Magnetic Anisotropy and Slow Magnetic Relaxation in Two Mononuclear Octahedral Cobalt(II) Complexes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of [Co(L)(NCS)2] (1)
2.3. Synthesis of [Co(L)(NCSe)2] (2)
2.4. X-Ray Single-Crystal Structure Determination
2.5. Magnetic Measurements
3. Results and Discussion
3.1. Structural Description
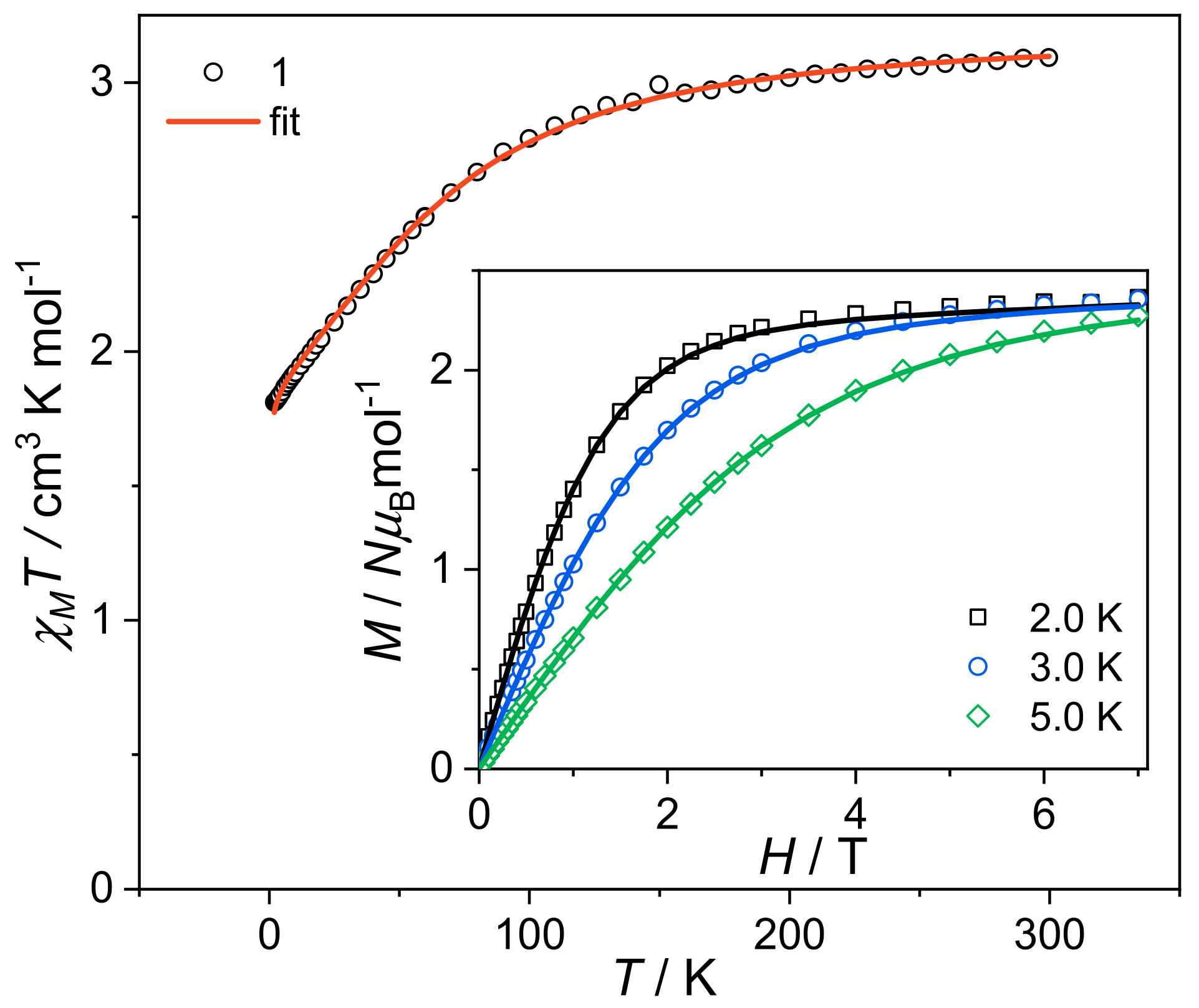
3.2. Static Magnetic Properties
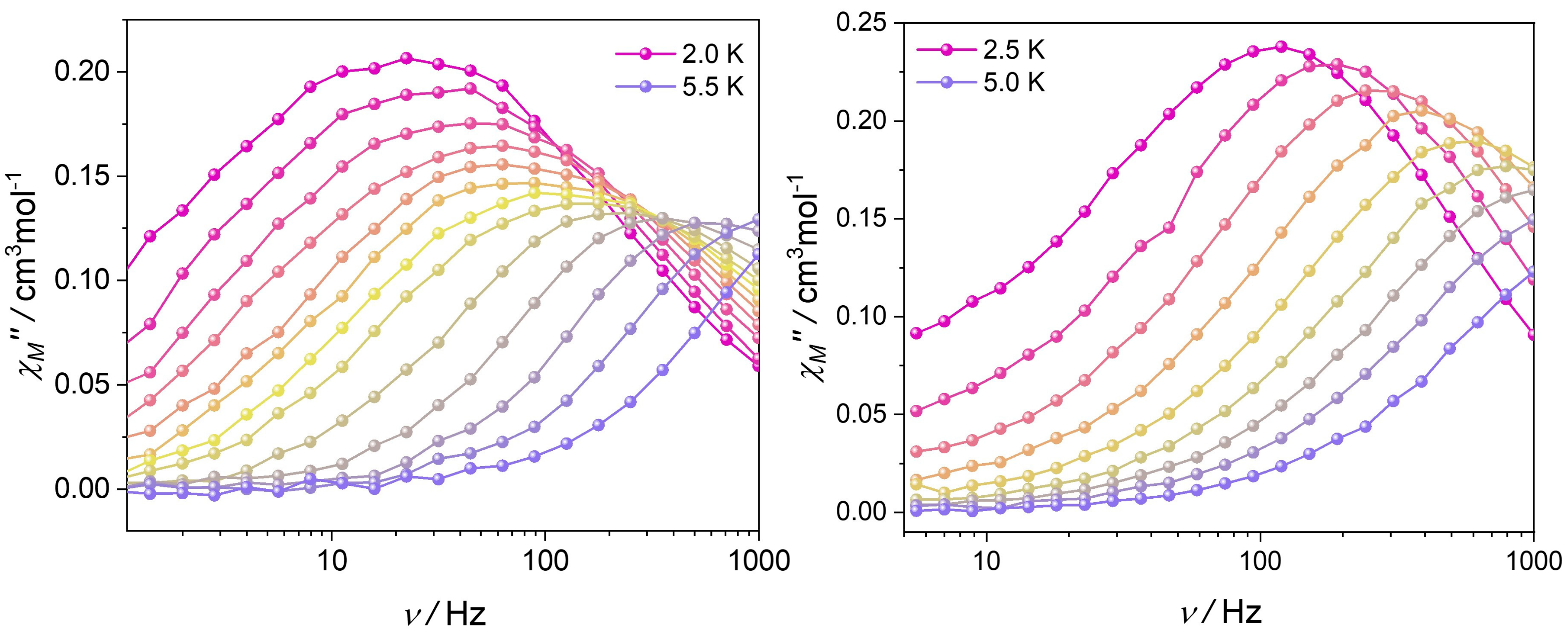
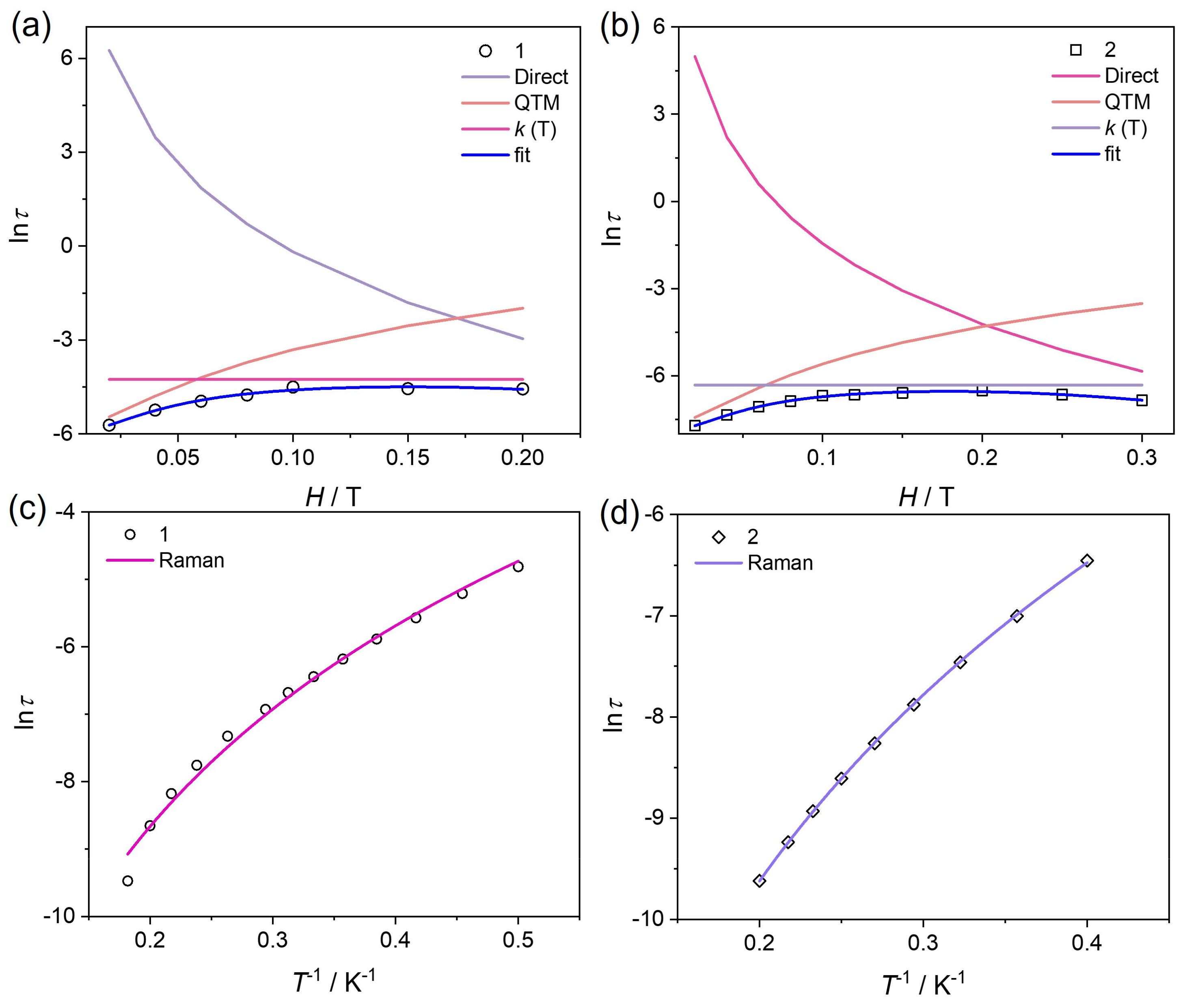
3.3. Dynamic Magnetic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gatteschi, D.; Sessoli, R.; Villain, J. Molecular Nanomagnets; Oxford University Press, Inc.: New York, NY, USA, 2006. [Google Scholar]
- Clemente-Juan, J.M.; Coronado, E.; Gaita-Arino, A. A magnetic polyoxometalates: From molecular magnetism to molecular spintronics and quantum computing. Chem. Soc. Rev. 2012, 41, 7464–7478. [Google Scholar] [CrossRef] [PubMed]
- Sanvito, S. Molecular spintronics. Chem. Soc. Rev. 2011, 40, 3336–3355. [Google Scholar] [CrossRef] [PubMed]
- Craig, G.A.; Murrie, M. 3d single-ion magnets. Chem. Soc. Rev. 2015, 44, 2135–2147. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Tong, M.-L. Single ion magnets from 3d to 5f: Developments and strategies. Chem. Eur. J. 2018, 24, 7574–7594. [Google Scholar] [CrossRef]
- Naoto, I.; Sugita, M.; Ishikawa, T.; Koshihara, A.S.; Kaizu, Y. Lanthanide double-decker complexes functioning as magnets at the single-molecular level. J. Am. Chem. Soc. 2003, 125, 8694–8695. [Google Scholar]
