Influence of Artificial Shading and SiO2 on Agastache mexicana subsp. mexicana’s Ability to Survive under Water Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Place and Amm Seeds
2.2. Preparation of Substrate and the Sown Seed
2.3. Preparation of Bio-Space
2.4. Calculating Water Irrigation
2.5. Statistics Analysis and Formulas
3. Results and Discussion
3.1. Statistical Results
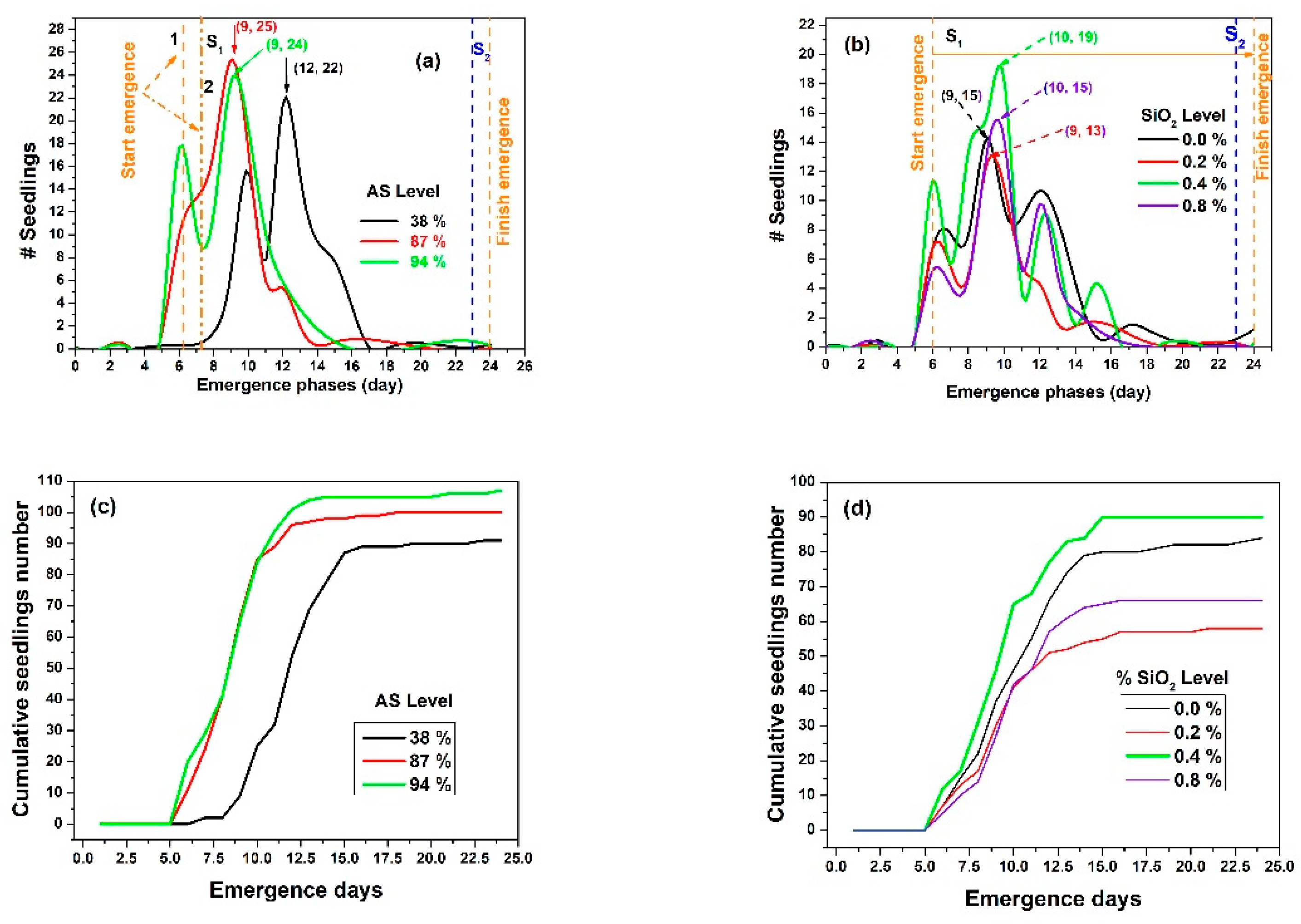
3.2. Emergence Results
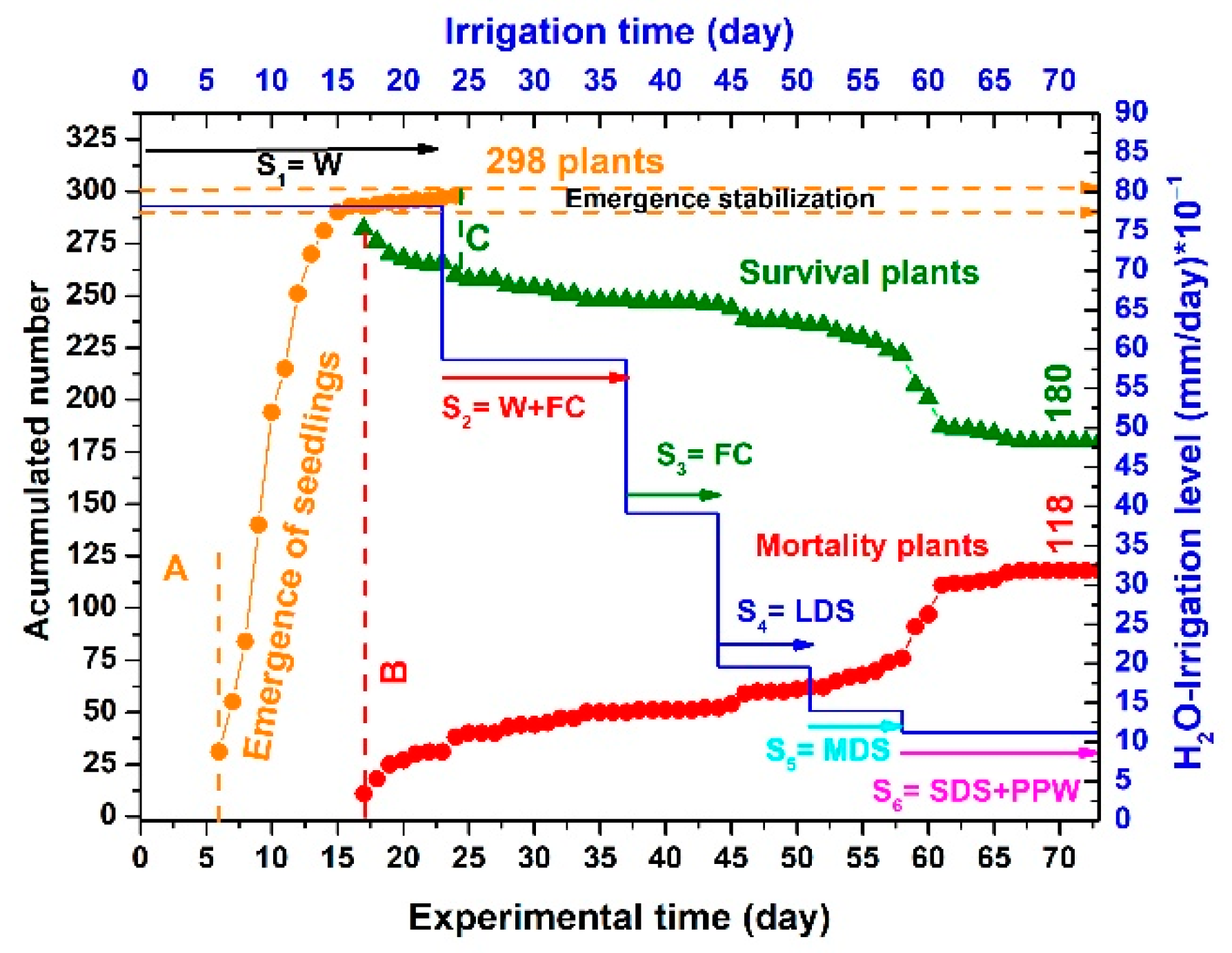
3.3. Emergence, Mortality, and Survival Results
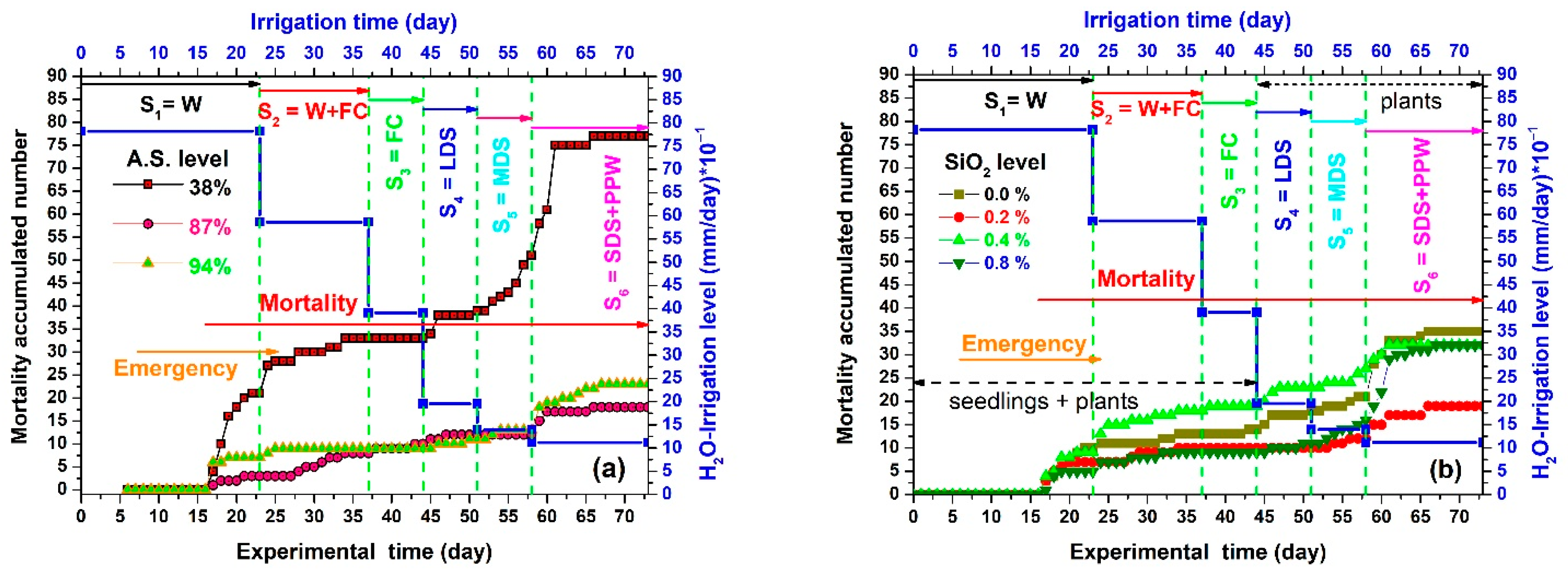
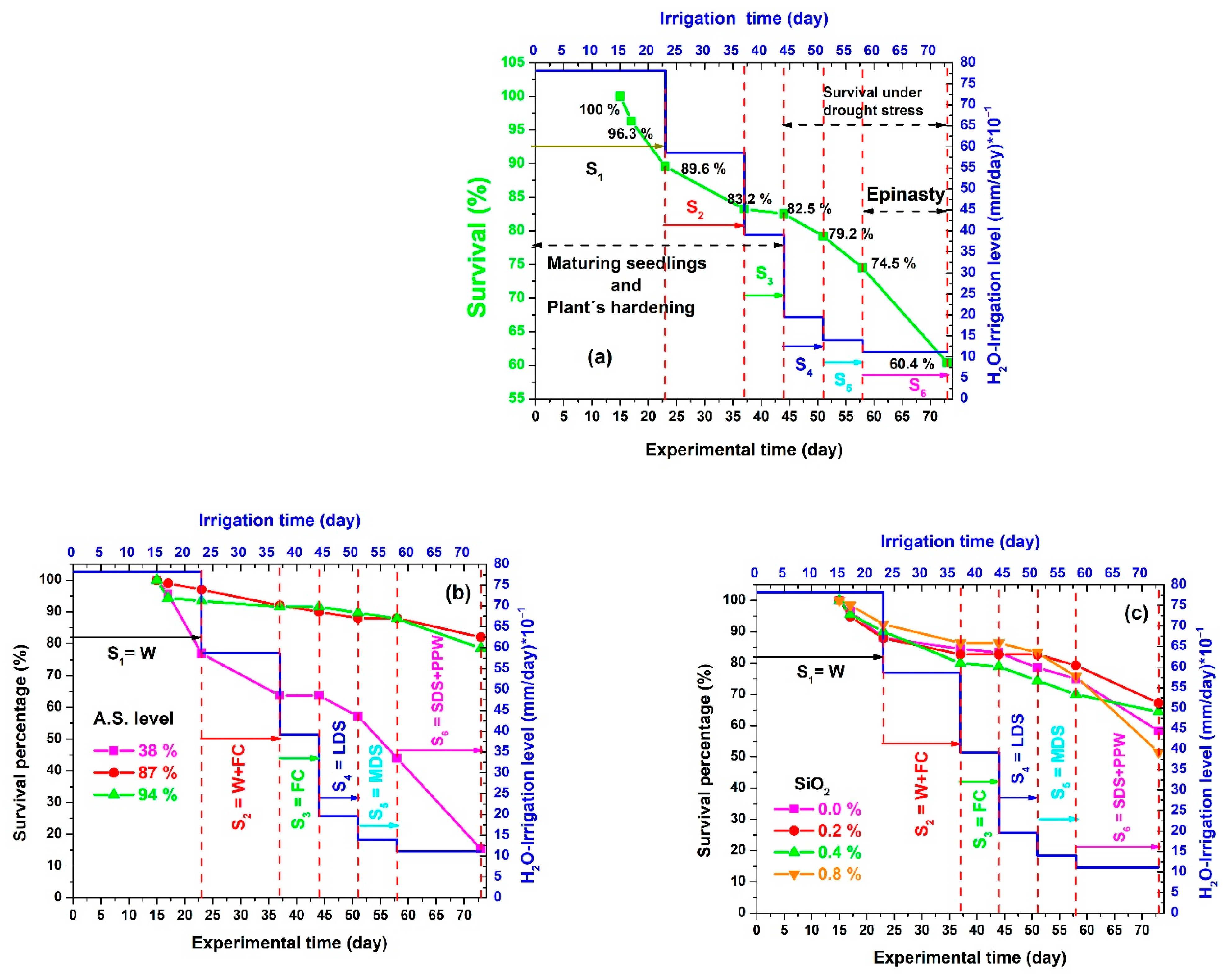
3.4. Hypothetical Analysis
3.4.1. Stage 1 (S1) Waterlogging
3.4.2. Stage 2 (S2) Latest Waterlogging and Early Field Capacity
3.4.3. Stage 3 (S3) Field Capacity
3.4.4. Stage 4 (S4) Low Drought Stress and Available Water Capacity
3.4.5. Stage 5 (S5) Moderate Drought Stress, an Incipient Permanent Wilting Point, and the Beginning of Epinasty
3.4.6. Stage 6 (S6) Severe Drought Stress, Permanent Wilting Point, Epinasty, and Final Survival
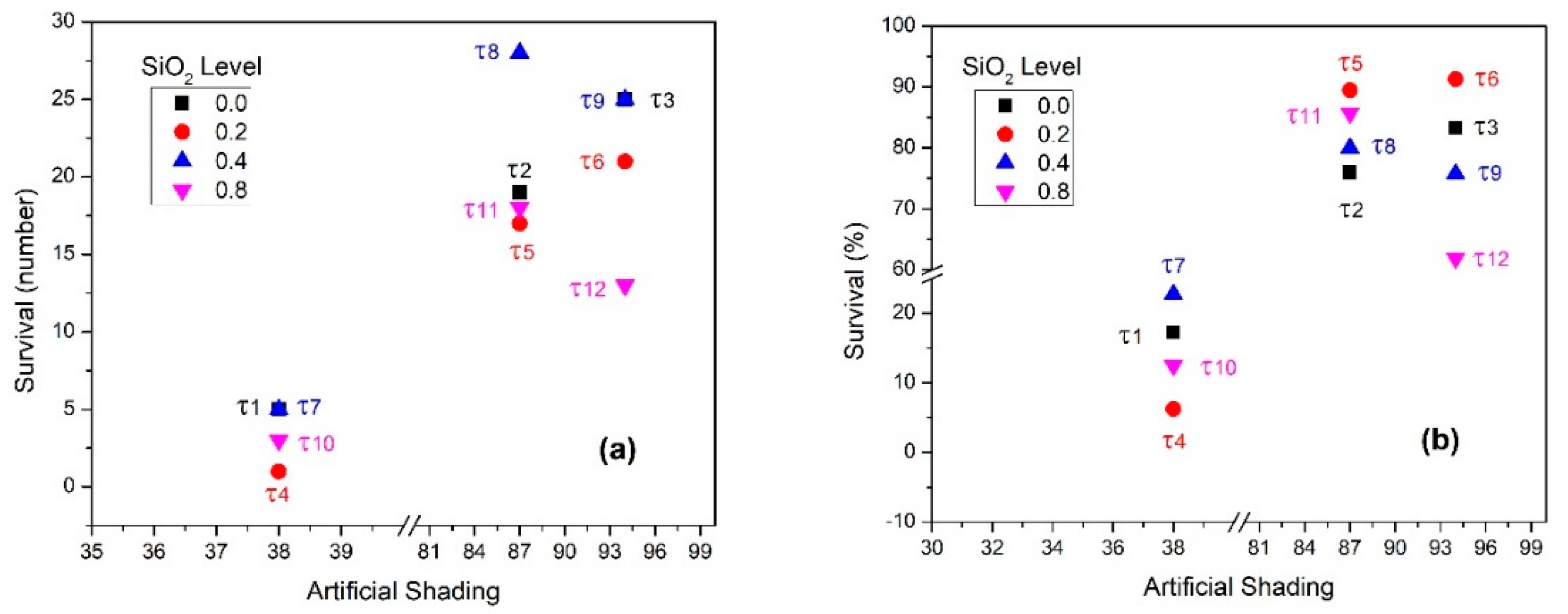
3.5. Results by Treatment
3.6. Future Researches
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- García-Sevilla, R. Respuesta a Estrés Hídrico de Agastache mexicana subsp. Mexicana (Toronjil morado), Debido al Uso de Dióxido de Silicio Amorfo, Sombra y Prefactibilidad Comercial. Bachelor´s Thesis, Facultad de Ciencias Agropecuarias y Ambientales, Universidad Autónoma de Guerrero, Chilpancingo, Mexico, 2022. [Google Scholar]
