, 7600–7606. [Google Scholar] [CrossRef] [PubMed]
- Bertaina, S.; Shim, J.H.; Gambarelli, S.; Malkin, B.Z.; Barbara, B. Spin-orbit qubits of rare-earth-metal ions in axially symmetric crystal fields. Phys. Rev. Lett. 2009, 103. [Google Scholar] [CrossRef] [PubMed]
- Fraval, E.; Sellars, M.J.; Longdell, J.J. Method of Extending Hyperfine Coherence Times in Pr3+: Y2SiO5. Phys. Rev. Lett. 2004, 92, 77601. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, N.; Mohan, R.K.; Kröll, S. Quantum computer hardware based on rare-earth-ion-doped inorganic crystals. Opt. Commun. 2002, 201, 71–77. [Google Scholar] [CrossRef]
- Longdell, J.J.; Sellars, M.J. Experimental demonstration of quantum-state tomography and qubit-qubit interactions for rare-earth-metal-ion-based solid-state qubits. Phys. Rev. A 2004, 69, 032307. [Google Scholar] [CrossRef]
- Rippe, L.; Nilsson, M.; Kröll, S.; Klieber, R.; Suter, D. Experimental demonstration of efficient and selective population transfer and qubit distillation in a rare-earth-metal-ion-doped crystal. Phys. Rev. A 2005, 71, 062328. [Google Scholar] [CrossRef]
- Fraval, E.; Sellars, M.J.; Longdell, J.J. Dynamic decoherence control of a solid-state nuclear-quadrupole qubit. Phys. Rev. Lett. 2005, 95, 030506. [Google Scholar] [CrossRef] [PubMed]
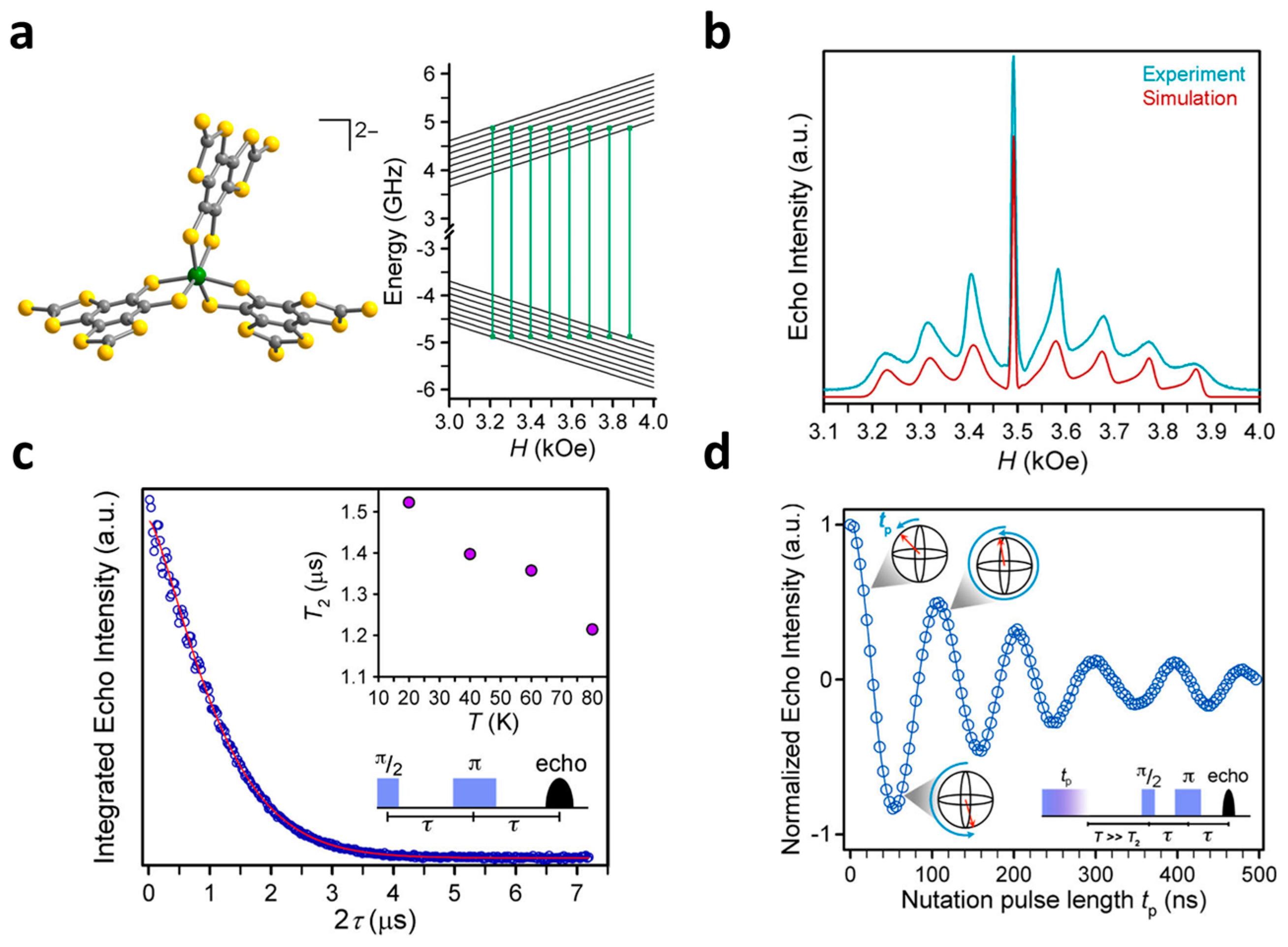
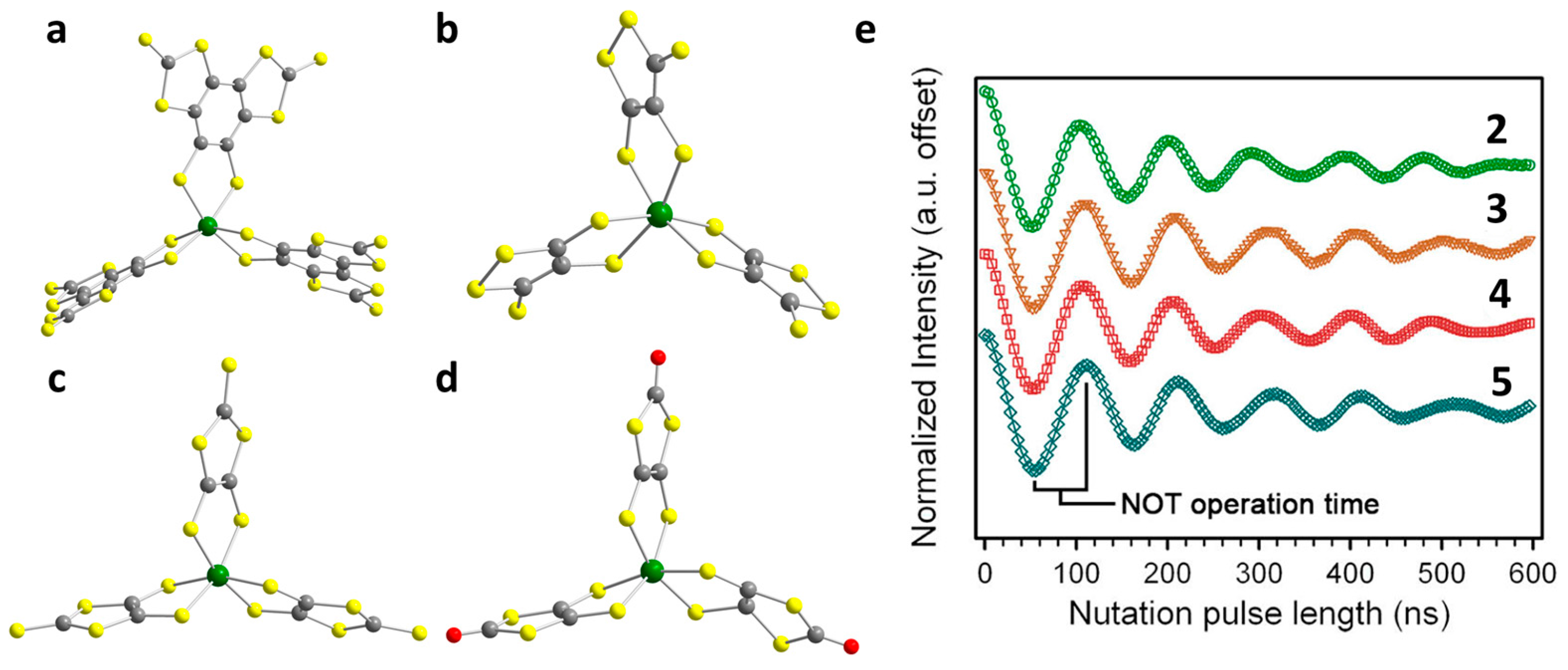

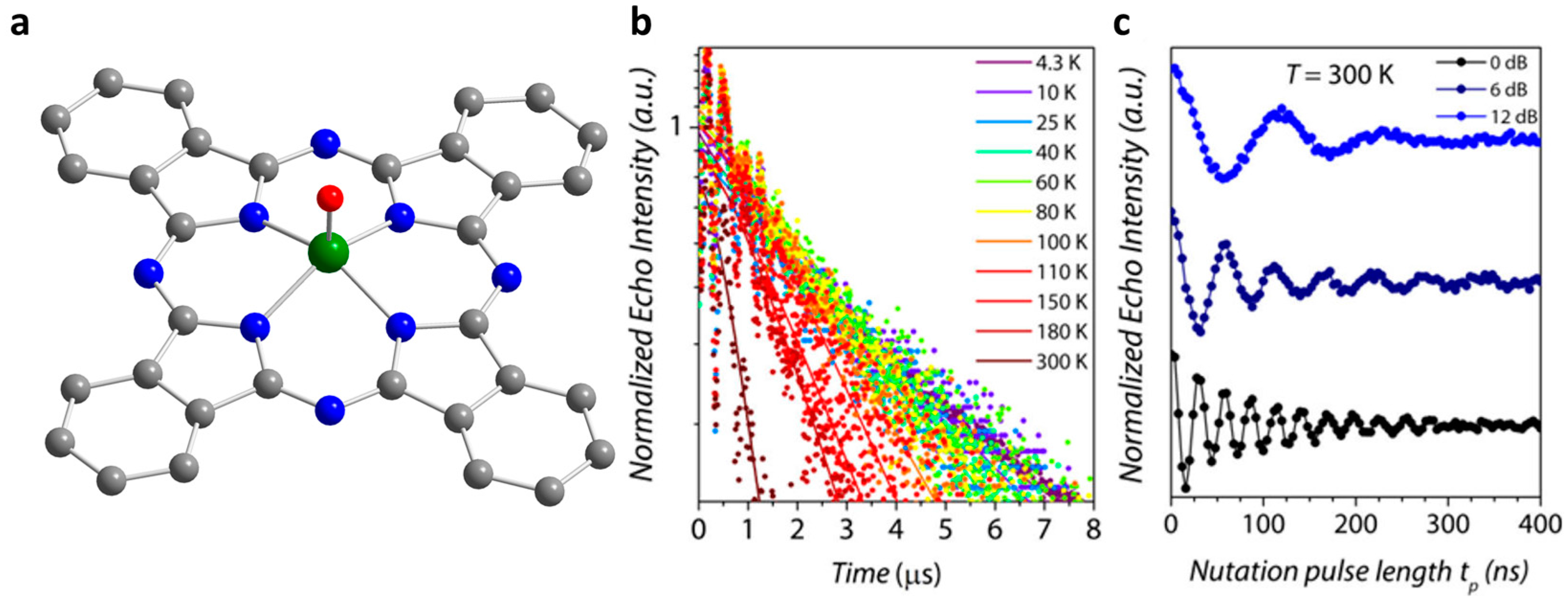
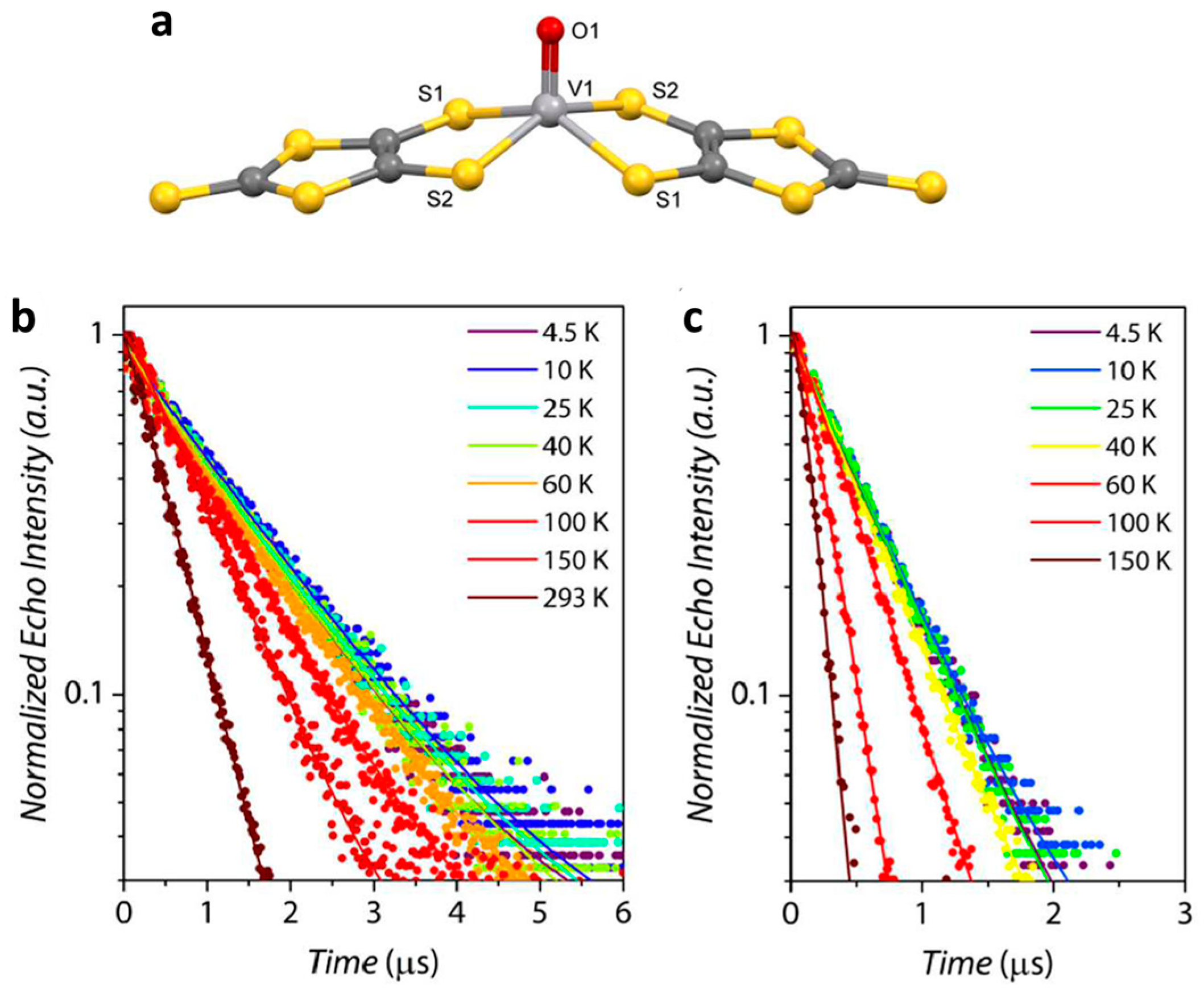

| Complex | T1/μs | T2/μs a | T2/μs b | TR/K c | Ref. |
