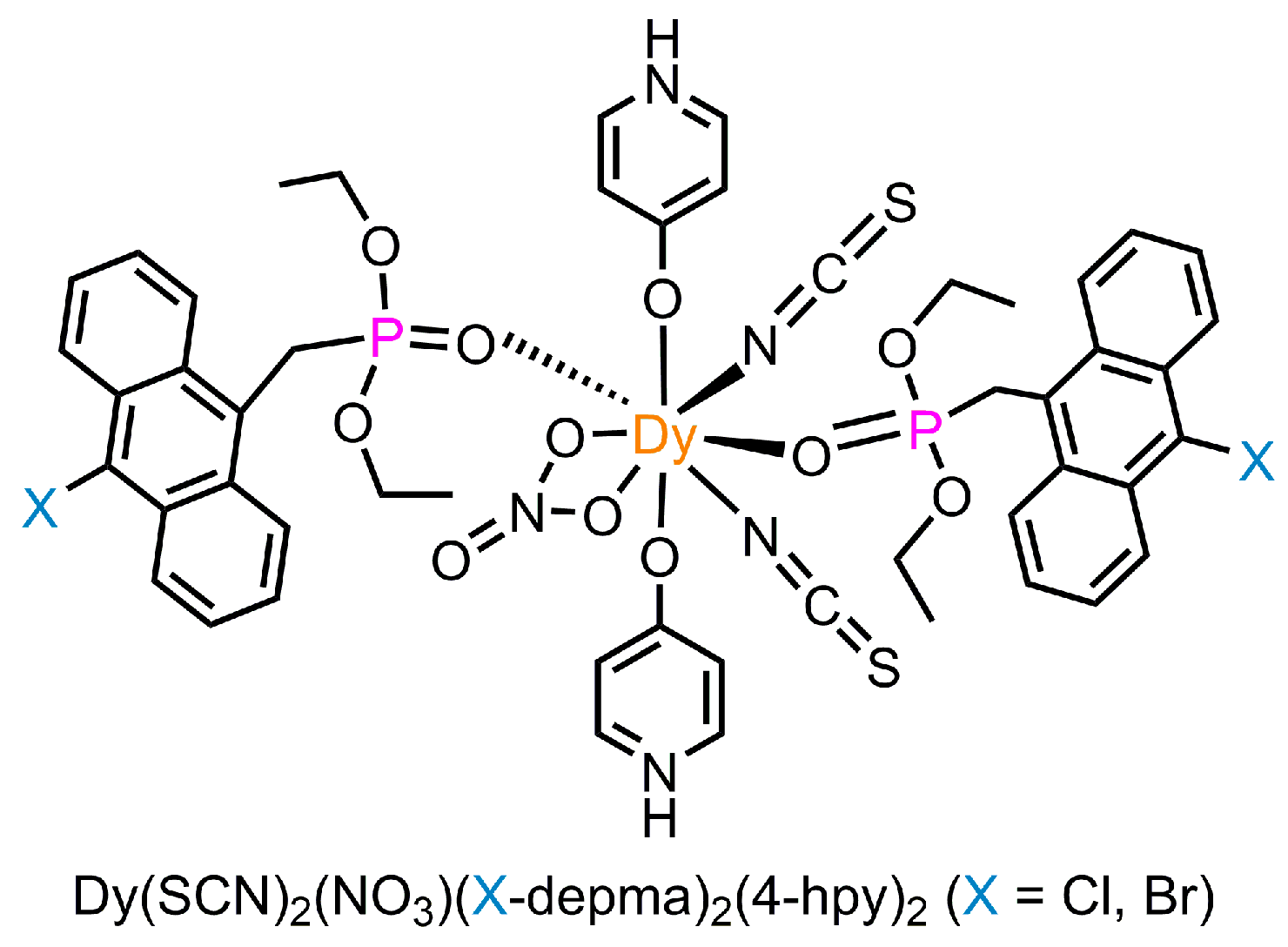
Dysprosium Complexes Incorporating Halogen-Substituted Anthracene: Piezochromism and Single-Molecule Magnet Properties
Abstract
1. Introduction
2. Experimental Section
2.1. General Information
2.2. Synthesis of Dy(SCN)2(NO3)(Cl-depma)2(4-hpy)2 (Dy-Cl)
2.3. Synthesis of Dy(SCN)2(NO3)(Br-depma)2(4-hpy)2 (Dy-Br)
2.4. Single-Crystal X-Ray Crystallography
2.5. High-Pressure Magnetic Measurement
3. Results and Discussion
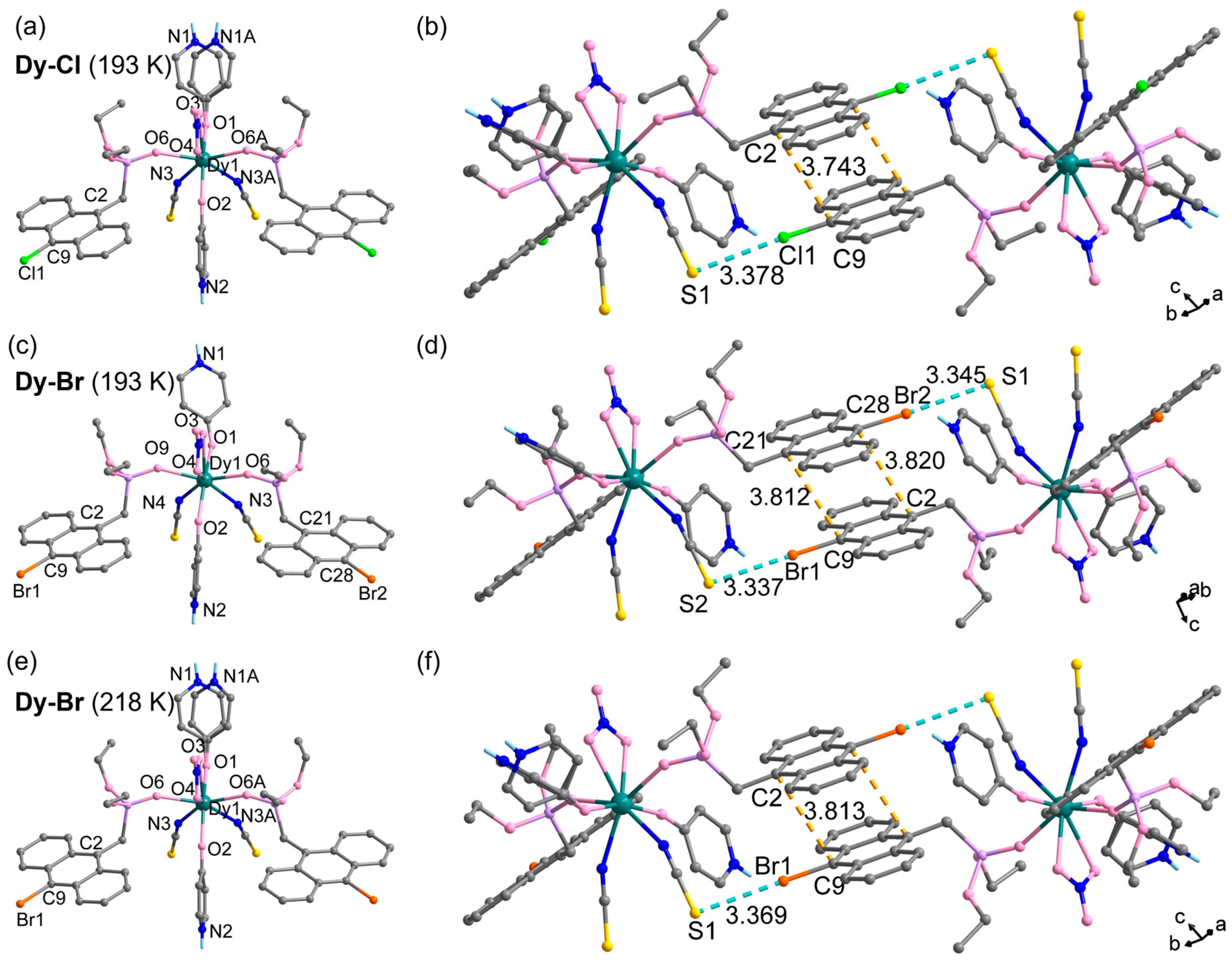
3.1. Crystal Structure
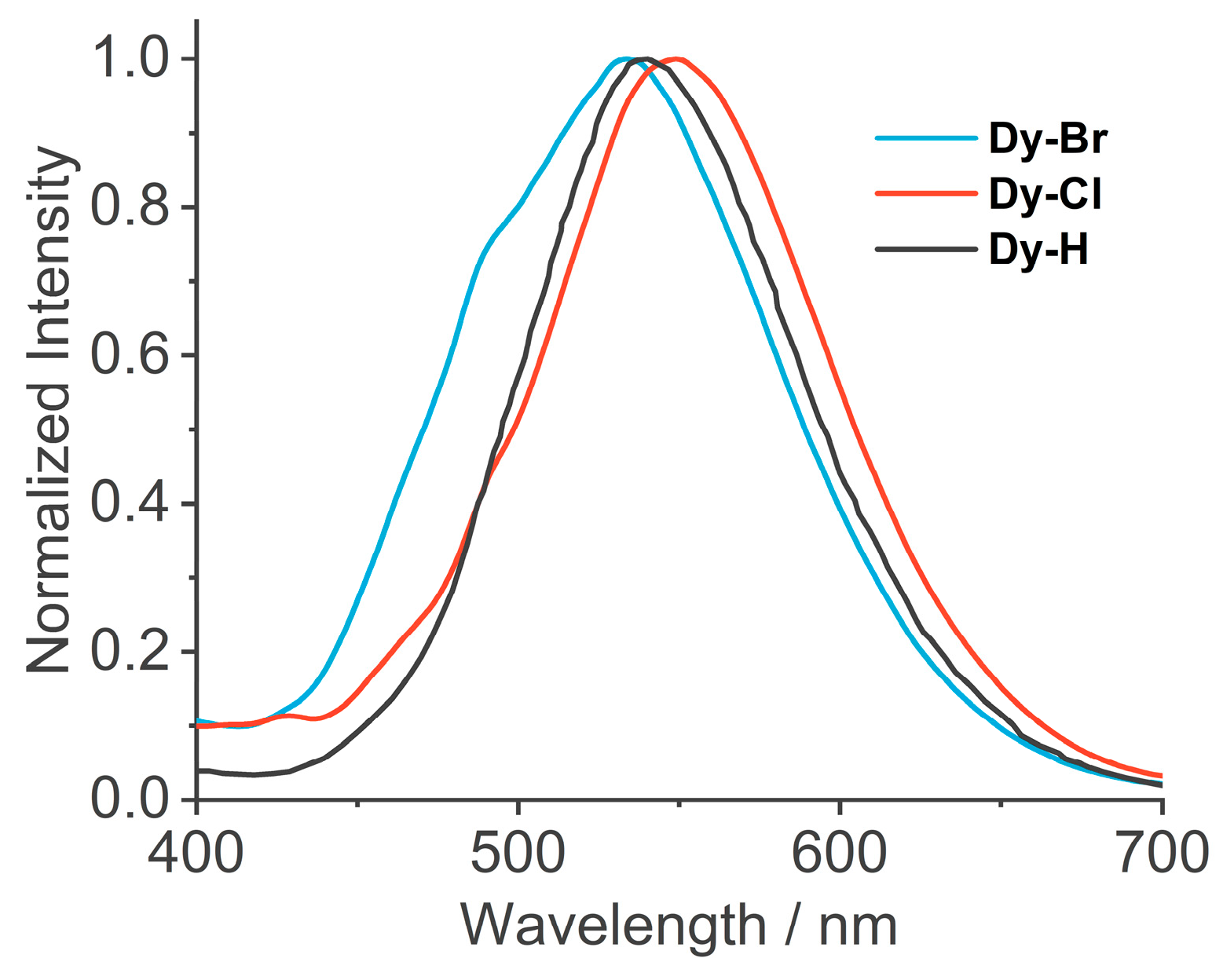
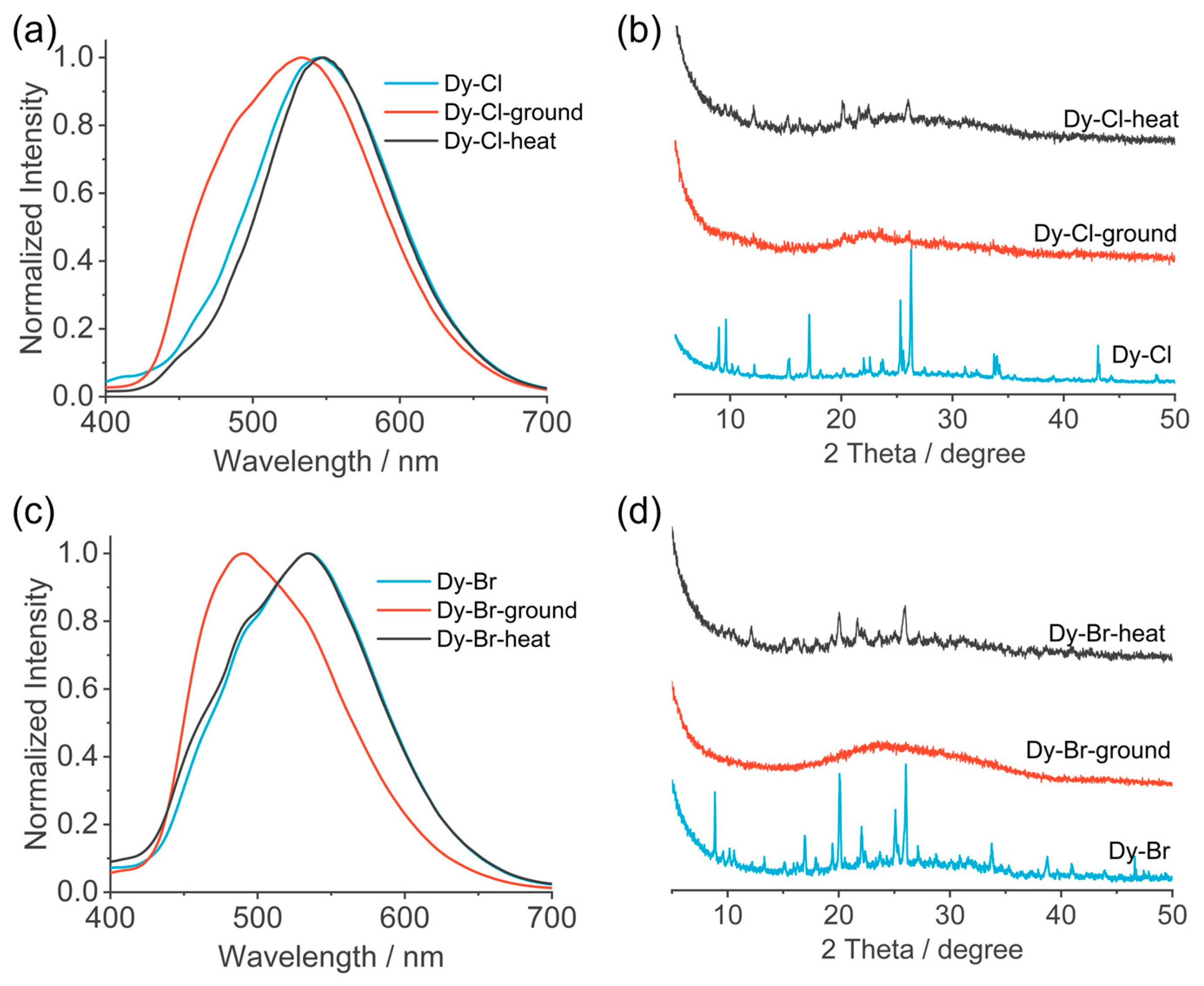
3.2. Photophysical Properties and External Stimulus Effects
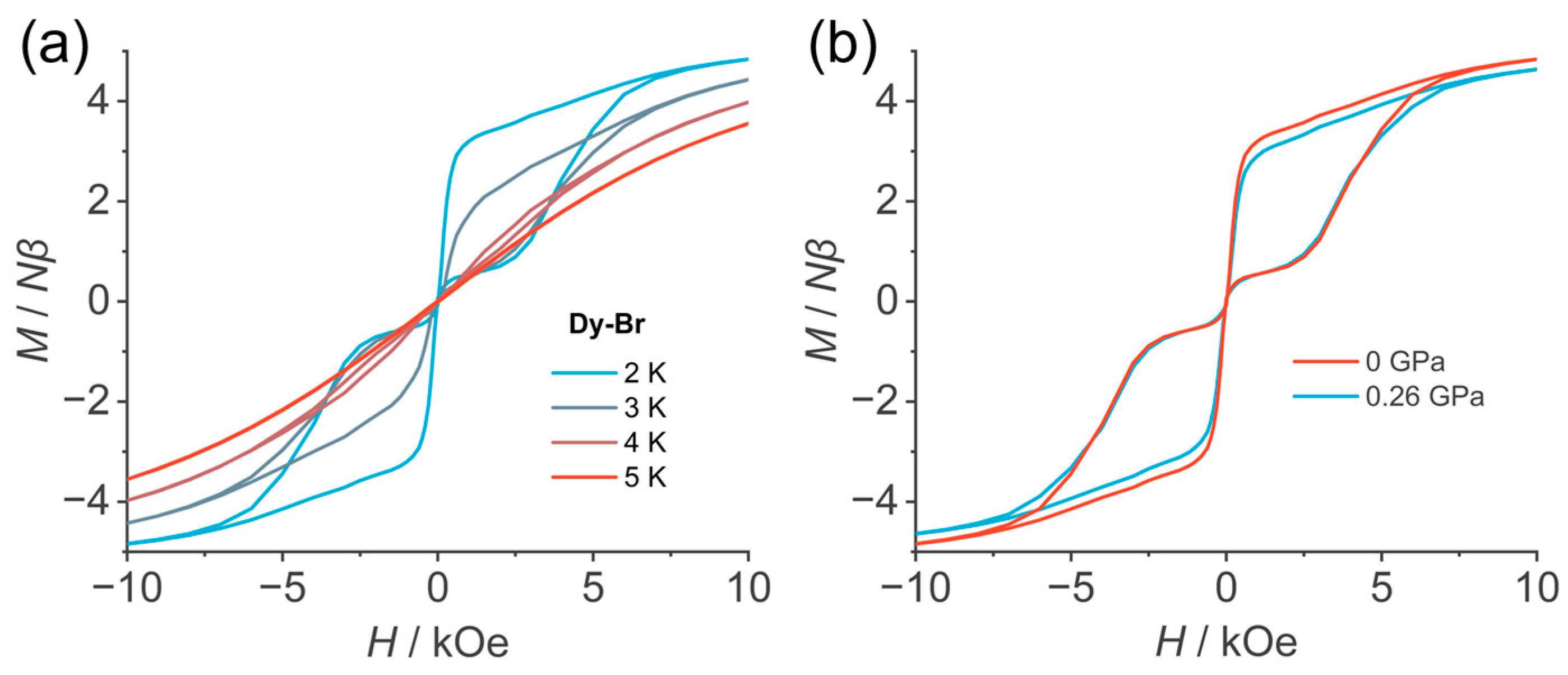
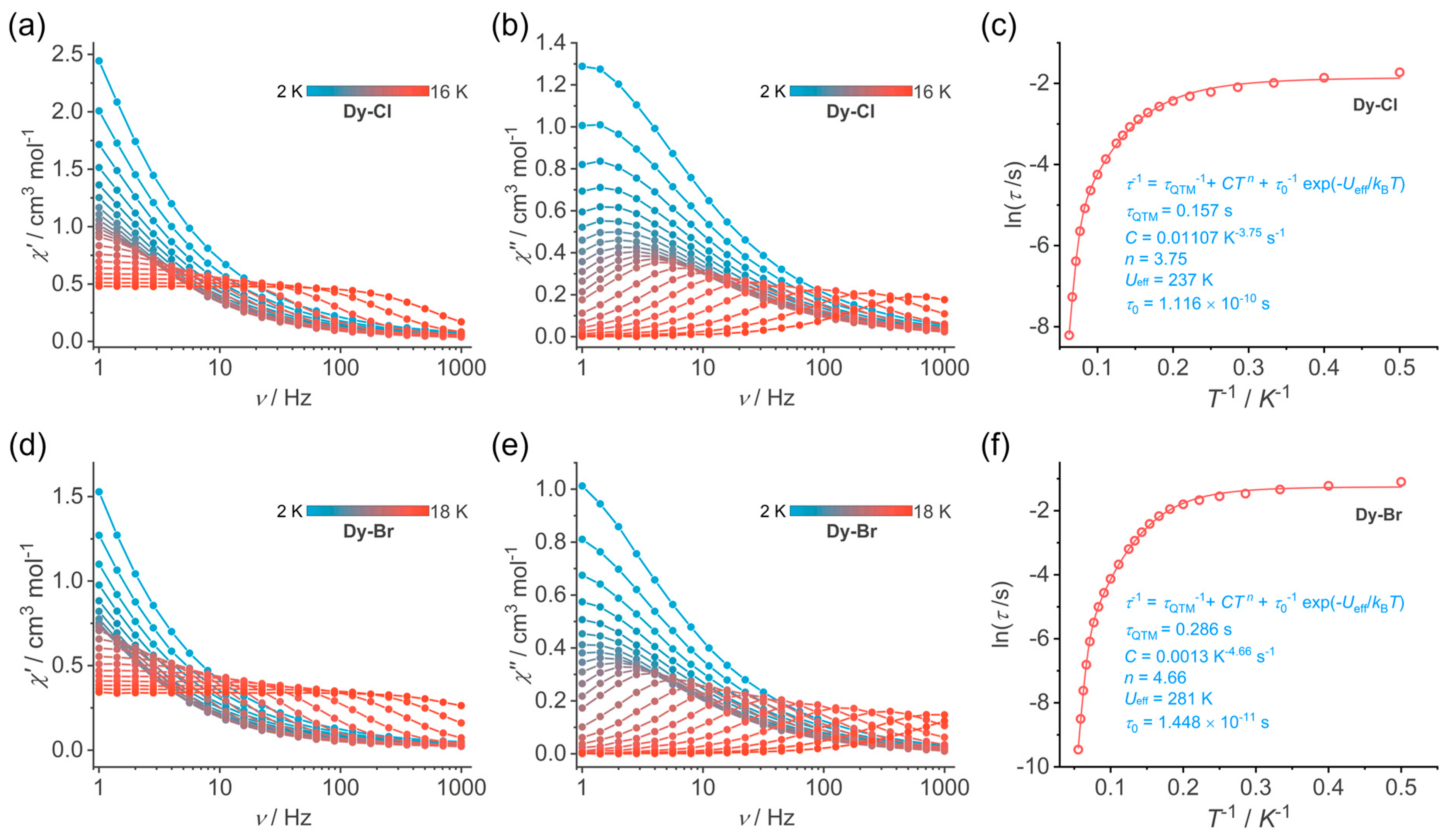
3.3. Magnetic Properties
3.4. Effect of High Pressure on the Magnetic Properties of Dy-Br
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corredoira-Vázquez, J.; González-Barreira, C.; Fondo, M.; García-Deibe, A.M.; Sanmartín-Matalobos, J.; Gómez-Coca, S.; Ruiz, E.; Brites, C.D.S.; Carlos, L.D. An Air-Stable High-Performance Single-Molecule Magnet Operating as a Luminescent Thermometer below Its Blocking Temperature. Inorg. Chem. Front. 2025, 12, 5506–5516. [Google Scholar] [CrossRef]
- Bispo, A.G., Jr.; Yeh, L.; Errulat, D.; Gálico, D.A.; Sigoli, F.A.; Murugesu, M. Improving the Performance of β-Diketonate-Based DyIII Single-Molecule Magnets Displaying Luminescence Thermometry. Chem. Commun. 2023, 59, 8723–8726. [Google Scholar] [CrossRef]
- Zanella, S.; Aragon-Alberti, M.; Brite, C.D.S.; Salles, F.; Carlos, L.D.; Long, J. Luminescent Single-Molecule Magnets as Dual Magneto-Optical Molecular Thermometers. Angew. Chem. Int. Ed. 2023, 62, e202306970. [Google Scholar] [CrossRef]
- Dhbaibi, K.; Grasser, M.; Douib, H.; Dorcet, V.; Cador, O.; Vanthuyne, N.; Riobé, F.; Maury, O.; Guy, S.; Bensalah-Ledoux, A.; et al. Multifunctional Helicene-Based Ytterbium Coordination Polymer Displaying Circularly Polarized Luminescence, Slow Magnetic Relaxation and Room Temperature Magneto-Chiral Dichroism. Angew. Chem. Int. Ed. 2023, 62, e202215558. [Google Scholar] [CrossRef]
- Zhang, Q.; Han, S.-D.; Li, Q.; Hu, J.-X.; Wang, G.-M. Electron Transfer Photochromism of Ln-Based (Ln = Dy, Tb) Coordinated Polymers for Reversibly Switching off/on Single-Molecule Magnetic Behavior. Sci. China Mater. 2022, 65, 788–794. [Google Scholar] [CrossRef]