- Karpiński, T.M. Essential oils of Lamiaceae family plants as antifungals. Biomolecules 2020, 10, 103. [Google Scholar] [CrossRef]
- Palma-Tenango, M.; Sánchez-Fernández, R.E.; Soto-Hernández, M. A systematic approach to Agastache mexicana research. Biology, Agronomy, Phytochemistry, and Bioactivity. Molecules 2021, 26, 3751. [Google Scholar] [CrossRef]
- Santillán-Ramírez, M.A.; López-Villafranco, M.; Aguilar-Rodríguez, S.; Aguilar-Contreras, A. Estudio etnobotánico, arquitectura foliar y anatomía vegetativa de Agastache mexicana ssp. mexicana y A. mexicana ssp. xolocotziana. Rev. Mex. Biodivers. 2008, 79, 513–524. [Google Scholar]
- Carrillo-Galván, G.; Bye, R.; Eguiarte, L.E.; Cristians, S.; Pérez-López, P.; Vergara-Silva, F.; Luna-Cavazos, M. Domestication of aromatic medicinal plants in México: Agastache (Lamiaceae)—An ethnobotanical, morpho-physiological, and phytochemical analysis. J. Ethnobiol. Etnomedicine 2020, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Reyes, R. Estudio Fitoquímico y Evaluación Neurofarmacológica de los “Toronjiles”, Clinopodium mexicanum, Dracocephalum moldavica y Agastache mexicana subespecie mexicana y subespecie Xolocotziana, Utilizados en la Medicina Tradicional Mexicana como Tranquilizantes. Ph.D. Thesis, Universidad Nacional Autónoma de México, Coyoacán, México, 2015. [Google Scholar]
- Estrada-Reyes, R.; López-Rubalcava, C.; Ferreyra-Cruz, O.A.; Dorantes-Barrón, A.M.; Heinze, G.; Aguilar, J.M.; Martínez-Vázquez, M. Central nervous system effects and chemical composition of two subspecies of Agastache mexicana; an ethnomedicine of México. J. Ethnopharmacol. 2014, 153, 98–110. [Google Scholar] [CrossRef]
