Positive and Negative Exchange Bias in N-, P- and Q-Type Ferri-Magnets of Niccolite Metal Formates [CH3NH2CH3]n[CrIII1−xFeIIIxFeII(HCO2)6]n
Abstract
1. Introduction
2. Materials and Methods
- Synthesis of [CH3NH2CH3]n[CrIII1−xFeIIIxFeII(HCO2)6]n
3. Results and Discussion
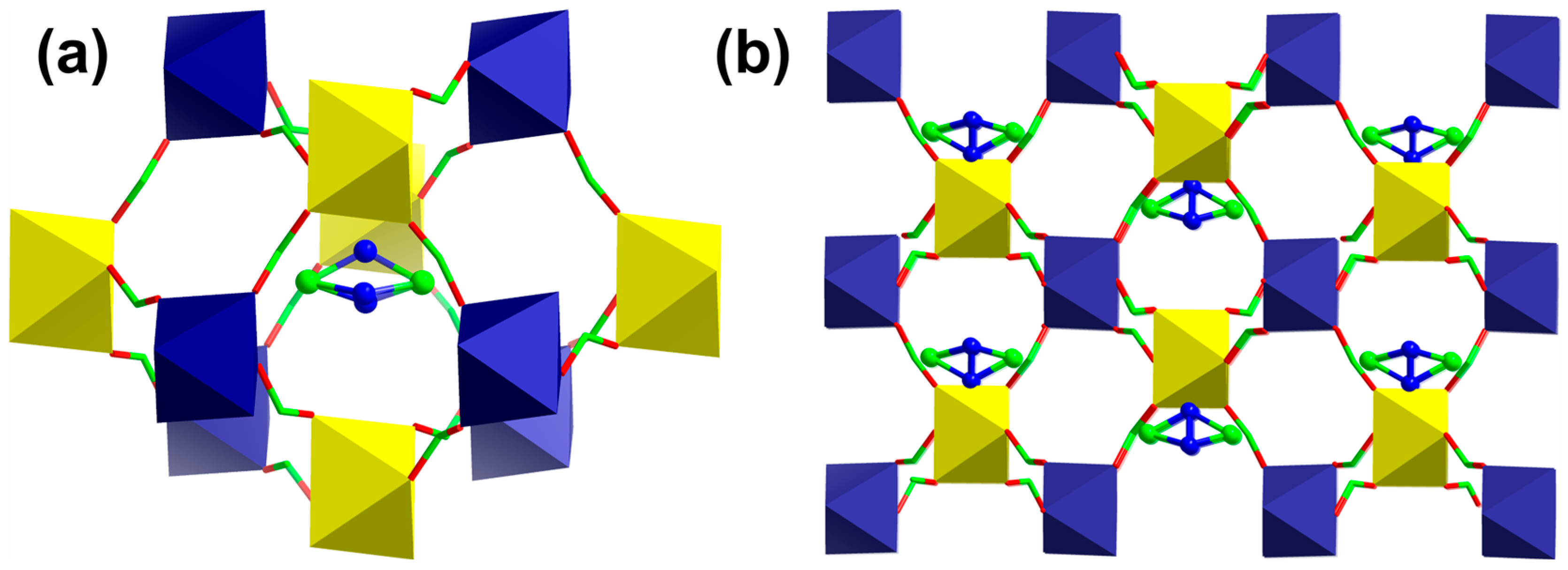
3.1. Syntheses and Crystal Structures
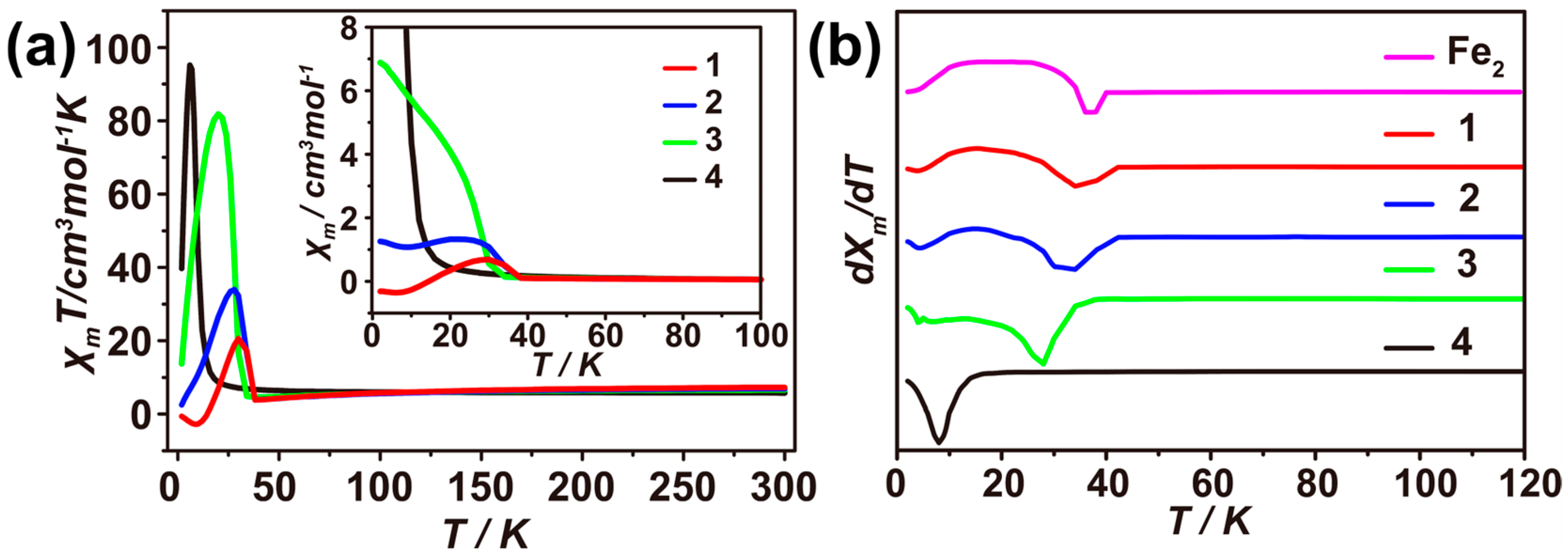
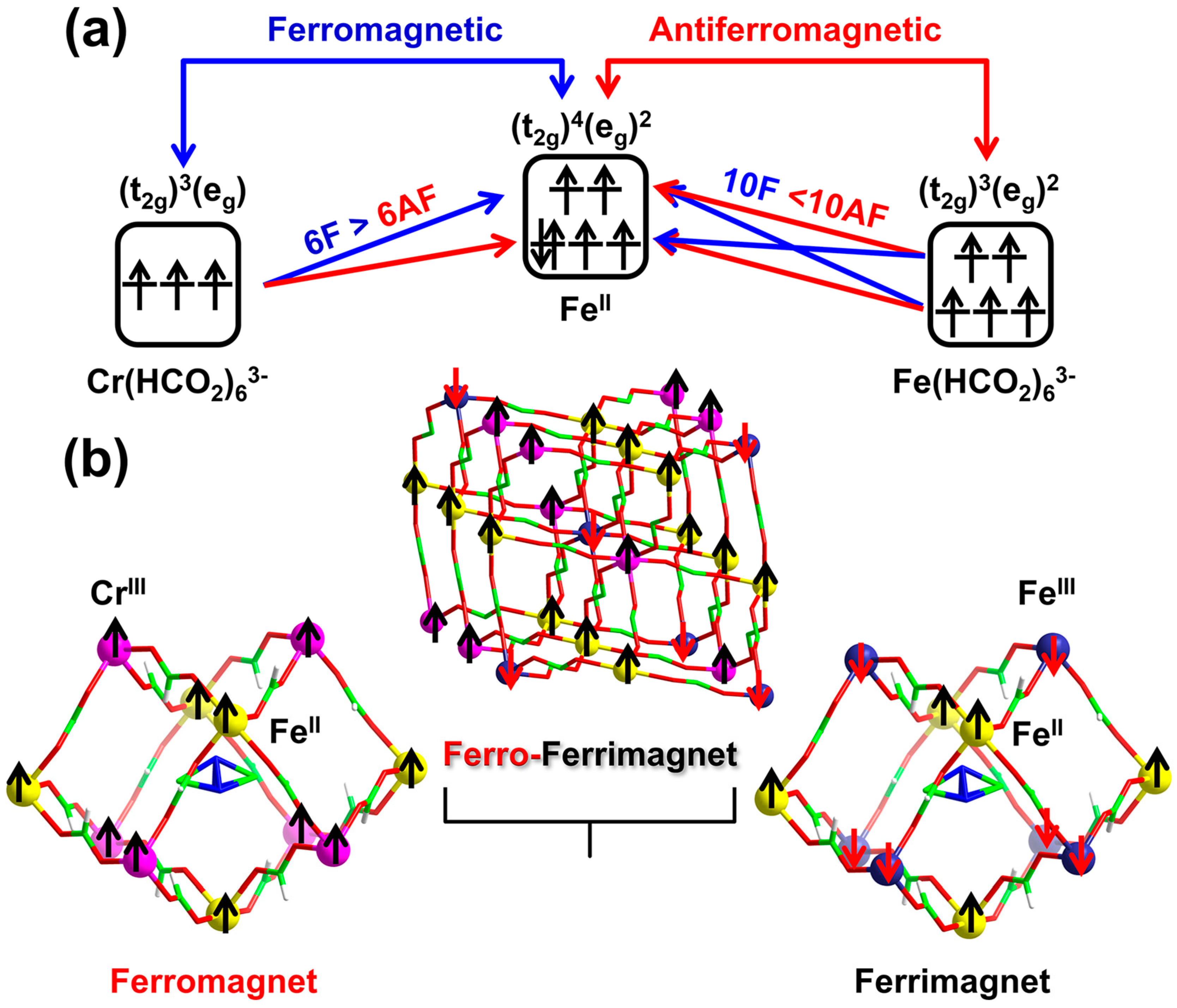
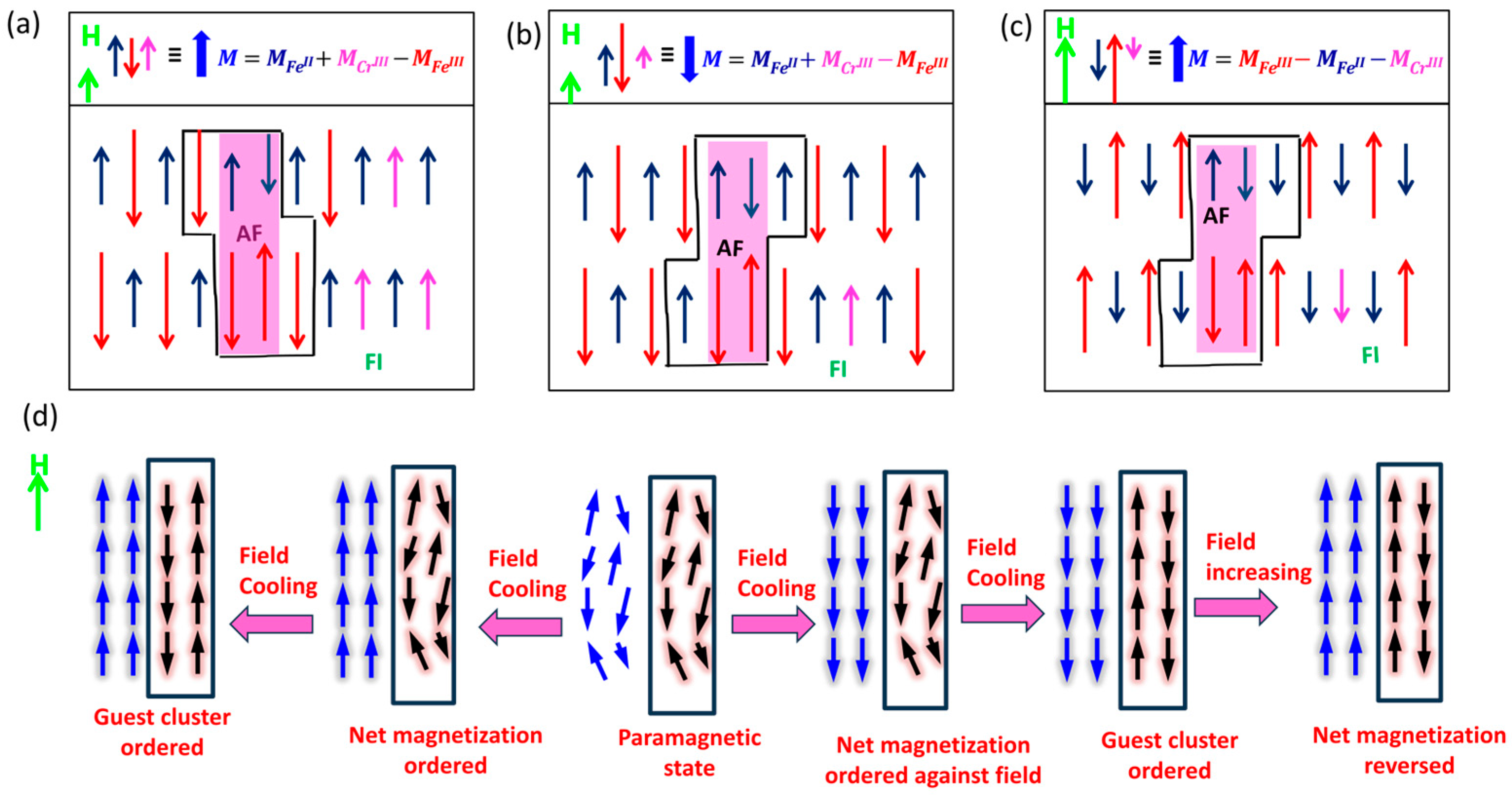
3.2. Magnetic Properties
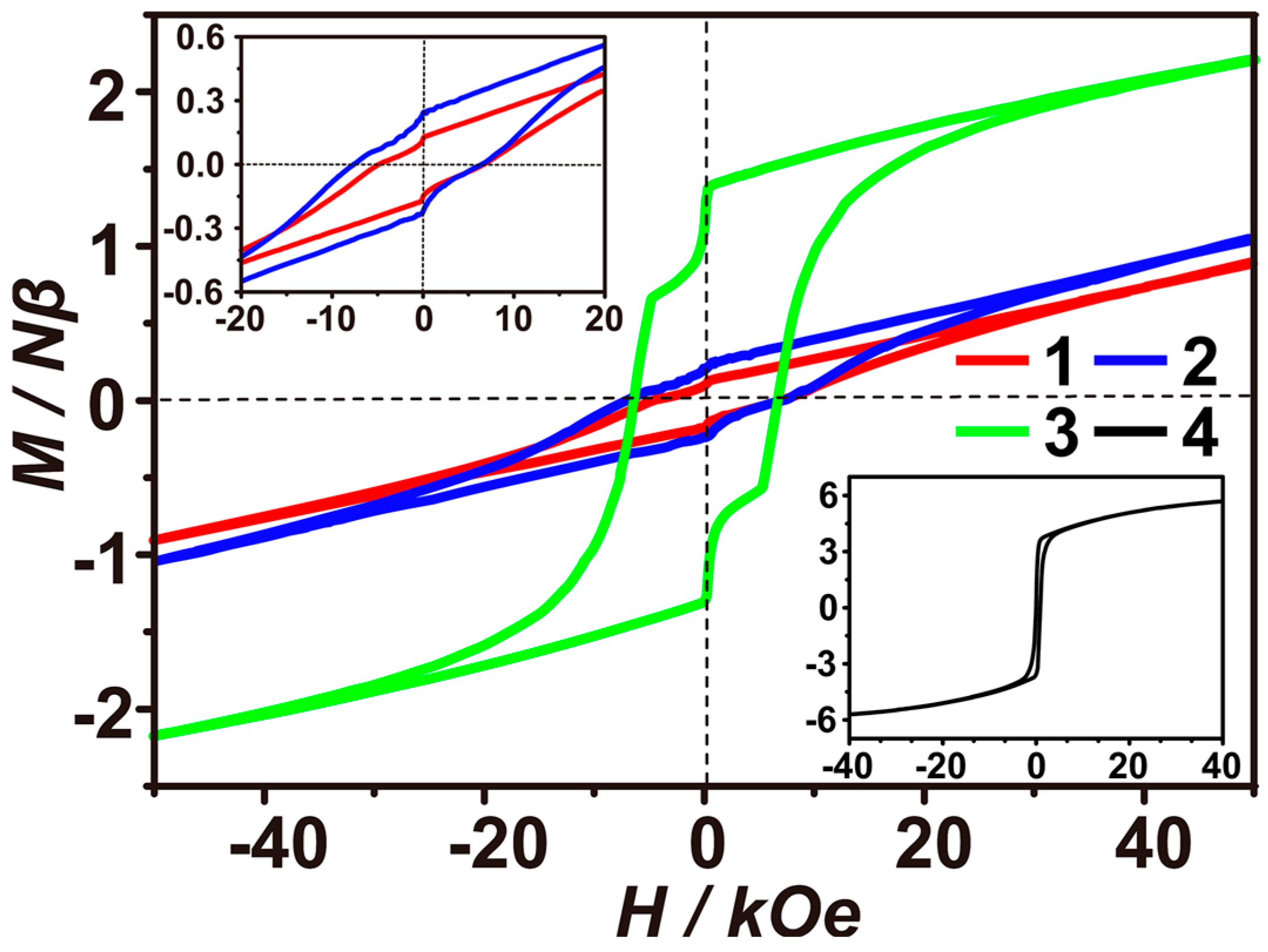
3.3. Exchange Bias
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| EB | Exchange Bias |
| FM | Ferromagnetic |
| AFM | Anti-ferromagnetic |
| TM | Transition metal |
| Tcomp | Compensated temperature |
| PXRD | Powder X-ray diffraction |
| ICP | Inductively coupled plasma |
| XPS | X-ray photoelectron spectroscopy |
| ZFCM | Zero field cooled magnetization |
| FCM | Field cooled magnetization |
References
- Nogués, J.; Schuller, I.K. Exchange bias. J. Magn. Magn. Mater. 1999, 192, 203–232. [Google Scholar] [CrossRef]