|---|---|---|---|---|---|
| (Bu4N)2[V(C8S8)3] (1) | - | 1.2 (80 K) | 1.52 (20 K) | 20 | [41] |
| (Ph4P)2[V(C8S8)3] (2) | 4.90 (120 K) | 1.18 (120 K) | 675(7) (10 K) | 20 | [42] |
| (Ph4P)2[V(β-C3S5)3] (3) | 2.24 (120 K) | 0.86 (120 K) | 6.13 (10 K) | 20 | [42] |
| (Ph4P)2[V(α-C3S5)3] (4) | 0.71 (150 K) | 0.19 (150 K) | 6.01 (10 K) | 60 | [42,47] |
| (Ph4P)2[V(C3S4O)3] (5) | 3.16 (120 K) | 0.96 (120 K) | 2.79 (10 K) | 20 | [42] |
| VO(dpm)2 (6) | 6 (110 K) | 0.1 (220 K) | 2.5 (10 K) | 4.3 | [45] |
| VO(acac)2 (7) | ≈102 (40 K) | - | - | - | [46] |
| VO(dbm)2 (8) | ≈102 (40 K) | - | - | - | [46] |
| VOPc (9) | 2.4 × 106 (7 K) | 22 (10 K) | - | - | [31] |
| VOPc (9) | 1.1 (300 K) | 0.83 (300 K) | - | 300 | [48] |
| [(Ph)4P]2[VO(α-C3S5)2] (10) | 3.37 (293 K) | 1.04 (293 K) | - | 293 | [47] |
