1. Introduction
Rotigotine, chemically named (6S)-6-{propyl [2-(2-thienyl)ethyl]amino}-5,6,7,8 -tetrahydro-1-naphthol, is a non-ergot selective dopamine receptor agonist. Rotigotine acts on all dopamine receptor subtypes, although it shows the highest affinity for and greatest agonistic effect on the D3 receptor. In comparison, its affinity for the D2, D4, and D5 receptors is 1/20 that of the D3 receptor and 1/100 that of the D1 receptor. Rotigotine also serves as an antagonist at α2B-adrenergic receptors and an agonist at 5-HT1A receptors [
1,
2,
3,
4]. Clinically, it is used to treat Parkinson’s disease and restless leg syndrome [
4,
5,
6].
When administered orally, rotigotine undergoes considerable first-pass metabolism, leading to very low bioavailability [
3,
4]. To address this issue, the German company Schwarz Pharma developed a rotigotine transdermal patch (Neupro), which was approved by the European Medicines Agency (EMA) in 2006 and the U.S. Food and Drug Administration (FDA) in 2007 [
7,
8,
9]. In 2008, Neupro was recalled from the U.S. market by Schwarz Pharma owing to crystallization issues, which could compromise its bioavailability and clinical efficacy. This led to the discovery of an alternative polymorph of rotigotine (polymorph II). Subsequently, Schwarz Pharma implemented cold-chain manufacturing, transport, and storage processes to prevent polymorph conversion, along with limiting the prescription shelf-life to 1 month. Following these improvements, the patch was reapproved by the EMA and FDA in 2012 [
9,
10,
11,
12,
13]. The rotigotine patch enables continuous drug delivery and helps maintain stable plasma concentrations for 24 h, alleviating motor and non-motor symptoms in patients with Parkinson’s disease [
14,
15,
16]. Its bioavailability varies depending on the application site, and daily application is required. Nevertheless, approximately 30% of patients experience application-site reactions, with up to 3% developing severe skin reactions [
12].
Luye Pharma Group (Shandong, China) recently developed a long-acting rotigotine microsphere formulation, which was approved for the Chinese market in June 2024. This formulation utilizes biodegradable poly(lactic-co-glycolic acid) as the microsphere matrix and is administered once weekly via intramuscular injection. Upon injection, the drug is released gradually, thereby achieving continuous dopaminergic stimulation [
17]. The formulation consists of rotigotine and the poly(lactic-co-glycolic acid) matrix, along with the excipients mannitol and stearic acid.
Quantitative nuclear magnetic resonance (qNMR) technology combines qualitative identification and quantitative analysis. Its advantages include the ability to perform quantitative analysis of substances, such as being nondestructive, having a high flux, and offering short analysis time. NMR spectroscopy has been included in the pharmacopoeiae of various countries. qNMR was first introduced in the United States Pharmacopeia 19th revision; the British Pharmacopoeia 1973, addendum 1975; the Japanese Pharmacopoeia 12th Edition; the European Pharmacopoeia 5th Edition; and the Pharmacopoeia of the People’s Republic of China 2010, and has since been officially included in their appendices. The technology is widely applied in pharmaceutical research [
18,
19], natural product assessments [
19,
20], reference standard research [
21,
22,
23], quality control, metabolic studies [
19,
24,
25,
26], food analysis [
19,
27], and environmental research [
28]. qNMR enables flexible adaptation of techniques and quantification methods based on sample properties; thus, it has broad application prospects.
Chromatography methods are among the most commonly used protocols for quality control. Compared with chromatography, qNMR does not require reference standards or separation of multi-component samples; thus, it enables simultaneous quantification of multiple components with simple sample preparation. It allows acquisition not only of qualitative (composition) but also of quantitative data on samples containing multiple components without a chromatographic separation step. Due to its unique physical principles, NMR technology can directly correlate the concentration of target substances through characteristic chemical shifts and peak areas, thus avoiding cumbersome separation steps. Thus, compared to chromatography, qNMR can substantially reduce solvent consumption and has proven to be an environmentally responsible alternative for chemical analysis [
18,
29]. Traditional quality control methods require establishing separate analytical methods for different components, while NMR can simultaneously determine the contents of multiple components through a single experiment, significantly simplifying the analytical process and improving the efficiency of analysis [
30,
31,
32]. qNMR also has many applications in complex drug delivery systems, such as in research on material morphology characterization, encapsulation efficiency, and release behavior [
33,
34,
35,
36,
37]. The one-test-for-multiple-evaluation approach allows qNMR to simultaneously monitor active pharmaceutical ingredients and excipients when performing quality control of complex drug delivery systems, which can significantly reduce comsumption of labor and resources. Its high efficiency, environmental friendliness, and high throughput mean that NMR technology offers unique advantages and good development potential in the quality control of multi-component and complex drug delivery systems.
