Digital Magnetic Sorting for Fractionating Cell Populations with Variable Antigen Expression in Cell Therapy Process Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Samples
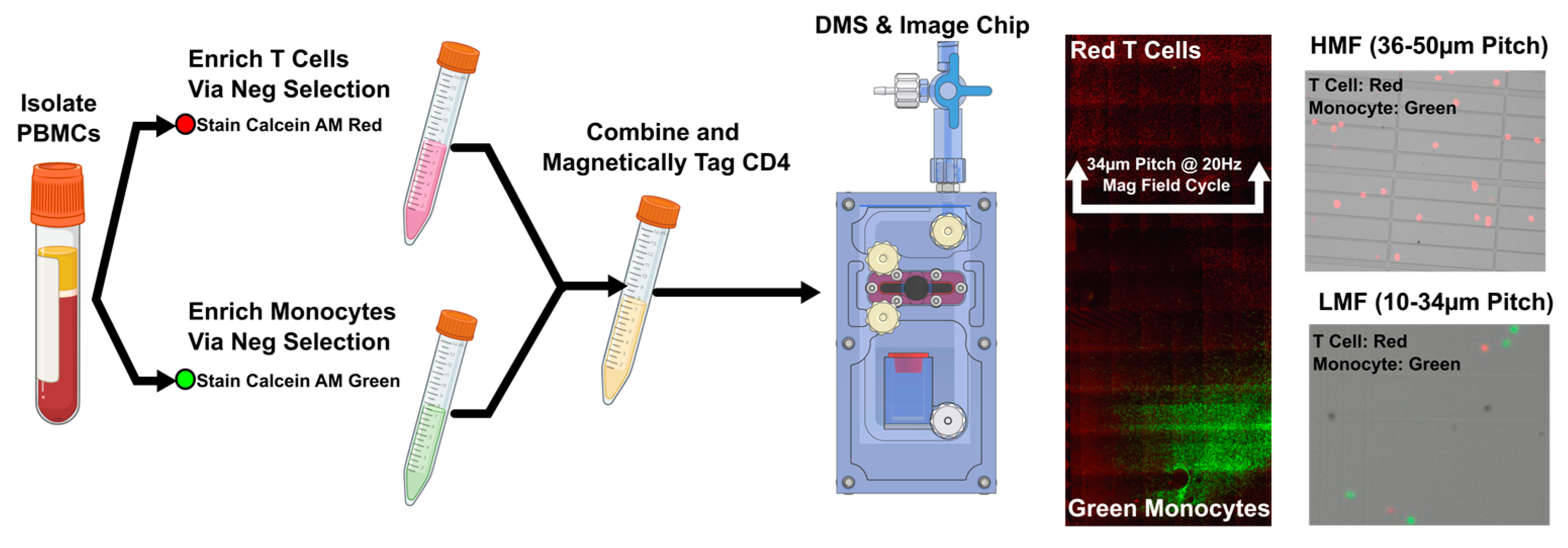
2.2. Magnetic Labeling CD4Hi CD4Low Populations
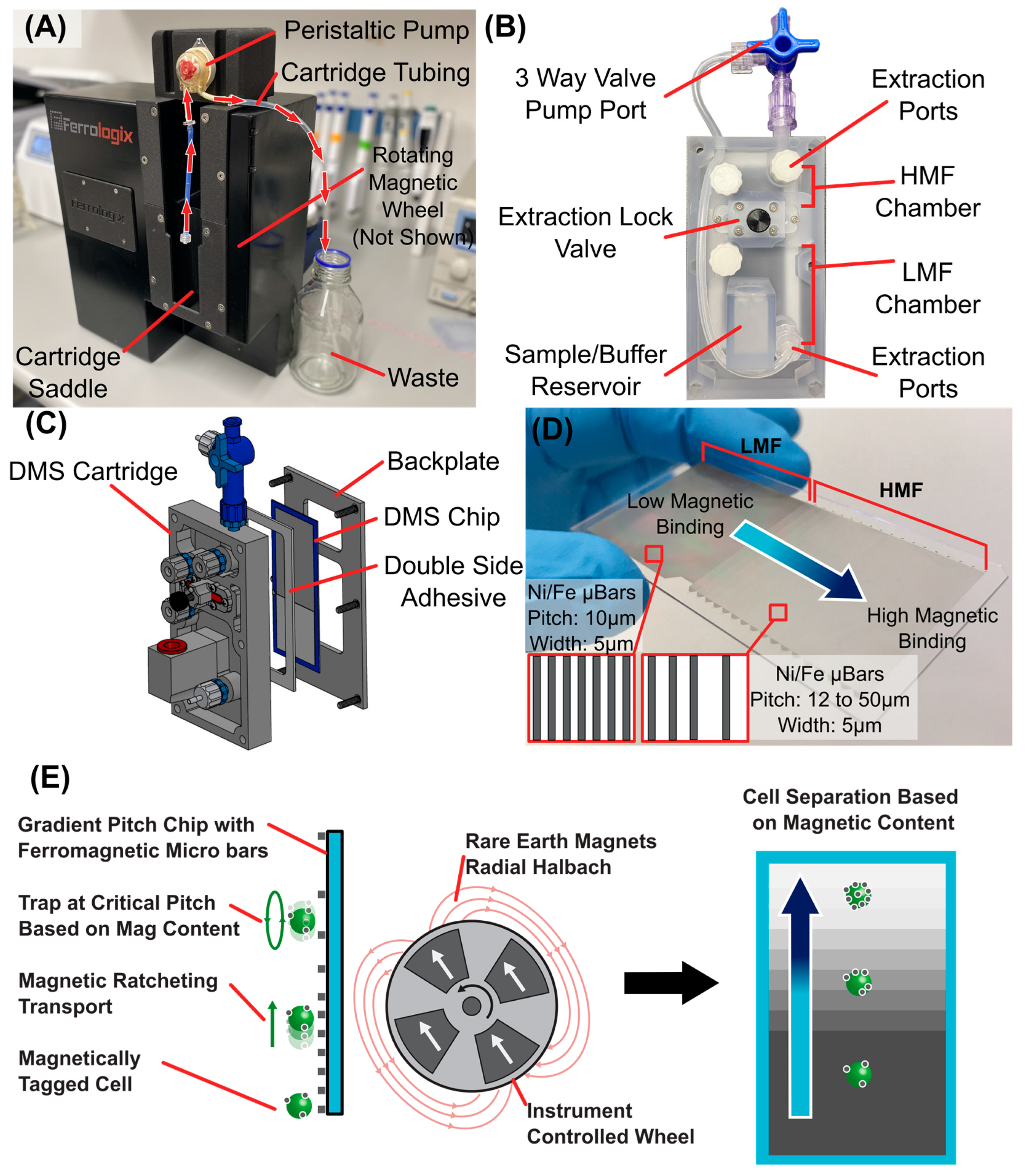
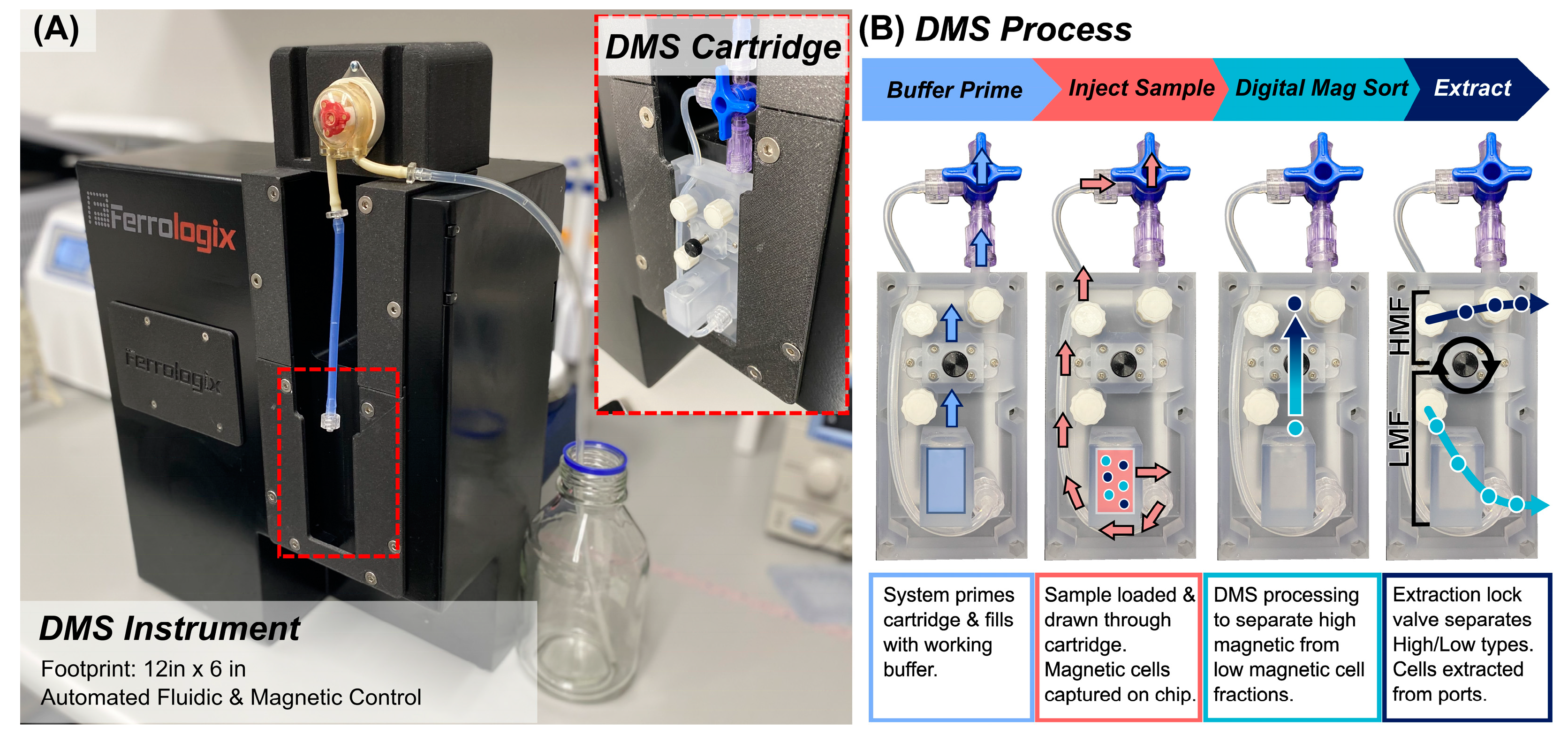
2.3. DMS of High and Low Populations
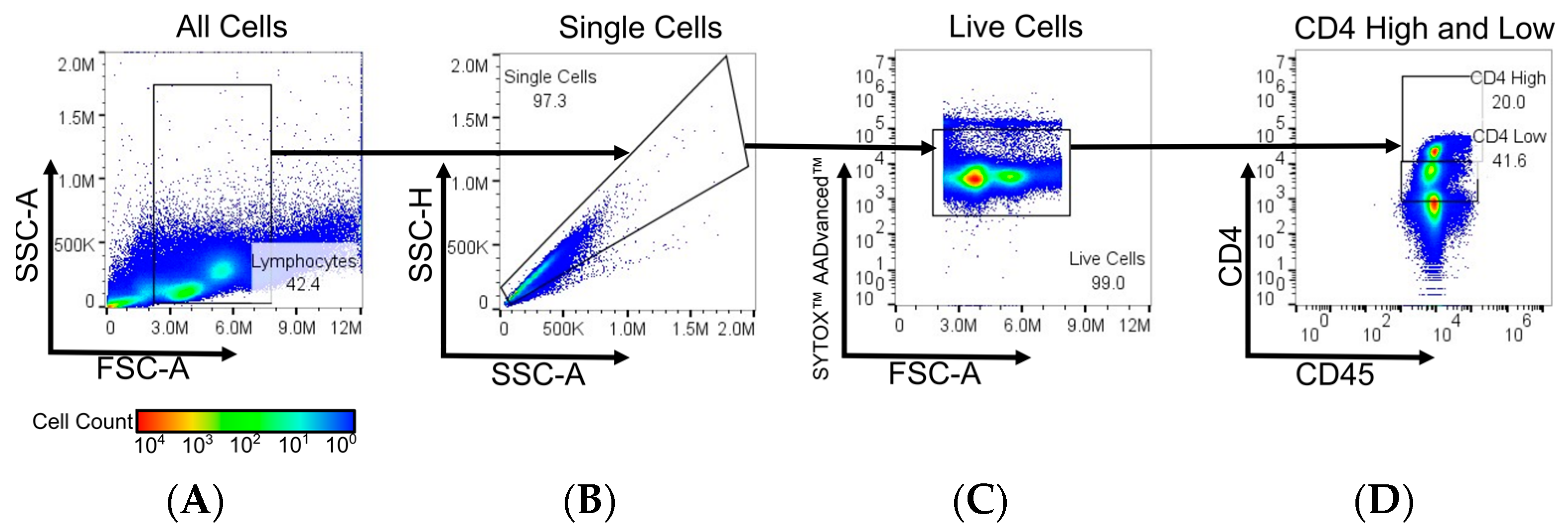
2.4. Flow Cytometry, Cell Counting, and Viability
2.5. Single-Cell RNA Seq of High and Low Populations
3. Results
3.1. DMS Workflow
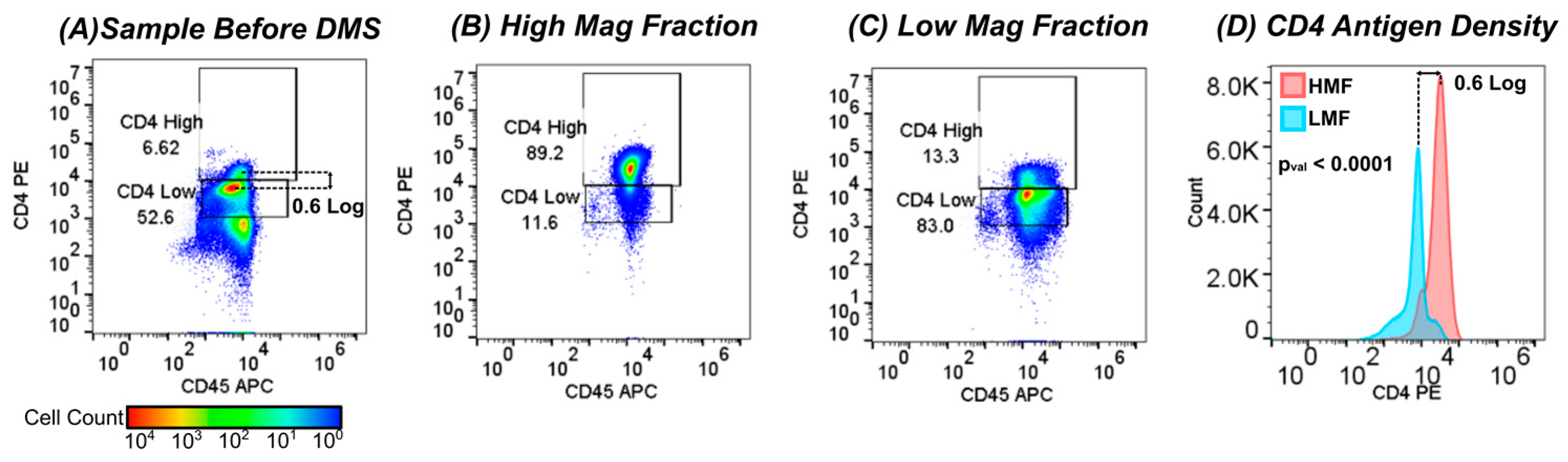
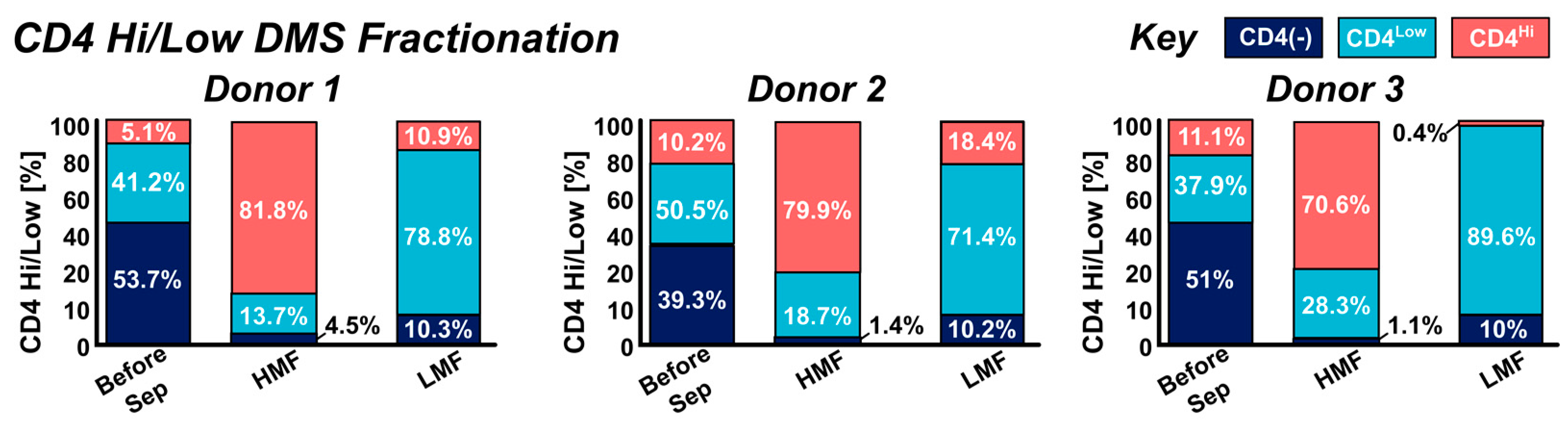
3.2. DMS Fractionation of CD4Hi and CD4Low Cell Types
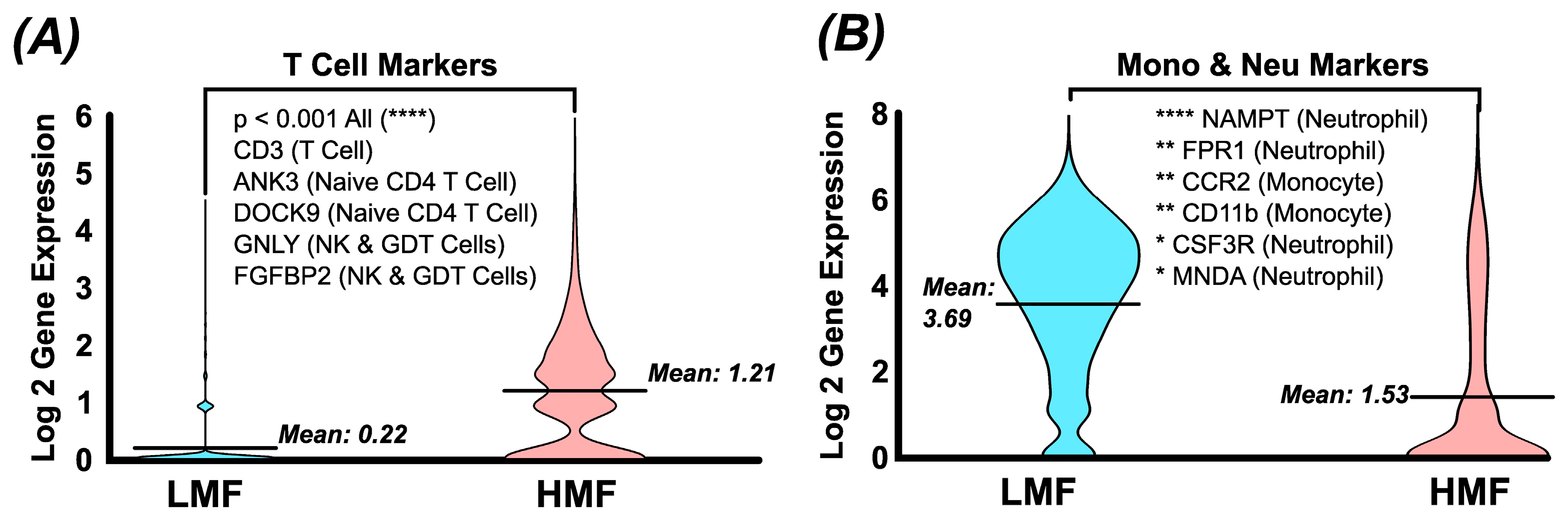
3.3. Single-Cell RNA Seq Confirms Antigen Density Separation
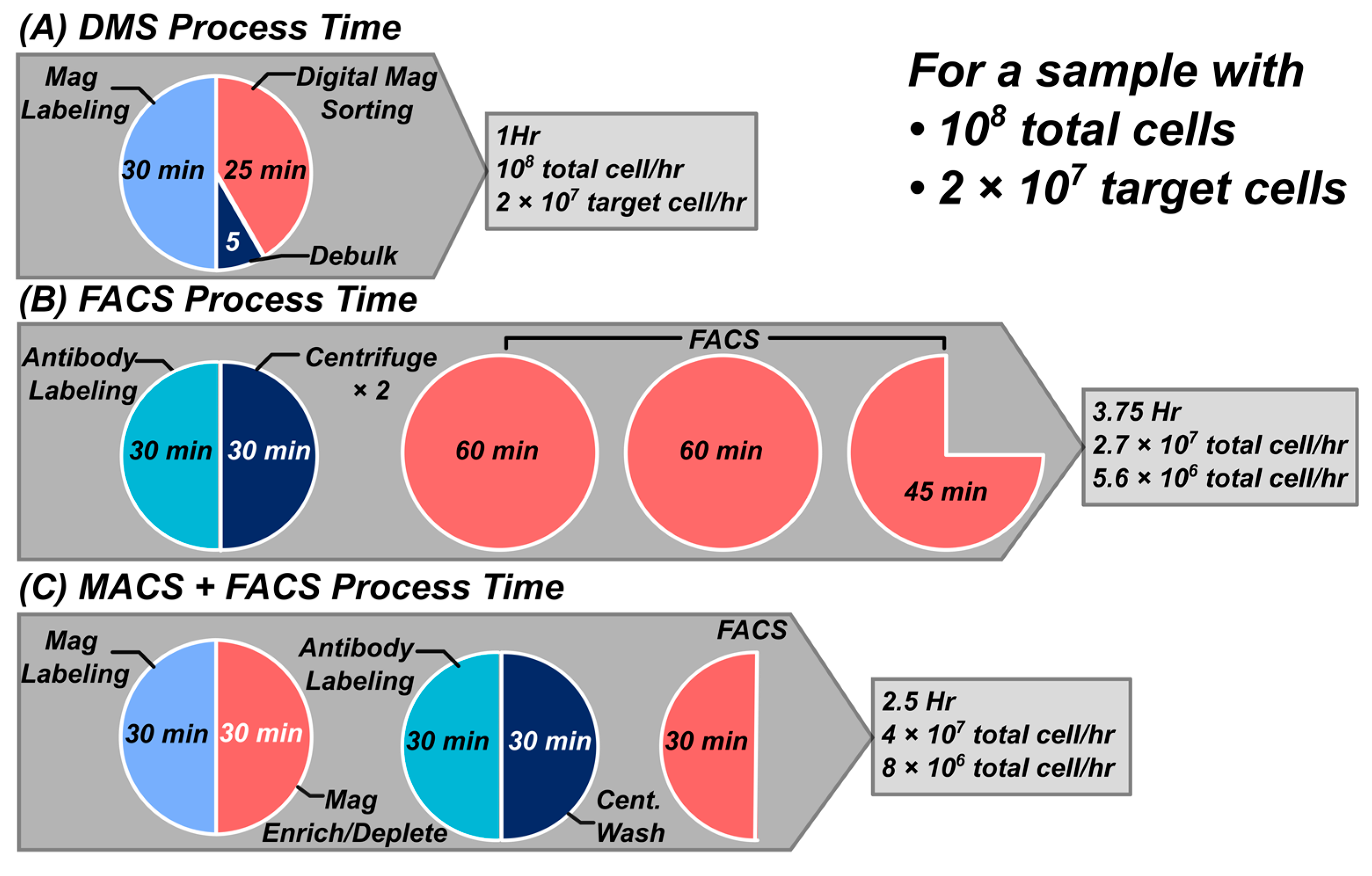
3.4. Cell-Processing Throughput
