Chemical and Structural Versatility in the Copper/2,2′-Bipyrimidine/Iodide System: A Regular Alternating Mixed-Valent Cu(II)-Cu(I) Chain Showing Unusually Similar Metal Coordination Environments †
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Complexes
2.2. X-Ray Data Collection and Structure Refinement
3. Results and Discussion
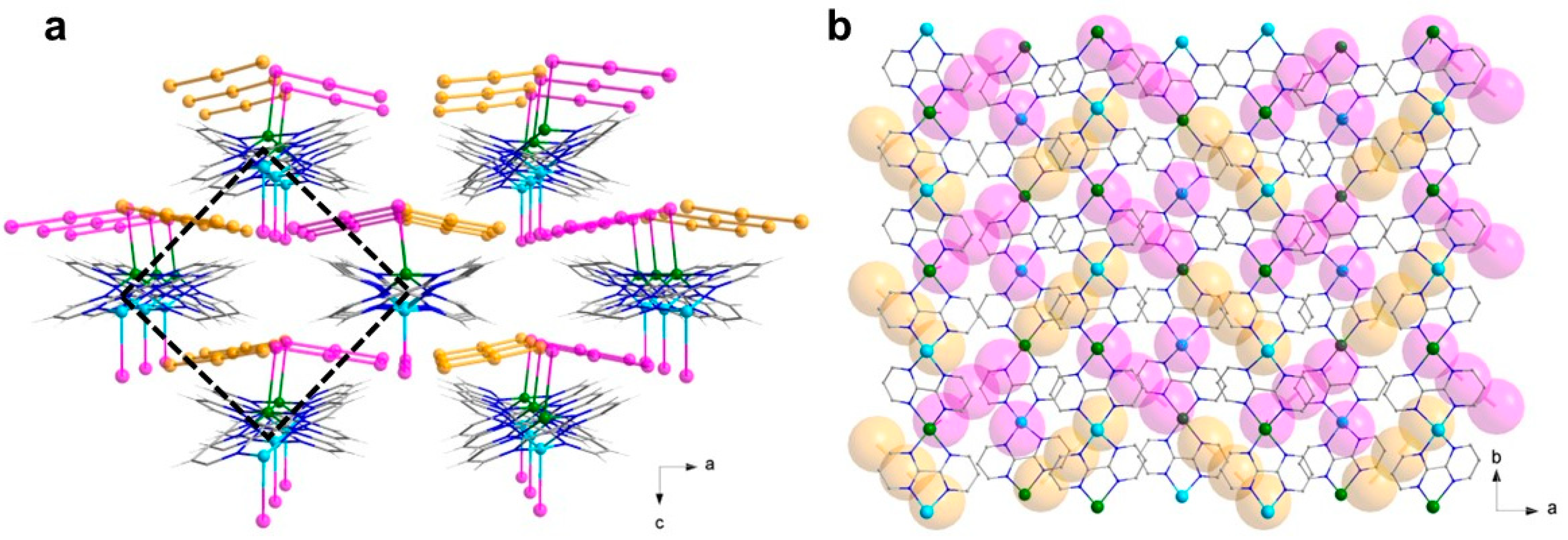
3.1. Description of the Structures
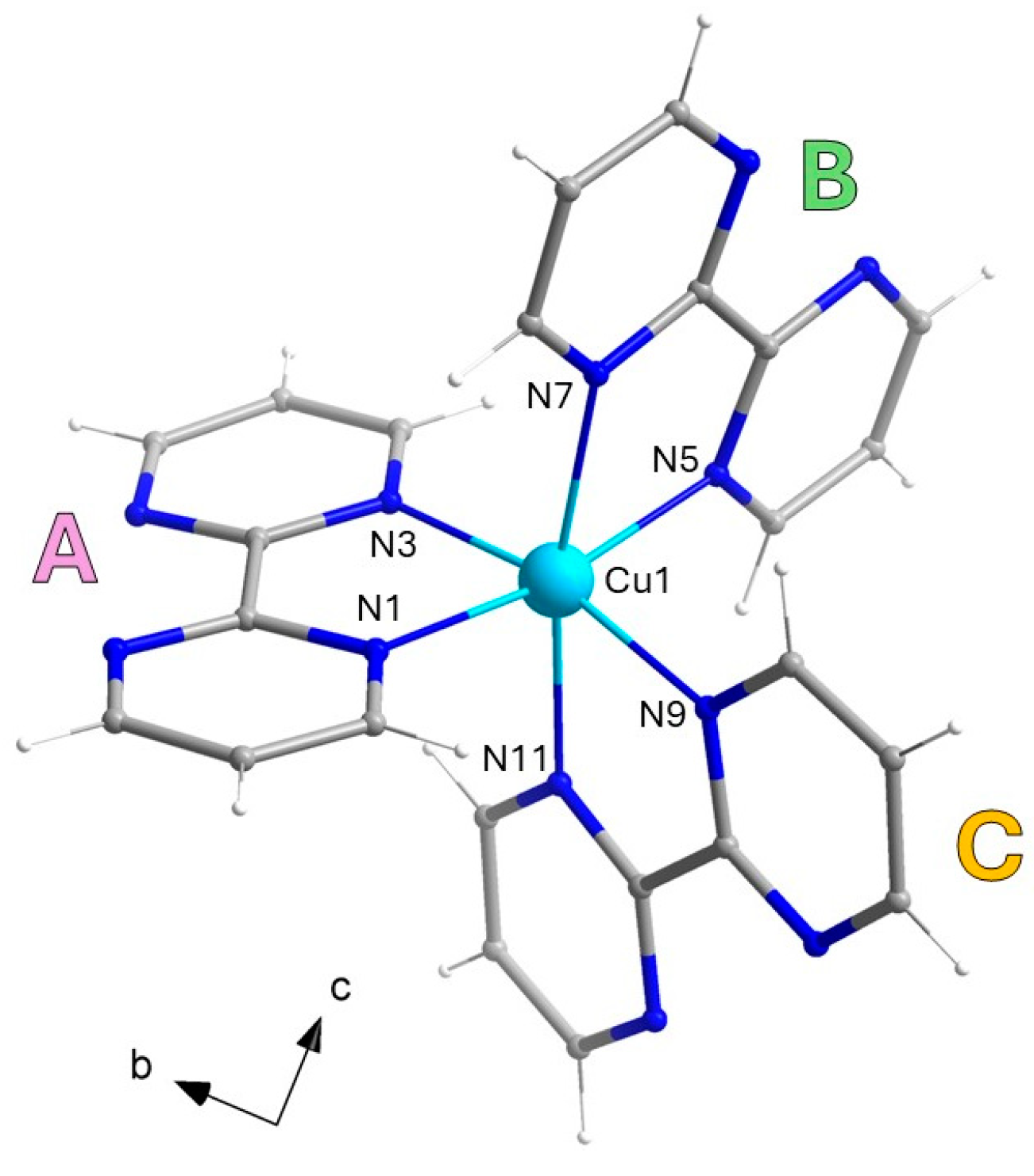
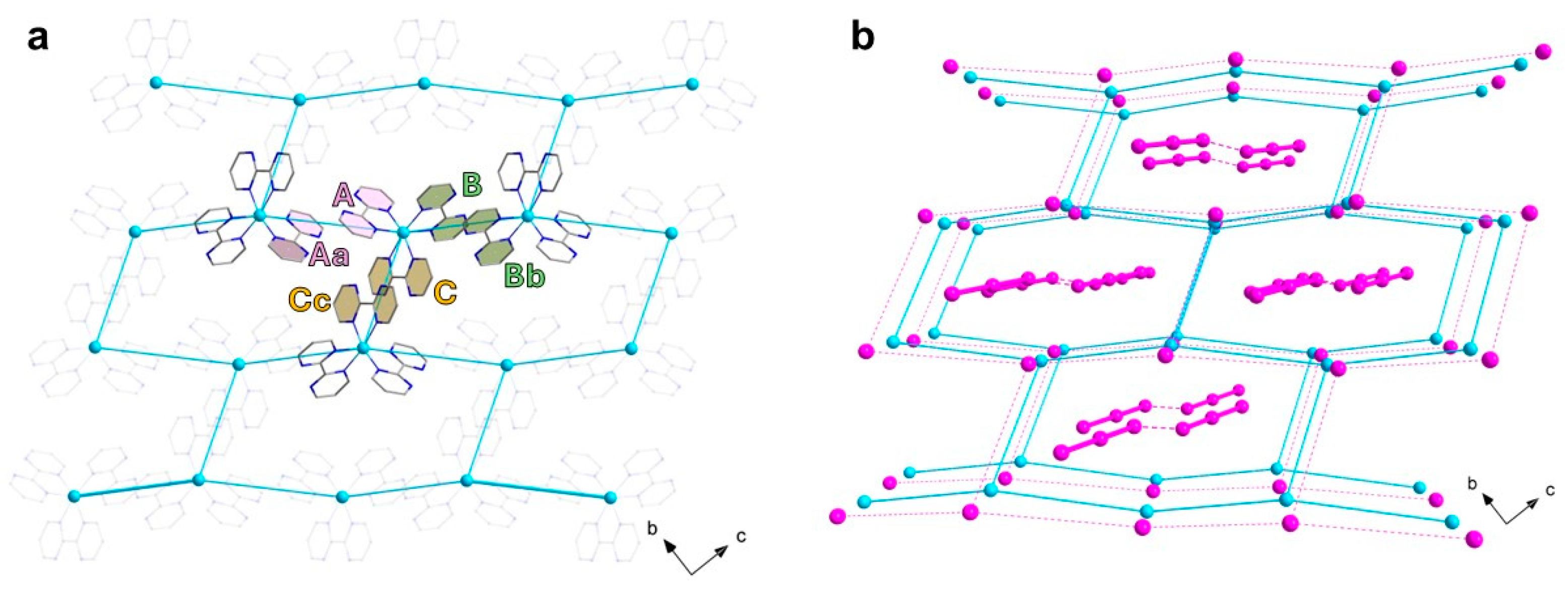
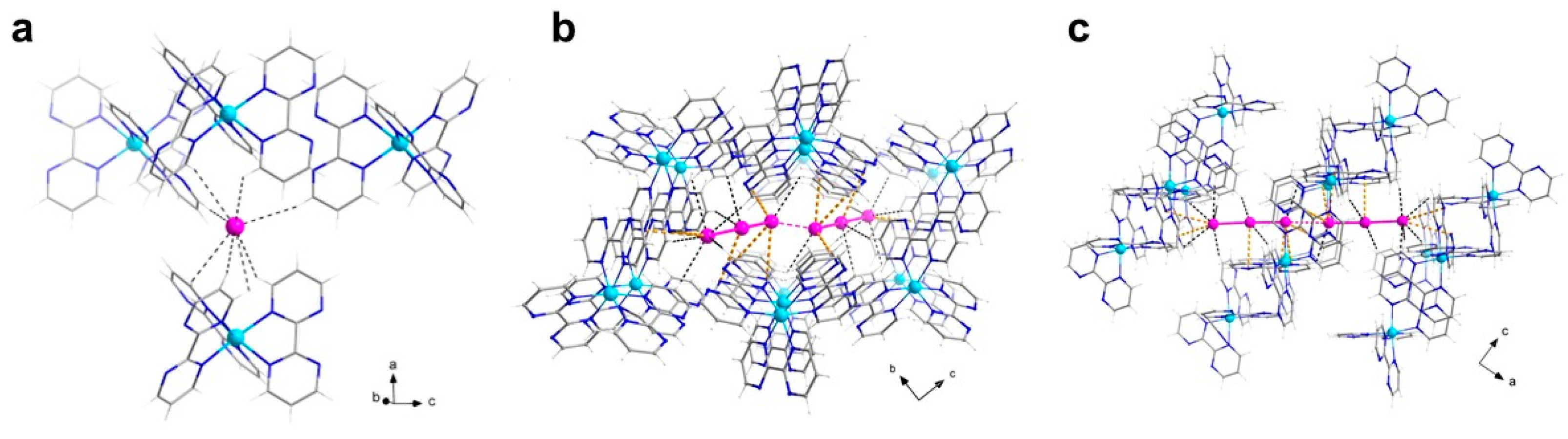
3.1.1. [CuII(bpm)3](I3)(I) (1)
| Cu1-N1 Cu1-N5 Cu1-N3 I1-I2 | 2.096(4) 2.236(4) 2.044(3) 2.9835(5) | Cu1-N7 Cu1-N9 Cu1-N11 I2-I3 | 2.108(4) 2.060(3) 2.244(3) 2.8679(5) |
| N3-Cu1-N9 N3-Cu1-N1 N9-Cu1-N1 N3-Cu1-N7 N9-Cu1-N7 N1-Cu1-N7 N3-Cu1-N5 N9-Cu1-N5 | 166.29(13) 78.84(13) 95.67(12) 95.05(13) 92.02(12) 169.86(12) 95.93(12) 97.16(12) | N1-Cu1-N5 N7-Cu1-N5 N3-Cu1-N11 N9-Cu1-N11 N1-Cu1-N11 N7-Cu1-N11 N5-Cu1-N11 I(1)-I(2)-I(3) | 96.31(14) 76.12(14) 91.34(12) 76.22(12) 92.20(12) 96.06(13) 169.73(13) 177.896(16) |
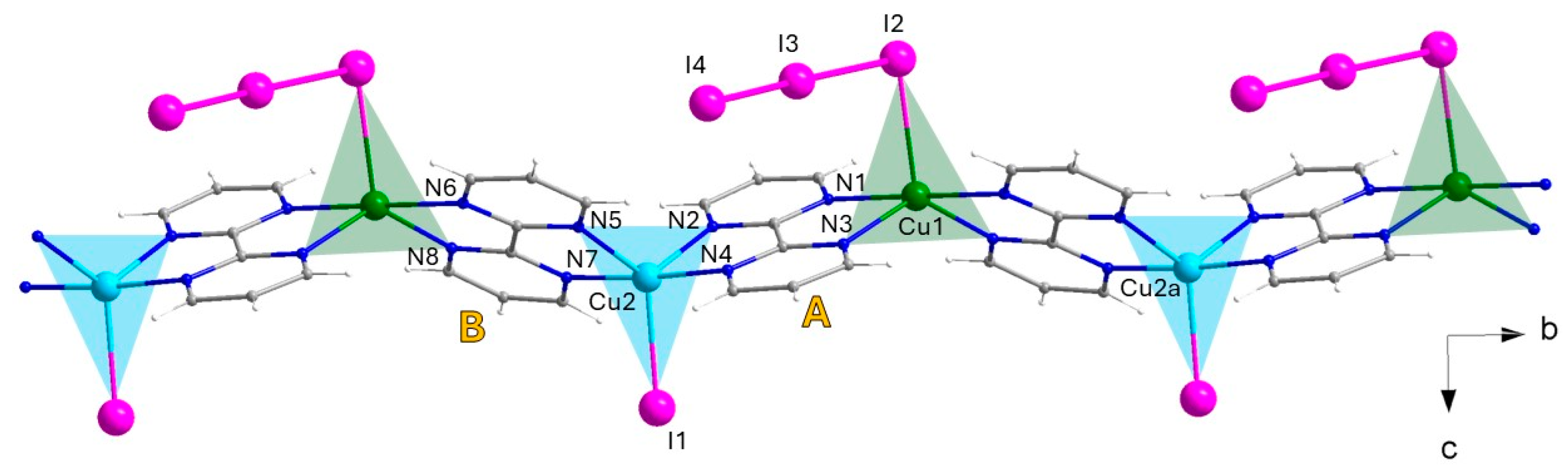
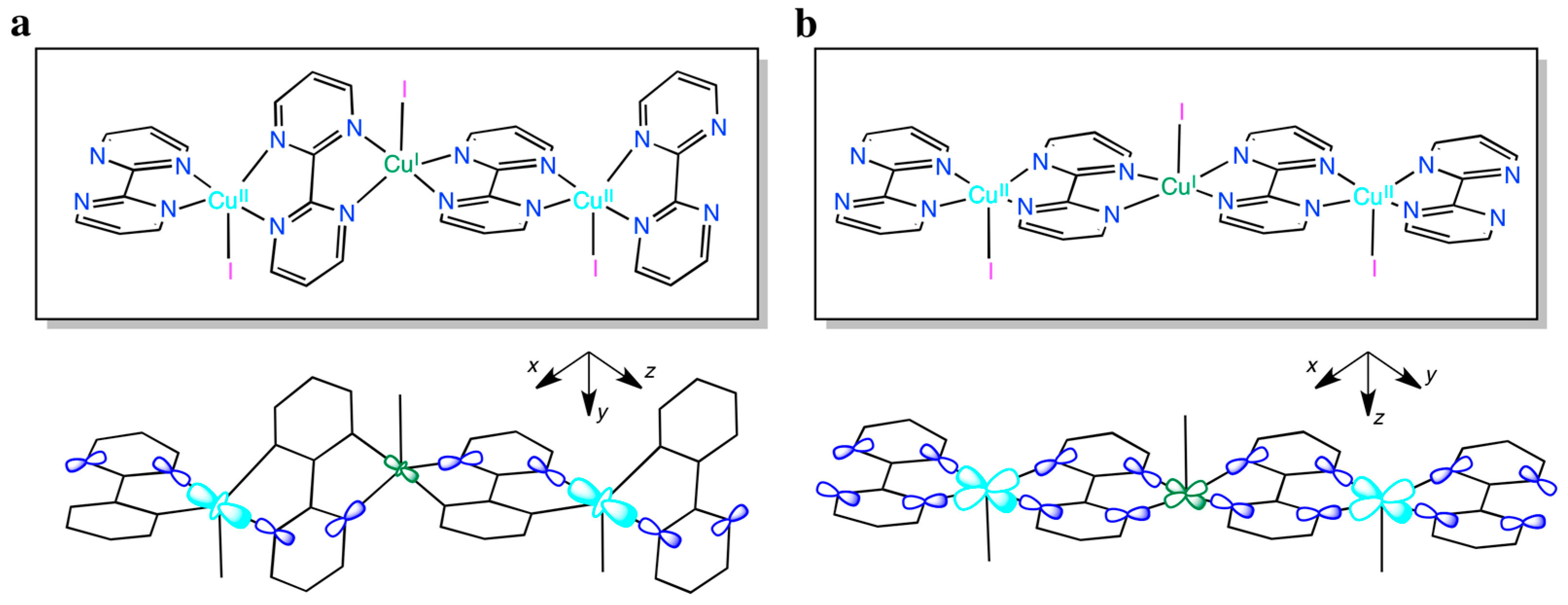
3.1.2. {[CuI(I3)CuII(I)(bpm)2](I3)}n (2)
| Cu1-N1 Cu1-N3 Cu1-N6a Cu1-N8a Cu1-I2 I2-I3 b I3-I4 b | 2.240(6) 2.106(6) 2.260(6) 2.097(5) 2.7907(16) 3.0321(11) 2.8704(11) | Cu2-N2 Cu2-N4 Cu2-N5 Cu2-N7 Cu2-I1 I5-I6 c I6-I7 c | 2.140(5) 1.993(5) 2.121(5) 1.985(5) 2.6179(10) 2.9291(10) 2.9448(10) |
| N8a-Cu1-N3 N8a-Cu1-N1 N3-Cu1-N1 N8a-Cu1-N6a N3-Cu1-N6a N1-Cu1-N6a N8a-Cu1-I2 N3-Cu1-I2 N1-Cu1-I2 N6a-Cu1-I2 I2-I3-I4 b | 128.8(2) 103.2(2) 77.7(2) 77.2(2) 101.6(2) 179.2(2) 123.84(17) 107.29(16) 89.59(17) 90.72(17) 176.71(3) | N5-Cu2-N2 N4-Cu2-N5 N4-Cu2-N2 N7-Cu2-N5 N7-Cu2-N2 N7-Cu2-N4 N5-Cu2-I1 N2-Cu2-I1 N4-Cu2-I1 N7-Cu2-I1 I5-I6-I7 c | 122.7(2) 98.1(2) 80.0(2) 80.0(2) 97.49(19) 175.5(2) 122.10(16) 115.23(15) 90.99(17) 93.52(16) 176.45(2) |
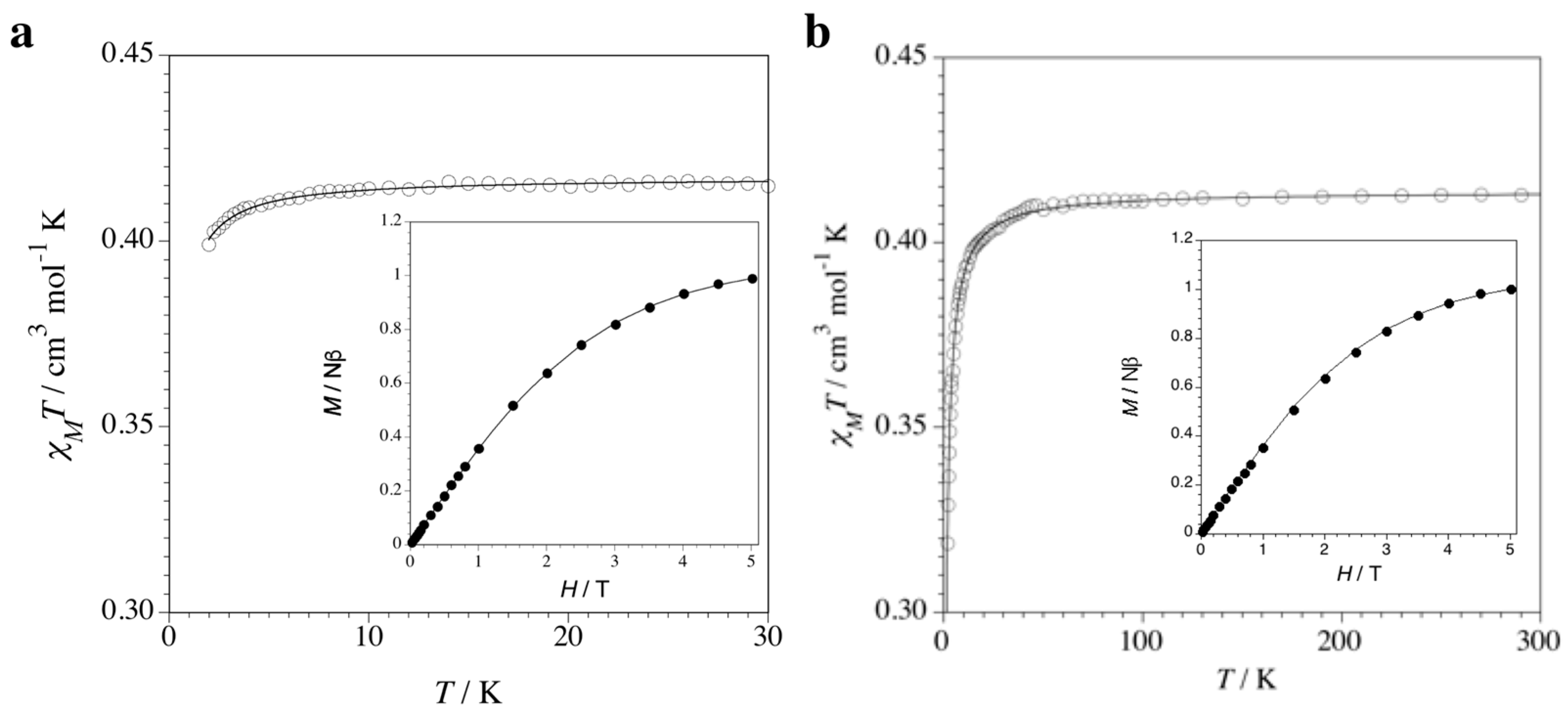
3.2. Magnetic Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrando-Soria, J.; Vallejo, J.; Castellano, M.; Martínez-Lillo, J.; Pardo, E.; Cano, J.; Castro, I.; Lloret, F.; Ruiz-García, R.; Julve, M. Molecular magnetism, quo vadis? A historical perspective from a coordination chemist viewpoint. Coord. Chem. Rev. 2017, 339, 17–103. [Google Scholar] [CrossRef]
- Marinescu, G.; Andruh, M.; Lloret, F.; Julve, M. Bis(oxalato)chromium(III) complexes: Versatile tectons in designing heterometallic coordination compounds. Coord. Chem. Rev. 2011, 255, 161–185. [Google Scholar] [CrossRef]
- Martínez-Lillo, J.; Faus, J.; Lloret, F.; Julve, M. Towards multifunctional magnetic systems through molecular-programmed self-assembly of Re(IV) metalloligands. Coord. Chem. Rev. 2015, 289–290, 215–237. [Google Scholar] [CrossRef]
