Abstract
The antibacterial activity of g-C3N4 and Cu-g-C3N4 was evaluated against E. coli, with their disinfection capabilities influenced by structural characteristics, photocatalytic properties, and modulation under a static magnetic field. The incorporation of Cu2+ does not significantly affect the (210) reflection in XRD analysis, indicating that the alignment of aromatic layers remains stable. However, the presence of copper enables complete disinfection, in contrast to graphitic carbon nitride, which achieves only partial disinfection. Cu2+ is likely positioned at N-aliphatic sites and coexists with hydroxylated species, which may influence photocatalytic performance by modifying reactant adsorption and ROS generation. SEM-EDS analysis confirmed that Cu2+ modification did not significantly alter the material’s morphology, although a 3% copper content was detected, suggesting a heterogeneous surface distribution. Thermodynamic analysis showed that exposure to a magnetic field increased the Gibbs free energy of adsorption from 6.34 J/m2 to 10.52 J/m2, reducing interactions with key reactants essential for ROS formation. As a result, both disinfection and photodegradation efficiency were significantly diminished. Additionally, the presence of a magnetic field was found to modify the surface properties of the material, affecting its photocatalytic performance. In Cu-C3N4 materials, a decrease in the contact angle suggests enhanced hydrophilicity, while an increase in surface tension may influence the adsorption of water and hydroxyl radicals. This study underscores the effect of a magnetic field on the photocatalytic behavior of materials deposited on polymeric substrates with intrinsic electronic properties, ultimately impacting overall disinfection efficiency.
1. Introduction
Photocatalysis has demonstrated significant potential in microbial disinfection, particularly in the inactivation of Escherichia coli, a commonly used model organism in water treatment research and a prevalent bacterium in hospital-acquired infections. The generation of hydroxyl (▪OH) radicals plays a vital role in disrupting microbial membranes and degrading intracellular components. However, the effectiveness of photocatalysis in disinfection is influenced by factors such as the photocatalyst’s surface characteristics, the type of light source, and environmental conditions, including the presence of external fields [1]. This process relies on forming reactive oxygen species (ROS), such as hydroxyl radicals and superoxide ions, under light exposure, contributing to the breakdown of pollutants and neutralizing pathogens [2].
Among the various photocatalytic materials studied, graphitic carbon nitride (g-C3N4) has gained considerable interest due to its chemical stability, affordability, and ability to absorb visible light. Despite these advantages, its relatively narrow conduction band limits ROS production, restricting its efficiency in advanced disinfection processes. To address these limitations, various modification strategies have been explored. One promising approach involves doping g-C3N4 with transition metals, such as copper ions (Cu2+), which enhances photocatalytic activity by improving electron/hole separation and expanding its absorption range within the visible light spectrum. These modifications increase ROS production, which is essential for degrading both organic pollutants and microbial contaminants [3]. Copper facilitates charge transfer processes and enhances the formation of hydroxyl radicals, which are crucial for effective disinfection [4].
Recent studies have also investigated the role of external magnetic fields in influencing photocatalytic reactions [5]. Magnetic fields can interact with the electronic states of photocatalytic materials, affecting charge carrier mobility and recombination dynamics. For example, they may induce spin polarization, thereby reducing electron/hole recombination and increasing the production of reactive species [6,7]. Additionally, magnetic fields can influence the adsorption of contaminants on the photocatalyst surface, potentially improving reaction efficiency. However, the impact of magnetic fields is highly dependent on the material used and the experimental conditions. While some studies suggest that magnetic fields enhance photocatalytic performance by modifying charge carrier behavior, others indicate potential drawbacks, such as increased charge recombination or altered surface chemistry. On the other hand, changes in surface tension and Gibbs free energy of adsorption can affect the availability of active sites for the reaction. It can modify the diffusion and reactivity of radicals, altering the contaminant degradation rate, and it may induce changes in the orientation and arrangement of molecules on the catalyst surface. In some cases, a magnetic field can induce changes in the crystalline structure of the material, affecting its ability to absorb light and generate electrons and holes [8].
In summary, the effects of a magnetic field on photocatalytic processes can be either positive or negative, depending on the material used, the field intensity, and the experimental conditions. This article synthesizes and tests a copper modification on carbon nitride, which is obtained at 550 °C through the polymerization of urea. The photocatalytic activity is compared against E. coli and a target molecule (dicloxacillin), analyzing the effect of the magnetic field on photocatalytic efficiency and its implications for enhancing or hindering the adsorption of the contaminant or microorganism. It was particularly interesting to relate these properties to spectroscopic characterization and to demonstrate, through contact angle, surface tension, and Gibbs free energy, how the material’s surface changes. This, in turn, contributes to a better understanding of the magnetic field phenomenon on substrates like PET.
2. Results
2.1. Bacterial Disinfection Efficiency
To assess the impact of the magnetic field on the efficiency of the photo-disinfectant material against gram-negative bacteria, an experiment was conducted using Cu-g-C3N4 (previously synthesized [4]) to target E. coli, both in the presence and absence of a 2000 mT magnetic field. As illustrated in Figure 1, the procedure included a 30-min period to establish adsorption equilibrium, followed by 60 min of exposure to a Xenon lamp. The unmodified carbon nitride demonstrated minimal disinfectant activity under visible light irradiation due to its absorption at 465 nm. Consequently, further experiments focused exclusively on copper-doped carbon nitride to gain deeper insights into the notable effects of the magnetic field on the material’s photoactivation.
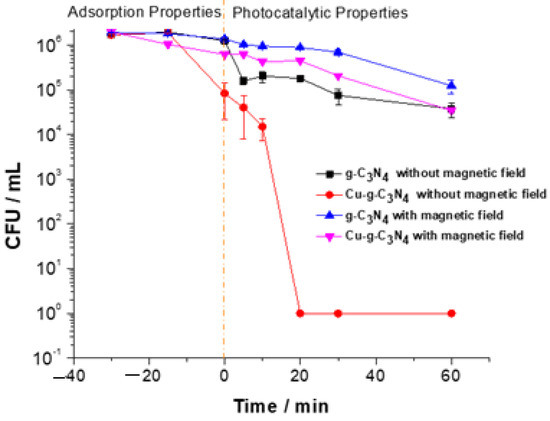
Figure 1.
