Effects of 1-Methylcyclopropene Treatment on the Quality and Malic Acid Metabolism of ‘Xiangjiao’ Plum under Low-Temperature Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Material and Treatments
2.2. Determination of CI Index
2.3. Determination of Sensory and Physiological Quality Indicators
2.4. Determination of Antioxidant Enzyme Activity
2.5. Determination of Malic Acid Content
2.6. Determination of Enzyme Activity Related to Malic Acid Metabolism
2.7. Determination of Crucial Enzyme Gene Expression in the Malic Acid Metabolism
2.8. Statistical Analysis
3. Results
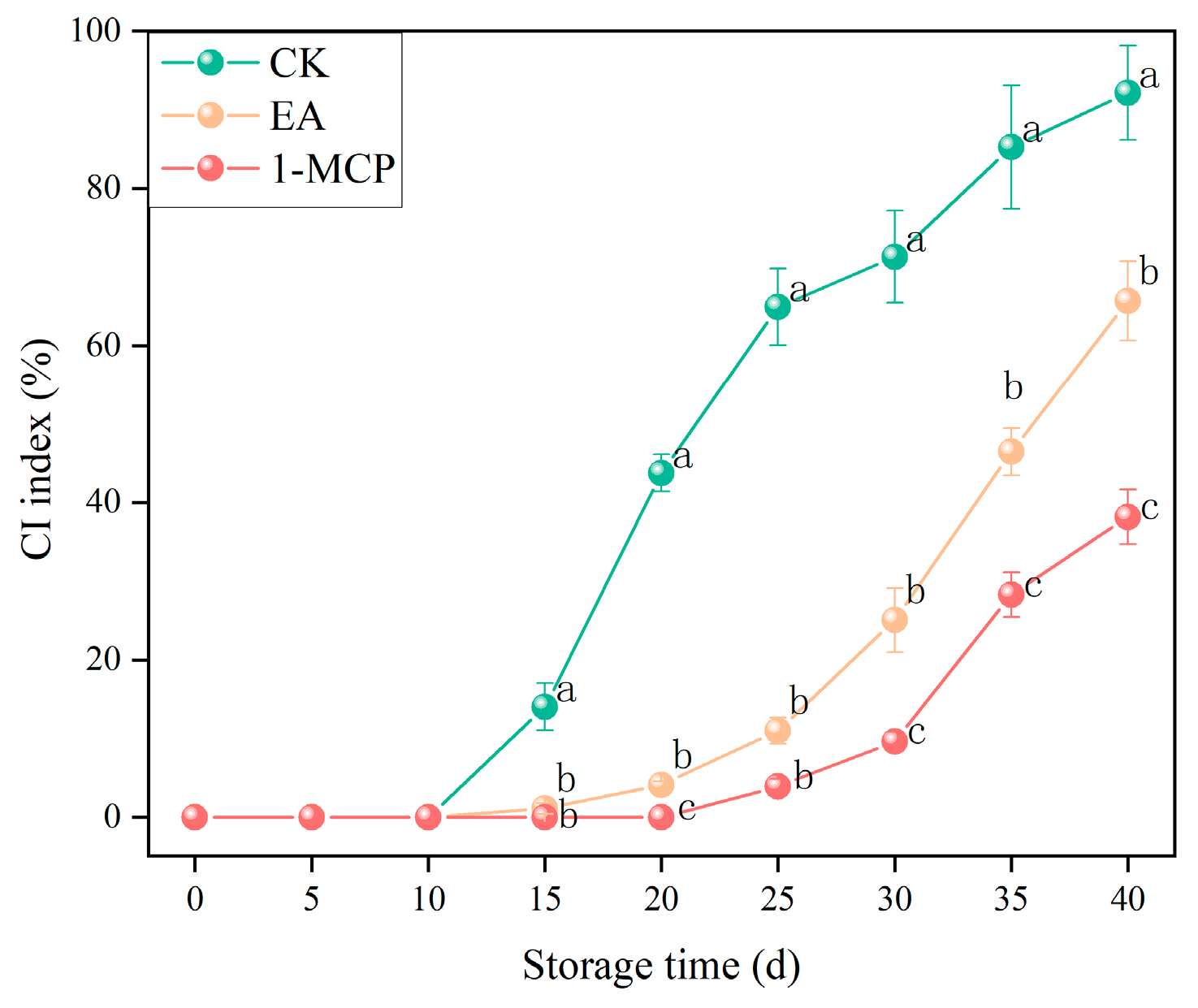
3.1. CI Index
3.2. Sensory and Physiological Quality Indicators
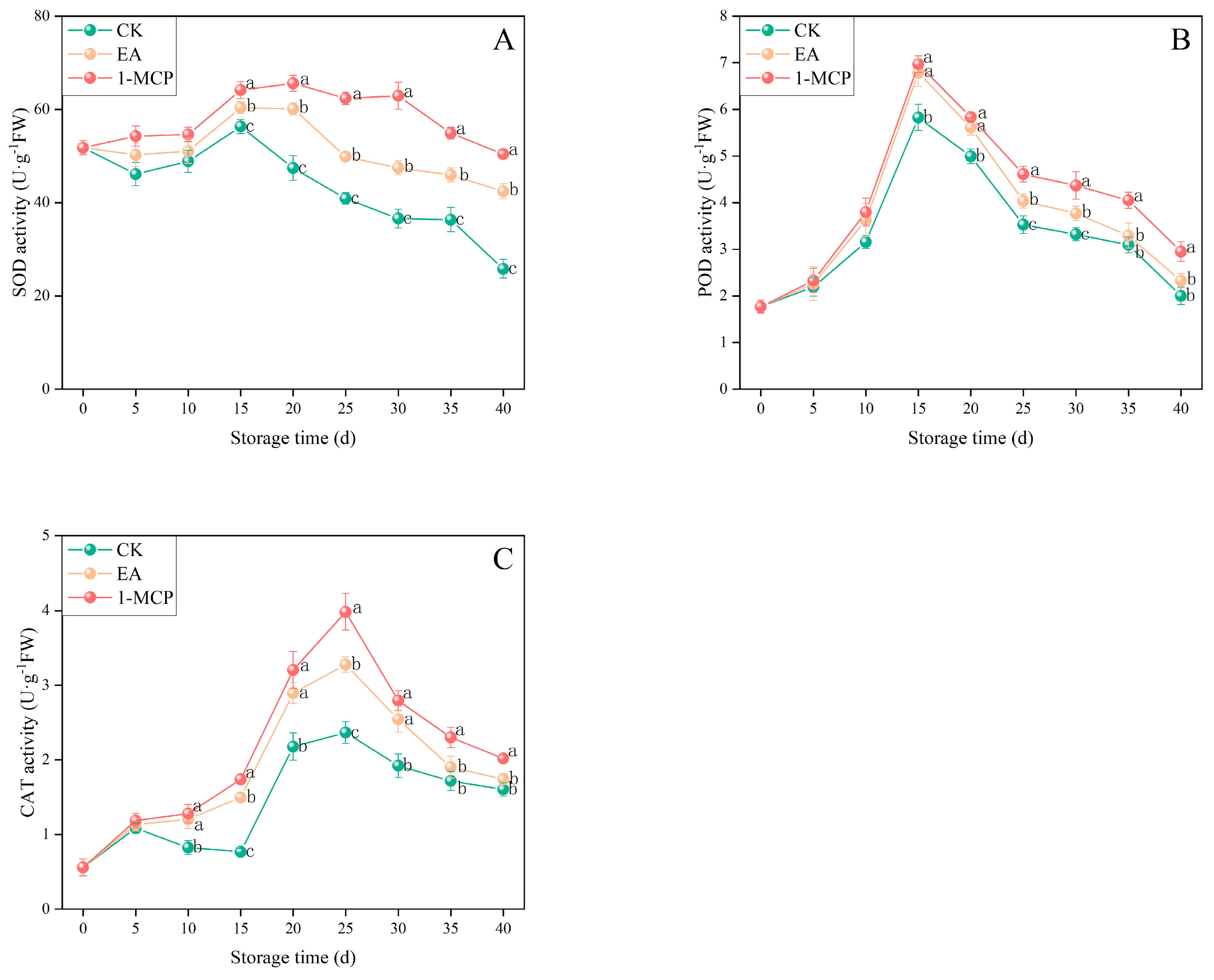
3.3. Antioxidant Enzyme Activity
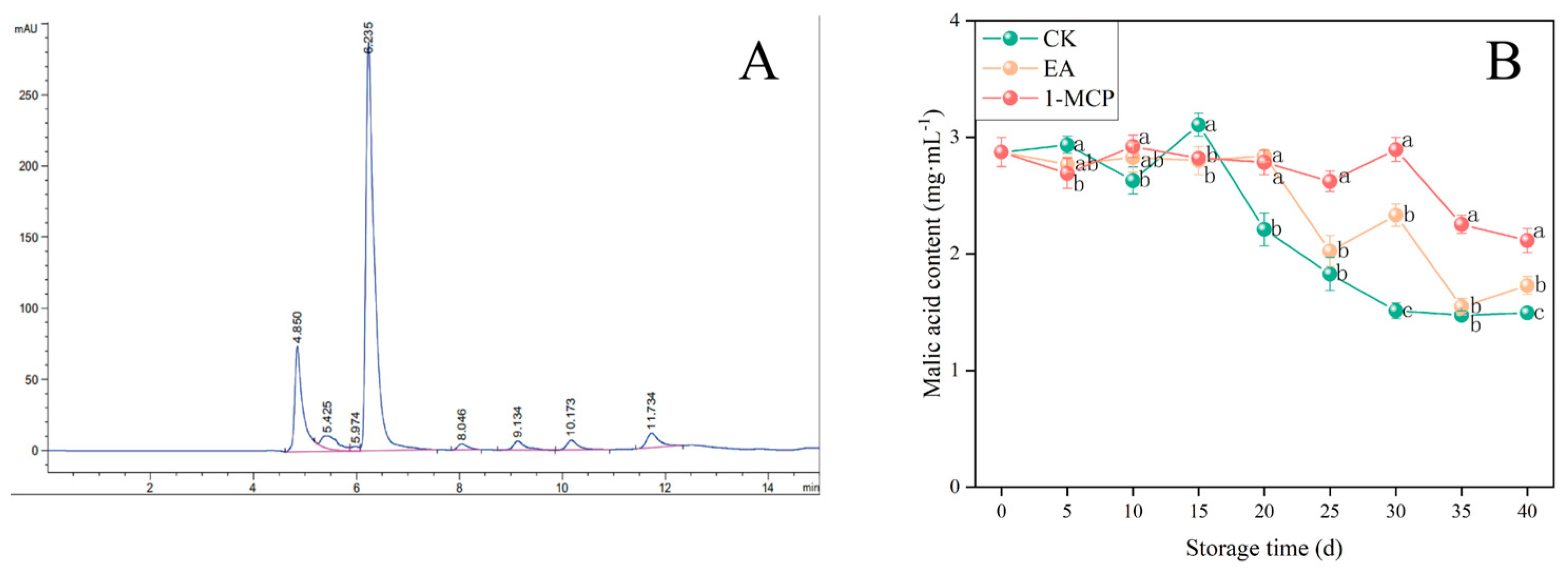
3.4. Malic Acid Content
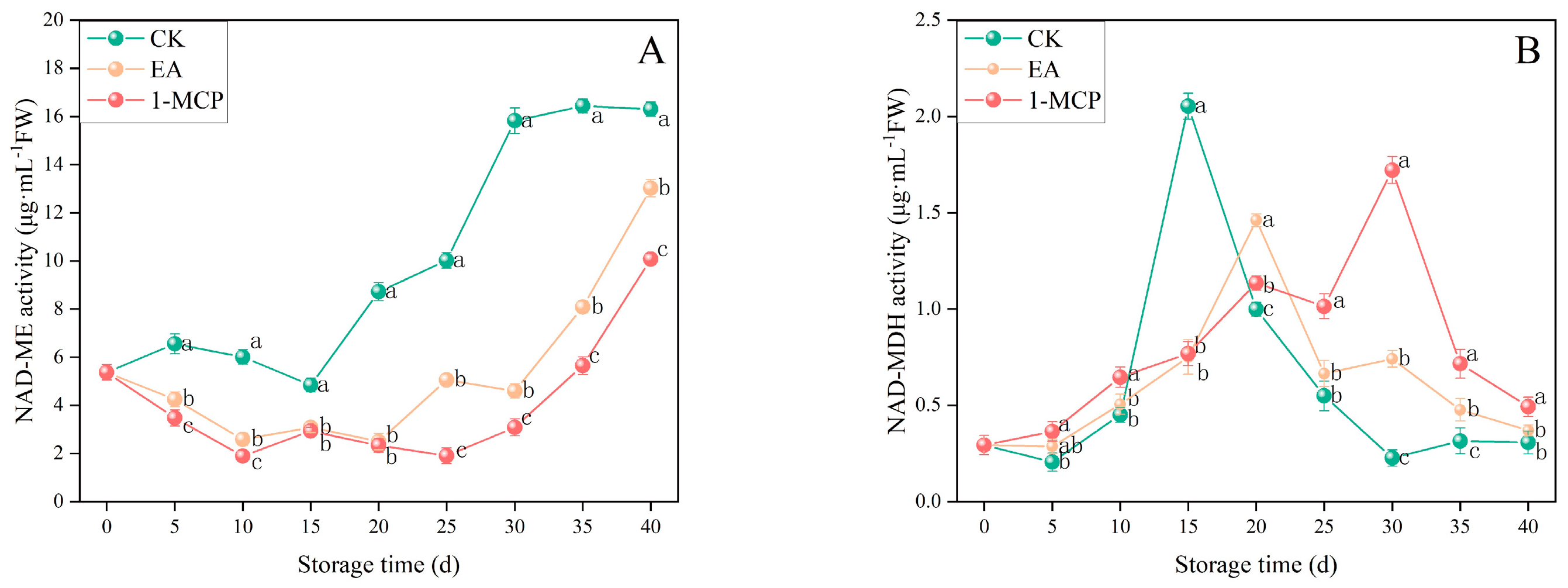
3.5. Malic Acid Metabolism-Related Enzyme Activity
3.6. Gene Expression of Malic Acid Metabolism-Related Enzyme
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Igwe, E.O.; Charlton, K.E. A Systematic Review on the Health Effects of Plums (Prunus domestica and Prunus salicina). Phytother. Res. 2016, 30, 701–731. [Google Scholar] [CrossRef]
- Wang, L.; Hong, K.; Xu, R.; Zhao, Z.; Cao, J. The Alleviation of Cold-Stimulated Flesh Reddening in ‘Friar’ Plum Fruit by the Elevated CO2 with Polyvinyl Chloride (PVC) Packaging. Sci. Hortic. 2021, 281, 109997. [Google Scholar] [CrossRef]
- Pan, H.; Wang, L.; Wang, R.; Xie, F.; Cao, J. Modifications of Cell Wall Pectin in Chilling-Injured ‘Friar’ Plum Fruit Subjected to Intermediate Storage Temperatures. Food Chem. 2018, 242, 538–547. [Google Scholar] [CrossRef]
- Lin, X.; Huang, S.; Huber, D.J.; Zhang, Q.; Wan, X.; Peng, J.; Luo, D.; Dong, X.; Zhu, S. Melatonin Treatment Affects Wax Composition and Maintains Storage Quality in ‘Kongxin’ Plum (Prunus salicina L. Cv) during Postharvest. Foods 2022, 11, 3972. [Google Scholar] [CrossRef]