- Castro, I.; Barros, W.P.; Calatayud, M.L.; Lloret, F.; Marino, N.; De Munno, G.; Stumpf, H.O.; Ruiz-García, R.; Julve, M. Dicopper(II) pyrazolenophanes: Ligand effects on their structures and magnetic properties. Coord. Chem. Rev. 2016, 315, 135–152. [Google Scholar] [CrossRef]
- Castro, I.; Calatayud, M.L.; Orts-Arroyo, M.; Marino, N.; De Munno, G.; Lloret, F.; Ruiz-García, R.; Julve, M. Oxalato as polyatomic coordination center and magnetic coupler in copper(II)-polypyrazole inverse polynuclear complexes and coordination polymers. Coord. Chem. Rev. 2022, 471, 214730–214737. [Google Scholar] [CrossRef]
- De Munno, G.; Julve, M.; Nicolò, F.; Lloret, F.; Faus, J.; Ruiz, R.; Sinn, E. 2,2′-Bipyrimidineoxalatocopper(II) Complexes: From the Mononuclear Complex to the 2D Sheetlike Polymer. Angew. Chem. Int. Ed. Engl. 1993, 32, 613–615. [Google Scholar] [CrossRef]
- Julve, M.; Lloret, F.; Faus, J.; De Munno, G.; Verdaguer, M.; Caneschi, A. Alternating Ferro- and Antiferromagnetic Interactions in a Chainlike CuII Coordination Polymer. Angew. Chem. Int. Ed. Engl. 1993, 32, 1046–1048. [Google Scholar] [CrossRef]
- Real, J.; De Munno, G.; Chiappetta, R.; Julve, M.; Lloret, F.; Journaux, Y.; Colin, J.-C.; Blondin, G. Structural and Magnetic Characterization of a Novel Heptanuclear Hydroxo-Bridged Copper(II) Cluster of the Dicubane Type. Angew. Chem. Int. Ed. Engl. 1994, 33, 1184–1186. [Google Scholar] [CrossRef]
- De Munno, G.; Julve, M.; Viau, G.; Lloret, F.; Faus, J.; Viterbo, D. Azido and 2,2′-Bipirimidine Ligands as Useful Tools in Designing Two- and Three-Dimensional Manganese(II) Networks. Angew. Chem. Int. Ed. Engl. 1996, 35, 1807–1810. [Google Scholar] [CrossRef]
- De Munno, G.; Poerio, T.; Viau, G.; Julve, M.; Lloret, F. Ferromagnetic Coupling in the Bis(μ-end-on-azido)iron(III) Dinuclear Complex Anion of [FeII(bpym)3]2[FeIII2(N3)10]⋅2H2O. Angew. Chem. Int. Ed. Engl. 1997, 36, 1459–1461. [Google Scholar] [CrossRef]
- Lloret, F.; De Munno, G.; Julve, M.; Cano, J.; Ruiz, R.; Caneschi, A. Spin Polarization and Ferromagnetism in 2D Sheetlike Cobalt(II) Polymers: [Co(L)2(SCN)2] (L = Pyrimidine or Pyrazine). Angew. Chem. Int. Ed. Engl. 1998, 37, 135–138. [Google Scholar] [CrossRef]
- De Munno, G.; Bruno, G. Catena-poly{μ-(2,2′-bypirimidine-N,N′,N″,N’‘‘)-[(nitrato-O,O’)copper(II)]-di-(μ-nitrato-μ-O)-[(nitrato-O,O’)copper(II)]}, [Cu2(NO3)4(C8H6N4)]. Acta Crystallogr. 1984, 40, 2030–2032. [Google Scholar] [CrossRef]
- Julve, M.; De Munno, G.; Bruno, G.; Verdaguer, M. Synthesis, Structure and Magnetic Properties of μ-(2,2′-Bipyrimidine-N,N′,N″,N’‘‘) Copper(II) Complexes. Inorg. Chem. 1988, 27, 3160–3165. [Google Scholar] [CrossRef]
- De Munno, G.; Bruno, G.; Julve, M.; Romeo, M. Synthesis and Structure of Aquabis(2,2′-bipyrimidine-N,N′)copper(II) hexafluorophosphate Dihydrate. Acta Crystallogr. 1990, 46, 1828–1830. [Google Scholar] [CrossRef]
