Parahydrogen-Based Hyperpolarization for the Masses at Millitesla Fields
Abstract
1. Introduction
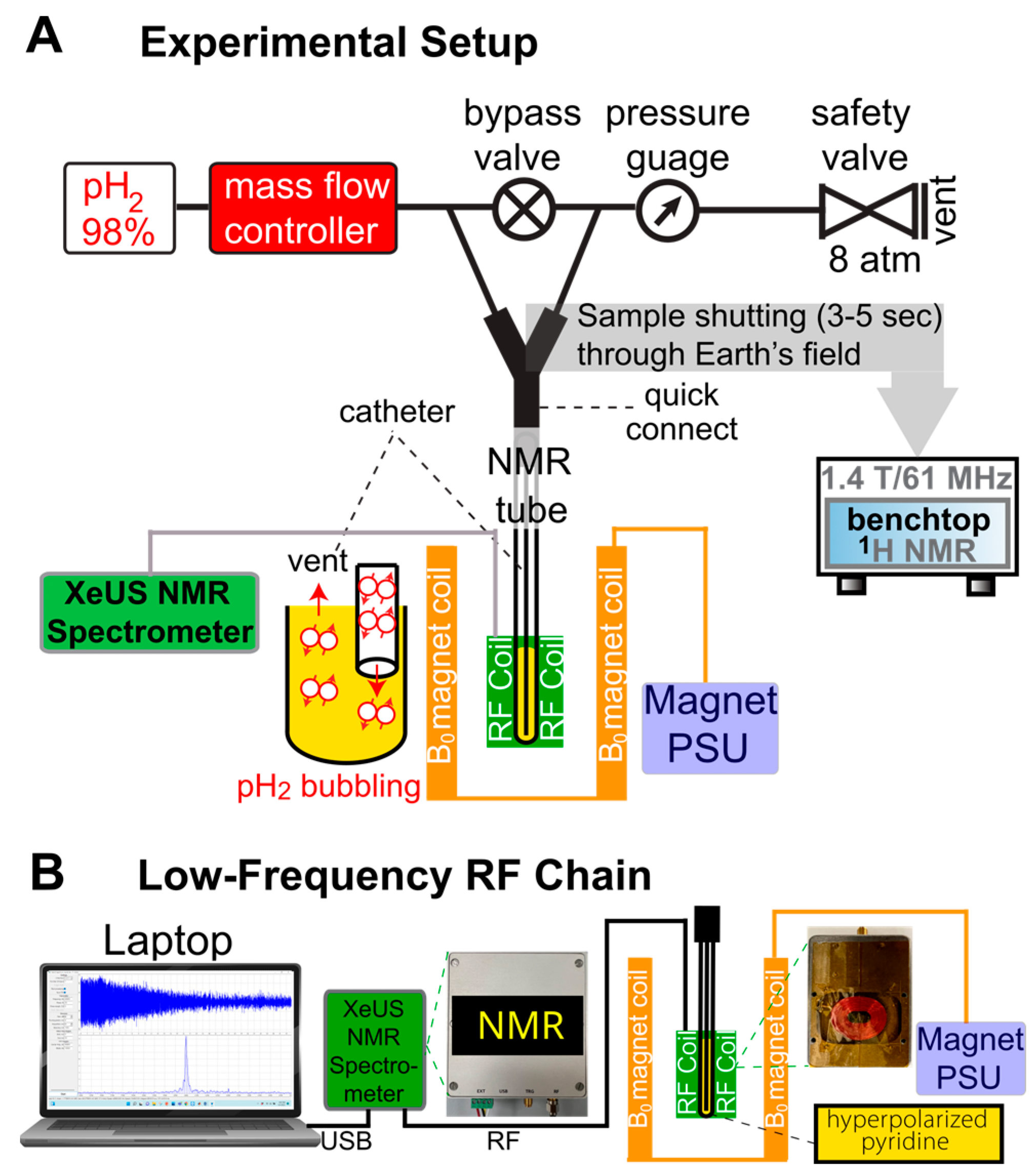
2. Materials and Methods
2.1. Setup Used for Winding of the Solenoid Coils
2.2. Winding of the Solenoid Coils
2.3. Simulation of Solenoid Electromagnetic Field
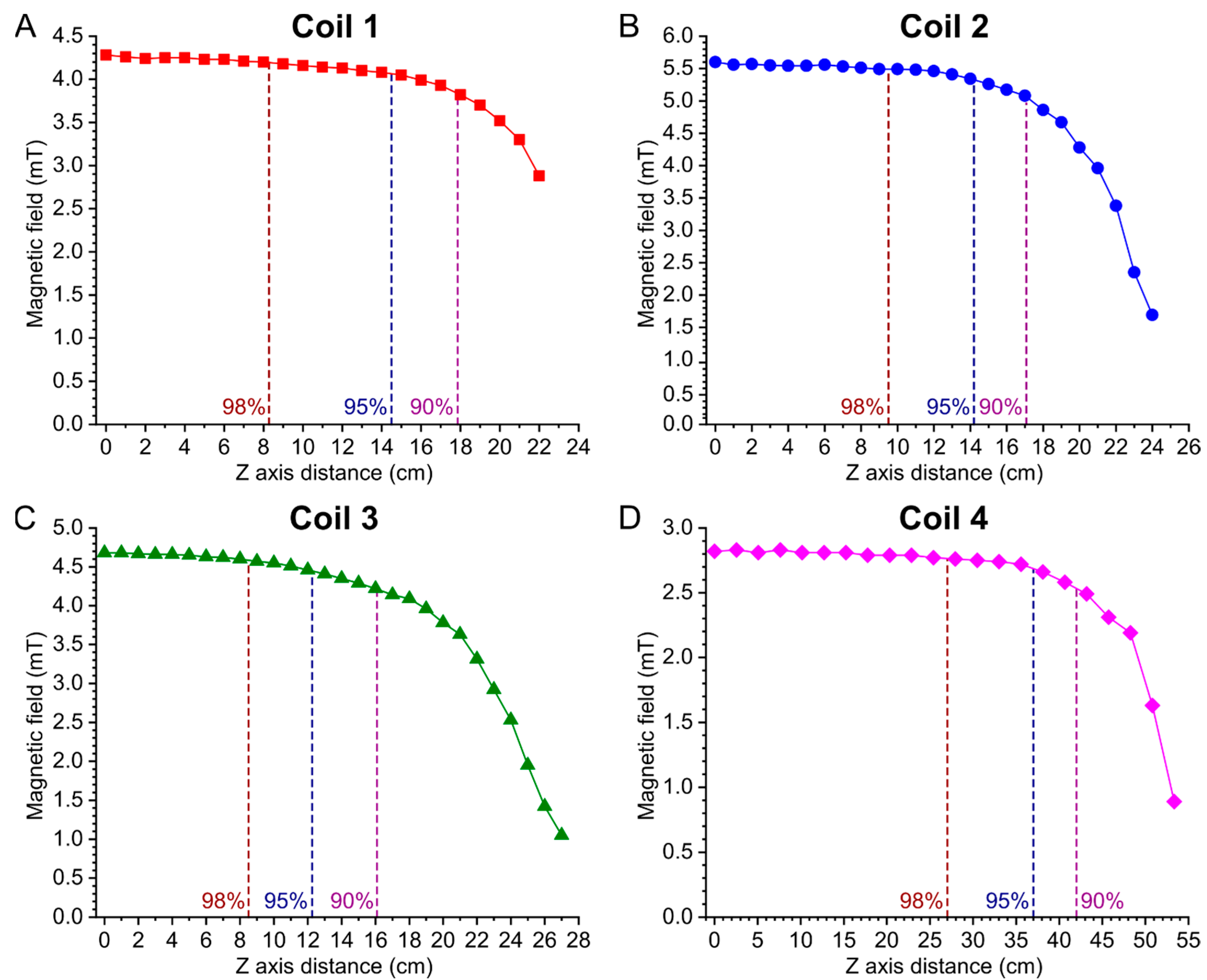
2.4. Magnetic Field Homogeneity Measurements of the Solenoid Coils
2.5. Sample Preparation
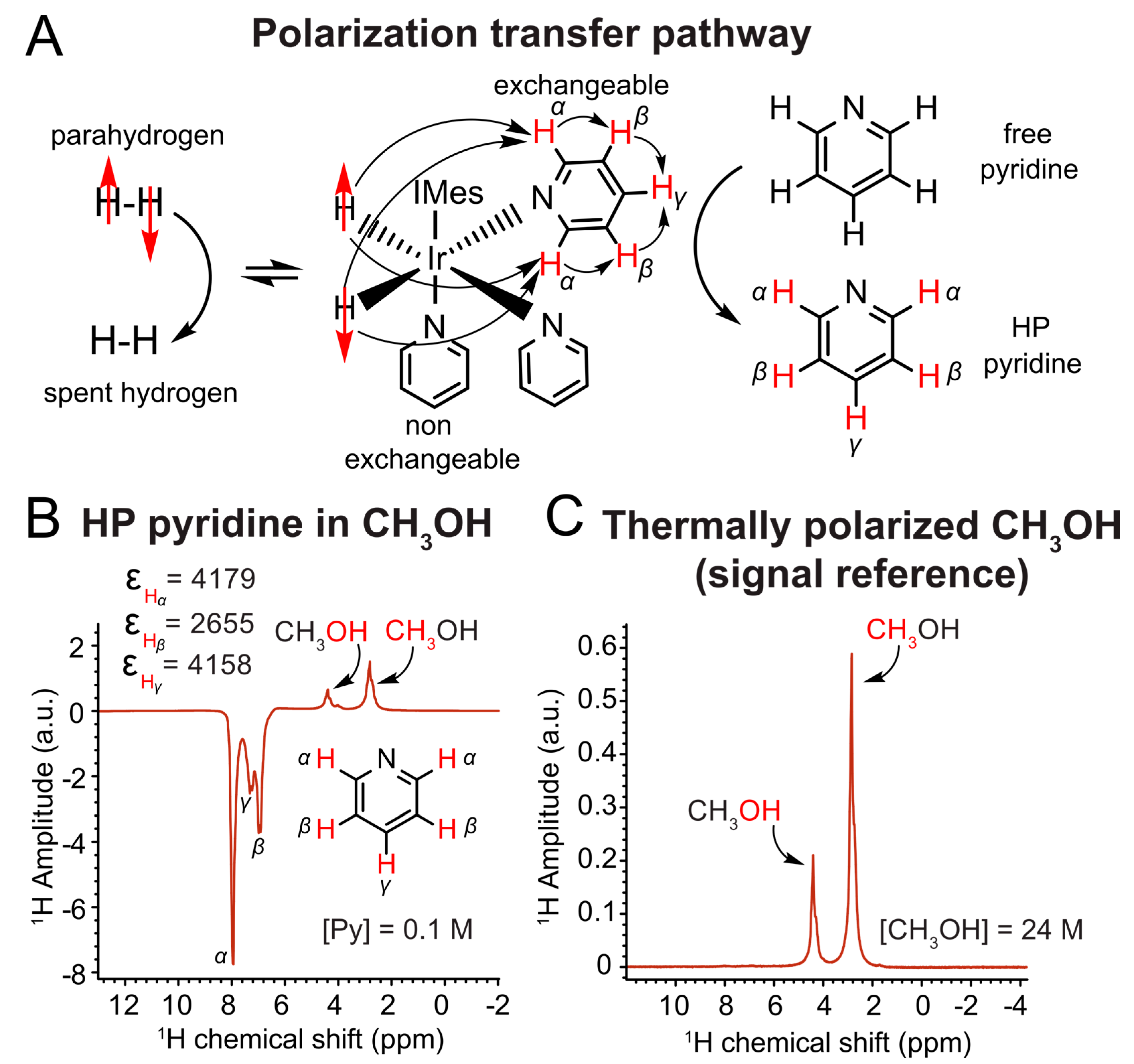
2.6. SABRE Experiments
3. Results and Discussion
3.1. Physical Properties of Constructed Solenoid Coils
3.2. Simulation of Solenoid Electromagnetic Field
3.3. Magnetic Field Homogeneity Measurements of the Solenoid Coils
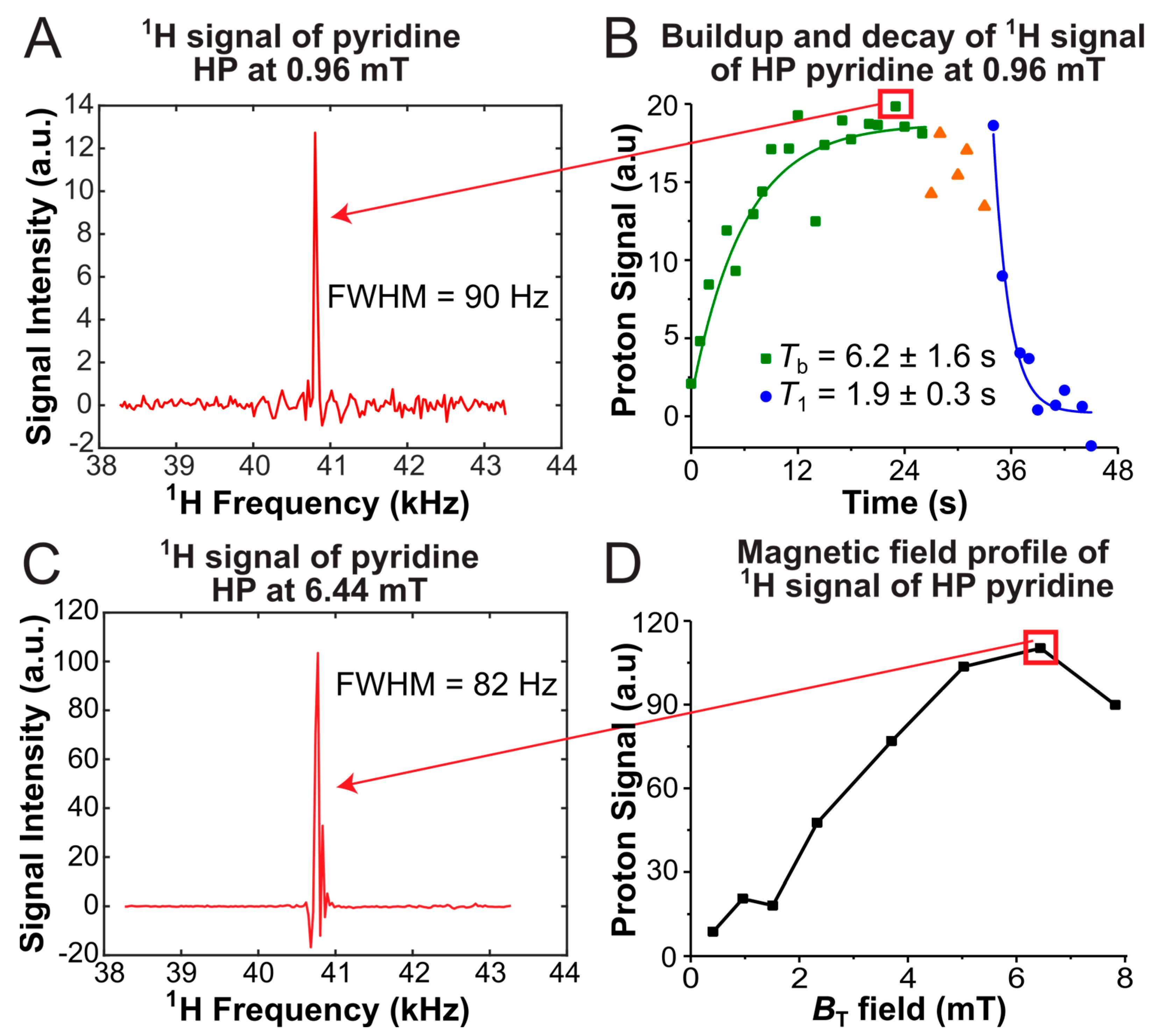
3.4. SABRE Hyperpolarization Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HP | Hyperpolarization |
| PHIP | Parahydrogen-Induced Polarization |
| SABRE | Signal Amplification By Reversible Exchange |
| d-DNP | Dissolution Dynamic Nuclear Polarization |
| NMR | Nuclear Magnetic Resonance |
| pH2 | Parahydrogen |
| PSU | Power Supply Unit |
| Py | Pyridine |
| IMes | 1,3-dimesitylimidazol-2-ylidene |
| COD | 1,5-cyclooctadiene |
| sccm | Standard Cubic Centimeters Per Minute |
References
- Eills, J.; Budker, D.; Cavagnero, S.; Chekmenev, E.Y.; Elliott, S.J.; Jannin, S.; Lesage, A.; Matysik, J.; Meersmann, T.; Prisner, T.; et al. Spin Hyperpolarization in Modern Magnetic Resonance. Chem. Rev. 2023, 123, 1417–1551. [Google Scholar] [CrossRef]
- Kovtunov, K.V.; Pokochueva, E.V.; Salnikov, O.G.; Cousin, S.F.; Kurzbach, D.; Vuichoud, B.; Jannin, S.; Chekmenev, E.Y.; Goodson, B.M.; Barskiy, D.A.; et al. Hyperpolarized NMR Spectroscopy: D-DNP, PHIP, and SABRE Techniques. Chem. Asian J. 2018, 13, 1857–1871. [Google Scholar] [CrossRef]
- Elliott, S.J.; Stern, Q.; Ceillier, M.; El Daraï, T.; Cousin, S.F.; Cala, O.; Jannin, S. Practical Dissolution Dynamic Nuclear Polarization. Prog. Nucl. Magn. Reson. Spectrosc. 2021, 126–127, 59–100. [Google Scholar] [CrossRef]
- Pinon, A.C.; Capozzi, A.; Ardenkjær-Larsen, J.H. Hyperpolarization via Dissolution Dynamic Nuclear Polarization: New Technological and Methodological Advances. Magn. Reson. Mater. Phys. Biol. Med. 2021, 34, 5–23. [Google Scholar] [CrossRef]
- Capozzi, A.; Patel, S.; Wenckebach, W.T.; Karlsson, M.; Lerche, M.H.; Ardenkjær-Larsen, J.H. Gadolinium Effect at High-Magnetic-Field DNP: 70% 13C Polarization of [U-13C] Glucose Using Trityl. J. Phys. Chem. Lett. 2019, 10, 3420–3425. [Google Scholar] [CrossRef]