- Larrigaudière, C.; Candan, A.P.; Ubach, D.; Graell, J. Physiological Response of ‘Larry Ann’ Plums to Cold Storage and 1-MCP Treatment. Postharvest Biol. Technol. 2009, 51, 56–61. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, S. Pre-Storage Cold Acclimation Maintained Quality of Cold-Stored Cucumber through Differentially and Orderly Activating ROS Scavengers. Postharvest Biol. Technol. 2017, 129, 1–8. [Google Scholar] [CrossRef]
- Shan, Y.; Zhang, D.; Luo, Z.; Li, T.; Qu, H.; Duan, X.; Jiang, Y. Advances in Chilling Injury of Postharvest Fruit and Vegetable: Extracellular ATP Aspects. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4251–4273. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Duan, W.; Li, W.; Wang, Q.; Li, J.; Song, H.; Xu, X. Melatonin Maintained Higher Contents of Unsaturated Fatty Acid and Cell Membrane Structure Integrity in Banana Peel and Alleviated Postharvest Chilling Injury. Food Chem. 2022, 397, 133836. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Q.; Tang, J.; Hu, K.-D.; Huang, Z.-Q.; Yang, F.; Zhang, H.-Y.; Hu, L.-Y.; Li, Y.-H.; Yao, G.-F.; Zhang, H. Antioxidative System in Sweet Potato Root Is Activated by Low-Temperature Storage. J. Sci. Food Agric. 2019, 99, 3824–3833. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, B.; Zhang, W.; Cao, J.; Jiang, W. Enhancement of Quality and Antioxidant Metabolism of Sweet Cherry Fruit by Near-Freezing Temperature Storage. Postharvest Biol. Technol. 2019, 147, 113–122. [Google Scholar] [CrossRef]
- Jiang, C.-C.; Fang, Z.-Z.; Zhou, D.-R.; Pan, S.-L.; Ye, X.-F. Changes in Secondary Metabolites, Organic Acids and Soluble Sugars during the Development of Plum Fruit Cv. “Furongli” (Prunus salicina Lindl). J. Sci. Food Agric. 2019, 99, 1010–1019. [Google Scholar] [CrossRef]