- De Munno, G.; Julve, M.; Verdaguer, M.; Bruno, G. Bonding Flexibility of 2,2′-Bipyrimidine (bpm): Symmetry and Magnetism of Three Copper(II) Complexes with Different Cu:bpm Ratios. Inorg. Chem. 1993, 32, 2215–2220. [Google Scholar] [CrossRef]
- De Munno, G.; Julve, M.; Lloret, F.; Cano, J.; Caneschi, A. Magneto-Structural Effects of the Jahn-Teller Distortions on 2,2′-Bipyrimidine (bpm)-Bridged Dinuclear Copper(II) Complexes: Crystal Structures and Magnetic Properties of [Cu2(bpm)(H2O)4(SO4)2]⋅3H2O and [Cu2(bpm)(H2O)8](SO4)2⋅2H2O. Inorg. Chem. 1995, 34, 2048–2053. [Google Scholar] [CrossRef]
- De Munno, G.; Poerio, T.; Viaux, G.; Julve, M.; Lloret, F.; Journaux, J.; Rivière, E. New magnetic behaviour of honeycomb layered compounds. Crystal structure of [M2(bipym)(N3)] (M = CoII, FeII; bipym = 2,2′-bipyrimidine). Chem. Commun. 1996, 22, 2587–2588. [Google Scholar] [CrossRef]
- De Munno, G.; Julve, M.; Real, J.A. Synthesis and crystal structure of the low-spin iron(II) complex [Fe(bpym)3](ClO4)2⋅1/4H2O. Inorganica Chim. Acta 1997, 255, 185–188. [Google Scholar] [CrossRef]
- De Munno, G.; Poerio, T.; Julve, M.; Lloret, F.; Faus, J.; Caneschi, A. Syntheses, crystal structures and magnetic properties of one-, two- and three-dimensional 2,2′-bipyrimidine-containig copper(II) complexes. J. Chem. Soc. Dalton Trans. 1998, 10, 1679–1685. [Google Scholar] [CrossRef]
- De Munno, G.; Ventura, W.; Viau, G.; Lloret, F.; Faus, J.; Julve, M. Structural Characterization and Magnetic Properties of the First 2,2′-Bipyrimidine (bpm)-containing Iron(III) Complexes. Inorg. Chem. 1998, 37, 1458–1464. [Google Scholar] [CrossRef]
- De Munno, G.; Armentano, D.; Julve, M.; Lloret, F.; Lescouëzec, R.; Faus, J. Two-dimensional Assembling of (2,2′-Bipirimidine)bis(oxalato)chromate(III) Units through Alkaline Cations. Inorg. Chem. 1999, 38, 2234–2237. [Google Scholar] [CrossRef] [PubMed]
- Armentano, D.; De Munno, G.; Lloret, F.; Julve, M. Inorg. Chem. Novel Three-Dimensional Cage Assembly of a µ4-Carbonato Bridged Cobalt(II) Compound [Co2(bpm)(H2O)2(CO3)(OH)]NO3⋅4H2O. Inorg. Chem. 1999, 38, 3744–3747. [Google Scholar] [CrossRef] [PubMed]
- Chiozzone, R.; Gonzáles, R.; Kremer, C.; Cerdá, M.F.; Armentano, D.; De Munno, G.; Martínez-Lillo, J.; Faus, J. A novel series of rhenium-bipyrimidine complexes: Synthesis, crystal structure and electrochemical properties. Dalton Trans. 2007, 6, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lillo, J.; Armentano, D.; De Munno, G.; Cano, J.; Lloret, F.; Julve, M.; Faus, J. First Magnetostructural Study on a Heterodinuclear 2,2′-Bipyrimidine-Bridged Complex. Inorg. Chem. 2011, 50, 12405–12407. [Google Scholar] [CrossRef]
