Molecular Nanomagnets with Photomagnetic Properties: Design Strategies and Recent Advances
Abstract
1. Introduction
2. Design Strategies for Molecular Nanomagnets with Photomagnetic Properties
2.1. Photoresponsive Spin-Crossover Units
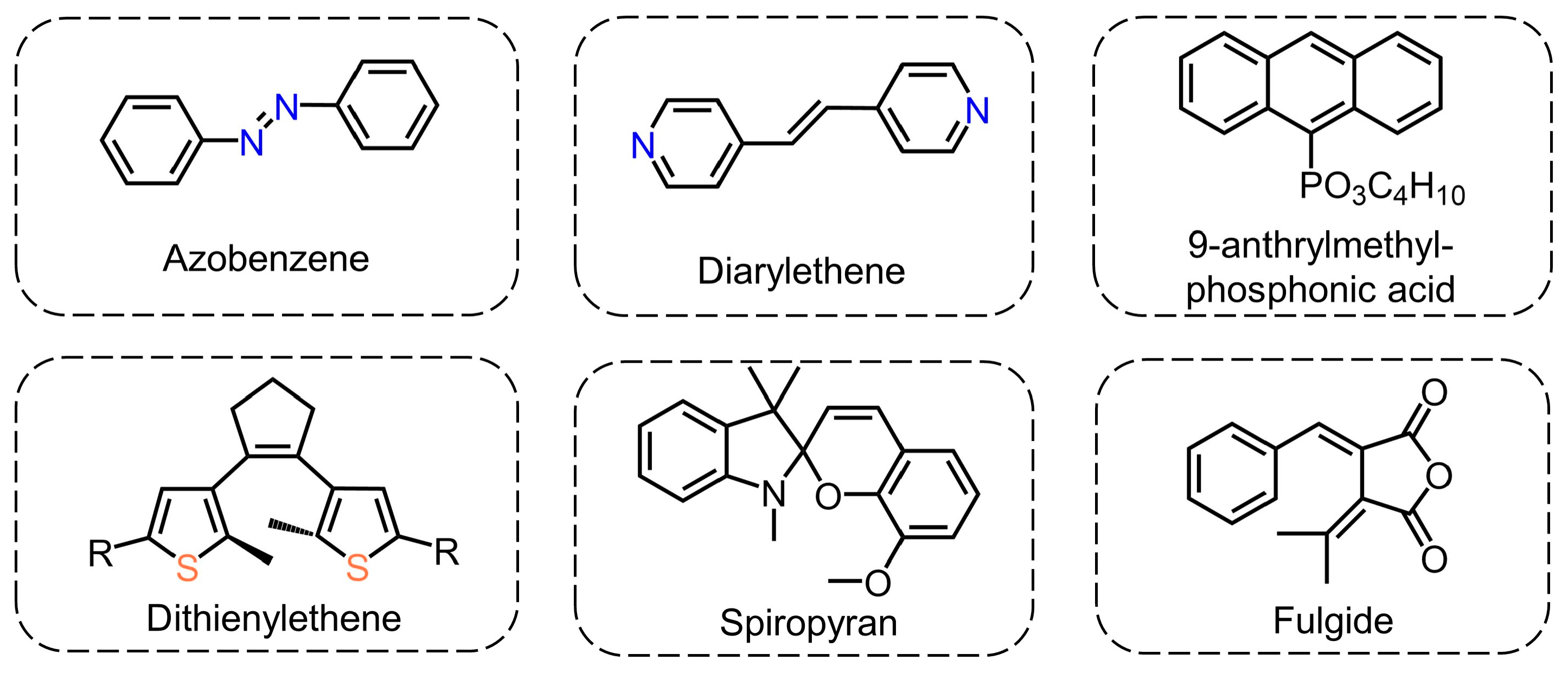
2.2. Photoinduced Structural Change in Organic Molecules
2.3. Photogenerated Organic Radicals
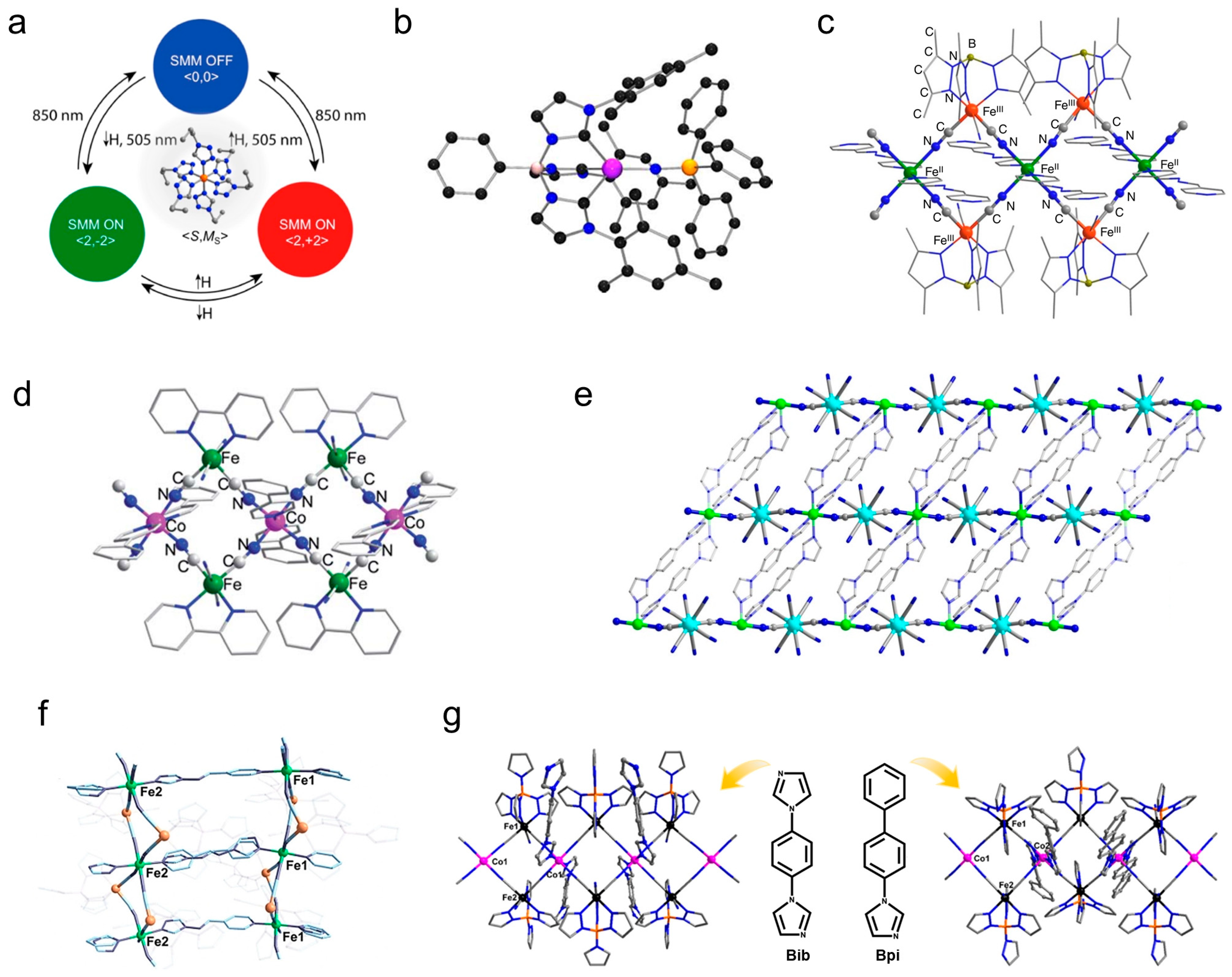
3. Photomagnetic Properties of Transition Metal Molecular Nanomagnets
3.1. Photomagnetic Properties of SCO-Type Transition Metal Molecular Nanomagnets
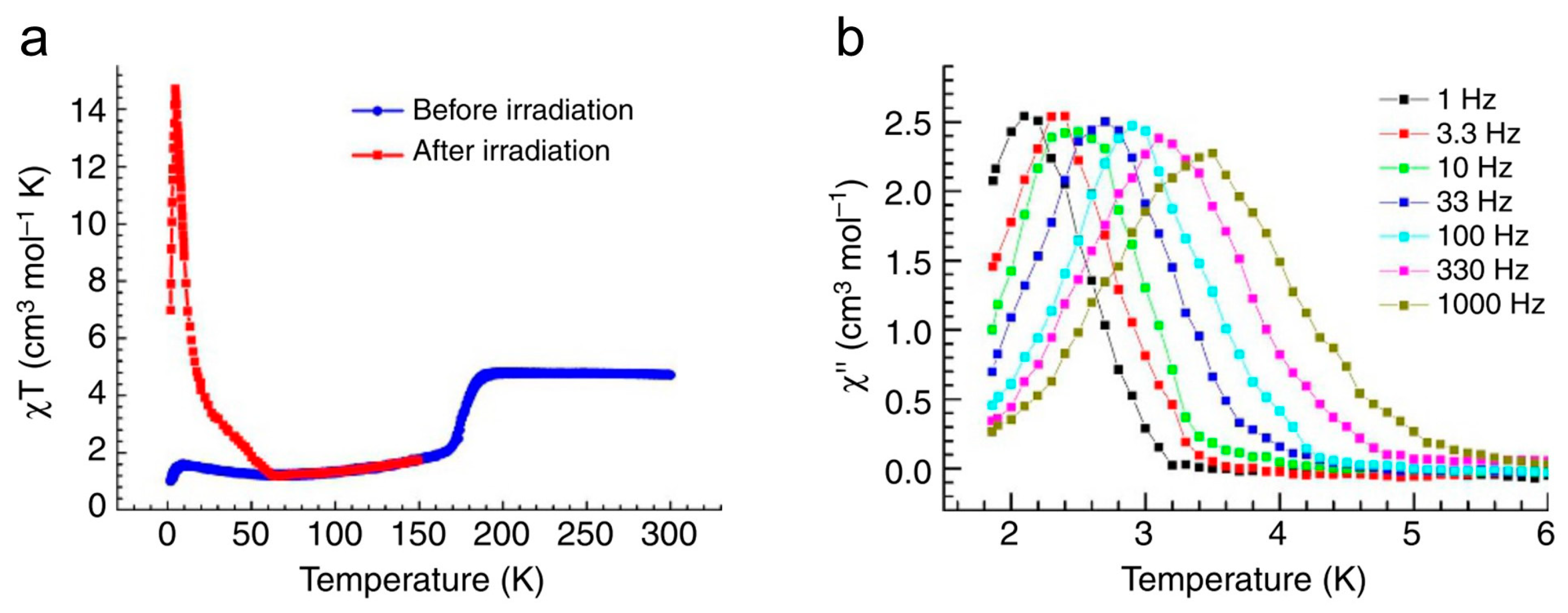
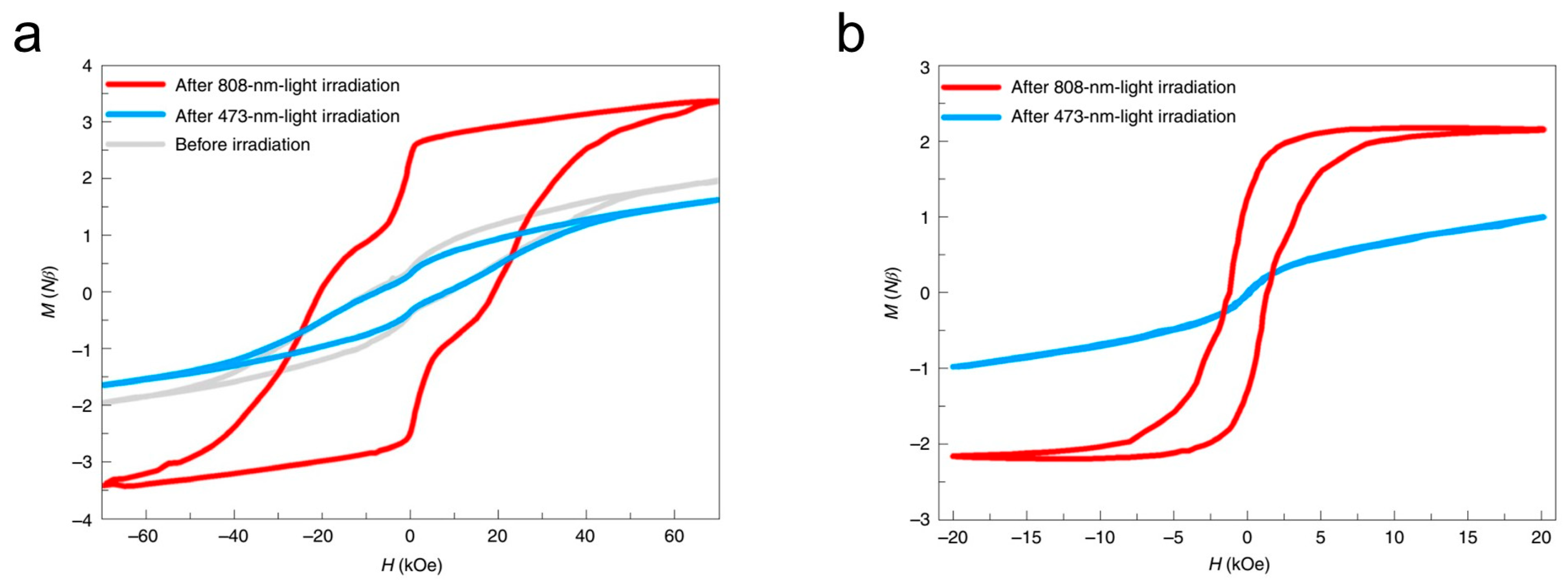
3.2. Photomagnetic Properties of Other Transition Metal Molecular Nanomagnets
4. Photomagnetic Properties of Lanthanide Molecular Nanomagnets
4.1. Photomagnetic Properties of Photocyclization-Type Lanthanide Molecular Nanomagnets
4.2. Photomagnetic Properties of Photogenerated Radical-Type Lanthanide Molecular Nanomagnets
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferreira, F.; Moreira, S.; Zhao, M.; Barriga, E.H. Stretch-induced endogenous electric fields drive directed collective cell migration in vivo. Nat. Mater. 2025, 24, 462–470. [Google Scholar] [CrossRef]
- Ghitani, N.; von Buchholtz, L.J.; MacDonald, D.I.; Falgairolle, M.; Nguyen, M.Q.; Licholai, J.A.; Ryba, N.J.P.; Chesler, A.T. A distributed coding logic for thermosensation and inflammatory pain. Nature 2025, 642, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, Q.-Q.; Chen, M.-H.; Xu, J.-J.; Chen, H.-Y.; Zhao, W.-W. Organic photoelectrochemical memtransistor. eScience 2025, 5, 100374. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Su, D.; Ma, Y.; Sun, Y.; Cheng, P. Electrical detection and modulation of magnetism in a Dy-based ferroelectric single-molecule magnet. Nat. Commun. 2023, 14, 7901. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Yan, S.-K.; Liang, S.; Zhao, Y.-R.; Zhang, J.; Wang, G.-M.; Hu, J.-X. Synergistic photomagnetic and photomechanical dynamics in a dysprosium-based smart molecule. Adv. Sci. 2025, e09088. [Google Scholar] [CrossRef] [PubMed]
- Raju, M.S.; Paillot, K.; Breslavetz, I.; Novitchi, G.; Rikken, G.L.J.A.; Train, C.; Atzori, M. Optical readout of single-molecule magnets magnetic memories with unpolarized light. J. Am. Chem. Soc. 2024, 146, 23616–23624. [Google Scholar] [CrossRef]
- Wang, J.; Zakrzewski, J.J.; Zychowicz, M.; Xin, Y.; Tokoro, H.; Chorazy, S.; Ohkoshi, S. Desolvation-induced highly symmetrical terbium(III) single-molecule magnet exhibiting luminescent self-monitoring of temperature. Angew. Chem. Int. Ed. 2023, 62, e202306372. [Google Scholar] [CrossRef]
- Magott, M.; Brzozowska, M.; Baran, S.; Vieru, V.; Pinkowicz, D. An intermetallic molecular nanomagnet with the lanthanide coordinated only by transition metals. Nat. Commun. 2022, 13, 2014. [Google Scholar] [CrossRef]
- Gatteschi, D.; Sessoli, R.; Villain, J. Molecular Nanomagnets; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Rodolphe, C.; Hitoshi, M.; Masahiro, Y.; Claude, C. Evidence for single-chain magnet behavior in a Mn(III)-Ni(II) chain designed with high spin magnetic units: A route to high temperature metastable magnets. J. Am. Chem. Soc. 2002, 124, 12837–12844. [Google Scholar] [CrossRef]
- Liu, X.; Feng, X.; Meihaus, K.R.; Meng, X.; Zhang, Y.; Li, L.; Liu, J.L.; Pedersen, K.S.; Keller, L.; Shi, W.; et al. Coercive fields above 6T in two cobalt(II)-radical chain compounds. Angew. Chem. Int. Ed. 2020, 59, 10610–10618. [Google Scholar] [CrossRef]
- Wu, Y.; Ruan, Z.-Y.; Zhang, C.; Chen, J.-N.; Wang, X.-Y.; Chen, Z.; Gou, X.; Lan, W.; Kong, X.-J.; Liu, J.-L.; et al. Strong and switchable magneto-optical effect in air-stable chiral DyIII complexes with magnetic anisotropy. J. Am. Chem. Soc. 2025, 147, 20799–20806. [Google Scholar] [CrossRef] [PubMed]
- Marin, R.; Brunet, G.; Murugesu, M. Shining new light on multifunctional lanthanide single-molecule magnets. Angew. Chem. Int. Ed. 2021, 60, 1728–1746. [Google Scholar] [CrossRef] [PubMed]
- Gou, X.; Wu, Y.; Wang, M.; Liu, N.; Lan, W.; Zhang, Y.-Q.; Shi, W.; Cheng, P. The influence of light on the field-induced magnetization dynamics of two Er(III) coordination polymers with different halogen substituents. Dalton Trans. 2024, 53, 148–152. [Google Scholar] [CrossRef]
- Zhao, L.; Meng, Y.S.; Liu, Q.; Sato, O.; Shi, Q.; Oshio, H.; Liu, T. Switching the magnetic hysteresis of an [Fe(II)-NC-W(V)]-based coordination polymer by photoinduced reversible spin crossover. Nat. Chem. 2021, 13, 698–704. [Google Scholar] [CrossRef]
- Errulat, D.; Harriman, K.L.M.; Gálico, D.A.; Kitos, A.A.; Mansikkamäki, A.; Murugesu, M. A trivalent 4f complex with two bis-silylamide ligands displaying slow magnetic relaxation. Nat. Chem. 2023, 15, 1100–1107. [Google Scholar] [CrossRef]
- Meihaus, K.R.; Minasian, S.G.; Lukens, W.W., Jr.; Kozimor, S.A.; Shuh, D.K.; Tyliszczak, T.; Long, J.R. Influence of pyrazolate vs N-heterocyclic carbene ligands on the slow magnetic relaxation of homoleptic trischelate lanthanide(III) and uranium(III) complexes. J. Am. Chem. Soc. 2014, 136, 6056–6068. [Google Scholar] [CrossRef]
- Gao, C.; Genoni, A.; Gao, S.; Jiang, S.; Soncini, A.; Overgaard, J. Observation of the asphericity of 4f-electron density and its relation to the magnetic anisotropy axis in single-molecule magnets. Nat. Chem. 2019, 12, 213–219. [Google Scholar] [CrossRef]