Influence of the magnetic field on g-C3N4 and Cu-g-C3N4 on their disinfectant power against E. coli ATCC 25922.
2.2. Photocatalytic Activity and Hydroxyl Radical Generation
Figure 2 presents the results of a photodegradation experiment of dicloxacillin using Cu2+-modified g-C3N4. This study compared the efficiency of organic molecule degradation under conditions with/ without the application of a constant magnetic field of 2000 mT.
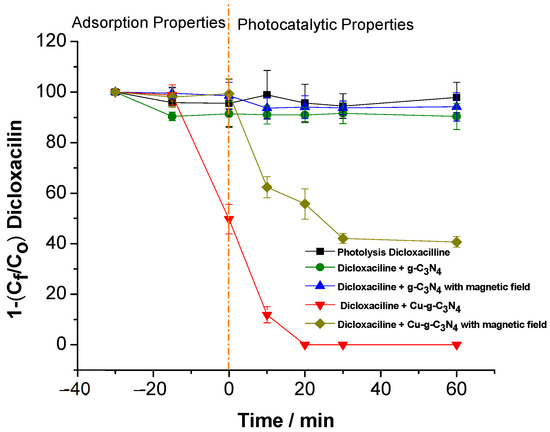
Figure 2.
Magnetic field effects on dicloxacillin degradation using g-C3N4 and Cu-modified g-C3N4. The reactor containing four sunlight lamps (Sylvania FT5T8 8500 K (40 cm length) has emissions length waves between 400 and 700 nm. The photocatalytic processes (60 W of light power, dicloxacillin 30.4 mM, catalyst 0.01 g).
Figure 3 displays the results of an experiment conducted to assess the influence of a magnetic field on hydroxyl radical (▪OH) generation within the photodegradation system. Methanol (MeOH) was utilized as a scavenger due to its high reactivity with ▪OH, effectively suppressing the degradation process. To ensure complete inhibition, MeOH was introduced in excess, at a concentration 10,000 times higher relative to dicloxacillin.
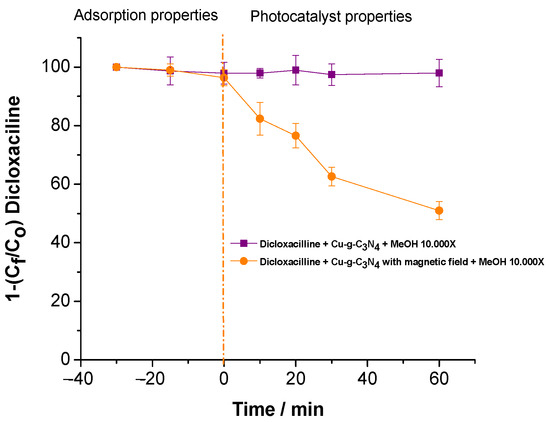
Figure 3.
Impact of magnetic field on hydroxyl radical generation in the presence of methanol as a scavenger.
To gain deeper insight into the impact of the magnetic field on photodegradation, an adsorption experiment was performed to evaluate the interaction of dicloxacillin with the photocatalytic material, both in the presence and absence of a magnetic field. The objective was to determine whether the magnetic field influences the drug’s adsorption onto the material’s surface, which could affect its availability for photodegradation and, consequently, the overall efficiency of the process (Figure 4).
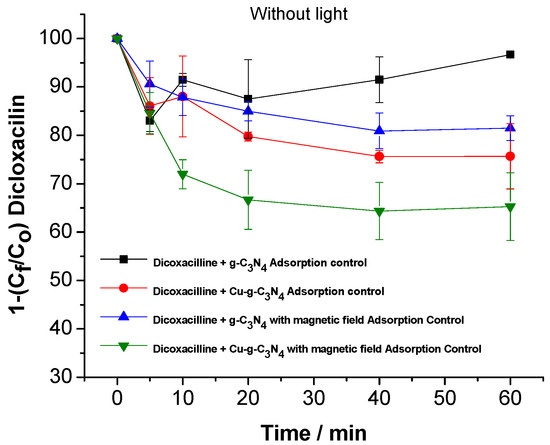
Figure 4.
Adsorption control of dicloxacillin on g-C3N4 and Cu-g-C3N4 under the influence of a magnetic field.
2.3. Scanning Electron Microscope (SEM) Analysis of Materials Deposited PET
The PET surface could impact both adsorption and the restriction of ROS species. Figure 5 illustrates an analysis conducted using scanning electron microscopy (COXEM, Daejeon, Republic of Korea) equipped with an EDS detector (EDAX) from Oxford Instruments (Abingdon, UK). This examination offers comprehensive insights into the material’s morphology and elemental composition.
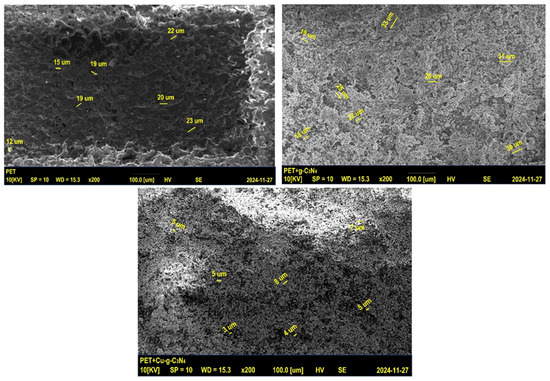
Figure 5.
SEM images of PET substrate (left), g-C3N4 deposited on PET (right), and Cu-g-C3N4 deposited on PET (bottom center).
