Clonal Selection of Autochthonous Grape Varieties in Badacsony, Hungary
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site, Vineyard and Growing Conditions
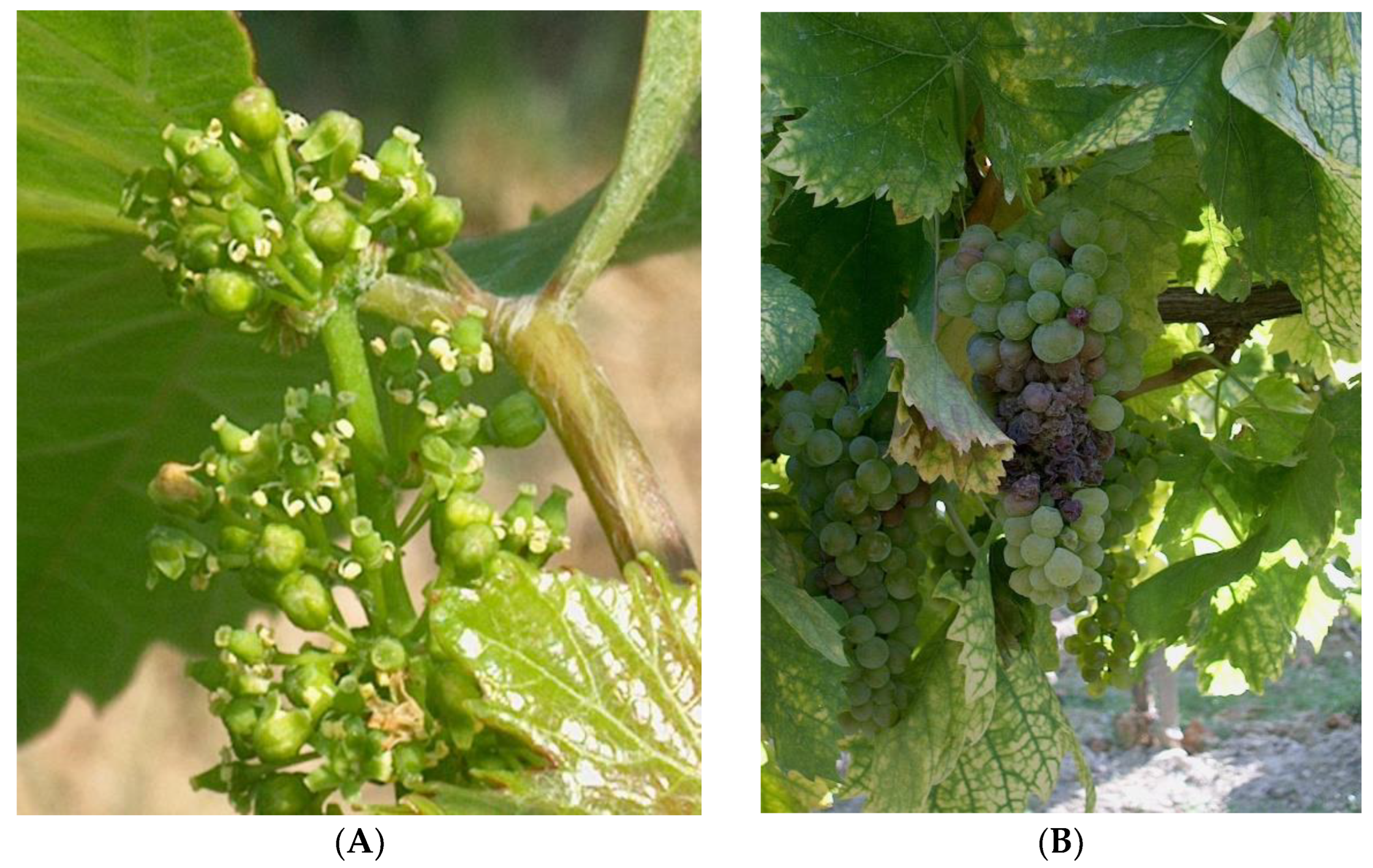
2.2. Experimental Harvest, Measures
2.3. Data Analysis
3. Results
3.1. Evaluation of the Meteorological Data and Indices
3.1.1. K-Means Clustering and Principal Component Analyses (PCA) of the Years
3.1.2. Evaluation of PCA Results of Agrometeorological Indices for ‘Kéknyelű’ Phenophases
- growing degree-days from budburst to the beginning of flowering (GDD1)
- growing degree-days from the end of flowering to veraison (GDD3),
- Huglin index from budburst to the beginning of flowering (HI1) and
- Huglin index from the end of flowering to veraison (HI3) defying the year group 3. This may mean, that the temperature in these periods has fundamental importance in the development of ‘Kéknyelű’ in years of group 3.
- hydrothermal coefficient during flowering (HTC2) and
- cumulative rainfall (precipitation) during flowering (P2) while the next indices have negative associations with both PC1 and PC2:
- hydrothermal coefficient from budburst to the beginning of flowering (HTC1),
- hydrothermal coefficient from the end of flowering to veraison (HTC3),
- Huglin index from veraison to maturity (HI4) and
- growing degree-days from veraison to maturity (GDD4).
- Huglin index during flowering (HI2),
- growing degree-days during flowering (GDD2) and
- hydrothermal coefficient during berry maturity (HTC4) defying the year group 2. In this group of years, the importance of the temperature during flowering could be emphasized.
3.1.3. Evaluation of PCA Results of Agrometeorological Indices for ‘Juhfark’
- growing degree-days from the end of flowering to veraison (GDD3) and
- Huglin index from the end of flowering to veraison (HI3)defying the year group 1 for ‘Juhfark’, probably meaning that the temperature in this phenological stage (berry development) has fundamental impact on the productivity of this variety in the years of group 1.
- growing degree-days from budburst to the beginning of flowering (GDD1),
- Huglin index from budburst to the beginning of flowering (HI1) and
- hydrothermal coefficient during flowering (HTC2) are in negative association with principal component 2 while
- Huglin index during berry maturity (HI4) and
- growing degree-days during berry maturity (GDD4) are in negative association with both principal components 1 or 2 of the years for ‘Juhfark’ defying group 3. This may mean, that in these years the most defying meteorological parameter from budburst to the beginning of flowering and during berry maturity was temperature, while rainfall also played an important role during flowering.
- Huglin index during flowering (HI2),
- growing degree-days during flowering (GDD2),
- hydrothermal coefficient from budburst to the beginning of flowering (HTC1),
- hydrothermal coefficient during berry maturity (HTC4) and
- cumulative rainfall (precipitation) during berry maturity (P4).