- Marino, N.; Armentano, D.; De Munno, G.; Cano, J.; Lloret, F.; Julve, M. Synthesis, Structure, and Magnetic Properties of Regular Alternating μ-bpm/di-μ-X Copper (II) Chains (bpm= 2, 2′-bipyrimidine; X= OH, F). Inorg. Chem. 2012, 51, 4323–4334. [Google Scholar] [CrossRef]
- Marino, N.; Armentano, D.; De Munno, G.; Lloret, F.; Cano, J.; Julve, M. Toward a better understanding of honeycomb alternating magnetic networks. Dalton Trans. 2015, 44, 11040–11051. [Google Scholar] [CrossRef]
- Martínez-Lillo, J.; Armentano, D.; Fortea-Pérez, F.R.; Stiriba, S.-E.; De Munno, G.; Lloret, F.; Julve, M.; Faus, J. A Chiral, Photoluminescent and Spin-Canted {CuIReIV2}n Branched Chain. Inorg. Chem. 2015, 54, 4594–4596. [Google Scholar] [CrossRef]
- Marino, N.; Mastropietro, T.F.; Armentano, D.; De Munno, G.; Doyle, R.P.; Lloret, F.; Julve, M. Spin canting in an unprecedented three-dimensional pyrophosphate- and 2,2′-bipyrimidine-bridged cobalt(II) framework. Dalton Trans. 2008, 54, 5152–5154. [Google Scholar] [CrossRef]
- Martínez-Lillo, J.; Mastropietro, T.F.; Lappano, R.; Madeo, A.; Alberto, M.E.; Russo, N.; Maggiolini, M.; De Munno, G. Rhenium(IV) compounds inducing apoptosis in cancer cells. Chem. Commun. 2011, 47, 5283–5285. [Google Scholar] [CrossRef]
- Betanzos-Lara, S.; Novakova, O.; Deeth, R.J.; Pizarro, A.M.; Clarkson, G.J.; Liskova, B.; Bravec, V.; PJSadler, P.J.; Habtemariam, A. Bipyrimidine ruthenium(II) arene complexes: Structure, reactivity and cytotoxicity. J. Biol. Inorg. Chem. 2012, 17, 1033–1051. [Google Scholar] [CrossRef]
- Kojima, T.; Morimoto, T.; Miyazaki, S.; Fukuzumi, S. Ruthenium(II) Pyridylamine Complexes with Diimine Ligands Showing Reversible Photochemical and Thermal Structural Change. Chem Eur. J. 2008, 14, 8904–8915. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, T.; Itogawa, M.; Shimomura, H.; Shiota, Y.; Kotani, H.; Yoshizawa, K.; Kojima, T. Redox properties of a bipyrimidine-bridged dinuclear ruthenium(II) complex. Inorg. Chem. Commun. 2020, 120, 108150. [Google Scholar] [CrossRef]
- Henke, W.C.; Kerr, T.A.; Sheridan, T.R.; Henling, L.M.; Takase, M.K.; Day, V.W.; Gray, H.B.; Blakemore, J.D. Synthesis, structural studies, and redox chemistry of bimetallic [Mn(CO)3] and [Re(CO)3] complexes. Dalton Trans. 2021, 50, 2746–2756. [Google Scholar] [CrossRef] [PubMed]
- Kaim, W.; Dogan, A.; Wanner, M.; Klein, A.; Tiritiris, I.; Schleid, T.; Stufkens, D.J.; Snoeck, T.L.; McInnes, E.J.L.; Fiedler, J.; et al. Reduced and Excited States of (bpym)[PtCl2]n (bpym = 2,2′-Bipyrimidine; n = 1, 2): Experiments and DFT Calculations. Inorg. Chem. 2002, 41, 4139–4148. [Google Scholar] [CrossRef]
- Real, J.A.; Gaspar, A.B.; Muñoz, M.C.; Gütlich, P.; Ksenofontov, V.; Spiering, H. Bipyrimidine-bridged dinuclear iron (II) spin crossover compounds. Top. Curr. Chem. 2004, 233, 167–193. [Google Scholar] [CrossRef]
