Splicing and Expression Regulation of fruitless Gene in Bemisia tabaci (Hemiptera: Aleyrodidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insect Strains
2.2. Sample Collection
2.3. RNA Extraction and cDNA Synthesis
2.4. Gene Cloning and Splice-Variant Detection
2.5. Phylogenetic Analysis
2.6. Real-Time Quantitative PCR
2.7. RNA Interference
3. Results
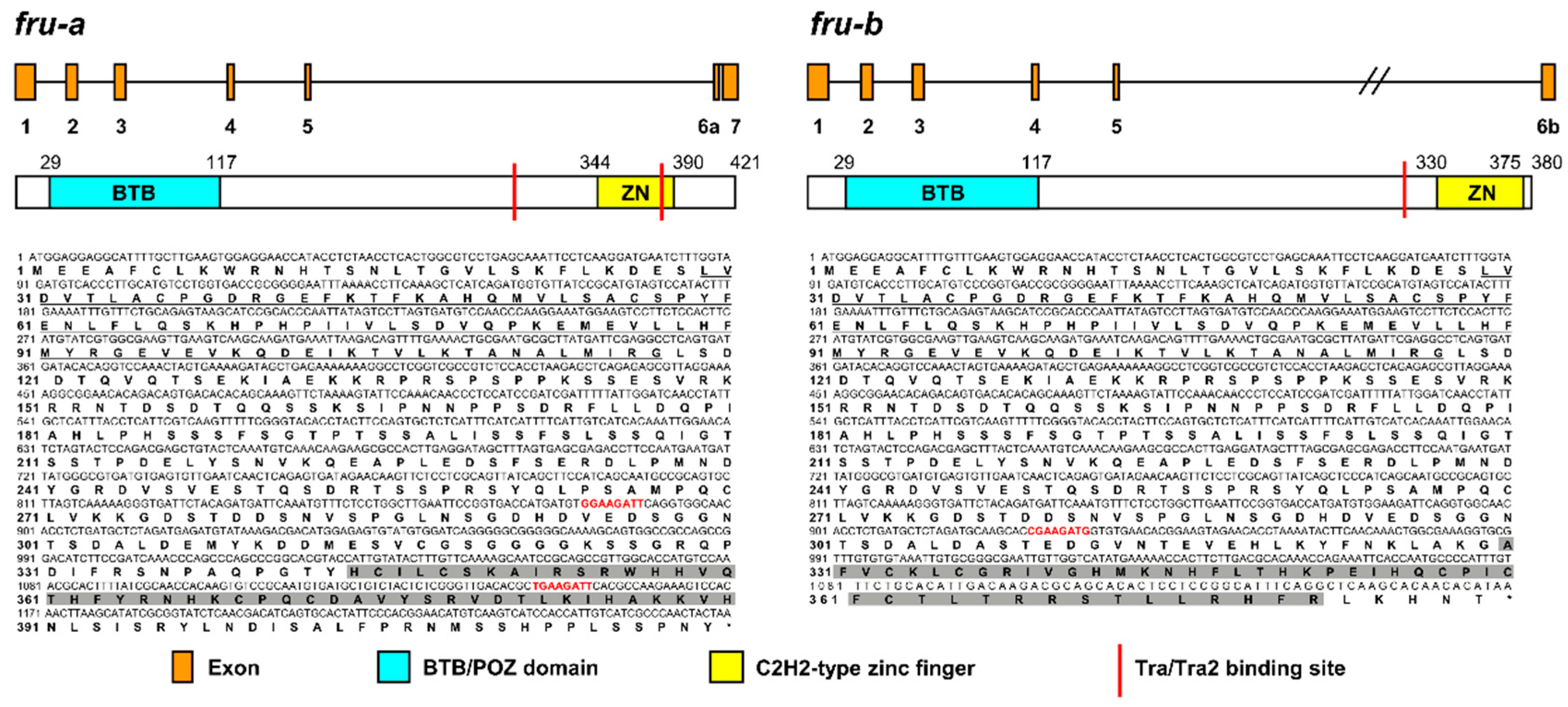
3.1. Characterization of Btfru
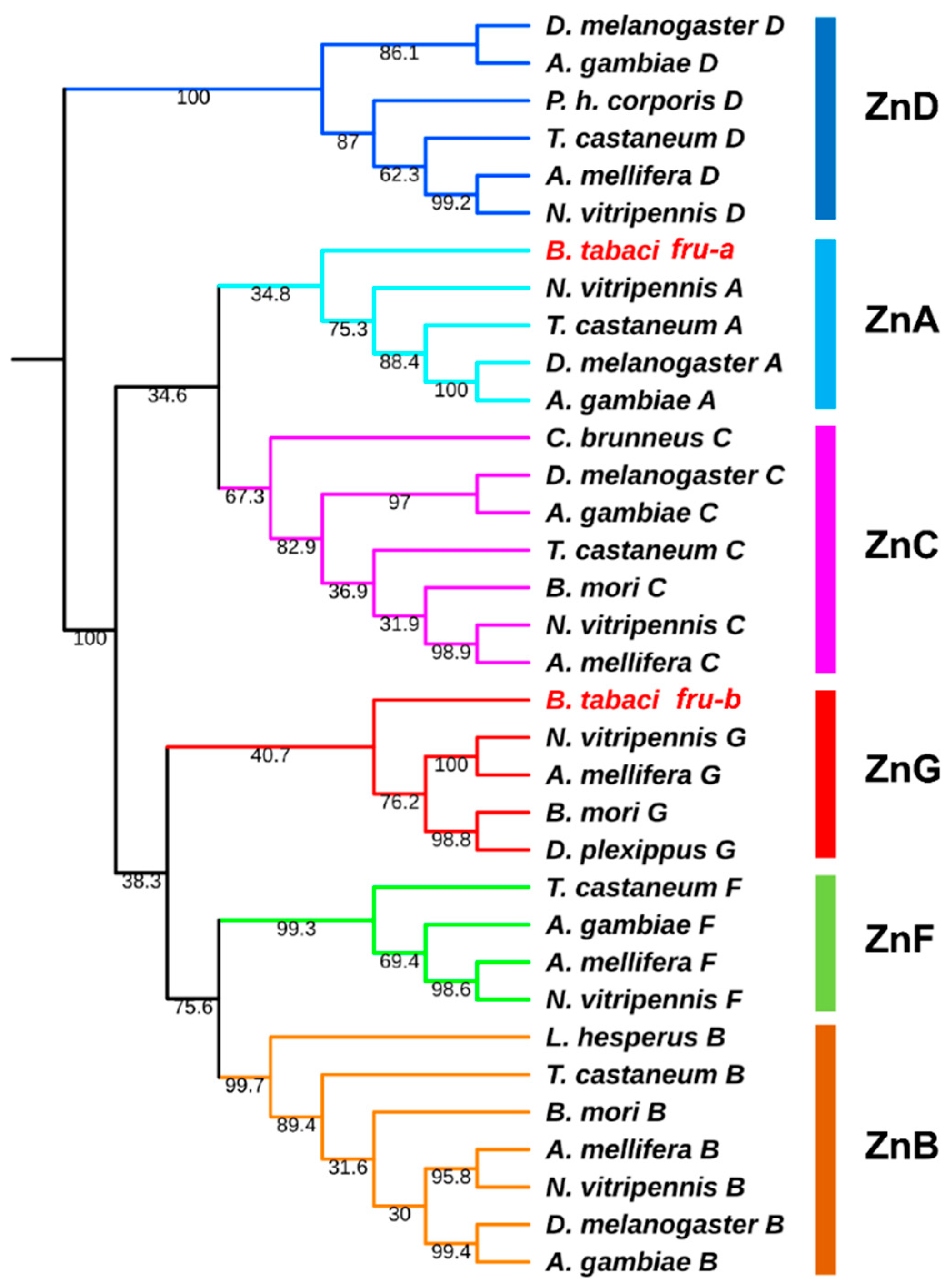
3.2. Phylogenetic Analysis of Btfru
3.3. Developmental Expression of Btfru
3.4. Analyses of Alternative Splicing Variants of Btfru
3.5. Analysis of the Interaction between Bttra and Btfru
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schütt, C.; Nöthiger, R. Structure, function and evolution of sex-determining systems in dipteran insects. Development 2000, 127, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, W.; Chen, Z.; Xie, W.; Zhang, Y. Research progress on sex determination cascade in insects. Acta Entomol. Sin. 2023, 66, 245–254. [Google Scholar]
- Peng, W.; Zhai, Z. Progress and prospects of insect sex determination mechanism. Chin. J. Biol. Control 2021, 37, 1313–1324. [Google Scholar]
- Shukla, J.N.; Nagaraju, J. Doublesex: A conserved downstream gene controlled by diverse upstream regulators. J. Genet. 2010, 89, 341–356. [Google Scholar] [CrossRef]
- Gailey, D.A.; Taylor, B.J.; Hall, J.C. Elements of the fruitless locus regulate development of the muscle of Lawrence, a male-specific structure in the abdomen of Drosophila melanogaster adults. Development 1991, 113, 879–890. [Google Scholar] [CrossRef]
- von Philipsborn, A.C.; Jörchel, S.; Tirian, L.; Demir, E.; Morita, T.; Stern, D.L.; Dickson, B.J. Cellular and behavioral functions of fruitless isoforms in Drosophila courtship. Curr. Biol. 2014, 24, 242–251. [Google Scholar] [CrossRef]
- Wohl, M.; Ishii, K.; Asahina, K. Layered roles of fruitless isoforms in specification and function of male aggression-promoting neurons in Drosophila. eLife 2020, 9, e52702. [Google Scholar] [CrossRef]
- Demir, E.; Dickson, B.J. fruitless splicing specifies male courtship behavior in Drosophila. Cell 2005, 121, 785–794. [Google Scholar] [CrossRef]
- Bertossa, R.C.; Zande, L.V.D.; Beukeboom, L.W. The fruitless gene in Nasonia displays complex sex-specific splicing and contains new zinc finger domains. Mol. Biol. Evol. 2009, 26, 1557–1569. [Google Scholar] [CrossRef]