- Vallejo, J.; Pascual-Álvarez, A.; Cano, J.; Castro, I.; Julve, M.; Lloret, F.; Krzystek, J.; De Munno, G.; Armentano, D.; Wernsdorfer, W.; et al. Field-induced hysteresis and quantum tunneling of the magnetization in a mononuclear manganese(III) complex. Angew. Chem. Int. Ed. 2013, 52, 14075–14079. [Google Scholar] [CrossRef]
- Zadrozny, J.M.; Atanasov, M.; Bryan, A.M.; Lin, C.-Y.; Rekken, B.D.; Power, P.P.; Neese, F.; Long, J.R. Slow magnetization dynamics in a series of two-coordinate iron(ii) complexes. Chem. Sci. 2013, 4, 125–130. [Google Scholar] [CrossRef]
- Bunting, P.C.; Atanasov, M.; Damgaard-Moller, E.; Perfetti, M.; Crassee, I.; Orlita, M.; Overgaard, J.; van Slageren, J.; Neese, F.; Long, J.R. A linear cobalt(II) complex with maximal orbital angular momentum from a non-Aufbau ground state. Science 2018, 362, eaat7319. [Google Scholar] [CrossRef]
- Rechkemmer, Y.; Breitgoff, F.D.; van der Meer, M.; Atanasov, M.; Hakl, M.; Orlita, M.; Neugebauer, P.; Neese, F.; Sarkar, B.; van Slageren, J. A fourcoordinate cobalt(II) single-ion magnet with coercivity and a very high energy barrier. Nat. Commun. 2016, 7, 10467. [Google Scholar] [CrossRef]
- Cui, H.-H.; Lu, F.; Chen, X.-T.; Zhang, Y.-Q.; Tong, W.; Xue, Z.-L. Zero-field slow magnetic relaxation and hysteresis loop in four-coordinate CoII single-ion magnets with strong easy-axis anisotropy. Inorg. Chem. 2019, 58, 12555–12564. [Google Scholar] [CrossRef]