- Yoshihara, H.A.I.; Can, E.; Karlsson, M.; Lerche, M.H.; Schwitter, J.; Comment, A. High-Field Dissolution Dynamic Nuclear Polarization of [1-13C]Pyruvic Acid. Phys. Chem. Chem. Phys. 2016, 18, 12409–12413. [Google Scholar] [CrossRef]
- Patel, S.; Pinon, A.C.; Lerche, M.H.; Karlsson, M.; Capozzi, A.; Ardenkjær-Larsen, J.H. UV-Irradiated 2-Keto-(1-13C)Isocaproic Acid for High-Performance 13C Hyperpolarized MR. J. Phys. Chem. C 2020, 124, 23859–23866. [Google Scholar] [CrossRef]
- Stern, Q.; Reynard-Feytis, Q.; Elliott, S.J.; Ceillier, M.; Cala, O.; Ivanov, K.; Jannin, S. Rapid and Simple 13C-Hyperpolarization by 1H Dissolution Dynamic Nuclear Polarization Followed by an Inline Magnetic Field Inversion. J. Am. Chem. Soc. 2023, 145, 27576–27586. [Google Scholar] [CrossRef]
- Pokochueva, E.V.; Le, N.H.; Guibert, S.; Gioiosa, C.; Stern, Q.; Tolchard, J.; Bocquelet, C.; Cala, O.; Cavaillès, M.; Veyre, L.; et al. Hybrid Polarizing Solids with Extended Pore Diameters for Dissolution Dynamic Nuclear Polarization. Chem.-Methods 2025, 5, e202400068. [Google Scholar] [CrossRef]
- Nikolaou, P.; Coffey, A.M.; Walkup, L.L.; Gust, B.M.; Whiting, N.; Newton, H.; Barcus, S.; Muradyan, I.; Dabaghyan, M.; Moroz, G.D.; et al. Near-Unity Nuclear Polarization with an Open-Source 129Xe Hyperpolarizer for NMR and MRI. Proc. Natl. Acad. Sci. USA 2013, 110, 14150–14155. [Google Scholar] [CrossRef]
- Nikolaou, P.; Coffey, A.M.; Walkup, L.L.; Gust, B.M.; LaPierre, C.D.; Koehnemann, E.; Barlow, M.J.; Rosen, M.S.; Goodson, B.M.; Chekmenev, E.Y. A 3D-Printed High Power Nuclear Spin Polarizer. J. Am. Chem. Soc. 2014, 136, 1636–1642. [Google Scholar] [CrossRef]
- Nikolaou, P.; Coffey, A.M.; Walkup, L.L.; Gust, B.M.; Whiting, N.; Newton, H.; Muradyan, I.; Dabaghyan, M.; Ranta, K.; Moroz, G.D.; et al. XeNA: An Automated “open-Source” 129Xe Hyperpolarizer for Clinical Use. Magn. Reson. Imaging 2014, 32, 541–550. [Google Scholar] [CrossRef]
- Birchall, J.R.; Irwin, R.K.; Nikolaou, P.; Coffey, A.M.; Kidd, B.E.; Murphy, M.; Molway, M.; Bales, L.B.; Ranta, K.; Barlow, M.J.; et al. XeUS: A Second-Generation Automated Open-Source Batch-Mode Clinical-Scale Hyperpolarizer. J. Magn. Reson. 2020, 319, 106813. [Google Scholar] [CrossRef]
- Korchak, S.; Mamone, S.; Glöggler, S. Over 50% 1H and 13C Polarization for Generating Hyperpolarized Metabolites—A Para-Hydrogen Approach. ChemistryOpen 2018, 7, 672–676. [Google Scholar] [CrossRef]
- Korchak, S.; Emondts, M.; Mamone, S.; Blümich, B.; Glöggler, S. Production of Highly Concentrated and Hyperpolarized Metabolites within Seconds in High and Low Magnetic Fields. Phys. Chem. Chem. Phys. 2019, 21, 22849–22856. [Google Scholar] [CrossRef]