- Clynen, E.; Ciudad, L.; Bellés, X.; Piulachs, M.D. Conservation of fruitless’ role as master regulator of male courtship behaviour from cockroaches to flies. Dev. Genes Evol. 2011, 221, 43–48. [Google Scholar] [CrossRef]
- Boerjan, B.; Tobback, J.; Vandersmissen, H.P.; Huybrechts, R.; Schoofs, L. Fruitless RNAi knockdown in the desert locust, Schistocerca gregaria, influences male fertility. J. Insect Physiol. 2012, 58, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T. Evolution of the neural sex-determination system in insects: Does fruitless homologue regulate neural sexual dimorphism in basal insects? Insect Mol. Biol. 2019, 28, 807–827. [Google Scholar] [CrossRef] [PubMed]
- Basrur, N.S.; de Obaldia, M.E.; Morita, T.; Herre, M.; von Heynitz, R.K.; Tsitohay, Y.N.; Vosshall, L.B. Fruitless mutant male mosquitoes gain attraction to human odor. eLife 2020, 9, e63982. [Google Scholar] [CrossRef] [PubMed]
- Laohakieat, K.; Isasawin, S.; Thanaphum, S. The transformer-2 and fruitless characterisation with developmental expression profiles of sex-determining genes in Bactrocera dorsalis and B. correcta. Sci. Rep. 2020, 10, 17938. [Google Scholar] [CrossRef]
- Xu, J.; Liu, W.; Yang, D.H.; Chen, S.Q.; Chen, K.; Liu, Z.L.; Yang, X.; Meng, J.; Zhu, G.H.; Dong, S.L.; et al. Regulation of olfactory-based sex behaviors in the silkworm by genes in the sex-determination cascade. PLoS Genet. 2020, 16, e1008622. [Google Scholar] [CrossRef]
- Salvemini, M.; Polito, C.; Saccone, G. Fruitless alternative splicing and sex behaviour in insects: An ancient and unforgettable love story? J. Genet. 2010, 89, 287–299. [Google Scholar] [CrossRef]
- Heinrichs, V.; Ryner, L.C.; Baker, B.S. Regulation of sex-specific selection of fruitless 5’ splice sites by transformer and transformer-2. Mol. Cell. Biol. 1998, 18, 450–458. [Google Scholar] [CrossRef]
- Sato, K.; Yamamoto, D. The mode of action of Fruitless: Is it an easy matter to switch the sex? Genes Brain Behav. 2020, 19, e12606. [Google Scholar] [CrossRef]
