A Cold Case—Glucosinolate Levels in Kale Cultivars Are Differently Influenced by Cold Temperatures
Abstract
:1. Introduction
| m/z [M-H] | Retention Time (min) | Post-Hydrolysis Derivatives (Bioactive Metabolites) | Main Activities in Conjunction with Human Consumption | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Trivial Name | Abbreviation | Structure | Molecular Formula | Exptl | Theor | Error (ppm) | Sensory Descriptor | |||
| aliphatic | gluconapin | GN | 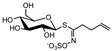 | C11H19N1O9S2 | 372.0399 | 372.0418 | 4.9 | 7.0 | 3-butenyl ITC | bitter taste, pungent | induction of cancer-cell apoptosis [40] |
| progoitrin | PR |  | C11H19N1O10S2 | 388.0368 | 388.0366 | 2.5 | 4.4 | goitrin (oxazolidine-2-thione) | bitter taste | taste [41], possibly causing goiter [42] | |
| glucoraphanin | GR | 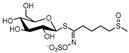 | C12H23N1O10S3 | 436.0396 | 436.0400 | 3.5 | 4.3 | sulforaphane (ITC) | no taste | anti-infective [43], antiviral [43], antagonizing angiogenesis and tumor-cell metastasis [44], apoptosis induction [45] | |
| sinigrin | SIN | 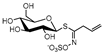 | C10H17N1O9S2 | 358.0339 | 358.0344 | 2.1 | 5.7 | allyl ITC (AITC), allyl TC | bitter taste, pungent | odor-active [41], taste [41], several therapeutic benefits [46] | |
| glucoiberin | GI | 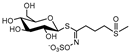 | C11H21N1O10S3 | 422.0325 | 422.0327 | 1.9 | 9.1 | iberin (ITC) | no taste | anticarcinogenic [47] | |
| aromatic | gluco- nasturtiin | GA |  | C15H21N1O9S2 | 422.0578 | 422.0574 | 1.7 | 17.0 | 2-phenylethyl ITC (PEITC) | pungent | anticarcinogenic [48], antibacterial [48] |
| indole | glucobrassicin | GB |  | C16H20N2O9S2 | 447.0496 | 447.0537 | 2.6 | 15.8 | indole-3-carbinol (I3C), 3,3-diindolylmethane (DIM) | bitter taste | high scavenging activity [49] |
2. Materials and Methods
2.1. Plant Material
2.2. Climate Chamber Experiment
2.3. Extraction of Glucosinolates from Plant Material
2.4. Quantification of Seven Glucosinolates with HPLC-ESI-qTOF-MS
2.5. Data Analysis
3. Results
Changes of Glucosinolate Patterns during Cold Acclimation
4. Discussion
4.1. Chilling from the Kale’s Perspective—Change of Glucosinolate Pattern in Cold-Treated Kale
4.2. Chilling from the Glucosinolates’ Perspective
4.3. Within-Cultivar Variation and the Issue of Low Glucosinolate Values
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Šamec, D.; Urlic, B.; Salopek-Sondi, B. Kale (Brassica oleracea var. acephala) as a superfood: Review of the scientific evidence behind the statement. Crit. Rev. Food Sci. Nutr. 2019, 59, 2411–2422. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.; Howard, N.P.; Albach, D.C. Different Shades of Kale—Approaches to Analyze Kale Variety Interrelations. Genes 2022, 13, 232. [Google Scholar] [CrossRef] [PubMed]
- Albach, D.; Mageney, V.; Hahn, C. Grünkohl—Ein zu wenig beachtetes Gemüse. Food-Lab 2017, 2, 6–10. [Google Scholar]
- Maggioni, L.; von Bothmer, R.; Poulsen, G.; Aloisi, K.H. Survey and genetic diversity of wild Brassica oleracea L. germplasm on the Atlantic coast of France. Genet. Resour. Crop Evol. 2020, 67, 1853–1866. [Google Scholar] [CrossRef]
- Thavarajah, D.; Thavarajah, P.; Abare, A.; Basnagala, S.; Lacher, C.; Smith, P.; Combs, G.F. Mineral micronutrient and prebiotic carbohydrate profiles of USA-grown kale (Brassica oleracea L. var. acephala). J. Food Compos. Anal. 2016, 52, 9–15. [Google Scholar] [CrossRef]
- Satheesh, N.; Workneh Fanta, S. Kale: Review on nutritional composition, bio-active compounds, anti-nutritional factors, health beneficial properties and value-added products. Cogent Food Agric. 2020, 6, 1811048. [Google Scholar] [CrossRef]