The SEM image of PET reveals a uniform surface morphology with an average particle size of approximately 15 μm, indicating a basic capacity for interaction with nitride materials. In contrast, the SEM image of g-C3N4-coated PET displays a significant increase in surface roughness and potential particle aggregation, with an average size of around 27 μm. These clusters contribute to an expanded surface area, enhancing drug adsorption.
In the SEM image of Cu-g-C3N4-coated PET, a distinct transformation is observed, characterized by a more refined morphology with smaller, uniformly distributed particles averaging 4 μm in size. This improved dispersion increases surface heterogeneity, enhancing dicloxacillin adsorption. Additionally, the finer particle distribution enhances interactions between catalytic active sites and visible light, leading to greater photoreactivity. The transition from larger, aggregated particles in g-C3N4 to smaller, well-dispersed ones in the Cu-modified material likely plays a crucial role in boosting adsorption capacity. The reduction in particle size and improved dispersion result in a larger effective surface area with more accessible active sites for drug molecules, aligning with the experimentally observed increase in adsorption. Furthermore, the smaller particle size in the Cu-modified material promotes charge carrier separation, which is essential for ROS generation under visible light. However, the application of a magnetic field appears to disrupt this balance, as evidenced by a decline in disinfection efficiency.
The increased adsorption observed under the influence of the magnetic field could interfere with effective charge separation, thereby reducing ROS production. To gain a comprehensive understanding of these effects, it is essential to explore the underlying thermodynamic mechanisms. This involves examining the correlation between adsorption-free energy and magnetic field-induced surface modifications to clarify how the field impacts the material’s affinity for the adsorbate and its role in charge transfer processes.
2.4. Energy Dispersive Spectroscopy (EDS) Analysis
The EDS analysis presents the elemental composition in weight percentage to provide deeper insight into the sample. Figure 6 illustrates the elemental distribution spectrum within the sample, while Table 1 displays the quantified values of each element.
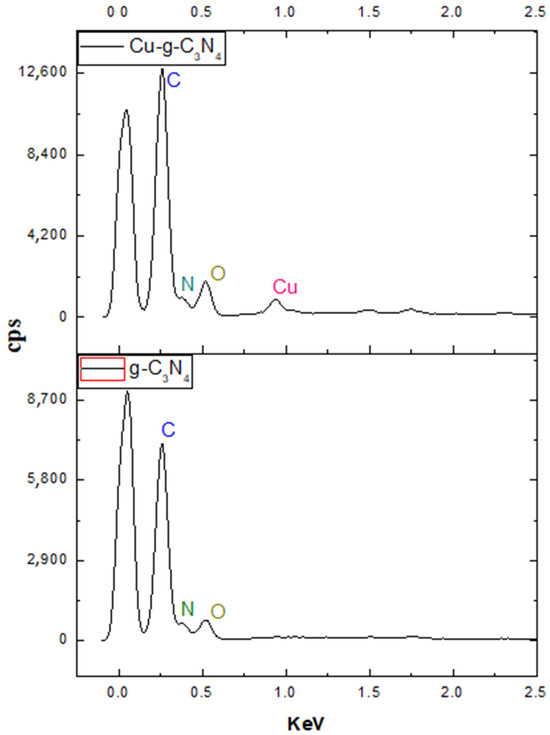
Figure 6.
Elemental distribution spectrum of material deposited on PET.

Table 1.
SEM-EDS elements C, N, O, Cu, Si.
Due to factors beyond the scope of this study, only the distribution of the materials could be measured, excluding that of the PET substrate. The analysis confirmed carbon, nitrogen, and oxygen as the primary components of the material. However, a key observation was the low copper content detected in the Cu2+-modified sample, measuring just 3%. This percentage does not accurately reflect the total metal content within the sample, likely due to the uneven distribution of copper on the surface. Since Cu2+ tends to form coordination bonds with nitrogen atoms at the nitride’s periphery, this heterogeneity could explain the discrepancy. These results indicate that the doping characteristics of the material are primarily influenced by the interactions between the metal and the nitride, governed by intermolecular forces at the material’s surface. To further investigate these interactions, the following section presents the findings of the X-ray diffraction analysis, offering deeper insight into the intermolecular forces that shape the material’s photocatalytic behavior.
2.5. X-Ray Diffraction (DRX) and Electronic Spectroscopic Characterization
Figure 7a illustrates the XRD analysis results, which were conducted to examine the structural modifications resulting from the incorporation of Cu2+ into the g-C3N4 network and to identify potential changes in the material’s structural arrangement. This analysis provides evidence of the dopant’s interaction with the polymeric matrix and enables the assessment of its impact on the nitride structure. These findings offer valuable insights into how these structural alterations may affect the material’s photocatalytic performance. Figure 7b shows the solid-state electronic spectrum of Cu-g-C3N4. This spectrum confirms the material’s absorption in the visible region and demonstrates how the spectrum of the lamp used for the photocatalysis process coincides with the material’s analysis region, allowing for optimal photocatalytic conditions.