| Complex | T1/μs | T2/μs a | T2/μs b | TR/K c | Ref. |
|---|---|---|---|---|---|
| CuPc (11) | 105 (7 K) | 1.0 (80 K) | 41 (7 K) | 5 | [31,49] |
| CuPcCl (12) | 105 (7 K) | 40 (7 K) | - | - | [31] |
| CuPcF (13) | 6 × 104 (7 K) | 40 (7 K) | - | - | [31] |
| (PPh4)2[Cu(mnt)2] (14) | 0.48 (294 K) | 0.6 (294 K) | 68 (7 K) | 15 | [50] |
| Complex | T1/μs | T2/μs a | T2/μs b | TR/K c | Ref. |
|---|---|---|---|---|---|
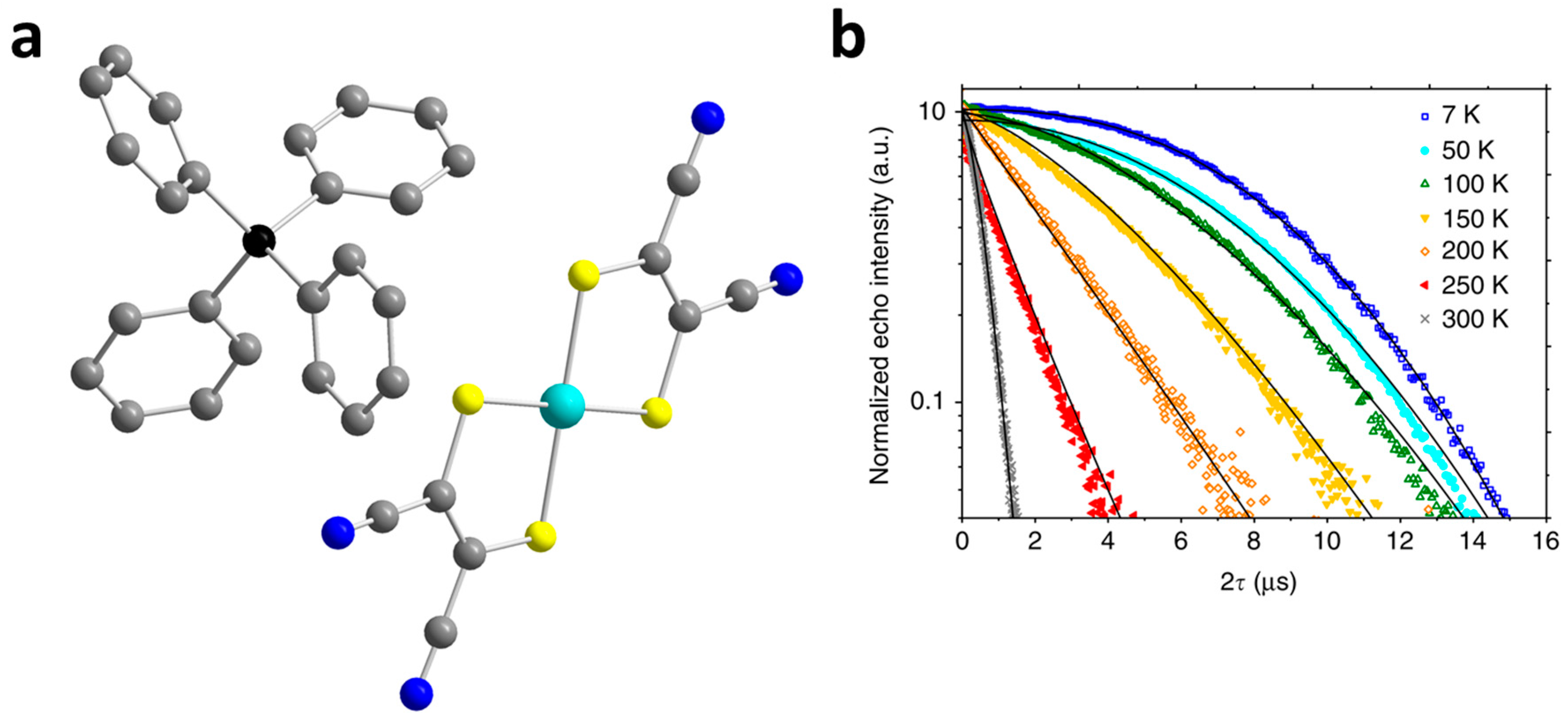
| (Ph4P)3[Cr(C3S5)3] (15) | 875 (30 K) | 0.82 (30 K) | 1.81 (5 K) | 5 K | [51] |
| K3[Cr(C2O4)3] (16) | - | 1.27 (22 K) | 2.79 (5 K) | - | [33] |
| K3[Fe(C2O4)3] (17) | - | 0.45 (22 K) | 1.83 (5 K) | - | [33] |
| (Ph4P)3[Fe(CN)6] (18) | - | 0.60 (22 K) | 2.38 (5 K) | - | [33] |