- Wang, J.; Zakrzewski, J.J.; Zychowicz, M.; Xin, Y.; Tokoro, H.; Chorazy, S.; Ohkoshi, S. Desolvation-Induced Highly Symmetrical Terbium(III) Single-Molecule Magnet Exhibiting Luminescent Self-Monitoring of Temperature. Angew. Chem. Int. Ed. 2023, 62, e202306372. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.-Y.; Liu, Y.; Ruan, Z.-Y.; Wang, H.-L.; Shi, C.-G.; Deng, W.; Wu, S.-G.; Jia, J.-H.; Tong, M.-L. Magnetic and Luminescent Dual Responses of Photochromic Hexaazamacrocyclic Lanthanide Complexes. Inorg. Chem. 2023, 62, 1075–1085. [Google Scholar] [CrossRef]
- Münzfeld, L.; Dahlen, M.; Hauser, A.; Mahieu, N.; Kuppusamy, S.K.; Moutet, J.; Tricoire, M.; Köppe, R.; La Droitte, L.; Cador, O.; et al. Molecular Lanthanide Switches for Magnetism and Photoluminescence. Angew. Chem. Int. Ed. 2023, 62, e202218107. [Google Scholar] [CrossRef] [PubMed]
- Cador, O.; Le Guennic, B.; Pointillart, F. Electro-Activity and Magnetic Switching in Lanthanide-Based Single-Molecule Magnets. Inorg. Chem. Front. 2019, 6, 3398–3417. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, X.-L.; Liu, S.; Tang, J. External Stimuli Modulate the Magnetic Relaxation of Lanthanide Single-Molecule Magnets. Inorg. Chem. Front. 2020, 7, 3315–3326. [Google Scholar] [CrossRef]
- Zakrzewski, J.J.; Liberka, M.; Wang, J.; Chorazy, S.; Ohkoshi, S. Optical Phenomena in Molecule-Based Magnetic Materials. Chem. Rev. 2024, 124, 5930–6050. [Google Scholar] [CrossRef]
- Bispo, A.G., Jr. Multifunctional Lanthanide(III) Single-Molecule Magnets Displaying Luminescence Thermometry: Progress, Challenges, and Future Directions. Coord. Chem. Rev. 2025, 537, 216685. [Google Scholar] [CrossRef]
- Huang, X.-D.; Xu, Y.; Fan, K.; Bao, S.; Kurmoo, M.; Zheng, L. Reversible SC-SC Transformation Involving [4+4] Cycloaddition of Anthracene: A Single-Ion to Single-Molecule Magnet and Yellow-Green to Blue-White Emission. Angew. Chem. Int. Ed. 2018, 57, 8577–8581. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-D.; Ma, X.; Zheng, L. Photo-responsive Single-Molecule Magnet Showing 0D to 1D Single-Crystal-to-Single-Crystal Structural Transition and Hysteresis Modulation. Angew. Chem. Int. Ed. 2023, 62, e202300088. [Google Scholar] [CrossRef]