- Wu, S.M.; Cybart, S.A.; Yu, P.; Rossell, M.D.; Zhang, J.X.; Ramesh, R.; Dynes, R.C. Reversible electric control of exchange bias in a multiferroic field-effect device. Nat. Mater. 2010, 9, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Meiklejohn, W.H.; Bean, C.P. A new magnetic anisotropy. IEEE Trans. Magn. 2001, 37, 3866–3876. [Google Scholar] [CrossRef]
- Nayak, A.K.; Nicklas, M.; Chadov, S.; Khuntia, P.; Shekhar, C.; Kalache, A.; Baenitz, M.; Skourski, Y.; Guduru, V.K.; Puri, A.; et al. Design of compensated ferrimagnetic Heusler alloys for giant tunable exchange bias. Nat. Mater. 2015, 14, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Kuch, W.; Chelaru, L.I.; Offi, F.; Wang, J.; Kotsugi, M.; Kirschner, J. Tuning the magnetic coupling across ultrathin antiferromagnetic films by controlling atomic-scale roughness. Nat. Mater. 2006, 5, 128–133. [Google Scholar] [CrossRef]
- Stamps, R.L. Mechanisms for exchange bias. J. Phys. D Appl. Phys. 2000, 33, R247–R268. [Google Scholar] [CrossRef]