| K3[Ru(C2O4)3] (19) | - | 0.41 (22 K) | 3.44 (5 K) | 5 | [33] |
| (Ph4P)3[Ru(CN)6] (20) | - | 1.29 (22 K) | 2.55 (5 K) | - | [33] |
| (Ph4P)3[Os(CN)6] (21) | - | 1.04 (22 K) | 4.12 (5 K) | - | [33] |
| (Ph4P)3[Fe(C5O5)3] (22) | 1.18 (15K) | 0.498 (40 K) | 1.63 (5 K) | 10 | [59] |
| MnPc (23) | 690 (7 K) | 14 (7 K) | - | - | [31] |
| CoPc (24) | 1.11 × 104 (7 K) | 9.44 (7 K) | - | - | [31] |
| Complex | T1/μs | T2/μs a | T2/μs b | TR/K c | Ref |
|---|---|---|---|---|---|
| [Gd(P5W30O110)] K14nH2O (25) | 1.6 (6 K) | 0.41 (6 K) | - | 5 | [70,71] |
| [Ho(W5O18P)2]9− (26) | 20 (5 K) | - | 8.4 (5 K) | - | [73] |
| TbPc2 (27) | - | - | 64 (40 mK) | 0.04 | [40] |
| Yb(trensal)3 (28) | 0.2 (20 K) | 0.17 (15 K) | 0.53 (2.7 K) | 5 | [76] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.-S.; Deng, Y.-F.; Zheng, Y.-Z. The Rise of Single-Ion Magnets as Spin Qubits. Magnetochemistry 2016, 2, 40. https://doi.org/10.3390/magnetochemistry2040040
Ding Y-S, Deng Y-F, Zheng Y-Z. The Rise of Single-Ion Magnets as Spin Qubits. Magnetochemistry. 2016; 2(4):40. https://doi.org/10.3390/magnetochemistry2040040
Chicago/Turabian StyleDing, You-Song, Yi-Fei Deng, and Yan-Zhen Zheng. 2016. "The Rise of Single-Ion Magnets as Spin Qubits" Magnetochemistry 2, no. 4: 40. https://doi.org/10.3390/magnetochemistry2040040
APA StyleDing, Y.-S., Deng, Y.-F., & Zheng, Y.-Z. (2016). The Rise of Single-Ion Magnets as Spin Qubits. Magnetochemistry, 2(4), 40. https://doi.org/10.3390/magnetochemistry2040040






