Abstract
The reaction in reagent grade acetone of copper(II) nitrate hexahydrate, 2,2′-bipyrimidine (bpm) and potassium iodide in a 1:2:2 molar ratio afforded three different products: an unreduced Cu(II) species, a fully reduced Cu(I) species and a mixed-valent Cu(II)/Cu(I) species. Of these, only the unreduced Cu(II) complex of formula [CuII(bpm)3](I3)(I) (1) could be structurally characterized, the other two products being initially only isolated as amorphous powders. X-ray quality, beautifully shaped, quasi-black prismatic crystals of compound 2, namely {[CuI(I3)CuII(I)(bpm)2](I3)}n, and brick-reddish parallelepipeds of compound 3, namely {[CuI2 (μ-I)2(bpm)]}n, were successively obtained through the slow diffusion in H-shaped tubes of aqueous solutions of the three reagents, after extensive optimization of the crystallization conditions. Compound 1 consists of a rare tris(2,2′-bipyrimidine)copper(II) monomeric dication, charge balanced by both iodide and triiodide anions. Compound 3, whose structure as well as optical and photocatalytic properties were recently disclosed, consists of a regular alternating μ-bpm/di-μ-iodide copper(I) chain. Finally, compound 2 consists of a rare, regular alternating mixed-valent Cu(II)-Cu(I) μ-bpm copper chain, showing unusual similarities in the metal coordination environment. The magnetic properties of compound 2 remarkably reveal a very weak antiferromagnetic coupling between the paramagnetic Cu(II) ions which are well separated both intra- and inter-chain.
1. Introduction
The interest in inorganic exchange–coupled systems has led to many synthetic endeavors and magnetostructural studies in the past decades [1]. In this field of research, the scientific contribution of Miguel Julve and Francesc Lloret is no less than outstanding [2,3,4,5].
The work presented herein aimed to give a modest tribute to these two brilliant researchers on the occasion of their retirement. Now that Miguel is dead, we would also like to remember him by briefly sharing some aspects of our personal journey together. There has been a very close scientific collaboration between the research group at the University of Calabria (De Munno) and that at the University of Valencia (Julve, Lloret), which lasted for almost forty years, also owing to the similar character of the protagonists and their true and profound friendship. Thanks to the pooling of different but complementary skills and backgrounds, such as structural chemistry and molecular magnetism, this collaboration has led to about 140 joint articles in peer-reviewed international scientific journals. It is hard not to remember with pride and nostalgia the early days, especially. In the period 1993–1998 alone, for example, six articles were published in Angewandte Chemie [6,7,8,9,10,11]. At the beginning, the scientific partnership was based on the magnetic and structural study of 2,2′-bipyrimidine (bpm) compounds. For this reason, the present commemorative contribution also focuses on bpm-containing species. As a bis-chelating ligand, its ability to mediate magnetic interactions between transition metal ions separated by distances ca. 5.5 A is now well known but, at the time the collaboration was born, there was only one structurally characterized bpm bridged compound, obtained in Prof. De Munno’s laboratory [12]. Over the years, more than 80 compounds containing bpm have been published by the two research groups. These comprise mononuclear, dinuclear and polynuclear species, as well as mono-, two- and three-dimensional polymers [6,7,8,9,10,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. In multiple cases, they have shown fascinating architecture and interesting magnetic features. Several reports are also known concerning the biological [29,30], photophysical, electrochemical and catalytical properties of bpm-containing compounds [23,31,32,33,34,35,36,37,38], proving the interest toward this widely used ligand is not limited to molecular magnetism.
At a certain point in time, we become involved in the study of halides and pseudohalides of paramagnetic metal ions containing bpm, whose synthesis and characterization appear very attractive from a structural and magnetic point of view. According to data reported in the literature, while the magnetic interaction through bpm is mostly antiferromagnetic, that mediated by bridging halogen/pseudoalogen can vary from antiferro- to weak or strong ferromagnetic [9,13,17,25,39,40,41,42,43,44]. Restricting ourselves to bridging bpm and halides Cu(II) systems, and excluding mixed-metal [45] or further mixed-ligand species [39], only three complexes result characterized to date, all displaying large antiferromagnetic behavior: two bidimensional polymers of general formula [Cu2(bpm)X4]n (X = Cl, Br) [13] and one chain compound of formula [{Cu2(μ-bpm)(H2O)2(μ-F)2F2}n [25]. With the iodide ion, to the best of our knowledge and according to a recent search within the Cambridge structural Database (CCDC 2024; ConQuest version 2024.3.0), no bpm complexes of Cu(II) have ever been reported, which prompted us to undertake the study presented herein. In this work, we focus on the versatility of the copper/2,2′-bipyrimidine/iodide system, which has led to the isolation and characterization of an unreduced Cu(II) species, a mixed-valent Cu(II)/Cu(I) species and a fully reduced Cu(I) species under the same, mild experimental conditions, starting from a Cu(II) salt, potassium iodide and bpm. The three complexes have formulae, respectively, [CuII(bpm)3](I3)(I) (1), {[CuII3 CuIII(bpm)2](I3)}n (2) and {[CuI2 (μ-I)2(bpm)]}n (3). A rationalized synthetic approach is presented, which proved pivotal for the obtaining of complexes 2 and 3, each as clean and almost quantitative product of two slightly distinct crystallization strategies. The crystal structure of complex 3, of broad interest for its optical and photocatalytic properties, has been recently evaluated by Hou et al. [38], CCDC refcode ZIJWEX. For this reason, only the new synthetic route to 3 is discussed herein. Although the leitmotiv of this work is both synthetic and structural, of course, we would not have achieved justice to honoring our friends Paco and Miguel without some magnetic measurements. They analyzed and conducted the magneto–structural correlation study themselves on compound 2, validating its nature as that of a quite unique, mixed-valent, perfectly alternating Cu(I)-Cu(II) chain based on bpm bridges.
2. Materials and Methods
All chemicals were purchased from commercial sources and used as received without further purification. Infrared spectra were recorded on a PerkinElmer 1750 FT-IR spectrometer (PerkinElmer, Waltham, MA, USA) as KBr pellets in the 4000−400 cm−1 region. The relative intensity of reported signals is defined as s = strong, br = broad, sh = sharp, m = medium, and w = weak. Elemental analysis (C, H, N) was performed by the Microanalytical Service of the Università della Calabria. Variable-temperature (2.0–300 K) magnetic susceptibility and variable-field (0–5 T) magnetization measurements were carried out with a SQUID magnetometer under an applied field of 0.1 T (T ≥ 25 K). A small magnetic field of 0.025 T was used in the low-temperature region (T < 25 K) to prevent saturation effects. The experimental magnetic susceptibility data were corrected for the diamagnetic contributions of the constituent atoms and the sample holder, as well as for the temperature-independent paramagnetism (tip) of the CuII ion (60 × 10−6 cm3 mol−1).
2.1. Preparation of the Complexes
[CuII(bpm)3](I3)(I) (1), {[CuI(I3)CuII(I)(bpm)2](I3)}n (2) and {[CuI2(μ-I)2(bpm)]}n (3).
Cu(NO3)2∙6H2O (0.2 mmol) and bpm (0.4 mmol) were dissolved in reagent grade acetone (50 mL), giving a dark green solution. To this solution, KI (0.4 mmol) was added as solid, slowly and under continuous stirring. Upon dissolution of KI, the solution color turned brown-yellowish, and a brick-red precipitate appeared simultaneously. This was filtered and the solution was left to evaporate slowly at room temperature. After a few hours, a new precipitate separated from the solution, exhibiting a darker color this time. The observation of this second precipitate fraction under the microscope revealed the presence of both brick-red and very dark, quasi black, grains in it. After filtering out this second solid fraction, amber-yellow parallelepipeds of compound 1 appeared in ca. 2 days on standing of the reaction solution at room temperature. Yield: ca. 20% based on Cu(II). Anal. Calcd. for C24H18CuI4N12 (1): C, 27.57; H, 1.74; N, 16.07. Found: C, 27.48; H, 1.65; N, 16.15. IR (KBr/cm−1): 3550 (br), 3410 (br), 3090 (w), 1568 (sh), 1552 (sh,s), 1449 (m), 1403 (sh,s), 1235 (m), 1145 (sh, m), 1098 (m), 1004 (sh, m), 802 (sh), 800 (sh), 750 (sh,s), 687 (sh), 653 (sh).
Various attempts to dissolve and recrystallize the two initial amorphous precipitate fractions failed, but their analysis through comparative infrared spectroscopy later revealed the first fraction being compound 3, while the second fraction being a mixture of compounds 2 and 3. In fact, single crystals of compounds 2 and 3 were successively obtained through slow diffusion in H-shaped tubes. Firstly, an H-shaped tube was prepared as follows. One arm was filled with a dark green aqueous solution (2 mL) of Cu(NO3)2∙6H2O (0.2 mmol) and bpm (0.1 mmol) and the other with a colorless aqueous solution of KI (0.4 mmol, so that, overall, the synthetic Cu(II):bpm:KI molar ratio was 2:1:4), layering both arms slowly with water. This method afforded, after a few weeks, very dark crystals of compound 2, their surface being extensively covered by much smaller, reddish crystals of compound 3, however. Clean, homogeneous batches of beautifully shaped, X-ray quality quasi-black prisms and reddish parallelepipeds of compound 2 and 3, respectively, could be finally grown in H-shaped tubes, following the same procedure indicated above, by adjusting the synthetic Cu(II):bpm:KI molar ratio to either 2:1:2 (for 2) or 2:1:1 (for 3), respectively. Yield: ca. 60% (2) and 75% (3) based on Cu(II). Anal. Calcd. for C16H12Cu2I7N8 (2): C, 14.43; H, 0.91; N, 8.41. Found: C, 14.20; H, 0.89; N, 8.50. Anal. Calcd. for C8H6Cu2I2N4 (3): C, 17.82; H, 1.12; N, 10.39. Found: C, 17.74; H, 1.05; N, 10.42. IR (KBr/cm−1) for 2: 3048 (m), 1595 (w), 1557 (sh, s), 1471 (w), 1403 (sh, s), 1329 (w), 1298 (w), 1215 (w), 1012 (m), 831 (sh), 818 (sh), 740 (sh,s), 668 (sh). IR (KBr/cm−1) for 3: 3050 (sh, m), 1558 (sh, s), 1407 (sh, s), 1140 (w), 1011 (sh, w), 832 (sh, m), 751 (sh, s), 681(sh, m), 667 (sh, m). Notes: sample purity of crystalline batches of 1–3 obtained through the optimized synthetic procedures was assessed first by eye and then confirmed by elemental analyses; spectroscopic analysis (FT-IR) was conducted over the entire course of the synthetic exploration briefly summarized above. The most characteristic band in the IR spectra of complex 1–3 is the strong and sharp band around 1580 cm−1, diagnostic of a coordinated bpm ligand. For 1, this band appears as a quasi-symmetric doublet, which is typically associated with the presence of terminal chelating bpm, while the same band appears as a sharp and strongly asymmetric doublet in the IR spectra of complexes 2 and 3, as expected [25,41]. Indeed, the IR spectra of 2 and 3 are very similar, although each band is sharper in the monovalent (3) vs. mixed-valent (2) species.