- Guo, F.S.; Day, B.M.; Chen, Y.C.; Tong, M.L.; Mansikkamäki, A.; Layfield, R.A. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef]
- Goodwin, C.A.P.; Ortu, F.; Reta, D.; Chilton, N.F.; Mills, D.P. Molecular magnetic hysteresis at 60 kelvin in dysprosocenium. Nature 2017, 548, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fang, Y.; Liu, S.; Hu, M.; Wang, W. Slow magnetic relaxation in a 3D dysprosium(III)-fluoro-oxalate framework containing zig-zag [Dy-F] chains. J. Rare Earths 2023, 41, 100–107. [Google Scholar] [CrossRef]
- Liao, X.; Chen, Y.; Xie, T.; Sun, R.J.; Yang, L.Z.; Liu, C.F.; Wang, R.; Klyatskaya, S.; Ruben, M.; Zhang, W.; et al. Spatially dependent f-pi exchange interaction within a single-molecule magnet TbPc2. Nat. Commun. 2025, 16, 6263. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, Q.; Jin, P.; Zhai, Y.; Zheng, Y. Fine tuning dynamic magnetism of dysprosiacarboranyl sandwiches. J. Rare Earths 2025, 43, 1205–1212. [Google Scholar] [CrossRef]
- Khariushin, I.V.; Ovsyannikov, A.S.; Islamov, D.R.; Samigullina, A.I.; Solovieva, S.E.; Zakrzewski, J.J.; Chorazy, S.; Ferlay, S. Tuning crystal packing and magnetic properties in a series of [Dy12] metallocubanes based on azobenzene derivatives of salicylic acid. Inorg. Chem. 2023, 62, 10548–10558. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-M.; Xu, F.; Han, S.-D.; Pan, J.; Wang, G.-M. Hybrid photochromic lanthanide phosphonate with multiple photoresponsive functionalities. Inorg. Chem. 2022, 61, 8379–8385. [Google Scholar] [CrossRef]
- Bartolomé, E.; Arauzo, A.; Luzón, J.; Gasque, L. Light-induced magnetic switching in a coumarin-based Tb single molecule magnet. J. Mater. Chem. C 2025, 13, 831–841. [Google Scholar] [CrossRef]
- Wang, L.-F.; Qiu, J.-Z.; Liu, J.-L.; Chen, Y.-C.; Jia, J.-H.; Jover, J.; Ruiz, E.; Tong, M.-L. Modulation of single-molecule magnet behaviour via photochemical [2 + 2] cycloaddition. Chem. Commun. 2015, 51, 15358–15361. [Google Scholar] [CrossRef]
- Huang, X.-D.; Xu, Y.; Fan, K.; Bao, S.-S.; Kurmoo, M.; Zheng, L.-M. Reversible SC-SC Transformation involving [4 + 4] cycloaddition of anthracene: A single-ion to single-molecule magnet and yellow-green to blue-white emission. Angew. Chem. Int. Ed. 2018, 57, 8577–8581. [Google Scholar] [CrossRef]
- Hojorat, M.; Al Sabea, H.; Norel, L.; Bernot, K.; Roisnel, T.; Gendron, F.; Guennic, B.L.; Trzop, E.; Collet, E.; Long, J.R.; et al. Hysteresis photomodulation via single-crystal-to-single-crystal isomerization of a photochromic chain of dysprosium single-molecule magnets. J. Am. Chem. Soc. 2020, 142, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-J.; Hu, J.-X.; Han, S.-D.; Pan, J.; Li, J.-H.; Wang, G.-M. Manipulating on/off single-molecule magnet behavior in a Dy(III)-based photochromic complex. J. Am. Chem. Soc. 2020, 142, 2682–2689. [Google Scholar] [CrossRef]
- Feng, X.; Mathoniere, C.; Jeon Ie, R.; Rouzieres, M.; Ozarowski, A.; Aubrey, M.L.; Gonzalez, M.I.; Clerac, R.; Long, J.R. Tristability in a light-actuated single-molecule magnet. J. Am. Chem. Soc. 2013, 135, 15880–15884. [Google Scholar] [CrossRef] [PubMed]
- Mathonière, C.; Lin, H.J.; Siretanu, D.; Clerac, R.; Smith, J.M. Photoinduced single-molecule magnet properties in a four-coordinate iron(II) spin crossover complex. J. Am. Chem. Soc. 2013, 135, 19083–19086. [Google Scholar] [CrossRef]
- Liu, T.; Zheng, H.; Kang, S.; Shiota, Y.; Hayami, S.; Mito, M.; Sato, O.; Yoshizawa, K.; Kanegawa, S.; Duan, C. A light-induced spin crossover actuated single-chain magnet. Nat. Commun. 2013, 4, 2826. [Google Scholar] [CrossRef]
- Jiang, W.; Jiao, C.; Meng, Y.; Zhao, L.; Liu, Q.; Liu, T. Switching single chain magnet behavior via photoinduced bidirectional metal-to-metal charge transfer. Chem. Sci. 2018, 9, 617–622. [Google Scholar] [CrossRef]
- Zhou, X.; Yan, F.; Lyubartsev, A.; Shen, B.; Zhai, J.; Conesa, J.C.; Hedin, N. Efficient production of solar hydrogen peroxide using piezoelectric polarization and photoinduced charge transfer of nanopiezoelectrics sensitized by carbon quantum dots. Adv. Sci. 2022, 9, 2105792. [Google Scholar] [CrossRef] [PubMed]
- Homer, M.K.; Kuo, D.-Y.; Dou, F.Y.; Cossairt, B.M. Photoinduced charge transfer from quantum dots measured by cyclic voltammetry. J. Am. Chem. Soc. 2022, 144, 14226–14234. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Wu, X.; Chen, N.; Cao, M.; Sartin, M.M.; Ren, B. Photoinduced charge transfer from a semiconductor to a metal probed at the single-nanoparticle level. ACS Energy Lett. 2021, 6, 3473–3480. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Lu, Z.; Duan, J.; Fry, H.C.; Gosztola, D.J.; Maindan, K.; Rajasree, S.S.; Deria, P. Photoinduced charge transfer with a small driving force facilitated by exciplex-like complex formation in metal-organic frameworks. J. Am. Chem. Soc. 2021, 143, 15286–15297. [Google Scholar] [CrossRef]
- Cammarata, M.; Zerdane, S.; Balducci, L.; Azzolina, G.; Mazerat, S.; Exertier, C.; Trabuco, M.; Levantino, M.; Alonso-Mori, R.; Glownia, J.M.; et al. Charge transfer driven by ultrafast spin transition in a CoFe Prussian blue analogue. Nat. Chem. 2020, 13, 10–14. [Google Scholar] [CrossRef]
- Ahmad, I.; Akhtar, M.S.; Ahmed, E.; Ahmad, M. Aluminium and cerium co-doped ZnO nanoparticles: Facile and inexpensive synthesis and visible light photocatalytic performances. J. Rare Earths 2021, 39, 151–159. [Google Scholar] [CrossRef]