- Knecht, S.; Blanchard, J.W.; Barskiy, D.; Cavallari, E.; Dagys, L.; Van Dyke, E.; Tsukanov, M.; Bliemel, B.; Münnemann, K.; Aime, S.; et al. Rapid Hyperpolarization and Purification of the Metabolite Fumarate in Aqueous Solution. Proc. Natl. Acad. Sci. USA. 2021, 118, e2025383118. [Google Scholar] [CrossRef]
- Dagys, L.; Korzeczek, M.C.; Parker, A.J.; Eills, J.; Blanchard, J.W.; Bengs, C.; Levitt, M.H.; Knecht, S.; Schwartz, I.; Plenio, M.B. Robust Parahydrogen-Induced Polarization at High Concentrations. Sci. Adv. 2024, 10, eado0373. [Google Scholar] [CrossRef]
- Marshall, A.; Salhov, A.; Gierse, M.; Müller, C.; Keim, M.; Lucas, S.; Parker, A.; Scheuer, J.; Vassiliou, C.; Neumann, P.; et al. Radio-Frequency Sweeps at Microtesla Fields for Parahydrogen-Induced Polarization of Biomolecules. J. Phys. Chem. Lett. 2023, 14, 2125–2132. [Google Scholar] [CrossRef]
- de Maissin, H.; Ivantaev, V.; Mohiuddin, O.; Berner, S.; von Elverfeldt, D.; Zaitsev, M.; Kiselev, V.; Schmidt, A.B. Overcoming the Challenges of Hyperpolarizing Substrates with Parahydrogen-Induced Polarization in an MRI System. Chem. Eur. J. 2025, 31, e202402911. [Google Scholar] [CrossRef]
- Rayner, P.J.; Norcott, P.; Appleby, K.M.; Iali, W.; John, R.O.; Hart, S.J.; Whitwood, A.C.; Duckett, S.B. Fine-Tuning the Efficiency of Para-Hydrogen-Induced Hyperpolarization by Rational N-Heterocyclic Carbene Design. Nat. Commun. 2018, 9, 4251. [Google Scholar] [CrossRef]
- Fekete, M.; Ahwal, F.; Duckett, S.B. Remarkable Levels of 15N Polarization Delivered Through SABRE into Unlabeled Pyridine, Pyrazine, or Metronidazole Enable Single Scan NMR Quantification at the mM Level. J. Phys. Chem. B 2020, 124, 4573–4580. [Google Scholar] [CrossRef]
- Rayner, P.J.; Burns, M.J.; Olaru, A.M.; Norcott, P.; Fekete, M.; Green, G.G.R.; Highton, L.A.R.; Mewis, R.E.; Duckett, S.B. Delivering Strong 1H Nuclear Hyperpolarization Levels and Long Magnetic Lifetimes through Signal Amplification by Reversible Exchange. Proc. Natl. Acad. Sci. USA 2017, 114, E3188–E3194. [Google Scholar] [CrossRef]
- Adelabu, I.; TomHon, P.; Kabir, M.S.H.; Nantogma, S.; Abdulmojeed, M.; Mandzhieva, I.; Ettedgui, J.; Swenson, R.E.; Krishna, M.C.; Theis, T.; et al. Order-Unity 13C Nuclear Polarization of [1-13C]Pyruvate in Seconds and the Interplay of Water and SABRE Enhancement. ChemPhysChem 2022, 23, 131–136. [Google Scholar] [CrossRef]