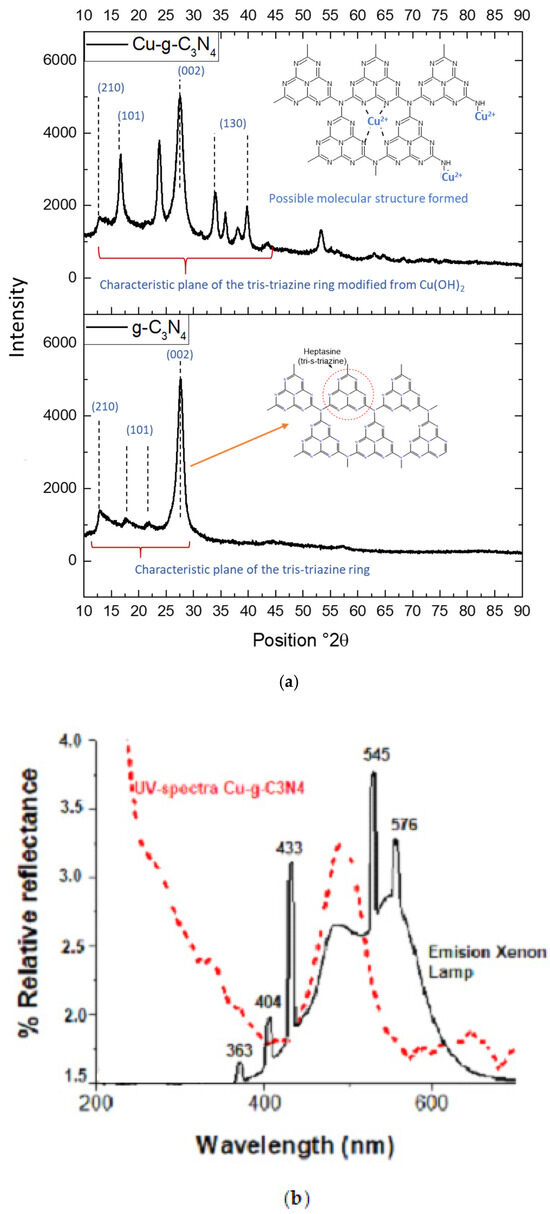
Figure 7.
(a). DRX patterns for g-C3N4 and its modification with Cu2+, (b). Solid electronic spectrum of Cu-g-C3N4.
The results indicate that the analyzed g-C3N4 exhibits a polymerized structure based on tri-s-triazine, aligning with the unit cell model proposed by Teter and Hemley [9]. The presence of characteristic reflections associated with this structural configuration, along with a slight shift toward lower angles, suggests alterations in the periodicity of the crystalline network. Specifically, the (210) reflection is observed at 2θ = 11.5°, the (101) at 2θ = 17.54°, and the (002) at 2θ = 27.78°. The latter is linked to the periodic arrangement of tris-triazine rings and the typical interplanar spacing found in graphitic g-C3N4 structures [10,11].
The analysis of the Cu2+-modified material shows that incorporating the metal ion does not significantly affect the (210) reflection, indicating that the alignment of aromatic layers remains stable. This finding aligns with previous studies suggesting that certain transition metals can interact with the structure without substantially disrupting its planar arrangement [12]. The slight variations observed in the diffraction patterns suggest that Cu2+ is likely positioned at the periphery of the g-C3N4 network, particularly at N-aliphatic sites, rather than being incorporated into the crystalline structure. This positioning would explain the minimal structural distortion observed [11].
Additionally, the (101) and (130) planes reveal the presence of small amounts of hydroxide, indicating that under the synthesis conditions, Cu2+ partially exists in its hydroxylated form. This observation is significant, as the coexistence of hydroxylated species on the material’s surface can impact its photocatalytic performance by either enhancing or restricting reactant adsorption and the generation of reactive oxygen species (ROS) [9,12]. These results suggest that while Cu2+ modification does not cause major disruptions to the structural periodicity of g-C3N4, its presence at the network’s periphery and its possible coexistence with hydroxylated species could influence surface interactions and, consequently, its photocatalytic activity.
2.6. Thermodynamic Evaluation
This thermodynamic approach focuses on the Gibbs free energy of adsorption, calculated using contact angle measurements, to analyze how magnetic fields modulate molecular adsorption and influence the production of ROS. Evaluating this interaction between surface properties and catalytic activity provides key insights into the magnetic field’s influence on molecular affinities and ROS generation processes.
2.6.1. Contact Angle Analysis
To evaluate the influence of the magnetic field on the surface properties of the material, contact angle measurements were performed using a drop tensiometer (SURFTENS-universal) under controlled conditions. In this experiment, a liquid droplet was carefully deposited on the material’s surface, and the contact angle was measured both with and without the presence of the magnetic field. Figure 8 shows how the evaluation was conducted.
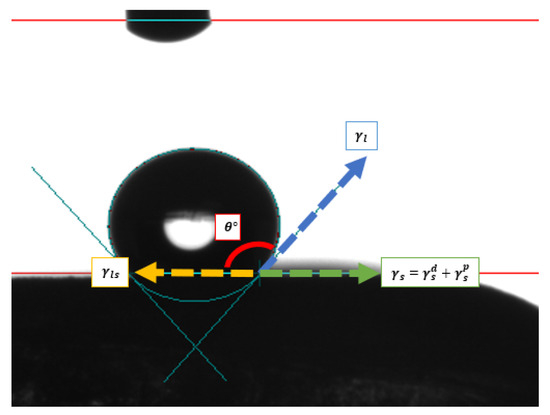
Figure 8.
Scheme contact angle analysis.
Where
= Dispersive contribution of the solid;
= Polar contribution of the solid;
= Surface tension of the solid;
= Surface tension of the liquid;
= Interfacial tension between the liquid and the solid;
= Contact angle.
The measurement is conducted to evaluate the interaction with a polar liquid, such as water, and glycerin as a non-polar liquid. The combination of both liquids in the measurement allows for the decomposition of the Gibbs free energy of adsorption into dispersive and polar components. The results are shown in Table 2 and Table 3.

Table 2.
Average Contact Angle with Water.

Table 3.
Average Contact Angle with Glycerin.
2.6.2. Gibbs Free Energy of Adsorption
To evaluate the interaction between the liquid and the surface of the material under different conditions, the mathematical expression of Young–Dupré (Equation (1)) and Owens–Wendt (Equation (2)) was utilized, which relates the work of adhesion to the contact angle and the surface tension of the liquid. This analysis enables the decomposition of the Gibbs free energy of adsorption into dispersive and polar components, providing key insights into the forces governing the photocatalytic activity of the material.
where
= Solid surface tension;
= Liquid surface tension;
= Contact angle;
= Adhesion work to temperature to 25 °C.
where
= Dispersive contribution solid;
= Dispersive contribution liquid;
= Solid polar contribution;
= Polar contribution liquid;
= Liquid surface tension;
= Contact angle;
= Adhesion work to temperature to 25 °C.