Typically, analyzing rotigotine, mannitol, and stearic acid in rotigotine prolonged-release microspheres for injection requires different chromatography methods for each component; they involve various chromatographic conditions, detection methods, and sample preparations for each component, which makes simultaneous quantification of rotigotine [
38,
39], stearic acid [
40,
41], and mannitol [
42] unfeasible. Additionally, these methods are often cumbersome, involve using separate reference standards, and consume large amounts of organic reagents.
In this study, we developed a 1H qNMR method for the quantitative analysis of the active pharmaceutical ingredient (API) and its related excipients in rotigotine prolonged-release microspheres. We aimed to provide an efficient and straightforward method to measure multiple components. The proposed method also serves as a reliable strategy for the quality control of microsphere formulations, offering a “one-test-multi-evaluation” approach.
As the water peak in the sample solution appears as a broad signal with a very wide range, it interferes with the mannitol signal in standard
1H NMR analysis. The NOESY experiment utilizes both pre-saturation and inherent solvent suppression through delays and phase cycling [
26]. The Bruker-developed noesygppr1d sequence, a 1D-
1H NOESY experiment with pre-saturation, uses gradient pulses for coherence selection and applies low-power continuous wave irradiation on the water resonance during both the pre-scan and mixing period [
43]. The noesygppr1d sequence, commonly used in metabolomics, includes pulsed field gradients for solvent suppression and phase cycling. When optimized with appropriate parameters (e.g., full relaxation delay, uniform excitation), it can yield accurate quantitative results [
21].
Pre-saturation is a crucial preliminary step in achieving water signal suppression. It selectively acts on the water resonance frequency using low-power, long pulses. To enhance suppression, the pulse action bandwidth may be expanded, which in turn can affect the surrounding peaks [
21,
26,
44]. Therefore, it is necessary to investigate whether the corresponding peaks can be accurately quantified. Furthermore, in accordance with the requirements for assay analysis specified in the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Guideline Q2, the specificity, linear range, accuracy, and precision of this method have been verified [
45].
2. Materials and Methods
2.1. Instruments
The instruments used in this study included an AVANCE III HD 600 MHz NMR spectrometer (Bruker, Billerica, MA, USA), an Agilent 1260 high-performance liquid chromatography system (Agilent Technologies, Santa Clara, CA, USA), an XPE26 microbalance (Mettler Toledo, Columbus, OH, USA), a centrifuge (Eppendorf, Hamburg, Germany), and an ultrasonic cleaner (Kunshan Ultrasonic Instruments Co., Ltd., Shanghai, China).
2.2. Reagents
The reagents employed in this study comprised rotigotine reference standard (99.8%; Luye Pharma Group, Shandong, China), stearic acid reference standard (99%; USP, North Bethesda, MD, USA), mannitol reference standard (99.5%; National Institutes for Food and Drug Control, Beijing, China), dimethyl terephthalate (DMT, 100%; National Institutes for Food and Drug Control, Beijing, China), maleic acid (MA, 99.4%; Dr. Ehrenstorfer, Wesel, Germany), and dimethylsulfoxide-d6 (DMSO-d6, Zancheng Science & Technology Co., Ltd., Tianjin, China).
Rotigotine prolonged-release microspheres for injection were produced by Luye Pharma Group, divided into three batches of 56 mg (A, B, C), one batch of 42 mg (D), one batch of 28 mg (E), and three batches of 14 mg (F, G, H).
2.3. Experimental Conditions
A pre-saturation experiment (zgpr pulse) was conducted under the following conditions: number of scans, 8; spectral width, 15 ppm; relaxation delay time (D1), 4 s; and temperature, 25 °C. This experiment identified the solvent peak position at 1975 Hz (3.291 ppm).
The longitudinal relaxation times (T1) of each component and the internal standard in the microsphere samples were measured using the inversion-recovery method for the D1 setting. In this experiment, the t1ir pulse sequence was applied. The basic pulse sequence consists of a 180° pulse that inverts the magnetization to the –z axis. During the following delay, relaxation along the longitudinal plane takes place. Magnetization comes back to the original equilibrium z-magnetization. A 90° pulse creates transverse magnetization. The experiment was repeated for a series of delay values taken from a variable delay list (vdlist), 0.01, 0.1, 0.2, 0.5, 1, 2, 4, 8, 16, and 30 s. The acquisition parameters were as follows: spectral width, 15 ppm; center frequency (O1), 1975 Hz; number of scans, 16; size of the fid (TD), 64 k; relaxation delay (D1), 30 s; and temperature, 25 °C.
Subsequently, a noesygppr1d pulse sequence was applied, including pr-saturation, volume selection, and a gradient pulse to effectively suppress large solvent peaks. The specific parameters and commands were as follows: pulse sequence, noesygppr1d; RF field strength for presaturation, 25 Hz; receiver gain, 14.2; spectral width, 15 ppm; center frequency (O1), 1975 Hz; number of scans, 32; TD, 128 k; zero filling, 128 k; acquisition time, 7.27 s; D1, 40 s; and temperature, 25 °C. Before conducting the quantitative experiments, an automatic pulse calibration is first performed by using the pulsecal command, followed by automatic tuning.