- Blackman, R.L.; Cahill, M. The karyotype of Bemisia tabaci (Hemiptera: Aleyrodidae). Bull. Entomol. Res. 1998, 88, 213–215. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, W.; Yang, X.; Guo, L.; Wang, S.; Wu, Q.; Yang, Z.; Zhou, X.; Zhang, Y. Molecular cloning of the sex-related gene PSI in Bemisia tabaci and its alternative splicing properties. Gene 2016, 580, 104–110. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, W.; Guo, L.; Yang, X.; Yang, J.; Wang, S.; Wu, Q.; Zhou, X.; Zhang, Y. Genome-wide dissection of sex determination genes in the highly invasive whitefly species Bemisia tabaci Q/MED. Insect Mol. Biol. 2019, 28, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, J.; Huo, Z.; Wang, S.; Wu, Q.; Zhou, X.; Xie, W.; Zhang, Y. Characteristic and functional study of intersex, a gene related to female fertility in Bemisia tabaci. Front. Physiol. 2020, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Xie, W.; Liu, Y.; Yang, Z.; Yang, X.; Xia, J.; Wang, S.; Wu, Q.; Zhang, Y. Identification and characterization of doublesex in Bemisia tabaci. Insect Mol. Biol. 2018, 27, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Huo, Z.; Liu, Y.; Yang, J.; Xie, W.; Wang, S.; Wu, Q.; Zhou, X.; Pang, B.; Zhang, Y. Transcriptomic analysis of mating responses in Bemisia tabaci MED females. Insects 2020, 11, 308. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Li, R.; Xie, W.; Wang, S.; Wu, Q.; Yang, N.; Yang, X.; Pan, H.; Zhou, X.; Bai, L.; Xu, B.; et al. Reference gene selection for qRT-PCR analysis in the sweetpotato whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). PLoS ONE 2013, 8, e53006. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Chandrashekar, K.; Thakur, N.; Verma, P.C.; Borgio, J.F.; Singh, P.K.; Tuli, R. RNA interference for the control of whiteflies (Bemisia tabaci) by oral route. J. Biosci. 2011, 36, 153–161. [Google Scholar] [CrossRef]
- Yang, X.; Xie, W.; Li, R.M.; Zhou, X.M.; Wang, S.L.; Wu, Q.J.; Yang, N.N.; Xia, J.X.; Yang, Z.Z.; Guo, L.T.; et al. RNA interference-mediated knockdown of the hydroxyacid-oxoacid transhydrogenase gene decreases thiamethoxam resistance in adults of the whitefly Bemisia tabaci. Sci. Rep. 2017, 7, 41201. [Google Scholar] [CrossRef]
- Ito, H.; Fujitani, K.; Usui, K.; Shimizu-Nishikawa, K.; Tanaka, S.; Yamamoto, D. Sexual orientation in Drosophila is altered by the satori mutation in the sex-determination gene fruitless that encodes a zinc finger protein with a BTB domain. Proc. Natl. Acad. Sci. USA 1996, 93, 9687–9692. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, A.M.; Sant’Angelo, D.B. The BTB-ZF family of transcription factors: Key regulators of lineage commitment and effector function development in the immune system. J. Immunol. 2011, 187, 2841–2847. [Google Scholar] [CrossRef] [PubMed]