- Trendafilova, A.; Ivanova, V.; Trusheva, B.; Kamenova-Nacheva, M.; Tabakov, S.; Simova, S. Chemical Composition and Antioxidant Capacity of the Fruits of European Plum Cultivar “Cacanska Lepotica” Influenced by Different Rootstocks. Foods 2022, 11, 2844. [Google Scholar] [CrossRef]
- Carrasco, B.; Meisel, L.; Gebauer, M.; Garcia-Gonzales, R.; Silva, H. Breeding in Peach, Cherry and Plum: From a Tissue Culture, Genetic, Transcriptomic and Genomic Perspective. Biol. Res. 2013, 46, 219–230. [Google Scholar] [CrossRef]
- Jiang, B.; Fang, X.; Fu, D.; Wu, W.; Han, Y.; Chen, H.; Liu, R.; Gao, H. Exogenous Salicylic Acid Regulates Organic Acids Metabolism in Postharvest Blueberry Fruit. Front. Plant Sci. 2022, 13, 1024909. [Google Scholar] [CrossRef]
- Morley, S.A.; Ma, F.; Alazem, M.; Frankfater, C.; Yi, H.; Burch-Smith, T.; Clemente, T.E.; Veena, V.; Nguyen, H.; Allen, D.K. Expression of Malic Enzyme Reveals Subcellular Carbon Partitioning for Storage Reserve Production in Soybeans. New Phytol. 2023, 239, 1834–1851. [Google Scholar] [CrossRef]
- Huang, X.-Y.; Wang, C.-K.; Zhao, Y.-W.; Sun, C.-H.; Hu, D.-G. Mechanisms and Regulation of Organic Acid Accumulation in Plant Vacuoles. Hortic. Res. 2021, 8, 227. [Google Scholar] [CrossRef] [PubMed]
- Piatek, A.M.; Bomble, Y.J.; Wiskur, S.L.; Anslyn, E.V. Threshold Detection Using Indicator-Displacement Assays: An Application in the Analysis of Malate in Pinot Noir Grapes. J. Am. Chem. Soc. 2004, 126, 6072–6077. [Google Scholar] [CrossRef]