- Meza Olmedo, A.J.M. Análisis de Flavonoides en Plantas Medicinales por Electroforesis Capilar y Determinación de su Actividad Biológica. Bachelor’s Thesis, Bioprocess Department Biotechnology Interdiscipline Unit, National Politechnique Institute, Ciudad de México, México, 2011. [Google Scholar]
- Navarrete, A.; Ávila-Rosas, N.; Majín-León, M.; Balderas-López, J.L.; Alfaro-Romero, A.; Tavares-Carvalho, J.C. Mechanism of action of relaxant effect of Agastache mexicana ssp. mexicana essential oil in guinea-pig trachea smooth muscle. Pharm. Biol. 2017, 55, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Kambizi, L.; Adebola, P.O.; Afolayan, A.J. Effects of temperature, pre-chilling and light on seed germination of Withania somnifera; a high value medicinal plant. S. Afr. J. Bot. 2006, 72, 11–14. [Google Scholar] [CrossRef]
- Benvenuti, S.; Andolfi, L.; Macchia, M. Light and temperature dependence for germination and emergence of white horehound (Marrubium vulgare L.) seeds. Seed Technol. 2001, 23, 138–144. [Google Scholar]
- Liang, H.; Liu, B.; Wu, C.; Zhang, X.; Wang, M.; Huang, X.; Tang, H. Effects of light intensity on the growth of Polygala fallax Hemsl. (Polygalaceae). Front. Plant Sci. 2022, 13, 3157. [Google Scholar] [CrossRef]
- Petritan, A.M.; Von Lüpke, B.; Petritan, I.C. Effects of shading on growth and mortality of maple (Acer pseudoplatanus), ash (Fraxinus excelsior) and beech (Fagus sylvatica) saplings. Forestry 2007, 80, 397–412. [Google Scholar] [CrossRef]
