Abrupt Spin Transition and Chiral Hydrogen-Bonded One-Dimensional Structure of Iron(III) Complex [FeIII(Him)2(hapen)]SbF6 (Him = imidazole, H2hapen = N,N′-bis(2-hydroxyacetophenylidene)ethylenediamine)
Abstract
:1. Introduction
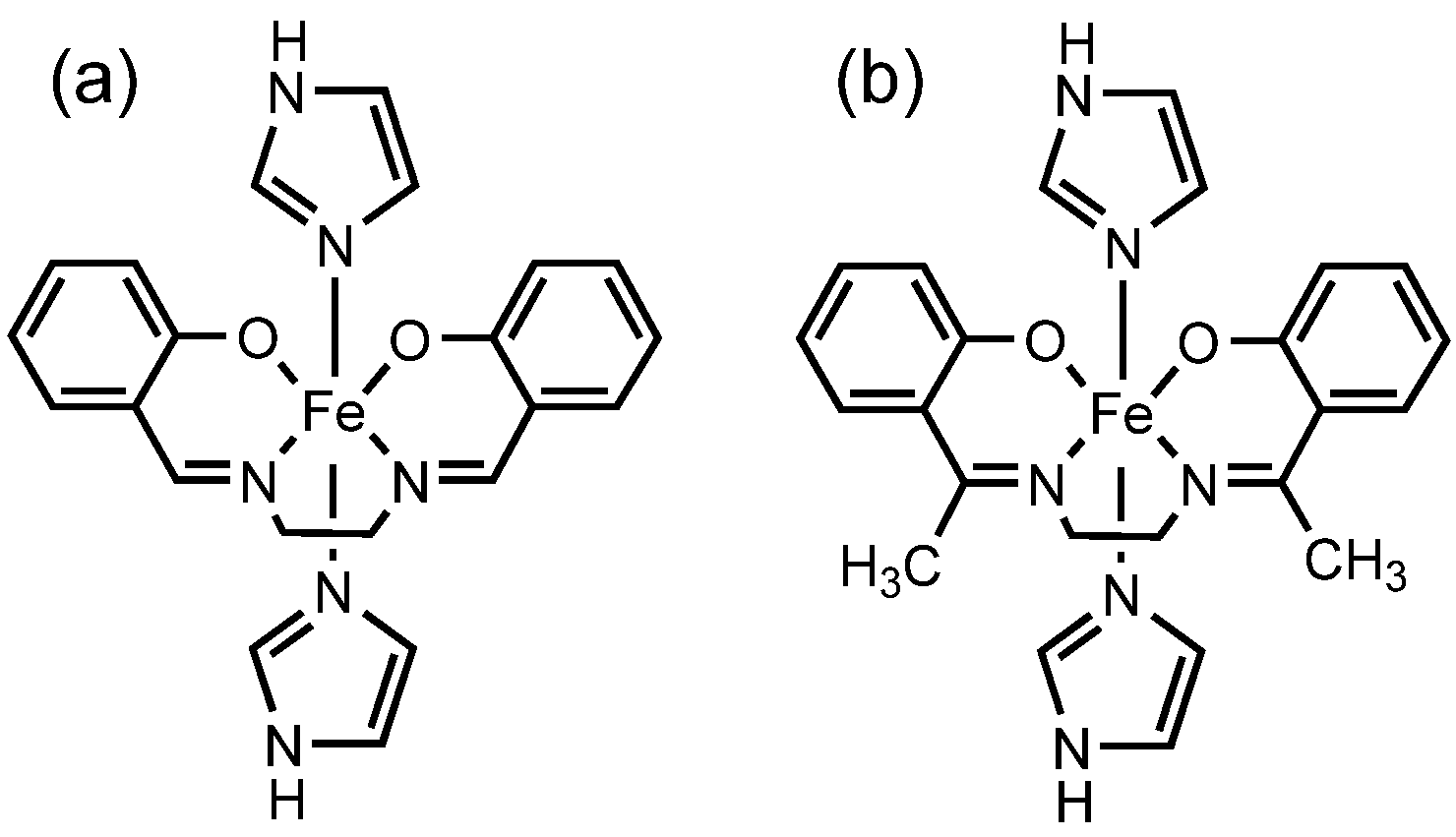
2. Results and Discussion
2.1. Synthesis and Characterization
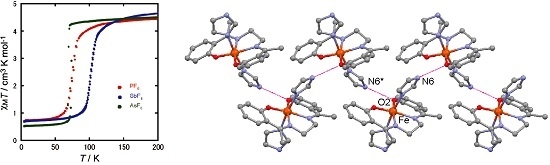
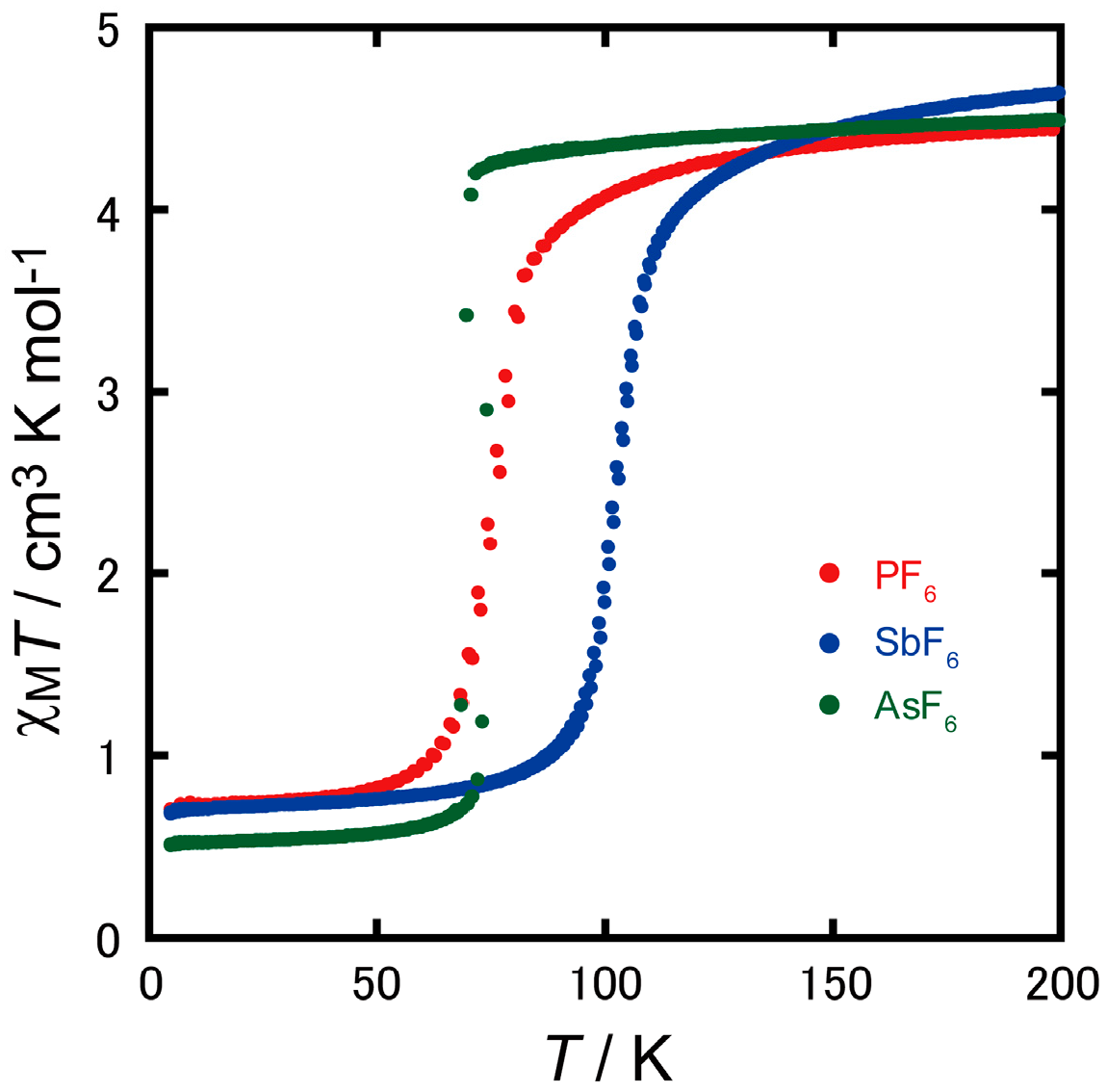
2.2. Magnetic Properties
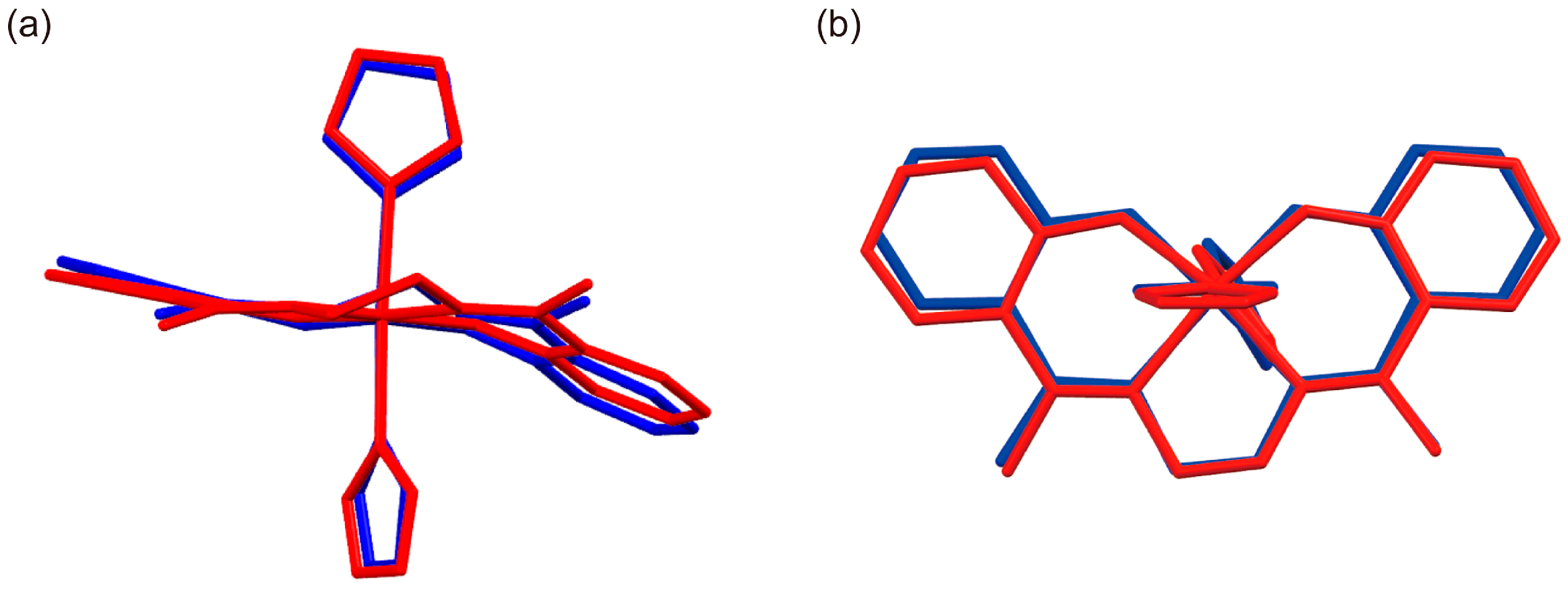
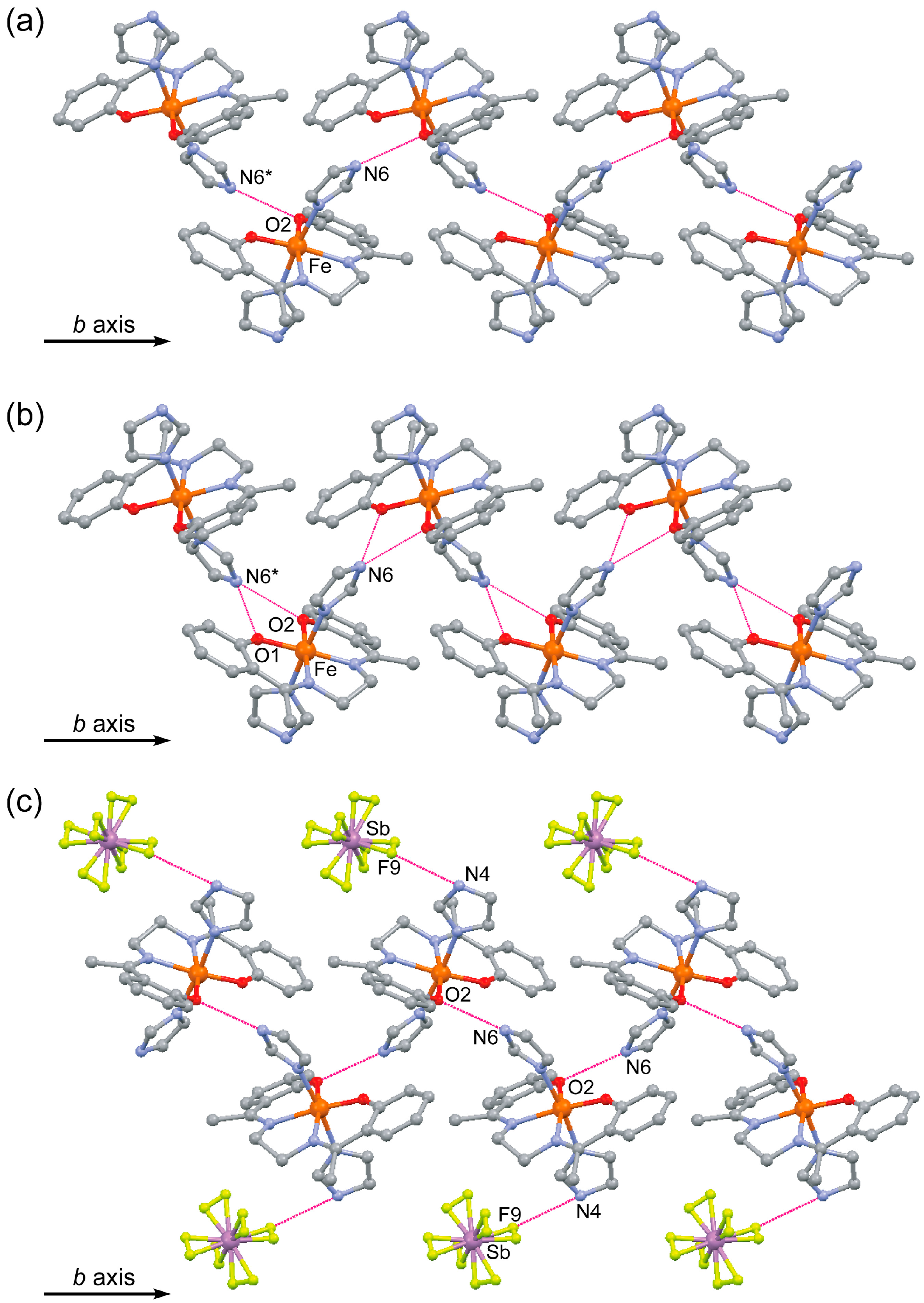
2.3. Crystal Structure of [FeIII(Him)2(hapen)]SbF6 at the HS and LS States
| Formula | C24H26N6O2FeSbF6 | ||
|---|---|---|---|
| formula weight | 722.10 | ||
| crystal system | monoclinic | ||
| space group | C2/c (No.15) | ||
| T, K | 296 | 100 | Δ, % |
| a, Å | 24.915(2) | 24.669(2) | 0.246, 0.98% |
| b, Å | 9.6553(7) | 9.2793(6) | 0.376, 3.9% |
| c, Å | 25.136(2) | 24.704(2) | 0.432, 1.7% |
| β, deg. | 112.256(2) | 109.991(2) | 2.266 |
| V, Å3 | 5596.5(7) | 5314.3(6) | 282.2, 5.0% |
| Z | 8 | 8 | - |
| Dcalcd, g cm−3 | 1.771 | 1.805 | - |
| μ, mm−1 | 1.558 | 1.637 | - |
| Ra, Rwb | 0.0992, 0.2712 | 0.0742, 0.1781 | - |
| Distance and angle | 296 K | 100 K | Δ (Å, °) |
|---|---|---|---|
| Bond lengths (Å) | |||