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Jackson, H.J.; Rafiq, S.; Brentjens, R.J. Driving CAR T-cells forward. Nat. Rev. Clin. Oncol. 2016, 13, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Sommermeyer, D.; Hudecek, M.; Kosasih, P.L.; Gogishvili, T.; Maloney, D.G.; Turtle, C.J.; Riddell, S.R. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia 2016, 30, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Koehl, U.; Esser, R.; Zimmermann, S.; Tonn, T.; Kotchetkov, R.; Bartling, T.; Sörensen, J.; Grüttner, H.-P.; Bader, P.; Seifried, E.; et al. Ex vivo expansion of highly purified NK cells for immunotherapy after haploidentical stem cell transplantation in children. Klin. Padiatr. 2005, 217, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Radtke, S.; Pande, D.; Cui, M.; Perez, A.M.; Chan, Y.Y.; Enstrom, M.; Schmuck, S.; Berger, A.; Eunson, T.; Adair, J.E.; et al. Purification of Human CD34+CD90+ HSCs Reduces Target Cell Population and Improves Lentiviral Transduction for Gene Therapy. Mol. Ther. Methods Clin. Dev. 2020, 18, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Zhen, A.; Krutzik, S.R.; Levin, B.R.; Kasparian, S.; Zack, J.A.; Kitchen, S.G. CD4 ligation on human blood monocytes triggers macrophage differentiation and enhances hiv infection. J. Virol. 2014, 88, 9934–9946. [Google Scholar] [CrossRef] [PubMed]
- Kazazi, F.; Mathijs, J.-M.; Foley, P.; Cunningham, A.L. Variations in CD4 expression by human monocytes and macrophages and their relationships to infection with the human immunodeficiency virus. J. Gen. Virol. 1989, 70 Pt 10, 2661–2672. [Google Scholar] [CrossRef]
- Biswas, P.; Mantelli, B.; Sica, A.; Malnati, M.; Panzeri, C.; Saccani, A.; Hasson, H.; Vecchi, A.; Saniabadi, A.; Lusso, P.; et al. Expression of CD4 on human peripheral blood neutrophils. Blood 2003, 101, 4452–4456. [Google Scholar] [CrossRef]
- Ziegler, S.; Weiss, E.; Schmitt, A.-L.; Schlegel, J.; Burgert, A.; Terpitz, U.; Sauer, M.; Moretta, L.; Sivori, S.; Leonhardt, I.; et al. CD56 Is a Pathogen Recognition Receptor on Human Natural Killer Cells. Sci. Rep. 2017, 7, 6138. [Google Scholar] [CrossRef]