- Vogler, C.; Hausen, H.-D.; Kaim, W.; Kohlmann, S.; Kramer, H.E.A.; Rieker, J. Copper(I)-Assisted Formation of an “Organic” Sandwich Structure: Structural Prerequisites for Luminescence of the Dinuclear Complexes [(μ-Bipyrimidine){Cu(PR3)2}2]X2. Angew. Chem. Int. Ed. 1989, 28, 1659–1660. [Google Scholar] [CrossRef]
- Sieger, M.; Vogler, C.; Klein, A.; Knödler, A.; Wanner, M.; Fiedler, J.; Záliš, S.; Snoeck, T.I.; Kaim, W. Geometrical and Electronic Structures of Dinuclear Complex Ions {(μ-bpym)[Cu(EAr3)2]2}2+ with Intramolecular “Organic Sandwich” Formation (E = P or As; Ar = Aryl; bpym = 2,2‘-Bipyrimidine). Inorg. Chem. 2005, 44, 4637–4643. [Google Scholar] [CrossRef]
- Hou, F.; Powell, M.; Dougherty, D.B.; Sommer, R.D.; Maggard, P.A. Tunable Optical and Photocatalytic Properties of Low-Dimensional Copper(I)-Iodide Hybrids Using Coordinating Organic Ligands. Cryst. Growth Des. 2018, 18, 5406–5416. [Google Scholar] [CrossRef]
- Decurtins, S.; Schmalle, H.W.; Schneuwly, P.; Zheng, L.-M.; Ensling, J.; Hauser, A. Synthesis, Crystal Structure, Optical and Magnetic Properties of a Novel Two-Dimensional Copper(II) Network formed conjointly with μ-Bipyrimidine, μ-Oxalato, and μ-Chloro Ligands. Inorg. Chem. 1995, 34, 5501–5506. [Google Scholar] [CrossRef]
- Ikotun, O.F.; Ouellette, W.; Lloret, F.; Julve, M.; Doyle, R.P. Synthesis, X-ray Structure, Thermal and Magnetic Behavior of [(bipy)2Ni2(μ-Cl)2Cl2(H2O)2]: The First Neutral Ferromagnetically Coupled Six-Coordinate Dichlorido-Bridged Nickel(II) Dimer. Eur. J. Inorg. Chem. 2007, 2007, 2083–2088. [Google Scholar] [CrossRef]
- Julve, M.; Verdaguer, M.; De Munno, G.; Real, J.A.; Bruno, G. Synthesis, crystal structure, and magnetic properties of (μ-bipyrimidine)(cyanato)copper(II) and -(thiocyanato)copper(II) complexes. Inorg. Chem. 1993, 32, 795–802. [Google Scholar] [CrossRef]
- Cortés, R.; Lezama, L.; Rojo, T.; Arriortua, M.I.; Pizarro, J.L.; Solans, X. Alternating Ferro- and Antiferromagnetic Interactions in a MnII Chain with Alternating End-On and End-to-End Bridging Azido Ligands. Angew. Chem. Int. Ed. Engl. 1994, 33, 2488–2489. [Google Scholar] [CrossRef]
- Cortés, R.; Drillon, M.; Solans, X.; Lezama, L.; Rojo, T. Alternating Ferromagnetic−Antiferromagnetic Interactions in a Manganese(II)−Azido One-Dimensional Compound: [Mn(bipy)(N3)2]. Inorg. Chem. 1997, 36, 677–683. [Google Scholar] [CrossRef]
- Viau, G.; Lombardi, M.G.; De Munno, D.; Julve, M.; Lloret, F.; Faus, J.; Caneschi, A.; Clemente-Juan, J.M. The azido ligand: A useful tool in designing chain compounds exhibiting alternating ferro- and antiferro-magnetic interactions. Chem. Commun. 1997, 13, 1195–1196. [Google Scholar] [CrossRef]