2.4. Solution Preparation
2.4.1. Preparation of Internal Standard Solutions
Internal standard solutions were prepared by weighing 50 mg of DMT into volumetric flasks and diluting it to specified volumes with DMSO-d6. Internal standard solutions 1, 2, and 3 were prepared with volumes of 10, 25, and 50 mL, respectively.
2.4.2. Preparation of Test Solutions
For 56- and 42 mg test solutions, one bottle of microspheres was used, with the aluminum cap and label removed and the bottle cleaned with ethanol. The bottle was then weighed. Subsequently, 2.8 mL of DMSO-d6 was added to the bottle, and the mixture was sonicated until fully dissolved. The resulting solution was then transferred to a 5 mL volumetric flask. The microsphere bottle was rinsed with 0.5 mL of DMSO-d6, and the rinse liquid was added to the flask, with the rinsing process repeated twice. Next, 1 mL of internal standard solution 1 was added to the 5 mL volumetric flask, the volume was adjusted to the mark, and the mixture was thoroughly mixed. A 0.6 mL aliquot of the supernatant was then extracted for NMR analysis. Additionally, the bottle and stopper were rinsed with anhydrous ethanol three times, dried at 40 °C for 2 h, and then cooled in a desiccator for 30 min at 25 °C. The bottle was then weighed again to calculate the weight of the test sample.
For 28 mg test solution, two bottles of microspheres were used, with 1.4 mL of DMSO-d6 added to each. The solutions were sonicated until fully dissolved, and then combined in a single 5 mL volumetric flask. After the transfer, each bottle was rinsed with 0.25 mL of solvent, and the rinse liquid was then added to the volumetric flask; the rinsing process was repeated twice. The remaining procedures were identical to those for the preparation of the 56- and 42 mg test solutions.
For 14 mg test solution, four bottles of microspheres were used, with 0.7 mL of DMSO-d6 added to each. The solutions were sonicated until fully dissolved, and then combined in a single 5 mL volumetric flask. After the transfer, each bottle was rinsed with 0.1 mL of solvent, and the rinse liquid was added to the volumetric flask; the rinsing process was repeated twice. The remaining procedures were identical to those for the preparation of the 56 and 42 mg test solutions.
2.4.3. Preparation of Calibration Solutions
Rotigotine reference standards, weighing 11, 15, 20, 25, 30, and 35 mg, were placed in separate 5 mL EP tubes. Mannitol reference standards, weighing 1, 2, 3, 4, 5, and 6 mg, were also placed in separate 5 mL EP tubes. Similarly, stearic acid reference standards, weighing 1, 1.5, 2, 2.5, 3, and 3.5 mg, were added to different 5 mL EP tubes. Each set of standards was dissolved in 2 mL of internal standard solution 3.
2.4.4. Preparation of Repeatability Solutions
Repeatability solution (1) was prepared using samples from batch A according to the method described in
Section 2.4.2. Six parallel samples were prepared, and the content of rotigotine, mannitol, and stearic acid was simultaneously determined.
Repeatability solution (2) was prepared using five bottles of batch A microspheres, the contents of which were ground thoroughly and transferred to 5 mL EP tubes. The microspheres were dispersed evenly by shaking. Subsequently, 55 mg of the sample was weighed into each 5 mL EP tube, and then 1 mL of internal standard solution 3 was added. The mixture was then sonicated for 30 min to ensure dissolution. A 0.6 mL aliquot of the supernatant was then extracted for NMR analysis, with six parallel samples prepared.
2.4.5. Preparation of Recovery Solutions
Rotigotine, stearic acid, and mannitol reference solutions were prepared using DMSO-d6 to achieve final concentrations of 12.6, 1.45, and 1.7 mg/mL, respectively.
For the rotigotine recovery solution, 55 mg of microspheres was combined with 1 mL of internal standard solution 2 and 1 mL of rotigotine reference solution, and sonicated until dissolved. Six parallel samples were prepared.
For the stearic acid recovery solution, 55 mg of microspheres was combined with 1 mL of internal standard solution 2 and 1 mL of stearic acid reference solution, and sonicated until dissolved. Six parallel samples were prepared.
For mannitol recovery solution, five bottles of batch A were extracted, ground thoroughly, placed in EP tubes, and shaken to ensure uniform dispersion of mannitol. Subsequently, 55 mg of the sample was accurately weighed into a 5 mL EP tube and combined with 1 mL of internal standard solution 2 and 1 mL of mannitol reference solution. The mixture was sonicated for 30 min and thoroughly shaken. A 0.6 mL aliquot was then extracted for NMR analysis.