2.2. X-Ray Data Collection and Structure Refinement
X-ray diffraction data on single crystals of 1–3 were collected at room temperature with a Bruker-Nonius X8-APEXII CCD area detector system by using graphite-monochromated Mo-Kα radiation (Bruker, Billerica, MA, USA) (λ = 0.71073 Å). The data were processed through the SAINT [46] reduction and SADABS [47] absorption software. The structures were solved by standard direct methods and subsequently completed by Fourier recycling by using the SHELXTL software packages [48]. The obtained models were refined with the 2019/3 version of SHELXL against F2 on all data by full-matrix least squares.
All non-hydrogen atoms of the bpm ligands were set in calculated positions and refined isotropically using the riding model. The final geometrical calculations and graphical manipulations were performed using the XP utility of SHELX 2019-3 [48] and the software Diamond (version 4.6.8) [49]. A summary of the crystallographic data and structure refinement for 1–3 is given in Table 1. Main bond lengths and angles for 1 and 2 are shown in Table 2 and Table 3, respectively (for a detailed structural description of compound 3 see ref. [38]). Crystallographic data have been deposited at the Cambridge Crystallographic Data Centre and have been assigned CCDC reference numbers 2419535 (1), 2419536 (2) and 2425651 (3). These data can be obtained free of charge from the Cambridge Crystallographic Data Center via https://www.ccdc.cam.ac.uk/structures/Home.

Table 1.
Summary of crystallographic data for [CuII(bpm)3](I3)(I) (1), {[CuII3 CuIII(bpm)2](I3)}n (2) and {[CuI2(μ-I)2(bpm)]}n (3).
3. Results and Discussion
3.1. Description of the Structures
3.1.1. [CuII(bpm)3](I3)(I) (1)
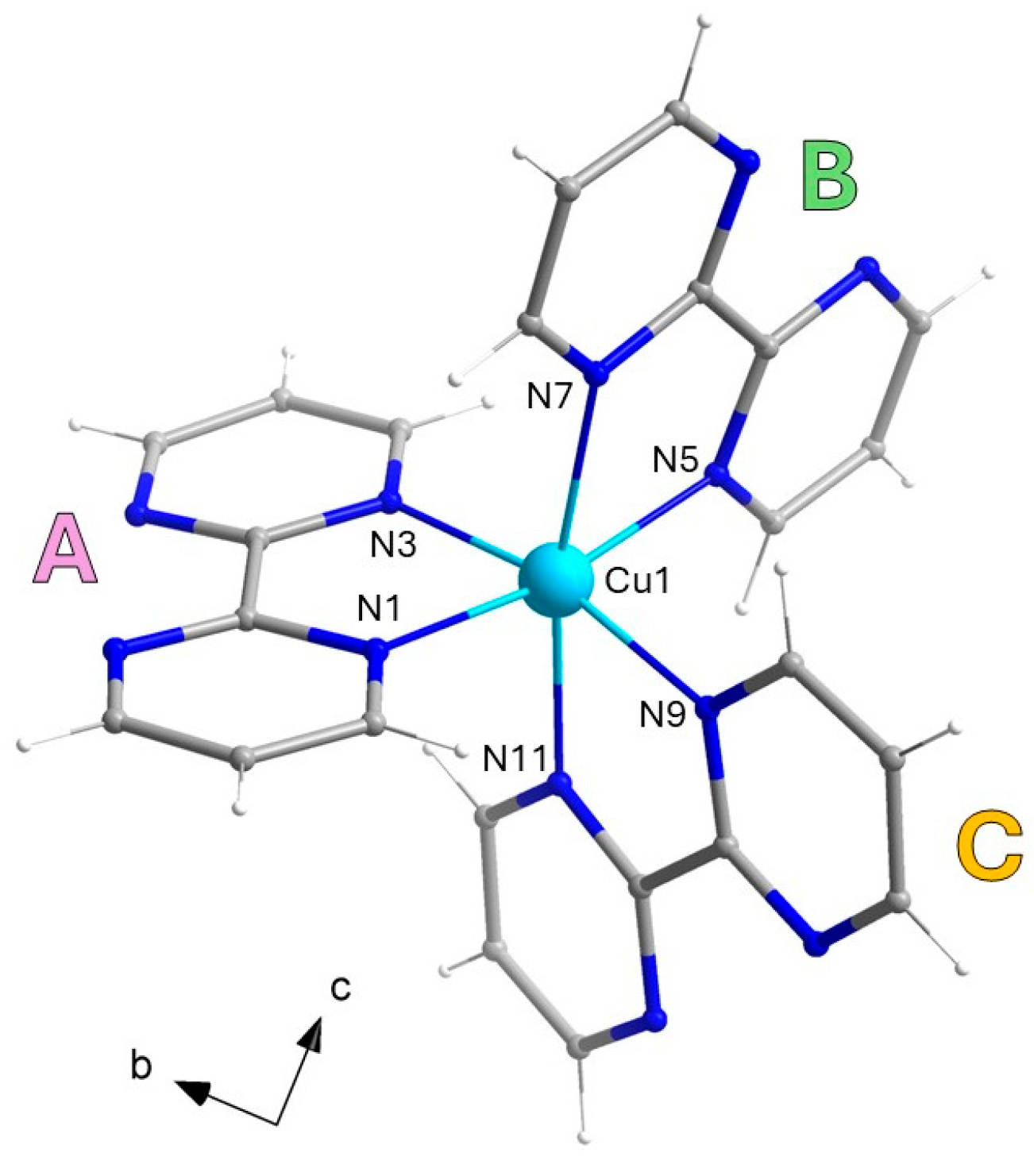
Compound 1 crystallizes in the triclinic space group P-1 and consists of a racemic mixture of Δ- and Λ-isomers of a monomeric [CuII(bpm)3]2+ cation (see Figure 1), charge-balanced by both iodide and triiodide anions. The metal ion in 1 is six-coordinate by the N atoms of three crystallographically independent bpm molecules (noted as A, B and C), adopting an axially elongated octahedral surrounding, as confirmed by continuous shape measures (CShM) [50] (see Table 2 for selected bond distances and angles).
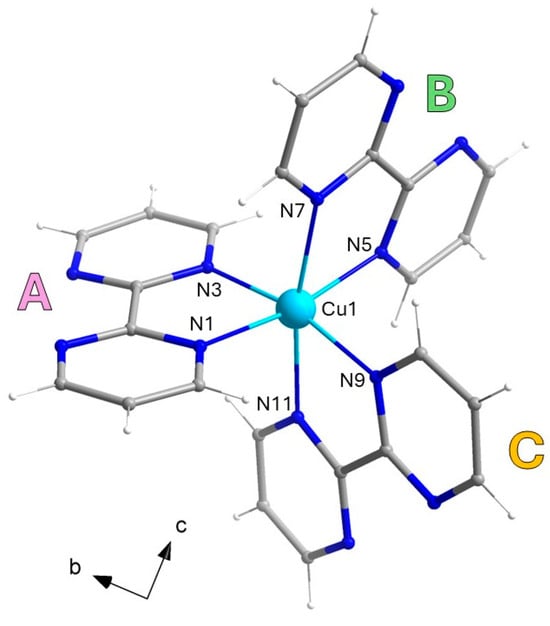
Figure 1.
Molecular structure of the monomeric [CuII(bpm)3]2+ cationic unit in 1, with selected labels. The Δ-isomer has been arbitrarily chosen as representative of the asymmetric unit; the Λ-isomer is generated by the inversion center. The three crystallographically independent bpm molecules are noted as A, B, and C. [Color codes: Cu(II), cyan; C, gray; N, blue].

Table 2.
Selected bond distances [Å] and angles [°] for 1.
Table 2.
Selected bond distances [Å] and angles [°] for 1.