- Bell, L.; Oloyede, O.O.; Lignou, S.; Wagstaff, C.; Methven, L. Taste and Flavor Perceptions of Glucosinolates, Isothiocyanates, and Related Compounds. Mol. Nutr. Food Res. 2018, 62, 1700990. [Google Scholar] [CrossRef]
- Cartea, M.E.; Velasco, P. Glucosinolates in Brassica foods: Bioavailability in food and significance for human health. Phytochem. Rev. 2007, 7, 213–229. [Google Scholar] [CrossRef]
- Wagner, A.E.; Terschluesen, A.M.; Rimbach, G. Health Promoting Effects of Brassica-Derived Phytochemicals: From Chemopreventive and Anti-Inflammatory Activities to Epigenetic Regulation. Oxid. Med. Cell. Longev. 2013, 2013, 964539. [Google Scholar] [CrossRef]
- Becker, T.M.; Juvik, J.A. The Role of Glucosinolate Hydrolysis Products from Brassica Vegetable Consumption in Inducing Antioxidant Activity and Reducing Cancer Incidence. Diseases 2016, 4, 22. [Google Scholar] [CrossRef]
- Sturm, C.; Wagner, A.E. Brassica-Derived Plant Bioactives as Modulators of Chemopreventive and Inflammatory Signaling Pathways. Int. J. Mol. Sci. 2017, 18, 1890. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.R.; Shafaei, A.; Balmer, L.; Lewis, J.R.; Hodgson, J.M.; Millar, A.H.; Blekkenhorst, L.C. Sulfur compounds: From plants to humans and their role in chronic disease prevention. Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef]
- Kliebenstein, D.J.; Gershenzon, J.; Mitchell-Olds, T. Comparative Quantitative Trait Loci Mapping of Aliphatic, Indolic and Benzylic Glucosinolate Production in Arabidopsis thaliana Leaves and Seeds. Genetics 2001, 159, 359–370. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.; Kim, H.; Chae, W.B.; Kim, S.J.; Lim, Y.P.; Oh, M.H. Brassinosteroids regulate glucosinolate biosynthesis in Arabidopsis thaliana. Physiol. Plant. 2018, 163, 450–458. [Google Scholar] [CrossRef]
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.P.T.; Stewart, J.; Lopez, M.; Ioannou, I.; Allais, F. Glucosinolates: Natural Occurrence, Biosynthesis, Accessibility, Isolation, Structures, and Biological Activities. Molecules 2020, 25, 4537. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, G.R.; Heaney, R.K. Glucosinolates and their breakdown products in cruciferous crops, foods and feedingstuffs. Food Chem. 1983, 11, 249–271. [Google Scholar] [CrossRef]
- Hahn, C.; Müller, A.; Kuhnert, N.; Albach, D. Diversity of Kale (Brassica oleracea var. sabellica): Glucosinolate Content and Phylogenetic Relationships. J. Agric. Food Chem. 2016, 64, 3215–3225. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Bones, A.M.; Rossiter, J.T. The myrosinase-glucosinolate system, its organisation and biochemistry. Physiol. Plant. 1996, 97, 194–208. [Google Scholar] [CrossRef]
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Bisht, N.C. Regulation of Glucosinolate Metabolism: From Model Plant Arabidopsis thaliana to Brassica Crops. In Glucosinolates; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 163–199. [Google Scholar] [CrossRef]
- Wittstock, U.; Kliebenstein, D.J.; Lambrix, V.; Reichelt, M.; Gershenzon, J. Glucosinolate hydrolysis and its impact on generalist and specialist insect herbivores. In Integrative Phytochemistry: From Ethnobotany to Molecular Ecology; Romeo, J.T., Ed.; Elsevier: Oxford, UK, 2003; Volume 37, pp. 101–125. [Google Scholar]
- Variyar, P.S.; Banerjee, A.; Akkarakaran, J.J.; Suprasanna, P. Role of Glucosinolates in Plant Stress Tolerance. In Emerging Technologies and Management of Crop Stress Tolerance; Ahmad, P., Rasool, S., Eds.; Elsevier: San Diego, CA, USA, 2014; Volume 1, pp. 271–291. [Google Scholar]
