Facile Synthesis of Core-Shell Magnetic Iron Oxide@SiO2-NH2 Nanoparticles and Their Application in Rapid Boron Removal from Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
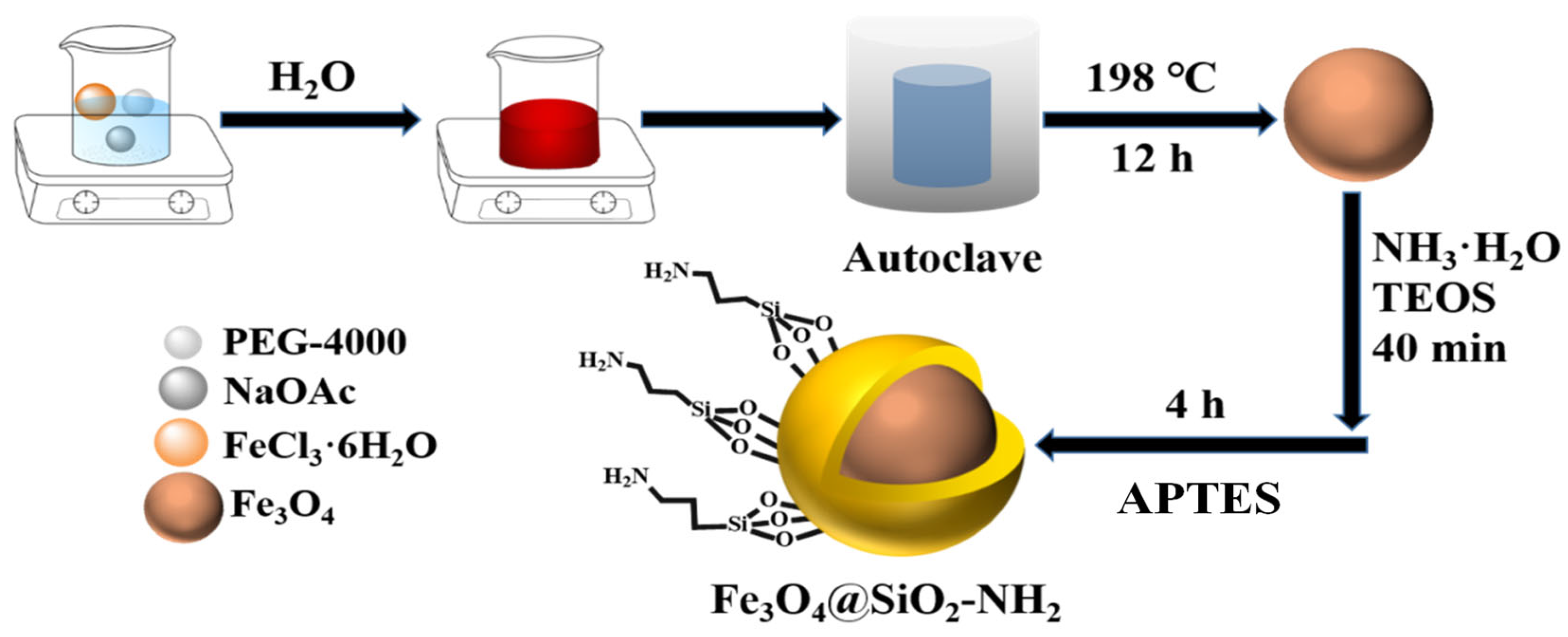
2.2. Synthesis of Iron Oxide MNPs
2.3. Synthesis of Iron Oxide@SiO2-NH2 MNPs
2.4. Characterization of Iron Oxide and Iron Oxide@SiO2-NH2 MNPs
2.5. Adsorption Experiments
2.6. Regeneration and Reuse Study
3. Results
3.1. Material Synthesis Process Optimization
3.2. Structural and Morphological Characterization
3.3. VSM and Zeta Potential Analyses
3.4. Adsorption Studies
3.4.1. Factors Affecting Adsorption
3.4.2. Adsorption Mechanism
3.4.3. Adsorption Kinetics, Isotherms, and Thermodynamic Study
3.5. Regeneration Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Figueira, M.; Reig, M.; de Labastida, M.F.; Cortina, J.L.; Valderrama, C. Boron recovery from desalination seawater brines by selective ion exchange resins. J. Environ. Manag. 2022, 314, 114984. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Mahasti, N.N.; Huang, Y.-H. Recent advances in adsorption and coagulation for boron removal from wastewater: A comprehensive review. J. Hazard. Mater. 2021, 407, 124401. [Google Scholar] [CrossRef] [PubMed]
- Duran, H.; Yavuz, E.; Sismanoglu, T.; Senkal, B. Functionalization of gum arabic including glycoprotein and polysaccharides for the removal of boron. Carbohydr. Polym. 2019, 225, 115139. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, H.; Juming, Z.; Ke-Mei, P. The physiological role of boron on health. Biol. Trace Elem. Res. 2018, 186, 31–51. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality, 4th ed.; WHO Press: Geneva, Switzerland, 2011; p. 178. [Google Scholar]
- Zhang, P.; Xiao, M.; Dai, Y.; Zhang, Z.; Liu, G.; Zhao, J. Evaluation of water quality of collected rainwater in the northeastern loess plateau. Sustainability 2022, 14, 10834. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Cheng, Z.; Su, Q.; Cao, Y. Health risk cause of water around landfill in hilly area and prevention and control countermeasures. J. Environ. Manag. 2023, 346, 119019. [Google Scholar] [CrossRef]
- Chen, M.; Dollar, O.; Shafer-Peltier, K.; Randtke, S.; Waseem, S.; Peltier, E. Boron removal by electrocoagulation: Removal mechanism, adsorption models and factors influencing removal. Water Res. 2020, 170, 115362. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, Y.; Lai, G.S.; Wang, R. Fabrication of fluorinated polyamide seawater reverse osmosis membrane with enhanced boron removal. J. Membr. Sci. 2022, 662, 121009. [Google Scholar] [CrossRef]
- Najid, N.; Kouzbour, S.; Ruiz-García, A.; Fellaou, S.; Gourich, B.; Stiriba, Y. Comparison analysis of different technologies for the removal of boron from seawater: A review. J. Environ. Chem. Eng. 2021, 9, 105133. [Google Scholar] [CrossRef]