3.2. Evaluation of the Harvest Results of ‘Kéknyelű’
3.3. Evaluation of the Results of the Harvest of the Variety ‘Juhfark’
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- van Leeuwen, C.; Darriet, P. The Impact of Climate Change on Viticulture and Wine Quality. J. Wine Econ. 2016, 11, 150–167. [Google Scholar] [CrossRef]
- van Leeuwen, C.; Destrac-Irvine, A. Modified Grape Composition under Climate Change Conditions Requires Adaptations in the Vineyard. Oeno One 2017, 51, 147–154. [Google Scholar] [CrossRef]
- Ollat, N.; van Leeuwen, C.; de Cortazar-Atauri, I.G.; Touzard, J.M. The Challenging Issue of Climate Change for Sustainable Grape and Wine Production. Oeno One 2017, 51, 59–60. [Google Scholar] [CrossRef]
- Németh, K. Klímaváltozás Hatása a Borszőlő Biológiai Jellemzőire, Termésmennyiségére, Minőségére. Értékálló Aranykorona 2012, 12, 6–7. [Google Scholar]
- Németh, K. Száraz Termőhelyen. Kert. Szolesz. 2015, 64, 20–21. [Google Scholar]
- Mesterházy, I.; Mészáros, R.; Pongrácz, R. The Effects of Climate Change on Grape Production in Hungary. Időjárás Q. J. Hung. Meteorol. Serv. 2014, 118, 193–206. [Google Scholar]
- Szenteleki, K.; Ladányi, M.; Gaál, M.; Zanathy, G.; Bisztray, G.Y. Climatic Risk Factors of Central Hungarian Grape Growing Regions. Appl. Ecol. Env. Res. 2011, 10, 87–105. [Google Scholar] [CrossRef]
- Tkhakushinov, E.K.; Hachemizova, E.A. Development Problems of the Regional Agro-Industrial Complex Management System. Int. J. Eng. Technol. 2018, 7, 329–332. [Google Scholar] [CrossRef]
- Ostroukhova, E.; Levchenko, S.; Vasylyk, I.; Volynkin, V.; Lutkova, N.; Boyko, V. Comparison of the Phenolic Complex of Crimean Autochthonous and Classic White-Berry Grape Cultivars. E3S Web Conf. 2020, 161, 01059. [Google Scholar] [CrossRef]
- Májer, J.; Győrffyné Jahnke, G. Autochton Szőlőfajták Optimális Termesztéstechnológiáját Megalapozó Kísérletek Eredményei Badacsonyban. Borászati Füzetek 2005, 17, 4–9. [Google Scholar]
- Somogyi, N.; Németh, K. A Kárpát-Medence Kincsei Határon Innen És Túl. Mezőhír: Országos Agrárinform. Szakl. 2018, 22, 44–46. [Google Scholar]
- Knolmajer-Szigeti, G.; Kocsis, L.; Hoffmann, S.; Májer, J.; Jahnke, G. Comparison of the Mid-Wire Cordon and the Umbrella Training System with the Grapevine Varieties “Olaszrizling” (‘Welschriesling’), “Szürkebarát” (‘Pinot Gris’) and “Kéknyelű” Varieties in Badacsony (Hungary). Mitt. Klosterneubg. Rebe Wein Obs. Fruchteverwert. 2014, 64, 44–53. [Google Scholar]
- Jahnke, G.G.; Májer, J. Results of the Experiments for the Improvement of the Fertilisation of the Functional Female Flowered Grapevine Cultivar “Kéknyelu”. Acta Hortic. 2003, 603, 767–773. [Google Scholar] [CrossRef]
- Goethe, H. Handbuch Der Ampelographie; Verlag Parely: Berlin, Germany, 1887. [Google Scholar]
- Németh, M. Ampelográfiai Album I. (Termesztett Borszőlőfajtáink 1.); Mezőgazdasági Kiadó: Budapest, Hungary, 1967. [Google Scholar]
- Jahnke, G.; Korbuly, J.; Májer, J.; Györffyné Molnár, J. Discrimination of the Grapevine Cultivars “Picolit” and “Kéknyelu” with Molecular Markers. Sci. Hortic. 2007, 114, 71–73. [Google Scholar] [CrossRef]
- Györffyné Jahnke, G.; Májer, J.; Korbuly, J. Distinguishing the Grapevine Cultivars “Picolit” and “Kéknyelü” with Isozymes and Microsatellite Markers. Acta Hortic. 2009, 827, 159–162. [Google Scholar] [CrossRef]
- Bakonyi, L.; Bényei, F.; Fazekas, I.; Hajdu, E.; Korbuly, J.; Lőrincz, A.; Marcinkó, F.; Pernesz, G.Y.; Romenda, R.; Zanathy, G. Borszőlőfajták, Csemegeszőlő-Fajták És Alanyok; Bényei, F., Lőrincz, A., Eds.; Mezőgazda Kiadó: Budapest, Hungary, 2005; ISBN 9789632865362. [Google Scholar]
- Varga, Z.; Bényei, F.; Lőrincz, A.; Ulz, A. Yields and Quality of Former Vitis-Cultivars at Tokaj-Hegyalja. Borászati Füzetek 2006, 16, 1–8. [Google Scholar]
- Varga, Z.; Ferenczy, A.; Bényei, F.; Zanathy, G. Examination of the Relations of Origin of Old Grape Vine Cultivars with Cluster Analysis in Tokaj-Hegyalja. Acta Hortic. 2009, 827, 169–176. [Google Scholar] [CrossRef]
- Halász, G.; Veres, A.; Kozma, P.; Kiss, E.; Balogh, A.; Galli, Z.; Szőke, A.; Hoffmann, S.; Heszky, L. Microsatellite Fingerprinting of Grapevine (Vitis Vinifera L.) Varieties of the Carpathian Basin. Vitis 2005, 44, 173–180. [Google Scholar]
- Lencsés, A.K.; Szőke, A.; Kozma, P.; Halász, G.; Katuláné Debreceni, D.; Veres, A.; Györffyné Jahnke, G.; Kiss, E. Mikroszatellit Markerek Alkalmazása a Magyarországi Szőlő Génforrások Megőrzésére. Kertgazdasag—Hortic. 2010, 42, 58–67. [Google Scholar]
- Jahnke, G.; Májer, J.; Lakatos, A.; Molnár, J.G.; Deák, E.; Stefanovits-Bányai, E.; Varga, P. Isoenzyme and Microsatellite Analysis of Vitis Vinifera, L. Varieties from the Hungarian Grape Germplasm. Sci. Hortic. 2009, 120, 213–221. [Google Scholar] [CrossRef]
- Jahnke, G.; Májer, J.; Varga, Z.; Deák, E.; Varga, P. An Acid Phosphatase Isoenzyme Pattern Is Characteristic for the Pontican Cultivars. Cereal Res. Commun. 2009, 37, 113–116. [Google Scholar] [CrossRef]
- Bodor, P.; Varga, Z.; Deák, T.; Pedryc, A.; Bisztray, G.D. Old Hungarian Grapevine Cultivars and Their Relations Characterized with Microsatellite Markers. Int. J. Hortic. Sci. 2008, 14, 27–31. [Google Scholar] [CrossRef]
- Kozma, P. A Szőlő Nemesítése. (Breeding of the Vine); Mezőgazdasági Kiadó: Budapest, Hungary, 1951. [Google Scholar]