- Thomas, A.L.; Applequist, W.L.; Rottinghaus, G.E.; Miller, J.S. Black cohosh rhizome and phytochemical production in response to shading, spacing, and age. Acta Hortic. 2010, 925, 175–183. [Google Scholar] [CrossRef]
- Gengmao, Z.; Shihui, L.; Xing, S.; Yizhou, W.; Zipan, C. The role of silicon in physiology of the medicinal plant (Lonicera japonica L.) under salt stress. Sci. Rep. 2015, 5, 12696. [Google Scholar] [CrossRef] [PubMed]
- Tubana, B.S.; Babu, T.; Datnoff, L.E. A review of silicon in soils and plants and its role in US agriculture: History and future perspectives. Soil Sci. 2016, 181, 393–411. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sharifi-Rad, M.; Teixeira da Silva, J.A. Morphological, physiological and biochemical responses of crops (Zea mays L., Phaseolus vulgaris L.), medicinal plants (Hyssopus officinalis L., Nigella sativa L.), and weeds (Amaranthus retroflexus L., Taraxacum officinale FH Wigg) exposed to SiO2 nanoparticles. J. Agric. Sci. Technol. 2016, 18, 1027–1040. [Google Scholar]
- Ashkavand, P.; Zarafshar, M.; Tabari, M.; Mirzaie, J.; Nikpour, A.; Bordbar, S.K.; Struve, D.; Striker, G.G. Application of SiO2 nanoparticles as pretreatment alleviates the impact of drought on the physiological performance of Prunus mahaleb (Rosaceae). Boletín Soc. Argent. Botánica 2018, 53, 207–219. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E. Silicon: Its manifold roles in plants. Ann. Appl. Biol. 2009, 155, 155–160. [Google Scholar] [CrossRef]
- Pérez, J.C.R.; Mancilla, C.L.A. El Papel del Silicio en los Organismos y Ecosistemas; Conciencia Tecnológica: Celaya Guanajuato, México, 2012; pp. 42–46. [Google Scholar]