- Peng, X.; Li, L.; Shi, D.; Zhang, L.; Li, H.; Nie, F.; Song, F. Recovery of boric acid from salt lake brines by solvent extraction with 2-butyl-1-n-octanol. Hydrometallurgy 2018, 177, 161–167. [Google Scholar] [CrossRef]
- Akdağ, S.; Keyikoğlu, R.; Karagunduz, A.; Keskinler, B.; Khataee, A.; Yoon, Y. Recent advances in boron species removal and recovery using layered double hydroxides. Appl. Clay Sci. 2023, 233, 106814. [Google Scholar] [CrossRef]
- Demirçivi, P.; Saygılı, G.N. Comparative study of modified expanded perlite with hexadecyltrimethylammonium- bromide and gallic acid for boron adsorption. J. Mol. Liq. 2018, 254, 383–390. [Google Scholar] [CrossRef]
- Kluczka, J.; Pudło, W.; Krukiewicz, K. Boron adsorption removal by commercial and modified activated carbons. Chem. Eng. Res. Des. 2019, 147, 30–42. [Google Scholar] [CrossRef]
- Sun, L.; Huang, J.; Liu, H.; Zhang, Y.; Ye, X.; Zhang, H.; Wu, A.; Wu, Z. Adsorption of boron by CA@ KH-550@EPH@ NMDG (CKEN) with biomass carbonaceous aerogels as substrate. J. Hazard. Mater. 2018, 358, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha Reddy, G.; Gerbec, J.A.; Shimizu, F.; Chmelka, B.F. Nanoscale surface compositions and structures influence boron adsorption properties of anion exchange resins. Langmuir 2019, 35, 15661–15673. [Google Scholar] [CrossRef] [PubMed]
- Heredia, A.l.C.; de la Fuente García-Soto, M.; Narros Sierra, A.; Mendoza, S.M.; Gómez Avila, J.; Crivello, M.n.E. Boron removal from aqueous solutions by synthetic MgAlFe mixed oxides. Ind. Eng. Chem. Res. 2019, 58, 9931–9939. [Google Scholar] [CrossRef]
- Wu, B.; Jiang, X.; Yu, S.; Bai, P.; Guo, X.; Lyu, J. Unveiling the nature of boric acid adsorption by metal-organic frameworks with hexanuclear clusters. Chem. Eng. J. 2022, 433, 133543. [Google Scholar] [CrossRef]
- Chen, T.; Wang, Q.; Lyu, J.; Bai, P.; Guo, X. Boron removal and reclamation by magnetic magnetite (Iron oxide) nanoparticle: An adsorption and isotopic separation study. Sep. Purif. Technol. 2020, 231, 115930. [Google Scholar] [CrossRef]
- Kamari, S.; Shahbazi, A. Biocompatible Iron oxide@SiO2-NH2 nanocomposite as a green nanofiller embedded in PES–nanofiltration membrane matrix for salts, heavy metal ion and dye removal: Long-term operation and reusability tests. Chemosphere 2020, 243, 125282. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lei, M.; Zeng, W.; Li, Y.; Li, B.; Liu, D.; Liu, C. Synthesis of magnetic Iron oxide@SiO2-(-NH2/-COOH) nanoparticles and their application for the removal of heavy metals from wastewater. Ceram. Int. 2023, 49, 20470–20479. [Google Scholar] [CrossRef]
- Ghorbani, F.; Kamari, S. Core-shell magnetic nanocomposite of Iron oxide@SiO2@NH2 as an efficient and highly recyclable adsorbent of methyl red dye from aqueous environ ments. Environ. Technol. Inno. 2019, 14, 100333. [Google Scholar] [CrossRef]