- Nogués, J.; Lederman, D.; Moran, T.J.; Schuller, I.K. Positive exchange bias in FeF2-Fe bilayers. Phys. Rev. Lett. 1996, 76, 4624–4627. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, D.A.; Olamit, J.; Dumas, R.K.; Kirby, B.J.; Grutter, A.J.; Maranville, B.B.; Arenholz, E.; Borchers, J.A.; Liu, K. Controllable positive exchange bias via redox-driven oxygen migration. Nat. Commun. 2016, 7, 11050. [Google Scholar] [CrossRef]
- Koon, N.C. Calculations of Exchange Bias in Thin Films with Ferromagnetic/Antiferromagnetic Interfaces. Phys. Rev. Lett. 1997, 78, 4865–4868. [Google Scholar] [CrossRef]
- Hong, T.M. Simple mechanism for a positive exchange bias. Phys. Rev. B 1998, 58, 97–100. [Google Scholar] [CrossRef]
- Kiwi, M. Exchange bias theory. J. Magn. Magn. Mater. 2001, 234, 584–595. [Google Scholar] [CrossRef]
- Zhao, J.P.; Hu, B.W.; Lloret, F.; Tao, J.; Yang, Q.; Zhang, X.F.; Bu, X.H. Magnetic Behavior Control in Niccolite Structural Metal Formate Frameworks [NH2(CH3)2][FeIIIMII(HCOO)6] (M = Fe, Mn, and Co) by Varying the Divalent Metal Ions. Inorg. Chem. 2010, 49, 10390–10399. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, K. Positive exchange bias from magnetization reversal in La1−xPrxCrO3 (x ∼ 0.7–0.85). Appl. Phys. Lett. 2011, 99, 142501. [Google Scholar] [CrossRef]
- Bora, T.; Ravi, S. Sign reversal of magnetization and tunable exchange bias field in NdCr1−xFexO3 (x = 0.05–0.2). J. Magn. Magn. Mater. 2015, 386, 85–91. [Google Scholar] [CrossRef]
- Néel, L. Propriétés magnétiques des ferrites; ferrimagnétisme et antiferromagnétisme. Ann. Phys. 1948, 12, 137–198. [Google Scholar] [CrossRef]
- Zhao, J.-P.; Han, S.-D.; Liu, F.-C. Tunable Ferromagnetic Strength in Niccolite Structural Heterometallic Formate Framework Based on Orthogonal Magnetic Orbital Interactions. Inorg. Chem. 2019, 58, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, J.; Pu, T.C.; Kong, M.; Zhang, J.; Song, Y. Study of the relationship between magnetic field and dielectric properties in two ferromagnetic complexes. RSC. Adv. 2017, 7, 47913–47919. [Google Scholar] [CrossRef]
- Pramanik, P.; Ghosh, S.; Yanda, P.; Joshi, D.C.; Pittala, S.; Sundaresan, A.; Mishra, P.K.; Thota, S.; Seehra, M.S. Magnetic ground state, field-induced transitions, electronic structure, and optical band gap of the frustrated antiferromagnet GeCo2O4. Phys. Rev. B 2019, 99, 134422. [Google Scholar] [CrossRef]
- Hagen, K.S.; Naik, S.G.; Huynh, B.H.; Masello, A.; Christou, G. Intensely Colored Mixed-Valence Iron(II) Iron(III) Formate Analogue of Prussian Blue Exhibits Néel N-Type Ferrimagnetism. J. Am. Chem. Soc. 2009, 131, 7516–7517. [Google Scholar] [CrossRef]
- Tian, Y.-Q.; Yao, S.-Y.; Guo, Y.-J.; Song, Y.; Zhang, G. Mixed Valence Iron Formate {[NH4][FeIIFeIII(CHOO)6]}∞: A Molecule-Based Ferrimagnet Showing Temperature Compensation Behavior. Chin. J. Inorg. Chem. 2010, 26, 385–390. [Google Scholar]
- Mączka, M.; Kucharska, E.; Gągor, A.; Pikul, A.; Hanuza, J. Synthesis, magnetic and vibrational properties of two novel mixed-valence iron(II)-iron(III) formate frameworks. J. Solid State Chem. 2018, 258, 163–169. [Google Scholar] [CrossRef]