- Epstein, E. Annual review of plant physiology and plant molecular biology. Silicon 1999, 50, 641–664. [Google Scholar]
- Villalón-Mendoza, H.; Castillo-Villarreal, M.A.; Garza-Ocañas, F.; Guevara-González, J.A.; Sánchez-Castillo, L. Dióxido de silicio como estimulante del índice de calidad de plantas de chile piquín (Capsicum annuum L. var. glabriusculum) producidas en vivero. Rev. Mex. Cienc. For. 2018, 9, 294–303. [Google Scholar] [CrossRef]
- Corzo, M. Experiencias experimentales del uso del Silicio como sustituto de fertilizantes en el cultivo de Palma de Aceite. In Memorias 1er Simposio Internacional Beneficios del Silicio en la Agricultura; Ibagué: Tolima, Colombia, 2013; pp. 6–17. [Google Scholar]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Pardos, J.A. Respuestas de las plantas al anegamiento del suelo. For. Syst. 2004, 13, 101–107. [Google Scholar] [CrossRef]
- Xie, L.J.; Zhou, Y.; Chen, Q.F.; Xiao, S. New insights into the role of lipids in plant hypoxia responses. Prog. Lipid Res. 2021, 81, 101072. [Google Scholar] [CrossRef]
- Murillo Gamboa, O. Variación en parámetros de germinación de una población natural de Alnus acuminata de Guatemala. Boletín Mejor. Genét. Semillas For. 1998, 19. [Google Scholar]
- Martínez, M.A.; Montechiarini, N.H.; Gosparini, C.O.; Arango, M.R.; Gallo, C.D.V.; Craviotto, R.M. Viabilidad, Vigor y Germinación de Semillas Verdes de Soja. 2019. Available online: https://ri.conicet.gov.ar/bitstream/handle/11336/175758/CONICET_Digital_Nro.d2c87edb-87fe-4c2a-a6f6-75da86711c0a_B.pdf?sequence=2&isAllowed=y (accessed on 3 August 2023).