- Majzner, R.G.; Rietberg, S.P.; Sotillo, E.; Dong, R.; Vachharajani, V.T.; Labanieh, L.; Myklebust, J.H.; Kadapakkam, M.; Weber, E.W.; Tousley, A.M.; et al. Tuning the Antigen Density Requirement for CAR T-cell Activity. Cancer Discov. 2020, 10, 702–723. [Google Scholar] [CrossRef]
- Hong, M.; Clubb, J.D.; Chen, Y.Y. Engineering car-T cells for next-generation cancer therapy. Cancer Cell 2020, 38, 473–488. [Google Scholar] [CrossRef]
- Wang, Z.; Ahmed, S.; Labib, M.; Wang, H.; Hu, X.; Wei, J.; Yao, Y.; Moffat, J.; Sargent, E.H.; Kelley, S.O. Efficient recovery of potent tumour-infiltrating lymphocytes through quantitative immunomagnetic cell sorting. Nat. Biomed. Eng. 2022, 6, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lyu, X.; Zhou, Z.; Yang, L.; Zeng, J.; Yang, Y.; Zhao, Z.; Chen, R.; Tong, X.; Li, J.; et al. Multifunctional Droplets Formed by Interfacially Self-Assembled Fluorinated Magnetic Nanoparticles for