- Establés-Ortiz, B.; Romero, P.; Ballester, A.-R.; González-Candelas, L.; Lafuente, M.T. Inhibiting Ethylene Perception with 1-Methylcyclopropene Triggers Molecular Responses Aimed to Cope with Cell Toxicity and Increased Respiration in Citrus Fruits. Plant Physiol. Biochem. 2016, 103, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Cocci, E.; Sacchetti, G.; Rocculi, P.; Dalla Rosa, M. Response of Pink Lady (R) Apples to Post-Harvest Application of 1-Methylcyclopropene as a Function of Applied Dose, Maturity at Harvest, Storage Time and Controlled Atmosphere Storage. J. Sci. Food Agric. 2014, 94, 2691–2698. [Google Scholar] [CrossRef] [PubMed]
- Selcuk, N.; Erkan, M. The Effects of 1-MCP Treatment on Fruit Quality of Medlar Fruit (Mespilus germanica L. Cv. Istanbul) during Long Term Storage in the Palliflex Storage System. Postharvest Biol. Technol. 2015, 100, 81–90. [Google Scholar] [CrossRef]
- Li, D.; Wu, X.; Li, L.; Wang, Y.; Xu, Y.; Luo, Z. Epibrassinolide Enhanced Chilling Tolerance of Postharvest Banana Fruit by Regulating Energy Status and Pyridine Nucleotide Homeostasis. Food Chem. 2022, 382, 132273. [Google Scholar] [CrossRef]
- Xu, F.; Lu, F.; Xiao, Z.; Li, Z. Influence of Drop Shock on Physiological Responses and Genes Expression of Apple Fruit. Food Chem. 2020, 303, 125424. [Google Scholar] [CrossRef]
- Xing, Y.; Li, X.; Xu, Q.; Yun, J.; Lu, Y.; Tang, Y. Effects of Chitosan Coating Enriched with Cinnamon Oil on Qualitative Properties of Sweet Pepper (Capsicum annuum L.). Food Chem. 2011, 124, 1443–1450. [Google Scholar] [CrossRef]
- Kou, X.; Wang, S.; Zhang, Y.; Guo, R.; Wu, M.; Chen, Q.; Xue, Z. Effects of Chitosan and Calcium Chloride Treatments on Malic Acid-Metabolizing Enzymes and the Related Gene Expression in Post-Harvest Pear Cv. “Huang Guan”. Sci. Hortic. 2014, 165, 252–259. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Vicente, A.R.; Crisosto, C.H.; Labavitch, J.M. Effect of Dips in a 1-Methylcyclopropene-Generating Solution on “Harrow Sun” Plums Stored under Different Temperature Regimes. J. Agric. Food Chem. 2007, 55, 7015–7020. [Google Scholar] [CrossRef]