| Cu1-N1 Cu1-N5 Cu1-N3 I1-I2 | 2.096(4) 2.236(4) 2.044(3) 2.9835(5) | Cu1-N7 Cu1-N9 Cu1-N11 I2-I3 | 2.108(4) 2.060(3) 2.244(3) 2.8679(5) |
| N3-Cu1-N9 N3-Cu1-N1 N9-Cu1-N1 N3-Cu1-N7 N9-Cu1-N7 N1-Cu1-N7 N3-Cu1-N5 N9-Cu1-N5 | 166.29(13) 78.84(13) 95.67(12) 95.05(13) 92.02(12) 169.86(12) 95.93(12) 97.16(12) | N1-Cu1-N5 N7-Cu1-N5 N3-Cu1-N11 N9-Cu1-N11 N1-Cu1-N11 N7-Cu1-N11 N5-Cu1-N11 I(1)-I(2)-I(3) | 96.31(14) 76.12(14) 91.34(12) 76.22(12) 92.20(12) 96.06(13) 169.73(13) 177.896(16) |
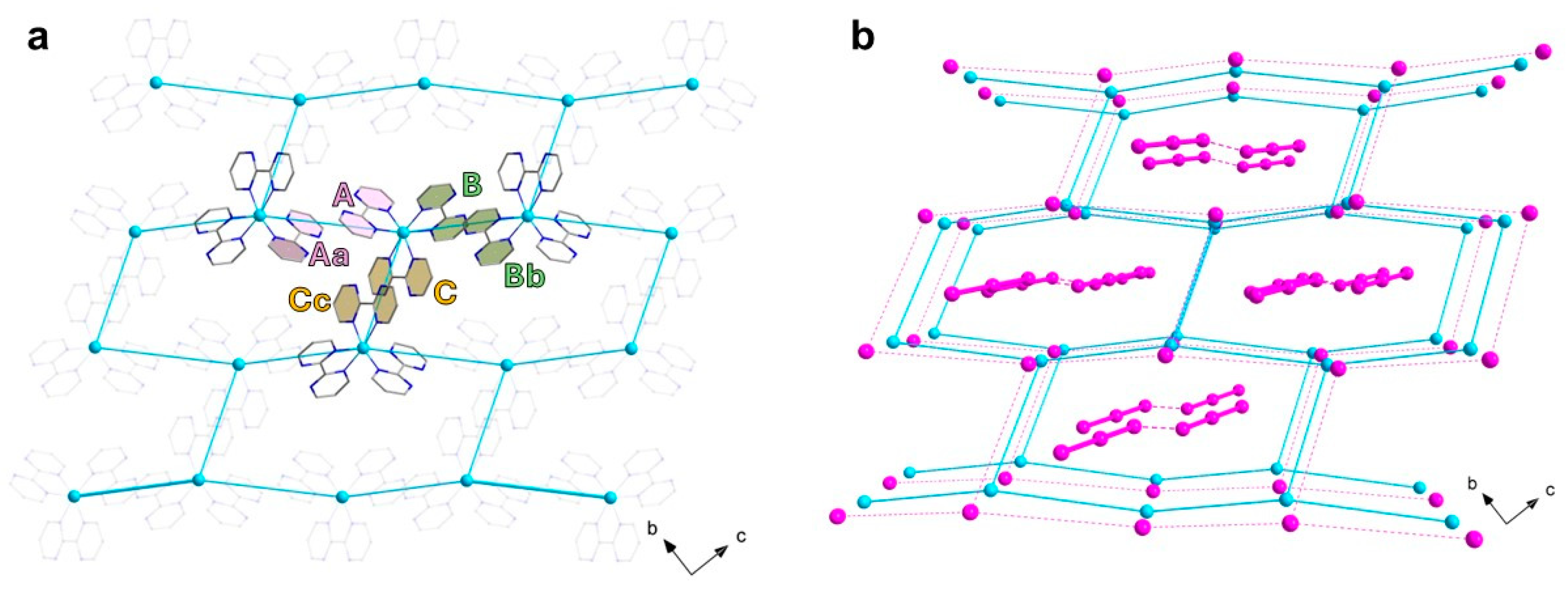
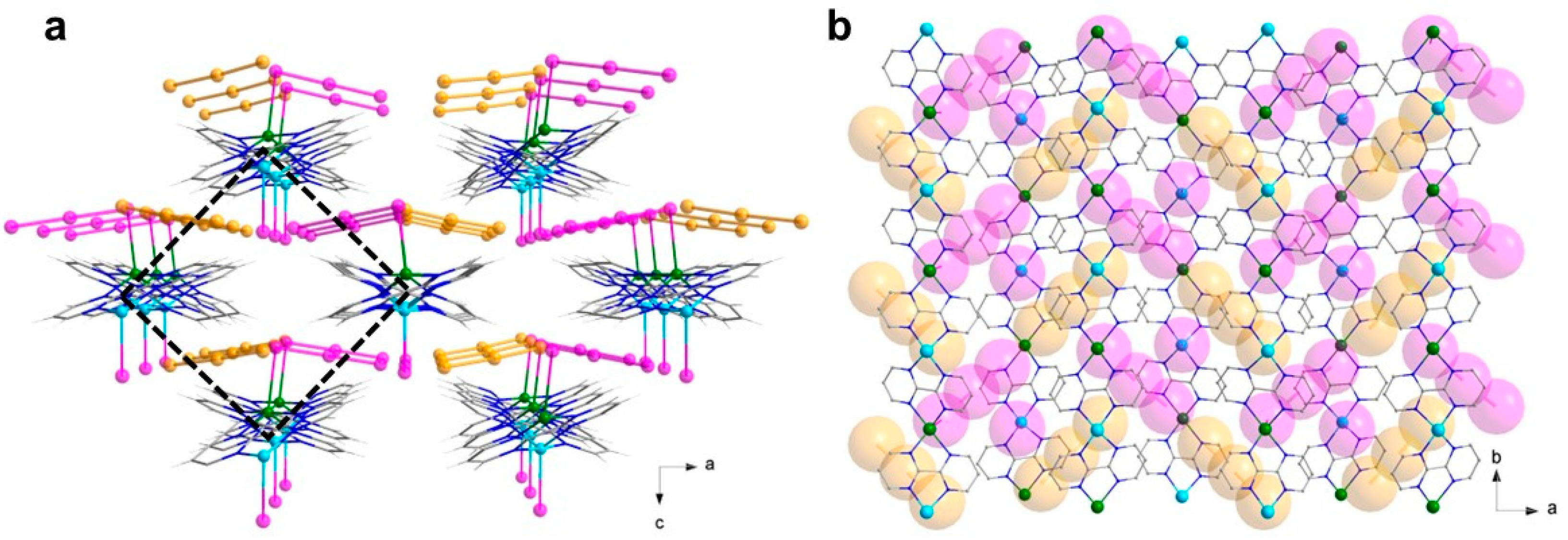
The three chelating bpm molecules are rather planar, with mean deviations from planarity laying in the range 0.020(4)–0.049(4) Å, and they form dihedral angles of 79.2(1), 86.6(1) and 85.9(1)° between each other. Each bpm self-interacts with a symmetry-equivalent molecule, following A∙∙∙Aa, B∙∙∙Bb and C∙∙∙Cc off-set π-π interactions paths [(a) −x, −y + 2, −z; (b) −x, −y + 1, −z + 1; (c) −x, −y + 1, -z, see Figure 2a], and establishing interplanar distances of ca. 3.35, 3.30 and 3.15 Å, respectively. In this way, each Δ-isomer is surrounded by three Λ- isomers and vice versa, with metal–metal distances in the range 8.3–9.4 Å [Cu1···Cu1a, 9.380(1); Cu1···Cu1b, 8.327(1); Cu1···Cu1c, 8.307(1) Å]. The resulting motif is overall half-way between the brick-wall and the honeycomb (6,3) net, as shown in Figure 2a, with the supramolecular layers growing parallel to the crystallographic bc plane. Interestingly, the iodide anions form an ideally identical 2D anionic net, with consistent I···I separations [8.105(1)–9.520(1) Å]. The cationic and anionic hexameric motifs are eclipsed and they alternate regularly along the crystallographic a axis, forming pseudo-hexagonal cavities (Figure 2b).
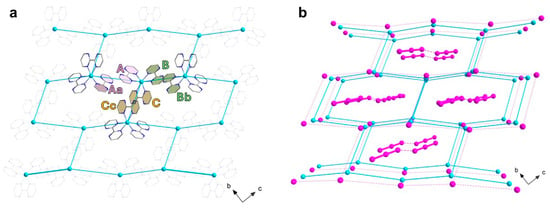
Figure 2.
(a) View along the crystallographic a axis of one cationic layer in 1, highlighting the bpm interaction paths and the topological 2D network of Cu(II) ions. (b) Perspective view of the alternating cationic and anionic topological 2D nets in 1, highlighting the content of the supramolecular pseudo-hexagonal cavities. [Color codes: Cu(II), cyan; C, gray; N, blue; I, pink. Solid and dotted lines are only a guide for the eyes].
A close look into the alternating layers reveals they are not well separated. Rather, numerous CH···I contacts between the bpm ligand and the iodide anions (with H···I distances in the range 3.0–3.5 Å) are observed, which likely contribute to the formation of the ordered anionic hexagonal net (Figure 3a). The triiodide anions, which are fairly symmetrical but deviate somewhat from linearity [I1-I2, 2.984(1) Å; I2-I3, 2.868(1) Å; I1-I2-I3, 162.1(1)°], form dimers reminiscent of a I62− anion [51,52,53], by means of I···I contacts that fall in the typical range of the so-called secondary halogen interactions [I3∙∙∙I3d, 3.698(7) Å; I2-I3∙∙∙I3d, 161.4(2)°; (d) = −x + 2, −y, −z + 1]. These anionic triiodide pairs are segregated within the supramolecular pseudo-hexagonal cavities by means of several, weak CH···I (in the range ~3.1–3.5 Å) and N···I (in the range ~3.8–4.0 Å) contacts (Figure 3b,c). No iodide-triiodide contacts are noted in the structure, instead.
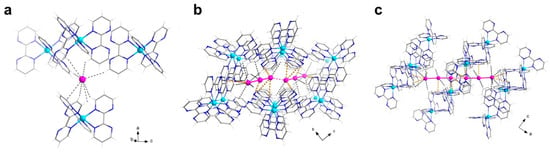
Figure 3.
(a) A view of the iodide anions close surrounding in 1, highlighting the several CH···I contacts (dashed black lines) established with the bpm molecules of vicinal complex cations. (b,c) Perspective views, along either the crystallographic a (b) or b (c) axes, of a portion of the 3D packing in 1, showing the triiodide pairs close surrounding within the pseudo-hexagonal cavities in 1. The weak CH···I and N···I contacts contributing to the anion segregation within the channels are drawn as black and yellow dashed lines, respectively. [Color codes: Cu(II), cyan; C, gray; N, blue; H, white; I, pink].
3.1.2. {[CuI(I3)CuII(I)(bpm)2](I3)}n (2)
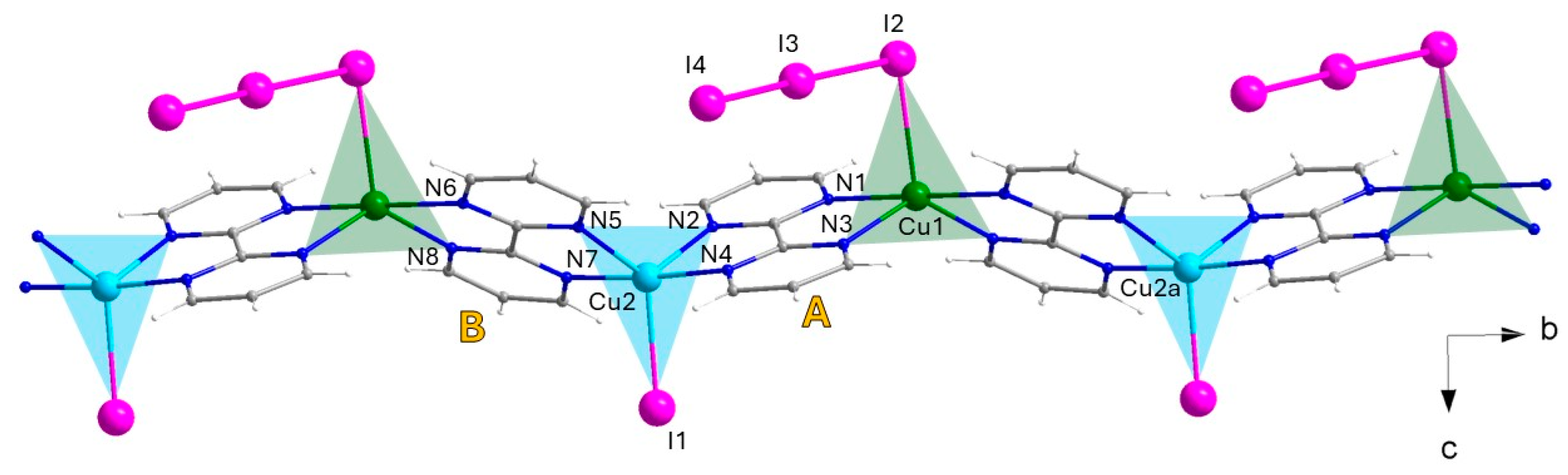
Compound 2 crystallizes in the orthorhombic space group Pna21 with two crystallographically independent copper atoms in the asymmetric unit, Cu1 and Cu2. It consists of chains of alternating Cu1 and Cu2 ions connected by two distinct bpm bridges (denoted A and B), with triiodide or iodide anions completing the coordination sphere of the two metal ions (Figure 4).