- Ishikawa, T.; Hayes, S.A.; Keskin, S.; Corthey, G.; Hada, M.; Pichugin, K.; Marx, A.; Hirscht, J.; Shionuma, K.; Onda, K.; et al. Direct observation of collective modes coupled to molecular orbital-driven charge transfer. Science 2015, 350, 1501–1505. [Google Scholar] [CrossRef]
- Kaneva, N.; Bachvarova-Nedelcheva, A. Photocatalytic efficiency of pure and palladium Co-catalytic modified binary system. Inorganics 2025, 13, 161. [Google Scholar] [CrossRef]
- Zoppellaro, G.; Medved, M.; Hrubý, V.; Zbořil, R.; Otyepka, M.; Lazar, P. Solvent controlled generation of spin active polarons in two-dimensional material under UV light irradiation. J. Am. Chem. Soc. 2024, 146, 15010–15018. [Google Scholar] [CrossRef] [PubMed]
- Widel, Z.X.W.; Alatis, J.A.; Stephenson, R.H.; Mastrocinque, F.; Wilcox, A.C.; Bullard, G.; Olivier, J.-H.; Bai, Y.; Zhang, P.; Beratan, D.N.; et al. Fluence-dependent photoinduced charge transfer dynamics in polymer-wrapped semiconducting single-walled carbon nanotubes. J. Am. Chem. Soc. 2024, 146, 31169–31176. [Google Scholar] [CrossRef] [PubMed]
- Shu, A.; Qin, C.; Li, M.; Zhao, L.; Shangguan, Z.; Shu, Z.; Yuan, X.; Zhu, M.; Wu, Y.; Wang, H. Electric effects reinforce charge carrier behaviour for photocatalysis. Energy Environ. Sci. 2024, 17, 4907–4928. [Google Scholar] [CrossRef]
- Royakkers, J.; Yang, H.; Gillett, A.J.; Eisner, F.; Ghosh, P.; Congrave, D.G.; Azzouzi, M.; Andaji-Garmaroudi, Z.; Leventis, A.; Rao, A.; et al. Synthesis of model heterojunction interfaces reveals molecular-configuration-dependent photoinduced charge transfer. Nat. Chem. 2024, 16, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Pearce, N.; Reynolds, K.E.A.; Kayal, S.; Sun, X.Z.; Davies, E.S.; Malagreca, F.; Schürmann, C.J.; Ito, S.; Yamano, A.; Argent, S.P.; et al. Selective photoinduced charge separation in perylenediimide-pillar[5]arene rotaxanes. Nat. Commun. 2022, 13, 415. [Google Scholar] [CrossRef]
- Zheng, F.; Dong, F.; Zhou, L.; Yu, J.; Luo, X.; Zhang, X.; Lv, Z.; Jiang, L.; Chen, Y.; Liu, M. Cerium and carbon-sulfur codoped mesoporous TiO2 nanocomposites for boosting visible light photocatalytic activity. J. Rare Earths 2023, 41, 539–549. [Google Scholar] [CrossRef]
- Pajuelo-Corral, O.; García, J.A.; Castillo, O.; Luque, A.; Rodríguez-Diéguez, A.; Cepeda, J. Single-ion magnet and photoluminescence properties of lanthanide(III) coordination polymers based on pyrimidine-4,6-dicarboxylate. Magnetochemistry 2021, 7, 8. [Google Scholar] [CrossRef]
- Fu, C.; Li, F.; Zhang, J.; Li, D.; Qian, K.; Liu, Y.; Tang, J.; Fan, F.; Zhang, Q.; Gong, X.Q.; et al. Site sensitivity of interfacial charge transfer and photocatalytic efficiency in photocatalysis: Methanol oxidation on anatase TiO2 nanocrystals. Angew. Chem. Int. Ed. 2021, 60, 6160–6169. [Google Scholar] [CrossRef] [PubMed]
- Graf, M.; Vonbun-Feldbauer, G.B.; Koper, M.T.M. Direct and broadband plasmonic charge transfer to enhance water oxidation on a gold electrode. ACS Nano 2021, 15, 3188–3200. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, G.; Guan, Z.; Yang, J.; Li, Q. Spatially separating redox centers and photothermal effect synergistically boosting the photocatalytic hydrogen evolution of ZnIn2S4 Nanosheets. Small 2021, 17, 2006952. [Google Scholar] [CrossRef]
- Delgado, T.; Pelé, A.-L. Measurement of reverse-light-induced excited spin state trapping in spin crossover systems: A study case with Zn1−xFex(6-mepy)3tren(PF6)2·CH3CN; x = 0.5%. Crystals 2024, 14, 210. [Google Scholar] [CrossRef]
- Zhu, H.-L.; Meng, Y.-S.; Hu, J.-X.; Oshio, H.; Liu, T. Photoinduced magnetic hysteresis in a cyanide-bridged two-dimensional [Mn2W] coordination polymer. Inorg. Chem. Front. 2022, 9, 4974–4981. [Google Scholar] [CrossRef]
- Su, S.-Q.; Wu, S.-Q.; Huang, Y.-B.; Xu, W.-H.; Gao, K.-G.; Okazawa, A.; Okajima, H.; Sakamoto, A.; Kanegawa, S.; Sato, O. Photoinduced persistent polarization change in a spin transition crystal. Angew. Chem. Int. Ed. 2022, 61, e202208771. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Cruddas, J.; Ruzzi, G.; Powell, B.J. Toward high-temperature light-induced spin-state trapping in spin-crossover materials: The interplay of collective and molecular effects. J. Am. Chem. Soc. 2022, 144, 9138–9148. [Google Scholar] [CrossRef] [PubMed]
- Benchohra, A.; Li, Y.; Chamoreau, L.M.; Baptiste, B.; Elkaïm, E.; Guillou, N.; Kreher, D.; Lescouëzec, R. The atypical hysteresis of [Fe(C6F5Tp)2]: Overlay of spin-crossovers and symmetry-breaking phase transition. Angew. Chem. Int. Ed. 2021, 60, 8803–8807. [Google Scholar] [CrossRef]
- Wen, W.; Meng, Y.-S.; Jiao, C.-Q.; Liu, Q.; Zhu, H.-L.; Li, Y.-M.; Oshio, H.; Liu, T. A mixed-valence {Fe13} cluster exhibiting metal-to-metal charge-transfer-switched spin crossover. Angew. Chem. Int. Ed. 2020, 59, 16393–16397. [Google Scholar] [CrossRef]
- Ossinger, S.; Näther, C.; Buchholz, A.; Schmidtmann, M.; Mangelsen, S.; Beckhaus, R.; Plass, W.; Tuczek, F. Spin transition of an iron(II) organoborate complex in different polymorphs and in vacuum-deposited thin films: Influence of cooperativity. Inorg. Chem. 2020, 59, 7966–7979. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ash, R.; Girolami, G.S.; Vura-Weis, J. Tracking the metal-centered triplet in photoinduced spin crossover of fe(phen)32+ with tabletop femtosecond M-Edge X-ray absorption Near-Edge structure spectroscopy. J. Am. Chem. Soc. 2019, 141, 17180–17188. [Google Scholar] [CrossRef]