- Hajdu, E. Grapevine Breeding in Hungary. In Grapevine Breeding Programs for the Wine Industry; Woodhead Publishing: Sawston, UK, 2015; pp. 103–134. [Google Scholar] [CrossRef]
- Németh, M. A Szőlő Klónszelekciós Nemesítéséről. (About the Clonal Selection of the Vine). Agrártudomány 1958, 10, 43–49. [Google Scholar]
- Lunz, O. A Klónszelekcióü Hazai Helyzete És Eredményei (National Situataion and Results of Clonal Selection). Szőlőtermesztés És Borászat 1990, 12, 2–7. [Google Scholar]
- Hajdu, E.; Korać, N.; Cindrić, P.; Medić, M. Genetical Variations in Vine (Mutation) The Importance of Clonal Selection of Grapevine and the Role of Selected Clones in Production of Healthy Propagating Stocks. Int. J. Hortic. Sci. 2011, 17, 15–24. [Google Scholar] [CrossRef]
- Hajdu, E. A Kertészeti Növények Nemesítése a Szőlő Példáján. (The Horticultural Plant Breeding on the Example of Grape. Gradus 2016, 3, 378–383. [Google Scholar]
- Szűgyi-Reiczigel, Z.; Ladányi, M.; Bisztray, G.D.; Varga, Z.; Bodor-Pesti, P. Morphological Traits Evaluated with Random Forest Method Explains Natural Classification of Grapevine (Vitis Vinifera L.) Cultivars. Plants 2022, 11, 3428. [Google Scholar] [CrossRef]
- Cousins, P.; Garris, A. Quality Improvement in “Vignoles” through Clonal Selection. Acta Hortic. 2014, 1046, 287–290. [Google Scholar] [CrossRef]
- Stover, E.; Aradhya, M.; Dangl, J.; Prins, B.; Cousins, P. Grape Genetic Resources and Research at the Davis California National Clonal Germplasm Repository. Acta Hortic. 2009, 827, 193–196. [Google Scholar] [CrossRef]
- Győrffyné Jahnke, G.; Knolmájerné Szigeti, G.; Németh, C.; Nagy, Z.A.; Májer, J. Kéknyelű És Juhfark Fajták Klónszelekciója Badacsonyban. Agrofórum Extra 2018, 46, 26–28. [Google Scholar]
- Jahnke, G.; Májer, J.; Varga, P.; Szöke, B. Analysis of Clones of Pinots Grown in Hungary by SSR Markers. Sci. Hortic. 2011, 129, 32–37. [Google Scholar] [CrossRef]
- Lemos, A.M.; Machado, N.; Egea-Cortines, M.; Barros, A.I. ATR-MIR Spectroscopy as a Tool to Assist ‘Tempranillo’ Clonal Selection Process: Geographical Origin and Year of Harvest Discrimination and Oenological Parameters Prediction. Food Chem. 2020, 325, 126938. [Google Scholar] [CrossRef] [PubMed]
- Zombardo, A.; Meneghetti, S.; Morreale, G.; Calò, A.; Costacurta, A.; Storchi, P. Study of Inter-and Intra-Varietal Genetic Variability in Grapevine Cultivars. Plants 2022, 11, 397. [Google Scholar] [CrossRef] [PubMed]
- Pejić, I.; Šimon, S.; Preiner, D.; Žulj Mihaljević, M.; Maletić, E.; Zdunić, G.; Petric, I.V.; Anhalt, U.; Forneck, A.; Ruehl, E. Estimate of Intravarietal Genetic Variation as a Prerequisite for Successful Clonal Selection in Grapevine. Acta Hortic. 2015, 1082, 105–112. [Google Scholar] [CrossRef]
- Doulati Baneh, H. Molecular Markers to Detect Genetic Variation in Superior Clones of Grapevine (Vitis Vinifera L. ’Keshmeshi’). Acta Hortic. 2015, 1082, 183–188. [Google Scholar] [CrossRef]
- Vezzulli, S.; Leonardelli, L.; Malossini, U.; Stefanini, M.; Velasco, R.; Moser, C. Assessing the Genetic Variability of Grape Clones. Acta Hortic. 2014, 1046, 357–362. [Google Scholar] [CrossRef]
- Anhalt, U.C.M.; Crespo Martínez, S.; Rühl, E.; Forneck, A. An AFLP-Marker Study of the Vitis Vinifera L. Cultivar “White Riesling” Comprising 86 Clones to Investigate the Stability of Clones. Acta Hortic. 2014, 1046, 681–684. [Google Scholar] [CrossRef]
- Lorenz, D.H.; Eichhorn, K.W.; Bleiholder, H.; Klose, R.; Meier, U.; Weber, E. Growth Stages of the Grapevine: Phenological Growth Stages of the Grapevine (Vitis Vinifera L. Ssp. Vinifera)—Codes and Descriptions According to the Extended BBCH Scale. Aust. J. Grape Wine Res. 1995, 1, 100–103. [Google Scholar] [CrossRef]
- Shahood, R.; Torregrosa, L.; Savoi, S.; Romieu, C. First Quantitative Assessment of Growth, Sugar Accumulation and Malate Breakdown in a Single Ripening Berry. OENO One 2020, 54, 1077–1092. [Google Scholar] [CrossRef]
- Antalick, G.; Šuklje, K.; Blackman, J.W.; Schmidtke, L.M.; Deloire, A. Performing Sequential Harvests Based on Berry Sugar Accumulation (Mg/Berry) to Obtain Specific Wine Sensory Profiles. OENO One 2021, 55, 131–146. [Google Scholar] [CrossRef]
- Lukácsy, G.; Szabó-Erdélyi, É.; Winkler, T.; Kelenfy, Z.B.; Zanathy, G.; Kocsis, L.; Bisztray, G.D. Determination of Sample Size at Harvest Sampling of Wine Grapes. Kertgazdasag Hortic. 2011, 43, 44–51. [Google Scholar]
- Arnold, C.Y. The Determination and Significance of the Base Temperature in a Linear Heat Unit System. Proc. Am. Soc. Hortic. Sci. 1959, 74, 430–445. [Google Scholar]
- Huglin, M.P. Nouveau Mode d’évaluation Des Possibilités Héliothermiques d’un Milieu Viticole. Comptes Rendus L’acad. D’gric. Fr. 1978, 64, 1117–1126. [Google Scholar]
- Selyaninov, G.L. About the Agricultural Evaluation of the Climate. Proc. Agric. Meteorol. 1928, 20, 177–185. [Google Scholar]
- Vlăduţ, A.; Nikolova, N.; Licurici, M. Aridity Assessment within Southern Romania and Northern Bulgaria “Climatic Conditions and Exposition Factor Influence on the Geomorphologic Slope Complexes”, 2013, Sofia University Scientific Fund View Project. Hrvat. Geogrfafski Glas. 2017, 79, 5–26. [Google Scholar] [CrossRef]
- Hartigan, J.A.; Wong, M.A. Algorithm AS 136: A K-Means Clustering Algorithm. Appl. Stat. 1979, 28, 100. [Google Scholar] [CrossRef]
- R: K-Means Clustering. Available online: http://127.0.0.1:10049/library/stats/html/kmeans.html (accessed on 13 July 2023).