- Ohkoshi, S.; Iyoda, T.; Fujishima, A.; Hashimoto, K. Magnetic properties of mixed ferro-ferrimagnets composed of Prussian blue analogs. Phys. Rev. B 1997, 56, 11642–11652. [Google Scholar] [CrossRef]
- Sun, Y.; Cong, J.Z.; Chai, Y.S.; Yan, L.Q.; Zhao, Y.L.; Wang, S.G.; Ning, W.; Zhang, Y.H. Giant exchange bias in a single-phase magnet with two magnetic sublattices. Appl. Phys. Lett. 2013, 102, 172406. [Google Scholar] [CrossRef]
- Fishman, R.S.; Reboredo, F.A. Magnetic anisotropy in the Fe(II)Fe(III) bimetallic oxalates. Phys. Rev. B 2008, 77, 144421. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, W.; Chai, Y.; Cong, J.; Shen, S.; Yan, L.; Wang, S.; Han, X.; Sun, Y. Quantum Tunneling of Magnetization in a Metal-Organic Framew. Phys. Rev. Lett. 2014, 112, 017202. [Google Scholar] [CrossRef]
- CrysAlisPro 1.171.39.7e, Empirical Absorption Correction Using Spherical Harmonics, Implemented in SCALE3 ABSPACK Scaling Algorithm; Rigaku Oxford Diffraction, 2015.
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A: Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Yao, Z.; Li, N.; Liu, F.; Zhao, J.; Bu, X. Positive and Negative Exchange Bias in