- Fatur, S.M.; Shepard, S.G.; Higgins, R.F.; Shores, M.P.; Damrauer, N.H. A synthetically tunable system to control MLCT excited-state lifetimes and spin states in iron(II) polypyridines. J. Am. Chem. Soc. 2017, 139, 4493–4505. [Google Scholar] [CrossRef]
- Wu, D.-Y.; Sato, O.; Einaga, Y.; Duan, C.-Y. A spin-crossover cluster of iron(II) exhibiting a mixed-spin structure and synergy between spin transition and magnetic interaction. Angew. Chem. Int. Ed. 2009, 48, 1475–1478. [Google Scholar] [CrossRef]
- Halder, G.J.; Chapman, K.W.; Neville, S.M.; Moubaraki, B.; Murray, K.S.; Létard, J.F.; Kepert, C.J. Elucidating the mechanism of a two-step spin transition in a nanoporous metal-organic framework. J. Am. Chem. Soc. 2009, 130, 17552–17562. [Google Scholar] [CrossRef] [PubMed]
- Agustí, G.; Ohtani, R.; Yoneda, K.; Gaspar, A.B.; Ohba, M.; Sánchez-Royo, J.F.; Muñoz, M.C.; Kitagawa, S.; Real, J.A. Oxidative addition of halogens on open metal sites in a microporous spin-crossover coordination polymer. Angew. Chem. Int. Ed. 2009, 48, 8944–8947. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yang, Q.; Fang, C.; Deng, S.; Xu, B. Advanced design for stimuli-reversible chromic wearables with customizable functionalities. Adv. Mater. 2024, 37, 2413665. [Google Scholar] [CrossRef]
- He, Y.; Dang, T.; Leach, A.G.; Zhang, Z.-Y.; Li, T. Photoswitchable azobispyrazole crystals achieving near-quantitative crystalline-state bidirectional E ⇆ Z conversions. J. Am. Chem. Soc. 2024, 146, 29237–29244. [Google Scholar] [CrossRef]
- Zhao, H.; Liao, J.; Fu, S.; Zi, Y.; Bai, X.; Ci, Y.; Zhang, Y.; Cai, X.; Li, Y.; Cun, Y.; et al. Direct 3D lithography of reversible photochromic patterns with tunable luminescence in amorphous transparent media. ACS Energy Lett. 2025, 10, 1235–1244. [Google Scholar] [CrossRef]
- Dong, G.; Lin, H.; Yang, H.; Hu, T.; Huang, Q.; Han, X.; Li, X.; Wang, P.; Xu, J.; Cheng, Y.; et al. Twin-perovskite structured Ba8Ti3Nb4O24: Pr3+: A fast photochromics with millisecond-level response speed for optical storage. ACS Energy Lett. 2024, 10, 484–495. [Google Scholar] [CrossRef]
- Hou, J.; Long, G.; Zhao, W.; Zhou, G.; Liu, D.; Broer, D.J.; Feringa, B.L.; Chen, J. Phototriggered complex motion by programmable construction of light-driven molecular motors in liquid crystal networks. J. Am. Chem. Soc. 2022, 144, 6851–6860. [Google Scholar] [CrossRef]
- Song, Y.-P.; Zhang, J.-N.; Wang, J.-R.; Li, K.; Yuan, Y.-X.; Li, B.; Zang, S.-Q. A fast responsive photochromic SCC-MOF for photoswitching and information encryption. Sci. China Mater. 2024, 67, 698–704. [Google Scholar] [CrossRef]
- Lu, H.; Xie, J.; Wang, X.-Y.; Wang, Y.; Li, Z.-J.; Diefenbach, K.; Pan, Q.-J.; Qian, Y.; Wang, J.-Q.; Wang, S.; et al. Visible colorimetric dosimetry of UV and ionizing radiations by a dual-module photochromic nanocluster. Nat. Commun. 2021, 12, 2798. [Google Scholar] [CrossRef]
- Roubinet, B.; Weber, M.; Shojaei, H.; Bates, M.; Bossi, M.L.; Belov, V.N.; Irie, M.; Hell, S.W. Fluorescent photoswitchable diarylethenes for biolabeling and single-molecule localization microscopies with optical superresolution. J. Am. Chem. Soc. 2017, 139, 6611–6620. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, L.; Samorì, P. Coupling carbon nanomaterials with photochromic molecules for the generation of optically responsive materials. Nat. Commun. 2016, 7, 11118. [Google Scholar] [CrossRef] [PubMed]
- Babii, O.; Afonin, S.; Garmanchuk, L.V.; Nikulina, V.V.; Nikolaienko, T.V.; Storozhuk, O.V.; Shelest, D.V.; Dasyukevich, O.I.; Ostapchenko, L.I.; Iurchenko, V.; et al. Direct photocontrol of peptidomimetics: An alternative to oxygen-dependent photodynamic cancer therapy. Angew. Chem. Int. Ed. 2016, 55, 5493–5496. [Google Scholar] [CrossRef] [PubMed]
- Rice, A.M.; Martin, C.R.; Galitskiy, V.A.; Berseneva, A.A.; Leith, G.A.; Shustova, N.B. Photophysics modulation in photoswitchable metal-organic frameworks. Chem. Rev. 2019, 120, 8790–8813. [Google Scholar] [CrossRef]
- Bisoyi, H.K.; Li, Q. Light-directing chiral liquid crystal nanostructures: From 1D to 3D. Acc. Chem. Res. 2014, 47, 3184–3195. [Google Scholar] [CrossRef]
- Kole, G.K.; Vittal, J.J. Solid-state reactivity and structural transformations involving coordination polymers. Chem. Soc. Rev. 2013, 42, 1755–1775. [Google Scholar] [CrossRef]
- Cao, L.-H.; Xu, X.-Q.; Tang, X.-H.; Yang, Y.; Liu, J.; Yin, Z.; Zang, S.-Q.; Ma, Y.-M. Controllable strategy for metal-organic framework light-driven [2 + 2] cycloaddition reactions via solvent-assisted linker exchange. Inorg. Chem. 2021, 60, 2117–2121. [Google Scholar] [CrossRef]
- Ou, Y.-C.; Liu, W.-T.; Li, J.-Y.; Zhang, G.-G.; Wang, J.; Tong, M.-L. Solvochromic and photodimerization behaviour of 1D coordination polymer via single-crystal-to-single-crystal transformation. Chem. Commun. 2011, 47, 9384–9386. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Wang, Z.-M.; Gao, S. A pillared layer MOF with anion-tunable magnetic properties and photochemical [2 + 2] cycloaddition. Chem. Commun. 2007, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, K.M.; Rupasinghe, T.P.; Ditzler, L.R.; Swenson, D.C.; Sander, J.R.G.; Baltrusaitis, J.; Tivanski, A.V.; MacGillivray, L.R. Nanocrystals of a metal-organic complex exhibit remarkably high conductivity that increases in a single-crystal-to-single-crystal transformation. J. Am. Chem. Soc. 2014, 136, 6778–6781. [Google Scholar] [CrossRef]