- Fraser, R.; Rutjes, F.P.J.T.; Feiters, M.C.; Tessari, M. Analysis of Complex Mixtures by Chemosensing NMR Using Para-Hydrogen-Induced Hyperpolarization. Acc. Chem. Res. 2022, 55, 1832–1844. [Google Scholar] [CrossRef]
- Plainchont, B.; Berruyer, P.; Dumez, J.N.; Jannin, S.; Giraudeau, P. Dynamic Nuclear Polarization Opens New Perspectives for NMR Spectroscopy in Analytical Chemistry. Anal. Chem. 2018, 90, 3639–3650. [Google Scholar] [CrossRef]
- Ribay, V.; Praud, C.; Letertre, M.P.M.; Dumez, J.-N.; Giraudeau, P. Hyperpolarized NMR Metabolomics. Curr. Opin. Chem. Biol. 2023, 74, 102307. [Google Scholar] [CrossRef]
- Silva Terra, A.I.; Taylor, D.A.; Halse, M.E. Hyperpolarised Benchtop NMR Spectroscopy for Analytical Applications. Prog. Nucl. Magn. Reson. Spectrosc. 2024, 144–145, 153–178. [Google Scholar] [CrossRef]
- Jimmink, B.O.; Negroni, M.; Posthumus, T.B.; Kentgens, A.P.M.; Tessari, M. Quantitative Trace Analysis of Dilute Mixtures Using a Benchtop NMR System with SABRE Hyperpolarization. Anal. Chem. 2025, 97, 10962–10965. [Google Scholar] [CrossRef]
- Dreisewerd, L.; Aspers, R.L.E.G.; Feiters, M.C.; Rutjes, F.P.J.T.; Tessari, M. NMR Discrimination of D- and L-α-Amino Acids at Submicromolar Concentration Via Parahydrogen-Induced Hyperpolarization. J. Am. Chem. Soc. 2023, 145, 1518–1523. [Google Scholar] [CrossRef]
- Ausmees, K.; Reimets, N.; Reile, I. Parahydrogen Hyperpolarization of Minimally Altered Urine Samples for Sensitivity Enhanced NMR Metabolomics. Chem. Commun. 2022, 58, 463–466. [Google Scholar] [CrossRef]
- Dey, A.; Charrier, B.; Martineau, E.; Deborde, C.; Gandriau, E.; Moing, A.; Jacob, D.; Eshchenko, D.; Schnell, M.; Melzi, R.; et al. Hyperpolarized NMR Metabolomics at Natural 13C Abundance. Anal. Chem. 2020, 92, 14867–14871. [Google Scholar] [CrossRef]
- Ribay, V.; Charrier, B.; Croyal, M.; Cariou, B.; Hadjadj, S.; Boccard, J.; Cannet, C.; Dumez, J.-N.; Letertre, M.P.M.; Giraudeau, P. Hyperpolarized 13C NMR Metabolomics of Urine Samples at Natural Abundance Applied to Chronic Kidney Disease. J. Am. Chem. Soc. 2025, 147, 644–650. [Google Scholar] [CrossRef]
- Kurhanewicz, J.; Vigneron, D.B.; Ardenkjaer-Larsen, J.H.; Bankson, J.A.; Brindle, K.; Cunningham, C.H.; Gallagher, F.A.; Keshari, K.R.; Kjaer, A.; Laustsen, C.; et al. Hyperpolarized 13C MRI: Path to Clinical Translation in Oncology. Neoplasia 2019, 21, 1–16. [Google Scholar] [CrossRef]
- Nelson, S.J.; Kurhanewicz, J.; Vigneron, D.B.; Larson, P.E.Z.; Harzstark, A.L.; Ferrone, M.; van Criekinge, M.; Chang, J.W.; Bok, R.; Park, I.; et al. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized [1-13C]Pyruvate. Sci. Transl. Med. 2013, 5, 198ra108. [Google Scholar] [CrossRef]