- Ma, X.-F.; Hou, X.-L.; Qin, Y.-H.; Teng, Q.; Bao, S.-S.; Tian, Y.-X.; Zheng, L.-M. A Thermally and Photoresponsive Luminescent Single-Molecule Magnet Based on Dysprosium–Anthracene: Effect of Temperature on Anthracene Photocycloaddition. Chem. Sci. 2025, 16, 10340–10348. [Google Scholar] [CrossRef]
- Huang, X.-D.; Ma, X.-F.; Shang, T.; Zhang, Y.-Q.; Zheng, L.-M. Photocontrollable Magnetism and Photoluminescence in a Binuclear Dysprosium Anthracene Complex. Inorg. Chem. 2023, 62, 1864–1874. [Google Scholar] [CrossRef]
- Ma, X.-F.; Huang, X.-D.; Zheng, L.-M. Tuning the Single-Molecule Magnet and Photoluminescence Properties of Binuclear Dysprosium Complexes by Light. Cryst. Growth Des. 2023, 23, 1095–1103. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Yan, S.-K.; Liang, S.; Zhao, Y.-R.; Zhang, J.; Wang, G.-M.; Hu, J.-X. Synergistic Photomagnetic and Photomechanical Dynamics in a Dysprosium-Based Smart Molecule. Adv. Sci. 2025, 12, e09088. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Gao, Z.-N.; Liang, S.; Wei, W.-J.; Han, S.-D.; Zhang, Y.-Q.; Hu, J.-X.; Wang, G.-M. Synergism of Light-Induced [4 + 4] Cycloaddition and Electron Transfer Toward Switchable Photoluminescence and Single-Molecule Magnet Behavior in a Dy4 Cubane. Research 2024, 7, 0411. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.-H.; Ma, X.-F.; Hou, X.; Huang, X.-D.; Bao, S.-S.; Tian, Y.; Zhang, Y.-Q.; Zheng, L.-M. Photoresponsive luminescent single-molecule magnets based on dysprosium-anthracene complexes: Regulating the de-dimerization temperature of the photocycloaddition product by co-ligand. Chem. Sci. 2025, 16, 19806–19819. [Google Scholar] [CrossRef]
- Huang, X.-D.; Hong, B.-K.; Wen, G.-H.; Li, S.-H.; Zheng, L.-M. Photo-Controllable Heterostructured Crystals of Metal–Organic Frameworks via Reversible Photocycloaddition. Chem. Sci. 2023, 14, 1852–1860. [Google Scholar] [CrossRef]
- Gao, R.; Zou, Q.; Su, Q.-Q.; Ma, X.-F.; Qin, Y.-H.; Liao, R.; Bao, S.-S.; Zheng, L.-M. Photoresponsive Lanthanide-Dianthracene Framework: Introduction of Photoactive Anthracene Pairs by Controlling the Synthesis Temperature. Chin. Chem. Lett. 2024, 36, 110404. [Google Scholar] [CrossRef]
- Abou-Hatab, S.; Spata, V.A.; Matsika, S. Substituent Effects on the Absorption and Fluorescence Properties of Anthracene. J. Phys. Chem. A 2017, 121, 1213–1222. [Google Scholar] [CrossRef]