With the surface tension values, the Gibbs free energy of adsorption of the material could be calculated considering the magnetic field and without magnetic fields, using the mathematical deduction shown in Equations (3)–(5).
where
= Gibs free energy of adsorption;
= Gas constant;
= Absolute temperature;
= Adsorption constant;
= Solid surface tension at any time of adsorption;
= Solid surface tension before adsorption occurs.
Since the calculation of surface tension from the contact angle is a time-based measurement, 5 contact angle measurements were made at times between 1 and 10 s (seg) to then calculate the value of when the time is close to 0 seg. Therefore, the values of and are shown in Table 4.

Table 4.
Surface tension values of the material.
Therefore, the values of the Gibbs free energy of adsorption are shown in Table 5.

Table 5.
Gibbs free energy of adsorption as a function of area J/m2.
3. Discussion
3.1. Bacterial Disinfection Efficiency Analysis
The findings presented in Figure 1 indicate a notable reduction in the disinfection efficiency of the Cu2+-modified photocatalytic material deposited on PET when subjected to a 2000 mT magnetic field. This decline suggests that the magnetic field directly impacts ROS generation mechanisms, potentially promoting charge carrier recombination and thereby limiting the production of ▪OH. Additionally, while PET is chemically stable, its low intrinsic conductivity may amplify the magnetic field’s effect on charge carrier dynamics within the system. To further explore the underlying mechanisms behind this response, the PET-based system was chosen for additional experiments, as it demonstrated relatively better performance and minimal interference with the intrinsic properties of the photocatalytic material.
3.2. Photocatalytic Activity and Hydroxyl Radical Generation Analysis
The results of the dicloxacillin photodegradation experiment underscore the crucial role of visible light and material modification in the catalytic process. The photolysis control confirms that no drug degradation occurs in the absence of visible light, highlighting the necessity of photoinduced activation. Additionally, when unmodified g-C3N4 is deposited on PET, no significant drug degradation is observed, regardless of the presence or absence of a magnetic field [13]. This indicates that the intrinsic catalytic properties of unmodified g-C3N4 are insufficient for effective degradation in this system.
In contrast, Cu2+ modification significantly enhances photocatalytic performance, achieving complete drug degradation (100%) within just 20 min under visible light, demonstrating the effectiveness of the modification in generating ROS such as hydroxyl radicals (Figure 2). However, applying a constant magnetic field of 2000 mT drastically reduces the degradation efficiency to approximately 60%. These findings suggest that the magnetic field disrupts ROS generation mechanisms in the modified material, potentially affecting charge transfer between Cu2+ and g-C3N4 or altering the behavior of hydroxyl radicals responsible for contaminant degradation. This interaction warrants further investigation to fully understand the magnetic field’s impact on the catalytic system.
Furthermore, PET serves not only as a mechanical support but may also influence the dielectric and electronic properties of the catalytic system. According to Talecka, J. (2024) [14,15], PET exhibits unique characteristics such as moderate resistance to electrostatic discharge (ESD) and limited conductivity, which could affect charge transfer between the modified g-C3N4 and adsorbed molecules [15]. These dielectric properties are particularly relevant as they may influence the distribution of local electric fields on the catalyst surface, ultimately impacting the efficiency of electron/hole pair separation.
The study examining the impact of a magnetic field on hydroxyl radical generation, using methanol as a scavenger, indicates that in the absence of a magnetic field, methanol completely inhibits degradation. This confirms that ▪OH radicals are the primary contributors to the photocatalytic process. These results reinforce the hypothesis that the Cu2+-modified material efficiently generates ▪OH under visible light (Figure 3 and Figure 4).
However, when subjected to a 2000 mT magnetic field, contaminant degradation is restricted to just 50%, suggesting that the magnetic field affects hydroxyl radical production or activity. This phenomenon may be linked to changes in charge transfer dynamics or modifications in the excited states of the catalytic system. Although PET substrates are known for their low conductivity, the presence of a magnetic field could promote charge carrier recombination induced by visible light, thereby decreasing the availability of electrons and holes necessary for ROS formation.
In the same context, PET is considered a dielectric material; its interaction with Cu-g-C3N4 can be modulated by the magnetic field, altering the charge transfer dynamics. Previous studies have highlighted how magnetic fields can modify the mobility and distribution of charge carriers in photocatalytic systems [16]. Furthermore, the results indicate that the deposition of Cu-g-C3N4 on PET not only acts as a physical support but can also influence the electronic interactions and catalytic efficiency. In particular, Song et al.’s study (2015) [15] on PET composites shows that the dielectric properties of this material may be relevant in applications where charge transfer is key, such as in photocatalysis. These properties could be amplified or altered under an intense magnetic field, affecting the dynamics of ROS generation.
3.3. SEM Analysis
The SEM analysis of PET reveals a uniform surface morphology with an average particle size of approximately 15 μm, indicating a fundamental capacity to interact with nitride materials. In contrast, the SEM image of PET coated with g-C3N4 exhibits a significant increase in surface roughness and potential particle aggregation, with an average particle size of around 27 μm. This aggregation forms notable clusters, enhancing the available surface area for drug adsorption. A distinct transformation is observed in the SEM image of PET coated with Cu-g-C3N4, where the surface morphology is characterized by smaller, more uniformly dispersed particles, averaging 4 μm in size. This refined dispersion increases surface heterogeneity, improving dicloxacillin adsorption efficiency. Additionally, the finer particle distribution enhances interactions between catalytic active sites and visible light, boosting photoreactivity. The shift from larger, aggregated particles in g-C3N4 to smaller, well-dispersed particles in the Cu-modified material likely plays a key role in increasing adsorption capacity. The reduction in particle size and improved dispersion contribute to a larger effective surface area and greater accessibility of active sites for drug molecules, which aligns with the experimentally observed increase in adsorption. Moreover, the smaller particle size of the Cu-modified material promotes charge carrier separation, which is essential for ROS generation under visible light. However, exposure to a magnetic field appears to disrupt this balance, as evidenced by the decline in disinfection efficiency.