- Siggs, O.M.; Beutler, B. The BTB-ZF transcription factors. Cell Cycle 2012, 11, 3358–3369. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.; Hiroki, I. Genomic structure of the sexual behaviour gene fruitless. Drosoph. Inf. Serv. 2001, 84, 65–66. [Google Scholar]
- Gailey, D.A.; Billeter, J.C.; Liu, J.H.; Bauzon, F.; Allendorfer, J.B.; Goodwin, S.F. Functional conservation of the fruitless male sex-determination gene across 250 Myr of insect evolution. Mol. Biol. Evol. 2006, 23, 633–643. [Google Scholar] [CrossRef]
- Meier, N.; Käppeli, S.C.; Hediger Niessen, M.; Billeter, J.C.; Goodwin, S.F.; Bopp, D. Genetic control of courtship behavior in the housefly: Evidence for a conserved bifurcation of the sex-determining pathway. PLoS ONE 2013, 8, e62476. [Google Scholar] [CrossRef]
- Salvemini, M.; D’Amato, R.; Petrella, V.; Aceto, S.; Nimmo, D.; Neira, M.; Alphey, L.; Polito, L.C.; Saccone, G. The orthologue of the fruitfly sex behaviour gene fruitless in the mosquito Aedes aegypti: Evolution of genomic organisation and alternative splicing. PLoS ONE 2013, 8, e48554. [Google Scholar] [CrossRef]
- Ryner, L.C.; Goodwin, S.F.; Castrillon, D.H.; Anand, A.; Villella, A.; Baker, B.S.; Hall, J.C.; Taylor, B.J.; Wasserman, S.A. Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell 1996, 87, 1079–1089. [Google Scholar] [CrossRef]
- De Barro, P.J.; Liu, S.S.; Boykin, L.M.; Dinsdale, A.B. Bemisia tabaci: A statement of species status. Annu. Rev. Entomol. 2011, 56, 1–19. [Google Scholar] [CrossRef]
- Salvemini, M.; Robertson, M.; Aronson, B.; Atkinson, P.; Polito, L.C.; Saccone, G. Ceratitis capitata transformer-2 gene is required to establish and maintain the autoregulation of Cctra, the master gene for female sex determination. Int. J. Dev. Biol. 2009, 53, 109–120. [Google Scholar] [CrossRef]
- Pane, A.; Salvemini, M.; Delli Bovi, P.; Polito, C.; Saccone, G. The transformer gene in Ceratitis capitata provides a genetic basis for selecting and remembering the sexual fate. Development 2002, 129, 3715–3725. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Whitworth, C.; Pozmanter, C.; Neville, M.C.; Van Doren, M. Doublesex regulates fruitless expression to promote sexual dimorphism of the gonad stem cell niche. PLoS Genet. 2021, 17, e1009468. [Google Scholar] [CrossRef] [PubMed]