- Liu, H.; Song, L.; You, Y.; Li, Y.; Duan, X.; Jiang, Y.; Joyce, D.C.; Ashraf, M.; Lu, W. Cold Storage Duration Affects Litchi Fruit Quality, Membrane Permeability, Enzyme Activities and Energy Charge during Shelf Time at Ambient Temperature. Postharvest Biol. Technol. 2011, 60, 24–30. [Google Scholar] [CrossRef]
- Cheng, S.; Yu, Y.; Guo, J.; Chen, G.; Guo, M. Effect of 1-Methylcyclopropene and Chitosan Treatment on the Storage Quality of Jujube Fruit and Its Related Enzyme Activities. Sci. Hortic. 2020, 265, 109281. [Google Scholar] [CrossRef]
- Chang, L.-Y.; Sargent, S.A.; Kim, J.; Brecht, J.K. Delaying Ripening Using 1-MCP Reveals Chilling Injury Symptom Development at the Putative Chilling Threshold Temperature for Mature Green Banana. Front. Plant Sci. 2022, 13, 966789. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Pei, H.; Jiao, J.; Jin, M.; Li, H.; Zhu, Q.; Ma, Y.; Rao, J. 1-Methylcyclopropene Treatment Followed with Ethylene Treatment Alleviates Postharvest Chilling Injury of “Xuxiang” Kiwifruit during Low-Temperature Storage. Food Control 2021, 130, 108340. [Google Scholar] [CrossRef]
- Zhao, Q.; Jin, M.; Guo, L.; Pei, H.; Nan, Y.; Rao, J. Modified Atmosphere Packaging and 1-Methylcyclopropene Alleviate Chilling Injury of “Youhou” Sweet Persimmon during Cold Storage. Food Packag. Shelf Life 2020, 24, 100479. [Google Scholar] [CrossRef]
- Valdenegro, M.; Huidobro, C.; Monsalve, L.; Bernales, M.; Fuentes, L.; Simpson, R. Effects of Ethrel, 1-MCP and Modified Atmosphere Packaging on the Quality of "Wonderful’ Pomegranates during Cold Storage. J. Sci. Food Agric. 2018, 98, 4854–4865. [Google Scholar] [CrossRef] [PubMed]
- Mattheis, J.P.; Rudell, D.R.; Hanrahan, I. Impacts of 1-Methylcyclopropene and Controlled Atmosphere Established during Conditioning on Development of Bitter Pit in “Honeycrisp” Apples. Hortscience 2017, 52, 132–137. [Google Scholar] [CrossRef]