3. Results and Discussion
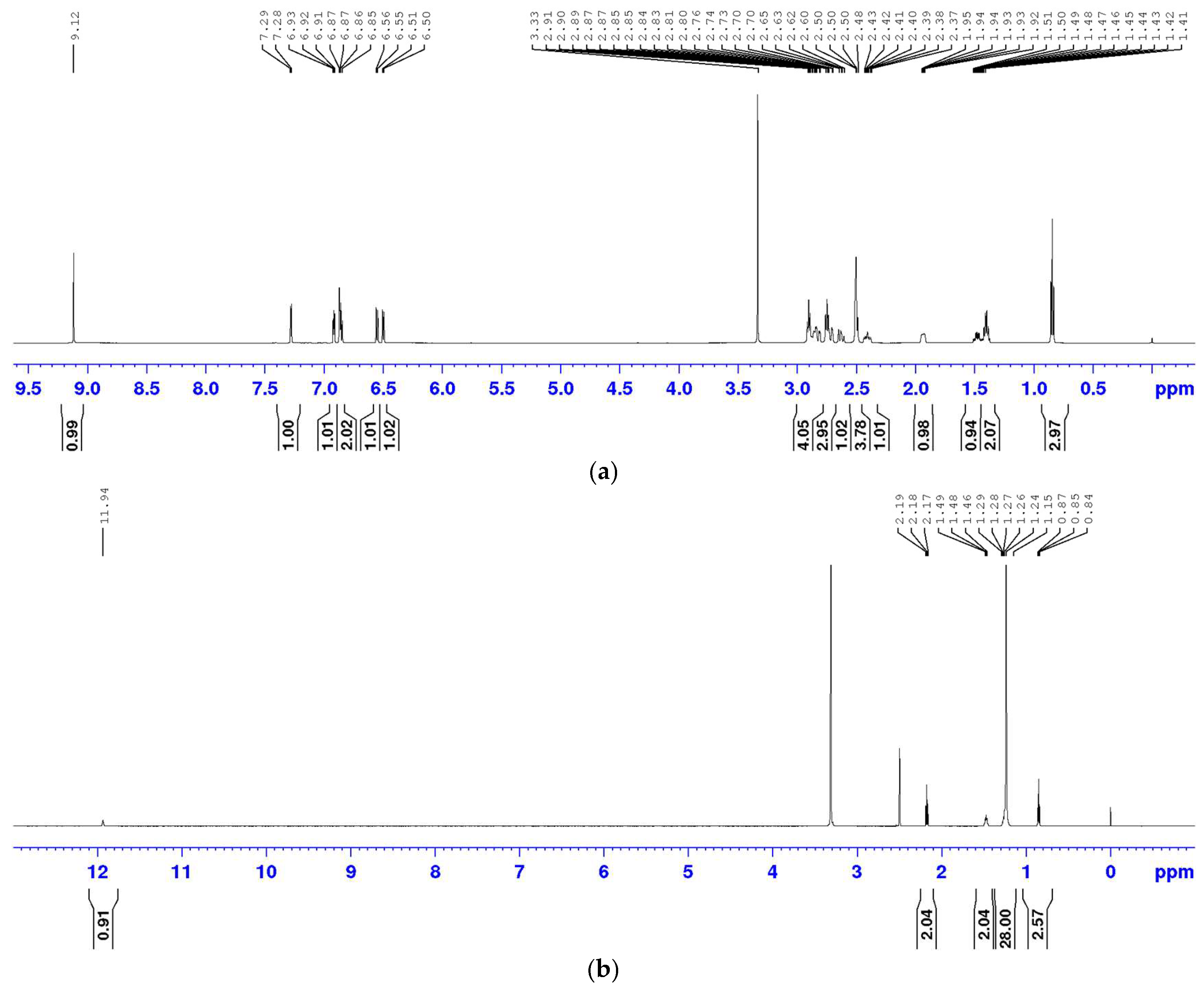
3.1. 1H NMR Spectra of Rotigotine, Stearic Acid, and Mannitol
The
1H NMR spectra of rotigotine, stearic acid, and mannitol reference standards were acquired (
Figure 1), and their corresponding signals were assigned. The structures of rotigotine, stearic acid, and mannitol are depicted in
Figure 2.
The
1H NMR spectrum of rotigotine, presented in
Figure 1a, is
1H NMR (DMSO-
d6, 600 M)
δ 9.13 (s, 1H, 1-OH), 7.28 (d,
J = 5.1Hz; 1H, 19-H), 6.92 (dd, J = 5.0, 3.5 Hz; 1H, 18-H), 6.89–6.83 (m, 2H, 17-H, 3-H), 6.55 (d, J = 7.9 Hz, 1H, 4-H), 6.50 (d, J = 7.6 Hz, 1H, 2-H), 2.96–2.78 (m, 4H, 15-H, 6-H, 8-H), 2.78–2.67 (m, 3H, 14-H, 5-H), 2.62 (dd, J = 16.1, 11.2 Hz; 1H, 5-H), 2.51–2.45 (m, 2H, 11-H), 2.45–2.33 (m, 1H, 8-H), 2.00–1.85 (m, 1H, 7-H), 1.48 (qd, J = 12.0, 5.6 Hz; 1H, 7-H), 1.40 (h, J = 7.3, 2H, 12-H), and 0.84 (t, J = 7.3 Hz, 3H, 13-H).