| Fe–N1 | 2.117(10) | 1.935(7) | −0.182 |
| Fe–N2 | 2.116(8) | 1.944(6) | −0.172 |
| Fe–N3 | 2.146(8) | 1.986(6) | −0.160 |
| Fe–N5 | 2.150(9) | 2.005(6) | −0.145 |
| Average <Fe–N> | 2.132 | 1.968 | −0.164 |
| Fe–O1 | 1.882(7) | 1.879(5) | −0.007 |
| Fe–O2 | 1.908(10) | 1.879(6) | −0.029 |
| Average <Fe–O> | 1.895 | 1.879 | −0.016 |
| Bond angles (°) | |||
| O1–Fe–O2 | 102.5(4) | 89.1(3) | −13.4 |
| O1–Fe–N1 | 88.0(4) | 92.3(3) | +4.3 |
| O2–Fe–N2 | 89.1(4) | 92.6(3) | +3.5 |
| N1–Fe–N2 | 80.5(4) | 86.1(3) | +5.6 |
| N1–Fe–N3 | 88.8(4) | 91.5(3) | +2.7 |
| N1–Fe–N5 | 89.1(4) | 91.0(3) | +1.9 |
| N2–Fe–N3 | 88.9(4) | 89.3(3) | −0.4 |
| N2–Fe–N5 | 85.5(4) | 88.3(3) | +2.8 |
| Hydrogen bond lengths (Å) | |||
| N6H∙∙∙O2 * | 2.95(1) | 2.902(8) | - |
| N6H∙∙∙O1 * | 3.28(2) | 3.03(3) | - |
| F3∙∙∙N4 | 3.10(3) | 3.104(14) | - |
| F9∙∙∙N4 | 3.00(2) | 2.891(14) | - |
| F9∙∙∙N4 * | 3.34(3) | 3.341(18) | - |

2.4. Spin Transition Profile and Temperature
3. Experimental Section
3.1. Synthesis of H2hapen and Precursor Iron(III) Complex [FeIIICl(hapen)]·0.5CH3OH
3.2. [FeIII(Him)2(hapen)]SbF6
3.3. Physical Measurements
3.4. X-Ray Crystallography
4. Conclusions
Author Contributions
Conflicts of Interest
References and Notes