- Schillmöller, T.; Ruth, P.N.; Herbst-Irmer, R.; Stalke, D. Three Colour Solid-State Luminescence from Positional Isomers of Facilely Modified Thiophosphoranyl Anthracenes. Chem. Commun. 2020, 56, 7479–7482. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, H.; Geng, T.; Duan, R.; Li, X.; Liu, Y.; Liu, W.; He, B.-G.; Sui, L.; Wang, K.; et al. Piezochromic Fluorescence of Anthracene Derivative Crystals with Different Stacking Patterns Designed around Excimers. J. Mater. Chem. C 2023, 11, 4892–4898. [Google Scholar] [CrossRef]
- Zhu, L.; Al-Kaysi, R.O.; Dillon, R.J.; Tham, F.S.; Bardeen, C.J. Crystal Structures and Photophysical Properties of 9-Anthracene Carboxylic Acid Derivatives for Photomechanical Applications. Cryst. Growth Des. 2011, 11, 4975–4983. [Google Scholar] [CrossRef]
- Shu, Y.; Sun, J.; Yue, Y.; Ye, K.; Lu, R. Visible-Light-Triggered Actuators Based on the Molecular Crystals of Anthracenecarbonitrile Undergoing Reversible [4+4] Cycloaddition. ChemPhotoChem 2022, 6, e202200006. [Google Scholar] [CrossRef]
- Gemen, J.; Białek, M.J.; Kazes, M.; Shimon, L.J.W.; Feller, M.; Semenov, S.N.; Diskin-Posner, Y.; Oron, D.; Klajn, R. Ternary Host-Guest Complexes with Rapid Exchange Kinetics and Photoswitchable Fluorescence. Chem 2022, 8, 2362–2379. [Google Scholar] [CrossRef]
- Yang, X.; Dai, Y.; Liu, H.; Wang, K.; Yan, H.-L.; Yu, X.; Xia, Z.-A.; Wu, M.; Zhang, S.; Xiao, G.; et al. Antagonistic Effects of Distance and Overlap toward Anomalous Pressure-Induced Blueshift of π–π Excimer Fluorescence in 9-(2,2-Diphenylvinyl)Anthracene Crystals. J. Am. Chem. Soc. 2025, 147, 5300–5309. [Google Scholar] [CrossRef]
- Chen, W.-T.; Chen, L.; Liang, Z.-Y.; Mo, Z.-W.; Ye, J.-W.; Chen, X.-M. Multiple Flexibilities Trigger Luminescent Piezochromism of Closely Packed Cu(I) Coordination Polymers. Adv. Opt. Mater. 2023, 11, 2202771. [Google Scholar] [CrossRef]
- Yu, J.-X.; Christou, G.; Cheng, H.-P. Analysis of Exchange Interactions in Dimers of Mn3 Single-Molecule Magnets, and Their Sensitivity to External Pressure. J. Phys. Chem. C 2020, 124, 14768–14774. [Google Scholar] [CrossRef]
- Craig, G.A.; Sarkar, A.; Woodall, C.H.; Hay, M.A.; Marriott, K.E.R.; Kamenev, K.V.; Moggach, S.A.; Brechin, E.K.; Parsons, S.; Rajaraman, G.; et al. Probing the Origin of the Giant Magnetic Anisotropy in Trigonal Bipyramidal Ni(II) under High Pressure. Chem. Sci. 2018, 9, 1551–1559. [Google Scholar] [CrossRef]