- Park, I.H.; Medishetty, R.; Kim, J.Y.; Lee, S.S.; Vittal, J.J. Distortional supramolecular isomers of polyrotaxane coordination polymers: Photoreactivity and sensing of nitro compounds. Angew. Chem. Int. Ed. 2014, 53, 5591–5595. [Google Scholar] [CrossRef]
- Zouev, I.; Cao, D.K.; Sreevidya, T.V.; Botoshansky, M.; Kaftory, M. Photodimerization of anthracene derivatives in their neat solid state and in solid molecular compounds. CrystEngComm 2011, 13, 4376–4381. [Google Scholar] [CrossRef]
- Becker, H.D. Unimolecular photochemistry of anthracenes. Chem. Rev. 1993, 93, 145–172. [Google Scholar] [CrossRef]
- Furlong, B.J.; Katz, M.J. Bistable dithienylethene-based metal-organic framework illustrating optically induced changes in chemical separations. J. Am. Chem. Soc. 2017, 139, 13280–13283. [Google Scholar] [CrossRef]
- Kobatake, S.; Takami, S.; Muto, H.; Ishikawa, T.; Irie, M. Rapid and reversible shape changes of molecular crystals on photoirradiation. Nature 2007, 446, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Du, M.; Chu, Z.; Li, C. Synchronizing multicolor changes and shape deformation into structurally homogeneous hydrogels via a single photochromophore. Adv. Mater. 2025, 37, 2500857. [Google Scholar] [CrossRef]
- Williams, D.E.; Martin, C.R.; Dolgopolova, E.A.; Swifton, A.; Godfrey, D.C.; Ejegbavwo, O.A.; Pellechia, P.J.; Smith, M.D.; Shustova, N.B. Flipping the switch: Fast photoisomerization in a confined environment. J. Am. Chem. Soc. 2018, 140, 7611–7622. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liao, W.-Q.; Li, Y.; Huang, C.-R.; Gan, T.; Chen, X.-G.; Lv, H.-P.; Song, X.-J.; Xiong, R.-G.; Wang, Z.-X. A homochiral fulgide organic ferroelectric crystal with photoinduced molecular orbital breaking. Angew. Chem. Int. Ed. 2023, 62, e202315189. [Google Scholar] [CrossRef]
- Du, Y.; Huang, C.-R.; Xu, Z.-K.; Hu, W.; Li, P.-F.; Xiong, R.-G.; Wang, Z.-X. Photochromic single-component organic fulgide ferroelectric with photo-triggered polarization response. JACS Au 2023, 3, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Schneider, S.; Yuan, Y.; Young, R.M.; Francese, T.; Mansoor, I.F.; Dudenas, P.J.; Lei, Y.; Gomez, E.D.; DeLongchamp, D.M.; et al. Twisted A-D-A type acceptors with thermally-activated delayed crystallization behavior for efficient nonfullerene organic solar cells. Adv. Energy Mater. 2022, 12, 2103957. [Google Scholar] [CrossRef]
- Pugliese, E.; Vo, N.T.; Boussac, A.; Banse, F.; Mekmouche, Y.; Simaan, J.; Tron, T.; Gotico, P.; Sircoglou, M.; Halime, Z.; et al. Photocatalytic generation of a non-heme Fe(III)-hydroperoxo species with O2 in water for the oxygen atom transfer reaction. Chem. Sci. 2022, 13, 12332–12339. [Google Scholar] [CrossRef]
- Hein, R.; Docker, A.; Davis, J.J.; Beer, P.D. Redox-switchable chalcogen bonding for anion recognition and sensing. J. Am. Chem. Soc. 2022, 144, 8827–8836. [Google Scholar] [CrossRef]
- Min, H.; Han, Z.; Sun, T.; Wang, K.; Xu, J.; Yao, P.; Yang, S.; Cheng, P.; Shi, W. Dynamic-static coupled sensing of trace biomarkers by molecularly imprinted metal-organic frameworks. Sci. China Chem. 2023, 66, 3511–3517. [Google Scholar] [CrossRef]
- Han, Z.; Wang, K.; Chen, Y.; Li, J.; Teat, S.J.; Yang, S.; Shi, W.; Cheng, P. A multicenter metal-organic framework for quantitative detection of multicomponent organic mixtures. CCS Chem. 2022, 4, 3238–3245. [Google Scholar] [CrossRef]
- Burton, S.T.; Lee, G.; Moore, C.E.; Sevov, C.S.; Turro, C. Cyclometallated Co(III) complexes with lowest-energy charge transfer excited states accessible with visible light. J. Am. Chem. Soc. 2025, 147, 13315–13327. [Google Scholar] [CrossRef]
- Martin, C.R.; Kittikhunnatham, P.; Leith, G.A.; Berseneva, A.A.; Park, K.C.; Greytak, A.B.; Shustova, N.B. Let the light be a guide: Chromophore communication in metal-organic frameworks. Nano Res. 2020, 14, 338–354. [Google Scholar] [CrossRef]
- Wang, S.; Sun, H.; Erdmann, T.; Wang, G.; Fazzi, D.; Lappan, U.; Puttisong, Y.; Chen, Z.; Berggren, M.; Crispin, X.; et al. A chemically doped naphthalenediimide-bithiazole polymer for n-type organic thermoelectrics. Adv. Mater. 2018, 30, 1801898. [Google Scholar] [CrossRef]
- Xiong, Z.; Li, Y.; Yuan, Z.; Liang, J.; Wang, S.; Yang, X.; Xiang, S.; Lv, Y.; Chen, B.; Zhang, Z. Switchable anisotropic/isotropic photon transport in a double-dipole metal-organic framework via radical-controlled energy transfer. Adv. Mater. 2024, 36, 2314005. [Google Scholar] [CrossRef]
- Liao, P.Y.; Li, J.X.; Liu, J.C.; Xiong, Q.; Ruan, Z.Y.; Li, T.; Deng, W.; Jiang, S.D.; Jia, J.H.; Tong, M.L. Radical-induced photochromic silver(I) metal-organic frameworks: Alternative topology, dynamic photoluminescence and efficient photothermal conversion modulated by anionic guests. Angew. Chem. Int. Ed. 2024, 63, e202401448. [Google Scholar] [CrossRef]
- Gong, T.; Sui, Q.; Li, P.; Meng, X.F.; Zhou, L.J.; Chen, J.; Xu, J.; Wang, L.; Gao, E.-Q. Versatile and switchable responsive properties of a lanthanide-viologen metal-organic framework. Small 2019, 15, 1803468. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hu, J.-X.; Meng, Y.-S.; Jiang, W.-J.; Wang, J.-L.; Wen, W.; Wu, Q.; Zhu, H.-L.; Zhao, L.; Liu, T. Asymmetric coordination toward a photoinduced single-chain magnet showing high coercivity values. Angew. Chem. Int. Ed. 2021, 60, 10537–10541. [Google Scholar] [CrossRef]