- Zaidi, P.H.; Rafique, S.; Singh, N.N. Response of maize (Zea mays L.) genotypes to excess soil moisture stress: Morpho-physiological effects and basis of tolerance. Eur. J. Agron. 2003, 19, 383–399. [Google Scholar] [CrossRef]
- Marinho, J.D.L.; Costa, D.S.D.; Carvalho, D.U.D.; Cruz, M.A.D.; Zucareli, C. Evaluation of vigor and tolerance of sweet corn seeds under hypoxia. J. Seed Sci. 2019, 41, 180–186. [Google Scholar] [CrossRef]
- Janislampi, K.W. Effect of Silicon on Plant Growth and Drought Stress Tolerance; Utah State University: Logan, UT, USA, 2012. [Google Scholar]
- Passioura, J.B. Roots and drought resistance. Dev. Agric. Manag. For. Ecol. 1983, 12, 265–280. [Google Scholar]
- Lichtenthaler, H.K. Vegetation stress: An introduction to the stress concept in plants. J. Plant Physiol. 1996, 148, 4–14. [Google Scholar] [CrossRef]
- Minguez, M.P.; Casanueva, G.M.; de Dios García, J.; Alonso, F.J.G.; Sainz, R.C. Respuesta temporal al ambiente lumínico y la sequía inducida en el regenerado de una masa mixta en el entorno mediterráneo. Cuadernos de la SECF 2019, 45, 1–18. [Google Scholar] [CrossRef]
- Padilla, F.M.; Pugnaire, F.I. Rooting depth and soil moisture control Mediterranean woody seedling survival during drought. Funct. Ecol. 2007, 21, 489–495. [Google Scholar] [CrossRef]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef]
- Chourasia, K.N. Resistance/Tolerance mechanism under water deficit (Drought) condition in plants. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 66–78. [Google Scholar]
| Treatments | ||||
|---|---|---|---|---|
| AS (%) | SiO2 (%) | |||
| 0.0 | 0.2 | 0.4 | 0.8 | |
| 38 | τ1 | τ4 | τ7 | τ10 |
| 87 | τ2 | τ5 | τ8 | τ11 |
| 94 | τ3 | τ6 | τ9 | τ12 |
| Stage | S1 | S2 | S3 | S4 | S5 | S6 |
|---|---|---|---|---|---|---|
| Duration (days) | 23 | 13 | 7 | 7 | 7 | 15 |
| Accumulated (days) | 24 | 37 | 44 | 51 | 58 | 73 |
| Water irrigation (mm/day) | 7.82 | 5.86 | 3.91 | 1.95 | 1.39 | 1.11 |
| Output Factor | Ratio |
|---|---|
| SE | # emergence of seeds/# total seeds per level |
| MP | (# dead plants/total emergence of seeds)*100 |
| SP | (# survival plants/total emergence of seeds)*100 |
| MI | # dead plants/# days by stage |
| Emergence peak value | Accumulated number of seed emergences/correspondent day |
| Output Factor Input Factor | Emergence | Mortality | Survival | ||||
|---|---|---|---|---|---|---|---|
| Level or Treatment | ** p-Value < 0.05 | Mean | p-Value < 0.05 | Mean | p-Value < 0.05 | Mean | |
| AS*SiO2 Interaction | Whole levels | 0.76 | --- | 0.82 | --- | 0.75 | --- |