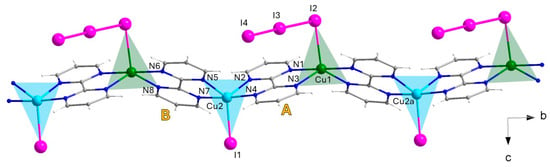
Figure 4.
A fragment of the cationic alternating copper(I)–copper(II) chain growing along the crystallographic b axis in 2, with selected labels, also showing the equatorial planes at both metal centers along the chain [Symmetry code: (a) x, 1 + y, z. Color codes: Cu(II), cyan; Cu(I), green; C, gray; N, blue; H, white; I, pink.].

Table 3.
Selected bond distances [Å] and angles [°] for 2a.
Table 3.
Selected bond distances [Å] and angles [°] for 2a.
| Cu1-N1 Cu1-N3 Cu1-N6a Cu1-N8a Cu1-I2 I2-I3 b I3-I4 b | 2.240(6) 2.106(6) 2.260(6) 2.097(5) 2.7907(16) 3.0321(11) 2.8704(11) | Cu2-N2 Cu2-N4 Cu2-N5 Cu2-N7 Cu2-I1 I5-I6 c I6-I7 c | 2.140(5) 1.993(5) 2.121(5) 1.985(5) 2.6179(10) 2.9291(10) 2.9448(10) |
| N8a-Cu1-N3 N8a-Cu1-N1 N3-Cu1-N1 N8a-Cu1-N6a N3-Cu1-N6a N1-Cu1-N6a N8a-Cu1-I2 N3-Cu1-I2 N1-Cu1-I2 N6a-Cu1-I2 I2-I3-I4 b | 128.8(2) 103.2(2) 77.7(2) 77.2(2) 101.6(2) 179.2(2) 123.84(17) 107.29(16) 89.59(17) 90.72(17) 176.71(3) | N5-Cu2-N2 N4-Cu2-N5 N4-Cu2-N2 N7-Cu2-N5 N7-Cu2-N2 N7-Cu2-N4 N5-Cu2-I1 N2-Cu2-I1 N4-Cu2-I1 N7-Cu2-I1 I5-I6-I7 c | 122.7(2) 98.1(2) 80.0(2) 80.0(2) 97.49(19) 175.5(2) 122.10(16) 115.23(15) 90.99(17) 93.52(16) 176.45(2) |
a Symmetry code: (a) x, 1 + y, z. b Coordinated triiodide anion. c Uncoordinated triiodide anion.
The presence of an uncoordinated extra triiodide anion in the asymmetric unit indicates that the chain is cationic, meaning 2 must be a mixed valence copper(I/II) compound. Both metal ions in 2 are five-coordinate, with four N atoms of two bridging bpm molecules and a I3− or I− anion building, in either case, a distorted {CuN4I} trigonal bipyramidal coordination arrangement (as confirmed by CShM [50]), where the triiodide (at Cu1) or iodide (at Cu2) anions occupies one of the three equatorial positions. Despite the striking likeliness of the two metal surroundings, the average Cu-N and Cu-I bond lengths are somewhat longer for Cu1 [2.176(6) and 2.791(2) Å, respectively] than for Cu2 [2.060(5) and 2.618(1) Å, respectively]. A closer look at the two coordination polyhedral shows that the two axial Cu-N bond lengths are longer than the two equatorial Cu-N bond lengths for Cu1, while it is the opposite for Cu2 (see Table 3). Both these findings suggest that Cu1 and Cu2 could be considered Cu(I) and Cu(II) centers [54,55], respectively, with crystallographic full occupancy.
The alternating Cu(I)/Cu(II) chains develop in the direction of the crystallographic b axis. The equatorial planes of adjacent Cu(I) and Cu(II) polyhedra are essentially coplanar, but rotated by about 174° (see Figure 4). The two crystallographically independent bis-chelating bpm molecules are reasonably planar and they form a dihedral angle of 47.9(1)° between each other. The metal–metal distance through either the A bpm or the B bpm corresponds to about 5.64 Å, which results in Cu1···Cu1a and Cu2···Cu2a intra-chain separations of ca. 10.844(1) Å [(a) x, 1 + y, z].
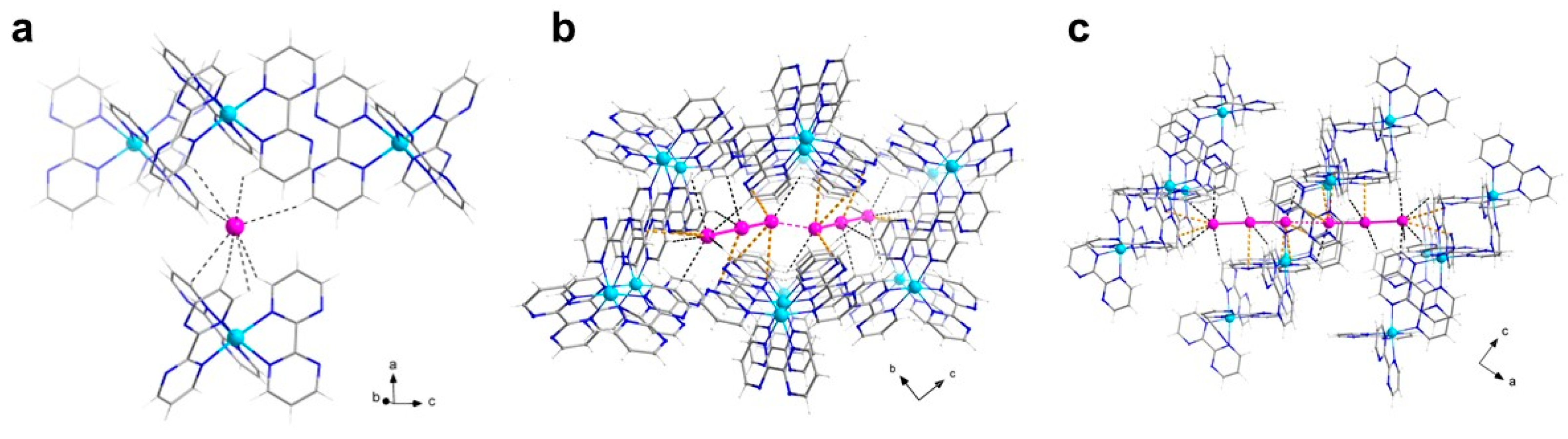
In the ac plane, the chains are arranged according to a quasi-ideal square net (Figure 5a), whose edges correspond to the smallest inter-chain Cu1-Cu1 and Cu2-Cu2 distances [Cu1···Cu1b, 8.127(2) Å; Cu1···Cu1c, 9.898(1) Å; Cu2···Cu2c, 9.810(1) Å; Cu2···Cu2d, 8.232(1) Å; (b) 1 − x, 2 − y, −0.5 + z; (c) 0.5 − x, 0.5 + y, 0.5 + z; (d) 1 − x, 1 − y, 0.5 + z]. Among those, the shorter separations [Cu1···Cu1b and Cu2···Cu2d, ca. 8 Å] are associated with weak off-set π-π stacking interactions established between an A bpm on one chain and a B bpm on the other (dihedral angle of 4.7°, interplanar distance of 3.4 Å); for the longer distances [Cu1···Cu1c and Cu2···Cu2c, ca. 10 Å], the aromatic interaction is even less relevant as the dihedral angle between the ligands would be 22° (Figure 5b).
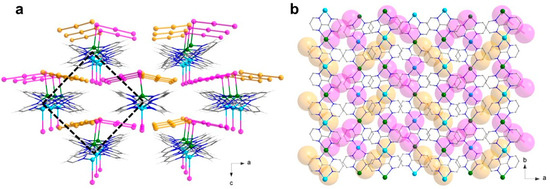
Figure 5.
(a) Perspective view, along the crystallographic b axis, of a fragment of the crystal packing of 2, highlighting the square arrangement of the copper(I/II) chains in the ac plane, and the apparent alternation between anionic and cationic regions along the c axis. (b) View of a fragment of the crystal packing of 2 along the c axis, showing the bpm-bpm contacts taking place through the ‘holes’ of the anionic layer (I atoms are depicted as van der Waals spheres). [Color codes: Cu(II), cyan; Cu(I), green; C, gray; N, blue; I(coordinated), pink; I(uncoordinated), gold].
Concerning the anions, the two crystallographically independent triiodide groups in 2 are structurally not equivalent. The uncoordinated anion is fairly linear (with a I5-I6-I7 angle of 176.45(2)°) and symmetrical (with I5-I6 and I6-I7 bond distances of 2.929(1) and 2.945(1) Å), while the Cu(I)-coordinated anion is linear (with a I2-I3-I4 angle of 176.71(3)°) but much less symmetrical (with I2-I3 and I3-I4 bond distances of 3.032(1) and 2.870(1) Å), as a consequence of coordination. They both participate in an extended, herringbone-like polyiodide 2D supramolecular network, with triiodide-triiodide contacts slightly below 4 Å, and weaker iodide-triiodide contacts in the range 4.2–4.3 Å. There is an apparent alternation of cationic (copper-bpm backbones) and anionic (coordinated iodide/coordinated and uncoordinated triiodide) regions in the crystal packing of 2, along the crystallographic c axis (Figure 5a). Truly, the copper chains are well separated along the a and c axes, but not halfway between them, where they interpenetrate the iodide-triiodide net through the mentioned moderate to weak off-set π-π contacts between bpm molecules placed above and beyond the anionic layer (Figure 5a,b).