- Gupta, S.K.; Rao, S.V.; Demeshko, S.; Dechert, S.; Bill, E.; Atanasov, M.; Neese, F.; Meyer, F. Air-stable four-coordinate cobalt(II) single-ion magnets: Experimental and ab initio ligand field analyses of correlations between dihedral angles and magnetic anisotropy. Chem. Sci. 2023, 14, 6355–6374. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.S.; Malta, J.F.; Bandeira, N.A.G.; Allgaier, A.; van Slageren, J.; Paixão, J.A.; Almeida, M.; Pereira, L.C.J.; Gomes, P.T. Enhancing SIM behaviour in a mononuclear tetrahedral [Co(N,N’-2-iminopyrrolyl)2] complex. Chem. Commun. 2022, 58, 9682–9685. [Google Scholar] [CrossRef]
- Cui, H.-H.; Xu, H.; Zhang, T.; Chen, Q.; Luo, S.; Wang, M.; Wang, J.; Chen, L.; Zhang, M.; Tang, Y. Magnetic anisotropy and relaxation in four-coordinate cobalt(II) single-ion magnets with a [CoIIO4] Core. Inorg. Chem. 2024, 63, 9050–9057. [Google Scholar] [CrossRef]
- Poulten, R.C.; Page, M.J.; Algarra, A.G.; Le Roy, J.J.; López, I.; Carter, E.; Llobet, A.; Macgregor, S.A.; Mahon, M.F.; Murphy, D.M.; et al. Synthesis, electronic structure, and magnetism of [Ni(6-Mes)2]+: A two-coordinate nickel(I) complex stabilized by bulky N-heterocyclic carbenes. J. Am. Chem. Soc. 2013, 135, 13640–13643. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.K.; Kharel, R.; Shome, S.; Goswami, S.; Konar, S. Understanding the unceasing evolution of Co(II) based single-ion magnets. Coord. Chem. Rev. 2023, 475, 214871. [Google Scholar]