| AS (%) | 38 | 0.62 | 5.68 a | 0.0001 * | 4.81 a | 0.0001 * | 0.87 b |
| 87 | 6.25 a | 1.12 b | 5.12 a | ||||
| 94 | 6.68 a | 1.43 b | 5.25 a | ||||
| SiO2 (%) | 0.0 | 0.09 | 7.0 a | 0.32 | 2.91 a | 0.15 | 4.08 a |
| 0.2 | 4.83 a | 1.58 a | 3.25 a | ||||
| 0.4 | 7.50 a | 2.66 a | 4.83 a | ||||
| 0.8 | 5.5 a | 2.66 a | 2.83 a | ||||
| Treatments | τ1 | 0.45 | 7.25 a | 0.0006 * | 6.0 a | 0.0003 * | 1.25 bc |
| τ2 | 6.25 a | 1.50 bac | 4.75 bac | ||||
| τ3 | 7.50 a | 1.25 bc | 6.25 ba | ||||
| τ4 | 4.00 a | 3.75 bac | 0.25 c | ||||
| τ5 | 4.75 a | 0.50 c | 4.25 bac | ||||
| τ6 | 5.75 a | 0.50 c | 5.25 bac | ||||
| τ7 | 5.50 a | 4.25 bac | 1.25 bc | ||||
| τ8 | 8.75 a | 1.75 bac | 7.0 a | ||||
| τ9 | 8.25 a | 2.0 bac | 6.25 ba | ||||
| τ10 | 6.0 a | 5.25 ba | 0.75 bc | ||||
| τ11 | 5.25 a | 0.75 bc | 4.50 bac | ||||
| τ12 | 5.25 a | 2.0 bac | 3.25 bac | ||||
| Source | DF | SS | MS | F | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E | M | S | E | M | S | E | M | S | E | M | S | |
| AS | 2 | 2 | 2 | 8.04 | 133.79 | 198.5 | 4.02 | 66.89 | 99.25 | 0.49 | 19.18 | 19.57 |
| SiO2 | 3 | 3 | 3 | 56.25 | 12.75 | 28.5 | 18.75 | 4.25 | 9.5 | 2.29 | 1.21 | 1.87 |
| AS*SiO2 | 6 | 6 | 6 | 27.62 | 9.87 | 17.5 | 4.6 | 1.64 | 2.91 | 0.56 | 0.47 | 0.57 |
| Error | - | - | - | 36 | 36 | 36 | 294 | 125.5 | 182.5 | 8.16 | 3.48 | 5.06 |
| Input Factor Level/Treatment | Total Seeds per Level/Treatment Sown (#) | Emergence Seeds (#) | SE (%) | Emergent Peak Value | Peak Day |
|---|---|---|---|---|---|
| 38% AS | 320 | 91 | 28.4 | 5.80 | 12 |
| 87% AS | 320 | 100 | 31.3 | 8.50 | 9 |
| 94% AS | 320 | 107 | 33.4 | 8.54 | 9 |
| 0.0% SiO2 | 240 | 84 | 35.0 | 5.69 | 9 |
| 0.2% SiO2 | 240 | 58 | 24.2 | 4.25 | 9 |
| 0.4% SiO2 | 240 | 90 | 37.5 | 6.41 | 10 |
| 0.8% SiO2 | 240 | 66 | 27.5 | 4.75 | 10 |
| τ1 | 80 | 29 | 36.2 | 1.86 | 12 |
| τ2 | 80 | 25 | 31.2 | 2.11 | 9 |
| τ3 | 80 | 30 | 37.5 | 2.33 | 9 |
| τ4 | 80 | 16 | 20.0 | 1.00 | 10 |
| τ5 | 80 | 19 | 23.7 | 1.60 | 9 |
| τ6 | 80 | 23 | 28.7 | 2.00 | 9 |
| τ7 | 80 | 22 | 27.5 | 1.47 | 13 |
| τ8 | 80 | 35 | 43.7 | 3.00 | 8 |
| τ9 | 80 | 33 | 41.2 | 2.90 | 6 |
| τ10 | 80 | 24 | 30.0 | 1.64 | 12 |
| τ11 | 80 | 21 | 26.2 | 1.90 | 9 |
| τ12 | 80 | 21 | 26.2 | 1.62 | 10 |
| Point | Day | Total Emergent Seedlings | Total Mortality | Total Survival | Description |
|---|---|---|---|---|---|
| A | 6 | 31 | 0 | 31 | Beginning emergence |
| B | 17 | 293 | 11 | 282 | Beginning mortality |
| C | 24 | 298 | 39 | 253 | Finishing emergence |
| Stage | Hypothetical Factor | Description of the Stage | Mortality Index (MI) (Plants/day) |
|---|---|---|---|