- Hu, J.-X.; Zhu, H.-L.; Meng, Y.-S.; Pang, J.; Li, N.; Liu, T.; Bu, X.-H. Ligand modified and light switched on/off single-chain magnets of {Fe2Co} coordination polymers via metal-to-metal charge transfer. CCS Chem. 2023, 5, 865–875. [Google Scholar] [CrossRef]
- Morimoto, M.; Miyasaka, H.; Yamashita, M.; Irie, M. Coordination assemblies of [Mn4] single-molecule magnets linked by photochromic ligands: Photochemical control of the magnetic properties. J. Am. Chem. Soc. 2009, 131, 9823–9835. [Google Scholar] [CrossRef] [PubMed]
- Fetoh, A.; Cosquer, G.; Morimoto, M.; Irie, M.; El-Gammal, O.; Abu El-Reash, G.M.; Breedlove, B.K.; Yamashita, M. Synthesis, structures, and magnetic properties of two coordination assemblies of Mn(III) single molecule magnets bridged via photochromic diarylethene ligands. Inorg. Chem. 2019, 58, 2307–2314. [Google Scholar] [CrossRef]
- Jiao, T.; Wu, C.-H.; Zhang, Y.-S.; Miao, X.; Wu, S.; Jiang, S.-D.; Wu, J. Solution-phase synthesis of Clar’s goblet and elucidation of its spin properties. Nat. Chem. 2025, 17, 924–932. [Google Scholar] [CrossRef]
- Eaton, S.S.; Yamabayashi, T.; Horii, Y.; Yamashita, M.; Eaton, G.R. Anisotropy of spin-lattice relaxation time (T1) for oxo-vanadium(IV) and nitrido chromium(V) porphyrins. J. Am. Chem. Soc. 2025, 147, 13815–13823. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.J.; Krzyaniak, M.D.; Wasielewski, M.R.; Freedman, D.E. Probing nuclear spin effects on electronic spin coherence via EPR measurements of vanadium(IV) complexes. Inorg. Chem. 2017, 56, 8106–8113. [Google Scholar] [CrossRef]
- Tesi, L.; Lucaccini, E.; Cimatti, I.; Perfetti, M.; Mannini, M.; Atzori, M.; Morra, E.; Chiesa, M.; Caneschi, A.; Sorace, L.; et al. Quantum coherence in a processable vanadyl complex: New tools for the search of molecular spin qubits. Chem. Sci. 2016, 7, 2074–2083. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.-T.; Zhao, L.; Yi, C.; Liu, Q.; Li, Y.-M.; Meng, Y.-S.; Oshio, H.; Liu, T. Manipulating selective metal-to-metal electron transfer to achieve multi-phase transitions in an asymmetric [Fe2Co]-assembled mixed-valence chain. Angew. Chem. Int. Ed. 2022, 61, e202115367. [Google Scholar] [CrossRef]
- Shang, M.-J.; Lu, H.-H.; Liu, Q.; Zhou, R.-H.; Sun, H.-Y.; Shao, Z.; Zhao, L.; Meng, Y.-S.; Liu, T. Reversible on–off switching of a single-chain magnet via single-crystal-to-single-crystal transition and light-induced metal-to-metal electron transfer. Inorg. Chem. Front. 2024, 11, 8704–8714. [Google Scholar] [CrossRef]
- Wang, L.-F.; Qiu, J.-Z.; Chen, Y.-C.; Liu, J.-L.; Li, Q.-W.; Jia, J.-H.; Tong, M.-L. [2 + 2] Photochemical modulation of the Dy(III) single-molecule magnet: Opposite influence on the energy barrier and relaxation time. Inorg. Chem. Front. 2017, 4, 1311–1318. [Google Scholar] [CrossRef]
- Huang, X.-D.; Wen, G.-H.; Bao, S.-S.; Jia, J.-G.; Zheng, L.-M. Thermo- and light-triggered reversible interconversion of dysprosium-anthracene complexes and their responsive optical, magnetic and dielectric properties. Chem. Sci. 2021, 12, 929–937. [Google Scholar] [CrossRef]
- Li, J.; Kong, M.; Yin, L.; Zhang, J.; Yu, F.; Ouyang, Z.-W.; Wang, Z.; Zhang, Y.-Q.; Song, Y. Photochemically tuned magnetic properties in an erbium(III)-based easy-plane single-molecule magnet. Inorg. Chem. 2019, 58, 14440–14448. [Google Scholar] [CrossRef]
- Zhang, Q.; Han, S.-D.; Li, Q.; Hu, J.-X.; Wang, G.-M. Electron transfer photochromism of Ln-based (Ln = Dy, Tb) coordinated polymers for reversibly switching off/on single-molecule magnetic behavior. Sci. China Mater. 2022, 65, 788–794. [Google Scholar] [CrossRef]
- Cai, L.-Z.; Guo, P.-Y.; Wang, M.-S.; Guo, G.-C. Photoinduced magnetic phase transition and remarkable enhancement of magnetization for a photochromic single-molecule magnet. J. Mater. Chem. C 2021, 9, 2231–2235. [Google Scholar] [CrossRef]
- Tian, H.; Su, J.-B.; Bao, S.-S.; Kurmoo, M.; Huang, X.-D.; Zhang, Y.-Q.; Zheng, L.-M. Reversible ON-OFF switching of single-molecule-magnetism associated with single-crystal-to-single-crystal structural transformation of a decanuclear dysprosium phosphonate. Chem. Sci. 2018, 9, 6424–6433. [Google Scholar] [CrossRef] [PubMed]
- Selvanathan, P.; Dorcet, V.; Roisnel, T.; Bernot, K.; Huang, G.; Le Guennic, B.; Norel, L.; Rigaut, S. Trans to cis photo-isomerization in merocyanine dysprosium and yttrium complexes. Dalton. Trans. 2018, 47, 4139–4148. [Google Scholar] [CrossRef] [PubMed]
- Cosquer, G.; Kamila, M.; Li, Z.-Y.; Breedlove, B.; Yamashita, M. Photo-modulation of single-molecule magnetic dynamics of a dysprosium dinuclear complex via a diarylethene bridge. Inorganics 2018, 6, 9. [Google Scholar] [CrossRef]
- Gou, X.; Chen, Z.; Jiang, J.; Liu, N.; Lan, W.; Wu, Y.; Cheng, P.; Shi, W. Influence of chelating ligands on photomagnetic properties of two erbium(III) coordination polymers. J. Rare Earths 2025, 43, 552–555. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Geng, Y.-W.; Wang, Z.; Xie, C.; Han, T.; Cheng, P. Two-dimensional metal-organic framework with high-performance single-molecule magnets as nodes showing magnetic coercivity photomodulation. J. Am. Chem. Soc. 2025, 147, 18044–18053. [Google Scholar] [CrossRef]