- Armentano, D.; Sanchis-Perucho, A.; Rojas-Dottib, C.; Martínez-Lillo, J. Halogen halogen interactions in the self-assembly of one-dimensional 2,2′-bipyrimidine-based CuIIReIV systems. CrystEngComm 2018, 20, 4575–4581. [Google Scholar] [CrossRef]
- SAINT, version 6.45; Bruker Analytical X-Ray Systems Inc.: Madison, WI, USA, 2003.
- SADABS, version 2.03; Bruker AXS Inc.: Madison, WI, USA, 2000.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Diamond (version 4.6.8)—Crystal and Molecular Structure Visualization, Crystal Impact—Dr. H. Putz & Dr. K. Brandenburg GbR, Kreuzherrenstr. 102, 53227 Bonn, Germany. Available online: https://www.crystalimpact.de/diamond (accessed on 30 January 2025).
- SHAPE, version 2.1; Electronic Structure Group, Universitat de Barcelona: Barcelona, Spain, 2013.
- Blake, A.J.; Li, W.-S.; Lippolis, V.; Schröder, M.; Devillanova, F.A.; Gould, R.O.; Parsons, S.; Radek, C. Template self-assembly of polyiodide networks. Chem. Soc. Rev. 1998, 27, 195–206. [Google Scholar] [CrossRef]
- Svensson, P.H.; Kloo, L. Synthesis, Structure, and Bonding in Polyiodide and Metal Iodide-Iodine Systems. Chem. Rev. 2003, 103, 1649–1684. [Google Scholar] [CrossRef]
- van Megen, M.; Reiss, G.J. I62− Anion Composed of Two Asymmetric Triiodide Moieties: A Competition between Halogen and Hydrogen Bond. Inorganics 2013, 1, 3–13. [Google Scholar] [CrossRef]
- Kulkarni, P.; Padhye, S.; Sinn, E.; Anson, C.E.; Powell, A.K. Comparative studies on copper(I) complexes: Synthesis, X-ray crystallography and electrochemical properties of [CuI (dafone)nX] complexes (dafone=/4,5-diaza-fluoren-9-one, X=/Br, I, SCN). Inorg. Chim. Acta 2002, 332, 167–175. [Google Scholar] [CrossRef]
- Horn, C.; Ali, B.; Dance, I.; Scudder, M.; Craig, D. Crystal supramolecularity: Extended aryl embraces in dimorphs of [Cu(1,10-phen)2I]I3. CrystEngComm 2000, 2, 6–15. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH Publishers: New York, NY, USA, 1993. [Google Scholar]
- Ruiz, R.; Julve, M.; Faus, J.; Lloret, F.; Muñoz, M.C.; Journaux, Y.; Bois, C. Ferromagnetic Coupling between Copper(II) Centers through the Diamagnetic Zinc(II) Ion: Crystal Structure and Magnetic Properties of [Cu2Zn(Hdmg)2(dmg)2(H2O)]·0.5H2dmg·H2O (H2dmg = Dimethylglyoxime). Inorg. Chem. 1997, 36, 3434–3439. [Google Scholar] [CrossRef] [PubMed]
- Buvaylo, E.A.; Kokozay, V.N.; Vassilyeva, O.Y.; Skelton, B.W.; Jezierska, J.; Brunel, L.C.; Ozarowski, A. Ozarowski. A Cu–Zn–Cu–Zn heterometallomacrocycle shows significant antiferromagnetic coupling between paramagnetic centres mediated by diamagnetic metal. Chem. Commun. 2005, 39, 4976–4978. [Google Scholar] [CrossRef] [PubMed]