| S1 | W | Waterlogging | 1.35 |
| S2 | W + FC | Waterlogging and beginning of field capacity | 1.46 |
| S3 | FC | Field capacity | 0.29 |
| S4 | LDS | Low drought stress | 1.43 |
| S5 | MDS | Moderate drought stress, an incipient permanent wilting point, and the beginning of epinasty | 2.0 |
| S6 | SDS + PPW | Severe drought stress, permanent wilting point, epinasty, and final survival | 2.8 |
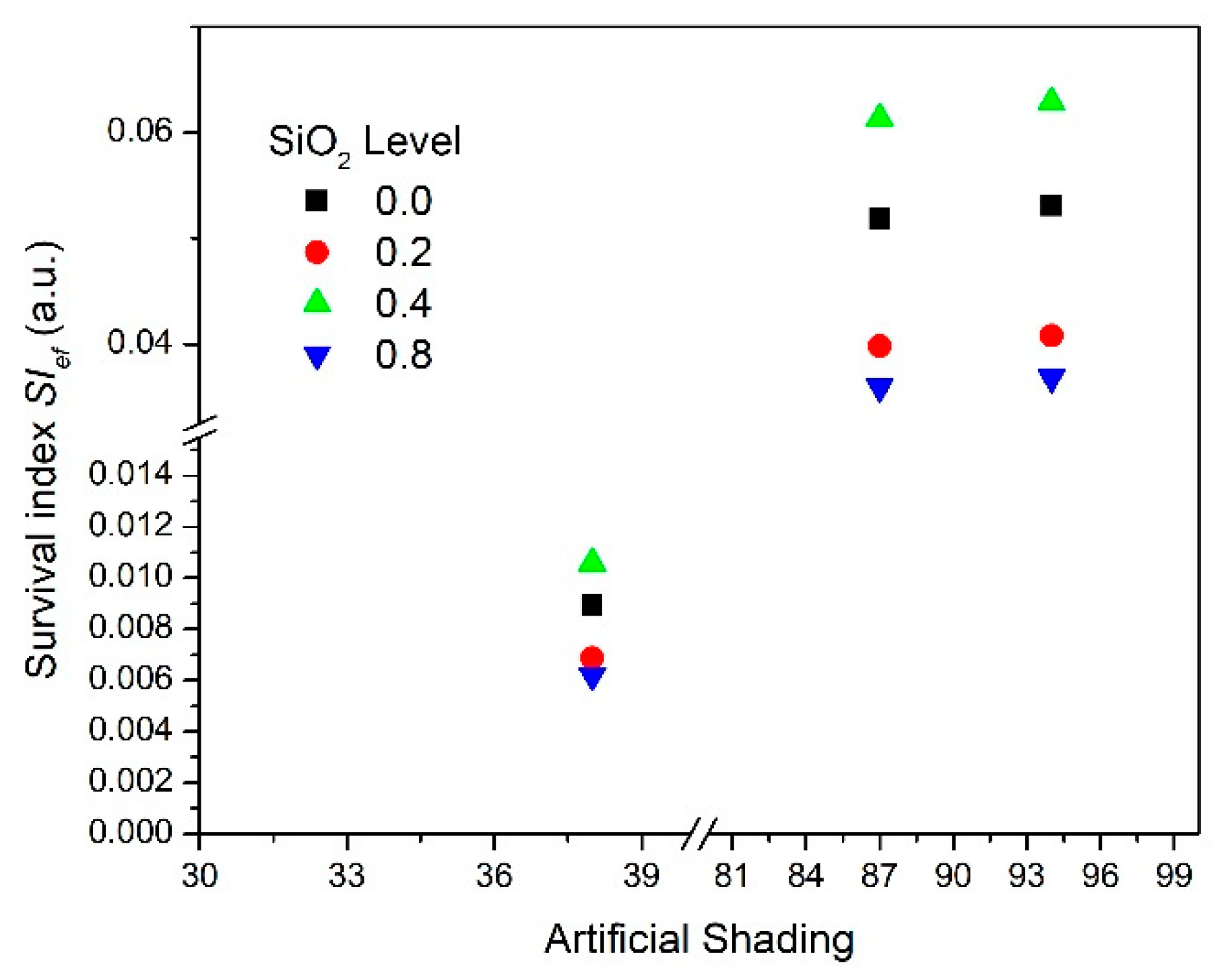
| Silicon Dioxide Effect | |||
| SiO2% | SP | SE | Sef1 |
| 0 | 0.58 | 0.35 | 0.20 |
| 0.2 | 0.67 | 0.23 | 0.15 |
| 0.4 | 0.64 | 0.38 | 0.24 |
| 0.8 | 0.52 | 0.28 | 0.14 |
| Artificial shading effect | |||
| AS% | SP | SE | Sef2 |
| 38 | 0.15 | 0.28 | 0.04 |
| 87 | 0.82 | 0.31 | 0.25 |
| 94 | 0.79 | 0.33 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reséndiz-Muñoz, J.; Cruz-Lagunas, B.; Fernández-Muñoz, J.L.; de Jesús Adame-Zambrano, T.; Delgado-Núñez, E.J.; Zagaceta-Álvarez, M.T.; Aguilar-Cruz, K.A.; Urbieta-Parrazales, R.; Miranda-Viramontes, I.; Morales-Barrera, J.; et al. Influence of Artificial Shading and SiO2 on Agastache mexicana subsp. mexicana’s Ability to Survive under Water Stress. Horticulturae 2023, 9, 995. https://doi.org/10.3390/horticulturae9090995
Reséndiz-Muñoz J, Cruz-Lagunas B, Fernández-Muñoz JL, de Jesús Adame-Zambrano T, Delgado-Núñez EJ, Zagaceta-Álvarez MT, Aguilar-Cruz KA, Urbieta-Parrazales R, Miranda-Viramontes I, Morales-Barrera J, et al. Influence of Artificial Shading and SiO2 on Agastache mexicana subsp. mexicana’s Ability to Survive under Water Stress. Horticulturae. 2023; 9(9):995. https://doi.org/10.3390/horticulturae9090995
Chicago/Turabian StyleReséndiz-Muñoz, Juan, Blas Cruz-Lagunas, José Luis Fernández-Muñoz, Tania de Jesús Adame-Zambrano, Edgar Jesús Delgado-Núñez, María Teresa Zagaceta-Álvarez, Karen Alicia Aguilar-Cruz, Romeo Urbieta-Parrazales, Isaias Miranda-Viramontes, Judith Morales-Barrera, and et al. 2023. "Influence of Artificial Shading and SiO2 on Agastache mexicana subsp. mexicana’s Ability to Survive under Water Stress" Horticulturae 9, no. 9: 995. https://doi.org/10.3390/horticulturae9090995
APA StyleReséndiz-Muñoz, J., Cruz-Lagunas, B., Fernández-Muñoz, J. L., de Jesús Adame-Zambrano, T., Delgado-Núñez, E. J., Zagaceta-Álvarez, M. T., Aguilar-Cruz, K. A., Urbieta-Parrazales, R., Miranda-Viramontes, I., Morales-Barrera, J., Sevilla-García, R., & Gruintal-Santos, M. A. (2023). Influence of Artificial Shading and SiO2 on Agastache mexicana subsp. mexicana’s Ability to Survive under Water Stress. Horticulturae, 9(9), 995. https://doi.org/10.3390/horticulturae9090995