- Gütlich, P.; Goodwin, H.A. “Spin Crossover in Transition Metal Compounds I–III”: Topics in Current Chemistry; Springer: New York, NY, USA, 2004. [Google Scholar]
- Weber, B.; Bauer, W.; Obel, J. An Iron (II) Spin-Crossover Complex with a 70 K Wide Thermal Hysteresis Loop. Angew. Chem. Int. Ed. 2008, 47, 10098–10101. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Hagiwara, H.; Torigoe, H.; Matsumoto, N.; Kojima, K.; Dahan, F.; Tuchagues, J.P.; Re, N.; Iijima, S. A Variety of Spin-Crossover Behaviors Depending on the Counter Anion: Two-Dimensional Complexes Constructed by NH Cl− Hydrogen Bonds, [FeIIH3LMe]Cl·X (X = PF6−, AsF6−, SbF6−, CF3SO3−; H3LMe = Tris[2-{[(2-methylimidazol-4-yl)methylidene]-amino}ethyl]amine). Chem. Eur. J. 2006, 12, 4536–4549. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Nishi, K.; Iijima, S.; Kojima, M.; Matsumoto, N. One-Step and Two-Step Spin-Crossover Iron(II) Complexes of ((2-Methylimidazol-4-yl)methylidene)histamine. Inorg. Chem. 2009, 48, 7211–7229. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Oshio, S.; Kida, S. Investigation on crossover complexes. Magnetic properties of iron(III) complexes with several Schiff bases. Chem. Lett. 1975, 79–80. [Google Scholar]