- Larson, P.E.Z.; Bernard, J.M.L.; Bankson, J.A.; Bøgh, N.; Bok, R.A.; Chen, A.P.; Cunningham, C.H.; Gordon, J.W.; Hövener, J.; Laustsen, C.; et al. Current Methods for Hyperpolarized [1-13C]Pyruvate MRI Human Studies. Magn. Reson. Med. 2024, 91, 2204–2228. [Google Scholar] [CrossRef]
- Khan, A.S.; Harvey, R.L.; Birchall, J.R.; Irwin, R.K.; Nikolaou, P.; Schrank, G.; Emami, K.; Dummer, A.; Barlow, M.J.; Goodson, B.M.; et al. Enabling Clinical Technologies for Hyperpolarized 129Xenon Magnetic Resonance Imaging and Spectroscopy. Angew. Chem. Int. Ed. 2021, 60, 22126–22147. [Google Scholar] [CrossRef]
- Ardenkjær-Larsen, J.H.; Fridlund, B.; Gram, A.; Hansson, G.; Hansson, L.; Lerche, M.H.; Servin, R.; Thaning, M.; Golman, K. Increase in Signal-to-Noise Ratio of >10,000 Times in Liquid-State NMR. Proc. Natl. Acad. Sci. USA 2003, 100, 10158–10163. [Google Scholar] [CrossRef]
- Hövener, J.-B.; Pravdivtsev, A.N.; Kidd, B.; Bowers, C.R.; Glöggler, S.; Kovtunov, K.V.; Plaumann, M.; Katz-Brull, R.; Buckenmaier, K.; Jerschow, A.; et al. Parahydrogen-Based Hyperpolarization for Biomedicine. Angew. Chem. Int. Ed. 2018, 57, 11140–11162. [Google Scholar] [CrossRef]
- Green, R.A.; Adams, R.W.; Duckett, S.B.; Mewis, R.E.; Williamson, D.C.; Green, G.G.R. The Theory and Practice of Hyperpolarization in Magnetic Resonance Using Parahydrogen. Prog. Nucl. Magn. Reson. Spectrosc. 2012, 67, 1–48. [Google Scholar] [CrossRef]
- Bowers, C.R.; Weitekamp, D.P. Transformation of Symmetrization Order to Nuclear-Spin Magnetization by Chemical Reaction and Nuclear Magnetic Resonance. Phys. Rev. Lett. 1986, 57, 2645–2648. [Google Scholar] [CrossRef]
- Bowers, C.R.; Weitekamp, D.P. Parahydrogen and Synthesis Allow Dramatically Enhanced Nuclear Alignment. J. Am. Chem. Soc. 1987, 109, 5541–5542. [Google Scholar] [CrossRef]
- Eisenschmid, T.C.; Kirss, R.U.; Deutsch, P.P.; Hommeltoft, S.I.; Eisenberg, R.; Bargon, J.; Lawler, R.G.; Balch, A.L. Para Hydrogen Induced Polarization in Hydrogenation Reactions. J. Am. Chem. Soc. 1987, 109, 8089–8091. [Google Scholar] [CrossRef]
- Adams, R.W.; Aguilar, J.A.; Atkinson, K.D.; Cowley, M.J.; Elliott, P.I.P.; Duckett, S.B.; Green, G.G.R.; Khazal, I.G.; López-Serrano, J.; Williamson, D.C. Reversible Interactions with Para-Hydrogen Enhance NMR Sensitivity by Polarization Transfer. Science 2009, 323, 1708–1711. [Google Scholar] [CrossRef]