- Ben Ammar, H.; Arena, D.; Treccarichi, S.; Di Bella, M.C.; Marghali, S.; Ficcadenti, N.; Lo Scalzo, R.; Branca, F. The Effect of Water Stress on the Glucosinolate Content and Profile: A Comparative Study on Roots and Leaves of Brassica oleracea L. Crops. Agronomy 2023, 13, 579. [Google Scholar] [CrossRef]
- Del Carmen Martínez-Ballesta, M.; Moreno, D.A.; Carvajal, M. The physiological importance of glucosinolates on plant response to abiotic stress in Brassica. Int. J. Mol. Sci. 2013, 14, 11607–11625. [Google Scholar] [CrossRef] [PubMed]
- Kushad, M.M.; Brown, A.F.; Kurilich, A.C.; Juvik, J.A.; Klein, B.P.; Wallig, M.A.; Jeffery, E.H. Variation of Glucosinolates in Vegetable Crops of Brassica oleracea. J. Agric. Food Chem. 1999, 47, 1541–1548. [Google Scholar] [CrossRef]
- Brown, P.D.; Tokuhisa, J.G.; Reichelt, M.; Gershenzon, J. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 2003, 62, 471–481. [Google Scholar] [CrossRef]
- Lin, H.; Sun, J.; Hu, Z.; Cheng, C.; Lin, S.; Zou, H.; Yan, X. Variation in Glucosinolate Accumulation among Different Sprout and Seedling Stages of Broccoli (Brassica oleracea var. italica). Plants 2022, 11, 1563. [Google Scholar] [CrossRef]
- Nilsson, J.; Olsson, K.; Engqvist, G.; Ekvall, J.; Olsson, M.; Nyman, M.; Åkesson, B. Variation in the content of glucosinolates, hydroxycinnamic acids, carotenoids, total antioxidant capacity and low-molecular-weight carbohydrates in Brassica vegetables. J. Sci. Food Agric. 2006, 86, 528–538. [Google Scholar] [CrossRef]
- Kissen, R.; Eberl, F.; Winge, P.; Uleberg, E.; Martinussen, I.; Bones, A.M. Effect of growth temperature on glucosinolate profiles in Arabidopsis thaliana accessions. Phytochemistry 2016, 130, 106–118. [Google Scholar] [CrossRef]
- Jurkow, R.; Wurst, A.; Kalisz, A.; Sekara, A.; Cebula, S. Cold stress modifies bioactive compounds of kale cultivars during fall-winter harvests. Acta Agrobot. 2019, 72, 1761. [Google Scholar] [CrossRef]
- Megías-Pérez, R.; Hahn, C.; Ruiz-Matute, A.I.; Behrends, B.; Albach, D.C.; Kuhnert, N. Changes in low molecular weight carbohydrates in kale during development and acclimation to cold temperatures determined by chromatographic techniques coupled to mass spectrometry. Food Res. Int. 2020, 127, 108727. [Google Scholar] [CrossRef]
- Cartea, M.E.; Velasco, P.; Obregon, S.; Padilla, G.; de Haro, A. Seasonal variation in glucosinolate content in Brassica oleracea crops grown in northwestern Spain. Phytochemistry 2008, 69, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Steindal, A.L.H.; Rødven, R.; Hansen, E.; Mølmann, J. Effects of photoperiod, growth temperature and cold acclimatisation on glucosinolates, sugars and fatty acids in kale. Food Chem. 2015, 174, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Ljubej, V.; Karalija, E.; Salopek-Sondi, B.; Šamec, D. Effects of Short-Term Exposure to Low Temperatures on Proline, Pigments, and Phytochemicals Level in Kale (Brassica oleracea var. acephala). Horticulturae 2021, 7, 341. [Google Scholar] [CrossRef]
- Ljubej, V.; Radojčić Redovniković, I.; Salopek-Sondi, B.; Smolko, A.; Roje, S.; Šamec, D. Chilling and Freezing Temperature Stress Differently Influence Glucosinolates Content in Brassica oleracea var. acephala. Plants 2021, 10, 1305. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Gomez-Carneros, C. Bitter taste, phytonutrients, and the consumer: A review. Am. J. Clin. Nutr. 2000, 72, 1424–1435. [Google Scholar] [CrossRef]
- The Royal Society of Chemistry. ChemSpider. Chemical Structure Database. Available online: http://www.chemspider.com/ (accessed on 15 April 2022).