- Nishida, Y.; Oshio, S.; Kida, S. Synthesis and magnetic properties of iron (III) complexes with several quadridentate Schiff bases. Bul. Chem. Soc. Jpn. 1977, 50, 119–122. [Google Scholar] [CrossRef]
- Matsumoto, N.; Kimoto, K.; Nishida, K.; Ohyoshi, A. Spin-State Equilibrium of Iron(III) Complex with Planar Unsymmertical Quadridentate Schiff Base in Solution. Chem. Lett. 1984, 479–480. [Google Scholar] [CrossRef]
- Matsumoto, N.; Kimoto, K.; Ohyoshi, A.; Maeda, Y.; Okawa, H. Synthesis and characterization of iron(III) complexes with unsymmetrical quadridentate Schiff bases, and spin equilibrium behavior in solution. Bull. Chem. Soc. Jpn. 1984, 57, 3307–3311. [Google Scholar] [CrossRef]
- Maeda, Y.; Takashima, Y.; Matsumoto, N.; Ohyoshi, A. Spectroscopic and Magnetic Properties of [FeL2(salacen)]PF6 (L = Imidazole or N-Methylimidazole): New Examples of Intermediate Electronic Relaxation between S=1/2 and S=5/2 States. X-Ray Crystal Structure of [Fe(Him)2(salacen)]PF6. J. Chem. Soc. Dalton Trans. 1986, 1115–1123. [Google Scholar] [CrossRef]