N-, P- and Q-Type Ferri-Magnets of Niccolite Metal Formates [CH3NH2CH3]n[CrIII1−xFeIIIxFeII(HCO2)6]n. Magnetochemistry 2025, 11, 10. https://doi.org/10.3390/magnetochemistry11020010
Zhou Y, Yao Z, Li N, Liu F, Zhao J, Bu X. Positive and Negative Exchange Bias in N-, P- and Q-Type Ferri-Magnets of Niccolite Metal Formates [CH3NH2CH3]n[CrIII1−xFeIIIxFeII(HCO2)6]n. Magnetochemistry. 2025; 11(2):10. https://doi.org/10.3390/magnetochemistry11020010
Chicago/Turabian StyleZhou, Yu, Zhaoquan Yao, Na Li, Fuchen Liu, Jiongpeng Zhao, and Xianhe Bu. 2025. "Positive and Negative Exchange Bias in N-, P- and Q-Type Ferri-Magnets of Niccolite Metal Formates [CH3NH2CH3]n[CrIII1−xFeIIIxFeII(HCO2)6]n" Magnetochemistry 11, no. 2: 10. https://doi.org/10.3390/magnetochemistry11020010
APA StyleZhou, Y., Yao, Z., Li, N., Liu, F., Zhao, J., & Bu, X. (2025). Positive and Negative Exchange Bias in N-, P- and Q-Type Ferri-Magnets of Niccolite Metal Formates [CH3NH2CH3]n[CrIII1−xFeIIIxFeII(HCO2)6]n. Magnetochemistry, 11(2), 10. https://doi.org/10.3390/magnetochemistry11020010