- Sarkar, A.; Dey, S.; Rajaraman, G. Role of coordination number and geometry in controlling the magnetic anisotropy in FeII, CoII, and NiII single-ion magnets. Chem. Eur. J. 2020, 26, 14036–14058. [Google Scholar] [CrossRef]
- Tripathi, S.; Dey, A.; Shanmugam, M.; Narayanan, R.S.; Chandrasekhar, V. Cobalt(II) Complexes as Single-Ion Magnets in Organometallic Magnets; Chandrasekhar, V., Pointillart, F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 35–75. [Google Scholar] [CrossRef]
- Juráková, J.; Šalitroš, I. Co(II) single-ion magnets: Synthesis, structure, and magnetic properties. Monatsh. Chem. 2022, 153, 1001–1036. [Google Scholar] [CrossRef]
- Vallejo, J.; Castro, I.; Ruiz García, R.; Cano, J.; Julve, M.; Lloret, F.; De Munno, G.; Wernsdorfer, W.; Pardo, E. Field-induced slow magnetic relaxation in a six-coordinate mononuclear cobalt(II) complex with a positive anisotropy. J. Am. Chem. Soc. 2012, 134, 15704–15707. [Google Scholar] [CrossRef]
- Gómez-Coca, S.; Urtizberea, A.; Cremades, E.; Alonso, P.J.; Camón, A.; Ruiz, E.; Luis, E. Origin of slow magnetic relaxation in kramers ions with non-uniaxial anisotropy. Nat. Commun. 2014, 5, 4300. [Google Scholar] [CrossRef]