- Gou, X.; Wu, Y.; Wen, H.; Li, L.; Lan, W.; Liu, N.; Zhang, S.; Ungur, L.; Cheng, P.; Shi, W. Modulating static and dynamic magnetizations of ytterbium(III) coordination polymers by light-induced radicals. CCS Chem. 2025, 1–9. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, Y.; Wu, F.; Xiao, W.; Hua, W.; Tang, Z.; Liu, W.; Chen, S.; Wang, Y.; Wu, W.; et al. Photoisomerization-mediated tunable pore size in metal organic frameworks for U(VI)/V(V) selective separation. Nat. Commun. 2025, 16, 2361. [Google Scholar] [CrossRef]
- Wu, L.; Liu, Y.; Yang, W.; Liu, Z.; Liu, C.; Yuan, X.; Zhang, L.; Ju, J.; Yao, X. In situ stimuli transfer in multi-environment shape-morphing hydrogels based on the copolymer between spiropyran and acrylic acid. Adv. Sci. 2025, 12, 2416173. [Google Scholar] [CrossRef]
- Tan, X.; Wang, Y.; Li, H.; Duan, Y.; Wen, B.; Zhao, J.; Kim, H.; Lee, J.Y.; Zhou, L.; Cheng, H.-B.; et al. Supramolecular synthesis of dithienylethene-albumin complexes for enhanced photoswitching in photoacoustic imaging-guided near-infrared photothermal therapy. Small 2025, 21, 2409027. [Google Scholar] [CrossRef]
- Jin, T.; Zhang, Z.; He, S.; Kaledin, A.L.; Xu, Z.; Liu, Y.; Zhang, P.; Beratan, D.N.; Lian, T. Shell thickness and heterogeneity dependence of triplet energy transfer between core-shell quantum dots and adsorbed molecules. J. Am. Chem. Soc. 2025, 147, 16282–16292. [Google Scholar] [CrossRef]
- Malkmus, S.; Koller, F.O.; Draxler, S.; Schrader, T.E.; Schreier, W.J.; Brust, T.; DiGirolamo, J.A.; Lees, W.J.; Zinth, W.; Braun, M. All-optical operation cycle on molecular bits with 250-GHz clock-rate based on photochromic fulgides. Adv. Funct. Mater. 2007, 17, 3657–3662. [Google Scholar] [CrossRef]
- Guo, F.-S.; Benjamin, M.D.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Richard, A.L. A Dysprosium metallocene single-molecule magnet functioning at the axial limit. Angew. Chem. Int. Ed. 2017, 56, 11445–11449. [Google Scholar] [CrossRef]
- Ou, R.; Zhang, H.; Zhao, C.; Hegab, H.M.; Jiang, L.; Truong, V.X.; Wang, H. Photoresponsive styrylpyrene-modified MOFs for gated loading and release of cargo molecules. Chem. Mater. 2020, 32, 10621–10627. [Google Scholar] [CrossRef]
- Arima, H.; Hiraide, S.; Nagano, H.; Abylgazina, L.; Senkovska, I.; Auernhammer, G.K.; Fery, A.; Kaskel, S.; Watanabe, S. Atomic force microscopy strategies for capturing guest-induced structural transitions in single flexible metal-organic framework particles. J. Am. Chem. Soc. 2025, 147, 14491–14503. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Zhang, R.; Jiang, J.; Chen, Z.; Ni, Y.; Xie, W.; Xu, J.; Zhou, Z.; Chen, J.; Cheng, P.; et al. High-efficiency lithium-ion transport in a porous coordination chain-based hydrogen-bonded framework. J. Am. Chem. Soc. 2023, 145, 10149–10158. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Li, J.; Lu, W.; Wang, K.; Chen, Y.; Zhang, X.; Lin, L.; Han, X.; Teat, S.J.; Frogley, M.D.; et al. A {Ni12}-wheel-based metal-organic framework for coordinative binding of sulphur dioxide and nitrogen dioxide. Angew. Chem. Int. Ed. 2022, 61, e202115585. [Google Scholar] [CrossRef]
- Sun, T.; Min, H.; Han, Z.; Wang, L.; Cheng, P.; Shi, W. Rapid detection of nanoplastic particles by a luminescent Tb-based coordination polymer. Chin. Chem. Lett. 2024, 35, 108718. [Google Scholar] [CrossRef]
- Han, Z.; Yan, Z.; Wang, K.; Kang, X.; Lv, K.; Zhang, X.; Zhou, Z.; Yang, S.; Shi, W.; Cheng, P. Observation of oxygen evolution over a {Ni12}-cluster-based metal-organic framework. Sci. China Chem. 2022, 65, 1088–1093. [Google Scholar] [CrossRef]
- Jing, P.; Wu, B.; Han, Z.; Shi, W.; Cheng, P. An efficient Ag/MIL-100(Fe) catalyst for photothermal conversion of CO2 at ambient temperature. Chin. Chem. Lett. 2021, 32, 3505–3508. [Google Scholar] [CrossRef]
- Gou, X.; Liu, N.; Wu, Y.; Lan, W.; Wang, M.; Shi, W.; Cheng, P. Modulation of magnetization dynamics of an Er(III) coordination polymer by the conversion of a ligand to a radical using UV light. Dalton Trans. 2023, 52, 10372–10377. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.-X.; Han, Z.-S.; Han, T.; Shi, W.; Cheng, P. Tuning the magnetization dynamics of TbIII-based single-chain magnets through substitution on the nitronyl nitroxide radical. Dalton Trans. 2019, 48, 8989–8994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, N.; Shi, W.; Wang, B.-W.; Cheng, P. The influence of an external magnetic field and magnetic-site dilution on the magnetization dynamics of a coordination network based on ferromagnetic coupled dinuclear dysprosium(III) units. Inorg. Chem. Front. 2018, 5, 432–437. [Google Scholar] [CrossRef]
- Wang, M.; Meng, X.; Song, F.; He, Y.; Shi, W.; Gao, H.; Tang, J.; Cheng, P. Reversible structural transformation induced switchable single-molecule magnet behavior in lanthanide metal-organic frameworks. Chem. Commun. 2018, 54, 10183–10186. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Shi, W.; Cheng, P. Rational design and synthesis of a chiral lanthanide-radical single-chain magnet. Inorg. Chem. 2018, 57, 13409–13414. [Google Scholar] [CrossRef]
- Song, F.; Zhang, Y.; Chen, J.; Han, T.; Cheng, P. Syntheses, crystal structures and magnetic properties of three lanthanide-nitronyl nitroxide complexes. J. Rare Earth 2017, 35, 24–27. [Google Scholar] [CrossRef]
- Zhang, S.; Li, H.; Duan, E.; Han, Z.; Li, L.; Tang, J.; Shi, W.; Cheng, P. A 3D heterometallic coordination polymer constructed by trimeric {NiDy2} single-molecule magnet units. Inorg. Chem. 2016, 55, 1202–1207. [Google Scholar] [CrossRef]