- R: Principal Components Analysis. Available online: http://127.0.0.1:10049/library/stats/html/prcomp.html (accessed on 13 July 2023).
- Wobbrock, J.O.; Findlater, L.; Gergle, D.; Higgins, J.J. The Aligned Rank Transform for Nonparametric Factorial Analyses Using Only ANOVA Procedures. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems, Vancouver, BC, Canada, 7–12 May 2011; pp. 143–146. [Google Scholar] [CrossRef]
- Elkin, L.A.; Kay, M.; Higgins, J.J.; Wobbrock, J.O. An Aligned Rank Transform Procedure for Multifactor Contrast Tests. In Proceedings of the 34th Annual ACM Symposium on User Interface Software and Technology, Virtual Event USA, 10–14 October 2021; pp. 754–768. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: http://www.r-project.org/ (accessed on 5 July 2023).
- Diófási, L.; Májer, J. A Kéknyelű Fajta Valós Értékeinek Feltárása Badacsonyban. Kertgazdasag 2001, 33, 1–9. [Google Scholar]
- Ollat, N.; Cookson, S.J.; Destrac-Irvine, A.; Lauvergeat, V.; Ouaked-Lecourieux, F.; Marguerit, E.; Barrieu, F.; Dai, Z.; Duchêne, E.; Gambetta, G.A.; et al. Grapevine Adaptation to Abiotic Stress: An Overview. Acta Hortic. 2019, 1248, 497–512. [Google Scholar] [CrossRef]
- Cornea, V.; Savin, G. Exploration and Revaluation of Old Autochthonous Varieties in the Republic of Moldova. VITIS J. Grapevine Res. 2015, 54, 115–119. [Google Scholar]
- Destrac-Irvine, A.; Gowdy, M.; Suter, B.; Goupil, W.; Thibon, C.; Ollat, N.; Darriet, P.; Parker, A.; Van Leeuwen, C. La diversité des cépages est une puissante ressource d’adaptation au changement climatique (The diversity of cultivars is a powerful resource for adapting to climate change). Rev. Française D’oenolog. Paris 2020, 2020, 16–21. [Google Scholar]
- Carámbula, C.; Moreno, M.T.; Riera, D.; Cretazzo, E.; Tomás, M.A.; Medrano, H. Selección Clonal de Las Principales Variedades Autóctonas de Baleares. In Proceedings of the XXIX Congreso Mundial de la Viña y del Vino, Logroño, Spain, 25–30 June 2006. [Google Scholar]
- Roby, J.P.; van Leeuwen, C. About the Need of Maintaining Simultaneously Mass and Clonal Selection for Conservation of Genetic Diversity in Both International and Autochtoneous Varieties. In Proceedings of the 2nd International Symposium. Exploitation of Autochthonous and More Common Vine Varieties. Genetic Pedigree and Phenotyping, Tolerance and Stress, Diseases to Control, Rootstocks. Oenoviti International Network, Geisenheim, Germany, 3 November 2014; pp. 93–97. [Google Scholar]
- Levchenko, S.; Vasylyk, I.; Volynkin, V.; Likhovskoy, V.; Polulyakh, A. Biological Characteristics of Native Grape Cultivars of Crimean Region and Availability of Their Use in Breeding. In Grapes Wine; BoD–Books on Demand: Paris, France, 2022. [Google Scholar] [CrossRef]
- Iliev, A.; Yankova, P. The Local Grape Varieties of Bulgaria. Vitic. Stud. 2021, 1, 21–28. [Google Scholar] [CrossRef]
- Cretazzo, E.; Sanz, P.M.; Lorenzi, S.; Benítez, M.L.; Velasco, L.; Emanuelli, F. Genetic Characterization by SSR Markers of a Comprehensive Wine Grape Collection Conserved at Rancho de La Merced (Andalusia, Spain). Plants 2022, 11, 1088. [Google Scholar] [CrossRef] [PubMed]
- Pankin, M.I.; Petrov, V.S.; Lukianova, A.A.; Ilnitskaya, E.T.; Nikulushkina, G.E.; Kovalenko, A.G.; Bolshakov, V.A. The Anapa Ampelographic Collection Is the Largest Center of Vine Gene Pool Accumulation and Research in Russia. Vavilovskii Z. Genet. Sel. 2018, 22, 54–59. [Google Scholar]
- Csepregi, P. “Hungarica” in Hungary’s grape variety collection. Kert. Tud. Hortic. Sci. 1994, 26, 64–67. [Google Scholar]
- Toth, S. Vineyard site—grape variety. Boraszati Lapok 1993. [Google Scholar]
- Kashirskaja-Tompa, A. Sculpture and Structure of Pollen Sporoderm in Different Grape-Flower Types. In Proceedings of the Giornate Piemontesi Del IV Simposio Internazionale Di Fisiologia Della Vite, Torino, Italy, 14–15 May 1992. [Google Scholar]
- Kara, Z.; Sabir, A.; Yazar, K.; Doğan, O.; Jalal, A.; Khaleel, K. Fertilization Biology of Ancient Grapevine Variety ‘Ekşi Kara’ (Vitis Vinifera L.). Selcuk J. Agric. Food Sci. 2017, 31, 92–97. [Google Scholar] [CrossRef]
- Kara, Z.; Sabir, A.; Eker, O. Ancient Grape Vitis Vinifera L. Cv ‘Ekşi Kara’ in Anatolia. Selcuk J. Agric. Food Sci. 2018, 32, 416–423. [Google Scholar] [CrossRef]