- Arora, R.; Kumar, R.; Mahajan, J.; Vig, A.P.; Singh, B.; Singh, B.; Arora, S. 3-Butenyl isothiocyanate: A hydrolytic product of glucosinolate as a potential cytotoxic agent against human cancer cell lines. J. Food Sci. Technol. 2016, 53, 3437–3445. [Google Scholar] [CrossRef]
- van Doorn, H.E.; van der Kruk, G.C.; van Holst, G.-J.; Raaijmakers-Ruijs, N.C.M.E.; Postma, E.; Groeneweg, B.; Jongen, W.H.F. The glucosinolates sinigrin and progoitrin are important determinants for taste preference and bitterness of Brussels sprouts. J. Sci. Food Agric. 1998, 78, 30–38. [Google Scholar] [CrossRef]
- Liu, Z.; Hirani, A.H.; McVetty, P.B.; Daayf, F.; Quiros, C.F.; Li, G. Reducing progoitrin and enriching glucoraphanin in Brassica napus seeds through silencing of the GSL-ALK gene family. Plant Mol. Biol. 2012, 79, 179–189. [Google Scholar] [CrossRef]
- Moon, J.K.; Kim, J.R.; Ahn, Y.J.; Shibamoto, T. Analysis and anti-Helicobacter activity of sulforaphane and related compounds present in broccoli (Brassica oleracea L.) sprouts. J. Agric. Food Chem. 2010, 58, 6672–6677. [Google Scholar] [CrossRef]
- Bayat Mokhtari, R.; Baluch, N.; Homayouni, T.S.; Morgatskaya, E.; Kumar, S.; Kazemi, P.; Yeger, H. The role of Sulforaphane in cancer chemoprevention and health benefits: A mini-review. J. Cell Commun. Signal. 2018, 12, 91–101. [Google Scholar] [CrossRef]
- Mi, L.; Xiao, Z.; Hood, B.L.; Dakshanamurthy, S.; Wang, X.; Govind, S.; Conrads, T.P.; Veenstra, T.D.; Chung, F.L. Covalent binding to tubulin by isothiocyanates. A mechanism of cell growth arrest and apoptosis. J. Biol. Chem. 2008, 283, 22136–22146. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, A.; Dwivedi, A.; du Plessis, J. Sinigrin and Its Therapeutic Benefits. Molecules 2016, 21, 416. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.-T.; Guo, Q.; Li, X.; Zhang, T.-N.; Liu, F.-H.; He, X.-H.; Lin, B.; Wu, Q.-J. Isothiocyanate Iberin inhibits cell proliferation and induces cell apoptosis in the progression of ovarian cancer by mediating ROS accumulation and GPX1 expression. Biomed. Pharmacother. 2021, 142, 111533. [Google Scholar] [CrossRef]
- Aires, A.; Carvalho, R.; Rosa, E.A.S.; Saavedra, M.J. Phytochemical characterization and antioxidant properties of baby-leaf watercress produced under organic production system. CyTA-J. Food 2013, 11, 343–351. [Google Scholar] [CrossRef]
- Vo, Q.V.; Mechler, A. In Silico Study of the Radical Scavenging Activities of Natural Indole-3-Carbinols. J. Chem. Inf. Model. 2020, 60, 316–321. [Google Scholar] [CrossRef]
- Šamec, D.; Ljubej, V.; Redovniković, I.R.; Fistanić, S.; Salopek-Sondi, B. Low Temperatures Affect the Physiological Status and Phytochemical Content of Flat Leaf Kale (Brassica oleracea var. acephala) Sprouts. Foods 2022, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Kruk, V.; Ivanišević, P. Influence of Seed Origin on Morphological Characteristics and Phytochemicals Levels in Brassica oleracea var. acephala. Agronomy 2019, 9, 502. [Google Scholar] [CrossRef]