- Donga, C.; Mishra, S.B.; Abd-El-Aziz, A.S.; Ndlovu, L.N.; Mishra, A.K.; Kuvarega, A.T. (3-Aminopropyl) triethoxysilane (APTES) functionalized magnetic nanosilica graphene oxide (MGO) nanocomposite for the comparative adsorption of the heavy metal [Pb (II), Cd (II) and Ni (II)] ions from aqueous solution. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2235–2248. [Google Scholar] [CrossRef]
- Liang, Y.; Jiang, L.; Xu, S.; Ju, W.; Tao, Z.; Yang, Y.; Peng, X.; Wei, G. Synthesis and characterization of Iron oxide nanoparticles Prepared by solvothermal method. J. Mater. Eng. Perform. 2023, 33, 6804–6815. [Google Scholar] [CrossRef]
- Yang, Y.; Miao, J.; Yin, Z.; Hao, W.; Shi, H.; Ma, L.; Shi, T. Synthesis of the magnetically nanoporous organic polymer Iron oxide@ SiO2-NH2-COP and its application in the determination of sulfonamide residues in surface water surrounding a cattle Farm. Bioinorg. Chem. Appl. 2022, 2022, 6453609. [Google Scholar] [CrossRef] [PubMed]
- Ajalli, N.; Pourmadadi, M.; Yazdian, F.; Rashedi, H.; Navaei-Nigjeh, M.; Díez-Pascual, A. Chitosan/Gamma-Alumina/Iron oxide@5-FU nanostructures as promising nanocarriers: Physiochemical characterization and toxicity activity. Molecules 2022, 27, 5369. [Google Scholar] [CrossRef] [PubMed]
- Sadegh, N.; Asfaram, A.; Javadian, H.; Haddadi, H.; Sharifpour, E. Ultrasound-assisted solid phase microextraction-HPLC method based on Iron oxide@SiO2-NH2-molecularly imprinted polymer magnetic nano-sorbent for rapid and efficient extraction of harmaline from peganum harmala extract. J. Chromatogr. B 2021, 1171, 122640. [Google Scholar] [CrossRef]
- Sarker, M.Z.; Rahman, M.M.; Minami, H.; Sarker, M.S.I.; Islam, M.S.; Ahmad, H. A potential recyclable catalyst: In situ growth of bimetallic Cu-Ag nanoalloy on the magnetic SiO2/Iron oxide-SiO2-NH2 nanocomposite support using a green approach. Colloids Surf. A 2023, 668, 131447. [Google Scholar] [CrossRef]
- Dong, S.; Wang, S.; Wang, X.; Zhai, L. Superparamagnetic nanocomposite Iron oxide@SiO2-NH2/CQDs as fluorescent probe for copper (II) detection. Mater. Lett. 2020, 278, 128404. [Google Scholar] [CrossRef]
- Kim, K.; Lee, K.; So, S.; Cho, S.; Lee, M.; You, K.; Moon, J.; Song, T. Communication-selective adsorption of PEG on SiO2 for high removal selectivity in tungsten CMP. ECS J. Solid State Sci. Technol. 2018, 7, 132–134. [Google Scholar] [CrossRef]
- Spessard, J.E. Investigations of borate equilibria in nuetral salt solutions. J. Inorg. Nucl. Chem. 1970, 36, 2607–2613. [Google Scholar] [CrossRef]
- Lynch, J.M.; Barbano, D.M. Kjeldahl nitrogen analysis as a reference method for protein determination in dairy products. J. Aoac Int. 1999, 82, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Moulder, F.J.; Stickle, F.W.; Sobol, E.P.; Bomben, D.K. Hand Book of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation Physical Electronics Division: Eden Prairie, MN, USA, 1992; pp. 42–43. [Google Scholar]