- Zhang, X.; Vieru, V.; Feng, X.; Liu, J.L.; Zhang, Z.; Na, B.; Shi, W.; Wang, B.-W.; Powell, A.K.; Chibotaru, L.F.; et al. Influence of guest exchange on the magnetization dynamics of dilanthanide single-molecule-magnet nodes within a metal-organic framework. Angew. Chem. Int. Ed. 2015, 54, 9861–9865. [Google Scholar] [CrossRef]
- Liu, K.; Li, H.; Zhang, X.; Shi, W.; Cheng, P. Constraining and tuning the coordination geometry of a lanthanide ion in metal-organic frameworks: Approach toward a single-molecule magnet. Inorg. Chem. 2015, 54, 10224–10231. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Li, H.; Shi, W.; Cheng, P. Influence of external magnetic field and magnetic-site dilution on the magnetic dynamics of a one-dimensional Tb(III)-radical complex. Chem. Commun. 2015, 51, 10933–10936. [Google Scholar] [CrossRef]
- Chen, D.-M.; Ma, X.-Z.; Zhang, X.-J.; Xu, N.; Cheng, P. Switching a 2D Co(II) layer to a 3D Co7-cluster-based metal-organic framework: Syntheses, crystal structures, and magnetic properties. Inorg. Chem. 2015, 54, 2976–2982. [Google Scholar] [CrossRef]
- Zou, J.-Y.; Shi, W.; Gao, H.-L.; Cui, J.-Z.; Cheng, P. Spin canting and metamagnetism in 3D pillared-layer homospin cobalt(II) molecular magnetic materials constructed via a mixed ligands approach. Inorg. Chem. Front. 2014, 1, 242–248. [Google Scholar] [CrossRef]
- Han, T.; Shi, W.; Niu, Z.; Na, B.; Cheng, P. Magnetic blocking from exchange interactions: Slow relaxation of the magnetization and hysteresis loop observed in a dysprosium-nitronyl nitroxide chain compound with an antiferromagnetic ground state. Chem. Eur. J. 2013, 19, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, B.; Xu, N.; Shi, W.; Gao, S.; Cheng, P. Ferromagnetic interactions in EO-azido-bridged binuclear transition metal(II) systems: Syntheses, crystal structures and magnetostructural correlations. Sci. China Chem. 2012, 55, 942–950. [Google Scholar] [CrossRef]




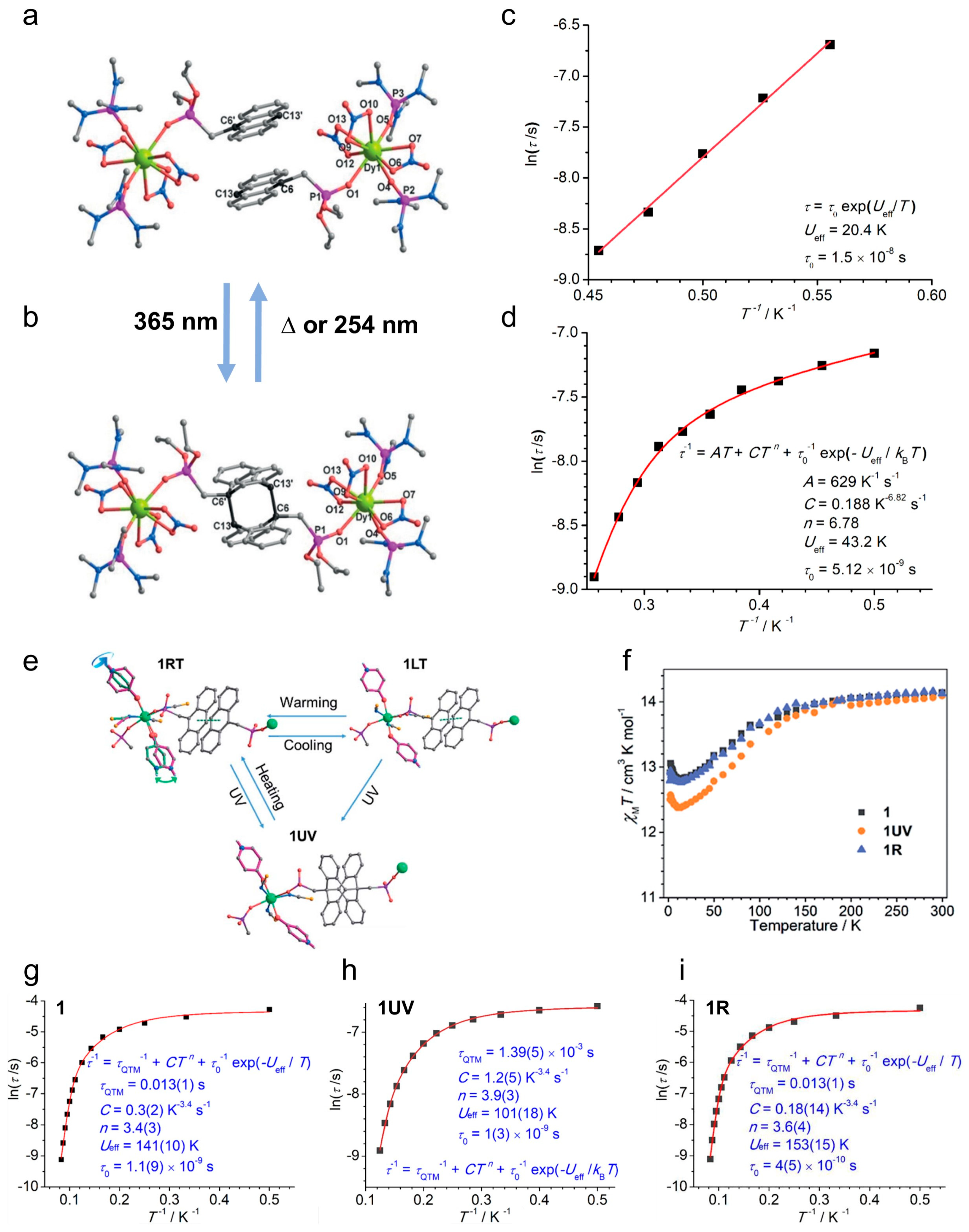
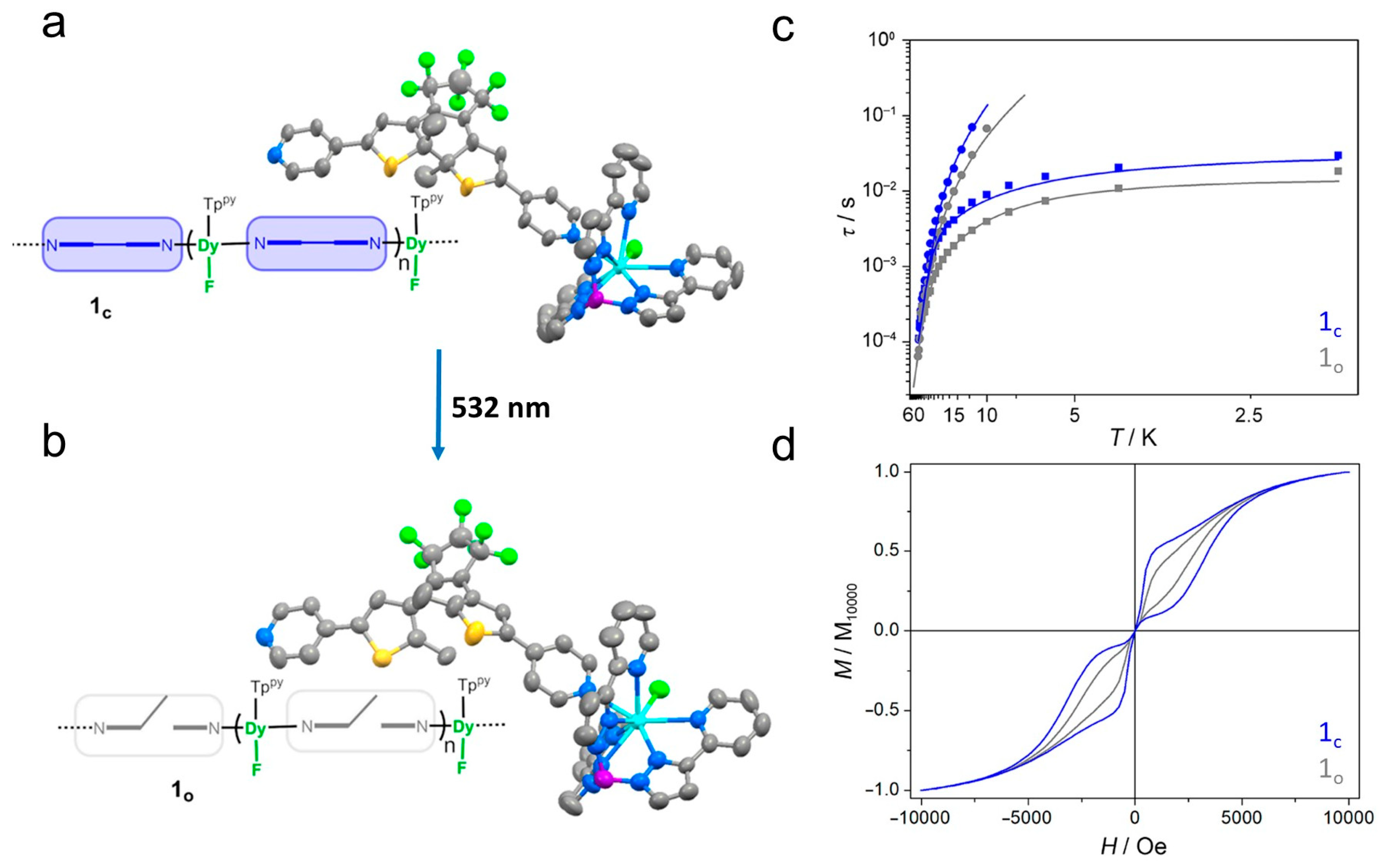
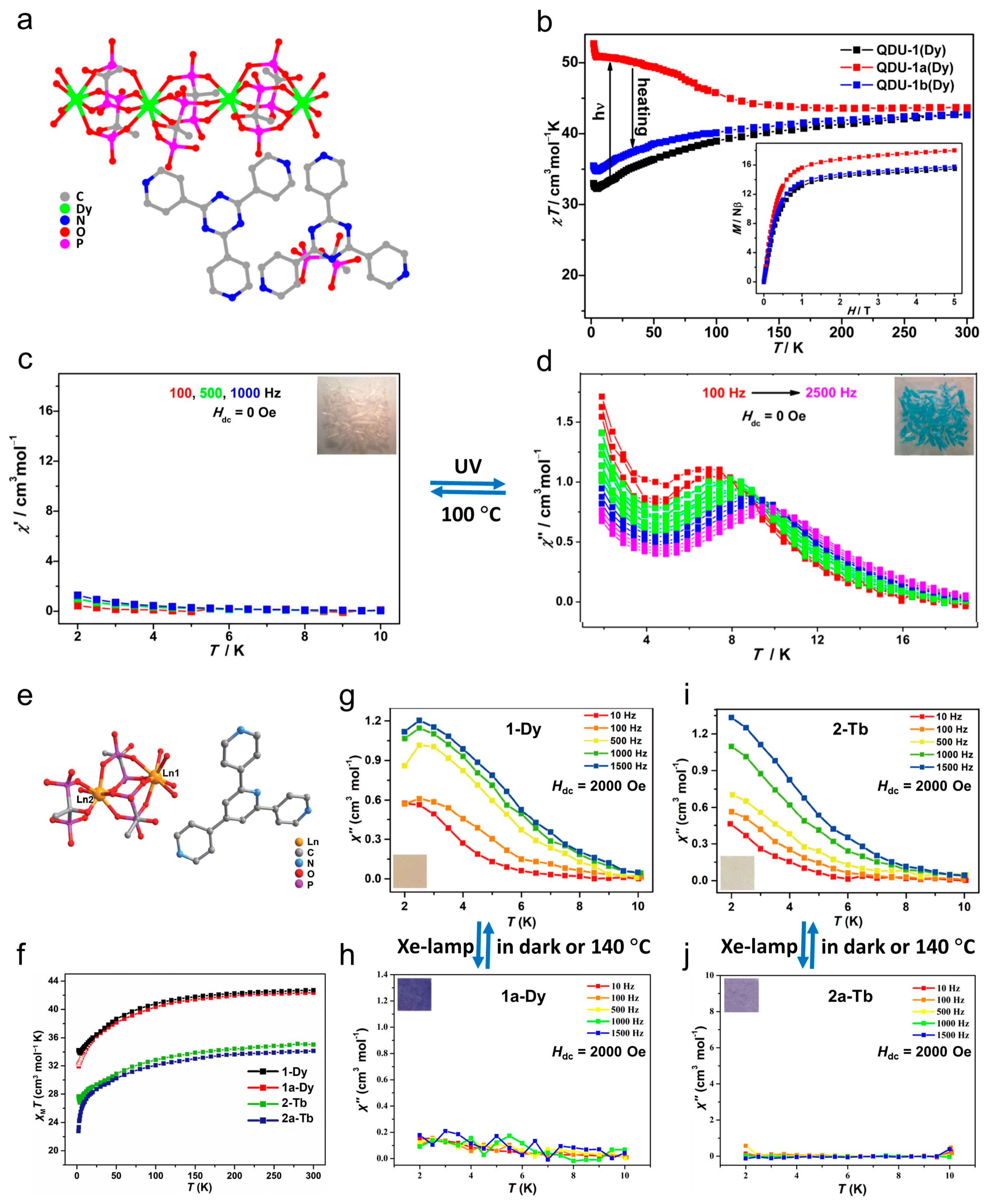
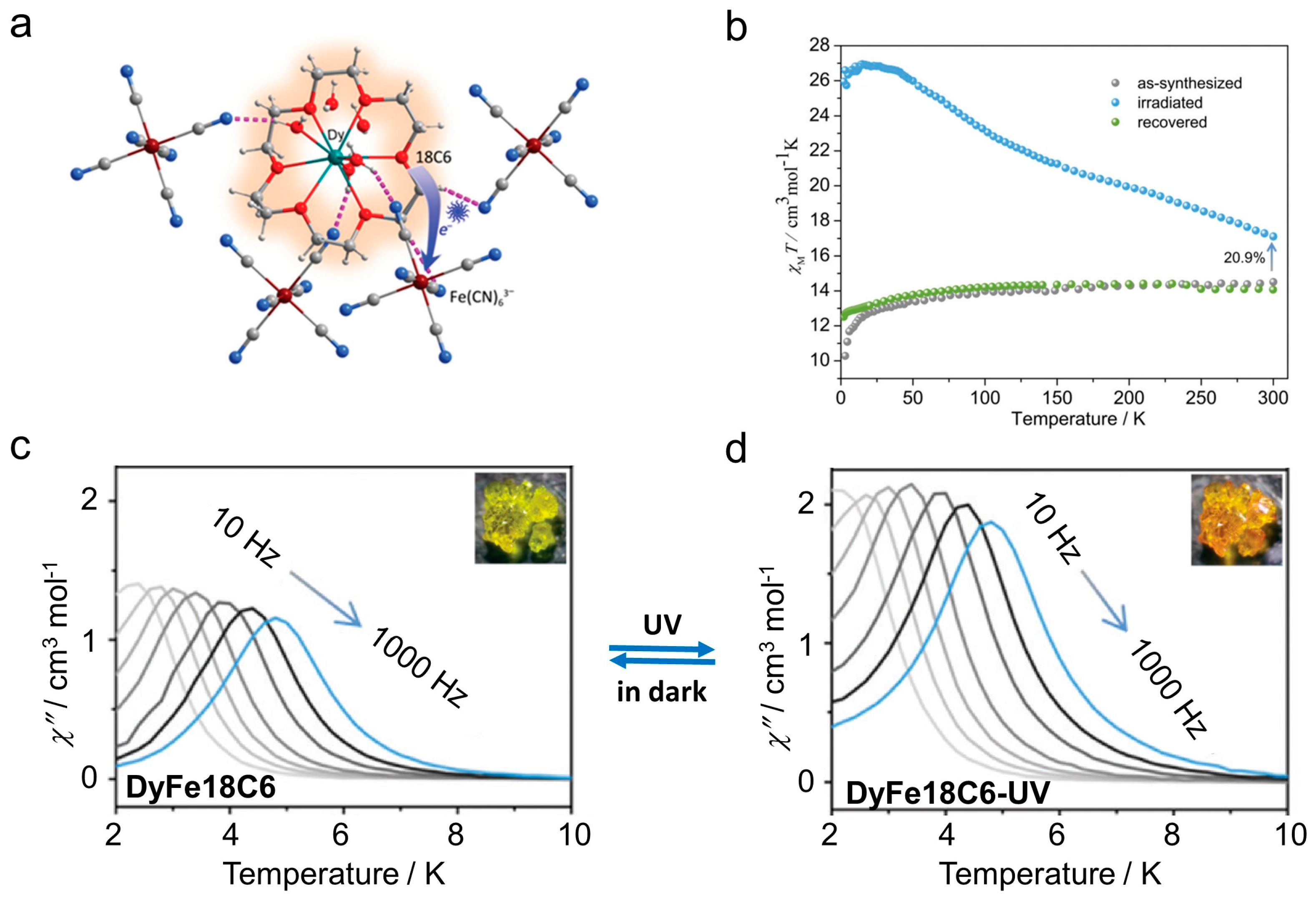

| Complex/ Photoproduct | Photochemical Reaction | χMT at RT cm3 mol−1 K | Hysteresis Loop at 2 K Hc (Oe) | Ueff (K) Hdc (Oe) | Ref. |
|---|---|---|---|---|---|
| [Dy(bpe)(H2O)4(NO3)2](NO3)·2bpe | [2,2] cycloaddition | 13.9 | - | - (slow magnetization relaxation) | [27] |
| [Dy2(tpcb)(H2O)8(NO3)4](NO3)2·2bpe·tpcb | 14.2 | - | - | ||
| (Hbpe)2[Dy(bpe)(H2O)(4-pyO)(NO3)(SCN)3]SCN with bpe | [2,2] cycloaddition | 13.9 | - (butterfly-shaped) | 153.8 (Hdc = 0) 201.9 (Hdc = 1500) | [112] |
| (Hbpe)2(H2tpcb)[Dy2(tpcb)2(H2O)2(4-pyO)2(NO3)2(SCN)6](SCN)2 | 13.9 | - (smaller butterfly-shaped) | 205.5 (Hdc = 0) 234.5 (Hdc = 1500) | ||
| DyIII(depma)(NO3)3(hmpa)2 | [4 + 4] cycloaddition | 13.8 | - | 20.4 (Hdc = 500) | [28] |
| DyIII2(depma2)(NO3)6(hmpa)4 | 14.1 | - | 43.2 (Hdc = 500) | ||
| DyIII(SCN)3(depma)2(4-hpy)2 (1) | [4 + 4] cycloaddition | 14.1 | - (butterfly-shaped) | 141 (Hdc = 0) | [113] |
| 1UV | 14.0 | - | 101 (Hdc = 0) | ||
| 1R | 14.1 | - (butterfly-shaped) | 153 (Hdc = 0) | ||
| [Dy(Tppy)F(Lc)]PF6 (1c) | ring opening | 13.9 | - (butterfly-shaped) | 225.9 (Hdc = 0) | [29] |
| 1o | 13.8 | - (smaller butterfly-shaped) | 225.9 (Hdc = 0) | ||
| [Dy3(H-HEDP)3(H2-HEDP)3]·2H3-TPT·H4-HEDP·10H2O (QDU-1) | photogenerated radical | 42.7 | - | -(SMM off) | [30] |
| QDU-1a | 43.7 | - | 108.1 (Hdc = 0) | ||
| QDU-1b | 42.7 | - | -(SMM off) | ||
| [Ln3(H-HEDP)2(H2-HEDP)2(H3-HEDP)2]·H3-TPP·11H2O (Ln = Dy (1-Dy)) | photogenerated radical | 42.6 | - | 16.9 (Hdc = 2000) | [115] |
| 1a-Dy | 41.8 | - | -(SMM off) | ||
| [Ln3(H-HEDP)2(H2-HEDP)2(H3-HEDP)2]·H3-TPP·11H2O (Ln = Tb (2-Tb)) | 35.2 | - | 12.1 (Hdc = 2000) | ||
| 2a-Tb | 33.3 | - | -(SMM off) | ||
| [DyIII(18C6)(H2O)3]FeIII(CN)6·2H2O (DyFe18C6) | photogenerated radical | 14.5 | - | 18.4 (Hdc = 800) | [116] |
| DyFe18C6-UV | 17.1 | - | 17.0 (Hdc = 800) | ||
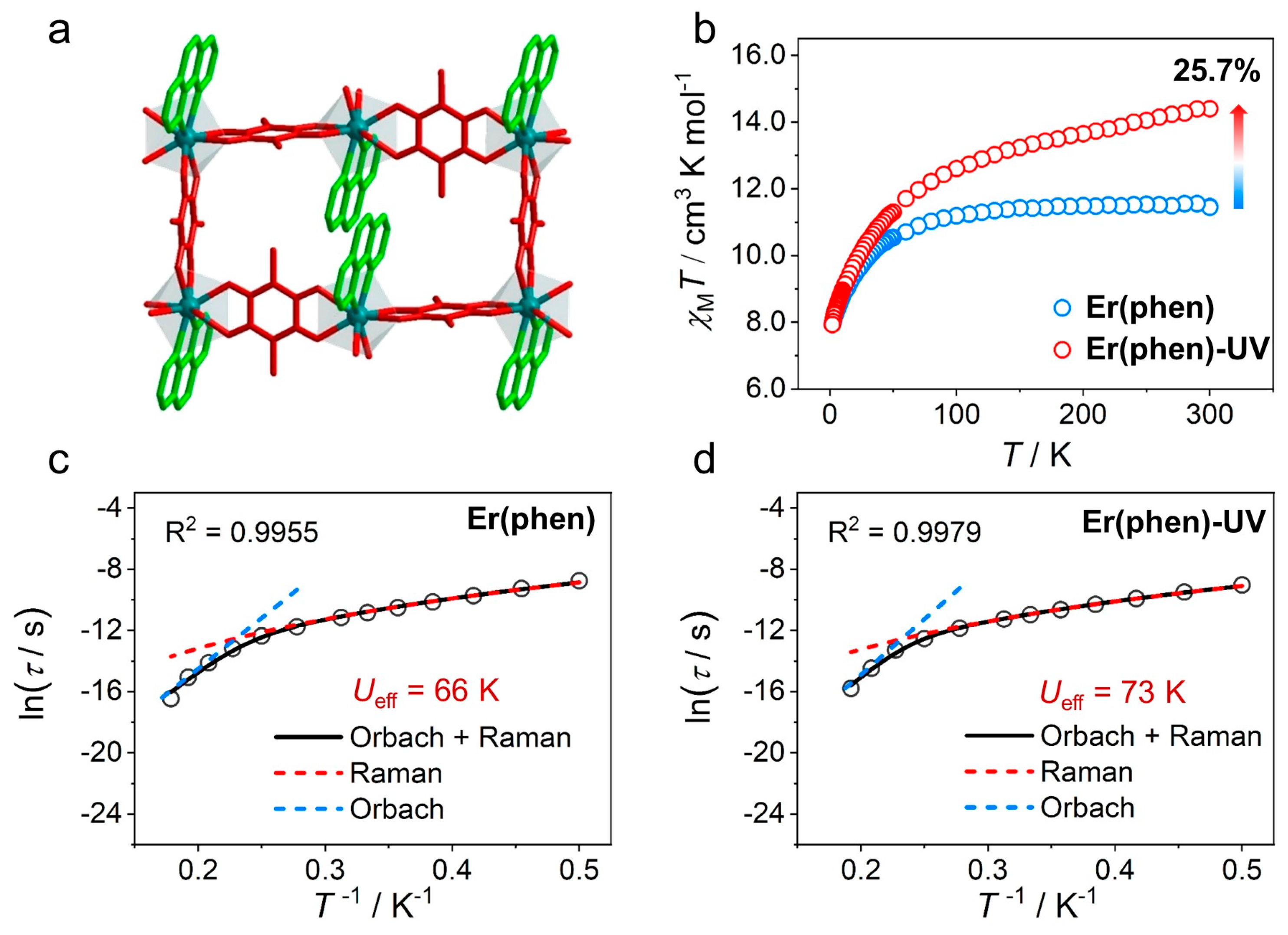
| [Er(CA)1.5(phen)(DMF)]n (Er(phen)) | photogenerated radical | 11.5 | - | 66.4 (Hdc = 600) | [120] |
| Er(phen)-UV | 14.4 | - | 72.7 (Hdc = 600) | ||
| {[Dy1.5(OPh)2Cl(BPy)3(THF)1.5][(BPh4)1.5]·0.5THF}n ((Dy(BPy)) | photogenerated radical | 20.57 | Hc = 4500 Oe | 822 (Hdc = 0, T = 12–40 K) 1048 (Hdc = 0, T = 53–71 K) | [121] |
| Dy(BPy)-UV | 20.63 | Hc = 1300 Oe | 641 (Hdc = 0, T = 12–40 K) 1000 (Hdc = 0, T = 53–71 K) | ||
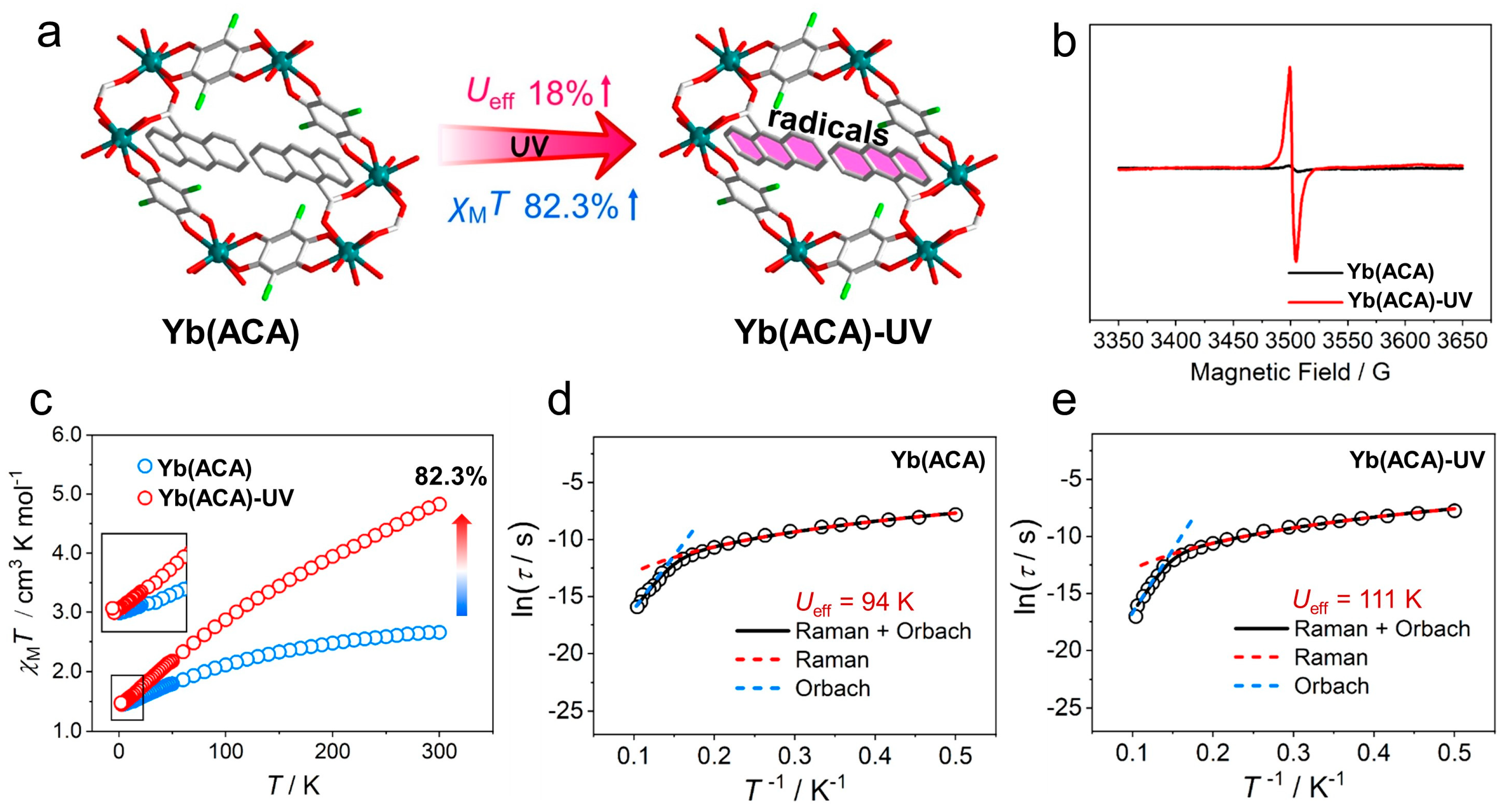
| [Yb(CA)(ACA)(DMF)(H2O)]n (Yb(ACA)) | photogenerated radical | 2.65 | - | 94 (Hdc = 800) | [122] |
| Yb@Y(ACA)-UV | 4.83 | - | 111 (Hdc = 800) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gou, X.; Sun, X.; Cheng, P.; Shi, W. Molecular Nanomagnets with Photomagnetic Properties: Design Strategies and Recent Advances. Magnetochemistry 2025, 11, 77. https://doi.org/10.3390/magnetochemistry11090077
Gou X, Sun X, Cheng P, Shi W. Molecular Nanomagnets with Photomagnetic Properties: Design Strategies and Recent Advances. Magnetochemistry. 2025; 11(9):77. https://doi.org/10.3390/magnetochemistry11090077
Chicago/Turabian StyleGou, Xiaoshuang, Xinyu Sun, Peng Cheng, and Wei Shi. 2025. "Molecular Nanomagnets with Photomagnetic Properties: Design Strategies and Recent Advances" Magnetochemistry 11, no. 9: 77. https://doi.org/10.3390/magnetochemistry11090077
APA StyleGou, X., Sun, X., Cheng, P., & Shi, W. (2025). Molecular Nanomagnets with Photomagnetic Properties: Design Strategies and Recent Advances. Magnetochemistry, 11(9), 77. https://doi.org/10.3390/magnetochemistry11090077