- Manoli, D.S.; Foss, M.; Villella, A.; Taylor, B.J.; Hall, J.C.; Baker, B.S. Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature 2005, 436, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, T.; Calhoun, R.M.; Bruch, K.; Moehring, A.J. The fruitless gene affects female receptivity and species isolation. Proc. R. Soc. B 2020, 287, 20192765. [Google Scholar] [CrossRef]
- Liu, G.Q.; Wu, Q.; Li, J.W.; Zhang, G.F.; Wan, F.H. RNAi-mediated knock-down of transformer and transformer 2 to generate male-only progeny in the oriental fruit fly, Bactrocera dorsalis (Hendel). PLoS ONE 2015, 10, e0128892. [Google Scholar] [CrossRef]
- Kyrou, K.; Hammond, A.M.; Galizi, R.; Kranjc, N.; Burt, A.; Beaghton, A.K.; Nolan, T.; Crisanti, A. A CRISPR-Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes. Nat. Biotechnol. 2018, 36, 1062–1066. [Google Scholar] [CrossRef]
- Xu, X.; Bi, H.; Wang, Y.; Li, X.; Xu, J.; Liu, Z.; He, L.; Li, K.; Huang, Y. Disruption of the ovarian serine protease (Osp) gene causes female sterility in Bombyx mori and Spodoptera litura. Pest Manag. Sci. 2020, 76, 1245–1255. [Google Scholar] [CrossRef]


| Application of Primers | Gene Name | Primer Name | Primer Sequence (5′-3′) |
|---|---|---|---|
| Cloning and AS analysis | fru-a | fru6255-F | CGTCTCTCCCCCAACCAG |
| fru-full-R | CCCTTAGCATCAATAGCGG | ||
| fru-b | fru-F5-full | ATGGAGGAGGCATTTTGTTTGAAG | |
| fru-R5-full | TTATGTGTTGTGCTTGAGCCTGAAA | ||
| qRT-PCR analysis | fru-a | qfru-A-F1 | AAGCAATCCGCAGCCGTT |
| qfru-A-R1 | CTGATGTCGTTGAGATACCGC | ||
| fru-b | qfru-G-F2 | ATGAAAAACCACTTCTTGACGC | |
| qfru-G-R2 | TATGTGTTGTGCTTGAGCCTGA | ||
| fru | BTB-qF | CATTCGTCAAGTTTTTCGGGTA | |
| BTB-qR | GGAAGGTCTCGCTCGCTAAA | ||
| tra | dsTra-qF2 | AAGTCCCTCTCCTCAGCCCA | |
| dsTra-qR2 | GCCACGGGTTAGACCTTTGA | ||
| SDHA | SDHA-qF | GCGACTGATTCTTCTCCTGC | |
| SDHA-qR | TGGTGCCAACAGATTAGGTGC | ||
| RNAi analysis | EGFP | dsEGFP-F | TAATACGACTCACTATAGGGAGACAGTGCTTCAGCCGCTAC |
| dsEGFP-R | TAATACGACTCACTATAGGGAGAGTTCACCTTGATGCCGTTC | ||
| tra | dsTra-F2 | GGATCCTAATACGACTCACTATAGGTTGAGACGAATCAGCAATCG | |
| dsTra-R3 | GGATCCTAATACGACTCACTATAGGGACCTTCGCAGGAACTTTTG |