- Gao, M.; Zhao, X.; Wang, W.; Zou, X.; Song, C. Preparation of fluorescently and biologically active chain-like chitosan nanocomposite and its use in separating MBP-tagged proteins and as fluorescent tracer of tobacco. Sens. Actuators B Chem. 2023, 381, 133371. [Google Scholar] [CrossRef]
- Liang, J.; Han, L.; Li, B.; Shi, Z.-Z.; Liu, X.-C.; Peng, L.-C.; Zhou, X. Fast and efficient immobilization behavior of bifunctional magnetic nano-amendment against multi-heavy metal. Chin. J. Inorg. Chem. 2021, 37, 1981–1990. [Google Scholar]
- Nadarajah, R.; Hossain, M.S.; Siddique, M.B.M.; Arafath, M.A.; Naushad, M.; Lim, J.-W.; Al-Gheethi, A.; Ahmad, H. A review on environ mental Chemodynamics, isothermal, kinetics, and thermodynamics modeling for the adsorptive removal of Cr (VI) from the industrial effluent using magnetic nanoparticles as a bio-sorbent. Environ. Sci. Water Res. Technol. 2023, 9, 1764–1782. [Google Scholar] [CrossRef]
- Chen, W.; Xie, H.; Jiang, N.; Guo, X.; Liu, Z. Synthesis of magnetic sodium lignosulfonate hydrogel (Iron oxide@LS) and its adsorption behavior for Cd2+ in wastewater. Int. J. Biol. Macromol. 2023, 245, 125498. [Google Scholar] [CrossRef]
- Chen, Y.; Lyu, J.; Wang, Y.; Chen, T.; Tian, Y.; Bai, P.; Guo, X. Synthesis, characterization, adsorption, and isotopic separation studies of pyrocatechol-modified MCM-41 for efficient boron removal. Ind. Eng. Chem. Res. 2019, 58, 3282–3292. [Google Scholar] [CrossRef]
- Abbasi, A.; Yahya, W.Z.N.; Nasef, M.M.; Moniruzzaman, M.; Ghumman, A.S.M.; Afolabi, H.K. Boron removal by glucamine-functionalized inverse vulcanized sulfur polymer. React. Funct. Polym. 2022, 177, 105311. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Q.; Zhang, M.; Peng, J.; Dong, Y.; Li, W.; Meng, L. Facile Synthesis of Core-Shell Magnetic Iron Oxide@SiO2-NH2 Nanoparticles and Their Application in Rapid Boron Removal from Aqueous Solutions. Magnetochemistry 2024, 10, 74. https://doi.org/10.3390/magnetochemistry10100074
Hu Q, Zhang M, Peng J, Dong Y, Li W, Meng L. Facile Synthesis of Core-Shell Magnetic Iron Oxide@SiO2-NH2 Nanoparticles and Their Application in Rapid Boron Removal from Aqueous Solutions. Magnetochemistry. 2024; 10(10):74. https://doi.org/10.3390/magnetochemistry10100074
Chicago/Turabian StyleHu, Qinqin, Manman Zhang, Jiaoyu Peng, Yaping Dong, Wu Li, and Lingzong Meng. 2024. "Facile Synthesis of Core-Shell Magnetic Iron Oxide@SiO2-NH2 Nanoparticles and Their Application in Rapid Boron Removal from Aqueous Solutions" Magnetochemistry 10, no. 10: 74. https://doi.org/10.3390/magnetochemistry10100074
APA StyleHu, Q., Zhang, M., Peng, J., Dong, Y., Li, W., & Meng, L. (2024). Facile Synthesis of Core-Shell Magnetic Iron Oxide@SiO2-NH2 Nanoparticles and Their Application in Rapid Boron Removal from Aqueous Solutions. Magnetochemistry, 10(10), 74. https://doi.org/10.3390/magnetochemistry10100074