- Pinkowicz, D.; Rams, M.; Mišek, M.; Kamenev, K.V.; Tomkowiak, H.; Katrusiak, A.; Sieklucka, B. Enforcing Multifunctionality: A Pressure-Induced Spin-Crossover Photomagnet. J. Am. Chem. Soc. 2015, 137, 8795–8802. [Google Scholar] [CrossRef]
- Handzlik, G.; Dziubek, K.F.; Hanfland, M.; Pinkowicz, D. Simultaneous Manipulation of Iron(II) Spin Crossover and LIESST Behaviour Using Pressure, Temperature and Light. Dalton Trans. 2024, 53, 7677–7681. [Google Scholar] [CrossRef]
- Liu, X.-L.; Zhao, Y.; Wang, T.; Wang, P.; Li, G.; Gao, B.-H.; Zhang, X.-L.; Wang, X.-Y. FeIII Spin Crossover Complexes with a Dual-Substituted Hqsal Ligand: Anion, Solvent, and Pressure Effects. Cryst. Growth Des. 2025, 25, 3518–3528. [Google Scholar] [CrossRef]
- Parmar, V.S.; Thiel, A.M.; Nabi, R.; Gransbury, G.K.; Norre, M.S.; Evans, P.; Corner, S.C.; Skelton, J.M.; Chilton, N.F.; Mills, D.P.; et al. Influence of Pressure on a Dysprosocenium Single-Molecule Magnet. Chem. Commun. 2023, 59, 2656–2659. [Google Scholar] [CrossRef]
- Pointillart, F.; Le Guennic, B.; Cador, O. Pressure-Induced Structural, Optical and Magnetic Modifications in Lanthanide Single-Molecule Magnets. Chem. Eur. J. 2024, 30, e202400610. [Google Scholar] [CrossRef] [PubMed]
- Norre, M.S.; Gao, C.; Dey, S.; Gupta, S.K.; Borah, A.; Murugavel, R.; Rajaraman, G.; Overgaard, J. High-Pressure Crystallographic and Magnetic Studies of Pseudo-D5h Symmetric Dy(III) and Ho(III) Single-Molecule Magnets. Inorg. Chem. 2020, 59, 717–729. [Google Scholar] [CrossRef]
- Parmar, V.S.; Thiel, A.M.; Norre, M.S.; Overgaard, J. High-Pressure Investigation of a Five-Coordinate Dy Single-Molecule Magnet. Cryst. Growth Des. 2023, 23, 6410–6417. [Google Scholar] [CrossRef]
- Pointillart, F.; Flores Gonzalez, J.; Douib, H.; Montigaud, V.; McMonagle, C.J.; Le Guennic, B.; Cador, O.; Pinkowicz, D.; Probert, M.R. Reversible Pressure-Magnetic Modulation in a Tetrathiafulvalene-Based Dyad Piezochromic Dysprosium Single-Molecule Magnet. Chem. Eur. J. 2023, 29, e202300445. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-B.; Chen, Y.-C.; Liu, J.-L.; Jia, J.-H.; Wang, L.-F.; Li, Q.-W.; Tong, M.-L. A Piezochromic Dysprosium(III) Single-Molecule Magnet Based on an Aggregation-Induced-Emission-Active Tetraphenylethene Derivative Ligand. Inorg. Chem. 2017, 56, 8730–8734. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, J.; Feng, Y.; Liu, H.; Zhang, X.; Hao, Y.; Wang, T.; Huang, X.; Hao, H. Multiple Stimuli-Responsive Flexible Crystal with 2D Elastic Bending, Plastic Twisting and Photoinduced Bending Capabilities. J. Mater. Chem. C 2021, 9, 16762–16770. [Google Scholar] [CrossRef]
- Koehne, I.; Pietschnig, R. Synthesis of Geminal Bis- and Tetrakisphosphonate Ester Derivatives and Their Coordination Behavior Towards Ca(II) Ions. Eur. J. Inorg. Chem. 2022, 2022, e202200194. [Google Scholar] [CrossRef]
- SAINT. Program for Data Extraction and Reduction, Version 8.40A; Bruker Nano. Inc.: Billerica, MA, USA, 2019. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Casanova, D.; Llunell, M.; Alemany, P.; Alvarez, S. The Rich Stereochemistry of Eight-Vertex Polyhedra: A Continuous Shape Measures Study. Chem. Eur. J. 2005, 11, 1479–1494. [Google Scholar] [CrossRef]
- Schmidt, G.M.J. Photodimerization in the Solid State. Pure Appl. Chem. 1971, 27, 647–678. [Google Scholar] [CrossRef]
- Pandiyan, B.V.; Deepa, P.; Kolandaivel, P. Do Resonance-Assisted Intramolecular Halogen Bonds Exist without a Charge Transfer and a σ-Hole? Phys. Chem. Chem. Phys. 2015, 17, 27496–27508. [Google Scholar] [CrossRef]
- Wu, X.; Guo, J.; Cao, Y.; Zhao, J.; Jia, W.; Chen, Y.; Jia, D. Mechanically Triggered Reversible Stepwise Tricolor Switching and Thermochromism of Anthracene-o-Carborane Dyad. Chem. Sci. 2018, 9, 5270–5277. [Google Scholar] [CrossRef]
- Suresh, S.K.; Raju, P.D.; Krishnan, A.; Ramachandran, L.M.; Suneesh, C.V. Supramolecular Interactions-Assisted Polymorphism and Unique Mechanofluorochromism in 9,10-Bis((E)-4-(Trifluoromethyl)Styryl)Anthracene. Chem. Eur. J. 2023, 29, e202204030. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Xu, B.; Zhang, J.; Tan, X.; Wang, L.; Chen, J.; Lv, H.; Wen, S.; Li, B.; Ye, L.; et al. Piezochromic Luminescence Based on the Molecular Aggregation of 9,10-Bis((E)-2-(Pyrid-2-Yl)Vinyl)Anthracene. Angew. Chem. Int. Ed. 2012, 51, 10782–10785. [Google Scholar] [CrossRef]
- Kannen, F.; Adachi, T.; Nishimura, M.; Yoza, K.; Kusukawa, T. Mechanofluorochromic Properties of 1,4-Diphenylanthracene Derivatives with Hypsochromic Shift. Molecules 2024, 29, 407. [Google Scholar] [CrossRef]