The increased adsorption under a magnetic field may impede optimal charge separation, thereby limiting ROS production. To gain a comprehensive understanding of these effects, it is essential to investigate the underlying thermodynamic mechanisms. Specifically, analyzing the relationship between adsorption-free energy and surface modifications induced by the magnetic field will help clarify its impact on material affinity for the adsorbate and its influence on charge transfer processes, as illustrated in Figure 5.
3.4. EDS Analysis
The EDS results show the distribution of elements such as carbon, nitrogen, and oxygen as the main components of the material. However, one of the most prominent results from the analysis is the low copper content detected in the Cu-modified sample, with only 3% reported. This percentage does not entirely represent the actual amount of metal present in the sample. However, this discrepancy could be due to the uneven distribution of copper on the surface, as Cu2+ commonly forms coordination bonds with nitrogen atoms on the nitride’s periphery [4], Table 1, Figure 6.
3.5. DRX and Electronic Analysis
The XRD analysis confirms the presence of copper affecting the periphery of the material but not the intercalation of the triazine units. Additionally, hydroxides are present in the material due to the pH increase during synthesis. On the other hand, for copper-modified carbon nitride, it is evident that the correct irradiation lamp was chosen for the analysis, as the material’s absorption spectrum falls within this range of electromagnetic radiation, shown in Figure 7a,b.
3.6. Analysis of Thermodynamic Properties
The thermodynamic results evidence that the presence of a magnetic field seems to affect the surface properties of the material, which, in turn, influences its photocatalytic capacity. The provided data on the contact angle, surface tension, and Gibbs free energy of adsorption explain how the magnetic field could influence the decrease in hydroxyl radical production of the material, especially the one modified with Cu2+ ion.
The contact angle with water (Table 2) and with glycerin (Table 3) shows a general trend of decreasing values when the magnetic field is present, especially in materials containing Cu-C3N4. PET + Cu-C3N4 experiences a decrease from 126.4° to 123.4° with water and from 121.7° to 136.4° with glycerin, suggesting that the surface becomes more hydrophilic under the influence of the magnetic field. The surface tension (Table 4) shows that, in the presence of a magnetic field, there is an increase in the surface tension of materials with Cu-C3N4. This indicates that the interaction between the material’s surface and the environment changes, which could modify the amount of water molecules or hydroxyl radicals adsorbed on the surface, altering its capacity to participate in photocatalytic reactions [17,18,19].
Consequently, the Gibbs free energy of adsorption shows that, without a magnetic field, materials with Cu-C3N4 have a value of 6.34 J/m2, whereas, with the magnetic field, this value increases to 10.52 J/m2. This increase in the free energy of adsorption indicates a decrease in the ease with which reactants, such as water or oxygen, are adsorbed on the material’s surface when the magnetic field is present. Higher values suggest that reactants are less likely to bind to the surface, which may reduce the number of hydroxyl radicals generated during the interaction, explaining the observed loss of photocatalytic efficiency with the magnetic field [20,21], as shown in Figure 8.
4. Materials and Methods
4.1. Reagents
Dicloxacillin (DCX) was obtained from Sinopharm Laboratories. Urea was sourced from Analytical Standards M&B Laboratory Chemicals (Cape Town, South Africa). Cupric chloride dihydrate was supplied by Fine Chemicals (Cape Town, South Africa). Sodium hydroxide pellets, fluorine-doped tin oxide sheets, perchloric acid, sodium sulfate, hydrogen peroxide, formic acid, and acetonitrile were acquired from Merk (Darmstadt, Germany).
4.2. Synthesis of Cu2+-Modified g-C3N4
Bulk g-C3N4 was prepared by pyrolyzing urea. Urea (10.0 g) was heated to 550 °C in an atmosphere-controlled oven at a heating rate of 100 °C h−1. This pyrolysis was conducted for 3 h, followed by cooling until reaching room temperature. The resulting powder was purified with water and dried at 80 °C. The modified g-C3N4 was prepared in a reflux system. Briefly, 0.067 g of the copper salt was diluted in 50 mL of distilled water and was put in reflux for 0.75 h at 100 °C. Subsequently, 0.2 g of g-C3N4 was added to the reflux system and allowed to react for 1 h. The reaction mixture was allowed to cool down and the pH was adjusted with concentrated NaOH equimolar until reaching a pH of 9. Finally, it was filtered by gravity and allowed to dry through paper (75 μm).
4.3. Photomagnetism Disinfection Against E. coli 25922 Using g-C3N4 and Modified with Copper II (Cu-g-C3N4)
4.3.1. Surface of PET-G for E. coli Disinfection System
A circular sheet with a 13 cm diameter made of polyethylene terephthalate glycol-modified (PET-G) was supplied by Distribuidora Biocientífica SAS, Medellín, Colombia. The sheet underwent a surface sanding process to increase roughness and enhance the adhesion of subsequent layers. The surface was then activated by acetone treatment to improve its chemical reactivity and promote the functionalization or adhesion of 150 mg/L of g-C3N4 and, in another experiment, Cu-g-C3N4. Finally, the circular sheet PET-G modified was inserted in a Petri dish and was left to dry at room temperature.