- Semenaka, V.V.; Nesterova, O.V.; Kokozay, V.N.; Dyakonenko, V.V.; Zubatyuk, R.I.; Shishkin, O.V.; Boča, R.; Jezierska, J.; Ozarowski, A. Ozarowski. CrIII−CrIII Interactions in Two Alkoxo-Bridged Heterometallic Zn2Cr2 Complexes Self-Assembled from Zinc Oxide, Reinecke’s Salt, and Diethanolamine. Inorg. Chem. 2010, 49, 5460–5471. [Google Scholar] [CrossRef]
- Oliveira, W.X.; Ribeiro, M.A.; Pinheiro, C.B.; da Costa, M.M.; Fontes, A.P.S.; Nunes, W.C.; Cangussu, D.; Julve, M.; Stumpf, H.O.; Pereira, C.L. Palladium(II)–Copper(II) Assembling with Bis(2-pyridylcarbonyl)amidate and Bis(oxamate) Type Ligands. Cryst. Growth Des. 2015, 15, 1325–1335. [Google Scholar] [CrossRef]
- Oliveira, W.X.C.; Ribeiro, M.A.; Pinheiro, C.B.; da Costa, M.M.; Fontes, A.P.S.; Nunes, W.C.; Cangussu, D.; Julve, M.; Stumpf, H.O.; Pereira, C.L.M. Experimental and Theoretical Investigation of the Anti-Ferromagnetic Coupling of CrIII Ions through Diamagnetic −O–NbV–O– Bridges. Inorg. Chem. 2017, 56, 6879–6889. [Google Scholar] [CrossRef]
- Jurić, M.; Dubraja, L.A.; Popović, J.; Molčanov, K.; Torić, F.; Pajić, D.; Lončarić, I. From a square core to square opening: Structural diversity and magnetic properties of the oxo-bridged [CrIIINbV] complexes. Dalton Trans. 2018, 47, 4183–4190. [Google Scholar] [CrossRef]
- Lu, J.Y. Crystal engineering of Cu-containing metal–organic coordination polymers under hydrothermal conditions. Coord. Chem. Rev. 2003, 246, 327–347. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Zheng, S.-L.; Huang, X.-C.; Chen, X.-M. Two Unprecedented 3-Connected Three-Dimensional Networks of Copper(I) Triazolates: In Situ Formation of Ligands by Cycloaddition of Nitriles and Ammonia. Angew. Chem. Int. Ed. 2004, 43, 206–209. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, Y.; Tong, M.-L.; Chen, X.-M.; Yuen, T.; Lin, C.L.; Huang, X.; Li, J. A mixed-valence copper coordination polymer generated by hydrothermal metal/ligand redox reactions. Chem. Commun. 2002, 13, 1342. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Tong, M.-L.; Chen, X.-M. Hydroxylation of N-Heterocycle Ligands Observed in Two Unusual Mixed-Valence CuI/CuII Complexes. Angew. Chem. Int. Ed. 2002, 41, 1029–1031. [Google Scholar] [CrossRef]
- Chamayou, J.A.A.-C.; Biswas, C.; Janiak, C.; Ghosh, A. Tris(2,2′-bipyridine-κ2N,N′)copper(II) bis(tetrafluoridoborate). Acta Cryst. 2007, 63, m1936–m1937. [Google Scholar] [CrossRef]
- Xu, F.; You, W.; Huang, W. Tris(2,20-bipyridyl-j2 N,N0)copper(II) sulfate 7.5-hydrate. Acta Cryst. 2009, 5, m129–m130. [Google Scholar] [CrossRef]
- Murphy, B.; Aljabri, M.; Ahmed, A.M.; Murphy, G.; Hathaway, B.J.; Light, M.E.; Geilbrich, T.; Hursthouse, M.B. Structural systematics of the [Cu(chelate)3][Y]2 series. An interesting crystallographic structural insight involving vibronic coupling and the Jahn–Teller effect (JTE). The syntheses and low temperature crystal structures of tris(2,2′bipyridyl)copper(ii) tetraphenylborate and tris(2,2′bipyridyl)zinc(ii) tetraphenylborate. Dalton Trans. 2006, 2, 357–367. [Google Scholar] [CrossRef]