The
1H NMR spectrum of stearic acid, shown in
Figure 1b, is
1H NMR (DMSO-
d6, 600 M)
δ 11.94 (s, 1H, 1-OH), 2.18 (t, J = 7.51 Hz, 2H, 2-H), 1.47 (t, J = 6.9 Hz, 2H, 3-H), 1.10–1.35 (m, 28H, 4–17-H), and 0.85 (t, J = 6.3 Hz, 3H, 18-H).
The
1H NMR spectrum of mannitol, depicted in
Figure 1c, is 4.40 (d, J = 5.55 Hz, 2H, 2,5-OH), 4.32 (t, J = 5.72 Hz, 2H, 1,6-OH), 4.13 (d, J = 7.08 Hz, 2H, 3,4-OH), 3.65–3.57 (m, 2H, 1,6-H), 3.54 (d, J = 7.8 Hz, 2H, 3,4-OH), 3.49–3.41 (m, 2H, 2,5-H), and 3.40–3.28 (m, 2H, 1,6-H).
3.2. Selection of Internal Standard and Signal Peaks
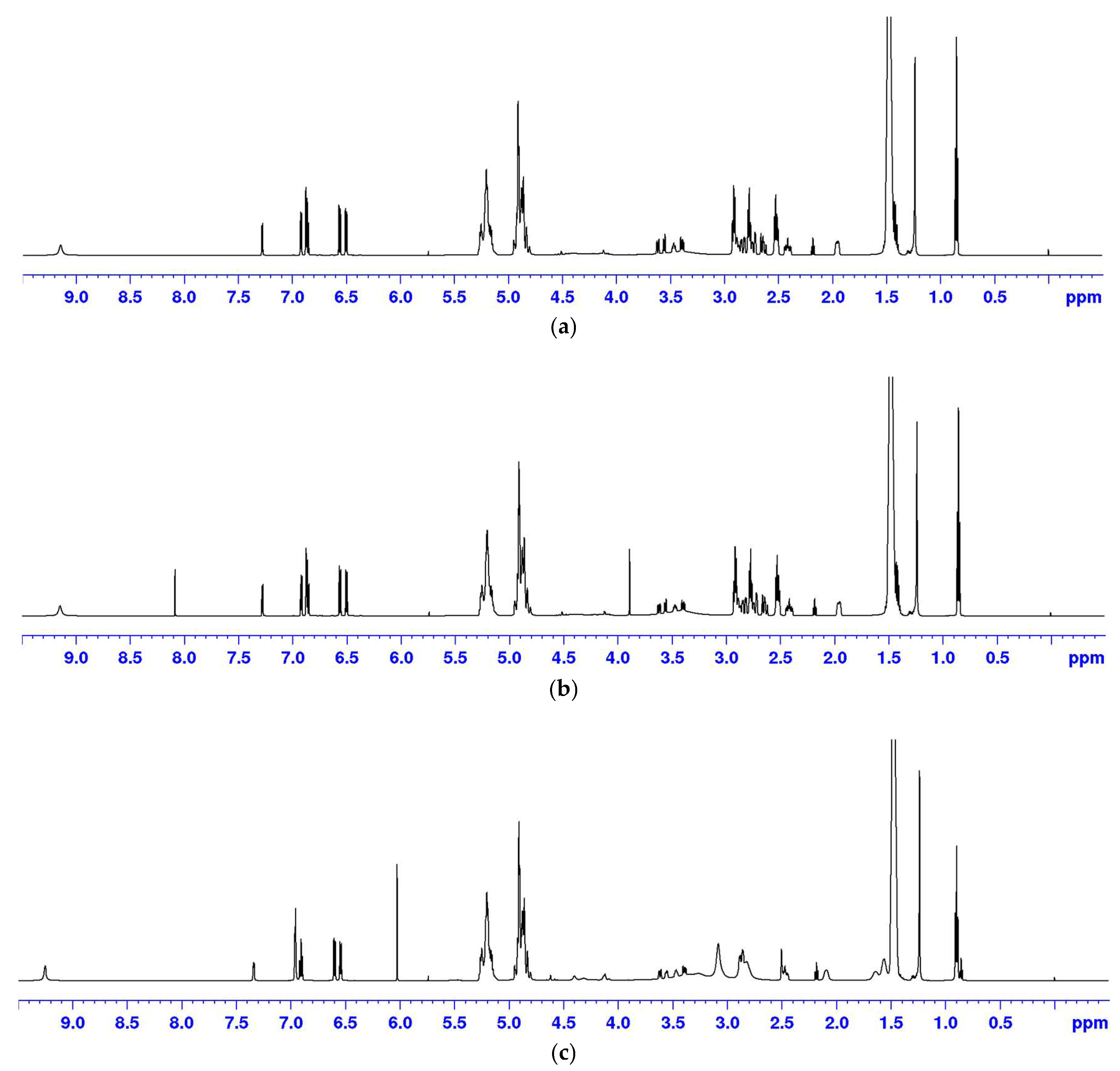
Using DMSO-d6 as the solvent, the suitability of DMT and MA as internal standards was evaluated. Specifically, microsphere samples were prepared with DMT and MA, and the corresponding 1H NMR spectrum was acquired.