3.2. Magnetic Properties
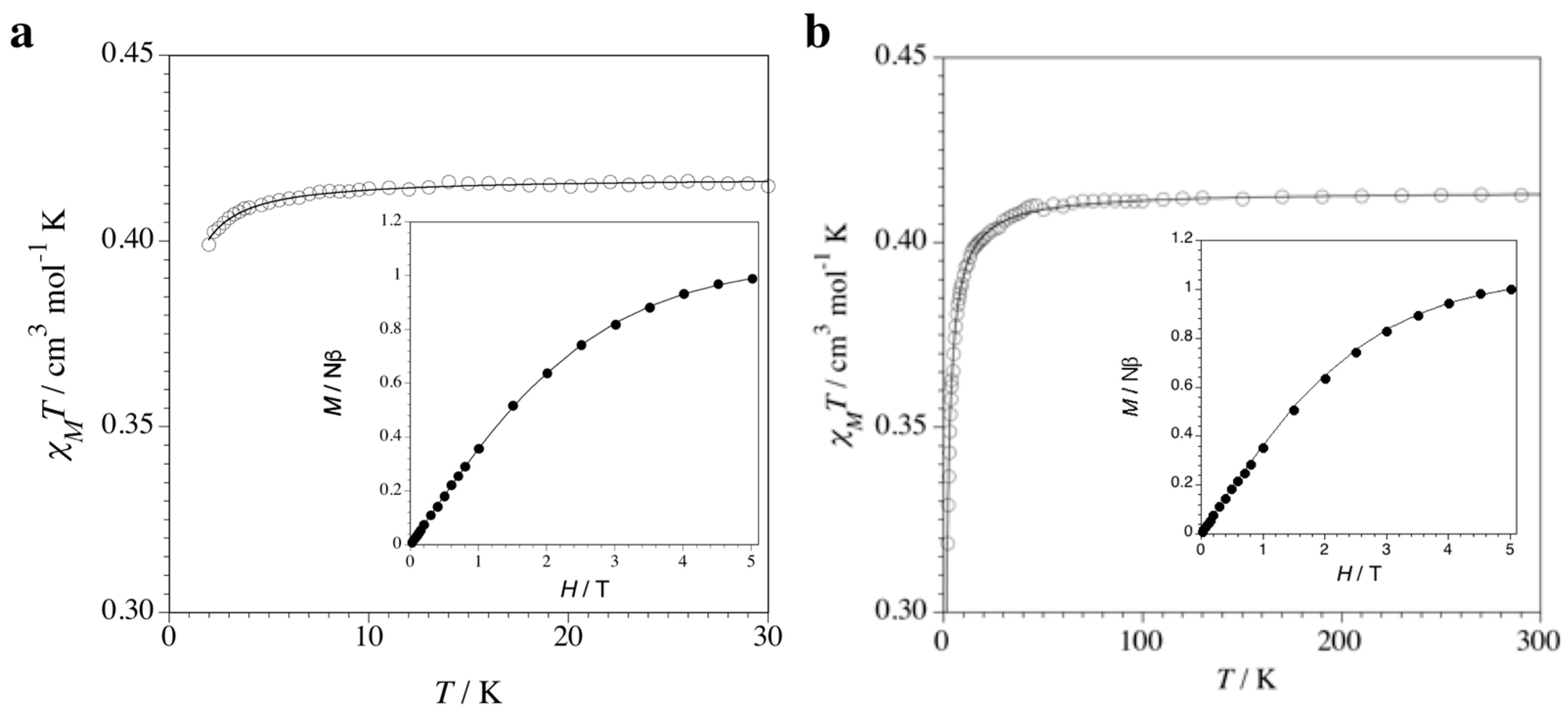
The magnetic properties of 1 and 2 in the form of the χMT vs. T plot (χM being the magnetic susceptibility per formula unit) are shown in Figure 6. At room temperature, the χMT value of 0.41 cm3 mol−1 K is that expected for a magnetically isolated spin doublet from the 3d9 Cu(II) ion (S = 1/2). This value remains constant upon cooling until 5 (1) and 50 K (2); then, it decreases slightly to reach 40 (1) and 0.32 cm3 mol−1 K (2) at 2.0 K. No maximum in the susceptibility curve is observed in any case. The magnetic behavior of 1 is typical of a mononuclear copper(II) complex with almost negligible intermolecular antiferromagnetic interactions, while that of 2 is indicative of the occurrence of a very weak, but non-negligible, antiferromagnetic interaction between the paramagnetic Cu(II) ions, which can be due to both inter- or intrachain interactions. In both cases, however, the closest Cu(II)-Cu(II) distances are very large (10.84 and 8.23 Å for the shortest intra- and inter-chain distances, respectively).
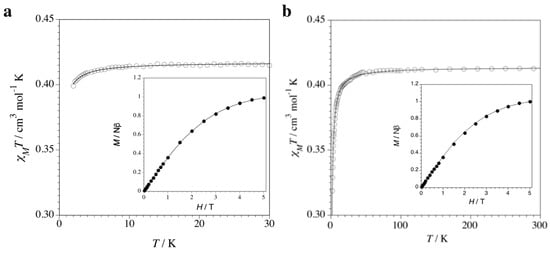
Figure 6.
Temperature dependence of χMT for 1 (a) and 2 (b). The solid lines are the best-fit curves through Equations (1) and (2), respectively (see text). The insets show the field dependence of M at 2.0 K. The solid lines are the simulated curves from the Brillouin function for an S = 1/2 with g = 2.16 (1) and 2.12 (2) (see text).
Hence, the fit of the magnetic susceptibility data for 1 and 2 through the Curie–Weiss law [Equation (1) with S = 1/2] gives θ = –0.08 (1) and –0.61 K (2) with g = 2.11 (1) and 2.12 (2), where θ is the Weiss temperature and g is the average Landé factor of the CuII ion. The absolute θ value for 1 is certainly negligible (|θ| < 0.1 K), while that of 2 is very small but non-negligible. This is in agreement with the M vs. H plots for 1 and 2 (M being the molar magnetization per formula unit and H the applied magnetic field) at 2.0 K (insets of Figure 6a,b). In fact, the isothermal magnetization curve exactly superposes for 1, but slightly deviates for 2, from the Brillouin function for one isolated doublet (S = 1/2) state with g = 2.11 (1) and 2.12 (2) obtained from the fit of the experimental data (solid line in the insets of Figure 6a,b).
χM = [Nβ2g2/3k(T − θ)]S(S + 1)
Because of the one-dimensional character of 2, and given the diamagnetic nature of the 3d10 Cu(I) ions, from a magnetic point of view, we can describe compound 2 as an antiferromagnetic coupled uniform chain of spin doublets through the spin Hamiltonian H = −J ∑i = 1−n Si · Si+1. So, we can alternatively analyze the magnetic susceptibility data through the numerical expression proposed by Bonner and Fisher [Equation (2) with x = |J|/kT] [56].
χMT = (Nβ2g2/k)(0.25 + 0.074975x + 0.075235x2)/(1.0 + 0.9931x + 0.172135x2 + 0.757825x3)
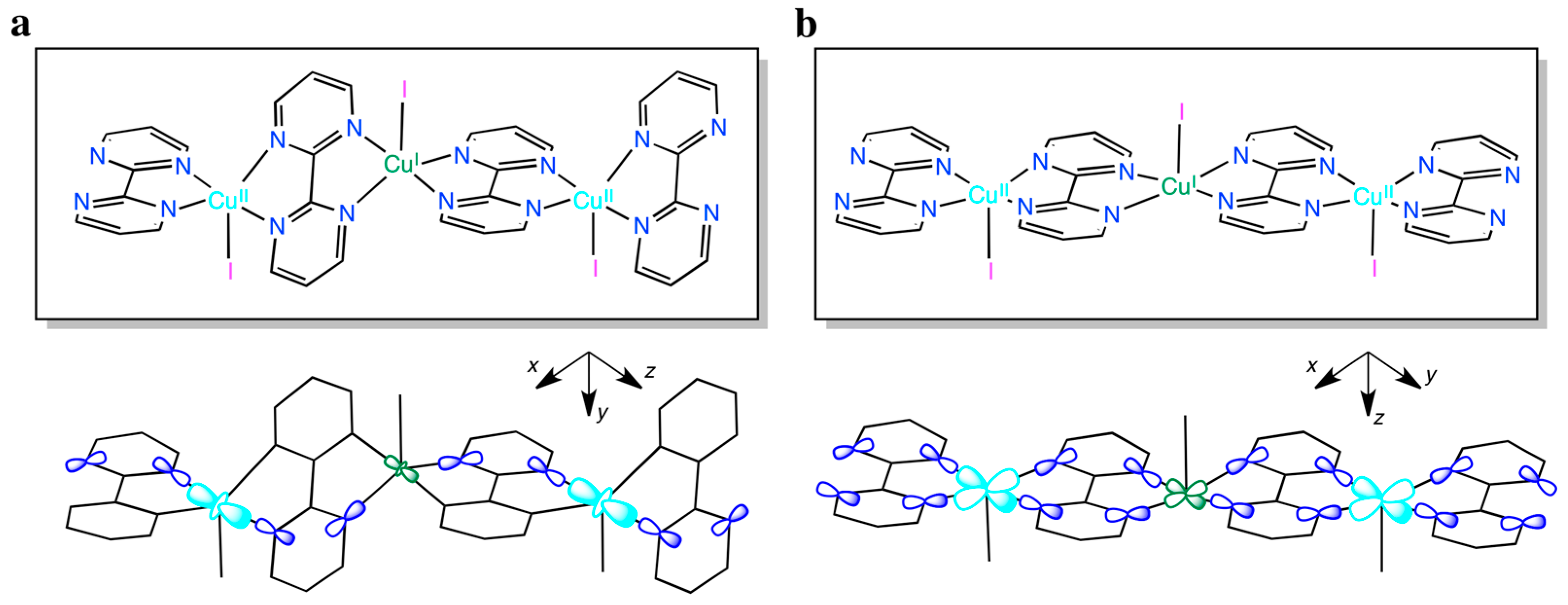
In such expression, |J| is the absolute value of the exchange coupling parameter between adjacent Cu(II) ions into the chain, g is the average Landé factor and N, β and k have their usual meaning. In the case of 2, neglecting the inter-chain interactions, least-squares fit of the magnetic susceptibility to Equation (2) leads to J = −0.650(3) cm−1 and g = 2.120(5). The calculated plot matches well the experimental data in the whole temperature range explored. The absolute J value is very small, as expected for such a long bridge: N-C-N(bpm)-Cu(I)-N-C-N(bpm). In fact, the antiferromagnetic exchange interactions between the unpaired electrons occupying the 3d(z2)-type magnetic orbitals of the paramagnetic trigonal bipyramidal Cu(II) ions, and partially delocalized on the N atoms of one of the two pyrimidine rings of the bpm bridges, are expected to be very weak, although not negligible, because of their small orbital overlap through the long orbital pathway involving the fully occupied 3d(z2)-type orbital of appropriate symmetry of the diamagnetic trigonal bipyramidal Cu(I) ions (Scheme 1a). Interestingly, a different magnetic behavior would result from a square pyramidal coordination environment at the metal atoms along the bipym-bridged mixed-valent copper(I)–copper(II) chain (Scheme 1b). In this hypothetical case, a stronger orbital overlap would occur between the unpaired electrons occupying the 3d(x2–y2)-type magnetic orbitals of the paramagnetic square pyramidal Cu(II) ions, and largely delocalized on the four N atoms of the two pyrimidine rings of the bpm bridges, across the orbital pathway involving the fully occupied 3d(x2–y2)-type orbital of appropriate symmetry of the diamagnetic square bipyramidal Cu(I) ions. This so-called “orbital reversal” phenomenon would instead lead to a moderate antiferromagnetic intrachain coupling.