- Salnikov, O.G.; Burueva, D.B.; Skovpin, I.V.; Koptyug, I.V. Parahydrogen-Based NMR Signal Amplification by Reversible Exchange (SABRE): Recent Advances and Applications. Mendeleev Commun. 2023, 33, 583–596. [Google Scholar] [CrossRef]
- Rayner, P.J.; Duckett, S.B. Signal Amplification by Reversible Exchange (SABRE): From Discovery to Diagnosis. Angew. Chem. Int. Ed. 2018, 57, 6742–6753. [Google Scholar] [CrossRef]
- Schmidt, A.B.; Bowers, C.R.; Buckenmaier, K.; Chekmenev, E.Y.; de Maissin, H.; Eills, J.; Ellermann, F.; Glöggler, S.; Gordon, J.W.; Knecht, S.; et al. Instrumentation for Hydrogenative Parahydrogen-Based Hyperpolarization Techniques. Anal. Chem. 2022, 94, 479–502. [Google Scholar] [CrossRef]
- Chapman, B.; Joalland, B.; Meersman, C.; Ettedgui, J.; Swenson, R.E.; Krishna, M.C.; Nikolaou, P.; Kovtunov, K.V.; Salnikov, O.G.; Koptyug, I.V.; et al. Low-Cost High-Pressure Clinical-Scale 50% Parahydrogen Generator Using Liquid Nitrogen at 77 K. Anal. Chem. 2021, 93, 8476–8483. [Google Scholar] [CrossRef]
- Ellermann, F.; Pravdivtsev, A.; Hövener, J.-B. Open-Source, Partially 3D-Printed, High-Pressure (50-Bar) Liquid-Nitrogen-Cooled Parahydrogen Generator. Magn. Reson. 2021, 2, 49–62. [Google Scholar] [CrossRef]
- Nantogma, S.; Chowdhury, M.R.H.; Kabir, M.S.H.; Adelabu, I.; Joshi, S.M.; Samoilenko, A.; de Maissin, H.; Schmidt, A.B.; Nikolaou, P.; Chekmenev, Y.A.; et al. MATRESHCA: Microtesla Apparatus for Transfer of Resonance Enhancement of Spin Hyperpolarization via Chemical Exchange and Addition. Anal. Chem. 2024, 96, 4171–4179. [Google Scholar] [CrossRef]
- Adams, R.W.; Duckett, S.B.; Green, R.A.; Williamson, D.C.; Green, G.G.R. A Theoretical Basis for Spontaneous Polarization Transfer in Non-Hydrogenative Parahydrogen-Induced Polarization. J. Chem. Phys. 2009, 131, 194505. [Google Scholar] [CrossRef]
- Pravdivtsev, A.N.; Ivanov, K.L.; Yurkovskaya, A.V.; Petrov, P.A.; Limbach, H.-H.; Kaptein, R.; Vieth, H.-M. Spin Polarization Transfer Mechanisms of SABRE: A Magnetic Field Dependent Study. J. Magn. Reson. 2015, 261, 73–82. [Google Scholar] [CrossRef]
- Chowdhury, M.R.H.; Ahmed, F.; Oladun, C.; Adelabu, I.; Abdurraheem, A.; Nantogma, S.; Birchall, J.R.; Gafar, T.A.; Chekmenev, Y.A.; Nikolaou, P.; et al. Low-Cost Purpose-Built Ultra-Low-Field NMR Spectrometer. Anal. Chem. 2024, 96, 16724–16734. [Google Scholar] [CrossRef]
- Cowley, M.J.; Adams, R.W.; Atkinson, K.D.; Cockett, M.C.R.; Duckett, S.B.; Green, G.G.R.; Lohman, J.A.B.; Kerssebaum, R.; Kilgour, D.; Mewis, R.E. Iridium N-Heterocyclic Carbene Complexes as Efficient Catalysts for Magnetization Transfer from Para-Hydrogen. J. Am. Chem. Soc. 2011, 133, 6134–6137. [Google Scholar] [CrossRef]