- Hellmiß, M. Kohl: Das Gesündeste Lebensmittel der Welt; Südwest Verlag: München, Germany, 2014; p. 160. [Google Scholar]
- Engelen-Eigles, G.; Holden, G.; Cohen, J.D.; Gardner, G. The effect of temperature, photoperiod, and light quality on gluconasturtiin concentration in watercress (Nasturtium officinale R. Br.). J. Agric. Food Chem. 2006, 54, 328–334. [Google Scholar] [CrossRef]
- Zang, Y.X.; Zhang, H.; Huang, L.H.; Wang, F.; Gao, F.; Lv, X.S.; Yang, J.; Zhu, B.; Hong, S.B.; Zhu, Z.J. Glucosinolate enhancement in leaves and roots of pak choi (Brassica rapa ssp chinensis) by methyl jasmonate. Hortic. Environ. Biotechnol. 2015, 56, 830–840. [Google Scholar] [CrossRef]
- Mellon, F.A.; Bennett, R.N.; Holst, B.; Williamson, G. Intact Glucosinolate Analysis in Plant Extracts by Programmed Cone Voltage Electrospray LC/MS: Performance and Comparison with LC/MS/MS Methods. Anal. Biochem. 2002, 306, 83–91. [Google Scholar] [CrossRef]
- Song, L.; Thornalley, P.J. Effect of storage, processing and cooking on glucosinolate content of Brassica vegetables. Food Chem. Toxicol. 2007, 45, 216–224. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing, Version 4.2.0; R Foundation for Statistical Computing: Vienna, Australia, 2022. [Google Scholar]
- Ferioli, F.; Giambanelli, E.; D’Antuono, L.F.; Costa, H.S.; Albuquerque, T.G.; Silva, A.S.; Hayran, O.; Koçaoglu, B. Comparison of leafy kale populations from Italy, Portugal, and Turkey for their bioactive compound content: Phenolics, glucosinolates, carotenoids, and chlorophylls. J. Sci. Food Agric. 2013, 93, 3478–3489. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Vives-Peris, V.; Gómez-Cadenas, A.; Arbona, V. A Fast and Precise Method To Identify Indolic Glucosinolates and Camalexin in Plants by Combining Mass Spectrometric and Biological Information. J. Agric. Food Chem. 2012, 60, 8648–8658. [Google Scholar] [CrossRef] [PubMed]
- Santarius, K.A. The protective effect of sugars on chloroplast membranes during temperature and water stress and its relationship to frost, desiccation and heat resistance. Planta 1973, 113, 105–114. [Google Scholar] [CrossRef]
- Shattuck, V.I.; Kakuda, Y.; Shelp, B.J. Effect of low temperature on the sugar and glucosinolate content of rutabaga. Sci. Hortic. 1991, 48, 9–19. [Google Scholar] [CrossRef]
- Rosa, E.A.S.; Heaney, R.K.; Portas, C.A.M.; Fenwick, G.R. Changes in Glucosinolate Concentrations in Brassica Crops (B. oleracea and B. napus) Throughout Growing Seasons. J. Sci. Food Agric. 1996, 71, 237–244. [Google Scholar] [CrossRef]
- Poorter, H.; Fiorani, F.; Pieruschka, R.; Wojciechowski, T.; van der Putten, W.H.; Kleyer, M.; Schurr, U.; Postma, J. Pampered inside, pestered outside? Differences and similarities between plants growing in controlled conditions and in the field. New Phytol. 2016, 212, 838–855. [Google Scholar] [CrossRef]
- Brown, A.F.; Yousef, G.G.; Reid, R.W.; Chebrolu, K.K.; Thomas, A.; Krueger, C.; Jeffery, E.; Jackson, E.; Juvik, J.A. Genetic analysis of glucosinolate variability in broccoli florets using genome-anchored single nucleotide polymorphisms. Theor. Appl. Genet. 2015, 128, 1431–1447. [Google Scholar] [CrossRef]