Figure 3a shows the
1H NMR spectrum of the test sample.
Figure 3b illustrates the spectrum with DMT as the internal standard. Finally,
Figure 3c depicts the spectrum with MA as the internal standard. When MA was used as the internal standard, large signal shifts were observed, particularly affecting the resolution of the quantitation peak for stearic acid at
δ 2.18 ppm. This shift was attributed to pH changes in the solution caused by the use of MA as the internal standard. Conversely, DMT did not affect the signal peaks of the sample, and its signal peaks exhibited no interference with the sample peaks. Therefore, DMT was selected as the internal standard for this study.
For rotigotine, the peak at δ 7.28 ppm was chosen as the quantitation peak. For stearic acid, the triplet at δ 2.18 ppm was selected. Both peaks showed good baseline separation and had simple peak shapes. The quantitation of mannitol focused on the regions of δ 3.69–3.60, δ 3.60–3.50, δ 3.51–3.45, and δ 3.45–3.36 ppm, corresponding to four groups of non-exchangeable hydrogens.
3.3. Optimization of Experimental Methods
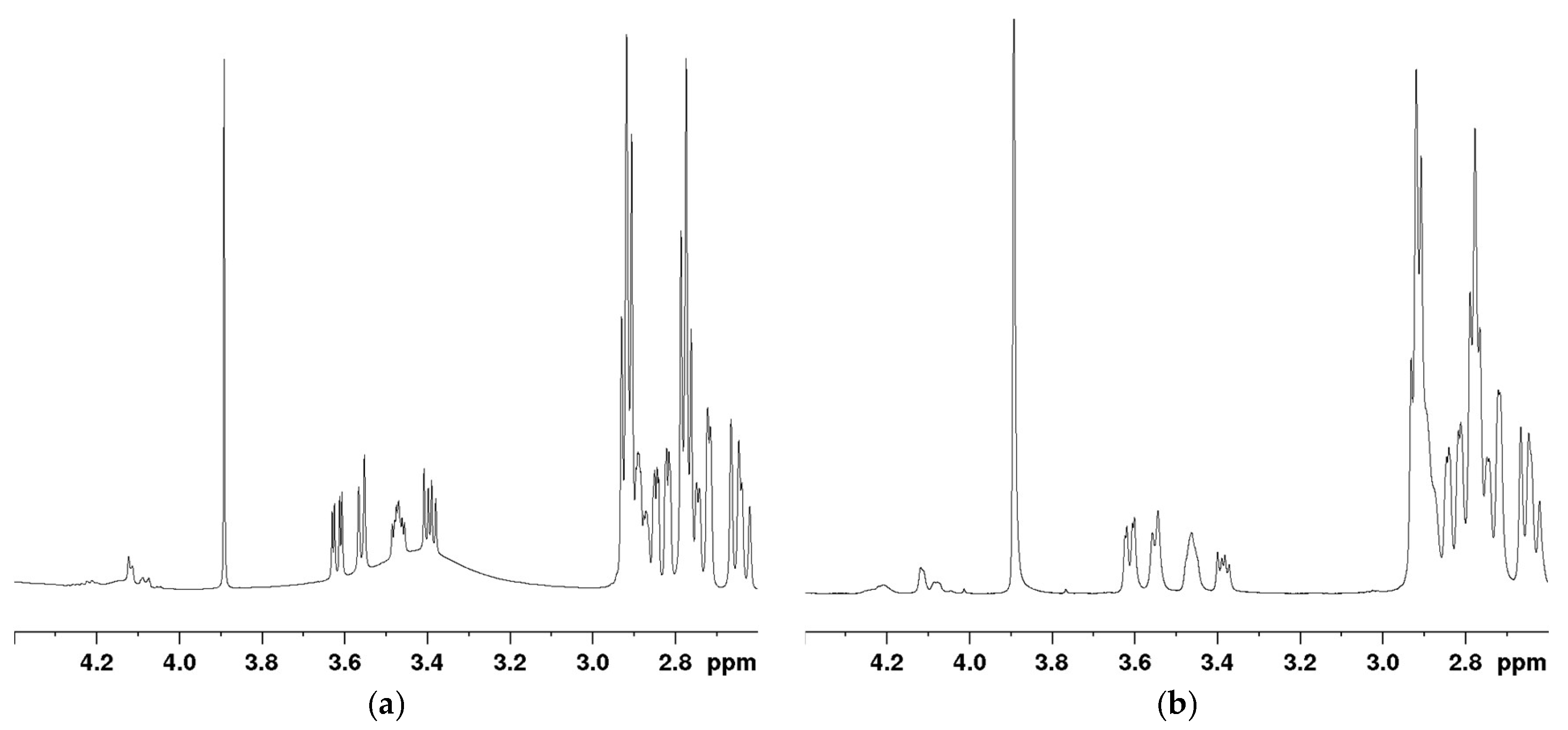
Conducting a standard
1H NMR analysis resulted in significant interference from the water peak within the mannitol signal peaks, making accurate quantitation of mannitol impossible (
Figure 4a). To address this issue, a solvent-suppressed
1H NMR analysis was instead performed using the noesygppr1d pulse sequence. This sequence includes pre-saturation, volume selection, and gradient pulse, making it particularly useful for suppressing large solvent peaks. The O1 was set to 1975 Hz (3.291 ppm). The spectrum obtained after solvent suppression is shown in
Figure 4b, where no interference was observed in the signal peaks of the API and stearic acid, and the baseline around the mannitol signals was improved. Consequently, this solvent-suppressed
1H NMR method was chosen for quantifying all components in the microsphere samples.