- Yu, H.; Li, M.; Chen, X.; Han, N.; Xu, Z.; Wang, M. Synthesis of Anthracene-Based Mechanofluorochromic Molecules and Their Differential Mechanochromic Effects Triggered by Nitrogen Atoms. Eur. J. Org. Chem. 2024, 27, e202400876. [Google Scholar] [CrossRef]
- Xu, W.-J.; Luo, Q.-C.; Li, Z.-H.; Zhai, Y.-Q.; Zheng, Y.-Z. Bis-Alkoxide Dysprosium(III) Crown Ether Complexes Exhibit Tunable Air Stability and Record Energy Barrier. Adv. Sci. 2024, 11, 2308548. [Google Scholar] [CrossRef]
- Li, D.-P.; Zhang, X.-P.; Wang, T.-W.; Ma, B.-B.; Li, C.-H.; Li, Y.-Z.; You, X.-Z. Distinct magnetic dynamic behavior for two polymorphs of the same Dy(III) complex. Chem. Commun. 2011, 47, 6867–6869. [Google Scholar] [CrossRef] [PubMed]
- Horii, Y.; Katoh, K.; Sugimoto, K.; Nakanishi, R.; Breedlove, B.K.; Yamashita, M. Detailed Analysis of the Crystal Structures and Magnetic Properties of a Dysprosium(III) Phthalocyaninato Sextuple-Decker Complex: Weak f–f Interactions Suppress Magnetic Relaxation. Chem. Eur. J. 2019, 25, 3098–3104. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Tolpygin, A.O.; Mamontova, E.; Lyssenko, K.A.; Liu, D.; Albaqami, M.D.; Chibotaru, L.F.; Guari, Y.; Larionova, J.; Trifonov, A.A. An Unusual Mechanism of Building up of a High Magnetization Blocking Barrier in an Octahedral Alkoxide Dy3+-Based Single-Molecule Magnet. Inorg. Chem. Front. 2021, 8, 1166–1174. [Google Scholar] [CrossRef]
- Cole, K.S.; Cole, R.H. Dispersion and Absorption in Dielectrics I. Alternating Current Characteristics. J. Chem. Phys. 1941, 9, 341–351. [Google Scholar] [CrossRef]
- Gu, L.; Wu, R. Origin of the Anomalously Low Raman Exponents in Single Molecule Magnets. Phys. Rev. B 2021, 103, 014401. [Google Scholar] [CrossRef]
- Ma, Y.; Zhai, Y.-Q.; Luo, Q.-C.; Ding, Y.-S.; Zheng, Y.-Z. Ligand Fluorination to Mitigate the Raman Relaxation of DyIII Single-Molecule Magnets: A Combined Terahertz, Far-IR and Vibronic Barrier Model Study. Angew. Chem. Int. Ed. 2022, 61, e202206022. [Google Scholar] [CrossRef]
| Compound | Dy-Cl | Dy-Br | Dy-H a | |
|---|---|---|---|---|
| Cell parameters | Temperature | 193 K | 193 K | 193 K |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | |
| Space group | P21/m | P21/n | P21/m | |
| a/Å | 9.736(1) | 9.754(1) | 9.605(3) | |
| b/Å | 25.723(1) | 25.833(1) | 25.654(10) | |