- Fedorina, J.; Tikhonova, N.; Ukhatova, Y.; Ivanov, R.; Khlestkina, E. Grapevine Gene Systems for Resistance to Gray Mold Botrytis Cinerea and Powdery Mildew Erysiphe Necator. Agronomy 2022, 12, 499. [Google Scholar] [CrossRef]
- Otto, M.; Geml, J.; Hegyi, Á.I.; Hegyi-Kaló, J.; Pierneef, R.; Pogány, M.; Kun, J.; Gyenesei, A.; Váczy, K.Z. Botrytis Cinerea Expression Profile and Metabolism Differs between Noble and Grey Rot of Grapes. Food Microbiol. 2022, 106, 104037. [Google Scholar] [CrossRef]
- Pogány, M.; Dankó, T.; Hegyi-Kaló, J.; Kámán-Tóth, E.; Szám, D.R.; Hamow, K.Á.; Kalapos, B.; Kiss, L.; Fodor, J.; Gullner, G.; et al. Redox and Hormonal Changes in the Transcriptome of Grape (Vitis Vinifera) Berries during Natural Noble Rot Development. Plants 2022, 11, 864. [Google Scholar] [CrossRef]
- Grimplet, J.; Ibáñez, S.; Baroja, E.; Tello, J.; Ibáñez, J. Phenotypic, Hormonal, and Genomic Variation among Vitis Vinifera Clones with Different Cluster Compactness and Reproductive Performance. Front. Plant Sci. 2019, 9, 408289. [Google Scholar] [CrossRef] [PubMed]
- Makra, L.; Vitányi, B.; Gál, A.; Mika, J.; Matyasovszky, I.; Hirsch, T. Wine Quantity and Quality Variations in Relation to Climatic Factors in the Tokaj (Hungary) Winegrowing Region. Am. J. Enol. Vitic. 2009, 60, 312–321. [Google Scholar] [CrossRef]
- Molitor, D.; Baus, O.; Hoffmann, L.; Beyer, M. Meteorological Conditions Determine the Thermal-Temporal Position of the Annual Botrytis Bunch Rot Epidemic on Vitis Vinifera L. Cv. Riesling Grapes. OENO One 2016, 50, 231–244. [Google Scholar] [CrossRef]
- Arrizabalaga-Arriazu, M.; Gomès, E.; Morales, F.; Irigoyen, J.J.; Pascual, I.; Hilbert, G. High Temperature and Elevated Carbon Dioxide Modify Berry Composition of Different Clones of Grapevine (Vitis Vinifera L.) Cv. Tempranillo. Front. Plant Sci. 2020, 11, 603687. [Google Scholar] [CrossRef] [PubMed]
- Pantelić, M.; Dabić Zagorac, D.; Natić, M.; Gašić, U.; Jović, S.; Vujović, D.; Popović Djordjević, J. Impact of Clonal Variability on Phenolics and Radical Scavenging Activity of Grapes and Wines: A Study on the Recently Developed Merlot and Cabernet Franc Clones (Vitis Vinifera L.). PLoS ONE 2016, 11, e0163823. [Google Scholar] [CrossRef] [PubMed]
- Ferrandino, A.; Guidoni, S. Anthocyanins, Flavonols and Hydroxycinnamates: An Attempt to Use Them to Discriminate Vitis Vinifera L. Cv “Barbera” Clones. Eur. Food Res. Technol. 2010, 230, 417–427. [Google Scholar] [CrossRef]
- Jovanović-Cvetković, T.; Grbić, R.; Bosančić, B.; Cvetković, M. Production and Oenological Potential of Riesling Variety Clones 49, 1091 and 1089. Turk. J. Agric. For. 2023, 47, 178–185. [Google Scholar] [CrossRef]
- Moret, F.; Lemaître-Guillier, C.; Grosjean, C.; Clément, G.; Coelho, C.; Negrel, J.; Jacquens, L.; Morvan, G.; Mouille, G.; Trouvelot, S.; et al. Clone-Dependent Expression of Esca Disease Revealed by Leaf Metabolite Analysis. Front. Plant Sci. 2019, 9, 427941. [Google Scholar] [CrossRef]
- Moret, F.; Clément, G.; Grosjean, C.; Lemaître-Guillier, C.; Morvan, G.; Trouvelot, S.; Adrian, M.; Fontaine, F. Metabolite Fingerprints of Chardonnay Grapevine Leaves Affected by Esca Is Both Clone- and Year-Dependent: Metabolic Fingerprints of Esca-Affected Clones Differs. Phytopathol. Mediterr. 2020, 59, 595–603. [Google Scholar] [CrossRef]
- Regner, F.; Philipp, C.; Reichl, M.; Hack, R.; Eder, P.; Rockenbauer, A.; Ferschl, E.; Endler, A. Klonenvergleich bei der Rebsorte ‘Weissburgunder’. Mitteilungen Klosterneubg. 2021, 37–53. [Google Scholar]
- Varela, A.; Ibañez, V.N.; Alonso, R.; Zavallo, D.; Asurmendi, S.; Gomez Talquenca, S.; Marfil, C.F.; Berli, F.J. Vineyard Environments Influence Malbec Grapevine Phenotypic Traits and DNA Methylation Patterns in a Clone-Dependent Way. Plant Cell Rep. 2020, 40, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Bonada, M.; Sadras, V.O. Review: Critical Appraisal of Methods to Investigate the Effect of Temperature on Grapevine Berry Composition. Aust. J. Grape Wine Res. 2015, 21, 1–17. [Google Scholar] [CrossRef]
- Boulton, R. The Relationships between Total Acidity, Titratable Acidity and PH in Grape Tissue. VITIS J. Grapevine Res. 1980, 19, 113. [Google Scholar] [CrossRef]
- Neethling, E.; Petitjean, T.; Quénol, H.; Barbeau, G. Assessing Local Climate Vulnerability and Winegrowers’ Adaptive Processes in the Context of Climate Change. Mitig. Adapt. Strateg. Glob. Change 2017, 22, 777–803. [Google Scholar] [CrossRef]