3.4. Setting D1 Value
In quantitative NMR, accurate integration requires that the D1 be at least five times the T1. T1 describes the recovery of nuclear longitudinal magnetization to its initial equilibrium state after excitation by a radiofrequency pulse. It is defined as the time required for the longitudinal magnetization vector (Mz) to recover to 63% of its equilibrium value. In science, the principle of 1D NMR quantification relies on the proportionality between peak area and the number of corresponding nuclei; complete recovery of longitudinal magnetization is essential. Therefore, T1 must be carefully considered.
The T1 of each component and the internal standard in the microsphere samples were determined using the inversion-recovery method. The T1 values for various signals of the components are presented in
Table 1. To ensure accurate quantitative results, D1 is set to be at least 5 times T
1 to allow full proton relaxation [
21,
25]. According to this criterion, the D1 setting for this experiment should be 30 s. However, to maximize the recovery of the macroscopic magnetization vector along the
Z-axis, D1 was ultimately set to 40 s, which was sufficient to meet the requirements for quantification.
3.5. Linearity and Range
A linear regression analysis was performed with concentration (x) as the independent variable and the ratio of the peak area of the analyte signal to that of the internal standard (y) as the dependent variable. For rotigotine at δ 7.28 ppm, the linear regression equation was y = 0.1481x – 0.0053, with an r value of 1.0000, indicating good linearity in the concentration range of 5.52–17.55 mg/mL.
For stearic acid at δ 2.18 ppm, the linear regression equation was y = 0.3339x − 0.0262, with an r value of 0.9989, indicating good linearity in the concentration range of 0.54–1.84 mg/mL.
For mannitol, at δ 3.69–3.60 ppm, the linear regression equation was y = 0.5077x − 0.0048, with an r value of 0.9999; at δ 3.60–3.50 ppm, the equation was y = 0.4933x − 0.0045, with an r value of 0.9994; at δ 3.51–3.45 ppm, the equation was y = 0.496x − 0.0097, with an r value of 0.9992; and at δ 3.45–3.36 ppm, the equation was y = 0.2832x − 0.0067, with an r value of 0.9987. Collectively, these results indicate good linearity for mannitol in the concentration range of 0.51–3.10 mg/mL.
The solvent suppression O1 value, set to 1975 Hz, was close to that of the mannitol signal at δ 3.45–3.36 ppm. Consequently, this signal was partially suppressed, as reflected in the lower slope of its linear regression equation than that of the other three signals, where each signal corresponds to two hydrogen atoms. This suppression suggests that calculations based on this signal would be inaccurate. Therefore, for the quantification of mannitol, the average of the peak areas at δ 3.69–3.60 ppm, δ 3.60–3.50 ppm, and δ 3.51–3.45 ppm was used.
3.6. Specificity
In the test solution, the internal standard peak at a chemical shift of δ 8.10 ppm was selected as the quantification peak. For rotigotine, the quantification peak was located at δ 7.28 ppm. Alternatively, stearic acid was quantified using the peak between δ 2.24 and 2.15 ppm. Lastly, mannitol was quantified using peaks at δ 3.69–3.60 ppm, δ 3.60–3.50 ppm, and δ 3.51–3.45 ppm. There was no interference between the internal standard peak and the signal peaks of the analytes, confirming good specificity.
3.7. Precision
The precision of the method was evaluated by analyzing the test solution of batch A six times. After integration, the relative standard deviation (RSD) of each component was calculated by comparing the peak area ratios of the analytes to the internal standard. The precision value for rotigotine, stearic acid, and mannitol was 0.3%, 1.0%, and 1.2%, respectively, all below the 2.0% threshold, indicating superior precision of the proposed method.