| 1 | 2 | 3 | |
|---|---|---|---|
| Formula | C24H18CuI4N12 | C16H12Cu2I7N8 | C8H6Cu2I2N4 |
| Fw/g mol−1 | 1045.64 | 1331.72 | 539.05 |
| Crystal system | triclinic | orthorhombic | monoclinic |
| Space group | P-1 | Pna21 | C2/m |
| a/Å | 10.7606(3) | 22.9658(6) | 12.7554(9) |
| b/Å | 11.3856(3) | 10.8435(3) | 8.1499(9) |
| c/Å | 13.6376(3) | 11.7187(3) | 6.2362(6) |
| α/° | 88.454(1) | 90 | 90 |
| β/° | 87.043(1) | 90 | 102.737(4) |
| γ/° | 72.620(1) | 90 | 90 |
| V/Å3 | 1592.29(7) | 2918.3(1) | 632.33(10) |
| Z | 2 | 4 | 2 |
| Dc/g cm−3 | 2.181 | 3.031 | 2.831 |
| F(000) | 974 | 2372 | 492 |
| μ(Mo-Kα)mm−1 | 4.600 | 8.884 | 8.222 |
| Refl. Collected | 22,922 | 60,622 | 5095 |
| Refl. Indep. [Rint] | 7847 [0.0248] | 9912 [0.0258] | 839 [0.0715] |
| Reflec. Obs. [I > 2σ(I)] | 5551 | 8616 | 586 |
| Data/restraints/parameters | 7847/0/370 | 9912/1/299 | 839/0/42 |
| Completeness to θ = 25.242° | 99.2% | 100% | 100% |
| GOOF c | 1.038 | 1.055 | 1.115 |
| R1 a [I > 2σ(I)] (all) | 0.0350(0.0559) | 0.0332(0.0411) | 0.0427(0.0507) |
| wR2 b [I > 2σ(I)] (all) | 0.0803(0.0901) | 0.0780(0.0819) | 0.1016(0.1083) |
| Largest diff. peak/hole/eÅ−3 | 1.202/−1.224 | 1.523/−1.431 | 2.384/−1.217 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marino, N.; Lloret, F.; Julve, M.; De Munno, G. Chemical and Structural Versatility in the Copper/2,2′-Bipyrimidine/Iodide System: A Regular Alternating Mixed-Valent Cu(II)-Cu(I) Chain Showing Unusually Similar Metal Coordination Environments. Magnetochemistry 2025, 11, 20. https://doi.org/10.3390/magnetochemistry11030020
Marino N, Lloret F, Julve M, De Munno G. Chemical and Structural Versatility in the Copper/2,2′-Bipyrimidine/Iodide System: A Regular Alternating Mixed-Valent Cu(II)-Cu(I) Chain Showing Unusually Similar Metal Coordination Environments. Magnetochemistry. 2025; 11(3):20. https://doi.org/10.3390/magnetochemistry11030020
Chicago/Turabian StyleMarino, Nadia, Francesc Lloret, Miguel Julve, and Giovanni De Munno. 2025. "Chemical and Structural Versatility in the Copper/2,2′-Bipyrimidine/Iodide System: A Regular Alternating Mixed-Valent Cu(II)-Cu(I) Chain Showing Unusually Similar Metal Coordination Environments" Magnetochemistry 11, no. 3: 20. https://doi.org/10.3390/magnetochemistry11030020
APA StyleMarino, N., Lloret, F., Julve, M., & De Munno, G. (2025). Chemical and Structural Versatility in the Copper/2,2′-Bipyrimidine/Iodide System: A Regular Alternating Mixed-Valent Cu(II)-Cu(I) Chain Showing Unusually Similar Metal Coordination Environments. Magnetochemistry, 11(3), 20. https://doi.org/10.3390/magnetochemistry11030020