- Novikov, V.V.; Pavlov, A.A.; Nelyubina, Y.V.; Boulon, M.E.; Varzatskii, O.A.; Voloshin, Y.Z.; Winpenny, R.E.P. A trigonal prismatic mononuclear cobalt(II) complex showing single-molecule magnet behavior. J. Am. Chem. Soc. 2015, 137, 9792–9795. [Google Scholar] [CrossRef]
- Zhu, Y.-Y.; Zhu, M.-S.; Yin, T.-T.; Meng, Y.-S.; Wu, Z.-Q.; Zhang, Y.-Q.; Gao, S. Cobalt(II) coordination polymer exhibiting single-ion-magnet-type field-induced slow relaxation behavior. Inorg. Chem. 2015, 54, 3716–3718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, J.; Yang, L.; Zhang, Y.-Q.; Song, Y. Magnetic anisotropy from trigonal prismatic to trigonal antiprismatic Co(II) complexes: Experimental observation and theoretical prediction. Inorg. Chem. 2018, 57, 3903–3912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Z.; Gómez-Coca, S.; Brown, A.J.; Saber, M.R.; Zhang, X.; Dunbar, K.R. Trigonal antiprismatic Co(II) single molecule magnets with large uniaxial anisotropies: Importance of Raman and tunneling mechanisms. Chem. Sci. 2016, 7, 6519–6527. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Song, J.; Yuan, A.; Wang, Z.; Chen, L.; Ouyang, Z.-W. Slow magnetic relaxation in two octahedral cobalt(II) complexes with positive axial anisotropy. Inorg. Chim. Acta. 2018, 479, 113–119. [Google Scholar] [CrossRef]
- SMART & SAINT Software Reference Manuals, version 6.45; Bruker Analytical X-ray Systems, Inc.: Madison, WI, USA, 2003.
- SAINT, Program for Data Extraction and Reduction; Siemens Analytical X-ray Instruments: Madison, WI, USA, 1996.