3.8. Stability of the Test Solution
The stability of the test solution was assessed over 60 h. The RSD values for rotigotine, mannitol, and stearic acid were 0.4%, 0.5%, and 0.7%, respectively, all remaining under 1.0%. This finding demonstrates that the test solution maintained its stability throughout the 60 h testing period.
3.9. Repeatability
A sample of batch A was prepared according to the method described in
Section 2.4.4 to evaluate the measurement repeatability of each component. Notably, mannitol was not incorporated within the microspheres but served as a lyophilization protectant on the outer surface of the microsphere, leading to its uneven distribution in the sample. Nevertheless, owing to the low mannitol content, the variation between vials did not affect the overall content uniformity of the product.
When following the preparation method described in
Section 2.4.4 for repeatability solution (1), where microspheres were dissolved directly in their original vials, slight variations in mannitol content between vials, caused by the solid-form packaging, led to high RSD values, as shown for Mannitol (1) in
Table 2. To mitigate this issue, the method was adjusted to instead follow the preparation method presented in
Section 2.4.4 for repeatability solution (2), where the sample was ground and shaken to distribute the mannitol more evenly before measuring its content. This modification significantly improved the RSD of repeatability measurements, as shown for Mannitol (2) in
Table 2. The slightly lower content than that observed in method (1) was because of mannitol adhering to the container walls. The results in
Table 2 confirm that the proposed method exhibited good repeatability, with the high RSD for Mannitol (1) attributed to the nature of the sample itself rather than the experimental procedure.
3.10. Recovery Rate
A sample of batch A was used to prepare the recovery rate solution according to the method described in
Section 2.4.5, and the recovery rates of each component were assessed. The results in
Table 3 indicate that the recovery rates for rotigotine, stearic acid, and mannitol were between 98% and 102%, demonstrating good accuracy.
3.11. Content Determination
The content of rotigotine prolonged-release microspheres for injection was determined for seven batches (B–H) of different specifications. The results are presented in
Table 4.
4. Conclusions
The primary challenge encountered in this study was the proximity of the mannitol signal peak to the water peak, which caused baseline distortions. To mitigate this challenge, we employed a solvent-suppressed 1H NMR technique, exercising caution while selecting signals not affected by these suppression pulses. Additionally, as mannitol was not uniformly dispersed within the microspheres, the measured mannitol content was influenced by the sampling method. To accurately determine the overall mannitol content, samples had to be dissolved in the drug vial and then transferred to a volumetric flask prior to the measurement. However, when using the method, the non-uniform distribution of mannitol led to potential inaccuracies in measurements. To address this issue, microspheres were ground and thoroughly shaken to achieve a more uniform distribution of mannitol before preparation for testing. This modification was subsequently employed when determining mannitol content, demonstrating that the high RSD value observed was because of sample variability rather than the method itself.
In this study, we developed and validated a qNMR method for measuring the main component rotigotine and its key excipients, mannitol and stearic acid, in rotigotine prolonged-release microspheres for injection using the solvent-suppressed 1H NMR technique. The proposed method showed favorable results and was applied to determine the content of these components in eight batches of four different microsphere specifications. The results confirmed that the method effectively quantifies rotigotine, mannitol, and stearic acid simultaneously.
HPLC methods for the analysis of rotigotine prolonged-release microspheres for injection require the detection of the three components: rotigotine, mannitol, and stearic acid. However, due to their distinct physicochemical properties, the standard HPLC method faces challenges in efficiency and resource consumption due to their distinct physicochemical properties. Conventionally, three separate HPLC methods are required (
Table 5). Each method demands not only specific sample preparation procedures and distinct chromatographic systems but also separate preparation of sample solutions for each component, resulting in a cumulative solvent consumption of about 120 mL, followed by analysis under respective chromatographic conditions, and analysis of three samples additionally consumes about 49 mL of mobile phase. Furthermore, each analysis strictly relies on specific reference standards (rotigotine, mannitol, stearic acid), necessitating independent preparation of reference solutions and parallel analysis as controls.
Our developed qNMR method demonstrates significant advantages in efficiency, economy, and sustainability. This approach requires only one sample and 5 mL of DMSO-d6 to simultaneously quantify all three components, with total solvent consumption substantially lower than that of HPLC. The entire detection process eliminates the need for mobile phases or additional reagents. Most strikingly, this method completely avoids the cumbersome preparation and analysis of specific reference standards (rotigotine, mannitol, stearic acid) and their solutions. Instead, only cost-effective DMT is needed as an internal standard, which is co-prepared with the sample to achieve accurate quantification.
Thus, the qNMR method is operationally simple and efficient, with its core advantage lying in synchronous multi-component analysis, significantly reducing labor costs and time expended. Critically, by entirely eliminating specific reference standards and drastically cutting solvent/reagent usage, this method effectively lowers economic costs and environmental impact, fully aligning with the principles of green analytical chemistry. This strategy can be extended to other microsphere systems, even other special drug delivery route drug systems and difficult-to-separate systems, offering unique value for the quality control of unconventional dosage forms.