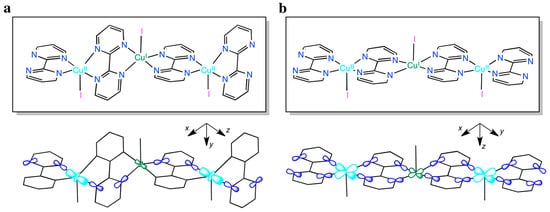
Scheme 1.
Illustration of the intra-chain orbital pathway for the electron exchange magnetic interactions operating in 2 for the actual trigonal bibyramidal (a) or hypothetical square pyramidal (b) coordination environment at the metal atoms along the bipym-bridged mixed-valent copper(I)–copper(II) chain, showing the orientation of the molecular axes centered on each metal atom.
To our knowledge, there are a few examples of polymetallic complexes with a non-negligible, weak to moderate magnetic coupling, either antiferro- or ferromagnetic, between paramagnetic metal centers (e.g., CuII or CrIII) mediated by a diamagnetic metal ion (e.g., ZnII, PdII, or NbV) [57,58,59,60,61,62].
4. Discussion
Cu(I) and Cu(I)-Cu(II) mixed-valent complexes have been generally obtained by using Cu(I) as precursor, while few examples are reported for the in situ total or partial reduction of Cu(II) to Cu(I) [63,64]. For instance, it was shown that pyridine derivatives under hydrothermal conditions can reduce the starting Cu(II) material to afford mixed-valent Cu(I)-Cu(II) species [65,66]. In general, the in situ generation of reagents is an attractive topic of coordination chemistry [63,64], especially if mild synthetic conditions can be used and, above all, when compounds whose preparation would be hampered otherwise become easily accessible instead.
In this work, we have explored the copper/2,2′-bipyrimidine/iodide system, in aqueous media and at room temperature, with the intent of combining the well-established reducing properties of the iodide ion towards copper(II) in water with the protective effect of ligand coordination, to make the metal oxidation state tunable at our wish. Our approach afforded systematically, with 100% reproducibility, compounds 2 and 3, at first together, and later as the only products, thanks to an extended optimization of the crystallization procedure. Our findings can be at least partially rationalized as follows.
When the reduction of Cu(II) to Cu(I) by iodide ions is conducted in dilute aqueous solution conditions and in defect of iodide, the formation of the triiodide anion (from 2Cu2+ + 4I− → 2 CuI(s) + I2 first, and then I2 + I− → I3−) is not competitive and, in the presence of the bpm ligand, the fully reduced compound 3 can be easily accessed, not just as microcrystalline precipitate, but rather as X-ray quality crystals, and in optimum yield (cfr. Ref. [38] for a previously described synthesis of 2 directly from CuI and bpm in acetonitrile or mixed water/acetonitrile/acetone media). When the formation of triiodide anions is not sufficiently hampered synthetically, they do form, consistently, and, in the presence of the bpm ligand, the reduction of the Cu(II) ions does not proceed to completeness. In this case, the mixed-valent species 2, whose crystal structure comprises two triiodide anions every Cu(I/II) metal pair, can be afforded instead. The peculiarities of compound 2 are numerous. First, the five-coordination geometry is not very common for the copper(I) ion; at present, it occurs in about the1.3% of all copper(I) compounds deposited in the Cambridge Structural Database (CCDC 2024; ConQuest version 2024.3.0), and the percentage drastically lowers if we exclude those comprising tetra- or penta-dentate ligands. More importantly, to the best of our knowledge, 2 represents the first example of structurally characterized alternating Cu(I)/Cu(II) chain, where the two metal ions exhibit the same coordination environment and a strikingly similar coordination chromophore [N4I]. Let us now focus on complex 1. Although the metal ion in 1 is in its +2 oxidation state, the presence of triiodide as co-counteranion requires that a partial Cu(II) → Cu(I) reduction process must happen in the reaction vessel. Thus, while 2 could be considered, in some way, a solid-state reaction intermediate, 1 cannot be considered a ‘precursor’ of compounds 2 and 3. We were never able to form 1 in pure water, even after extensive optimization of the synthetic parameters. Rather, the compound was obtained from acetone, only after the formation of 2 and 3 as insoluble powders. This suggests that, when the reaction media is not ideal for the slow self-assembly of the mixed valent, Cu(I)/Cu(II) chain of 2, complex 1 becomes accessible, thanks to the simultaneous presence in solution of an excess of the bpm ligand as well as of residual iodide and triiodide anions. Interestingly, several attempts to intentionally form a tris(bpm)copper(II) dication with all sort of different counteranions, made over years also by us, were never successful. Thus, while the [bis(bpm)copper(II)H2O]2+ species is somewhat known [14,15], and the analog tris(2,2′-bipyridine)copper(II) species are well documented [67,68,69], to the best of our knowledge the complex cation in 1 represents the first tris(bpm)copper(II) species reported to date.
5. Conclusions
In this work, we have demonstrated the possibility to obtain three different products, i.e., the complexes of formulae [CuII(bpm)3](I3)(I) (1), {[CuI(I3)CuII(I)(bpm)2](I3)}n (2) and {[CuI2(μ-I)2(bpm)]}n (3), by using copper(II)nitrate hexahydrate, 2,2′-bipyrimidine (bpm) and potassium iodide as starting reagents, in mild conditions, in reagent grade acetone or aqueous media. To our knowledge, Cu(I)- or mixed Cu(I)/Cu(II)-halides frameworks, prepared by the in situ reduction of Cu(II) precursors with the bpm ligand and subsequent self-assembly, have not been reported to date. Compound 2 was of the most interesting nature. X-ray single crystal analysis revealed for it the occurrence of two crystallographically distinct copper centers, regularly alternating within a μ-bpm bridged chain structure, charge balanced by three monovalent anions (two of which coordinated). Despite both metal centers having quasi-identical pentacoordination surroundings, it was possible to assign undoubtedly formal +1 oxidation state to one copper ion and +2 to the other, based on the Cu-N and Cu-I bond distances. The magnetic analysis of 2 revealed a very weak antiferromagnetic coupling between the Cu(II) ions, which can be due to both inter- or intrachain interactions [the intrachain Cu(II)-Cu(II) separation, through the diamagnetic Cu(I) center, is 10.8435(3) Å; the shortest interchain Cu(II)-Cu(II) distance is 8.232(1) Å, and it is associated with moderate off-set π-π stacking interactions between bpm ligands]. In either case, the magnetic measurements were in agreement with the hypothesis, based on the structural observations, that in 2 the Cu(I) and Cu(II) metal sites alternate regularly; i.e., the Cu(II) ions are well isolated from each other’s. Compound 2 can be regarded as a solid-state reaction intermediate. The numerous I···I contacts established between the coordinated and uncoordinated iodide and triiodide anions reasonably account for its great stability and striking synthetic reproducibility.
Author Contributions
N.M. and G.D.M. conceived the project and designed the experiments; N.M. synthesized and characterized the complexes, and carried out the crystallographic study; F.L. and M.J. performed the magnetic study; N.M., G.D.M., F.L. and M.J. discussed the results and agreed on their interpretation, exactly as discussed herein; N.M., G.D.M., F.L. and M.J. wrote the manuscript (original draft); N.M. and G.D.M. revised and edited the manuscript. All authors have read and agreed to the published version of the manuscript, except for M.J., who sadly is no longer with us.
Funding
This work was supported by the Italian MUR through the ElectroLight4Value project (PRIN 2020, 2020927WY3).