- Jin, P.; Shang, H.; Chen, J.; Zhu, H.; Zhao, Y.; Zheng, Y. Effect of 1-Methylcyclopropene on Chilling Injury and Quality of Peach Fruit during Cold Storage. J. Food Sci. 2011, 76, S485–S491. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Chen, S.; Xu, R.; Tu, S.; Tu, K. Interactions among Chilling Tolerance, Sucrose Degradation and Organic Acid Metabolism in UV-C-Irradiated Peach Fruit during Postharvest Cold Storage. Acta Physiol. Plant. 2019, 41, 79. [Google Scholar] [CrossRef]
- Zhu, J.; Li, C.; Fan, Y.; Qu, L.; Huang, R.; Liu, J.; Zhang, C.; Ge, Y. γ-Aminobutyric Acid Regulates Mitochondrial Energy Metabolism and Organic Acids Metabolism in Apples during Postharvest Ripening. Postharvest Biol. Technol. 2022, 186, 111846. [Google Scholar] [CrossRef]
- Fan, Y.; Li, C.; Zhu, J.; Sun, L.; Huang, R.; Guo, M.; Wu, Y.; Ge, Y. Organic Acids Metabolism and GABA Shunt Involved in Maintaining Quality of Malus Domestica by Methyl Jasmonate Treatment. Food Res. Int. 2022, 160, 111741. [Google Scholar] [CrossRef]
- Yao, Y.-X.; Dong, Q.-L.; Zhai, H.; You, C.-X.; Hao, Y.-J. The Functions of an Apple Cytosolic Malate Dehydrogenase Gene in Growth and Tolerance to Cold and Salt Stresses. Plant Physiol. Biochem. 2011, 49, 257–264. [Google Scholar] [CrossRef]
- Wei, Q.-J.; Ma, Q.-L.; Zhou, G.-F.; Liu, X.; Ma, Z.-Z.; Gu, Q.-Q. Identification of Genes Associated with Soluble Sugar and Organic Acid Accumulation in ‘Huapi’ Kumquat (Fortunella crassifolia Swingle) via Transcriptome Analysis. J. Sci. Food Agric. 2021, 101, 4321–4331. [Google Scholar] [CrossRef]