- Neethling, E.; Barbeau, G.; Coulon-Leroy, C.; Quénol, H. Spatial Complexity and Temporal Dynamics in Viticulture: A Review of Climate-Driven Scales. Agric. For. Meteorol. 2019, 15, 107618. [Google Scholar] [CrossRef]
- Szenteleki, K.; Horváth, L.; Ladányi, M. Climate Risk and Climate Analogies in Hungarian Viticulture. Int. Conf. Future Environ. Energy 2012, 28, 250–254. [Google Scholar]
- Navrátilová, M.; Beranová, M.; Severová, L.; Šrédl, K.; Svoboda, R.; Abrhám, J. The Impact of Climate Change on the Sugar Content of Grapes and the Sustainability of Their Production in the Czech Republic. Sustainability 2021, 13, 222. [Google Scholar] [CrossRef]
- Roby, J.-P.; van Leeuwen, C.; Gonçalves, E.; Graça, A.; Martins, A. The Preservation of Genetic Resources of the Vine Requires Cohabitation between Institutional Clonal Selection, Mass Selection and Private Clonal Selection. BIO Web Conf. 2014, 3, 01018. [Google Scholar] [CrossRef]
- Prusky, D. Pathogen Quiescence in Postharvest Diseases. Annu. Rev. Phytopathol. 2003, 34, 413–434. [Google Scholar] [CrossRef]
- Prusky, D.; Alkan, N.; Mengiste, T.; Fluhr, R. Quiescent and Necrotrophic Lifestyle Choice During Postharvest Disease Development. Annu. Rev. Phytopathol. 2013, 51, 155–176. [Google Scholar] [CrossRef]
- Padgett, M.; Morrison, J.C. Changes in Grape Berry Exudates during Fruit Development and Their Effect on Mycelial Growth of Botrytis Cinerea. J. Am. Soc. Hortic. Sci. 1990, 115, 269–273. [Google Scholar] [CrossRef]






| Year/Phenophase-BBCH Code | Budburst 9 | Beginning of Flowering—61 | End of Flowering—69 | Veraison 81 | Maturity/ Harvest—89 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Date | DOY | Date | DOY | Date | DOY | Date | DOY | Date | DOY | |
| 2011 | 13 April | 102 | 30 May | 149 | 13 June | 163 | 30 July | 210 | 21 September | 263 |
| 2012 | 11 April | 101 | 29 May | 149 | 7 June | 158 | 27 July | 208 | 6 September | 249 |
| 2013 | 23 April | 112 | 7 June | 157 | 17 June | 167 | 8 July | 219 | 1 October | 273 |
| 2014 | 7 April | 96 | 4 June | 154 | 15 June | 165 | 5 August | 216 | 22 September | 264 |
| 2015 | 20 April | 109 | 4 June | 154 | 13 June | 163 | 4 August | 215 | 12 September | 334 |
| 2017 | 6 April | 95 | 8 June | 158 | 19 June | 169 | 1 August | 212 | 20 September | 262 |
| 2018 | 16 April | 105 | 21 May | 140 | 28 May | 147 | 16 July | 196 | 20 September | 262 |
| 2019 | 12 April | 101 | 6 June | 156 | 20 June | 170 | 29 July | 209 | 2 October | 274 |
| 2020 | 9 April | 99 | 4 June | 155 | 12 June | 163 | 4 August | 216 | 22 September | 265 |
| 2021 | 23 April | 112 | 13 June | 163 | 23 June | 173 | 6 August | 217 | 30 September | 272 |
| 2022 | 14 April | 103 | 2 June | 152 | 9 June | 159 | 29 July | 209 | 22 September | 264 |
| Year/Phenophase-BBCH Code | Budburst 09 | Beginning of Flowering—61 | End of Flowering—69 | Veraison 81 | Maturity/ Harvest-89 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Date | DOY | Date | DOY | Date | DOY | Date | DOY | Date | DOY | |
| 2011 | 11 April | 100 | 27 May | 146 | 8 June | 158 | 25 July | 205 | 15 September | 257 |
| 2012 | 4 April | 94 | 24 May | 144 | 6 June | 157 | 23 July | 204 | 10 September | 253 |
| 2013 | 18 April | 107 | 4 June | 154 | 14 June | 164 | 27 July | 207 | 27 September | 269 |
| 2014 | 4 April | 93 | 27 May | 146 | 9 June | 159 | 30 July | 210 | 16 September | 258 |
| 2015 | 18 April | 107 | 2 June | 152 | 6 June | 156 | 31 July | 211 | 9 September | 251 |
| 2017 | 4 April | 93 | 6 June | 156 | 15 June | 165 | 25 July | 205 | 13 September | 255 |
| 2018 | 12 April | 101 | 18 May | 137 | 25 May | 144 | 9 July | 189 | 5 September | 247 |
| 2020 | 30 March | 89 | 29 May | 149 | 9 June | 160 | 24 July | 205 | 10 September | 253 |
| 2021 | 17 April | 106 | 11 June | 161 | 18 June | 168 | 29 July | 209 | 9 September | 251 |
| 2022 | 9 April | 98 | 25 May | 144 | 2 June | 152 | 19 July | 199 | 1 September | 243 |
| (A) | |||||||||||||
| Year/Climate index | Growing Degree Days | Huglin Index | Hydrothermal Coefficient | Precipitation (mm) Rainfall ** | |||||||||
| Phenophases/BBCH * | 9–61 | 61–69 | 69–81 | 81–89 | 09–61 | 61–69 | 69–81 | 81–89 | 09–61 | 61–69 | 69–81 | 81–89 | 61–69 |
| 2011 | 298.53 | 165.55 | 543.61 | 651.80 | 450.89 | 217.43 | 734.16 | 877.33 | 0.20 | 0.28 | 0.82 | 0.46 | 8.60 |