- Kennedy, B.J.; McGrath, A.C.; Murray, K.S.; Skelton, B.W.; White, A.H. Variable-temperature magnetic, spectral, and x-ray crystallographic studies of “spin-crossover” iron(III) Schiff-base-Lewis-base adducts. Influence of non-coordinated anions on spin-state interconversion dynamics in [Fe(salen)(imd)2]Y species (Y = ClO4−, BF4−, PF6−, BPh4−; imd = imidazole). Inorg. Chem. 1987, 26, 483–495. [Google Scholar] [CrossRef]
- Ross, T.M.; Neville, S.M.; Innes, D.S.; Turner, D.R.; Moubaraki, B.; Murray, K.S. Spin crossover in iron(III) Schiff-base 1-D chain complexes. Dalton Trans. 2010, 39, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Hernandenz-Molia, R.; Mederis, A.; Dominguez, S.; Gili, P.; Ruiz-Perez, C.; Castineiras, A.; Solans, X.; Lloret, F.; Real, J.A. Different ground spin states in iron (III) complexes with quadridentate Schiff bases: Synthesis, crystal structures, and magnetic properties. Inorg. Chem. 1998, 37, 5102–5108. [Google Scholar] [CrossRef]
- Halcrow, M.A. Structure:function relationships in molecular spin-crossover complexes. Chem. Soc. Rev. 2011, 40, 4119–4142. [Google Scholar] [CrossRef] [PubMed]
- Koike, M.; Murakami, K.; Fujinami, T.; Nishi, K.; Matsumoto, N.; Sunatsuki, Y. Syntheses, three types of hydrogen-bonded assembly structures, and magnetic properties of [FeIII(Him)2(hapen)]Y·solvent (Him = imidazole, hapen = N,N′-bis(2-hydroxyacetophenylidene)ethylenediamine, Y = BPh4−, CF3SO3−, PF6−, ClO4−, and BF4−). Inorg. Chim. Acta 2013, 399, 185–192. [Google Scholar] [CrossRef]