Biocompatible Single Cell Culture and Magnet-Driven Manipulation. ACS Appl. Mater. Interfaces 2023, 15, 17324–17334. [Google Scholar] [CrossRef] [PubMed]
- Abedini-Nassab, R.; Adibi, E.; Ahmadiasl, S. Characterization of AI-enhanced magnetophoretic transistors operating in a tri-axial magnetic field for on-chip bioparticle sorting. Sci. Rep. 2024, 14, 23381. [Google Scholar] [CrossRef]
- Shanehband, N.; Naghib, S.M. Microfluidics-assisted Tumor Cell Separation Approaches for Clinical Applications: An Overview on Emerging Devices. Comb. Chem. High Throughput Screen. 2024, 28, 202–225. [Google Scholar] [CrossRef]
- Hewlin, R.L., Jr.; Edwards, M.; Schultz, C. Design and Development of a Traveling Wave Ferro-Microfluidic Device and System Rig for Potential Magnetophoretic Cell Separation and Sorting in a Water-Based Ferrofluid. Micromachines 2023, 14, 889. [Google Scholar] [CrossRef]
- Bshara-Corson, S.; Vukovic, L.; Verhagen, A.; Tiemann, T.; Murray, C. Rare Cell Purification Using Ferrologix’s Digital Magnetic Sorting. In Proceedings of the Annual Conference of the Society for Laboratory Automation and Screening, Boston, MA, USA, 6 February 2022. [Google Scholar]
- Adeyiga, O.B.; Murray, C.; Muñoz, H.E.; Escobar, A.; Di Carlo, D. Magnetic microparticle concentration and collection using a mechatronic magnetic ratcheting system. PLoS ONE 2021, 16, e0246124. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.; Miwa, H.; Dhar, M.; Park, D.E.; Pao, E.; Martinez, J.; Kaanumale, S.; Loghin, E.; Graf, J.; Rhaddassi, K.; et al. Unsupervised capture and profiling of rare immune cells using multi-directional magnetic ratcheting. Lab Chip 2018, 18, 2396–2409. [Google Scholar] [CrossRef]
- Murray, C.; Pao, E.; Tseng, P.; Aftab, S.; Kulkarni, R.; Rettig, M.; Di Carlo, D. Quantitative Magnetic Separation of Particles and Cells Using Gradient Magnetic Ratcheting. Small 2016, 12, 1891–1899. [Google Scholar] [CrossRef]
- Murray, C.; Pao, E.; Jann, A.; Park, D.E.; Di Carlo, D. Continuous and Quantitative Purification of T-Cell Subsets for Cell Therapy Manufacturing Using Magnetic Ratcheting Cytometry. SLAS Technol. 2018, 23, 326–337. [Google Scholar] [CrossRef]
- Ferrologix, Inc. Digital Magnetic Sorting. synthesia.io. Available online: https://share.synthesia.io/8704ea27-e45b-46e4-ae29-5d36bf204dfe (accessed on 16 October 2024).
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Yang, C.; Rong, R. Discrepant mRNA and Protein Expression in Immune Cells. Curr. Genom. 2020, 21, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Flow Cytometry University of Iowa Helathcare. Cell Sorting|Flow Cytometry. (n.d.). Available online: https://medicine.uiowa.edu/flowcytometry/cell-sorting (accessed on 31 October 2023).
- Miltenyi. (n.d.). autoMACS® Pro Separator User Manual. Available online: https://static.miltenyibiotec.com/asset/150655405641/document_smosus3i9h7jf8mkpdpb52bn0u?content-disposition=inline (accessed on 31 October 2023).