4.3.2. Setup of the Photomagnetism Disinfection Experiments
To establish the contribution of the magnetic field in the photo-disinfection process, an aluminum photoreaction system equipped with temperature control composed of 2.2 kW Xenon lamps (Sylvania FT5T8 8500 K 40 cm length, Medellín, Colombia) that emitted wavelengths between 400 and 700 nm to 60 W of light power and two vortex systems equipped with a Petri dish were used. This system allows for a high interaction with light and materials. Between the vortex and the Petri dish were installed four magnets generating a magnetic field of 2000 milliTesla (mT). These values are related to the work conducted by Farhan et al., where they experimented with the use of different magnetic fields for the disinfection of pathogenic bacteria in water and found that the most efficient is 1900 mT [9]. The system is shown in Figure 9.
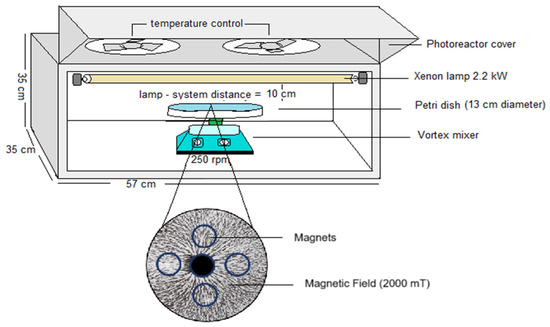
Figure 9.
Photomagnetism disinfection system.
4.3.3. Preparation and Inoculation of E. coli 25922 Cultures
The E. coli ATCC 25922 bacterial strain utilized in this study is part of the microbiological collection of the Research Group in Basic and Applied Microbiology (MICROBA) from the University of Antioquia, Medellín, Colombia. Before each experiment, the bacterial growth culture medium was prepared by dispersing 23.5 g of HIMEDIA Plate Count Agar (PCA) in 1 L of distilled millipore water. This PCA medium was dissolved, heated to boiling, and sterilized by autoclaving at 15 psi of pressure for 20 min. Subsequently, the medium was cooled to 57 ± 2 °C, and 19 ± 1 mL portions were poured into 100 × 15 mm sterile Petri dishes. Immediately, the culture medium underwent sterilization under UV radiation for 10 min. Then, a stock suspension of 5 × 108 Forming Colony Units (FCU)/mL was prepared as follows: E. coli (ATCC 25922) was retrieved from glycerol at −20 °C using a loop. It was then streaked on agar contained in a Petri dish, which was incubated for 18 h at 37 °C. Afterward, one colony was suspended in 5 mL of H2O and stirred using a vortex mixer. This dispersion was analyzed at 540 nm using a Shimadzu spectrophotometer at 568 nm. A concentration of 108 FCU/mL was adjusted to an absorbance of 0.7.
4.3.4. Conditions and Parameters for E. coli 25922 Photomagnetism Experiments
Initially, the photodisinfection system was tested under dark conditions, first without and then with the application of a magnetic field, as previously described. A total of 150 mL of distilled water was mixed with 1 mL of the bacterial suspension, resulting in a final concentration of 5 × 106 CFU/mL, which was experimentally confirmed. The mixture was then applied to the surface of the modified PET-G system, as detailed in Section 2.2 and stirred at 250 rpm. The samples were collected in the dark at 15 and 30 min (t = −15, −30). Subsequently, the lamp was turned on, and sampling was carried out at various intervals of 0, 5, 10, 20, 30, and 60 min. Samples were serially diluted (1/10) in saline, when necessary, to obtain microorganism counts between 102 and 105 CFU. In all cases, the bacterial suspensions were plated on count agar and incubated for 24 h. The number of CFUs was manually counted, and CFU/mL was calculated. Each experiment was performed at least in duplicate with and without the application of a magnetic field.
4.3.5. Dicloxacillin Degradation
Dicloxacillin was quantitatively analyzed using a UHPLC Thermo Scientific Dionex UltiMate 3000 (Waltham, MA, USA) system equipped with an Acclaim 120 RP C18 column (5 μm, 4.6 × 150 mm) and a photodiode array detector. Detection was performed at a wavelength of 270 nm, with an injection volume of 20 μL. The mobile phase consisted of a 50:50 mixture of formic acid (10 mmol L−1, pH 3.0) and acetonitrile, with a flow rate of 0.7 mL min−1.
4.4. Spectroscopic Characterization
The crystal structures were characterized using an X-ray powder diffractometer (Model XPert pro-MPD, Medellín, Colombia). Copper X-ray diffraction (XRD) patterns were recorded within a 5° to 80° range at a scan speed of 4° min−1, utilizing an incident wavelength of 0.15406 nm (Cu-Kα). Scanning electron microscopy (SEM) followed ASTM E1508-12 and ASTM E766-14 standards under the supervision of Committee E04 on Metallography and Subcommittee E04.11 on X-ray and Electron Metallography (USP<1181>). The solid-state diffuse reflectance spectrum was obtained using a Shimadzu UV-2401 PC spectrophotometer (Shimadzu, Tokyo, Japan) and an Agilent 8453 diode array spectrophotometer (Agilent, Santa Clara, CA, USA), respectively.
5. Conclusions
The influence of the magnetic field on Cu-C3N4 materials alters their surface properties, reducing the adsorption capacity of reactants and affecting photocatalytic efficiency. This effect may be due to the reduction in hydroxyl radical production. The induction of changes in the material’s surface properties by the magnetic field affects its adsorption capacity and interaction with other reactants present in the system, decreasing the material’s ability to perform disinfection and degradation of organic pollutants.