- van Weerdenburg, B.J.A.; Glöggler, S.; Eshuis, N.; Engwerda, A.H.J.; Smits, J.M.M.; de Gelder, R.; Appelt, S.; Wymenga, S.S.; Tessari, M.; Feiters, M.C.; et al. Ligand Effects of NHC-Iridium Catalysts for Signal Amplification by Reversible Exchange (SABRE). Chem. Commun. 2013, 49, 7388–7390. [Google Scholar] [CrossRef]
- Lloyd, L.S.; Asghar, A.; Burns, M.J.; Charlton, A.; Coombes, S.; Cowley, M.J.; Dear, G.J.; Duckett, S.B.; Genov, G.R.; Green, G.G.R.; et al. Hyperpolarisation Through Reversible Interactions with Parahydrogen. Catal. Sci. Technol. 2014, 4, 3544–3554. [Google Scholar] [CrossRef]
- Appleby, K.M.; Mewis, R.E.; Olaru, A.M.; Green, G.G.R.; Fairlamb, I.J.S.; Duckett, S.B. Investigating Pyridazine and Phthalazine Exchange in a Series of Iridium Complexes in Order to Define Their Role in the Catalytic Transfer of Magnetisation from Para-Hydrogen. Chem. Sci. 2015, 6, 3981–3993. [Google Scholar] [CrossRef]
- TomHon, P.M.; Han, S.; Lehmkuhl, S.; Appelt, S.; Chekmenev, E.Y.; Abolhasani, M.; Theis, T. A Versatile Compact Parahydrogen Membrane Reactor. ChemPhysChem 2021, 22, 2526–2534. [Google Scholar] [CrossRef]
- Fekete, M.; Rayner, P.J.; Green, G.G.R.; Duckett, S.B. Harnessing Polarisation Transfer to Indazole and Imidazole through Signal Amplification by Reversible Exchange to Improve Their NMR Detectability. Magn. Reson. Chem. 2017, 55, 944–957. [Google Scholar] [CrossRef]
- Iali, W.; Rayner, P.J.; Alshehri, A.; Holmes, A.J.; Ruddlesden, A.J.; Duckett, S.B. Direct and Indirect Hyperpolarisation of Amines Using Para Hydrogen. Chem. Sci. 2018, 9, 3677–3684. [Google Scholar] [CrossRef]
- Mewis, R.E.; Green, R.A.; Cockett, M.C.R.; Cowley, M.J.; Duckett, S.B.; Green, G.G.R.; John, R.O.; Rayner, P.J.; Williamson, D.C. Strategies for the Hyperpolarization of Acetonitrile and Related Ligands by SABRE. J. Phys. Chem. B 2015, 119, 1416–1424. [Google Scholar] [CrossRef]
- Smith, I.; Terkildsen, N.; Bender, Z.; Abdurraheem, A.; Nantogma, S.; Samoilenko, A.; Gyesi, J.; Kovtunova, L.M.; Salnikov, O.G.; Koptyug, I.V.; et al. SABRE Ir-IMes Catalysis for the Masses. Molecules 2025, in press. [Google Scholar]
- Kownacki, I.; Kubicki, M.; Szubert, K.; Marciniec, B. Synthesis, Structure and Catalytic Activity of the First Iridium(I) Siloxide versus Chloride Complexes with 1,3-Mesitylimidazolin-2-Ylidene Ligand. J. Organomet. Chem. 2008, 693, 321–328. [Google Scholar] [CrossRef]