- Sheldrick, G.M. SADABS: Software for Empirical Absorption. Correction, version 2.05; University of Göttingen: Göttingen, Germany, 2002.
- Sheldrick, G.M. SHELXL14: Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Bain, G.A.; Berry, J.F. Diamagnetic corrections and Pascal’s constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE, version 2.1; Universitat de Barcelona: Barcelona, Spain, 2013.
- Alvarez, S.; Alemany, P.; Casanova, D.; Cirera, J.; Llunell, M.; Avnir, D. Shape maps and polyhedral interconversion paths in transition metal chemistry. Coord. Chem. Rev. 2005, 249, 1693–1708. [Google Scholar] [CrossRef]
- Kurdziel, K.; Głowiak, T. X-ray and spectroscopic characterization of octahedral cobalt(II) and nickel(II) complexes with 1-(prop-2-en-1-yl)-1H-imidazole in the solid state and electron-donor properties of the latter in aqueous solution. Polyhedron 2000, 19, 2183–2188. [Google Scholar] [CrossRef]
- Maslejova, A.; Uhrinova, S.; Mroziriski, J.; Zurowska, B.; Munoz, M.C.; Julve, M. Study of the mutual influence of ligands in cobalt(II) complexes containing thiocyanate and imidazole derivatives. Inorg. Chimi. Acta 1997, 255, 343–349. [Google Scholar] [CrossRef]
- Osipov, N.G.; Faraonov, M.A.; Yakushev, I.A.; Denisov, N.N.; Otsuka, A.; Kitagawa, H.; Konarev, D.V. Slow magnetic relaxation in a complex of photochromic spiropyran in a merocyanine form and cobalt(II) hexafluoroacetylacetonate. Dalton Trans. 2024, 53, 3159–3166. [Google Scholar] [CrossRef]
- Hu, Z.-B.; Jing, Z.-Y.; Li, M.-M.; Yin, L.; Gao, Y.-D.; Yu, F.; Hu, T.-P.; Wang, Z.; Song, Y. Important role of intermolecular interaction in cobalt(II) single-ion magnet from single slow relaxation to double slow relaxation. Inorg. Chem. 2018, 57, 10761–10767. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Cui, H.-H.; Zhang, Y.-Q.; Wang, Z.; Ouyang, Z.-W.; Chen, L.; Yan, H.; Xue, Z.-L. Magnetic anisotropy and relaxation behavior of six-coordinate tris(pivalato)-Co(II) and -Ni(II) complexes. Dalton Trans. 2018, 47, 10162–10171. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.P.S.; Bowling, G.; Ariciu, A.