| 2012 | 284.50 | 82.69 | 695.46 | 590.05 | 441.66 | 119.49 | 910.64 | 782.54 | 0.85 | 0.42 | 0.75 | 0.04 | 7.20 |
| 2013 | 297.59 | 118.33 | 754.67 | 436.35 | 448.66 | 159.35 | 995.75 | 647.92 | 1.12 | 0.39 | 0.42 | 1.28 | 8.60 |
| 2014 | 274.50 | 151.52 | 604.13 | 414.16 | 441.32 | 198.78 | 807.10 | 591.50 | 1.03 | 0.00 | 1.07 | 3.50 | 0.00 |
| 2015 | 299.63 | 125.32 | 670.72 | 488.73 | 449.31 | 162.34 | 886.58 | 665.45 | 0.98 | 0.00 | 0.32 | 0.81 | 0.00 |
| 2016 | 304.80 | 63.50 | 697.20 | 471.00 | 479.17 | 90.67 | 916.86 | 661.61 | 1.39 | 1.11 | 0.94 | 0.60 | 14.80 |
| 2017 | 350.57 | 124.98 | 592.36 | 547.79 | 516.65 | 169.15 | 787.37 | 750.64 | 0.64 | 0.11 | 0.94 | 0.99 | 2.60 |
| 2018 | 298.11 | 80.03 | 556.93 | 882.49 | 419.64 | 107.75 | 753.81 | 1216.37 | 1.30 | 0.84 | 1.26 | 1.17 | 12.60 |
| 2019 | 237.70 | 201.50 | 513.80 | 708.70 | 362.78 | 259.04 | 683.71 | 992.30 | 2.05 | 0.25 | 1.07 | 0.77 | 8.50 |
| 2020 | 274.50 | 71.70 | 637.00 | 568.80 | 463.10 | 102.80 | 857.69 | 783.14 | 0.74 | 1.58 | 1.18 | 0.62 | 24.00 |
| 2021 | 275.20 | 149.80 | 636.10 | 526.40 | 432.23 | 195.72 | 829.08 | 762.04 | 0.85 | 0.00 | 0.69 | 0.51 | 0.00 |
| 2022 | 300.50 | 80.50 | 688.10 | 613.50 | 443.73 | 106.26 | 906.52 | 830.66 | 1.28 | 2.34 | 0.57 | 0.83 | 35.20 |
| Average | 291.34 | 117.95 | 632.51 | 574.98 | 445.76 | 157.4 | 839.11 | 796.79 | 1.04 | 0.61 | 0.84 | 0.97 | 10.18 |
| (B) | |||||||||||||
| Year / Climate index | Growing degree days | Huglin index | Hydrothermal coefficient | Precipitation (mm) rainfall | |||||||||
| phenophases/BBCH | 09–61 | 61–69 | 69–81 | 81–89 | 09–61 | 61–69 | 69–81 | 81–89 | 09–61 | 61–69 | 69–81 | 81–89 | 81–89 |
| 2011 | 280.37 | 135.97 | 551.99 | 645.11 | 427.78 | 182.48 | 742.82 | 863.10 | 0.19 | 0.40 | 0.49 | 0.72 | 83.80 |
| 2012 | 256.88 | 108.36 | 653.20 | 693.47 | 401.71 | 159.26 | 854.12 | 922.30 | 1.06 | 0.33 | 0.72 | 0.11 | 12.80 |
| 2013 | 306.02 | 93.10 | 582.11 | 651.14 | 464.79 | 129.41 | 773.54 | 917.78 | 1.19 | 0.50 | 0.52 | 0.77 | 97.60 |
| 2014 | 239.56 | 113.87 | 608.82 | 448.57 | 387.33 | 163.94 | 811.13 | 625.89 | 1.26 | 0.02 | 1.07 | 3.12 | 289.60 |
| 2015 | 273.06 | 54.75 | 711.22 | 531.04 | 418.80 | 71.01 | 937.93 | 712.71 | 1.03 | 0.00 | 0.31 | 0.75 | 69.60 |
| 2016 | 274.60 | 75.50 | 632.30 | 484.10 | 436.59 | 107.10 | 835.80 | 668.80 | 1.19 | 1.56 | 0.97 | 0.77 | 70.20 |
| 2017 | 337.33 | 97.22 | 541.70 | 615.51 | 498.93 | 133.05 | 721.19 | 830.92 | 0.66 | 0.05 | 0.95 | 0.63 | 70.40 |
| 2018 | 298.68 | 66.44 | 513.95 | 799.28 | 420.36 | 91.89 | 690.87 | 1082.17 | 1.29 | 0.92 | 1.18 | 1.43 | 200.50 |
| 2019 | 234.60 | 187.50 | 515.90 | 643.40 | 356.53 | 238.93 | 688.70 | 870.35 | 2.22 | 0.00 | 1.07 | 0.87 | 102.50 |
| 2020 | 256.40 | 75.50 | 509.80 | 587.90 | 435.12 | 115.03 | 698.62 | 790.76 | 0.64 | 1.44 | 1.06 | 1.01 | 107.40 |
| 2021 | 255.70 | 84.70 | 624.10 | 466.80 | 411.50 | 112.88 | 810.18 | 648.69 | 1.19 | 0.32 | 0.30 | 0.90 | 79.90 |
| 2022 | 249.60 | 58.90 | 599.40 | 630.40 | 377.74 | 83.58 | 794.85 | 819.68 | 1.17 | 1.40 | 0.96 | 0.60 | 64.70 |
| Average | 271.90 | 95.98 | 587.04 | 599.73 | 419.77 | 132.38 | 779.98 | 812.76 | 1.09 | 0.58 | 0.80 | 0.97 | 104.08 |
| Clone * | Year | Yield kg/m2 | Sugar Content of the Juice °Bx | Titratable Acid Content of the Juice g/L (in Tartaric Acid) | pH | Botrytis Infection % |
|---|---|---|---|---|---|---|
| B.1. | 2011 | 0.88 | 20.20 | 7.25 | 3.08 | 10.00 |
| B.2. | 2011 | 1.26 | 17.40 | 7.09 | 3.07 | 10.00 |
| Base | 2011 | 0.99 | 18.60 | 6.59 | 3.38 | 10.00 |
| B.1. | 2012 | 0.95 | 18.30 | 6.52 | 3.55 | 0.00 |
| B.2. | 2012 | 1.10 | 17.50 | 6.36 | 3.67 | 0.00 |
| Base | 2012 | 1.08 | 18.50 | 4.64 | 3.47 | 0.00 |
| B.1. | 2013 | 1.39 | 18.90 | 11.15 | 3.26 | 0.00 |
| B.2. | 2013 | 1.61 | 17.70 | 10.36 | 3.31 | 0.00 |
| Base | 2013 | 1.21 | 21.20 | 9.60 | 3.56 | 0.00 |
| B.1. | 2014 | 1.01 | 18.10 | 16.60 | 3.19 | 30.00 |
| B.2. | 2014 | 0.91 | 18.00 | 17.91 | 3.25 | 30.00 |
| Base | 2014 | 0.72 | 19.50 | 15.57 | 3.24 | 30.00 |
| B.1. | 2015 | 1.07 | 18.40 | 7.82 | 3.39 | 0.00 |
| B.2. | 2015 | 1.20 | 18.00 | 6.83 | 3.40 | 0.00 |
| Base | 2015 | 0.97 | 18.20 | 6.98 | 3.51 | 0.00 |
| B.1. | 2017 | 1.15 | 18.10 | 6.71 | 3.25 | 0.00 |
| B.2. | 2017 | 1.33 | 17.70 | 5.72 | 3.38 | 0.00 |
| Base | 2017 | 0.92 | 18.20 | 6.74 | 3.36 | 0.00 |
| B.1. | 2018 | 1.23 | 17.70 | 8.26 | 3.43 | 3.00 |
| B.2. | 2018 | 1.90 | 18.20 | 6.96 | 3.39 | 0.00 |