Author Contributions
Conceptualization: E.D.C.C. and Y.P.Á.-T. Methodology: E.D.C.C. and Y.P.Á.-T. Software and instrumental methodology: S.C. Investigation: Y.P.Á.-T. Resources: Y.P.Á.-T. Data curation: E.D.C.C. and Y.P.Á.-T. Writing—original draft preparation: E.D.C.C. Writing—review and editing: Y.P.Á.-T. Supervision: Y.P.Á.-T. Project administration: Y.P.Á.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Universidad de Antioquia—financial GRANT—SGR (Sistema.General de Regalías) Minciencias 2020000100587.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Corrales, J.; Acosta, J.; Castro, S.; Riascos, H.; Serna-Galvis, E.; Torres-Palma, R.A.; Ávila-Torres, Y. Manganese Dioxide Nanoparticles Prepared by Laser Ablation as Materials with Interesting Electronic, Electrochemical, and Disinfecting Properties in Both Colloidal Suspensions and Deposited on Fluorine-Doped Tin Oxide. Nanomaterials 2022, 12, 4061. [Google Scholar] [CrossRef] [PubMed]
- Cabral, J.; Rodrigues, A.G. Blue Light Disinfection in Hospital Infection Control: Advantages, Drawbacks, and Pitfalls. Antibiotics 2019, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Serna-Galvis, E.A.; Martínez-Mena, Y.L.; Porras, J.; Ávila-Torres, Y.; Silva-Agredo, J.; Torres-Palma, R.A. Understanding the Role of Complexation of Fluoroquinolone and β-Lactam Antibiotics with Iron (III) on the Photodegradation under Solar Light and UVC Light. Water 2021, 13, 2603. [Google Scholar] [CrossRef]
- Lasso-Escobar, A.V.; Castrillon, E.D.C.; Acosta, J.; Navarro, S.; Correa-Penagos, E.; Rojas, J.; Ávila-Torres, Y.P. Modulation of Electronic Availability in g-C3N4 Using Nickel (II), Manganese (II), and Copper (II) to Enhance the Disinfection and Photocatalytic Properties. Molecules 2024, 29, 3775. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Guo, J.; Gao, C.; Yin, H.; Xie, Y.; Lin, Z. Recent Advances in Efficient Photocatalysis via Modulation of Electric and Magnetic Fields and Reactive Phase Control. Adv. Mater. 2023, 35, 914. [Google Scholar] [CrossRef]
- Shu, A.; Qin, C.; Li, M.; Zhao, L.; Shangguan, Z.; Shu, Z.; Yuan, X.; Zhu, M.; Wu, Y.; Wang, H. Electric effects reinforce charge carrier behaviour for photocatalysis. Energy Environ. Sci. 2024, 17, 4907–4928. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Wu, C.; Tsang, S.C.E. Electric-/magnetic-field-assisted photocatalysis: Mechanisms and design strategies. Joule 2022, 6, 1798–1825. [Google Scholar] [CrossRef]
- Zhang, G.; Li, X.; Liu, Y.; Du, G.; Pang, H. Metal–organic framework derived micro-/nano-materials: Precise synthesis and clean energy applications. Inorg. Chem. Front. 2024, 11, 6275–6306. [Google Scholar] [CrossRef]
- Teter, D.M.; Hemley, R.J. Low-Compressibility Carbon Nitrides. Science 1996, 271, 53–55. [Google Scholar] [CrossRef]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Müller, J.O.; Schlögl, R.; Carlsson, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893. [Google Scholar] [CrossRef]
- Algara-Siller, G.; Severin, N.; Chong, S.Y.; Björkman, T.; Palgrave, R.G.; Laybourn, A.; Antonietti, M.; Khimyak, Y.Z.; Krasheninnikov, A.V.; Rabe, J.P.; et al. Triazine-Based Graphitic Carbon Nitride: A Two-Dimensional Semiconductor. Angew. Chem. Int. Ed. 2014, 53, 7450–7455. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M.G.; Mahal, J.D.; Mohammed, A.B. Evaluate the efficiency of treatment by using electromagnetic field in reducing bacterial contamination in wells water. Tikrit J. Pure Sci. 2017, 22, 11–19. [Google Scholar]
- Talecka, J.; Kluczyński, J.; Jasik, K.; Szachogłuchowicz, I.; Torzewski, J. Strength and Electrostatic Discharge Resistance Analysis of Additively Manufactured Polyethylene Terephthalate Glycol (PET-G) Parts for Potential Electronic Application. Materials 2024, 17, 4095. [Google Scholar] [CrossRef]
- Song, J.; Yuan, Q.; Zhang, H.; Huang, B.; Fu, F. Elevated conductivity and electromagnetic interference shielding effectiveness of PVDF/PETG/carbon fiber composites through incorporating carbon black. J. Polym. Res. 2015, 22, 158. [Google Scholar] [CrossRef]
- Xu, H.; Wang, M.; Yu, Z.-G.; Wang, K.; Hu, B. Magnetic field effects on excited states, charge transport, and electrical polarization in organic semiconductors in spin and orbital regimes. Adv. Phys. 2019, 68, 49–121. [Google Scholar] [CrossRef]
- Staniscia, F.; Guzman, H.V.; Kanduč, M. Tuning Contact Angles of Aqueous Droplets on Hydrophilic and Hydrophobic Surfaces by Surfactants. J. Phys. Chem. B 2022, 126, 3374–3384. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.; Kumar, T.; Ojha, K.; Mandal, A. Adsorption of surfactants on sand surface in enhanced oil recovery: Isotherms, kinetics and thermodynamic studies. Appl. Surf. Sci. 2013, 284, 87–99. [Google Scholar] [CrossRef]
- Sigal, G.B.; Mrksich, M.; Whitesides, G.M. Effect of Surface Wettability on the Adsorption of Proteins and Detergents. J. Am. Chem. Soc. 1998, 120, 3464–3473. [Google Scholar] [CrossRef]
- Oosterlaken, B.M.; van den Bruinhorst, A.; de With, G. On the Use of Probe Liquids for Surface Energy Measurements. Langmuir 2023, 39, 16701–16711. [Google Scholar] [CrossRef]
- Iwamatsu, M. Free-energy barrier of filling a spherical cavity in the presence of line tension: Implication to the energy barrier between the Cassie and Wenzel state on a superhydrophobic surface with spherical cavities. Langmuir 2016, 32, 9475–9483. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).