- Blanchard, J.W.; Ripka, B.; Suslick, B.A.; Gelevski, D.; Wu, T.; Münnemann, K.; Barskiy, D.A.; Budker, D. Towards Large-Scale Steady-State Enhanced Nuclear Magnetization with in Situ Detection. Magn. Reson. Chem. 2021, 59, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Barskiy, D.A.; Kovtunov, K.V.; Koptyug, I.V.; He, P.; Groome, K.A.; Best, Q.A.; Shi, F.; Goodson, B.M.; Shchepin, R.V.; Coffey, A.M.; et al. The Feasibility of Formation and Kinetics of NMR Signal Amplification by Reversible Exchange (SABRE) at High Magnetic Field (9.4 T). J. Am. Chem. Soc. 2014, 136, 3322–3325. [Google Scholar] [CrossRef]
- Vázquez-Serrano, L.D.; Owens, B.T.; Buriak, J.M. Catalytic Olefin Hydrogenation Using N-Heterocyclic Carbene-Phosphine Complexes of Iridium. Chem. Commun. 2002, 21, 2518–2519. [Google Scholar] [CrossRef]


| Coil # | Length, cm | Length (Coiled Wire), cm | Inner Diameter, cm | Thickness of Tube Base, cm | Wire Diameter, mm |
|---|---|---|---|---|---|
| Coil 1 | 51.2 | 46.4 | 7.6 | 0.165 | 0.812 |
| Coil 2 | 49.2 | 44.3 | 7.6 | 0.165 | 0.511 |
| Coil 3 | 56.1 | 48.9 | 7.6 | 0.158 | 1.024 |
| Coil 4 | 106.7 | 101.8 | 15.2 | 0.275 | 0.812 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wibbels, G.L.; Oladun, C.; O’Hara, T.Y.; Adelabu, I.; Robinson, J.E.; Ahmed, F.; Bender, Z.T.; Samoilenko, A.; Gyesi, J.; Kovtunova, L.M.; et al. Parahydrogen-Based Hyperpolarization for the Masses at Millitesla Fields. Magnetochemistry 2025, 11, 80. https://doi.org/10.3390/magnetochemistry11090080
Wibbels GL, Oladun C, O’Hara TY, Adelabu I, Robinson JE, Ahmed F, Bender ZT, Samoilenko A, Gyesi J, Kovtunova LM, et al. Parahydrogen-Based Hyperpolarization for the Masses at Millitesla Fields. Magnetochemistry. 2025; 11(9):80. https://doi.org/10.3390/magnetochemistry11090080
Chicago/Turabian StyleWibbels, Garrett L., Clementinah Oladun, Tanner Y. O’Hara, Isaiah Adelabu, Joshua E. Robinson, Firoz Ahmed, Zachary T. Bender, Anna Samoilenko, Joseph Gyesi, Larisa M. Kovtunova, and et al. 2025. "Parahydrogen-Based Hyperpolarization for the Masses at Millitesla Fields" Magnetochemistry 11, no. 9: 80. https://doi.org/10.3390/magnetochemistry11090080
APA StyleWibbels, G. L., Oladun, C., O’Hara, T. Y., Adelabu, I., Robinson, J. E., Ahmed, F., Bender, Z. T., Samoilenko, A., Gyesi, J., Kovtunova, L. M., Salnikov, O. G., Koptyug, I. V., Goodson, B. M., Snow, W. M., Chekmenev, E. Y., & Shchepin, R. V. (2025). Parahydrogen-Based Hyperpolarization for the Masses at Millitesla Fields. Magnetochemistry, 11(9), 80. https://doi.org/10.3390/magnetochemistry11090080