- Fujinami, T.; Koike, M.; Matsumoto, N.; Sunatsuki, Y.; Okazawa, A.; Kojima, N. Abrupt spin transition with thermal hysteresis of iron(III) complex [FeIII(Him)2(hapen)]AsF6 (Him = imidazole, H2hapen = N,N′-bis(2-hydroxyacetophenylidene)ethylenediamine). Inorg. Chem. 2014, 53, 2254–2259. [Google Scholar] [CrossRef] [PubMed]
- Fujinami, T.; Ikeda, M.; Koike, M.; Matsumoto, N.; Oishi, T.; Sunatsuki, Y. Syntheses, hydrogen-bonded assembly structures, and spin crossover properties of [FeIII(Him)2(n-MeOhapen)]PF6 (Him = imidazole and n-MeOhapen = N,N′-bis(n-methoxy-2-oxyacetophenylidene)ethylenediamine); n = 4, 5, 6). Inorg. Chim. Acta 2015, 432, 89–95. [Google Scholar] [CrossRef]
- Spartan; Version 10; Wavefunction Inc.: Irvine, CA, USA, 2011.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueno, T.; Miyano, K.; Hamada, D.; Ono, H.; Fujinami, T.; Matsumoto, N.; Sunatsuki, Y. Abrupt Spin Transition and Chiral Hydrogen-Bonded One-Dimensional Structure of Iron(III) Complex [FeIII(Him)2(hapen)]SbF6 (Him = imidazole, H2hapen = N,N′-bis(2-hydroxyacetophenylidene)ethylenediamine). Magnetochemistry 2015, 1, 72-82. https://doi.org/10.3390/magnetochemistry1010072