| Type | Variants | Exons Included | Size (bp) | Female (3) a | Male (3) a | Domain |
|---|---|---|---|---|---|---|
| fru-a | 1 | 1,2,3,4,5,6a,7 | 1263 | 3 | 3 | BTB + Zn |
| 2 | 1-198,160-7 | 278 | 1 | 0 | BTB | |
| 3 | 1-169,178-7 | 289 | 1 | 0 | BTB | |
| 4 | 1-210,110-7 | 316 | 1 | 0 | ||
| 5 | 1-194,88-7 | 354 | 1 | 0 | BTB | |
| 6 | 1-308,48-6a,7 | 357 | 1 | 0 | Zn | |
| 7 | 1-200,78-7 | 358 | 1 | 0 | BTB | |
| 8 | 1-279,71-6a,7 | 363 | 0 | 1 | Zn | |
| 9 | 1-161,85-7 | 390 | 1 | 0 | BTB | |
| 10 | 1-197,38-7 | 401 | 1 | 0 | BTB | |
| 11 | 1-132,98-7 | 406 | 1 | 0 | BTB | |
| 12 | 1-346,55-5,6a,7 | 444 | 1 | 0 | Zn | |
| 13 | 1-131,9-7 | 496 | 1 | 0 | BTB | |
| 14 | 1-305,37-5,6a,7 | 503 | 1 | 0 | ||
| 15 | 1,2,3-107,244-7 | 667 | 1 | 0 | BTB | |
| 16 | 1,2,3-7,79-7 | 895 | 0 | 1 | BTB | |
| 17 | 1,2,3,4,5-86,163-7 | 935 | 1 | 0 | BTB | |
| 18 | 1,2,3,4-73,21-6a,7 | 1074 | 0 | 1 | BTB + Zn | |
| 19 | 1,2,3,4,5,6a-66,82-7 | 1114 | 1 | 0 | BTB | |
| 20 | 1,2,3,4,5-56,7 | 1130 | 1 | 0 | BTB | |
| 21 | 1-10,98-2,3,4,5,6a,7 | 1155 | 1 | 0 | BTB + Zn | |
| fru-b | 1 | 1,2,3,4,5,6b | 1143 | 3 | 3 | BTB + Zn |
| 2 | 1-185,155-6b | 253 | 1 | 0 | BTB | |
| 3 | 1-284,5,6b | 348 | 1 | 0 | ||
| 4 | 1-292,5,6b | 396 | 1 | 0 | BTB | |
| 5 | 1,2-81,178-6b | 517 | 0 | 1 | BTB | |
| 6 | 1-51,2,3,4,5,6b | 1090 | 1 | 0 | BTB + Zn |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Xie, J.; Wang, W.; Lei, Y.; Zhou, X.; Zhang, Y.; Xie, W. Splicing and Expression Regulation of fruitless Gene in Bemisia tabaci (Hemiptera: Aleyrodidae). Horticulturae 2023, 9, 962. https://doi.org/10.3390/horticulturae9090962
Liu Y, Xie J, Wang W, Lei Y, Zhou X, Zhang Y, Xie W. Splicing and Expression Regulation of fruitless Gene in Bemisia tabaci (Hemiptera: Aleyrodidae). Horticulturae. 2023; 9(9):962. https://doi.org/10.3390/horticulturae9090962
Chicago/Turabian StyleLiu, Yating, Jinxi Xie, Wenlu Wang, Yanyuan Lei, Xuguo Zhou, Youjun Zhang, and Wen Xie. 2023. "Splicing and Expression Regulation of fruitless Gene in Bemisia tabaci (Hemiptera: Aleyrodidae)" Horticulturae 9, no. 9: 962. https://doi.org/10.3390/horticulturae9090962
APA StyleLiu, Y., Xie, J., Wang, W., Lei, Y., Zhou, X., Zhang, Y., & Xie, W. (2023). Splicing and Expression Regulation of fruitless Gene in Bemisia tabaci (Hemiptera: Aleyrodidae). Horticulturae, 9(9), 962. https://doi.org/10.3390/horticulturae9090962