-M.; Jailani, N.F.M.; Chilton, N.F.; Waddell, P.G.; Collison, D.; Tuna, F.; Higham, L.J. Evidence of slow magnetic relaxation in Co(AcO)2(py)2(H2O)2. Magnetochemistry 2016, 2, 23. [Google Scholar] [CrossRef]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Cole, K.S.; Cole, R.H. Dispersion and absorption in dielectrics I. Alternating current characteristics. J. Chem. Phys. 1941, 9, 341–351. [Google Scholar] [CrossRef]
- Smolko, L.; Černák, J.; Dušek, M.; Titiš, J.; Boča, R. Tetracoordinate Co(II) complexes containing bathocuproine and single molecule magnetism. New J. Chem. 2016, 40, 6593–6598. [Google Scholar] [CrossRef]
- Smolko, L.; Černák, J.; Kuchár, J.; Rajnák, C.; Titiš, J.; Boča, R. Field-induced slow magnetic relaxation in mononuclear tetracoordinate cobalt(II) complexes containing a neocuproine ligand. Eur. J. Inorg. Chem. 2017, 2017, 3080–3086. [Google Scholar] [CrossRef]
- Hu, Z.-B.; Gui, L.-A.; Li, L.-H.; Xiao, T.-T.; Hand, A.-T.; Tin, P.; Ozerov, M.; Peng, Y.; Ouyang, Z.; Wang, Z.; et al. CoII single-ion magnet and its multi-dimensional aggregations: Influence of the structural rigidity on magnetic relaxation process. Chinese Chem. Lett. 2025, 36, 109600. [Google Scholar] [CrossRef]
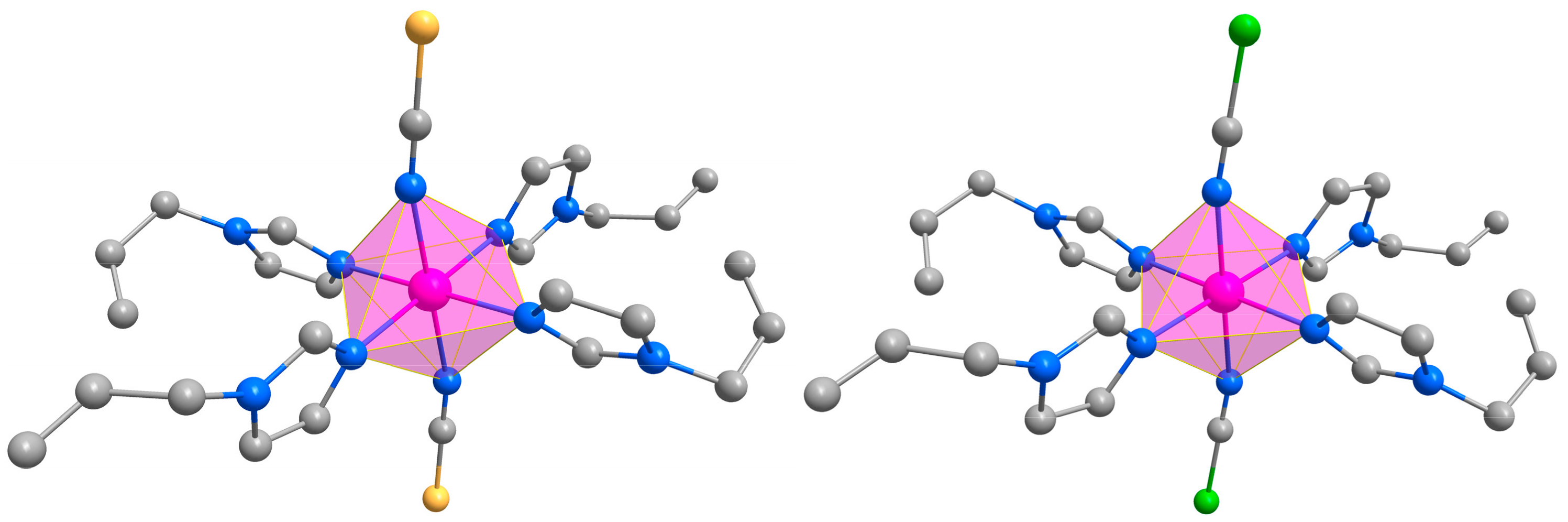
| Compound | 1 | 2 |
|---|---|---|
| Molecular formular | C26H32CoN10S2 | C26H32CoN10Se2 |
| M (g mol−1) | 607.66 | 701.46 |
| Temperature (K) | 296 | 296 |
| Crystal system | Triclinic | Triclinic |
| Space group | P-1 | P-1 |
| a/Å | 8.875 (4) | 8.9168 (16) |
| b/Å | 9.620 (4) | 9.7118 (17) |
| c/Å | 10.632 (4) | 10.6394 (19) |
| α (°) | 69.923 (7) | 107.091 (3) |
| β (°) | 65.435 (6) | 113.791 (3) |
| γ (°) | 86.636 (6) | 94.205 (3) |
| V/Å3 | 771.6 (6) | 786.2 (2) |
| Z | 1 | 1 |
| ρcalc, g/cm3 | 1.308 | 1.482 |
| μ/mm−1 | 0.724 | 2.893 |
| F (000) | 317.0 | 353.0 |
| θ range [°] | 4.5/55.176 | 6.47/55.372 |
| Reflns collected | 4743 | 4896 |
| Rint | 0.0523 | 0.1411 |
| Indep. reflns | 3388 | 3508 |
| Data/restr./paras | 3388/0/186 | 3508/0/178 |
| Goodness-of-fit on F2 | 1.216 | 1.057 |
| R1, wR2 [I > 2σ (I)] a | 0.0410/0.1155 | 0.0676/0.1698 |
| R1, wR2 [all data] a | 0.0459/0.1277 | 0.0776/0.1794 |
| 1 | 2 | ||
|---|---|---|---|