Ueno T, Miyano K, Hamada D, Ono H, Fujinami T, Matsumoto N, Sunatsuki Y. Abrupt Spin Transition and Chiral Hydrogen-Bonded One-Dimensional Structure of Iron(III) Complex [FeIII(Him)2(hapen)]SbF6 (Him = imidazole, H2hapen = N,N′-bis(2-hydroxyacetophenylidene)ethylenediamine). Magnetochemistry. 2015; 1(1):72-82. https://doi.org/10.3390/magnetochemistry1010072
Chicago/Turabian StyleUeno, Takahiro, Kyohei Miyano, Daisuke Hamada, Hiromasa Ono, Takeshi Fujinami, Naohide Matsumoto, and Yukinari Sunatsuki. 2015. "Abrupt Spin Transition and Chiral Hydrogen-Bonded One-Dimensional Structure of Iron(III) Complex [FeIII(Him)2(hapen)]SbF6 (Him = imidazole, H2hapen = N,N′-bis(2-hydroxyacetophenylidene)ethylenediamine)" Magnetochemistry 1, no. 1: 72-82. https://doi.org/10.3390/magnetochemistry1010072
APA StyleUeno, T., Miyano, K., Hamada, D., Ono, H., Fujinami, T., Matsumoto, N., & Sunatsuki, Y. (2015). Abrupt Spin Transition and Chiral Hydrogen-Bonded One-Dimensional Structure of Iron(III) Complex [FeIII(Him)2(hapen)]SbF6 (Him = imidazole, H2hapen = N,N′-bis(2-hydroxyacetophenylidene)ethylenediamine). Magnetochemistry, 1(1), 72-82. https://doi.org/10.3390/magnetochemistry1010072