- Hirani, A.H.; Li, G.; Zelmer, C.D.; McVetty, P.B.; Asif, M.; Goyal, A. Molecular Genetics of Glucosinolate Biosynthesis in Brassicas: Genetic Manipulation and Application Aspects. In Crop Plant; Goyal, A., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 189–216. [Google Scholar] [CrossRef]
- Iahtisham-Ul-Haq; Khan, S.; Awan, K.A.; Iqbal, M.J. Sulforaphane as a potential remedy against cancer: Comprehensive mechanistic review. J. Food Biochem. 2022, 46, e13886. [Google Scholar] [CrossRef]
- Chin, H.W.; Zeng, Q.L.; Lindsay, R.C. Occurrence and flavor properties of sinigrin hydrolysis products in fresh cabbage. J. Food Sci. 1996, 61, 101–104. [Google Scholar] [CrossRef]
- Engel, E.; Baty, C.; Le Corre, D.; Souchon, I.; Martin, N. Flavor-active compounds potentially implicated in cooked cauliflower acceptance. J. Agric. Food Chem. 2002, 50, 6459–6467. [Google Scholar] [CrossRef] [PubMed]
- Felker, P.; Bunch, R.; Leung, A.M. Concentrations of thiocyanate and goitrin in human plasma, their precursor concentrations in brassica vegetables, and associated potential risk for hypothyroidism. Nutr. Rev. 2016, 74, 248–258. [Google Scholar] [CrossRef]
- Lietzow, J. Biologically Active Compounds in Mustard Seeds: A Toxicological Perspective. Foods 2021, 10, 2089. [Google Scholar] [CrossRef] [PubMed]
- Charron, C.S.; Saxton, A.M.; Sams, C.E. Relationship of climate and genotype to seasonal variation in the glucosinolate-myrosinase system. I. Glucosinolate content in ten cultivars of Brassica oleracea grown in fall and spring seasons. J. Sci. Food Agric. 2005, 85, 671–681. [Google Scholar] [CrossRef]
- Ciska, E.; Martyniak-Przybyszewska, B.; Kozlowska, H. Content of glucosinolates in cruciferous vegetables grown at the same site for two years under different climatic conditions. J. Agric. Food Chem. 2000, 48, 2862–2867. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.; Kiraga, S.; Islam, M.N.; Ali, M.; Reza, M.N.; Lee, W.-H.; Chung, S.-O. Effects of Temperature, Relative Humidity, and Carbon Dioxide Concentration on Growth and Glucosinolate Content of Kale Grown in a Plant Factory. Foods 2021, 10, 1524. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Shaker, K.; Heinzel, N.; Ralph, J.; Gális, I.; Baldwin, I.T. Environmental Stresses of Field Growth Allow Cinnamyl Alcohol Dehydrogenase-Deficient Nicotiana attenuata Plants to Compensate for their Structural Deficiencies. Plant Physiol. 2012, 159, 1545–1570. [Google Scholar] [CrossRef]


| Glucosinolate | Slope | Axis Intercept | R2 | RSD (%) | LOQ (mg/L) | |
|---|---|---|---|---|---|---|
| GN | gluconapin | 0.0036 | 2.7525 | 0.9963 | 3.97 | 3.49 |
| PR | progoitrin | 0.0048 | 0.5658 | 0.9997 | 3.01 | 1.33 |
| GR | glucoraphanin | 0.0074 | 0.2895 | 0.9996 | 4.16 | 1.25 |
| SIN | sinigrin | 0.0046 | 0.3270 | 0.9989 | 3.84 | 1.25 |
| GI | glucoiberin | 0.0034 | 0.2931 | 0.9993 | 3.91 | 0.99 |
| GA | gluconasturtiin | 0.0030 | −1.0210 | 0.9975 | 4.37 | 0.01 |