- Hurth, M.A.; Suh, S.J.; Kretzschmar, T.; Geis, T.; Bregante, M.; Gambale, F.; Martinoia, E.; Neuhaus, H.E. Impaired PH Homeostasis in Arabidopsis Lacking the Vacuolar Dicarboxylate Transporter and Analysis of Carboxylic Acid Transport across the Tonoplast. Plant Physiol. 2005, 137, 901–910. [Google Scholar] [CrossRef] [PubMed]



| Gene | Upstream Primer (5′–3′) | Downstream Primer (5′–3′) | Product Length (bp) |
|---|---|---|---|
| NADP-ME | TGCTTGCTTGCCTGTAAC | TCCTGTCCAGTAGCCCTC | 102 |
| NAD-MDH | TCCTTGTTACTGGAGCCG | AGAGGGAAAGCAGCATCG | 172 |
| PEPC | CACTCCAGCGTTTCACAG | GGACGACTCCCAATGTTC | 199 |
| tDT | CTGGCAGTGCTTGTTTGG | TGGATCTCCGCAGAATAG | 249 |
| 18S | GTTACTTTTAGGACTCCGCCA | ATTCCTTTAAGTTTCAGCCTTG | 97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Zhu, Z.; Han, Y.; Ji, S.; Cheng, S.; Zhou, Q.; Zhou, X.; Li, M.; Wei, B. Effects of 1-Methylcyclopropene Treatment on the Quality and Malic Acid Metabolism of ‘Xiangjiao’ Plum under Low-Temperature Storage. Horticulturae 2023, 9, 952. https://doi.org/10.3390/horticulturae9090952
Wu S, Zhu Z, Han Y, Ji S, Cheng S, Zhou Q, Zhou X, Li M, Wei B. Effects of 1-Methylcyclopropene Treatment on the Quality and Malic Acid Metabolism of ‘Xiangjiao’ Plum under Low-Temperature Storage. Horticulturae. 2023; 9(9):952. https://doi.org/10.3390/horticulturae9090952
Chicago/Turabian StyleWu, Shutong, Zhiqiang Zhu, Yunze Han, Shujuan Ji, Shunchang Cheng, Qian Zhou, Xin Zhou, Meilin Li, and Baodong Wei. 2023. "Effects of 1-Methylcyclopropene Treatment on the Quality and Malic Acid Metabolism of ‘Xiangjiao’ Plum under Low-Temperature Storage" Horticulturae 9, no. 9: 952. https://doi.org/10.3390/horticulturae9090952
APA StyleWu, S., Zhu, Z., Han, Y., Ji, S., Cheng, S., Zhou, Q., Zhou, X., Li, M., & Wei, B. (2023). Effects of 1-Methylcyclopropene Treatment on the Quality and Malic Acid Metabolism of ‘Xiangjiao’ Plum under Low-Temperature Storage. Horticulturae, 9(9), 952. https://doi.org/10.3390/horticulturae9090952