- Miltenyi. (n.d.). CliniMACS Prodigy® User Manual. Available online: https://static.miltenyibiotec.com/asset/150655405641/document_h0fd9i4al17q32uhfuf4h7lu25?content-disposition=inline (accessed on 31 October 2023).
- Valli, H.; Sukhwani, M.; Dovey, S.L.; Peters, K.A.; Donohue, J.; Castro, C.A.; Chu, T.; Marshall, G.R.; Orwig, K.E. Fluorescence- and magnetic-activated cell sorting strategies to isolate and enrich human spermatogonial stem cells. Fertil. Steril. 2014, 102, 566–580.e7. [Google Scholar] [CrossRef] [PubMed]
- Pigeau, G.M.; Csaszar, E.; Dulgar-Tulloch, A. Commercial Scale Manufacturing of Allogeneic Cell Therapy. Front. Med. 2018, 5, 233. [Google Scholar] [CrossRef]
- Ham, R.M.T.; Hövels, A.M.; Hoekman, J.; Frederix, G.W.; Leufkens, H.G.; Klungel, O.H.; Jedema, I.; Veld, S.A.; Nikolic, T.; Van Pel, M.; et al. What does cell therapy manufacturing cost? A framework and methodology to facilitate academic and other small-scale cell therapy manufacturing costings. Cytotherapy 2020, 22, 388–397. [Google Scholar] [CrossRef]
- Ferrologix, Inc. High Throughput & Quantitative Cell Purification for Immunotherapy Manufacture (SBIR Grant No. 5R44CA228844-03); U.S. Department of Health and Human Services: Rockville, MD, USA, 2021. [Google Scholar]
- Song, H.W.; Prochazkova, M.; Shao, L.; Traynor, R.; Underwood, S.; Black, M.; Fellowes, V.; Shi, R.; Pouzolles, M.; Chou, H.-C.; et al. CAR-T cell expansion platforms yield distinct T cell differentiation states. Cytotherapy 2024, 26, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Abdo, L.d.M.; Barros, L.R.C.; Viegas, M.S.; Marques, L.V.C.; Ferreira, P.d.S.; Chicaybam, L.; Bonamino, M.H. Development of CAR-T cell therapy for B-ALL using a point-of-care approach. OncoImmunology 2020, 9, 1752592. [Google Scholar] [CrossRef]
- Ghassemi, S.; Durgin, J.S.; Nunez-Cruz, S.; Patel, J.; Leferovich, J.; Pinzone, M.; Shen, F.; Cummins, K.D.; Plesa, G.; Cantu, V.A.; et al. Rapid manufacturing of non-activated potent CAR T cells. Nat. Biomed. Eng. 2022, 6, 118–128. [Google Scholar] [CrossRef]
- Poli, A.; Michel, T.; Thérésine, M.; Andrès, E.; Hentges, F.; Zimmer, J. CD56bright natural killer (NK) cells: An important NK cell subset. Immunology 2009, 126, 458–465. [Google Scholar] [CrossRef]
- Wagner, J.A.; Rosario, M.; Romee, R.; Berrien-Elliott, M.M.; Schneider, S.E.; Leong, J.W.; Sullivan, R.P.; Jewell, B.A.; Becker-Hapak, M.; Schappe, T.; et al. CD56bright NK cells exhibit potent antitumor responses following IL-15 priming. J. Clin. Investig. 2017, 127, 4042–4058. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bshara-Corson, S.; Burwell, A.; Tiemann, T.; Murray, C. Digital Magnetic Sorting for Fractionating Cell Populations with Variable Antigen Expression in Cell Therapy Process Development. Magnetochemistry 2024, 10, 81. https://doi.org/10.3390/magnetochemistry10110081
Bshara-Corson S, Burwell A, Tiemann T, Murray C. Digital Magnetic Sorting for Fractionating Cell Populations with Variable Antigen Expression in Cell Therapy Process Development. Magnetochemistry. 2024; 10(11):81. https://doi.org/10.3390/magnetochemistry10110081
Chicago/Turabian StyleBshara-Corson, Savannah, Andrew Burwell, Timothy Tiemann, and Coleman Murray. 2024. "Digital Magnetic Sorting for Fractionating Cell Populations with Variable Antigen Expression in Cell Therapy Process Development" Magnetochemistry 10, no. 11: 81. https://doi.org/10.3390/magnetochemistry10110081
APA StyleBshara-Corson, S., Burwell, A., Tiemann, T., & Murray, C. (2024). Digital Magnetic Sorting for Fractionating Cell Populations with Variable Antigen Expression in Cell Therapy Process Development. Magnetochemistry, 10(11), 81. https://doi.org/10.3390/magnetochemistry10110081