| GB | glucobrassicin | 0.0068 | −0.5779 | 0.9986 | 3.23 | 0.17 |
| Cultivar | Treatment | Sampling | Glucosinolates (mg 100 g−1 FW) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| GN | PR | GR | SIN | GI | GA | GB | |||
| F | warm (day 0) | nd | 0.83 (0.83) | 0.65 (0.48–1.02) a | 0.63 (0.48–0.81) | nd | nd | 30.30 (6.12–57.52) | |
| cold | 12 h | nd | 0.47 (0.45–0.49) | 0.85 (0.57–1.18) ab | 0.56 (0.44–0.68) | nd | nd | 37.68 (12.23–69.37) | |
| 7d | nd | 0.77 (0.40–1.44) | 1.21 (0.80–2.00) bc | 0.85 (0.52–1.41) | nd | nd | 31.26 (18.75–56.93) | ||
| control (warm) | day 0 | nd | nd | nd | nd | nd | nd | 40.74 (19.55–67.94) | |
| 12 h | nd | nd | nd | nd | nd | nd | 48.39 (12.77–104.51) | ||
| 7d | nd | nd | nd | nd | nd | nd | 41.33 (19.30–92.03) | ||
| B | warm (day 0) | nd | nd | 1.82 (0.66–5.93) a | nd | nd | nd | 53.37 (2.69–139.16) | |
| cold | 12 h | nd | nd | 1.83 (0.91–3.95) a | nd | nd | nd | 34.57 (4.21–73.69) | |
| 7d | nd | nd | 6.10 (0.70–19.45) b | nd | nd | nd | 61.18 (7.12–130.56) | ||
| control (warm) | day 0 | nd | nd | nd | nd | nd | nd | 41.01 (5.92–93.91) | |
| 12 h | nd | nd | nd | nd | nd | nd | 50.63 (22.05–126.03) | ||
| 7d | nd | nd | nd | nd | nd | nd | 67.42 (35.45–119.66) | ||
| W | warm (day 0) | 10.50 (0.52–34.75) | 17.57 (2.27–40.19) | 4.20 (0.59–12.96) | 4.63 (0.84–12.15) | nd | nd | 90.11 (11.50–192.31) | |
| cold | 12 h | 7.90 (0.84–20.37) | 20.78 (6.73–46.59) | 15.46 (0.77–51.55) | 3.39 (3.39) | nd | nd | 86.83 (18.91–182.14) | |
| 7d | 8.23 (0.53–30.79) | 17.37 (6.99–34.00) | 16.32 (0.82–70.92) | 3.82 (0.61–7.02) | nd | nd | 60.81 (24.77–150.33) | ||
| control (warm) | day 0 | 1.04 (0.45–3.97) | 6.19 (0.51–21.31) | 4.53 (0.40–11.98) | 3.58 (0.63–7.17) | nd | nd | 150.67 (17.44–356.33) | |
| 12 h | 0.80 (0.39–1.28) | 5.48 (0.87–20.93) | 3.50 (0.45–6.55) | 3.39 (1.47–6.70) | nd | nd | 149.69 (26.84–329.89) | ||
| 7d | 1.16 (0.44–1.75) | 6.73 (1.23–19.83) | 5.84 (0.56–9.20) | 2.86 (0.55–7.80) | nd | nd | 125.79 (16.53–268.24) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hahn, C.; Müller, A.; Kuhnert, N.; Albach, D.C. A Cold Case—Glucosinolate Levels in Kale Cultivars Are Differently Influenced by Cold Temperatures. Horticulturae 2023, 9, 953. https://doi.org/10.3390/horticulturae9090953
Hahn C, Müller A, Kuhnert N, Albach DC. A Cold Case—Glucosinolate Levels in Kale Cultivars Are Differently Influenced by Cold Temperatures. Horticulturae. 2023; 9(9):953. https://doi.org/10.3390/horticulturae9090953
Chicago/Turabian StyleHahn, Christoph, Anja Müller, Nikolai Kuhnert, and Dirk C. Albach. 2023. "A Cold Case—Glucosinolate Levels in Kale Cultivars Are Differently Influenced by Cold Temperatures" Horticulturae 9, no. 9: 953. https://doi.org/10.3390/horticulturae9090953
APA StyleHahn, C., Müller, A., Kuhnert, N., & Albach, D. C. (2023). A Cold Case—Glucosinolate Levels in Kale Cultivars Are Differently Influenced by Cold Temperatures. Horticulturae, 9(9), 953. https://doi.org/10.3390/horticulturae9090953