Data Availability Statement
The data used in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
Special thanks are due to Rafael Ruiz-García (ICMol, Valencia) for his unselfish support with the magnetic measurements on compound 1 and with the revision of Section 3.2.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Ferrando-Soria, J.; Vallejo, J.; Castellano, M.; Martínez-Lillo, J.; Pardo, E.; Cano, J.; Castro, I.; Lloret, F.; Ruiz-García, R.; Julve, M. Molecular magnetism, quo vadis? A historical perspective from a coordination chemist viewpoint. Coord. Chem. Rev. 2017, 339, 17–103. [Google Scholar] [CrossRef]
- Marinescu, G.; Andruh, M.; Lloret, F.; Julve, M. Bis(oxalato)chromium(III) complexes: Versatile tectons in designing heterometallic coordination compounds. Coord. Chem. Rev. 2011, 255, 161–185. [Google Scholar] [CrossRef]
- Martínez-Lillo, J.; Faus, J.; Lloret, F.; Julve, M. Towards multifunctional magnetic systems through molecular-programmed self-assembly of Re(IV) metalloligands. Coord. Chem. Rev. 2015, 289–290, 215–237. [Google Scholar] [CrossRef]
- Castro, I.; Barros, W.P.; Calatayud, M.L.; Lloret, F.; Marino, N.; De Munno, G.; Stumpf, H.O.; Ruiz-García, R.; Julve, M. Dicopper(II) pyrazolenophanes: Ligand effects on their structures and magnetic properties. Coord. Chem. Rev. 2016, 315, 135–152. [Google Scholar] [CrossRef]
- Castro, I.; Calatayud, M.L.; Orts-Arroyo, M.; Marino, N.; De Munno, G.; Lloret, F.; Ruiz-García, R.; Julve, M. Oxalato as polyatomic coordination center and magnetic coupler in copper(II)-polypyrazole inverse polynuclear complexes and coordination polymers. Coord. Chem. Rev. 2022, 471, 214730–214737. [Google Scholar] [CrossRef]
- De Munno, G.; Julve, M.; Nicolò, F.; Lloret, F.; Faus, J.; Ruiz, R.; Sinn, E. 2,2′-Bipyrimidineoxalatocopper(II) Complexes: From the Mononuclear Complex to the 2D Sheetlike Polymer. Angew. Chem. Int. Ed. Engl. 1993, 32, 613–615. [Google Scholar] [CrossRef]
- Julve, M.; Lloret, F.; Faus, J.; De Munno, G.; Verdaguer, M.; Caneschi, A. Alternating Ferro- and Antiferromagnetic Interactions in a Chainlike CuII Coordination Polymer. Angew. Chem. Int. Ed. Engl. 1993, 32, 1046–1048. [Google Scholar] [CrossRef]
- Real, J.; De Munno, G.; Chiappetta, R.; Julve, M.; Lloret, F.; Journaux, Y.; Colin, J.-C.; Blondin, G. Structural and Magnetic Characterization of a Novel Heptanuclear Hydroxo-Bridged Copper(II) Cluster of the Dicubane Type. Angew. Chem. Int. Ed. Engl. 1994, 33, 1184–1186. [Google Scholar] [CrossRef]
- De Munno, G.; Julve, M.; Viau, G.; Lloret, F.; Faus, J.; Viterbo, D. Azido and 2,2′-Bipirimidine Ligands as Useful Tools in Designing Two- and Three-Dimensional Manganese(II) Networks. Angew. Chem. Int. Ed. Engl. 1996, 35, 1807–1810. [Google Scholar] [CrossRef]
- De Munno, G.; Poerio, T.; Viau, G.; Julve, M.; Lloret, F. Ferromagnetic Coupling in the Bis(μ-end-on-azido)iron(III) Dinuclear Complex Anion of [FeII(bpym)3]2[FeIII2(N3)10]⋅2H2O. Angew. Chem. Int. Ed. Engl. 1997, 36, 1459–1461. [Google Scholar] [CrossRef]
- Lloret, F.; De Munno, G.; Julve, M.; Cano, J.; Ruiz, R.; Caneschi, A. Spin Polarization and Ferromagnetism in 2D Sheetlike Cobalt(II) Polymers: [Co(L)2(SCN)2] (L = Pyrimidine or Pyrazine). Angew. Chem. Int. Ed. Engl. 1998, 37, 135–138. [Google Scholar] [CrossRef]
- De Munno, G.; Bruno, G. Catena-poly{μ-(2,2′-bypirimidine-N,N′,N″,N’‘‘)-[(nitrato-O,O’)copper(II)]-di-(μ-nitrato-μ-O)-[(nitrato-O,O’)copper(II)]}, [Cu2(NO3)4(C8H6N4)]. Acta Crystallogr. 1984, 40, 2030–2032. [Google Scholar] [CrossRef]
- Julve, M.; De Munno, G.; Bruno, G.; Verdaguer, M. Synthesis, Structure and Magnetic Properties of μ-(2,2′-Bipyrimidine-N,N′,N″,N’‘‘) Copper(II) Complexes. Inorg. Chem. 1988, 27, 3160–3165. [Google Scholar] [CrossRef]
- De Munno, G.; Bruno, G.; Julve, M.; Romeo, M. Synthesis and Structure of Aquabis(2,2′-bipyrimidine-N,N′)copper(II) hexafluorophosphate Dihydrate. Acta Crystallogr. 1990, 46, 1828–1830. [Google Scholar] [CrossRef]
- De Munno, G.; Julve, M.; Verdaguer, M.; Bruno, G. Bonding Flexibility of 2,2′-Bipyrimidine (bpm): Symmetry and Magnetism of Three Copper(II) Complexes with Different Cu:bpm Ratios. Inorg. Chem. 1993, 32, 2215–2220. [Google Scholar] [CrossRef]
- De Munno, G.; Julve, M.; Lloret, F.; Cano, J.; Caneschi, A. Magneto-Structural Effects of the Jahn-Teller Distortions on 2,2′-Bipyrimidine (bpm)-Bridged Dinuclear Copper(II) Complexes: Crystal Structures and Magnetic Properties of [Cu2(bpm)(H2O)4(SO4)2]⋅3H2O and [Cu2(bpm)(H2O)8](SO4)2⋅2H2O. Inorg. Chem. 1995, 34, 2048–2053. [Google Scholar] [CrossRef]
- De Munno, G.; Poerio, T.; Viaux, G.; Julve, M.; Lloret, F.; Journaux, J.; Rivière, E. New magnetic behaviour of honeycomb layered compounds. Crystal structure of [M2(bipym)(N3)] (M = CoII, FeII; bipym = 2,2′-bipyrimidine). Chem. Commun. 1996, 22, 2587–2588. [Google Scholar] [CrossRef]
- De Munno, G.; Julve, M.; Real, J.A. Synthesis and crystal structure of the low-spin iron(II) complex [Fe(bpym)3](ClO4)2⋅1/4H2O. Inorganica Chim. Acta 1997, 255, 185–188. [Google Scholar] [CrossRef]
- De Munno, G.; Poerio, T.; Julve, M.; Lloret, F.; Faus, J.; Caneschi, A. Syntheses, crystal structures and magnetic properties of one-, two- and three-dimensional 2,2′-bipyrimidine-containig copper(II) complexes. J. Chem. Soc. Dalton Trans. 1998, 10, 1679–1685. [Google Scholar] [CrossRef]
- De Munno, G.; Ventura, W.; Viau, G.; Lloret, F.; Faus, J.; Julve, M. Structural Characterization and Magnetic Properties of the First 2,2′-Bipyrimidine (bpm)-containing Iron(III) Complexes. Inorg. Chem. 1998, 37, 1458–1464. [Google Scholar] [CrossRef]
- De Munno, G.; Armentano, D.; Julve, M.; Lloret, F.; Lescouëzec, R.; Faus, J. Two-dimensional Assembling of (2,2′-Bipirimidine)bis(oxalato)chromate(III) Units through Alkaline Cations. Inorg. Chem. 1999, 38, 2234–2237. [Google Scholar] [CrossRef] [PubMed]
- Armentano, D.; De Munno, G.; Lloret, F.; Julve, M. Inorg. Chem. Novel Three-Dimensional Cage Assembly of a µ4-Carbonato Bridged Cobalt(II) Compound [Co2(bpm)(H2O)2(CO3)(OH)]NO3⋅4H2O. Inorg. Chem. 1999, 38, 3744–3747. [Google Scholar] [CrossRef] [PubMed]
- Chiozzone, R.; Gonzáles, R.; Kremer, C.; Cerdá, M.F.; Armentano, D.; De Munno, G.; Martínez-Lillo, J.; Faus, J. A novel series of rhenium-bipyrimidine complexes: Synthesis, crystal structure and electrochemical properties. Dalton Trans. 2007, 6, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lillo, J.; Armentano, D.; De Munno, G.; Cano, J.; Lloret, F.; Julve, M.; Faus, J. First Magnetostructural Study on a Heterodinuclear 2,2′-Bipyrimidine-Bridged Complex. Inorg. Chem. 2011, 50, 12405–12407. [Google Scholar] [CrossRef]
- Marino, N.; Armentano, D.; De Munno, G.; Cano, J.; Lloret, F.; Julve, M. Synthesis, Structure, and Magnetic Properties of Regular Alternating μ-bpm/di-μ-X Copper (II) Chains (bpm= 2, 2′-bipyrimidine; X= OH, F). Inorg. Chem. 2012, 51, 4323–4334. [Google Scholar] [CrossRef]
- Marino, N.; Armentano, D.; De Munno, G.; Lloret, F.; Cano, J.; Julve, M. Toward a better understanding of honeycomb alternating magnetic networks. Dalton Trans. 2015, 44, 11040–11051. [Google Scholar] [CrossRef]
- Martínez-Lillo, J.; Armentano, D.; Fortea-Pérez, F.R.; Stiriba, S.-E.; De Munno, G.; Lloret, F.; Julve, M.; Faus, J. A Chiral, Photoluminescent and Spin-Canted {CuIReIV2}n Branched Chain. Inorg. Chem. 2015, 54, 4594–4596. [Google Scholar] [CrossRef]
- Marino, N.; Mastropietro, T.F.; Armentano, D.; De Munno, G.; Doyle, R.P.; Lloret, F.; Julve, M. Spin canting in an unprecedented three-dimensional pyrophosphate- and 2,2′-bipyrimidine-bridged cobalt(II) framework. Dalton Trans. 2008, 54, 5152–5154. [Google Scholar] [CrossRef]
- Martínez-Lillo, J.; Mastropietro, T.F.; Lappano, R.; Madeo, A.; Alberto, M.E.; Russo, N.; Maggiolini, M.; De Munno, G. Rhenium(IV) compounds inducing apoptosis in cancer cells. Chem. Commun. 2011, 47, 5283–5285. [Google Scholar] [CrossRef]
- Betanzos-Lara, S.; Novakova, O.; Deeth, R.J.; Pizarro, A.M.; Clarkson, G.J.; Liskova, B.; Bravec, V.; PJSadler, P.J.; Habtemariam, A. Bipyrimidine ruthenium(II) arene complexes: Structure, reactivity and cytotoxicity. J. Biol. Inorg. Chem. 2012, 17, 1033–1051. [Google Scholar] [CrossRef]
- Kojima, T.; Morimoto, T.; Miyazaki, S.; Fukuzumi, S. Ruthenium(II) Pyridylamine Complexes with Diimine Ligands Showing Reversible Photochemical and Thermal Structural Change. Chem Eur. J. 2008, 14, 8904–8915. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, T.; Itogawa, M.; Shimomura, H.; Shiota, Y.; Kotani, H.; Yoshizawa, K.; Kojima, T. Redox properties of a bipyrimidine-bridged dinuclear ruthenium(II) complex. Inorg. Chem. Commun. 2020, 120, 108150. [Google Scholar] [CrossRef]
- Henke, W.C.; Kerr, T.A.; Sheridan, T.R.; Henling, L.M.; Takase, M.K.; Day, V.W.; Gray, H.B.; Blakemore, J.D. Synthesis, structural studies, and redox chemistry of bimetallic [Mn(CO)3] and [Re(CO)3] complexes. Dalton Trans. 2021, 50, 2746–2756. [Google Scholar] [CrossRef] [PubMed]