| Base | 2018 | 1.76 | 17.70 | 6.85 | 3.55 | 5.00 |
| B.1. | 2019 | 1.39 | 18.70 | 8.70 | 3.28 | 0.00 |
| B.2. | 2019 | 1.46 | 18.60 | 3.29 | 3.80 | 0.00 |
| Base | 2019 | 1.27 | 18.70 | 7.56 | 3.44 | 5.00 |
| B.1. | 2020 | 1.26 | 17.70 | 8.68 | 3.14 | 0.00 |
| B.2. | 2020 | 1.31 | 18.20 | 7.60 | 3.43 | 0.00 |
| Base | 2020 | 1.03 | 18.80 | 8.57 | 3.55 | 0.00 |
| B.1. | 2021 | 1.28 | 18.90 | 8.62 | 3.29 | 0.00 |
| B.2. | 2021 | 1.24 | 20.20 | 7.40 | 3.25 | 0.00 |
| Base | 2021 | 1.04 | 20.20 | 9.38 | 3.37 | 0.00 |
| B.1. | 2022 | 2.79 | 16.50 | 7.40 | 3.19 | 0.00 |
| B.2. | 2022 | 2.88 | 16.30 | 6.10 | 3.41 | 0.00 |
| Base | 2022 | 2.03 | 17.00 | 6.20 | 3.58 | 0.00 |
| B.1. | 2011–2022 | 1.31 | 18.32 | 8.88 | 3.28 | 3.91 |
| B.2. | 2011–2022 | 1.47 | 17.98 | 7.78 | 3.40 | 3.64 |
| Base | 2011–2022 | 1.18 | 18.78 | 8.06 | 3.46 | 4.55 |
| Mean | 1.32 | 18.36 | 8.24 | 3.50 | 4.03 |
| Clone * | Year | Yield kg/m2 | Sugar Content of the Juice °Bx | Titratable Acid Contant of the Juice g/L (in Tartaric Acid) | pH | Botrytis Infection % | |
|---|---|---|---|---|---|---|---|
| B.1. | 2011 | 0.28 | 20.80 | 7.47 | 3.13 | 50.00 | |
| B.2. | 2011 | 0.64 | 20.20 | 6.86 | 2.96 | 35.00 | |
| Base | 2011 | 0.87 | 20.70 | 8.55 | 2.96 | 30.00 | |
| B.1. | 2012 | 1.38 | 17.40 | 8.31 | 3.59 | 0.00 | |
| B.2. | 2012 | 1.34 | 17.40 | 7.21 | 3.42 | 0.00 | |
| Base | 2012 | 1.06 | 20.10 | 7.16 | 3.55 | 0.00 | |
| B.1. | 2013 | 1.35 | 20.70 | 12.07 | 3.37 | 3.00 | |
| B.2. | 2013 | 1.56 | 19.90 | 10.82 | 3.33 | 2.00 | |
| Base | 2013 | 1.08 | 19.80 | 11.50 | 3.21 | 5.00 | |
| B.1. | 2014 | 0.73 | 17.40 | 19.84 | 3.28 | 60.00 | |
| B.2. | 2014 | 0.21 | 16.50 | 17.52 | 3.18 | 80.00 | |
| Base | 2014 | 0.21 | 15.10 | 16.41 | 3.20 | 85.00 | |
| B.1. | 2015 | 1.01 | 19.40 | 8.62 | 3.52 | 3.00 | |
| B.2. | 2015 | 1.21 | 18.20 | 8.04 | 3.47 | 5.00 | |
| Base | 2015 | 1.46 | 17.80 | 9.91 | 3.50 | 10.00 | |
| B.1. | 2017 | 1.31 | 17.70 | 7.71 | 3.36 | 5.00 | |
| B.2. | 2017 | 1.28 | 18.40 | 7.45 | 3.33 | 5.00 | |
| Base | 2017 | 1.57 | 17.50 | 9.44 | 3.28 | 5.00 | |
| B.1. | 2018 | 2.26 | 18.00 | 7.29 | 3.46 | 7.00 | |
| B.2. | 2018 | 1.99 | 17.40 | 8.48 | 3.47 | 5.00 | |
| Base | 2018 | 2.02 | 17.90 | 9.94 | 3.35 | 10.00 | |
| B.1. | 2019 | nd. | nd. | nd. | nd. | nd. | |
| B.2. | 2019 | nd. | nd. | nd. | nd. | nd. | |
| Base | 2019 | 1.43 | 20.80 | 12.55 | 3.42 | 40.00 | |
| B.1. | 2020 | 1.17 | 20.40 | 11.88 | 3.41 | 20.00 | |
| B.2. | 2020 | 1.06 | 19.90 | 11.08 | 3.34 | 25.00 | |
| Base | 2020 | 1.55 | 17.20 | 12.40 | 3.29 | 20.00 | |
| B.1. | 2021 | 1.12 | 19.60 | 14.20 | 3.14 | 20.00 | |
| B.2. | 2021 | 1.09 | 19.00 | 12.40 | 3.18 | 20.00 | |
| Base | 2021 | 1.48 | 15.10 | 18.80 | 3.01 | 20.00 | |
| B.1. | 2022 | 0.96 | 17.70 | 7.47 | 3.37 | 10.00 | |
| B.2. | 2022 | nd. | nd. | nd. | nd. | nd. | |
| Base | 2022 | 2.25 | 17.10 | 10.54 | 3.14 | 10.00 | |
| B.1. | 2011–2022 | 1.16 1 | 18.91 1 | 10.49 1 | 3.36 1 | 17.80 1 | 8.50 2 |
| B.2. | 2011–2022 | 1.15 1 | 18.54 1 | 9.98 1 | 3.30 1 | 19.67 1 | 5.00 2 |
| Base | 2011–2022 | 1.36 1 | 18.10 1 | 11.56 1 | 3.26 1 | 21.36 1 | 10.00 2 |
| Mean | - | 1.23 1 | 18.5 | 10.73 | 3.31 | 19.67 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farkas, E.A.; Jahnke, G.; Szőke, B.; Deák, T.; Oláh, R.; Oláh, K.; Knolmajerné Szigeti, G.; Németh, C.; Nyitrainé Sárdy, D.Á. Clonal Selection of Autochthonous Grape Varieties in Badacsony, Hungary. Horticulturae 2023, 9, 994. https://doi.org/10.3390/horticulturae9090994
Farkas EA, Jahnke G, Szőke B, Deák T, Oláh R, Oláh K, Knolmajerné Szigeti G, Németh C, Nyitrainé Sárdy DÁ. Clonal Selection of Autochthonous Grape Varieties in Badacsony, Hungary. Horticulturae. 2023; 9(9):994. https://doi.org/10.3390/horticulturae9090994
Chicago/Turabian StyleFarkas, Eszter Alexandra, Gizella Jahnke, Barna Szőke, Tamás Deák, Róbert Oláh, Krisztina Oláh, Gyöngyi Knolmajerné Szigeti, Csaba Németh, and Diána Ágnes Nyitrainé Sárdy. 2023. "Clonal Selection of Autochthonous Grape Varieties in Badacsony, Hungary" Horticulturae 9, no. 9: 994. https://doi.org/10.3390/horticulturae9090994
APA StyleFarkas, E. A., Jahnke, G., Szőke, B., Deák, T., Oláh, R., Oláh, K., Knolmajerné Szigeti, G., Németh, C., & Nyitrainé Sárdy, D. Á. (2023). Clonal Selection of Autochthonous Grape Varieties in Badacsony, Hungary. Horticulturae, 9(9), 994. https://doi.org/10.3390/horticulturae9090994