| Co1–N21 | 2.1656 (18) | Co1–N21 | 2.163 (3) |
| Co1–N2 | 2.1656 (18) | Co1–N2 | 2.163 (3) |
| Co1–N4 | 2.140 (2) | Co1–N4 | 2.131 (3) |
| Co1–N41 | 2.1401 (19) | Co1–N41 | 2.131 (3) |
| Co1–N3 | 2.163 (2) | Co1–N3 | 2.149 (3) |
| Co1–N31 | 2.163 (2) | Co1–N31 | 2.149 (3) |
| N21–Co1–N2 | 180.0 | N21–Co1–N2 | 180.00 (12) |
| N4–Co1–N2 | 87.23 (7) | N4–Co1–N2 | 92.70 (11) |
| N41–Co1–N2 | 92.77 (7) | N41–Co1–N2 | 87.30 (11) |
| N2–Co1–N3 | 91.29 (8) | N2–Co1–N3 | 88.81 (12) |
| N4–Co1–N41 | 180.0 | N4–Co1–N41 | 180.0 |
| N31–Co1–N3 | 180.0 | N31–Co1–N3 | 180.0 |
| Fittings of the dc Magnetic Data with Equation (1) | |||||
|---|---|---|---|---|---|
| D (cm−1) | |E| (cm−1) | gx,y | gz | zj (cm−3) | |
| 1 | +70.4(7) | 0 (1) | 2.53 (1) | 2.74 (1) | −0.013 (2) |
| 2 | +64.0(2) | 0 (8) | 2.60 (1) | 2.66 (1) | / |
| A (s−1 T−4 K−1) | B1 (s−1) | B2 (T−2) | k (T) (s−1) | |
|---|---|---|---|---|
| 1 | 6016.3 | 340.6 | 1145.8 | 70.7 |
| 2 | 21,346.5 | 2167.2 | 707.4 | 554.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, H.-H.; Wang, D.-Z.; Li, S.; Wang, L.; Yu, X.; Liu, X.; Wang, J.; Wang, M.; Tang, Y. Magnetic Anisotropy and Slow Magnetic Relaxation in Two Mononuclear Octahedral Cobalt(II) Complexes. Magnetochemistry 2025, 11, 11. https://doi.org/10.3390/magnetochemistry11020011
Cui H-H, Wang D-Z, Li S, Wang L, Yu X, Liu X, Wang J, Wang M, Tang Y. Magnetic Anisotropy and Slow Magnetic Relaxation in Two Mononuclear Octahedral Cobalt(II) Complexes. Magnetochemistry. 2025; 11(2):11. https://doi.org/10.3390/magnetochemistry11020011
Chicago/Turabian StyleCui, Hui-Hui, Dou-Zun Wang, Shixiang Li, Leixin Wang, Xinrui Yu, Xiancong Liu, Jin Wang, Miao Wang, and Yanfeng Tang. 2025. "Magnetic Anisotropy and Slow Magnetic Relaxation in Two Mononuclear Octahedral Cobalt(II) Complexes" Magnetochemistry 11, no. 2: 11. https://doi.org/10.3390/magnetochemistry11020011
APA StyleCui, H.-H., Wang, D.-Z., Li, S., Wang, L., Yu, X., Liu, X., Wang, J., Wang, M., & Tang, Y. (2025). Magnetic Anisotropy and Slow Magnetic Relaxation in Two Mononuclear Octahedral Cobalt(II) Complexes. Magnetochemistry, 11(2), 11. https://doi.org/10.3390/magnetochemistry11020011