- Kaim, W.; Dogan, A.; Wanner, M.; Klein, A.; Tiritiris, I.; Schleid, T.; Stufkens, D.J.; Snoeck, T.L.; McInnes, E.J.L.; Fiedler, J.; et al. Reduced and Excited States of (bpym)[PtCl2]n (bpym = 2,2′-Bipyrimidine; n = 1, 2): Experiments and DFT Calculations. Inorg. Chem. 2002, 41, 4139–4148. [Google Scholar] [CrossRef]
- Real, J.A.; Gaspar, A.B.; Muñoz, M.C.; Gütlich, P.; Ksenofontov, V.; Spiering, H. Bipyrimidine-bridged dinuclear iron (II) spin crossover compounds. Top. Curr. Chem. 2004, 233, 167–193. [Google Scholar] [CrossRef]
- Vogler, C.; Hausen, H.-D.; Kaim, W.; Kohlmann, S.; Kramer, H.E.A.; Rieker, J. Copper(I)-Assisted Formation of an “Organic” Sandwich Structure: Structural Prerequisites for Luminescence of the Dinuclear Complexes [(μ-Bipyrimidine){Cu(PR3)2}2]X2. Angew. Chem. Int. Ed. 1989, 28, 1659–1660. [Google Scholar] [CrossRef]
- Sieger, M.; Vogler, C.; Klein, A.; Knödler, A.; Wanner, M.; Fiedler, J.; Záliš, S.; Snoeck, T.I.; Kaim, W. Geometrical and Electronic Structures of Dinuclear Complex Ions {(μ-bpym)[Cu(EAr3)2]2}2+ with Intramolecular “Organic Sandwich” Formation (E = P or As; Ar = Aryl; bpym = 2,2‘-Bipyrimidine). Inorg. Chem. 2005, 44, 4637–4643. [Google Scholar] [CrossRef]
- Hou, F.; Powell, M.; Dougherty, D.B.; Sommer, R.D.; Maggard, P.A. Tunable Optical and Photocatalytic Properties of Low-Dimensional Copper(I)-Iodide Hybrids Using Coordinating Organic Ligands. Cryst. Growth Des. 2018, 18, 5406–5416. [Google Scholar] [CrossRef]
- Decurtins, S.; Schmalle, H.W.; Schneuwly, P.; Zheng, L.-M.; Ensling, J.; Hauser, A. Synthesis, Crystal Structure, Optical and Magnetic Properties of a Novel Two-Dimensional Copper(II) Network formed conjointly with μ-Bipyrimidine, μ-Oxalato, and μ-Chloro Ligands. Inorg. Chem. 1995, 34, 5501–5506. [Google Scholar] [CrossRef]
- Ikotun, O.F.; Ouellette, W.; Lloret, F.; Julve, M.; Doyle, R.P. Synthesis, X-ray Structure, Thermal and Magnetic Behavior of [(bipy)2Ni2(μ-Cl)2Cl2(H2O)2]: The First Neutral Ferromagnetically Coupled Six-Coordinate Dichlorido-Bridged Nickel(II) Dimer. Eur. J. Inorg. Chem. 2007, 2007, 2083–2088. [Google Scholar] [CrossRef]
- Julve, M.; Verdaguer, M.; De Munno, G.; Real, J.A.; Bruno, G. Synthesis, crystal structure, and magnetic properties of (μ-bipyrimidine)(cyanato)copper(II) and -(thiocyanato)copper(II) complexes. Inorg. Chem. 1993, 32, 795–802. [Google Scholar] [CrossRef]
- Cortés, R.; Lezama, L.; Rojo, T.; Arriortua, M.I.; Pizarro, J.L.; Solans, X. Alternating Ferro- and Antiferromagnetic Interactions in a MnII Chain with Alternating End-On and End-to-End Bridging Azido Ligands. Angew. Chem. Int. Ed. Engl. 1994, 33, 2488–2489. [Google Scholar] [CrossRef]
- Cortés, R.; Drillon, M.; Solans, X.; Lezama, L.; Rojo, T. Alternating Ferromagnetic−Antiferromagnetic Interactions in a Manganese(II)−Azido One-Dimensional Compound: [Mn(bipy)(N3)2]. Inorg. Chem. 1997, 36, 677–683. [Google Scholar] [CrossRef]
- Viau, G.; Lombardi, M.G.; De Munno, D.; Julve, M.; Lloret, F.; Faus, J.; Caneschi, A.; Clemente-Juan, J.M. The azido ligand: A useful tool in designing chain compounds exhibiting alternating ferro- and antiferro-magnetic interactions. Chem. Commun. 1997, 13, 1195–1196. [Google Scholar] [CrossRef]
- Armentano, D.; Sanchis-Perucho, A.; Rojas-Dottib, C.; Martínez-Lillo, J. Halogen halogen interactions in the self-assembly of one-dimensional 2,2′-bipyrimidine-based CuIIReIV systems. CrystEngComm 2018, 20, 4575–4581. [Google Scholar] [CrossRef]
- SAINT, version 6.45; Bruker Analytical X-Ray Systems Inc.: Madison, WI, USA, 2003.
- SADABS, version 2.03; Bruker AXS Inc.: Madison, WI, USA, 2000.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Diamond (version 4.6.8)—Crystal and Molecular Structure Visualization, Crystal Impact—Dr. H. Putz & Dr. K. Brandenburg GbR, Kreuzherrenstr. 102, 53227 Bonn, Germany. Available online: https://www.crystalimpact.de/diamond (accessed on 30 January 2025).
- SHAPE, version 2.1; Electronic Structure Group, Universitat de Barcelona: Barcelona, Spain, 2013.
- Blake, A.J.; Li, W.-S.; Lippolis, V.; Schröder, M.; Devillanova, F.A.; Gould, R.O.; Parsons, S.; Radek, C. Template self-assembly of polyiodide networks. Chem. Soc. Rev. 1998, 27, 195–206. [Google Scholar] [CrossRef]
- Svensson, P.H.; Kloo, L. Synthesis, Structure, and Bonding in Polyiodide and Metal Iodide-Iodine Systems. Chem. Rev. 2003, 103, 1649–1684. [Google Scholar] [CrossRef]
- van Megen, M.; Reiss, G.J. I62− Anion Composed of Two Asymmetric Triiodide Moieties: A Competition between Halogen and Hydrogen Bond. Inorganics 2013, 1, 3–13. [Google Scholar] [CrossRef]
- Kulkarni, P.; Padhye, S.; Sinn, E.; Anson, C.E.; Powell, A.K. Comparative studies on copper(I) complexes: Synthesis, X-ray crystallography and electrochemical properties of [CuI (dafone)nX] complexes (dafone=/4,5-diaza-fluoren-9-one, X=/Br, I, SCN). Inorg. Chim. Acta 2002, 332, 167–175. [Google Scholar] [CrossRef]
- Horn, C.; Ali, B.; Dance, I.; Scudder, M.; Craig, D. Crystal supramolecularity: Extended aryl embraces in dimorphs of [Cu(1,10-phen)2I]I3. CrystEngComm 2000, 2, 6–15. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH Publishers: New York, NY, USA, 1993. [Google Scholar]
- Ruiz, R.; Julve, M.; Faus, J.; Lloret, F.; Muñoz, M.C.; Journaux, Y.; Bois, C. Ferromagnetic Coupling between Copper(II) Centers through the Diamagnetic Zinc(II) Ion: Crystal Structure and Magnetic Properties of [Cu2Zn(Hdmg)2(dmg)2(H2O)]·0.5H2dmg·H2O (H2dmg = Dimethylglyoxime). Inorg. Chem. 1997, 36, 3434–3439. [Google Scholar] [CrossRef] [PubMed]
- Buvaylo, E.A.; Kokozay, V.N.; Vassilyeva, O.Y.; Skelton, B.W.; Jezierska, J.; Brunel, L.C.; Ozarowski, A. Ozarowski. A Cu–Zn–Cu–Zn heterometallomacrocycle shows significant antiferromagnetic coupling between paramagnetic centres mediated by diamagnetic metal. Chem. Commun. 2005, 39, 4976–4978. [Google Scholar] [CrossRef] [PubMed]
- Semenaka, V.V.; Nesterova, O.V.; Kokozay, V.N.; Dyakonenko, V.V.; Zubatyuk, R.I.; Shishkin, O.V.; Boča, R.; Jezierska, J.; Ozarowski, A. Ozarowski. CrIII−CrIII Interactions in Two Alkoxo-Bridged Heterometallic Zn2Cr2 Complexes Self-Assembled from Zinc Oxide, Reinecke’s Salt, and Diethanolamine. Inorg. Chem. 2010, 49, 5460–5471. [Google Scholar] [CrossRef]
- Oliveira, W.X.; Ribeiro, M.A.; Pinheiro, C.B.; da Costa, M.M.; Fontes, A.P.S.; Nunes, W.C.; Cangussu, D.; Julve, M.; Stumpf, H.O.; Pereira, C.L. Palladium(II)–Copper(II) Assembling with Bis(2-pyridylcarbonyl)amidate and Bis(oxamate) Type Ligands. Cryst. Growth Des. 2015, 15, 1325–1335. [Google Scholar] [CrossRef]
- Oliveira, W.X.C.; Ribeiro, M.A.; Pinheiro, C.B.; da Costa, M.M.; Fontes, A.P.S.; Nunes, W.C.; Cangussu, D.; Julve, M.; Stumpf, H.O.; Pereira, C.L.M. Experimental and Theoretical Investigation of the Anti-Ferromagnetic Coupling of CrIII Ions through Diamagnetic −O–NbV–O– Bridges. Inorg. Chem. 2017, 56, 6879–6889. [Google Scholar] [CrossRef]
- Jurić, M.; Dubraja, L.A.; Popović, J.; Molčanov, K.; Torić, F.; Pajić, D.; Lončarić, I. From a square core to square opening: Structural diversity and magnetic properties of the oxo-bridged [CrIIINbV] complexes. Dalton Trans. 2018, 47, 4183–4190. [Google Scholar] [CrossRef]
- Lu, J.Y. Crystal engineering of Cu-containing metal–organic coordination polymers under hydrothermal conditions. Coord. Chem. Rev. 2003, 246, 327–347. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Zheng, S.-L.; Huang, X.-C.; Chen, X.-M. Two Unprecedented 3-Connected Three-Dimensional Networks of Copper(I) Triazolates: In Situ Formation of Ligands by Cycloaddition of Nitriles and Ammonia. Angew. Chem. Int. Ed. 2004, 43, 206–209. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, Y.; Tong, M.-L.; Chen, X.-M.; Yuen, T.; Lin, C.L.; Huang, X.; Li, J. A mixed-valence copper coordination polymer generated by hydrothermal metal/ligand redox reactions. Chem. Commun. 2002, 13, 1342. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Tong, M.-L.; Chen, X.-M. Hydroxylation of N-Heterocycle Ligands Observed in Two Unusual Mixed-Valence CuI/CuII Complexes. Angew. Chem. Int. Ed. 2002, 41, 1029–1031. [Google Scholar] [CrossRef]
- Chamayou, J.A.A.-C.; Biswas, C.; Janiak, C.; Ghosh, A. Tris(2,2′-bipyridine-κ2N,N′)copper(II) bis(tetrafluoridoborate). Acta Cryst. 2007, 63, m1936–m1937. [Google Scholar] [CrossRef]
- Xu, F.; You, W.; Huang, W. Tris(2,20-bipyridyl-j2 N,N0)copper(II) sulfate 7.5-hydrate. Acta Cryst. 2009, 5, m129–m130. [Google Scholar] [CrossRef]
- Murphy, B.; Aljabri, M.; Ahmed, A.M.; Murphy, G.; Hathaway, B.J.; Light, M.E.; Geilbrich, T.; Hursthouse, M.B. Structural systematics of the [Cu(chelate)3][Y]2 series. An interesting crystallographic structural insight involving vibronic coupling and the Jahn–Teller effect (JTE). The syntheses and low temperature crystal structures of tris(2,2′bipyridyl)copper(ii) tetraphenylborate and tris(2,2′bipyridyl)zinc(ii) tetraphenylborate. Dalton Trans. 2006, 2, 357–367. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).