| c/Å | 11.251(1) | 21.114(1) | 11.161(4) | |
| β/° | 111.4(1) | 94.739(1) | 112.1(1) | |
| V/Å3 | 2623.2(2) | 5302.0(4) | 2548.5(16) | |
| Z | 2 | 4 | 2 | |
| CCDC number | 2475934 | 2475970 | 2233761 | |
| Structural parameters | Dy1-O(axial)/Å | 2.251(5)/2.246(5) | 2.260(4)/2.235(5) | 2.248(8)/2.213(9) |
| Dy1-O(equatorial)/Å | 2.337(4)-2.572(5) | 2.328(3)-2.574(4) | 2.319(7)-2.571(8) | |
| Dy1-N/Å | 2.450(5) | 2.455(4)/2.440(4) | 2.426(8) | |
| O1-Dy1-O2(axial)/° | 163.9(2) | 163.7(2) | 163.9(4) | |
| dDy–Dy/Å | 9.736 | 9.754 | 9.605 | |
| dcc/Å | 3.758 | 3.810 | 3.753 | |
| dpp/Å | 3.372 | 3.408 | 3.409 | |
| slip angle/° | 23.10 | 23.61/23.06 | 22.36 | |
| dC2–C9A/Å | 3.743 | - | 3.754 | |
| dC2–C28, dC9-C21/Å | - | 3.819/3.811 | - | |
| PL properties | Excimer peak/nm | 550 | 535 | 540 |
| Lifetime/ns | 54.1 | 7.11 | 41.7 | |
| PLQY/% | 10.31 | 1.76 | 12.69 | |
| Magnetic properties | χMT/cm3 K mol−1 | 14.77 | 14.92 | 14.18 |
| M/Nβ (70 kOe) | 5.65 | 5.54 | 5.22 | |
| Ueff/kB/K | 259(12) | 264(9) | 277 | |
| τ0/s | 2.77(222) × 10−11 | 4.46(243) × 10−11 | 7.16 × 10−11 | |
| C/K−n s−1 | 2.1(5) × 10−3 | 9.1(7) × 10−4 | 1.17 × 10−3 | |
| n | 4.37(10) | 4.80(8) | 4.42 | |
| τQTM/s | 0.241(7) | 0.316(8) | 0.188 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.-H.; Su, Q.-Q.; Bao, S.-S.; Zheng, L.-M. Dysprosium Complexes Incorporating Halogen-Substituted Anthracene: Piezochromism and Single-Molecule Magnet Properties. Magnetochemistry 2025, 11, 102. https://doi.org/10.3390/magnetochemistry11120102
Qin Y-H, Su Q-Q, Bao S-S, Zheng L-M. Dysprosium Complexes Incorporating Halogen-Substituted Anthracene: Piezochromism and Single-Molecule Magnet Properties. Magnetochemistry. 2025; 11(12):102. https://doi.org/10.3390/magnetochemistry11120102
Chicago/Turabian StyleQin, Ye-Hui, Qian-Qian Su, Song-Song Bao, and Li-Min Zheng. 2025. "Dysprosium Complexes Incorporating Halogen-Substituted Anthracene: Piezochromism and Single-Molecule Magnet Properties" Magnetochemistry 11, no. 12: 102. https://doi.org/10.3390/magnetochemistry11120102
APA StyleQin, Y.-H., Su, Q.-Q., Bao, S.-S., & Zheng, L.-M. (2025). Dysprosium Complexes Incorporating Halogen-Substituted Anthracene: Piezochromism and Single-Molecule Magnet Properties. Magnetochemistry, 11(12), 102. https://doi.org/10.3390/magnetochemistry11120102








